Abstract
Chitins and chitosans are among the most widespread and versatile functional biopolymers, with interesting biological activities and superior material properties. While chitins are evolutionary ancient and present in many eukaryotes except for higher plants and mammals, the natural distribution of chitosans, i.e. extensively deacetylated derivatives of chitin, is more limited. Unequivocal evidence for its presence is only available for fungi where chitosans are produced from chitin by the action of chitin deacetylases. However, neither the structural details such as fraction and pattern of acetylation nor the physiological roles of natural chitosans are known at present. We hypothesise that the chitin deacetylases are generating chitins and chitosans with specific acetylation patterns and that these provide information for the interaction with specific chitin- and chitosan-binding proteins. These may be structural proteins involved in the assembly of the complex chitin- and chitosan-containing matrices such as fungal cell walls and insect cuticles, chitin- and chitosan-modifying and -degrading enzymes such as chitin deacetylases, chitinases, and chitosanases, but also chitin- and chitosan-recognising receptors of the innate immune systems of plants, animals, and humans. The acetylation pattern, thus, may constitute a kind of ‘ChitoCode’, and we are convinced that new in silico, in vitro, and in situ analytical tools as well as new synthetic methods of enzyme biotechnology and organic synthesis are currently offering an unprecedented opportunity to decipher this code. We anticipate a deeper understanding of the biology of chitin- and chitosan-containing matrices, including their synthesis, assembly, mineralisation, degradation, and perception. This in turn will improve chitin and chitosan biotechnology and the development of reliable chitin- and chitosan-based products and applications, e.g. in medicine and agriculture, food and feed sciences, as well as cosmetics and material sciences.
Chitosans as a versatile family of functional biopolymers
Chitins and, in particular, chitosans, their partially deacetylated derivatives, are among the most versatile functional biopolymers. In nature, they are integral parts of complex matrices, such as arthropod cuticles or fungal cell walls, which can be highly flexible or extremely durable, even translucent or bioactive, making them outstanding biomaterials. They have gel-, film-, and fibre-forming properties; they can be nano-formulated into nanoparticles, nanocapsules, or nanofibers; they have metal ion- and protein-binding capacities; they have antibacterial and antifungal activities; they can promote plant growth and development; they can induce plant disease resistance and abiotic stress tolerance; they have blood-coagulating and wound healing properties, etc. In different countries, they are already being used for waste and drinking water purification or the clearing of beverages such as juices, beer, and wine; as a cosmetics and health care ingredient; as a probiotic animal feed additive or human food supplement; as a plant strengthener or biopesticide; and as haemostatic or wound-healing dressing in veterinary or human medicine. Potential future uses in different stages of development are as pharmaceutical drug, gene, or vaccine delivery vehicle and diverse other medical applications, e.g. in cancer treatment. Other promising uses would be possible e.g. in the paper and textile industries or as a bioplastic, but these would require larger scale production facilities than currently available. And: chitosans are environment-friendly and consumer-safe, non-toxic and non-allergenic, biodegradable and biocompatible in many systems [1].
Of course, not all chitosans possess all of these properties and bioactivities. The functional diversity is based on an equally broad structural diversity. Chitosans are linear co-polymers of glucosamine (GlcN) and N-acetylglucosamine (GlcNAc) residues, differing in (i) their degree of polymerisation (DP), i.e. the number of monomeric units in the molecule, (ii) their fraction of acetylation (FA), i.e. the relative abundance of GlcNAc residues, and (iii) their pattern of acetylation (PA), i.e. the sequence of GlcN and GlcNAc residues within the chain. All three parameters are known to deeply influence the physico-chemical properties and biological activities of the chitosans [2, 3]. In fact, the solubility in slightly acidic media (below ca. pH 6) is generally accepted as the defining criterion for the distinction between acid-insoluble chitins and acid-soluble chitosans. Importantly, chitosan samples are always mixtures of molecules more or less strongly differing in these parameters, so that their dispersities (Đ) will also significantly influence their functionalities [4].
‘First generation’ commercial chitosans were poorly defined mixtures of chitosans with often large batch-to-batch variability, but research of the past decades enabled the production of ‘second generation’ chitosans which are well-defined in terms of DP and FA, and have known random PA [5–8]. And at lab scale, even better defined, less disperse chitosans with non-random PAs are beginning to be reported, e.g. based on biotechnological production or modification processes, raising expectations for ‘third generation’ chitosans with further improved functionalities [2, 3, 9, 10].
Why studying fungal chitosans?
The market availability of second generation chitosans with well-defined properties and functionalities and low batch-to-batch variability has led to a surge in market interest so that for the first time, demands are exceeding supplies [11]. However, chitosan production from crustacean chitin is limited by the availability of shell wastes [12, 13]. Fungal cell walls are an interesting additional source of chitin, available in more constant quality with no seasonal variation from almost sterile waste streams of the food and biotechnology industries and, at least potentially, in a more sustainable way [14]. An additional advantage of fungal chitin over shellfish chitin is its non-animal origin which is preferred for e.g. cosmetic applications or by customers who follow a vegan lifestyle.
The main drawback of fungi as a source of chitin is the fact that in fungal cell walls, chitin is covalently linked to glucans, making extraction more demanding and the product, at least potentially, less pure [15]. Clearly, more research into the biosynthesis, cross-linking, and degradation of chitin in fungal cell walls is required as a basis to develop yield- and cost-efficient protocols for its extraction [16, 17]. And of course, a fundamental understanding of chitin metabolism and its physiological role in the growth and differentiation of fungi is an exciting scientific goal in itself.
But fungi cannot only serve as a source of chitin for chemical conversion into chitosan. Fungi are also the only confirmed source of natural chitosans [18, 19]. This biogenic chitosan is believed to originate from chitin by the action of chitin deacetylases acting in concert with chitin synthases [20]. And it is highly likely that enzymatic deacetylation yields chitosans with different, specific patterns of acetylation, while chemically produced chitosans are invariably characterised by random acetylation patterns. As the distribution of acetyl groups along the linear chitosan chain has recently been proven to critically influence the physico-chemical properties and biological activities of chitosan oligomers and polymers [9, 10, 21–23], the study of natural chitosans and their original in muro pattern of acetylation becomes highly promising, potentially opening up completely new possibilities for chitosan-based applications [2, 3].
And of course again, aiming at a fundamental understanding of fungal chitosans is an exciting and promising research area in itself. Why do some fungi under some conditions convert some of their chitin into chitosans? And how do they do it? Why do chitin deacetylases typically occur in multigene families [24, 25]? What is the DP, FA, and PA of these natural chitosans? What are their physico-chemical properties and how do they contribute to the properties of the fungal cell walls? What is the physiological role of these chitosans in growth or morphogenesis of the fungi, or in the interaction with e.g. host organisms? Plenty of questions which await answers.
Three advances of research in the last decade
Bioanalytics and bioinformatics offer new tools to study and model structures and functions of chitosans
The progress from first to second generation chitosans was largely driven by the improvement of analytical tools for their structural characterisation: HPSEC-RI-MALLS for DP and ĐDP determination, 1H- and 13C-NMR for FA and PA analysis [5, 26–28]. Currently, new methods are being developed, most prominently based on mass spectrometry (MS), first for chitosan oligomers, and then, in the form of enzymatic-mass spectrometric fingerprinting (EMS-FP), also for chitosan polymers [4, 29, 30]. MS allows the accurate determination of DP and FA of chitosan oligomers up to a DP of ca. 15, and of PA up to DP 6, for the entire range of FA-values [29]. Also, MS techniques automatically give insight into the dispersities in both DP (ĐDP) and FA (ĐFA), so that mixtures of chitosan oligomers can be comprehensively described, as long as they are not too complex and the DP is not too high [31]. EMS-FP, i.e. the MS analysis of chitosan oligomer mixtures obtained upon enzymatic hydrolysis of a chitosan polymer using enzymes with known subsite preferences, allows a more accurate determination of FA and a more detailed analysis of PA than can be achieved using NMR, and this even with sub-microgram quantities of material compared to multi-milligram amounts required for NMR [10, 30, 32]. Capillary electrophoresis (CE) emerges as a potential tool to analyse ĐFA of chitosan polymer samples, a parameter that was hitherto not accessible to any analytical technique [33, 34]. Solid state NMR allows non-destructive insights into complex biological matrices into which chitin is intrinsically embedded, such as fungal cell walls or insect cuticles [35–40]. Unexpectedly, these studies revealed a significant role of α-1,3-glucans and, at least in Aspergillus fumigatus, of galactomannans in the rigid chitin/ß-1,3-glucan complex of the fungal cell wall. Interestingly, depleting the cell wall of galactomannans led to a five-fold increase in chitin content [41, 42], an observation of potentially significant impact for the biotechnological production of fungal chitin and chitosan, given that the chitin content in fungal cell walls rarely exceeds 15%.
These improvements in methods of analytical biochemistry are paralleled and supported by tremendous advances in bioinformatic tools. Among them, one of the most fascinating is the ab initio modelling of protein structures using the program AlphaFold which allows the accurate prediction of the 3D structures with atomic accuracy even of proteins for which no crystal structures exist, as a basis for docking and molecular dynamics studies for e.g. chitin- or chitosan-protein interactions [43]. These are relevant for our understanding of the biosynthesis and assembly of chitin/chitosan-containing biological matrices as well as their enzymatic modification and biodegradation and, eventually, the interaction of oligomeric breakdown products with targets and receptors e.g. of the immune systems of plants and animals [44–48].
Solid state synthesis and recombinant enzymes offer access to fully defined chitosan oligomers
This progress in analytical tools is currently driving the development of even better characterised third generation chitosans, either biotechnologically using enzymes or chemically using automated solid-state synthesis.
The biotechnological approach makes use of the increasing number of well-characterised, regio-selective chitin deacetylases and sequence-dependent chitosan hydrolases [49–55]. The latter can yield mixtures of chitosan oligomers with partially defined acetylation patterns, as the subsite preferences of the hydrolases determine the residues at and near the reducing and non-reducing ends of the oligomeric products. The former, acting in forward or reverse mode, can yield fully defined, partially acetylated chitosan oligomers [22]. Interestingly, these enzymes can also convert high-FA chitosan polymers into low-FA chitosans or, in reverse mode, polyglucosamines into low-FA-, then also high-FA chitosan polymers with, depending on the enzyme used, different PAs [9, 10].
By sequentially adding monosaccharide building blocks to a solid support, automated glycan synthesis allows the production of practically any chitosan oligomer with fully defined sequence, limited only by solubility issues particularly of high-DP, high-FA oligomers [56, 57].
Unlike the enzymatic approach which is limited in the oligomers that can be targeted by the sequence preferences of the (still few) available enzymes, chemical synthesis can yield any oligomer. On the other hand, solid-state synthesis only yields low amounts of products in the mg-range, while enzymatic production processes can be scaled up, potentially to yield kg-amounts.
Physicochemical properties and biological activities of chitosans are influenced by the pattern of acetylation
Theoretical considerations had long predicted that PA should play a crucial role in determining both physico-chemical properties and biological activities of chitosans, and first experimental evidence starts accumulating to verify this hypothesis.
The physico-chemical properties of chitosan polymers have been predicted to be influenced by PA based on comparison with other natural biopolymers or synthetic copolymers in which the pattern of substitution or the monomer sequence is known to influence e.g. solubility, phase behaviour, solution rheology, gelling, crystallinity, or conformation. Studies using chitosan polymers prepared using recombinant chitin deacetylases and, therefore, differing in their PA now showed that PA indeed influences the rheological properties of chitosans, including their suitability for nano-formulation [9, 10].
The influence of PA on biological activities of chitosans has been predicted based on the assumption that the sequence of more hydrophobic GlcNAc and positively charged GlcN residues will determine their interaction with hydrophobic and negatively charged patches on the surface of proteins [58]. These might be chitinases or chitosanases processing the chitosan polymers applied to a target tissue, yielding specific oligomeric products. And it was recently shown that the bioactivity of chitosan oligomers depends on their monomer sequence [21, 23, 44, 59]. The reason for this PA dependency of biological activities of chitosans presumably lies in the presence of chitosan-specific receptors involved in the cellular perception of chitosans e.g. by the immune system of animals and plants [60].
Three areas ripe for development
Biotechnological production of chitosans in biorefineries and cell factories
Waste streams of the fisheries and food production are limited resources that cannot be expected to satisfy the increasing demand for well-defined chitosans [12]. There are a plethora of markets, with different requirements concerning quality and purity, different price sensitivities, and different needs in terms of volumes. While only rather small amounts (perhaps in the range of tens of kg/a) will be required for biomedical, pharmaceutical, or biocosmetic applications, the amounts required for agricultural purposes or food and feed applications are much higher (in the range of hundreds of t/a), and the demands for material applications such as for paper and textile finishing, bioplastics, or green batteries are completely beyond reach even if fungal and insect waste streams would be exploited (in the range of thousands of t/a, a single paper factory may require roughly the current global annual chitosan production of perhaps 5,000 t/a). Consequently, biotechnological production processes will be needed, also to protect biological sources from over-exploitation.
Chitosan polymers may best be produced by fungal fermentation given that fungi are natural producers of chitosans, but fungal chitosan is covalently embedded into the cell wall, making extraction difficult [15]. Genetic engineering may be employed to reduce or eliminate chitin-glucan crosslinks and the mutants might still be viable under the controlled conditions of a fermenter but so far, this approach has not yet been successful [61]. Alternatively, organisms not naturally producing chitin or chitosan may be developed into transgenic production systems secreting the polymers into the medium. Again, this has not yet been achieved at any reasonable scale [62]. Still, giving our increasing knowledge on chitin synthases and chitin deacetylases, both approaches would seem like challenges that can be met within the next decade.
Biotechnological production of defined chitosan oligomers might be more easily achieved, using microbial cell factories. Proof of principle for this approach was already shown decades ago with the recombinant expression of a bacterial chitin oligomer synthase yielding chitin pentamer in E. coli [63]. Co-expression with a bacterial chitin deacetylase yielded the mono-deacetylated chitosan pentamer with the GlcN unit exclusively located at the non-reducing end [64]. Varying or engineering the enzymes expressed might yield chitin and chitosan oligomers with different DP, FA, and PA. Eventually, this approach could give access to monoclonal chitosan oligosaccharides, and the process would be rather easily scalable [65].
Interactions of bioactive chitosans with receptors and targets to understand their modes of action
Two different hypotheses have been put forward to explain the modes of action of bioactive chitosans, the ‘receptor hypothesis’ and the ‘target hypothesis’. Chitosan oligomers are likely to be recognised by specific receptors, inducing specific cellular responses, whereas polymers may rather interact non-specifically with target structures, such as polyanionic cell surfaces, unless they are quickly degraded to oligomers [44, 58, 66]. The antimicrobial activities of chitosan polymers appear to rely on target interactions with e.g. teichoic acids of Gram-positive bacteria or phospholipid membranes of fungal cells [67, 68]. However, it is unclear why such an effect seems to be lacking in other eukaryotic cells. In both plant and human cells, chitin oligomer receptors or binding proteins have been described, such as CERK1/LYK4/LYK5/CEBiP in Arabidopsis and rice plants or TLR2 in human cells, but a chitosan oligomer receptor is still unknown, though likely to exist [44, 45, 69–71]. The availability of structurally well-defined chitosan oligomers and polymers with non-random PA will enable studies into the molecular and cellular modes of action of chitosans in these different biological systems. And this fundamental knowledge will eventually lead to more specifically targeted and more reliably effective biotechnological applications of chitosans in many fields, including biomedical and pharmaceutical applications, human food and animal feed applications, biopesticide and biostimulant agricultural applications, etc.
Biosynthesis, assembly, biodegradation, and structure–function relationships of chitin/chitosan-containing biomaterials
While chitin-containing biomaterials are wide-spread in nature, e.g. in insect cuticles and fungal cell walls, chitosan-containing biomaterials so far are known only from fungi [72–74]. Chitin-containing matrices cover the entire body of insects, fulfilling a broad diversity of roles, from flexible joints and hard cuticles to translucent lenses. How these different matrices are synthesised and assembled is far from being understood. And even less is known about the much rarer chitosan-containing fungal cell walls in which chitosan typically is a very minor component only, with unknown functions. In some pathogenic fungi, conversion of chitin to chitosan has been hypothesised to serve as a sort of stealth strategy to hide from the chitin-triggered immune system of the plant and animal hosts [18]. The only fungi thought to contain substantial amounts of chitosan in their cell walls are zygomycetes fungi, but structural details of this chitosan and how it is embedded in the complex cell wall matrix are still scarce [75]. Chitin polymers are synthesised by transmembrane chitin synthases, and the nascent chitin chains emanating from the synthases may be partially deacetylated by chitin deacetylases acting in concert with the synthases [20]. Extensive deacetylation would yield chitosan polymers, but even chitin polymers are thought to be slightly deacetylated [39, 76]. Little is known about the cooperation and regulation of these processes, and even less is known on the assembly and, sometimes, biomineralization of the complex chitin-containing matrices. Clearly, chitin-binding structural proteins will be involved in this process, and it is tempting to speculate that local deacetylations may play a crucial role in the chitin-protein interaction, given that a deacetylated GlcN unit or perhaps a small partially deacetylated domain with a specific sequence of GlcN and GlcNAc units within an otherwise fully acetylated chitin chain would represent an unmistakable marker for specific interactions. At this stage, this is fully hypothetical, but the tools and materials are rapidly becoming available to critically test such a hypothesis.
Conclusions
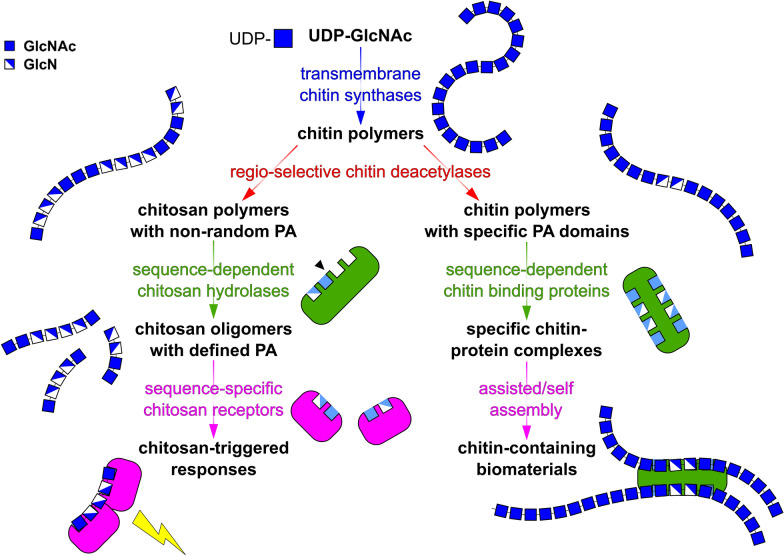
Chitins and chitosans and the matrices of which they are integral components are so much more than simple structural protections for delicate cells and organisms. They are functional biomaterials as well as bioactive biologics. In both respects, their versatility is expressed by specific interactions with proteins which are responsible for their biosynthesis, modification, assembly, perception, and degradation. The PA of chitosans may act like a ‘chito-code’, ‘written’ by trans-membrane chitin synthases in conjunction with regio-selective chitin deacetylases, ‘read’ by sequence-dependent chitin binding proteins and chitosan hydrolases and, possibly, ‘understood’ by a proteinaceous assembly machinery and pattern recognition receptors (Fig. 1). Today, this is just a hypothesis, but one that can and will be tested in the foreseeable future.
Fig. 1.
The Chito-Code: fiction today, fact tomorrow? While chitosan-specific receptors for the perception of chitosan oligomers with defined patterns of acetylation (PA) acting as functional biologics triggering specific responses in target cells have not yet been described, and while the involvement of partially deacetylated domains with specific PAs within otherwise fully acetylated chitins in the protein-assisted assembly of functional biomaterials is fully hypothetical, regio-selective chitin deacetylases and sequence-dependent chitosan hydrolases and chitin-binding proteins are well known
Acknowledgements
The authors gratefully acknowledge the financial support for their work on chitosans through different national, European, and international research projects, particularly the FunChi project (grant # 22031315 of BMEL-FNR) of the „Industrial Biotechnology“ programme ERA-IB-15-080, and the lignoLIPP project (Grant # 031B0933A of BMBF-PTJ) of the “Bioeconomy in the North” programme BiN, both of the European Union. We would like to thank all partners involved in these projects for critical discussions. We are also grateful for comments on market aspects concerning second generation chitosans to Dr. Katja Richter (Heppe Medical Chitosan GmbH, Halle, Germany), Dr. Hélène Liette Lauzon (Primex ehf, Siglufjordur, Iceland), Dr. Dominique Gillet (Gillet Chitosan SASU, Plumaudan, France), Dr. Jean-Pierre Say (France Chitine SAS, Orange, France), and Dr. Siegfried Bank (ChiPro GmbH, Bremen, Germany). We also wish to thank Martin Bonin for the graphics in the figure. We would like to thank all of our past and present group members as well as the partners of our past and present collaborative research projects for stimulating discussions on the future of chitosans.
Authors' contributions
Both authors contributed equally to planning the manuscript; SCL performed the literature searches; BM wrote the manuscript. Both authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study received funding by Fachagentur Nachwachsende Rohstoffe with Grant number 22031315 and by Bundesministerium für Bildung und Forschung with Grant number 031B0933A.
Availability of data and materials
N/a.
Declarations
Ethics approval and consent to participate
N/a.
Consent for publication
N/a.
Competing interests
N/a.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Broek LAM, Boeriu CG, editors. Chitin and Chitosan. Chichester, UK: Wiley; 2019. 10.1002/9781119450467.
- 2.Wattjes J, Sreekumar S, Richter C, Cord-Landwehr S, Singh R, El Gueddari NE, et al. Patterns matter part 1: Chitosan polymers with non-random patterns of acetylation. React Funct Polym. 2020;151(Special issue: Chitosan for the future):104583. doi: 10.1016/j.reactfunctpolym.2020.104583. [DOI] [Google Scholar]
- 3.Cord-Landwehr S, Richter C, Wattjes J, Sreekumar S, Singh R, Basa S, et al. Patterns matter part 2: Chitosan oligomers with defined patterns of acetylation. React Funct Polym. 2020;151(Special issue: Chitosan for the future):104577. doi: 10.1016/j.reactfunctpolym.2020.104577. [DOI] [Google Scholar]
- 4.Cord-Landwehr S, Niehues A, Wattjes J, Moerschbacher BM. New developments in the analysis of partially acetylated chitosan polymers and oligomers. In: van den Broek LAM, Boeriu CG, editors. Chitin and Chitosan: properties and applications. Chichester, UK: Wiley; 2019. p. 81–95. 10.1002/9781119450467.ch4.
- 5.Vårum KM, Anthonsen MW, Grasdalen H, Smidsrød O. 13C-N.m.r. studies of the acetylation sequences in partially N-deacetylated chitins (chitosans) Carbohydr Res. 1991;217:19–27. doi: 10.1016/0008-6215(91)84113-S. [DOI] [PubMed] [Google Scholar]
- 6.El Gueddari NE, Moerschbacher BM. A bioactivity matrix for chitosans as elicitors of disease resistance reactions in wheat. Adv Chitin Sci. 2004;VII:56–59. [Google Scholar]
- 7.Weinhold MX, Sauvageau JCMM, Kumirska J, Thöming J. Studies on acetylation patterns of different chitosan preparations. Carbohydr Polym. 2009;78:678–684. doi: 10.1016/j.carbpol.2009.06.001. [DOI] [Google Scholar]
- 8.Schatz C, Viton C, Delair T, Pichot C, Domard A. Typical physicochemical behaviors of chitosan in aqueous solution. Biomacromol. 2003;4:641–648. doi: 10.1021/bm025724c. [DOI] [PubMed] [Google Scholar]
- 9.Wattjes J, Sreekumar S, Niehues A, Mengoni T, Mendes ACL, Morris ER, et al. Biotechnology-derived chitosans with non-random patterns of acetylation differ from conventional chitosans in their properties and activities. ChemRxiv Prepr. 2021 doi: 10.26434/chemrxiv.14356088.v1. [DOI] [Google Scholar]
- 10.Wattjes J, Niehues A, Cord-Landwehr S, Hoßbach J, David L, Delair T, et al. Enzymatic production and enzymatic-mass spectrometric fingerprinting analysis of chitosan polymers with different nonrandom patterns of acetylation. J Am Chem Soc. 2019;141:3137–3145. doi: 10.1021/jacs.8b12561. [DOI] [PubMed] [Google Scholar]
- 11.Mandon P, Prasad E. Chitosan Market by Source (Shrimp, Squid, Crab, Krill, and Others) and Application (Water Treatment, Biomedical & Pharmaceutical, Cosmetics, Food & Beverage, and Others): Global Opportunity Analysis and Industry Forecast, 2020–2027. 2020. https://www.alliedmarketresearch.com/chitosan-market.
- 12.Yan N, Chen X. Sustainability: don’t waste seafood waste. Nature. 2015;524:155–157. doi: 10.1038/524155a. [DOI] [PubMed] [Google Scholar]
- 13.Muñoz I, Rodríguez C, Gillet D, Moerschbacher BM. Life cycle assessment of chitosan production in India and Europe. Int J Life Cycle Assess. 2018;23:1151–1160. doi: 10.1007/s11367-017-1290-2. [DOI] [Google Scholar]
- 14.Ghormade V, Pathan EK, Deshpande MV. Can fungi compete with marine sources for chitosan production? Int J Biol Macromol. 2017;104:1415–1421. doi: 10.1016/j.ijbiomac.2017.01.112. [DOI] [PubMed] [Google Scholar]
- 15.Gow NARR, Latge J-P, Munro CA. The fungal cell wall: structure, biosynthesis, and function. Am Soc Microbiol. 2017;5:267–292. doi: 10.1128/9781555819583.ch12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown D. Method for Chitosan Production. WO 2015/085429 A1. 2015;39.
- 17.Dhillon GS, Kaur S, Brar SK, Verma M. Green synthesis approach: extraction of chitosan from fungus mycelia. Crit Rev Biotechnol. 2013;33:379–403. doi: 10.3109/07388551.2012.717217. [DOI] [PubMed] [Google Scholar]
- 18.El Gueddari NE, Rauchhaus U, Moerschbacher BM, Deising HB. Developmentally regulated conversion of surface-exposed chitin to chitosan in cell walls of plant pathogenic fungi. New Phytol. 2002;156:103–112. doi: 10.1046/j.1469-8137.2002.00487.x. [DOI] [Google Scholar]
- 19.Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- 20.Davis LL, Bartnicki-Garcia S. Chitosan synthesis by the tandem action of chitin synthetase and chitin deacetylase from Mucor rouxii. Biochemistry. 1984;23:1065–1073. doi: 10.1021/bi00301a005. [DOI] [Google Scholar]
- 21.Basa S, Nampally M, Honorato T, Das SN, Podile AR, El Gueddari NE, et al. The pattern of acetylation defines the priming activity of chitosan tetramers. J Am Chem Soc. 2020;142:1975–1986. doi: 10.1021/jacs.9b11466. [DOI] [PubMed] [Google Scholar]
- 22.Hembach L, Cord-Landwehr S, Moerschbacher BM. Enzymatic production of all fourteen partially acetylated chitosan tetramers using different chitin deacetylases acting in forward or reverse mode. Sci Rep. 2017;7:17692. doi: 10.1038/s41598-017-17950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cord-Landwehr S, Melcher RLJ, Kolkenbrock S, Moerschbacher BM. A chitin deacetylase from the endophytic fungus Pestalotiopsis sp. efficiently inactivates the elicitor activity of chitin oligomers in rice cells. Sci Rep. 2016;6:38018. doi: 10.1038/srep38018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noorifar N, Savoian M, Ram A, Lukito Y, Hassing B, Weikert T, et al. Chitin deacetylases are required for epichloë festucae endophytic cell wall remodelling during establishment of a mutualistic symbiotic interaction with Lolium perenne. Mol Plant Microbe Interact. 2021 doi: 10.1094/MPMI-12-20-0347-R. [DOI] [PubMed] [Google Scholar]
- 25.Rizzi YS, Happel P, Lenz S, Urs MJ, Bonin M, Cord-Landwehr S, et al. Chitosan and chitin deacetylase activity are necessary for development and virulence of Ustilago maydis. MBio. 2021;12. 10.1128/mBio.03419-20. [DOI] [PMC free article] [PubMed]
- 26.Horn SJ, Sikorski P, Cederkvist JB, Vaaje-Kolstad G, Sørlie M, Synstad B, et al. Costs and benefits of processivity in enzymatic degradation of recalcitrant polysaccharides. Proc Natl Acad Sci. 2006;103:18089–18094. doi: 10.1073/pnas.0608909103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vårum KM, Anthonsen MW, Grasdalen H, Smidsrød O. Determination of the degree of N-acetylation and the distribution of N-acetyl groups in partially N-deacetylated chitins (chitosans) by high-field n.m.r. spectroscopy. Carbohydr Res. 1991;211:17–23. doi: 10.1016/0008-6215(91)84142-2. [DOI] [PubMed] [Google Scholar]
- 28.Kumirska J, Weinhold MX, Steudte S, Thöming J, Brzozowski K, Stepnowski P. Determination of the pattern of acetylation of chitosan samples: comparison of evaluation methods and some validation parameters. Int J Biol Macromol. 2009;45:56–60. doi: 10.1016/j.ijbiomac.2009.04.002. [DOI] [Google Scholar]
- 29.Cord-Landwehr S, Ihmor P, Niehues A, Luftmann H, Moerschbacher BM, Mormann M. Quantitative mass-spectrometric sequencing of chitosan oligomers revealing cleavage sites of chitosan hydrolases. Anal Chem. 2017;89:2893–2900. doi: 10.1021/acs.analchem.6b04183. [DOI] [PubMed] [Google Scholar]
- 30.Niehues A, Wattjes J, Bénéteau J, Rivera-Rodriguez GR, Moerschbacher BM. Chitosan analysis by enzymatic/mass spectrometric fingerprinting and in silico predictive modeling. Anal Chem. 2017;89:12602–12608. doi: 10.1021/acs.analchem.7b04002. [DOI] [PubMed] [Google Scholar]
- 31.Haebel S, Bahrke S, Peter MG. Quantitative sequencing of complex mixtures of heterochitooligosaccharides by vMALDI-linear ion trap mass spectrometry. Anal Chem. 2007;79:5557–5566. doi: 10.1021/ac062254u. [DOI] [PubMed] [Google Scholar]
- 32.Wattjes J, Niehues A, Moerschbacher BM. Robust enzymatic-mass spectrometric fingerprinting analysis of the fraction of acetylation of chitosans. Carbohydr Polym. 2020;231:115684. doi: 10.1016/j.carbpol.2019.115684. [DOI] [PubMed] [Google Scholar]
- 33.Mnatsakanyan M, Thevarajah JJ, Roi RS, Lauto A, Gaborieau M, Castignolles P. Separation of chitosan by degree of acetylation using simple free solution capillary electrophoresis. Anal Bioanal Chem. 2013;405:6873–6877. doi: 10.1007/s00216-013-7126-4. [DOI] [PubMed] [Google Scholar]
- 34.Thevarajah JJ, Van Leeuwen MP, Cottet H, Castignolles P, Gaborieau M. Determination of the distributions of degrees of acetylation of chitosan. Int J Biol Macromol. 2017;95:40–48. doi: 10.1016/j.ijbiomac.2016.10.056. [DOI] [PubMed] [Google Scholar]
- 35.Gullion JD, Gullion T. Solid-state NMR study of the cicada wing. J Phys Chem B. 2017;121:7646–7651. doi: 10.1021/acs.jpcb.7b05598. [DOI] [PubMed] [Google Scholar]
- 36.Eddy S, Gullion T. Characterization of Insect Wing Membranes by 13 C CPMAS NMR. J Phys Chem C. 2021;125:931–936. doi: 10.1021/acs.jpcc.0c08423. [DOI] [Google Scholar]
- 37.Ehren HL, Appels FVW, Houben K, Renault MAM, Wösten HAB, Baldus M. Characterization of the cell wall of a mushroom forming fungus at atomic resolution using solid-state NMR spectroscopy. Cell Surf. 2020;6:100046. doi: 10.1016/j.tcsw.2020.100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao W, Fernando LD, Kirui A, Deligey F, Wang T. Solid-state NMR of plant and fungal cell walls: a critical review. Solid State Nucl Magn Reson. 2020;107:101660. doi: 10.1016/j.ssnmr.2020.101660. [DOI] [PubMed] [Google Scholar]
- 39.Kang X, Kirui A, Muszyński A, Widanage MCD, Chen A, Azadi P, et al. Molecular architecture of fungal cell walls revealed by solid-state NMR. Nat Commun. 2018;9:2747. doi: 10.1038/s41467-018-05199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chrissian C, Lin CP-C, Camacho E, Casadevall A, Neiman AM, Stark RE. Unconventional constituents and shared molecular architecture of the melanized cell wall of C. neoformans and Spore Wall of S. cerevisiae. J Fungi. 2020;6:329. 10.3390/jof6040329. [DOI] [PMC free article] [PubMed]
- 41.Henry C, Li J, Danion F, Alcazar-Fuoli L, Mellado E, Beau R, et al. Two KTR mannosyltransferases are responsible for the biosynthesis of cell wall mannans and control polarized growth in Aspergillus fumigatus. MBio. 2019;10. 10.1128/mBio.02647-18. [DOI] [PMC free article] [PubMed]
- 42.Chakraborty A, Fernando LD, Fang W, DickwellaWidanage MC, Wei P, Jin C, et al. A molecular vision of fungal cell wall organization by functional genomics and solid-state NMR. Nat Commun. 2021;12:6346. doi: 10.1038/s41467-021-26749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gubaeva E, Gubaev A, Melcher RLJ, Cord-Landwehr S, Singh R, El Gueddari NE, et al. ‘Slipped Sandwich’ model for chitin and chitosan perception in Arabidopsis. Mol Plant-Microbe Interact. 2018;31:1145–1153. doi: 10.1094/MPMI-04-18-0098-R54. [DOI] [PubMed] [Google Scholar]
- 45.Fuchs K, Cardona Gloria Y, Wolz O, Herster F, Sharma L, Dillen CA, et al. The fungal ligand chitin directly binds TLR2 and triggers inflammation dependent on oligomer size. EMBO Rep. 2018;19. 10.15252/embr.201846065. [DOI] [PMC free article] [PubMed]
- 46.Gong B-Q, Wang F-Z, Li J-F. Hide-and-seek: chitin-triggered plant immunity and fungal counterstrategies. Trends Plant Sci. 2020;25:805–816. doi: 10.1016/j.tplants.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Cheval C, Samwald S, Johnston MG, de Keijzer J, Breakspear A, Liu X, et al. Chitin perception in plasmodesmata characterizes submembrane immune-signaling specificity in plants. Proc Natl Acad Sci. 2020;117:9621–9629. doi: 10.1073/pnas.1907799117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziatabar S, Zepf J, Rich S, Danielson BT, Bollyky PI, Stern R. Chitin, chitinases, and chitin lectins: emerging roles in human pathophysiology. Pathophysiology. 2018;25:253–262. doi: 10.1016/j.pathophys.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Aam BB, Heggset EB, Norberg AL, Sørlie M, Vårum KM, Eijsink VGH. Production of chitooligosaccharides and their potential applications in medicine. Mar Drugs. 2010;8:1482–1517. doi: 10.3390/md8051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonin M, Sreekumar S, Cord-Landwehr S, Moerschbacher BM. Preparation of defined chitosan oligosaccharides using chitin deacetylases. Int J Mol Sci. 2020;21:7835. doi: 10.3390/ijms21217835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh R, Weikert T, Basa S, Moerschbacher BM. Structural and biochemical insight into mode of action and subsite specificity of a chitosan degrading enzyme from Bacillus spec. MN. Sci Rep. 2019;9. [DOI] [PMC free article] [PubMed]
- 52.Weikert T, Niehues A, Cord-Landwehr S, Hellmann MJ, Moerschbacher BM. Reassessment of chitosanase substrate specificities and classification. Nat Commun. 2017;8:1698. doi: 10.1038/s41467-017-01667-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grifoll-Romero L, Pascual S, Aragunde H, Biarnés X, Planas A. Chitin deacetylases: structures, specificities, and biotech applications. Polymers (Basel) 2018;10:352. doi: 10.3390/polym10040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrés E, Albesa-Jové D, Biarnés X, Moerschbacher BM, Guerin ME, Planas A. Structural basis of chitin oligosaccharide deacetylation. Angew Chemie Int Ed. 2014;53:6882–6887. doi: 10.1002/anie.201400220. [DOI] [PubMed] [Google Scholar]
- 55.Vaaje-Kolstad G, Tuveng TR, Mekasha S, Eijsink VGH. Enzymes for modification of chitin and chitosan. In: van den Broek LAM, Boeriu CG, editors. Chitin and chitosan: properties and applications. Chichester, UK: Wiley; 2019. pp. 189–228. [Google Scholar]
- 56.Delbianco M, Kononov A, Poveda A, Yu Y, Diercks T, Jiménez-Barbero J, et al. Well-defined oligo- and polysaccharides as ideal probes for structural studies. J Am Chem Soc. 2018;140:5421–5426. doi: 10.1021/jacs.8b00254. [DOI] [PubMed] [Google Scholar]
- 57.Tyrikos-Ergas T, Bordoni V, Fittolani G, Chaube MA, Grafmüller A, Seeberger PH, et al. Systematic structural characterization of chitooligosaccharides enabled by automated glycan assembly. Chemistry. 2021;27:2321–2325. doi: 10.1002/chem.202005228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El Gueddari NE, Schaaf A, Kohlhoff M, Gorzelanny C, Moerschbacher BM. Substrates and products of chitin and chitosan modifying enzymes. Adv Chitin Sci. 2007;X:119–126. [Google Scholar]
- 59.Hembach L, Bonin M, Gorzelanny C, Moerschbacher BM. Unique subsite specificity and potential natural function of a chitosan deacetylase from the human pathogen Cryptococcus neoformans. Proc Natl Acad Sci. 2020;117:3551–3559. doi: 10.1073/pnas.1915798117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El Gueddari NE, Kolkenbrock S, Schaaf A, Chilukoti N, Brunel F, Gorzelanny C, et al. Chitin and chitosan modifying enzymes: versatile novel tools for the analysis of structure–function relationship of partially acetylaed chitosans. In: Filho SPC, Beppu MM, Fiamingo A, editors. Adv Chitin Sci. 2014; 40–7.
- 61.van Leeuwe TM, Wattjes J, Niehues A, Forn-Cuní G, Geoffrion N, Mélida H, et al. A seven-membered cell wall related transglycosylase gene family in Aspergillus niger is relevant for cell wall integrity in cell wall mutants with reduced α-glucan or galactomannan. Cell Surf. 2020;6:100039. doi: 10.1016/j.tcsw.2020.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salgado-Lugo H, Sánchez-Arreguín A, Ruiz-Herrera J. Heterologous expression of an active chitin synthase from Rhizopus oryzae. Fungal Genet Biol. 2016;97:10–17. doi: 10.1016/j.fgb.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Samain E, Drouillard S, Heyraud A, Driguez H, Geremia RA. Gram-scale synthesis of recombinant chitooligosaccharides in Escherichia coli. Carbohydr Res. 1997;302:35–42. doi: 10.1016/S0008-6215(97)00107-9. [DOI] [PubMed] [Google Scholar]
- 64.Cottaz S, Samain E. Genetic engineering of Escherichia coli for the production of N I, NII-diacetylchitobiose (chitinbiose) and its utilization as a primer for the synthesis of complex carbohydrates. Metab Eng. 2005;7:311. doi: 10.1016/j.ymben.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Naqvi S, Moerschbacher BM. The cell factory approach toward biotechnological production of high-value chitosan oligomers and their derivatives: an update. Crit Rev Biotechnol. 2017;37:11–25. doi: 10.3109/07388551.2015.1104289. [DOI] [PubMed] [Google Scholar]
- 66.Kauss H, Jeblick W, Domard A. The degrees of polymerization and N-acetylation of chitosan determine its ability to elicit callose formation in suspension cells and protoplasts of Catharanthus roseus. Planta. 1989;178:385–392. doi: 10.1007/BF00391866. [DOI] [PubMed] [Google Scholar]
- 67.Raafat D, Sahl H-G. Chitosan and its antimicrobial potential—a critical literature survey. Microb Biotechnol. 2009;2:186–201. doi: 10.1111/j.1751-7915.2008.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raafat D, von Bargen K, Haas A, Sahl H-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol. 2008;74:3764–3773. doi: 10.1128/AEM.00453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Zhao Y, Tan H, Chi N, Zhang Q, Du Y, et al. Characterisation of a chitinase from Pseudoalteromonas sp. DL-6, a marine psychrophilic bacterium. Int J Biol Macromol. 2014;70:455–462. doi: 10.1016/j.ijbiomac.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 70.Hadwiger LA. Multiple effects of chitosan on plant systems: solid science or hype. Plant Sci. 2013;208:42–49. doi: 10.1016/j.plantsci.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Lopez-Moya F, Suarez-Fernandez M, Lopez-Llorca LV. Molecular mechanisms of chitosan interactions with fungi and plants. Int J Mol Sci. 2019;20:332. doi: 10.3390/ijms20020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rinaudo M. Chitin and chitosan: properties and applications. Elsevier. 2007 doi: 10.1016/j.progpolymsci.2006.06.001. [DOI] [Google Scholar]
- 73.Peter MG. Chitin and Chitosan in Fungi. In: Vandamme EJ, De Baets S, Steinbüchel A, editors. Biopolymers Online. Wiley; 2002. 10.1002/3527600035.bpol6005.
- 74.Peter MG. Chitin and chitosan from animal sources. In: Vandamme EJ, De Baets S, Steinbüchel A, editors. Biopolymers online. Wiley; 2002. 10.1002/3527600035.bpol6015.
- 75.Fernando LD, Dickwella Widanage MC, Penfield J, Lipton AS, Washton N, Latgé J-P, et al. Structural polymorphism of chitin and chitosan in fungal cell walls from solid-state nmr and principal component analysis. Front Mol Biosci. 2021;8. 10.3389/fmolb.2021.727053. [DOI] [PMC free article] [PubMed]
- 76.Einbu A, Vårum KM. Characterization of chitin and its hydrolysis to GlcNAc and GlcN. Biomacromol. 2008;9:1870–1875. doi: 10.1021/bm8001123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/a.