Abstract
The novel coronavirus SARS-CoV-2 uses the angiotensin-converting enzyme 2 receptor as an entry point to the cell. Cardiovascular disease (CVD) is a risk factor for COVID-19 with poor outcomes. We tested the hypothesis that the rate of angiotensin-converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) use is associated with the rate of COVID-19–confirmed cases and deaths. We conducted a geospatial, ecological study using publicly available county-level data. The Medicare ACEI and ARB prescription rate was exposure. The COVID-19–confirmed case and death rates were outcomes. Spatial autoregression models were adjusted for the rate of births and deaths; Group Quarters population; percentage of female; percentage of Native American, Pacific Islander, Hispanic, and Black; percentage of children and older (>65 years) adults; percentage of uninsured; percentage of those living in poverty; percentage of those who are obese, smoking, admitting insufficient sleep, and those with at least some college degree; median household income; air quality index; CVD hospitalization rate in Medicare beneficiaries; and CVD death rate in a total county population. After adjustment for confounders, the ACEI use rate did not associate with COVID-19–confirmed case rate (direct county-own effect + 0.027%; 95% confidence interval [CI] −1.080 to 1.134; p = 0.962; indirect spillover effect + 0.26%; 95% CI −70.0 to 70.5; p = 0.994). Similarly, the ARB use rate was not associated with COVID-19–confirmed case rate (direct effect + 0.029%; 95% CI −0.803 to 0.862; p = 0.945; indirect effect + 0.19%; 95% CI −52.8 to 53.2; p = 0.994). In both unadjusted and adjusted Bayesian zero inflation Poisson analysis, neither ACEI nor ARB use rates were associated with COVID-19 death rates. In conclusion, ACEI and ARB use rates were not associated with COVID-19 infectivity and death rate in this ecological study.
Despite available vaccines, the pandemic of the novel COVID-19 caused by the SARS-CoV-2 virus remains a threat.1 It has been shown that SARS-CoV-2 uses the angiotensin-converting enzyme (ACE) 2 receptor as an entry point into a cell.2 With the ACE2 receptor acting as a binding site for SARS-CoV-2, the renin-angiotensin-aldosterone system, and the medications affecting it become important points of discussion.3 ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are 2 classes of medications widely used in patients with hypertension, diabetes mellitus, cardiovascular disease (CVD), and latent or manifest left ventricular dysfunction, who are at risk for severe COVID-19 cases and deaths.4 Previous experiments showed that ACE2 expression was associated with susceptibility to SARS-CoV-2 infection.5 The consensus is reached about the importance of the continuation of ACEI and ARB use in the COVID-19 pandemic.6 , 7 However, controversy remains whether clinically indicated use of ACEI and ARB improves or worsens infectivity or the course of COVID-19. To address this need, we conducted an ecological study. We hypothesized that in the geospatial analysis, the rate of ACEI and ARB use is associated with the number of confirmed COVID-19 cases and deaths in the United States.
Methods
An individual county in the United States was an observation unit in this study. We used a Federal Information Processing Standard (FIPS) county code to link the data. Data with missing FIPS codes were excluded from the study. Geographic information about each county was obtained from the cartographic boundary files (shapefiles) provided by the United States Census Bureau's MAF/TIGER (Master Address File/Topologically Integrated Geographic Encoding and Referencing) geographic database.8
We used the 2018 Centers for Medicare & Medicaid Services public dataset, the Medicare Provider Utilization and Payment Data: Part D Prescriber Public Use File, with information on prescription drugs prescribed by individual physicians and other health care providers and paid for under the Medicare Part D Prescription Drug Program in 2018.9 The dataset included the total number of prescriptions that were dispensed (total day supply), which include original prescriptions and any refills, and therefore reflects ACEI and ARB usage.
The Medicare dataset only includes city and state information and not county information. To map the Medicare prescription data to their corresponding FIPS Code, we used the Google Geocoding application programming interface.10 We then loaded the Medicare data and geocoded data into the SQLite database to produce the final datasets with prescription counts per county. Prescriptions from county-equivalents (independent cities) were manually matched with their corresponding FIPS code. Medicare prescriptions with misspelled cities or prescriptions that lacked valid city and state descriptions, if unable to be assigned, were excluded. Excluded prescriptions accounted for <0.01% of the data.
We calculated a drug class use rate as a sum of the total day supply in a county for all drugs comprising a particular class (Supplementary Table 1), normalized by the total county population estimate. We used the United States Census Annual Resident Population Estimates for July 1, 2019.
The primary outcome was confirmed COVID-19 cases. The secondary outcome was COVID-19 deaths. We imported the raw COVID-19 data from the Johns Hopkins GitHub repository.11 The number of confirmed COVID-19 cases and deaths in each county as reported for February 6, 2021, was divided by the total population in each county (2019 county population estimate) and multiplied by 100,000 to convert to cases and deaths per 100,000 population. We decided to use February 6, 2021, COVID-19 data because, by that date, the pandemic had sufficiently played out without the significant confounding effect of the vaccination, which began at the end of December 2020.
We used the United States Census County Population Estimates, released in March 2020, and included reported deaths and births from July 1, 2018, to June 30, 2019.12 Because of the known negative impact of COVID-19 on the population of nursing homes and prisons/jails, we included July 1, 2019, Group Quarters total population estimate. Group Quarters Facilities include correctional facilities for adults, nursing homes, college/university student housing, military quarters, and group homes. Group Quarters data were gathered from an estimated 20,000 randomly selected facilities. Data were then collected through resident interviews of these selected facilities using the American Community Survey conducted by the United States Census Bureau.12 The total 2019 county population estimate12 normalized all demographic characteristics.
To characterize socioeconomic characteristics, we used the 2018 median household income expressed as a percent of the state total and percent of the total population in poverty, as reported by the Economic Research Service of the United States Department of Agriculture.13 We also used the data compiled by the County Health Rankings & Roadmaps program, which is a collaboration between the Robert Wood Johnson Foundation and the University of Wisconsin Population Health Institute.14
To characterize CVD prevalence and severity, we used the Centers for Disease Control and Prevention (CDC) estimates15 of total CVD death rate per 100,000 population (2016 to 2018), total CVD hospitalizations (2015 to 2017) per 1,000 Medicare beneficiaries, heart failure (HF) death rate per 100,000 population (2016 to 2018), HF hospitalization rate per 1,000 Medicare beneficiaries (2015 to 2017), coronary heart disease (CHD) death rate per 100,000 population (2016 to 2018), CHD hospitalization rate per 1,000 Medicare beneficiaries (2015 to 2017), and age-adjusted diabetes mellitus percentage in adults (age >20 years). These data were obtained from the Interactive Atlas of Heart Disease and Stroke, published by the CDC. Within this atlas, death rates were gathered from the National Vital Statistics Program (Deaths), hospitalization rates were gathered from the CMS Medicare Provider Analysis and Review File Part A, and diabetes mellitus percentages were collected from the Division of Diabetes Translation.15
To characterize the use of cardiovascular (CV) medications, we calculated the rate of CV medications use, which included original prescriptions and any refills (total day supply), as reported in the 2018 Centers for Medicare & Medicaid Services Part D Medicare Prescriber Public Use File.9 We considered the total day supply data for 20 medication groups (Supplementary Table 1): ACEI; ARB; beta blockers; alpha and beta blockers; alpha blockers; Class I, III, and V antiarrhythmic medications; dihydropyridine and nondihydropyridine calcium channel blockers; aldosterone antagonists; central acting antihypertensive medications; vasodilators; diuretics; lipid-lowering drugs; insulins; oral hypoglycemic agents; anticoagulants; and antiplatelet medications. We normalized the CV medications’ day supply for each county by the 2019 county population estimate.12
A detailed description of statistical analysis methods is provided in the Supplementary Material. To model an ecological association of exposure with outcomes, we used different models for COVID-19–confirmed case rate and death rate to obtain the best fit and satisfy the assumptions. As COVID-19 is a contagious disease, confirmed cases and deaths in neighboring counties are spatially correlated. Therefore, we used the spatial autoregression model16 that allows modeling the spatial dependence in the outcomes, covariates, and unobserved errors.17 The spatial autoregression model used the generalized spatial 2-stage (method-of-moment), least-squares estimator.18 , 19 As we confirmed (Supplementary Figure 1) nearly normal distribution of standardized confirmed case rates, which had no 0 values, we used Gaussian likelihood. The model included spatial lags of the outcome variable, spatial lags of covariates, and spatially autoregressive errors. The lag operator was a spatial weighting (inverse-distance) matrix, which summarized spatial relations between counties based on the distance between county centroids. The weighting matrix was scaled so that its largest eigenvalue is 1, which guarantees nonsingularity in the model estimation. We constructed cross-sectional spatial autoregressive models. The estimator treated the errors as heteroskedastic, thus relaxing the assumption that errors represent identically distributed disturbance. We conducted the Moran test to determine whether exposure, outcome, and covariates are spatially dependent.
First, we constructed unadjusted spatial autoregression models, to investigate a geospatial association of the county population characteristics with the ACEI and ARB use rate. Next, we constructed unadjusted and bivariate spatial autoregression models to evaluate a geospatial association of the county population characteristics with the rate of COVID-19–confirmed cases. We constructed adjusted spatial autoregression models to answer the main study question: whether there is an independent association of ACEI or ARB use rates with COVID-19–confirmed case rate. The selection of covariates for adjustment was guided by confounders observed in this study and model fit. Models with COVID-19–confirmed case rate outcome were adjusted for the rate of births and deaths; Group Quarters population; percentage of female, Native American, Pacific Islander, Hispanic, and Black non-Hispanic county residents; percentage of county residents younger than 18 and older than 65 years of age; percentage of uninsured, living in poverty, residents with at least some college degree; median household income as a percent of the state total; percentage of obese, smoking, admitting insufficient sleep residents; air quality index; CDC-reported CVD hospitalization rate in Medicare beneficiaries; and CVD death rate in a total county population.
Because there were counties without documented COVID-19 deaths, to account for multimodal distribution (mixture of distributions) of COVID-19 death outcome, we constructed Bayesian zero-inflated Poisson models20 , 21 with noninformative prior. Models were adjusted for the rate of births and deaths, Group Quarters population, percentage of Hispanic residents, living in poverty, with at least some college degree, and admitting insufficient sleep, as well as air quality index, CDC-reported CVD hospitalization rate in Medicare beneficiaries, and CVD death rate in a total county population.
Cross-sectional geospatial analysis is susceptible to reverse causality bias. It is well-documented that patients with CVD and CV risk factors have a higher rate of COVID-19–confirmed cases and deaths. The rate of ACEI and ARB use indirectly indicates CVD prevalence and severity. While we adjusted our models for the broad range of confounders, including CVD mortality in a total county population, and CVD hospitalization rate in Medicare beneficiaries, reverse causality remained a concern. To assess the possibility and extent of reverse causality bias, we constructed the models for the use rate of other CV medications for each class of drugs separately, one by one. Statistical analyses were performed using STATA MP 16.1 (Stata Statistical Software: Release 16. (College Station, Texas. StataCorp LLC.). The study dataset and STATA code are provided at https://github.com/Tereshchenkolab/geospatial.
Results
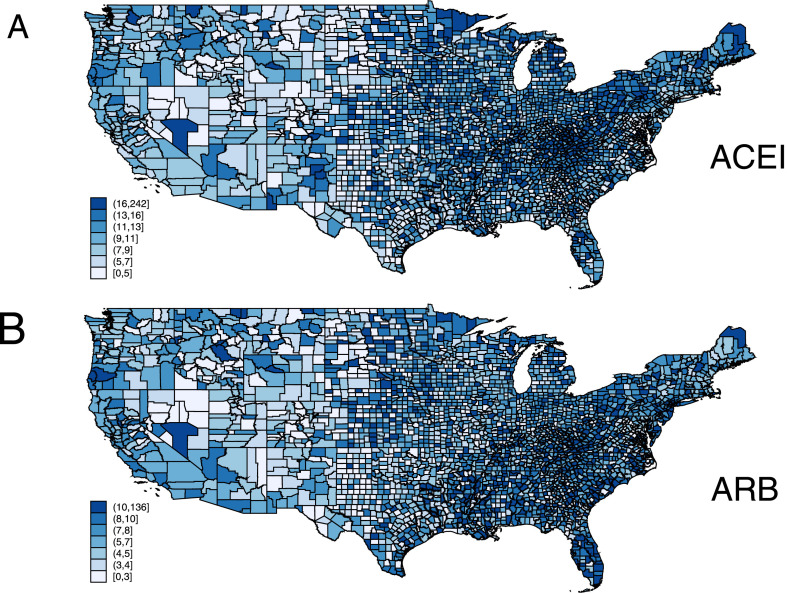
We analyzed the data of 3,141 counties and county-equivalents in the 50 states and the District of Columbia. The ACEI group was the second most ubiquitous medication, surpassed only by lipid-lowering drugs (Supplementary Table 1). Average county characteristics are reported in Table 1 . Figure 1 shows the ACEI and ARB total day supply rates across the United States. On average, the total daily supply rate was higher for ACEI than for ARB The Moran test indicated that the rates of ACEI and ARB use were spatially dependent (p <0.0001).
Table 1.
Average characteristics of counties
| Characteristic | Mean ± SD |
|---|---|
| Population in 2019 | 104,502 ± 333,504 |
| Births in 2019 per 100,000 population | 1,098.7 ± 240.5 |
| Deaths in 2019 per 100,000 population | 1,041.3 ± 269.8 |
| Group Quarters population in 2019 per 100,000 population | 3,375.5 ± 4,411.8 |
| % poverty | 15.2 ± 6.1 |
| Median household income as % of state total | 89.4 ± 20.1 |
| % Adults with self-reported poor or fair health | 17.9 ± 4.7 |
| % Adult smoking | 17.5 ± 3.6 |
| % Adult obesity | 32.9 ± 5.4 |
| % Physical inactivity | 27.4 ± 5.7 |
| % Excessive drinking | 17.5 ± 3.1 |
| % uninsured (all) | 11.5 ± 5.1 |
| % with some college education | 57.9 ± 11.8 |
| Air pollution index | 8.98 ± 2.01 |
| % of households with high housing costs | 11.1 ± 3.7 |
| % Food insecurity | 13.2 ± 4.0 |
| % insufficient sleep | 33.0 ± 4.2 |
| % population age <18 years | 22.1 ± 3.5 |
| % population age >65 years | 19.3 ± 4.7 |
| % non-Hispanic Black | 9.0 ± 14.3 |
| % Native Americans | 2.3 ± 7.7 |
| % Asians | 1.6 ± 3.0 |
| % Pacific Islanders | 0.1 ± 0.4 |
| % Hispanics | 9.7 ± 13.8 |
| % non-Hispanic White | 76.0 ± 20.2 |
| % female | 49.9 ± 2.2 |
| CVD hospitalization rate per 1,000 Medicare beneficiaries | 59.5 ± 16.7 |
| CVD death rate per 100,000 population | 239.9 ± 51.5 |
| Heart failure hospitalization rate per 1,000 Medicare beneficiaries | 15.2 ± 6.5 |
| Heart failure death rate per 100,000 population | 107.9 ± 25.8 |
| CHD hospitalization rate per 1,000 Medicare beneficiaries | 13.1 ± 4.0 |
| CHD death rate per 100,000 population | 102.7 ± 32.1 |
| Diabetes mellitus age-adjusted percentage (age > 20 years) | 10.4 ± 3.8 |
CHD = coronary heart disease; CVD = cardiovascular disease.
Figure 1.
ACEI (A) and ARB (B) total day supply rate.
In unadjusted spatial autoregression analysis (Supplementary Table 2), as expected, CVD prevalence, general demographic characteristics, uninsured rate, and air quality were associated with the use of both ACEI and ARB. A higher percentage of the total population in poverty and the lower Group Quarters population were associated with higher use of ACEI, but not ARB. A higher percentage of residents with at least some college degree was associated with the use of ARB, but not ACEI.
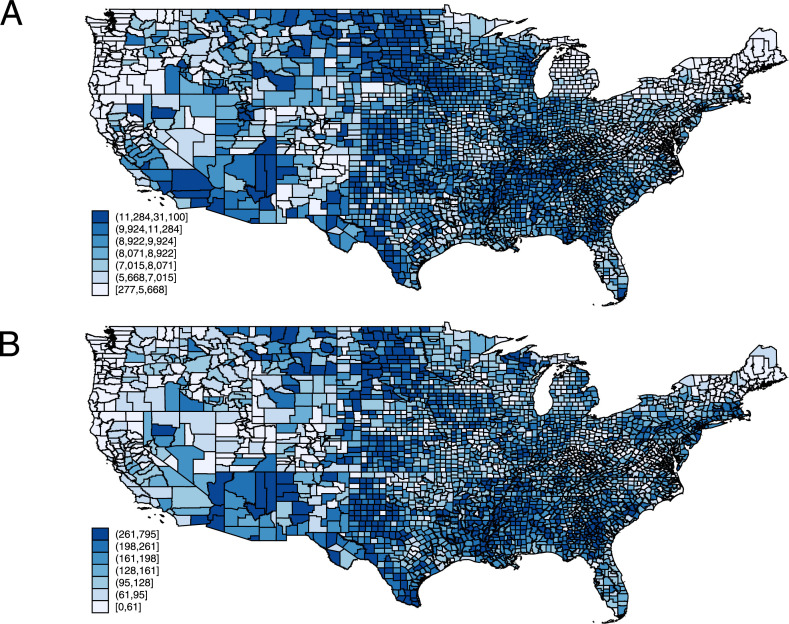
COVID-19–confirmed case and death rates (Figure 2 ) had similar geographic distributions. As of February 6, 2021, in an average county, there was a median of 8,498.1 (interquartile range 6,742.6 to 10,201.6) confirmed cases per 100,000 population. Every county had confirmed cases. The lowest rate of confirmed cases was 250.4 per 100,000 population.
Figure 2.
Confirmed COVID-19 cases (A) and deaths (B) in the United States adjusted for a county population size. Data of February 6, 2021.
In unadjusted spatial autoregression analysis (Table 2 ), higher CVD, CHD, and HF hospitalization rates in Medicare beneficiaries; higher CVD and HF mortality; a higher percentage of Black, Hispanic, and Native American residents; greater proportions of children (age <18 years); a higher percentage of residents admitting insufficient sleep, physical inactivity, smoking, obesity, and poor health; a higher percentage of uninsured, poverty, and Group Quarters population; and worse air quality were associated with a higher rate of confirmed COVID-19 cases. In contrast, a higher median household income, a larger proportion of county residents with some college education, a greater percentage of adults above 65 years of age, females, and non-Hispanic White residents were associated with a lower rate of confirmed COVID-19 cases. As expected, we observed significant indirect (spillover) effects of socioeconomic factors coming from neighboring counties on confirmed COVID-19 case rates to a given county.
Table 2.
Unadjusted ecological association of a county's sociodemographic characteristics with COVID-19–confirmed case rate
| Impact factor | Direct (county-own) effect |
Indirect (spillover) effect |
||
|---|---|---|---|---|
| per 1% rate increase | Marginal effect (95% CI) | p value | Marginal effect (95% CI) | p value |
| All CVD drugs use | +0.016 (0.007 to 0.026) | 0.001 | −0.98 (−1.45 to −0.50) | <0.0001 |
| Births in 2019 | +0.45 (0.36 to 0.55) | <0.0001 | −7.09 (−9.05 to −5.14) | <0.0001 |
| Deaths in 2019 | −0.08 (−0.16 to −0.005) | 0.037 | −0.41 (−1.26 to 0.43) | 0.337 |
| GQ Population 2019 | +0.02 (0.002 to 0.03) | 0.027 | 0.24 (−0.34 to 0.81) | 0.424 |
| Poverty | +0.01 (0.009 to 0.015) | <0.0001 | −0.05 (−0.10 to −0.013) | 0.01 |
| Median HH income | −0.001 (−0.002 to −0.01) | 0.004 | −0.17 (−0.90 to 0.57) | 0.658 |
| Poor/fair health | +2.84 (2.43 to 3.25) | <0.0001 | −21.44 (−31.34 to −11.54) | <0.0001 |
| Smoking | +2.83 (2.30 to 3.36) | <0.0001 | −77.0 (−130.5 to −23.6) | 0.005 |
| Obesity | +1.04 (0.75 to 1.34) | <0.0001 | −7.02 (−16.40 to 2.35) | 0.142 |
| Physical inactivity | +0.88 (0.59 to 1.16) | <0.0001 | +0.36 (−5.05 to 5.76) | 0.897 |
| Drinking | +0.21 (−0.51 to 0.93) | 0.573 | −1.29 (−8.71 to 6.14) | 0.734 |
| Uninsured (all) | +1.07 (0.55 to 1.59) | <0.0001 | +2.52 (−1.04 to 6.08) | 0.165 |
| Some college | −0.67 (−0.82 to −0.52) | <0.0001 | +2.58 (0.99 to 4.17) | 0.001 |
| Air pollution index | +0.07 (0.06 to 0.09) | <0.0001 | −1.22 (−1.75 to −0.69) | <0.0001 |
| HH with high cost | +0.01 (−0.50 to 0.51) | 0.984 | −34.2 (−41.5 to −26.9) | <0.0001 |
| Food insecurity | +0.18 (−0.31 to 0.67) | 0.474 | −8.48 (−14.68 to −2.64) | 0.005 |
| Insufficient sleep | +2.61 (2.06 to 3.17) | <0.0001 | −47.5 (−65.9 to −29.2) | <0.0001 |
| Population age <18 | +2.45 (1.89 to 3.02) | <0.0001 | +18.05 (16.67 to 19.43) | <0.0001 |
| Population age >65 | −2.38 (−2.76 to −2.00) | <0.0001 | +13.56 (9.07 to 18.04) | <0.0001 |
| Non-Hispanic Black | +0.49 (0.38 to 0.60) | <0.0001 | −2.73 (−3.94 to −1.51) | <0.0001 |
| Native Americans | +0.70 (0.48 to 0.92) | <0.0001 | +31.3 (26.7 to 35.6) | <0.0001 |
| Asians | −0.26 (−1.25 to 0.73) | 0.602 | −9.18 (−25.5 to 7.1) | 0.270 |
| Pacific Islanders | −8.1 (−15.7 to −0.6) | 0.034 | −369.1 (−656.2 to −81.9) | 0.012 |
| Hispanics | +1.09 (0.93 to 1.25) | <0.0001 | −4.6 (−6.8 to −2.3) | <0.0001 |
| Non-Hispanic White | −0.71 (−0.82 to −0.61) | <0.0001 | +1.34 (0.47 to 2.21) | 0.003 |
| Female | −2.31 (−3.02 to −1.61) | <0.0001 | +32.6 (25.9 to 39.2) | <0.0001 |
| CVD hospitalizations | +0.004 (0.003 to 0.006) | <0.0001 | −0.09 (−0.11 to −0.06) | <0.0001 |
| CVD death | +0.001 (0.0008 to 0.002) | <0.0001 | −0.01 (−0.018 to −0.003) | 0.006 |
| HF hospitalizations | +0.01 (0.009 to 0.014) | <0.0001 | −0.17 (−0.21 to −0.14) | <0.0001 |
| HF death | +0.0009 (0.0003 to 0.0015) | 0.005 | +0.001 (−0.008 to 0.010) | 0.830 |
| CHD hospitalizations | +0.013 (0.007 to 0.018) | <0.0001 | −0.39 (−0.59 to −0.19) | <0.0001 |
| CHD death | +0.001 (0.0009 to 0.002) | <0.0001 | −0.01 (−0.02 to −0.0009) | 0.031 |
| Diabetes mellitus | +0.01 (0.009 to 0.02) | <0.0001 | −0.15 (−0.27 to −0.04) | 0.010 |
CHD = coronary heart disease; CI = confidence interval; CVD = cardiovascular disease; HF = heart failure; HH = household.
Statistically significant findings (p<0.05) are highlighted by Bold.
In unadjusted analysis (Supplementary Table 3), both ACEI and ARB use rates were associated with confirmed COVID-19 case rates. The associations of ACEI and ARB use with COVID-19–confirmed cases were fully explained by confounders (Table 3 ). Of note, adjustment fully explained the indirect (spillover) impact of ACEI and ARB use rates coming from all neighboring counties on COVID-19–confirmed case rates to a given county, whereas all models confirmed strong spatial dependence (Moran test of spatial terms for all models p <0.00001). Marginal analysis showed no significant differences in outcomes if ACEI and ARB use rates would change in the same direction (either increase or decrease) in all counties (Supplementary Figure 2).
Table 3.
Adjusted ecological association of the rate of CV medications use with COVID-19 confirmed case rate
| Impact factor | Direct (county-own) effect |
Indirect (spillover) effect |
||
|---|---|---|---|---|
| Marginal effect (95% CI) | p value | Marginal effect (95% CI) | p value | |
| ACEI | +0.027 (−1.080 to 1.134) | 0.962 | +0.26 (−70.0 to 70.5) | 0.994 |
| ARB | +0.029 (−0.803 to 0.862) | 0.945 | +0.19 (−52.8 to 53.2) | 0.994 |
| Lipid-lowering drugs | +0.007 (−1.459 to 1.474) | 0.992 | −0.76 (−93.5 to 92.0) | 0.987 |
| CCB dihydropyridine | +0.019 (0.0008 to 0.037) | 0.041 | −0.21 (−1.79 to 1.37) | 0.796 |
| CCB nondihydropyridine | +0.014 (0.005 to 0.023) | 0.002 | −0.20 (−0.90 to 0.50) | 0.574 |
| Beta blockers | +0.020 (−0.070 to 0.110) | 0.666 | −0.09 (−6.23 to 6.05) | 0.977 |
| Alpha blockers | +0.015 (0.006 to 0.024) | 0.001 | −0.221 (−0.796 to 0.353) | 0.450 |
| Alpha and beta blockers | +0.017 (0.005 to 0.028) | 0.005 | −0.23 (−1.29 to 0.83) | 0.675 |
| Aldosterone antagonists | +0.016 (−0.005 to 0.036) | 0.138 | −0.34 (−1.96 to 1.28) | 0.680 |
| Anticoagulants | +0.019 (0.010 to 0.028) | <0.0001 | −0.21 (−0.84 to 0.41) | 0.503 |
| Antiplatelets | +0.019 (−0.020 to 0.058) | 0.331 | −0.17 (−3.05 to 2.71) | 0.909 |
| AAD class I | +0.009 (0.003 to 0.015) | 0.002 | −0.06 (−0.57 to 0.45) | 0.817 |
| AAD class III | +0.013 (−0.028 to 0.054) | 0.538 | −0.31 (−3.25 to 2.63) | 0.837 |
| AAD class V | +0.013 (−0.030 to 0.055) | 0.553 | −0.19 (−3.07 to 2.69) | 0.897 |
| Vasodilators | +0.009 (0.005 to 0.014) | <0.0001 | −0.14 (−0.70 to 0.42) | 0.615 |
| Central | +0.014 (−0.0005 to 0.029) | 0.058 | −0.17 (−1.43 to 1.09) | 0.793 |
| Loop diuretics | +0.015 (−0.043 to 0.073) | 0.615 | −0.35 (−4.42 to 3.72) | 0.866 |
| Thiazides, other diuretics | +0.019 (0.006 to 0.032) | 0.005 | −0.28 (−1.54 to 0.98) | 0.661 |
| Insulins | +0.018 (0.008 to 0.028) | <0.0001 | −0.25 (−1.03 to 0.53) | 0.532 |
| Oral hypoglycemic drugs | +0.022 (−0.268 to 0.311) | 0.881 | −0.003 (−18.7 to 18.7) | 1.000 |
AAD = antiarrhythmic drug; ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; CCB = calcium channel blocker; CI = confidence interval.
Statistically significant findings (p<0.05) are highlighted by Bold.
In unadjusted analysis, the use of CV medications reflects CVD prevalence. As expected, the use of all types of CV medications was associated with a slightly higher COVID-19–confirmed case rate (Supplementary Table 3), similar for different medications. In adjusted analysis (Table 3), the rate of use of most CV medications had no statistically significant association with COVID-19–confirmed case rate, with few exceptions. The rate of insulin use, and the rates of using medications with pronounced vasodilating effect (vasodilators, calcium channel blockers, alpha blockers), class I antiarrhythmic drugs, anticoagulants, and thiazides remained associated with the COVID-19–confirmed case rate.
As of February 6, 2021, in an average county, there was a median of 142.4 (interquartile range 84.4 to 209.3) deaths per 100,000 population (Figure 2). There were 58 counties with zero COVID-19 deaths. In both an unadjusted and adjusted Bayesian zero inflation Poisson analysis (Supplementary Table 4), neither ACEI nor ARB use rates were associated with COVID-19 death rates. Similarly, no other CV medications were statistically significantly associated with the COVID-19 death rate.
Discussion
Our study established that there was no statistically significant ecological association between the rate of ACEI/ARB use and COVID-19–confirmed case and death rate. Our results highlight the safety of ACEI/ARB use for patients with clinical indications for ACEI/ARB in the COVID-19 era, consistently with small patient-level studies.22, 23, 24, 25
Our results also highlight how socioeconomic factors and medical co-morbidities affect the COVID-19–confirmed case rate. A recent study26 showed increased odds of COVID-19 infection in patients with hypertension not on antihypertensive therapy in comparison to similar patients taking commonly prescribed antihypertensive medications, suggesting that barriers to receiving medications, such as being uninsured or being from a racial minority, are more important than the use of a specific hypertensive drug class during COVID-19. Our results also indicated that the populations with a significant prevalence of comorbidities might have an increased risk of COVID-19 infection. Reverse causality bias likely at least partially explains an association of some classes of CV medication use with the COVID-19–confirmed case rate.
Although the Medicare Part D Prescriber Public Use File has a wealth of information, the dataset has several limitations. The data may not be representative of a physician's entire practice or all of Medicare, as it only includes information on beneficiaries enrolled in the Medicare Part D prescription drug program (approximately two-thirds of all Medicare beneficiaries). In addition, available data were for the year 2018 and did not reflect the most recent use of medications in 2020. Notably, we measured exposure before the outcome, which is essential for the interpretation and supports the validity of the study findings. However, in our earlier analysis,27 we used the only available data for earlier exposure (2017) and outcome (June 11, 2020) at the time of preprint writing, which exacerbated biases.
Furthermore, we did not adjust for adherence to medications. Nevertheless, a recent geospatial study of ACEI/ARB adherence28 showed a relatively consistent geographic distribution of ACEI/ARB adherence across the United States. An observational cross-sectional ecological study is susceptible to reverse causality bias. To address this limitation, we analyzed all classes of CV medications, which helped identify and assess the strength of the reverse causality bias. In addition, unobserved confounding was likely present in this observational study. The most apparent missing data included the rate of COVID-19 testing. Therefore, observed effect sizes have to be interpreted with caution. Finally, ecological bias because of within-county variability in exposures and confounders was likely present in this ecological study. Notably, the observed county-level ecological associations should not be interpreted at the individual level. However, these ecological study results add to the totality of evidence supporting the safety of ACEI and ARB medications. Ecological study design complements other, more traditional methods in CV research, playing an increasingly important role by informing policy makers and health system managers and providing a unique insight into scientific questions.29 The strength of ecological studies includes avoiding the “individualistic fallacy” of assuming that individuals are unaffected by the community, geographical region, or health care system's characteristics.
In conclusion, in this ecological study, ACEI and ARB use rates were not associated with COVID-19 infectivity and death rate. Our findings support the safety of ACEI and ARB use in patients with CVD in the COVID-19 era. Significant ecological association of numerous socioeconomic characteristics of counties with COVID-19–confirmed case rates underscores the importance of public health policies to minimize the impact of COVID-19 on socioeconomically disadvantaged neighborhoods.
Disclosures
Dr. Tereshchenko reports receiving financial support from the National Heart, Lung, and Blood Institute (HL118277). The remaining authors have no conflicts of interest to declare.
Footnotes
This work was partially supported by the National Institutes of Health (HL118277), Medical Research Foundation of Oregon (Portland, OR), and Dr. Tereshchenko received funding support from Oregon Health & Science University President Bridge funding (Portland, OR).
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2021.10.050.
Appendix. Supplementary materials
References
- 1.Tereshchenko LG. Monitoring the spread of SARS-CoV-2 is an important public health task. Am J Public Health. 2021;111:1387–1388. doi: 10.2105/AJPH.2021.306392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin-converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofmann H, Geier M, Marzi A, Krumbiegel M, Peipp M, Fey GH, Gramberg T, Pöhlmann S. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin-converting enzyme 2 and infection can be blocked by soluble receptor. Biochem Biophys Res Commun. 2004;319:1216–1221. doi: 10.1016/j.bbrc.2004.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States Census Bureau. Cartographic boundary files - shapefile. 2018. Available at: https://www.census.gov/geographies/mapping-files/time-series/geo/carto-boundary-file.html. Accessed on May 5, 2020.
- 9.Centers for Medicare & Medicaid Services. The Medicare provider utilization and payment data: Part D prescriber public use file. 2017. Available at:https://data.cms.gov/Medicare-Part-D/Medicare-Provider-Utilization-and-Payment-Data-201/77gb-8z53/data. Accessed on May 18, 2020.
- 10.Google Maps platform. Google Geocoding API. Available at:https://developers.google.com/maps/documentation/geocoding/start. Accessed on May 18, 2020.
- 11.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United States Census Bureau. Population division. CO-EST2019-alldata: annual resident population estimates, estimated components of resident population change, and rates of the components of resident population change for states and counties: April 1, 2010 to July 1, 2019. File: 7/1/2019 County Population Estimates. Release Date. Updated October 8, 2021. Available at:https://www2.census.gov/programs-surveys/popest/datasets/2010-2019/counties/totals/. Group Quarters Information. Accessed on May 18, 2020.
- 13.The United States Department of Agriculture, Economic Research Service. County-level data sets. Available at: https://www.ers.usda.gov/data-products/county-level-data-sets/. Accessed on May 18, 2020.
- 14.County Health Rankings & Roadmaps. The Robert Wood Johnson Foundation and the University of Wisconsin population Health Institute. County Health Rankings & Roadmaps program. Available at:https://www.countyhealthrankings.org/explore-health-rankings/rankings-data-documentation. Accessed on May 18, 2020.
- 15.The Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division for Heart Disease and Stroke Prevention. Data Sources. Available at:https://www.cdc.gov/dhdsp/maps/atlas/data-sources.html#dataControls. Accessed on May 18. 2020.
- 16.Drukker DM, Egger P, Prucha IR. On two-step estimation of a spatial autoregressive model with autoregressive disturbances and endogenous regressors. Econ Rev. 2013;32:686–733. [Google Scholar]
- 17.Lee LF. Asymptotic distributions of quasi-maximum likelihood estimators for spatial autoregressive models. Econometrica. 2004;72:1899–1925. [Google Scholar]
- 18.Kelejian HH, Prucha IR. Specification and estimation of spatial autoregressive models with autoregressive and heteroskedastic disturbances. J Econom. 2010;157:53–67. doi: 10.1016/j.jeconom.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badinger H, Egger P. Estimation of higher-order spatial autoregressive cross-section models with heteroscedastic disturbances. Pap Reg Sci. 2011;90:213–235. [Google Scholar]
- 20.Lawson AB. 3rd ed. CRC Press; Boca Raton: 2018. Bayesian Disease Mapping: Hierarchical Modeling in Spatial Epidemiology. [Google Scholar]
- 21.Aregay M, Lawson AB, Faes C, Kirby RS, Carroll R, Watjou K. Zero-inflated multiscale models for aggregated small area health data. Environmetrics. 2018;29:e2477. doi: 10.1002/env.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Abajo FJ, Rodríguez-Martín S, Lerma V, Mejía-Abril G, Aguilar M, García-Luque A, Laredo L, Laosa O, Centeno-Soto GA, Ángeles Gálvez M, Puerro M, González-Rojano E, Pedraza L, de Pablo I, Abad-Santos F, Rodríguez-Mañas L, Gil M, Tobías A, Rodríguez-Miguel A, Rodríguez-Puyol D, MED-ACE2-COVID19 study group Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395:1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bean DM, Kraljevic Z, Searle T, Bendayan R, Kevin O, Pickles A, Folarin A, Roguski L, Noor K, Shek A, Zakeri R, Shah AM, Teo JTH, Dobson RJB. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur J Heart Fail. 2020;22:967–974. doi: 10.1002/ejhf.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales DR, Conover MM, You SC, Pratt N, Kostka K, Duarte-Salles T, Fernández-Bertolín S, Aragón M, DuVall SL, Lynch K, Falconer T, van Bochove K, Sung C, Matheny ME, Lambert CG, Nyberg F, Alshammari TM, Williams AE, Park RW, Weaver J, Sena AG, Schuemie MJ, Rijnbeek PR, Williams RD, Lane JCE, Prats-Uribe A, Zhang L, Areia C, Krumholz HM, Prieto-Alhambra D, Ryan PB, Hripcsak G, Suchard MA. Renin-angiotensin system blockers and susceptibility to COVID-19: an international, open science, cohort analysis. Lancet Digit Health. 2021;3:e98–e114. doi: 10.1016/S2589-7500(20)30289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semenzato L, Botton J, Drouin J, Baricault B, Vabre C, Cuenot F, Penso L, Herlemont P, Sbidian E, Weill A, Dray-Spira R, Zureik M. Antihypertensive drugs and COVID-19 risk: a cohort study of 2 million hypertensive patients. Hypertension. 2021;77:833–842. doi: 10.1161/HYPERTENSIONAHA.120.16314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An J, Wei R, Zhou H, Luong TQ, Gould MK, Mefford MT, Harrison TN, Creekmur B, Lee MS, Sim JJ, Brettler JW, Martin JP, Ong-Su AL, Reynolds K. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers use and COVID-19 infection among 824 650 patients with hypertension from a US Integrated Healthcare System. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson K, Khayyat-Kholghi M, Johnson B, Tereshchenko LG. The association between the rate of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers use and the number of Covid-19 confirmed cases and deaths in the United States: geospatial study. medRxiv Preprint posted online June 20, 2020. doi:10.1101/2020.05.31.20118802. [DOI] [PMC free article] [PubMed]
- 28.Han Y, Saran R, Erickson SR, Hirth RA, He K, Balkrishnan R. Environmental and individual predictors of medication adherence among elderly patients with hypertension and chronic kidney disease: a geospatial approach. Res Social Adm Pharm. 2020;16:422–430. doi: 10.1016/j.sapharm.2019.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu JV, Ko DT. Ecological studies and cardiovascular outcomes research. Circulation. 2008;118:2588–2593. doi: 10.1161/CIRCULATIONAHA.107.729806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.