Abstract
Objectives
Wound infection is the most common complication associated with percutaneous endoscopic gastrostomy (PEG) placement, with an incidence between 4% and 30%. In this study, we compared the characteristics of PEG site infection between the head and neck cancer (HNC) group and the non‐HNC group.
Methods
This study was conducted at Kangdong Sacred Heart Hospital at the Ilsong Head and Neck Cancer Center. We retrospectively collected and analyzed data on patients who underwent PEG insertion from October 2003 to May 2019 to evaluate the risk factors and microbiological etiologies of PEG site infection.
Results
A total of 316 (HNC group [n = 129] and non‐HNC group [n = 187]) patients undergoing PEG insertion were included in this study. Moreover, 67 episodes of PEG site infection were diagnosed, with an overall prevalence of 21.2%. PEG site infections were significantly higher in the HNC group than in the non‐HNC group (32.6% vs 13.4%, P <.001). Pseudomonas aeruginosa is the most common pathogen associated with a PEG site infection. Multidrug‐resistant (MDR) P aeruginosa was more frequent in the HNC group than in the non‐HNC group (78.6% vs 25.0%, P = .006).
Conclusions
For appropriate treatment, P aeruginosa, especially MDR P aeruginosa, should be considered when selecting empirical antibiotics for PEG site infection in patients with HNC.
Level of Evidence: 4
Keywords: head and neck cancer, multidrug resistance, percutaneous endoscopic gastrostomy site infection, Pseudomonas aeruginosa
1. INTRODUCTION
Percutaneous endoscopic gastrostomy (PEG) is the preferred route of feeding and nutritional support in patients with swallowing difficulties who require long‐term enteral nutrition. Head and neck cancer (HNC) is the sixth most common type of cancer, accounting for an estimated 650 000 new cancer cases and 350 000 cancer deaths worldwide every year. 1 More recently, the incidence of oropharyngeal cancer in the younger population has been increasing. 2 Swallowing difficulty in patients with HNC is a common complication, and as a result, PEG placement is more likely performed in HNC than in other diseases. 3 , 4
PEG site infection is the most common complication associated with PEG placement, with an incidence between 4% and 30%. 5 , 6 Infection prevention and prompt treatment using appropriate antimicrobial agents are recommended. In the era of multidrug‐resistant (MDR) bacteria, selecting an appropriate empirical antibiotic for the treatment of PEG infection is significantly important. Newer therapeutic options may be necessary based on local microbiology and susceptibility‐resistance patterns. Patients with HNC may have different risk factors and causes of infection due to chemotherapy and radiation therapy compared to patients with no HNC.
We conducted this study to investigate the nature of different PEG site infections in the HNC group compared to the non‐HNC group and identified the current microbiology of PEG site infections. The primary objective was to compare the incidence of PEG site infection between the HNC and non‐HNC groups. The secondary objectives were to identify the causative organism and susceptibility‐resistance patterns and to optimize therapeutic strategies.
2. MATERIALS AND METHODS
2.1. Study design and patients
This study was conducted at Kangdong Sacred Heart Hospital at the Ilsong Head and Neck Cancer Center, a university‐affiliated hospital with 640 beds in Seoul, South Korea. This retrospective review included patient medical records from October 2003 to May 2019 and microbiology laboratory databases. The case group included patients aged 20 years or older who were diagnosed with HNC and implanted with a PEG tube. The control group included patients with no HNC aged 20 years or older who were implanted with a PEG tube. In cases of repeat PEG site infection, only the initial episode was enrolled in the study.
The patient data collected included age, sex, underlying disease, severity of underlying diseases as classified by McCabe and Jackson criteria, 7 and any antimicrobial therapy prior to the onset of the infection. The presence of any of the following comorbid conditions was documented: chronic liver diseases, lung diseases, heart diseases, diabetes mellitus (DM), neurologic diseases, solid cancer, chronic renal diseases, neutropenia, recent surgical procedure within the prior 3 months, corticosteroid use within the prior 1 month, and immunosuppressive therapy within the prior 1 month. The severity of comorbid conditions was assessed based on the Charlson Comorbidity Index score. 8
Because the study was retrospective, the attending physician determined the indications for cultures, other tests, and treatments based on each patient's individual clinical status. The study was approved by the Institutional Review Board of the Kangdong Sacred Heart Hospital, Seoul, Korea. Written informed consent was not required because of the retrospective design of the study.
2.2. Insertion technique of percutaneous endoscopic gastrostomy
In our hospital, a “pull” technique was used to insert a PEG tube. 9 With the patients placed in the supine position, the fasting stomach was insufflated with air by endoscopy. The optical puncture position was confirmed endoscopically by transillumination and clear visualization of the stomach indentation by external palpitation on the marked point. A small incision was made with a surgical blade, and a 14‐G needle with a cannula was inserted through the abdominal wall. The guidewire was passed through the cannula. A snare was passed through the endoscope to catch the guidewire, which was brought out through the mouth. The PEG tube was then pulled through a marked point on the abdominal wall. The PEG tube was secured using an outer flange. Patients received tube feeding 24‐48 hours later.
2.3. Microbial identification and susceptibility testing
All cultures isolated from the peristomal wound were identified using the VITEK 2 automated system (bioMerieux Inc., Hazelwood, Missouri) in a microbiology laboratory. Antimicrobial susceptibility testing was performed using the gram‐negative identification and gram‐positive identification cards in a VITEK 2 automated system according to the Clinical and Laboratory Standards Institute guidelines. The susceptibility to each tested antimicrobial agent in the antibiogram was reported as susceptible, intermediate, or resistant. Resistance was defined as testing nonsusceptible (reported as resistant or intermediate).
2.4. Definitions
PEG site infection was defined as peristomal erythema and/or purulent discharge with positive microbiological evidence of wound culture. Early PEG site infection was defined as infection within day 7 after PEG insertion, and late PEG site infection was defined as infection 7 days after PEG insertion. Steroid use was defined as daily use of at least 20 mg of prednisone for at least 2 weeks. Immunocompromised patients included those who had undergone immunosuppressive treatments (chemotherapy, radiation therapy, or immunosuppressive agent exposure). Neutropenia was defined as an absolute neutrophil count <500/mm3. Prior antibiotic therapy was defined as the receipt of any systemic antibiotics more than 48 hours in the preceding 30 days. Gram‐negative isolates that have acquired nonsusceptibility to at least one agent in three or more antimicrobial categories are considered MDR. 10 , 11
2.5. Statistical analyses
Normally distributed continuous variables are reported as mean ± SD and compared using Student's t‐test. Non‐normally distributed continuous variables are reported as medians with interquartile ranges and compared using the Mann‐Whitney U test. Categorical variables are reported as percentages and compared using chi‐squared tests or Fisher's exact test, as appropriate. Variables with P values <.05 in the univariate analyses and clinically significant factors were candidates for inclusion in the multivariate analysis. Odds ratios were calculated at 95% confidence intervals. All reported P values were two‐tailed, and P <.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences version 26 (IBM Corp., Armonk, New York).
3. RESULTS
3.1. Clinical characteristics of the study population
During the study period, 316 (187 with no HNC and 129 with HNC) patients had undergone PEG insertion. Baseline patient characteristics are shown in Table 1. The mean ages of the patients were 67.33 ± 14.71 and 59.75 ± 11.00 years in the non‐HNC group and the HNC group, respectively. There were 113 (60.4%) and 106 (82.2%) male patients in the non‐HNC group and the HNC group (P <.001), respectively.
TABLE 1.
Baseline characteristics and demographics of patients who underwent percutaneous endoscopic gastrostomy tube insertion
| Variables | Nonhead and neck cancer | Head and neck cancer | Total | P |
|---|---|---|---|---|
| n = 187 (%) | n = 129 (%) | n = 316 (%) | ||
| Male | 113 (60.4) | 106 (82.2) | 219 (69.3) | <.001 |
| Age (years), mean ± SD | 67.33 ± 14.71 | 59.75 ± 11.00 | 64.24 ± 13.81 | <.001 |
| Underlying disease | ||||
| Neurologic diseases | 128 (68.4) | 8 (6.2) | 166 (52.5) | .000 |
| Solid cancer | 15 (8.0) | 129 (100) | 144 (52.5) | .000 |
| Diabetes mellitus | 50 (26.7) | 22 (17.1) | 72 (22.8) | .044 |
| Heart diseases | 24 (12.8) | 5 (3.9) | 29 (9.2) | .007 |
| Chronic lung diseases | 10 (5.3) | 3 (2.3) | 13 (4.1) | .184 |
| Chronic renal diseases | 10 (5.3) | 3 (2.3) | 13 (4.1) | .184 |
| Chronic liver diseases | 4 (2.1) | 1 (0.8) | 5 (1.5) | .652 |
| PEG site infection | 25 (13.4) | 42 (32.6) | 67 (21.2) | <.001 |
| Early infection | 8 (32.0) | 11 (26.2) | 19 (28.4) | .610 |
| Late infection | 17 (68.0) | 31 (73.8) | 48 (71.6) | |
| Prophylactic antibiotics | 180 (96.3) | 124 (96.1) | 304 (96.2) | 1.000 |
| Prior antibiotic therapy | 89 (47.6) | 99 (75.2) | 188 (59.5) | <.001 |
| Penicillin | 47 (25.1) | 43 (33.3) | 90 (28.5) | .128 |
| Cephalosporin | 14 (7.5) | 28 (21.7) | 42 (13.3) | <.001 |
| Fluoroquinolone | 22 (11.8) | 18 (14.0) | 40 (12.7) | .565 |
| Carbapenems a | 16 (8.6) | 14 (10.9) | 30 (9.5) | .494 |
| Glycopeptide | 16 (8.6) | 20 (15.5) | 36 (11.4) | .056 |
Note: Values are presented as no. (%) unless otherwise indicated.
Abbreviations: PEG, percutaneous endoscopic gastrostomy; SD, standard deviation.
Includes imipenem and meropenem.
Among the underlying diseases, neurologic diseases, DM, and heart diseases were significantly higher in the non‐HNC group, which was associated with a higher incidence of neurologic diseases in the non‐HNC group (68.4%), than in the HNC group. There were no differences in lung diseases, liver diseases, and renal diseases between patients with and without HNC (P >.05). First‐generation cephalosporin was the most commonly used prophylactic antibiotic (59.4% in the non‐HNC group vs 35.7% in the HNC group). The use of another class of antibiotics for prophylactic treatment was higher in the HNC group than in the non‐HNC group (59.7% vs 32.6%). Since patients on antibiotics for other indications maintained the same antibiotics, administration of prior antibiotics was significantly higher in the HNC group than in the non‐HNC group (75.2% vs 47.6%).
3.2. Characteristics of infection and antimicrobial susceptibility
A total of 67 episodes of PEG site infection were diagnosed during the study period. Table 1 shows the comparison incidence of PEG site infection between the HNC and non‐HNC groups. The overall prevalence of PEG site infection was 21.2%. PEG site infections were significantly higher in the HNC group than in the non‐HNC group (32.6% vs 13.4%, P <.001). Only 19 (28.4%) infections at the PEG site occurred within 7 days (26.2% in the HNC group vs 32.0% in the non‐HNC group). There was no mortality related to PEG site infection.
Clinical characteristics were compared according to the presence or absence of HNC among patients with PEG site infection (Table 2). The age of the HNC group was significantly lower than that of the non‐HNC group (P = .001). Neurologic diseases were more frequent in the non‐HCN group than in the HCN group. The Charlson Comorbidity Index score, an index of underlying disease severity, was also higher in the non‐HNC group than in the HNC group (Table 2). However, multivariate analysis was performed, and there were no significant differences between the two groups, except for neurologic diseases (Table 2).
TABLE 2.
Clinical characteristics of patients with percutaneous endoscopic gastrostomy site infection with and without head and neck cancer
| Variables | Nonhead and neck cancer | Head and neck cancer | Total | P | Adjusted ORs (95% CI) | P |
|---|---|---|---|---|---|---|
| n = 25 (%) | n = 42 (%) | n = 67 (%) | ||||
| Male | 17 (68.0) | 33 (78.6) | 50 (74.6) | .391 | ||
| Age (years), mean ± SD | 64.10 ± 14.00 | 58.74 ± 8.02 |
62.90 ± 11.86 |
.001 | 0.932 (0.859‐1.012) | .093 |
| Underlying disease | ||||||
| Neurologic diseases | 21 (84.0) | 1 (2.4) | 22 (32.8) | <0.001 | 0.023 (0.002‐0.324) | .005 |
| Solid cancer | 2 (8.0) | 42 (100) | 44 (65.6) | <0.001 | ||
| Diabetes mellitus | 6 (24.0) | 4 (9.5) | 10 (14.9) | .157 | ||
| Heart diseases | 4 (16.0) | 2 (4.8) | 6 (9.0) | .186 | ||
| Chronic lung diseases | 2 (8.0) | 0 (0.0) | 2 (3.0) | .136 | ||
| Chronic renal diseases | 2 (8.0) | 3 (2.3%) | 13 (4.1) | .184 | ||
| Chronic liver diseases | 0 (0.0) | 1 (2.4) | 1 (1.5) | 1.000 | ||
| Charlson Comorbidity Index score | 3.04 ± 1.24 | 1.48 ± 0.74 | 2.06 ± 1.22 | <0.001 | 0.451 (0.191‐1.068) | .070 |
| Late infection | 17 (68.0) | 31 (73.8) | 48 (71.6) | .780 | ||
| Prophylactic antibiotics | 23 (92.0) | 41 (97.6) | 64 (95.5) | .230 | ||
| Prior antibiotic therapy | 14 (56.0) | 33 (78.6) | 47 (70.1) | .051 | 1.598 (0.288‐8.854) | .591 |
| Penicillin | 4 (16.0) | 15 (35.7) | 19 (24.8) | .083 | ||
| Cephalosporin | 3 (12.0) | 10 (23.8) | 13 (19.4) | .237 | ||
| Fluoroquinolone | 6 (24.0) | 5 (11.9) | 11 (16.4) | .196 | ||
| Carbapenems a | 3 (12.0) | 5 (11.9) | 8 (11.9) | .991 | ||
| Glycopeptide b | 5 (20.0 | 8 (19.0) | 13 (19.4) | .924 |
Note: Values are presented as no. (%) unless otherwise indicated.
Abbreviations: CI, confidence interval; OR, odds ratio; SD, standard deviation.
Includes imipenem, meropenem.
Includes vancomycin and teicoplanin.
Prior antibiotics also tended to be significantly administered to the HNC group, but the difference was not statistically significant (78.6% in the HNC group vs 56.0% in the non‐HNC group, P = .051).
Among the 67 patients with PEG site infection, 15 had polymicrobial infection. The microbiological details of these infections are summarized in Table 3. A total of 82 organisms were isolated from these 67 patients. The dominant pathogens were Pseudomonas aeruginosa (n = 25) followed by Klebsiella pneumoniae (n = 21) and Staphylococcus aureus (n = 16). Of the 16 S aureus isolates, 14 were methicillin‐resistant S aureus (MRSA). There were no differences in the pathogens of PEG site infection between the two groups (Table 3).
TABLE 3.
Major microbiological details of percutaneous endoscopic gastrostomy site infection with and without head and neck cancer
| Bacterial agents | Nonhead and neck cancer | Head and neck cancer | P |
|---|---|---|---|
| n = 25 (%) | n = 42 (%) | ||
| Pseudomonas aeruginosa | 11 (44.0) | 14 (33.3) | .383 |
| Klebsiella pneumonia | 10 (40.0) | 11 (26.2) | .239 |
| Staphylococcus aureus | 4 (16.0) | 12 (28.6) | .375 |
| Acinetobacter baumannii | 4 (16.0) | 3 (7.1) | .411 |
| Escherichia coli | 2 (8.0) | 2 (4.8) | .626 |
| Serratia sp. | 1 (4.0) | 2 (4.8) | 1.000 |
| Enterobacter spp. | 1 (4.0) | 2 (4.8) | 1.000 |
| Enterococcus faecium | 1 (4.0) | 1 (2.4) | 1.000 |
| Streptococcus | 0 (0.0) | 1 (2.4) | 1.000 |
Note: Values are presented as no. (%) unless otherwise indicated. Polymicrobial infection was included in each pathogen number.
The antimicrobial susceptibility of gram‐negative bacteria in the HNC and non‐HNC groups is shown in Figure 1. Among all identified gram‐negative bacteria, 31 were MDR organisms (overall 33.3%; 52.8% of PEG site infection in HNC, 36.4% of PEG site infection in non‐HNC, P = .207), of which P aeruginosa (45.2%) was the most prevalent pathogen among the MDR isolates. When the antimicrobial susceptibility to gram‐negative pathogens was compared with the HNC and non‐HNC groups, the resistance rates to meropenem and imipenem were significantly higher in the HNC group than in the non‐HNC group (Figure 1).
FIGURE 1.
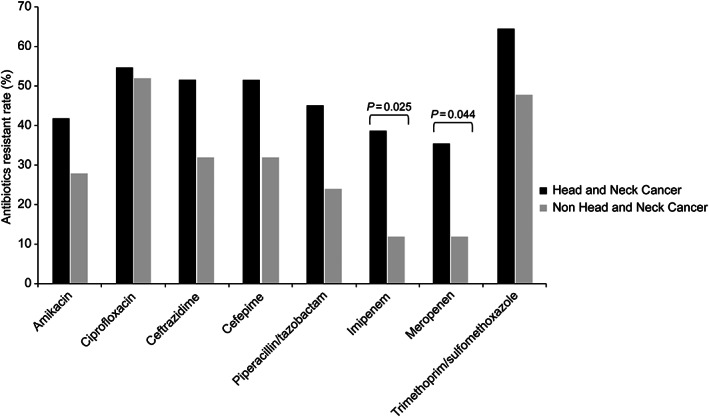
Antimicrobial susceptibility of gram‐negative bacteria in the head and neck cancer group and the nonhead and neck cancer group
The antimicrobial susceptibility patterns of P aeruginosa isolates are shown in Figure 2. Of the 25 clinical isolates of P aeruginosa, the overall drug resistance to all antipseudomonal drugs tested was higher in the HNC group than in the non‐HNC group (amikacin 57.1% vs 16.7%, ceftazidime 64.3% vs 16.7%, ciprofloxacin 71.4% vs 50.0%, cefepime 71.4% vs 25.0%, meropenem 57.1% vs 16.7%, imipenem 57.1% vs 16.7%, piperacillin/tazobactam 64.3% vs 8.3%). In P aeruginosa, antibiotic resistance was significantly higher in the HNC group than in the non‐HNC group (Figure 2). MDR P aeruginosa was more frequent in the HNC group than in the non‐HNC group (78.6% vs 25.0%, P = .006). Only 47.6% of the patients with HNC received proper antibiotics for empirical treatment.
FIGURE 2.
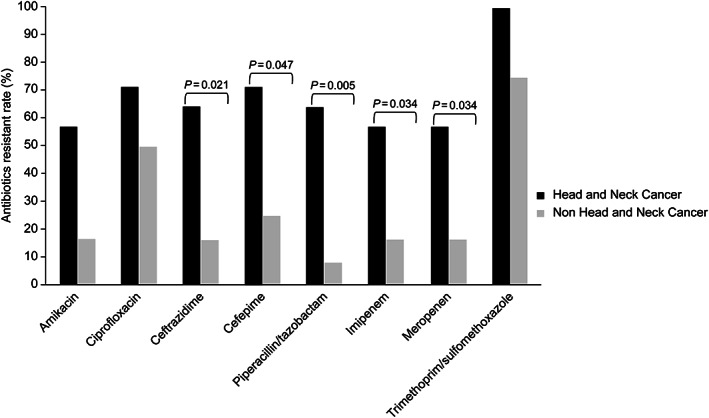
Comparison of antimicrobial resistance of Pseudomonas aeruginosa between the head and neck cancer and nonhead and neck cancer groups
4. DISCUSSION
This retrospective study of PEG site infection at a single center over a period of 16 years found that there was a significant difference on sexual distribution between the two groups (P <.001), because males generally had a higher incidence of HNC than females (male‐to‐female ratios ranging from 4:1 to 2:1). 12 This study found that the overall incidence rate of infection was 21.2%. PEG site infections were significantly higher in the HNC group than in the non‐HNC group (13.4% non‐HNC group vs 32.6% HNC group). P aeruginosa, K pneumoniae, and MRSA were the major causative agents in the study population. Of all gram‐negative isolates, no significant difference was observed in the antimicrobial resistant rate between the two groups, except for imipenem and meropenem (P = .025 and P = .044, respectively). However, although there was no statistical significance between the two groups, the antimicrobial resistance rate was higher in the HNC group than in the non‐HNC group. PEG wound isolates included P aeruginosa (33.3%), S aureus (28.6%) and K pneumoniae (26.2%) in patients with HNC. The high prevalence of MDR pathogens, especially P aeruginosa, in patients with HNC was one of the most important findings of this study. Prior antibiotic use was significantly higher in the HNC group than in the non‐HNC group (75.2% vs 47.6%). The use of broad‐spectrum antibiotics before PEG insertion could explain the high prevalence of MDR pathogens in the HNC group.
A single dose of cephalosporin was recommended as a first‐choice prophylaxis by the British Society of Gastroenterology. 13 , 14 Cephalosporin is not susceptible to P aeruginosa and MRSA, which are common pathogens of PEG site infection in this study. However, only 28.4% of patients (32.2% in the non‐HNC group vs 26.2% in the HNC group) developed PEG site infection within 7 days after PEG insertion. This indicates that most infections were not procedure‐related. For this reason, it is better to consider patient‐related factors than change a single dose of cephalosporin for broad‐spectrum prophylactic antibiotics to prevent and treat PEG site infection.
Peristomal infection following PEG insertion is associated with several risk factors, such as underlying malignant disease, institutional factors, experience of the endoscopic team, and PEG tube size. 15 Age, DM, smoking, poor hygiene, malnutrition, and change in immunity due to chemoradiotherapy are patient‐related factors that could influence PEG site infections. Many of these factors are present in patients with HNC. 16
Gram‐positive bacteria remain the predominant pathogens isolated from surgical site infection. It is well known that S aureus is the most commonly implicated organism in PEG site infection. 17 , 18 P aeruginosa was also a common pathogen of PEG site infection in previous studies. 19 , 20 , 21 In our study, gram‐negative bacteria, especially P aeruginosa, were more prominent. Of the 42 patients with HNC with PEG infection, 32 (76.2%) were considered to have a late infection. Moreover, 37.5% of these patients had isolated P aeruginosa, but only 21.4% of these patients had proper antibiotics as empirical treatment. This result supports the necessity of antipseudomonal coverage for PEG site infections in patients with HNC.
This study has several limitations. First, due to the retrospective nature of the study, the possibility of a limitation in precluding accurate comparisons should be considered. The present study was observational; thus, unknown factors might have been unequally distributed between the two groups. Second, we defined PEG site infection only when causative bacteria were identified. It is possible that there was a selection bias according to our definition of PEG site infection. There may be a PEG site infection clinically, but it is possible that it was excluded from the infection case because the causative pathogen was not identified. Third, this was a 16‐year retrospective study with a limited study population. Thus, despite our efforts, a selection bias is possible. Fourth, we conducted the study based on microbiological culture results, but some isolates might have been due to colonization.
5. CONCLUSION
We found that PEG site infection was more prominent in patients with HNC than in patients with no HNC. Different from other surgical site infections, gram‐negative bacteria, especially P aeruginosa, are the major causative pathogens of PEG site infection in patients with HNC. PEG site infections caused by MDR pathogens were common, especially P aeruginosa, in the HNC group, which received inappropriate therapy more frequently. It is better to initiate broad‐spectrum empirical therapy followed by de‐escalation if it is considered a late PEG site infection in the HNC group. Clinicians' judgment is necessary in the identification of patients who should receive broad‐spectrum antibiotics concerning MDR‐Pseudomonas based on locally obtained microbiological data.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Oh J, Park SY, Lee JS, Park J‐Y, Lee SH. Clinical characteristics and pathogens in percutaneous endoscopic gastrostomy site infection in patients with head and neck cancer: A 16‐year retrospective study. Laryngoscope Investigative Otolaryngology. 2021;6(6):1325‐1331. doi: 10.1002/lio2.666
BIBLIOGRAPHY
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 2. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489‐501. [DOI] [PubMed] [Google Scholar]
- 3. Atasoy BM, Yonal O, Demirel B, et al. The impact of early percutaneous endoscopic gastrostomy placement on treatment completeness and nutritional status in locally advanced head and neck cancer patients receiving chemoradiotherapy. Eur Arch Otorhinolaryngol. 2012;269:275‐282. [DOI] [PubMed] [Google Scholar]
- 4. Arends J, Bodoky G, Bozzetti F, et al. ESPEN guidelines on enteral nutrition: non‐surgical oncology. Clin Nutr. 2006;25:245‐259. [DOI] [PubMed] [Google Scholar]
- 5. Safadi BY, Marks JM, Ponsky JL. Percutaneous endoscopic gastrostomy. Gastrointest Endosc Clin N Am. 1998;8:551‐568. [PubMed] [Google Scholar]
- 6. Ahmad I, Mouncher A, Abdoolah A, et al. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy—a prospective, randomised, double‐blind trial. Aliment Pharmacol Ther. 2003;18:209‐215. [DOI] [PubMed] [Google Scholar]
- 7. Perl TM, Dvorak L, Hwang T, Wenzel RP. Long‐term survival and function after suspected gram‐negative sepsis. JAMA. 1995;274:338‐345. [PubMed] [Google Scholar]
- 8. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 9. Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15:872‐875. [DOI] [PubMed] [Google Scholar]
- 10. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268‐281. [DOI] [PubMed] [Google Scholar]
- 11. Hirsch EB, Tam VH. Impact of multidrug‐resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev Pharmacoecon Outcomes Res. 2010;10:441‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perdomo S, Martin Roa G, Brennan P, Forman D, Sierra MS. Head and neck cancer burden and preventive measures in Central and South America. Cancer Epidemiol. 2016;44(Suppl 1):S43‐s52. [DOI] [PubMed] [Google Scholar]
- 13. Khashab MA, Chithadi KV, Acosta RD, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81‐89. [DOI] [PubMed] [Google Scholar]
- 14. Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58:869‐880. [DOI] [PubMed] [Google Scholar]
- 15. Zopf Y, Konturek P, Nuernberger A, et al. Local infection after placement of percutaneous endoscopic gastrostomy tubes: a prospective study evaluating risk factors. Can J Gastroenterol. 2008;22:987‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rolston KV, Mihu C, Tarrand JJ. Current microbiology of percutaneous endoscopic gastrostomy tube (PEG tube) insertion site infections in patients with cancer. Support Care Cancer. 2011;19:1267‐1271. [DOI] [PubMed] [Google Scholar]
- 17. Hull M, Beane A, Bowen J, Settle C. Methicillin‐resistant Staphylococcus aureus infection of percutaneous endoscopic gastrostomy sites. Aliment Pharmacol Ther. 2001;15:1883‐1888. [DOI] [PubMed] [Google Scholar]
- 18. Mainie I, Loughrey A, Watson J, Tham TC. Percutaneous endoscopic gastrostomy sites infected by methicillin‐resistant Staphylococcus aureus: impact on outcome. J Clin Gastroenterol. 2006;40:297‐300. [DOI] [PubMed] [Google Scholar]
- 19. Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14:45‐51. [DOI] [PubMed] [Google Scholar]
- 20. Vizhi K, Rao HB, Venu RP. Percutaneous endoscopic gastrostomy site infections‐Incidence and risk factors. Indian J Gastroenterol. 2018;37:103‐107. [DOI] [PubMed] [Google Scholar]
- 21. Krishna S, Singh S, Dinesh KR, Kp R, Siyad I, Karim S. Percutaneous endoscopic gastrostomy (PEG) site infections: a clinical and microbiological study from university teaching hospital, India. J Infect Prev. 2015;16:113‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]


