Abstract
A nonchromogenic Mycobacterium species was isolated from an AIDS patient with acute lymphadenitis. On the basis of the results of conventional tests, the strain appeared to be an atypical nonphotochromogenic Mycobacterium kansasii strain. Sequencing of the 16S rRNA gene revealed a unique nucleic acid sequence, suggesting that the isolate represents an undescribed pathogenic species.
CASE REPORT
In December 1993, a 47-year-old human immunodeficiency virus-infected man was admitted with new-onset left axillar adenopathy. His past medical history included syphilis, gonorrhea, and chronic active hepatitis B. The patient had been treated with zidovudine since 1991, when his CD4+-cell count had dropped to below 300 × 106/liter. In 1993 he developed Kaposi's sarcoma and chronic oral candidiasis. Physical examination revealed a firm, painful left axillar adenopathy (1.5 by 1.5 cm). The patient had no fever, pulmonary signs, or enteric disorders. The white cell count was 3,800 × 106/liter, with 50% neutrophils and 30% lymphocytes, and his CD4+-cell count was 24 × 106/liter. Chest X rays showed no abnormalities. Surgical ablation of the adenopathy was performed. Histopathology showed granulomatous inflammation with focal noncaseating necrosis and diffuse acid-fast bacilli (>100/field). The excised tissue was cultured for mycobacteria and was inoculated into Middlebrook 7H12 medium (BACTEC 12B; Becton Dickinson Diagnostic Instruments, Sparks, Md.). Therapy consisted of isoniazid, rifampin, and ethambutol for 3 months, followed by isoniazid and rifampin for only 2 months because of nausea. The patient died of Pseudomonas aeruginosa pulmonary infection 4 months after the therapy was discontinued. At that time there was no new adenopathy, and blood and sputum remained negative for acid-fast bacilli.
Microbiological investigation.
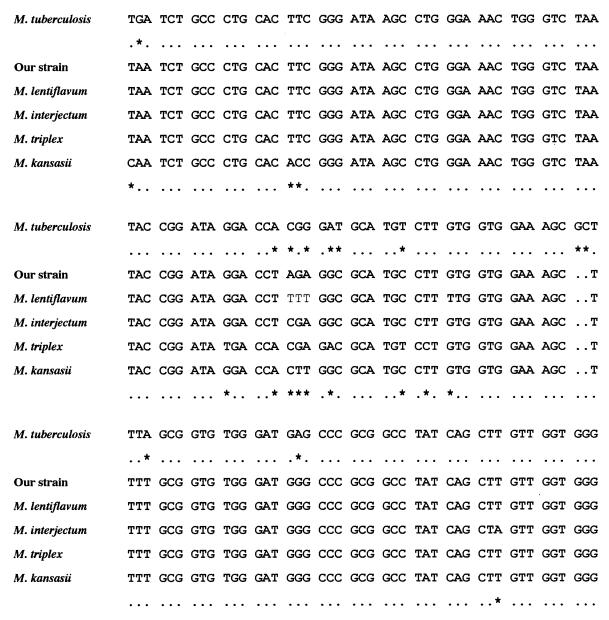
Mycobacterial growth was detected by the BACTEC radiometric method (Becton Dickinson, Towson, Md.) after 7 days of incubation. Since culture confirmation testing with use of a commercially available DNA probe for the Mycobacterium avium-M. intracellulare complex (Accu-Probe; Gen Probe Inc., San Diego, Calif.) was negative, identification was performed by biochemical tests (13), mycolic acid analysis (13), PCR-restriction fragment length polymorphism analysis (PRA) of the amplified hsp-65 gene (10), and 16S rRNA gene sequencing (7). The isolate grew as smooth, nonphotochromogenic colonies on Lowenstein-Jensen medium within 28 days at temperatures of 22 and 37°C but failed to grow at 41°C. Colonies grew on thiophen-2 carboxylic acid hydrazide and p-nitrobenzoate but not on hydroxylamine or NaCl (5%). The test for niacin production was negative, and the tests for heat-stable catalase, nitrate reductase, Tween hydrolysis, and arylsulfatase were positive (Table 1). The strain was susceptible to rifampin, ethambutol, and pyrazinamide and was resistant to isoniazid and streptomycin as determined with a radiometric system (Becton Dickinson). The mycolate pattern was I, III, and IV. According to these phenotypic data, the strain was considered most like an unusual M. kansasii strain with no photochromogenic ability. However, the molecular markers did not confirm this identification. The polymorphism of the hsp-65 gene, which encodes the 65-kDa heat shock protein, was investigated by PRA. The PRA pattern matched the M. triplex (1) and M. lentiflavum (8) PRA patterns. The 16S rRNA gene sequence within hypervariable region A was unique and differed from all previously published mycobacterial sequences in the EMBL nucleotide sequence database. The closest sequence was that of M. interjectum (6), with two nucleotide differences (Fig. 1). The sequences of the hypervariable regions A of M. kansasii, M. triplex, and M. lentiflavum showed six, six, and four different nucleotides, respectively (Fig. 1). It must be noted that the PRA pattern of our strain was distinct from the PRA pattern of M. interjectum. The combined results of the phenotypic and genotypic analyses indicate that our strain does not belong to any described Mycobacterium species.
TABLE 1.
Comparison of conventional biochemical and cultural results, mycolate pattern, and susceptibility pattern for our isolate and some of the more related speciesa
| Characteristic | Our strain | M. triplex | M. kansasii | M. interjectum | M. lentiflavum |
|---|---|---|---|---|---|
| Growth in >7 days | + | + | + | + | + |
| Growth at: | |||||
| 22°C | + | − | + | − | + |
| 37°C | + | + | + | + | + |
| 41°C | − | − | + | − | − |
| Pigmentation | − | − | + | + | + |
| Tolerance to: | |||||
| p-Nitrobenzoate | + | V | + | ||
| NaCl (5%) | − | − | − | − | |
| Hydroxylamide (500 μg/ml) | − | V | V | ||
| Thiophen-2-carboxylic acid hydrazide | + | + | + | + | |
| Enzymatic activity | |||||
| Urease | + | + | + | + | − |
| Tween hydrolysis | + | − | + | − | − |
| Nitrate reductase | + | + | + | − | − |
| Arylsulfatase, 10 days | + | V | + | − | − |
| Thermostable catalase | + | + | + | + | + |
| Niacin | − | − | − | − | − |
| Mycolate patternb | I, III, IV | I, II, IV | I, III, IV | I, IV, VI | I, II, IV |
| Susceptibility to: | |||||
| Isoniazid | R | R | R | R | R |
| Streptomycin | R | R | R | V | R |
| Ethambutol | S | R | S | R | R |
| Rifampin | S | R | S | S | R |
−, negative; +, positive; V, variable; S, susceptible; R, resistant.
I, α-mycolate; II α′-mycolate; III, methoxy-mycolate; IV, keto-mycolate; VI, dicarboxy-mycolate.
FIG. 1.
Partial alignment within hypervariable region A of selected mycobacterial 16S rRNA sequences. The sequence from M. tuberculosis was used as the reference sequence. Only nucleotides different from those in the M. tuberculosis sequence are shown. The first nucleotide corresponds to Escherichia coli position 40 or M. tuberculosis position 129 (7). Asterisks indicate nucleotide differences within the sequences of M. tuberculosis and our strain (second row) and within the sequences of our strain and any other species included in the comparative analysis (last row).
Discussion.
Previous reports have already described disagreements between phenotypic and genotypic features (11, 12), suggesting that the strains were new Mycobacterium species. During routine application of 16S rRNA sequence determination for the identification of mycobacteria, it has become clear that the genus Mycobacterium is much more diverse than was previously anticipated and harbors a significant number of yet to be described pathogens. Lymphadenitis represents the most frequent extrapulmonary disease due to mycobacteria other than tubercle bacillus infection in children (2, 5). Recently, the incidence of the disease appeared to be increasing, along with a shift in the spectrum of mycobacterial species involved. Since 1996 the list of species that cause lymphadenitis was extended with the description of M. lentiflavum, M. triplex, M. heidelbergense, and M. interjectum (1, 3, 4, 9). In all reported cases, infections due to these agents often occurred in the cervical lymph nodes of children. The pathogenic role of our strain was strongly supported by the site of isolation, the presence of acid-fast bacilli on direct examination, and histopathological observation. The clinical case reported here shows that other mycobacterial species that lack a former description may cause lymphadenitis in AIDS patients.
Acknowledgments
We thank A. Varnerot for sequencing analysis.
REFERENCES
- 1.Floyd M M, Guthertz L S, Silcox V A, Duffey P S, Jang Y, Desmond E P, Crawford J T, Butler W R. Characterization of an SAV organism and proposal of Mycobacterium triplex sp. nov. J Clin Microbiol. 1996;34:2963–2967. doi: 10.1128/jcm.34.12.2963-2967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grange J M, Yates M, Pozniak A. Bacteriologically confirmed non tuberculous mycobacterial lymphadenitis in southeast England: a recent increase in number of cases. Arch Dis Child. 1995;75:516–517. doi: 10.1136/adc.72.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas W H, Butler W R, Kirschner P, Plikaytis B P, Coyle M B, Amthor B, Steigerwalt A G, Brenner D J, Salfinger M, Crawford J T, Böttger B C, Bremer H J. A new agent of mycobacterial lymphadenitis in children: Mycobacterium heidelbergense sp. nov. J Clin Microbiol. 1997;35:3203–3209. doi: 10.1128/jcm.35.12.3203-3209.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haase G, Kentrup H, Skopnik H, Springer B, Böttger E C. Mycobacterium lentiflavum: an etiologic agent of cervical lymphadenitis. Clin Infect Dis. 1997;25:1245–1246. doi: 10.1086/516958. [DOI] [PubMed] [Google Scholar]
- 5.Hazra R, Robson C D, Perez-Atayde A R, Husson R N. Lymphadenitis due to nontuberculous mycobacteria in children and response to therapy. Clin Infect Dis. 1999;28:123–129. doi: 10.1086/515091. [DOI] [PubMed] [Google Scholar]
- 6.Lumb R, Goodwin A, Ratcliff R, Stapledon R, Holland A, Bastian I. Phenotypic and molecular characterization of three clinical isolates of Mycobacterium interjectum. J Clin Microbiol. 1997;35:2782–2785. doi: 10.1128/jcm.35.11.2782-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogall T, Flohr T, Böttger E C. Differentiation of Mycobacterium species by direct sequencing of amplified DNA. J Gen Microbiol. 1993;136:1915–1920. doi: 10.1099/00221287-136-9-1915. [DOI] [PubMed] [Google Scholar]
- 8.Springer B, Wu W, Bodmer T, Haase G, Pfyffer G E, Kroppenstedt R M, Schröder K H, Emler S, Kilburn J O, Kirschner P, Telenti A, Coyle M B, Böttger E C. Isolation and characterization of a unique group of slowly growing mycobacteria: description of Mycobacterium lentiflavum sp. nov. J Clin Microbiol. 1996;34:1100–1107. doi: 10.1128/jcm.34.5.1100-1107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Springer B, Kirschner P, Rost-Meyer G, Schröder K H, Kroppenstedt R M, Böttger E C. Mycobacterium interjectum, a new species isolated from patient with chronic lymphadenitis. J Clin Microbiol. 1993;32:3083–3089. doi: 10.1128/jcm.31.12.3083-3089.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Telenti A, Marchesi F, Balz M, Böttger E C, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tortoli E, Kischner P, Bartoloni A, Burrini C, Manfrin V, Mantella A, Scagnelli M, Scarparo C, Simonetti M T, Böttger E C. Isolation of an unusual mycobacterium from an AIDS patient. J Clin Microbiol. 1996;34:2316–2319. doi: 10.1128/jcm.34.9.2316-2319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tortoli E, Kischner P, Bartoloni A, Burrini C, Mantella A, Scagnelli M, Scarparo C, Simonetti M T, Böttger E C. Cervical lymphadenitis due to an unusual mycobacterium. Eur J Clin Microbiol Infect Dis. 1997;16:308–311. doi: 10.1007/BF01695636. [DOI] [PubMed] [Google Scholar]
- 13.Vincent Lévy-Frébault V, Portaels F. Proposed minimal standards for the genus Mycobacterium and for the description of slowly-growing bacteria. Int J Syst Bacteriol. 1992;42:315–323. doi: 10.1099/00207713-42-2-315. [DOI] [PubMed] [Google Scholar]