Abstract
We describe a 66-year-old woman with infective endocarditis due to Cardiobacterium hominis whose condition, complicated by severe aortic regurgitation and congestive heart failure, necessitated aortic valve replacement despite treatment with ceftriaxone followed by ciprofloxacin. The blood isolate of C. hominis produced β-lactamase and exhibited high-level resistance to penicillin (MIC, ≧256 μg/ml) and reduced susceptibility to vancomycin (MIC, 8 μg/ml).
CASE REPORT
A 66-year-old woman who had been well except for having diabetes mellitus was admitted to a district hospital in the city of Taipei, Taiwan, on 4 April 1998 because of chest discomfort and fatigability that worsened during the course of a day. She was not febrile on admission. Dental caries were noted. Grade II systolic and diastolic murmurs were heard at the cardiac apex and at the left lower and right upper sternal borders. Otherwise, the physical examination was unremarkable. The white blood cell count was 8,500 cells/mm3, with 75% neutrophils, 20% lymphocytes, and 5% monocytes. The hemoglobin level was 9.9 g/dl, and the platelet count was 199,000/mm3. The remaining laboratory results were within normal limits.
Transthoracic and transesophageal echocardiography revealed vegetation at the aortic valve in addition to severe aortic regurgitation and dilation of the left ventricle and atrium. Infective endocarditis was suspected, and treatment with ampicillin (2 g every [q] 4 h) and gentamicin (60 mg q 12 h), administered intravenously, was begun after blood specimens were obtained for culture. Blood cultures showed no growth after incubation for a week. The patient's symptoms were aggravated, and exertional dyspnea developed after treatment for 14 days; she was then referred to National Taiwan University Hospital. On admission, her temperature was 37.5°C. A repeat echocardiography revealed findings similar to those obtained previously, but without perivalvular abscess.
Ampicillin and gentamicin were replaced by ceftriaxone (2 g q 12 h) on 20 April 1998. Because skin rashes developed 3 weeks later, ceftriaxone was replaced by intravenous ciprofloxacin (400 mg q 12 h), which was continued for 1 week. On 13 May 1998, the patient developed worsening dyspnea on mild exertion and orthopnea. She underwent aortic valve replacement because of progressive heart failure on 21 May 1998. Perforation of the noncoronary cusp of the aortic valve was found. Cultures of blood and the aortic valves were negative. The postoperative course was uneventful and she remained well during the 8 months of follow-up care.
Microbiology.
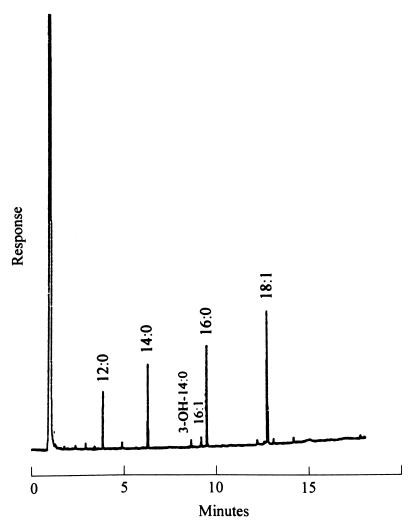
Ten milliliters of blood was obtained from the patient upon her admission to the first hospital. A 5-ml volume of the original blood sample was added to each of two blood cultures (BACTEC 6A aerobic and BACTEC 7A anaerobic bottles; Becton Dickinson, Sparks, Md.) by using the BACTEC 860 System (Becton Dickinson). After a 10-day incubation (prolonged incubation because of clinical suspicion of infective endocarditis caused by organisms of the group [Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Capnocytophaga spp., Eikenella corrodens, and Kingella species]), HACEK a terminal subculture of the BACTEC 6A aerobic bottle grew a gram-negative bacillus and the microorganism was reported as Pasteurella multocida. The isolate was sent to National Taiwan University Hospital for further identification. A tiny, glistening, opaque colony was observed on trypticase soy agar supplemented with 5% sheep blood (BBL Microbiology Systems, Cockeysville, Md.) after culture at 37°C in an atmosphere of 5% carbon dioxide for more than 2 days. No hemolysis was observed on blood agar. Rods with one or both ends swollen were seen observed after Gram staining of the colony. The isolate was oxidase-positive, catalase-negative, nitrate-negative, indole-positive, urease-negative, and nonmotile. The organisms fermented glucose, sucrose, and lactose. The biochemical profiles of the isolate were in accord with the identification of C. hominis (2). Cellular fatty acid chromatograms of the isolate, obtained as previously described (3), corresponded to the pattern of C. hominis which contained a high percentage (≥3%) of 18:1 ω7c/ω9t/ω12t fatty acid methyl ester (FAME), 16:0 FAME, 14:0 FAME, 12:0 FAME, and 16:1 ω7c/15-2-OH FAME (Fig. 1) (17).
FIG. 1.
Gas chromatography of FAMES of C. hominis. The designations refer to the number of carbon atoms (number before the colon) and the number of double bonds (number after the colon). 3-OH, hydroxyl group at carbon 3 (Microbial Identification System; Microbial ID Inc., Newark, Del.).
The MICs of 11 antimicrobial agents were determined by using the broth dilution method with cation-adjusted Mueller-Hinton broth with 5% lysed horse blood as described by the National Committee for Clinical Laboratory Standards for susceptibility testing for Streptococcus pneumoniae (11), and the results were read after 24 h of incubation at 37°C in an ambient air. S. pneumoniae ATCC 49619 was used as control strain. The MICs of the 11 antimicrobial agents for the control strain were within the ranges provided by the National Committee for Clinical Laboratory Standards (11). The MICs for the C. hominis isolate were as follows: penicillin, >256 μg/ml; ampicillin, >256 μg/ml; amoxicillin-clavulanic acid, 0.5 μg/ml; cephalothin, 4.0 μg/ml; cefotaxime, 1.0 μg/ml; ceftriaxone, 1.0 μg/ml; tetracycline, 4 μg/ml; gentamicin, 0.5 μg/ml; ciprofloxacin, 0.5 μg/ml; trimethoprim-sulfamethoxazole, 0.25 μg/ml; and vancomycin, 8 μg/ml. The isolate produced β-lactamase by means of the nitrocefin-based test (Cefinase disk; BBL Microbiology Systems).
Discussion.
C. hominis is a member of the HACEK group, exhibiting the common characteristics of a slow growth rate and a requirement of CO2 for optimal growth and causing similar clinical syndromes (9, 10). Nearly one-third of the isolates of Haemophilus aphrophilus and 40% of A. actinomycetemcomitans were reported to be resistant to penicillin in 1961 (6). Compared with the other members of HACEK group, C. hominis is rarely reported to be resistant to penicillin, and penicillin or ampicillin has been considered the drug of choice for treating infective endocarditis caused by this organism (1, 4, 9, 10, 13, 17, 18). Although a strain of penicillin-resistant C. hominis was reported as early as in 1977 (J. J. Rahal, Jr., and M. S. Simberkoff, Program Abstr. 17th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 5, 1977), infection with a β-lactamase-producing strain was not reported until 1994 (8) and there was no further report of such a β-lactamase producer. Herein, we have reported another case of infective endocarditis complicated with severe congestive heart failure due to β-lactamase-producing C. hominis.
It is not unusual for C. hominis to be misidentified (7). The first case of endocarditis due to C. hominis in the Kingdom of Saudi Arabia was diagnosed as Brucella endocarditis (5). The isolate we reported was the first isolate of C. hominis in Taiwan and was initially misidentified as P. multocida. Interestingly, C. hominis was described as a Pasteurella-like organism when it was first isolated in 1962. The resemblance between C. hominis and several other organisms may explain why this bacterium, one of the upper respiratory tract flora (4), had never been described until 1962 (14).
For correct identification of C. hominis in clinical laboratory, it should be distinguished from other members of the HACEK group and from Pasteurella, Brucella, Streptobacillus moniliformis, and Bordetella parapertussis. The main characteristics of C. hominis distinguishing it from other closely related organisms are absence of catalase activity, positive oxidase reaction, production of indole, and absence of nitrate production (14). For example, Pasteurella is distinguished from C. hominis by catalase positivity. The laboratory diagnosis of the HACEK group of organisms requires a high index of suspicion. Organisms of this group should be suspected in all cases of endocarditis in which a gram-negative coccobacillary organism is isolated, an organism which has failed to grow on MacConkey agar or any medium selective for Enterobacteriaceae, shows characteristic rosette formation upon Gram staining, and has a stellate colonial appearance.
The American Heart Association suggested that trimethoprim-sulfamethoxazole, a fluoroquinolone, and aztreonam could be considered as alternative regimens in patients unable to tolerate β-lactam therapy (17). Our patient developed an allergic reaction to ceftriaxone, and ciprofloxacin was used to complete the 4-week antimicrobial course. Bacteriologic cure was documented with negative culture results from the excised aortic valve and from a blood specimen after therapy.
Serious complications, such as embolic events and congestive heart failure, among patients with C. hominis endocarditis during the course of therapy are not uncommon (44% for each complication), although bacteriologic cure may be achieved (10, 18). Severe cardiac failure demanding surgical intervention developed in our case in spite of successful ceftriaxone and then ciprofloxacin therapy to eradicate the pathogen.
Our case is the second reported case of endocarditis due to β-lactamase-producing C. hominis. The first reported β-lactamase-producing C. hominis isolated in France had an antibiogram different from ours (8). That isolate was susceptible to vancomycin and that patient was successfully treated, with no sequels, with vancomycin and rifampin for 4 weeks, followed by amoxicillin-clavulanate for 2 weeks. The MICs of gentamicin and trimethoprim-sulfamethoxazole for our isolate were 0.5 and 0.25 μg/ml, respectively, whereas the previously reported isolate was resistant to the two antimicrobial agents.
In vitro susceptibility testing may be difficult to perform and interpret because of the fastidious nature and slow growth of C. hominis (7). There are no breakpoints for this organism, nor have the correct medium or atmosphere and time of incubation been established (11). Penicillin or ampicillin has been the antibiotic of choice for C. hominis until now (1, 4, 10, 13, 17, 18). In contrast to other members of the HACEK group with a high frequency of penicillin resistance, C. hominis has rarely been reported to be penicillin-resistant (5, 8, 9; J. J. Rahal, Jr., and M. S. Simberkoff, 17th ICAAC). Because of difficulties inherent in performing antimicrobial susceptibility testing, in 1995 the American Heart Association recommended that HACEK microorganisms should be considered ampicillin-resistant and the third-generation cephalosporins should be considered the antibiotics of choice (15). The facts that the MICs of cefotaxime and ceftriaxone for this isolate were both elevated (1.0 μg/ml) and that the previous β-lactamase-producing C. hominis was resistant to cefotaxime suggested that we should be alert to the occurrence of β-lactamase-producing C. hominis, noting its susceptibility to penicillin and the third-generation cephalosporins.
Susceptibility of C. hominis to vancomycin is variable (10). Our isolate showed reduced susceptibility to vancomycin (MIC, 8 μg/ml). It should be noted that vancomycin and erythromycin are recommended for prophylaxis of infective endocarditis in patients allergic to penicillins. Failure of prophylaxis with erythromycin for dental extractions may occur in the face of C. hominis resistant to erythromycin and vancomycin (12). A therapy regimen including vancomycin as did the previous successful treatment for β-lactamase-producing C. hominis was not suitable for our patient.
In summary, our case report has provided evidence that C. hominis, like the other HACEK microorganisms, can produce β-lactamase, and our results support the recommendation of American Heart Association that the third-generation cephalosporins should be considered the antibiotic of choice for the treatment of HACEK endocarditis.
REFERENCES
- 1.Christin R D. Cardiobacterium hominis endocarditis in a patient with a hypersensitivity reaction to penicillin. Successful treatment with partial resection of the posterior mitral valve leaflet and antibiotic therapy with cefazolin. Infection. 1990;18:291–293. doi: 10.1007/BF01647009. [DOI] [PubMed] [Google Scholar]
- 2.Holmes B, Pickett M J, Hollis D G. Unusual gram-negative bacteria, including Capnocytophaga, Eikenella, Pasteurella, and Streptobacillus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 499–503. [Google Scholar]
- 3.Hsueh P-R, Teng L-J, Yang P-C, Ho S-W, Luh K-T. Bacteremia caused by Arcobacter cryaerophilus 1B. J Clin Microbiol. 1997;35:489–491. doi: 10.1128/jcm.35.2.489-491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joklik W K, Willet H P, Amos D B, Wilfert C M, editors. Zinsser microbiology. 20th ed. Norwalk, Conn: Appleton & Lange; 1992. p. 605. [Google Scholar]
- 5.Khizzi N E, Kasab S A, Osoba A O. HACEK group endocarditis at the Riyadh Armed Forces Hospital. J Infect. 1997;34:69–74. doi: 10.1016/s0163-4453(97)80013-8. [DOI] [PubMed] [Google Scholar]
- 6.King E O, Tatum H W. Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. J Infect Dis. 1961;111:85–94. doi: 10.1093/infdis/111.2.85. [DOI] [PubMed] [Google Scholar]
- 7.Lane T, MacGregor R R, Wright D, Hollander J. Cardiobacterium hominis: an elusive cause of endocarditis. J Infect. 1983;6:75–80. doi: 10.1016/s0163-4453(83)95776-6. [DOI] [PubMed] [Google Scholar]
- 8.Le Quellec A, Bessis D, Perez C, Ciurana A J. Endocarditis due to beta-lactamase-producing Cardiobacterium hominis. Clin Infect Dis. 1994;19:994–995. doi: 10.1093/clinids/19.5.994. [DOI] [PubMed] [Google Scholar]
- 9.Maury S, Leblanc T, Rousselot P, Legrand P, Arlet G, Cordonnier C. Bacteremia due to Capnocytophaga species in patients with neutropenia: high frequency of beta-lactamase-producing strains. Clin Infect Dis. 1999;28:1172–1174. doi: 10.1086/517772. [DOI] [PubMed] [Google Scholar]
- 10.McGowan J E, Jr, Steinberg J P. Other Gram negative bacilli. In: Mandell G L, Bennett J E, Dolin R, editors. Mandell, Douglas and Bennett's principles and practice of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1995. pp. 2108–2109. [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing: ninth informational supplement M100-S9. Wayne, Pa: National Committee for Laboratory Standards; 1999. [Google Scholar]
- 12.Prior R B, Spagna V A, Perkins R L. Endocarditis due to strain of Cardiobacterium hominis resistant to erythromycin and vancomycin. Chest. 1979;75:85–86. doi: 10.1378/chest.75.1.85. [DOI] [PubMed] [Google Scholar]
- 13.Rechtman D J, Nadler J P. Abdominal abscess due to Cardiobacterium hominis and Clostridium bifermentans. Rev Infect Dis. 1991;13:418–419. doi: 10.1093/clinids/13.3.418. [DOI] [PubMed] [Google Scholar]
- 14.Savage D D, Kagan R L, Young N A, Horvath A E. Cardiobacterium hominis endocarditis: description of two patients and characterization of the organism. J Clin Microbiol. 1977;5:75–80. doi: 10.1128/jcm.5.1.75-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tucker D N, Slotnick I J, King E O, Tynes B, Nicholson J, Crevassee L. Endocarditis caused by a Pasturella-like organism. N Engl J Med. 1962;267:913–916. doi: 10.1056/NEJM196211012671804. [DOI] [PubMed] [Google Scholar]
- 16.Wallace P L, Hollis D G, Weaver R E, Moss C W. Cellular fatty acid composition of Kingella species, Cardiobacterium hominis, and Eikenella corrodens. J Clin Microbiol. 1988;26:1592–1594. doi: 10.1128/jcm.26.8.1592-1594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson W R, Karchmer A W, Dajani A S, Taubert K A, Bayer A, Kaye D, Bisno A L, Ferrieri P, Shulman S T, Durack D T. Antibiotic treatment of adults with infective endocarditis due to streptococci, enterococci, staphylococci, and HACEK microorganisms. JAMA. 1995;274:1706–1713. [PubMed] [Google Scholar]
- 18.Wormser G P, Bottone E J. Cardiobacterium hominis: review of microbiologic and clinical features. Rev Infect Dis. 1983;5:680–691. doi: 10.1093/clinids/5.4.680. [DOI] [PubMed] [Google Scholar]