Abstract
In severe asthma with type 2 (T2) inflammation, biologics targeting key mediators of T2 inflammation, including interleukin (IL)-5, IL-4/IL-13, and immunoglobulin (Ig)E, remarkably improve the management of severe asthma, providing new insights into the clinical course of asthma such as disease modification and broad modulation of T2 inflammation. Once severe asthma has become a “controllable” condition, the question of discontinuation of biologics arises due to cost and side effects. The studies on discontinuing biologics in asthma demonstrate that some of patients successfully discontinue biologics, indicating that it is a feasible option in a subset of patients. Incorporating the evidence of discontinuation, we propose the criteria for the discontinuation of biologics. Our proposed criteria for the discontinuation of biologics consist of an absence of asthma symptoms (asthma control questionnaire [ACQ] score < 1.5 or asthma control test [ACT] score > 19), no asthma exacerbations, no use of oral corticosteroids, normalized spirometry (forced exhaled volume in 1 second [FEV1] ≥ 80%), suppressed T2 inflammation (blood eosinophil counts < 300 μL and fractional exhaled nitric oxide [FeNO] < 50 ppb), and control of asthma comorbidities. Real-world evidence verified a subset of patients achieving highly well-controlled conditions after use of biologics, namely super-responders, who are candidates for the discontinuation of biologics. If super-responders meet all of the criteria, they are allowed to discontinue biological therapies. Our proposed algorithm may support physicians’ treatment decisions for patients receiving biologics.
Keywords: biologics, discontinuation, severe asthma, super-responder
Introduction
Asthma is among the most common chronic respiratory diseases worldwide for all age groups.1 Severe asthma, a condition with uncontrolled symptoms and higher exacerbation rates despite medium- or high-dose inhaled treatments with a second controller (GINA step 4–5) with good adherence and inhaler technique, affects 3.7% of patients with asthma.2 An estimated 70–80% of patients with severe asthma show evidence of type 2 (T2) inflammation,3,4 which is clinically characterized by increased blood and airway eosinophils, elevated serum immunoglobulin (Ig)E, and fractional exhaled nitric oxide (FeNO) levels.
Biologics targeting mediators of T2 inflammation (eg, IgE, interleukin [IL]-5, and IL-4/IL-13) have dramatically improved the management of severe asthma during the past decade, decreasing exacerbations and oral corticosteroid use.5–10 In current guidelines, severe asthma patients with the T2 phenotype are eligible for biologics, accounting for 85% of severe asthma patients.11 Recent advances in asthma management, such as the advent of biologics, have provided new insight into the clinical course of asthma such as disease modification and broad modulation of T2 inflammation.12
However, responses to biologics vary among patients.13–17 Some patients respond to biologics, while others do not, and predictors of a response remain to be elucidated. The high cost of biologics creates an economic problem in patients and healthcare systems, and some patients may have difficulty continuing them as a result.18 Nonetheless, current guidelines provide no information about which patients with severe asthma should stop biologics despite addressing when and how they should be initiated.1
A management goal in patients with severe asthma is well-controlled condition, which is defined as the absence of symptoms, optimized lung function, no use of oral corticosteroids (OCS), and no exacerbations. Although this goal is not achievable for all the patients receiving biologics, there may be cases in which biologics can be discontinued if tight asthma control is maintained over time.
In our institution, we experienced two cases with severe asthma who achieved extremely well-controlled conditions after benralizumab (no exacerbations, no OCS use, absence of significant asthma symptoms, normalized lung function, suppressed T2 inflammation assessed by blood eosinophils, and fractional exhaled nitric oxide [FeNO]). Two patients discontinued benralizumab following the dramatic improvement by the biologic agent. One of our two cases achieved a well-controlled status even after discontinuation of the biologic agent, which stresses the hypothesis that stopping biologics is a feasible option in some patients with well-controlled asthma. In fact, many previous reports demonstrated that some cases of severe asthma were dramatically improved with biologics.13–17 However, no algorithm or criteria have been proposed to determine which patients should be considered for biologics discontinuation. Here we discuss how discontinuing biologics can be approached to both maintain tight asthma control and reduce the health care economic burden.
Discontinuation of Biologics in Patients with Severe Asthma
Here we review the current evidence about discontinuing biologics in cases of severe asthma. Six studies related to the discontinuation of biologics in severe asthma have been published to date (Table 1).19–24 Of them, two were related to the anti-IgE biologic omalizumab.19,20 Three studies were related to the anti-interleukin 5 (anti-IL-5) biologic mepolizumab.21–23 The remaining study was on all kinds of biologics (omalizumab, mepolizumab, benralizumab, reslizumab, and dupilumab).24
Table 1.
Summary of Discontinuation of Biologics Among Patients with Severe Asthma
| Biologic | Patient Number Discontinued/Continued | Study Design | Main Results | References |
|---|---|---|---|---|
| Omalizumab | 88/88 | Randomized, placebo-controlled, double-blind trial | An increase in the rate of asthma exacerbations by 20.0%. | [19] |
| Omalizumab | 49/0 | Non-controlled, observational | The rate of maintained asthma control, defined as patients without exacerbations, were 75.5% at 1 year and 60% at 4 years. | [20] |
| Mepolizumab | 27/0 | Non-controlled, observational | Worsening of asthma symptoms (mean increase in ACQ, 0.59 points). | [21] |
| Mepolizumab | 592/0 | Non-controlled, observational | Deterioration of asthma symptoms (mean increase in ACQ, 0.35 points). | [22] |
| Mepolizumab | 151/144 | Randomized, placebo-controlled, double-blind trial | An increase rate of significant exacerbations by 14%, but the rate of exacerbations requiring ED visit and hospitalization was not elevated. | [23] |
| Omalizumab Dupilumab Mepolizumab Benralizumab Reslizumab |
1247/1247 | Controlled (propensity score matched), Observational | No risk of asthma exacerbation requiring ED visit or administration of systemic corticosteroid. | [24] |
Abbreviations: ACQ, asthma control questionnaire; ED, emergency department.
The Xolair Persistency Of Response After Long-Term Therapy (XPORT) study, a 52-week multicenter randomized double-blind study, evaluated the effects of discontinuing omalizumab in patients with severe asthma.19 The XPORT trial randomly assigned 88 patients as the discontinued group (placebo) and 88 patients as the continued group (omalizumab) for 1 year. The primary endpoint was asthma exacerbations defined as a clinically significant deterioration of asthma requiring a systemic corticosteroid, a hospitalization, or an emergency department (ED) visit. Although the exacerbation rate was higher in the discontinuation group (52.3%) than in the continuation group (33.0%), the conditions of 47.7% of subjects who discontinued the biologics remained well-controlled regardless. Notably, the mean baseline blood eosinophil count in the discontinuation group was significantly lower among patients without exacerbations than in those with exacerbations. Moreover, in the discontinued group without exacerbations, FeNO levels were not increased after the withdrawal of omalizumab. This trial provides two important insights into the discontinuation of biologics. First, the conditions of nearly half of the patients who discontinued the biologic agent remained well-controlled. Second, patients without exacerbations post-withdrawal showed lower peripheral eosinophil counts during biologic treatment and no increase in FeNO level compared to those with exacerbations.
In an open prospective study, Vennera et al reported the efficacy of omalizumab for 4 years after its discontinuation among 49 patients with severe asthma.20 The study showed that the effects of the long-term use of omalizumab persisted for at least 4 years after treatment discontinuation in 60% of patients. There tended to be more patients with chronic rhinosinusitis, nasal polyps, and non-steroidal anti-inflammatory drug intolerance in the failure group (those who experienced exacerbations after discontinuation) than in the success group, although the difference was not statistically significant (P = 0.09). This finding indicates that the presence of comorbidities may be a potential predictor of failure after discontinuation.
Halder et al evaluated asthma outcomes after stopping mepolizumab in 27 patients with severe asthma.21 In this study, the discontinuation of mepolizumab led to more exacerbations (rate increased from 0.56/patient to 1.2/patient over 6 months). Twelve months after the discontinuation of mepolizumab, the mean score on the modified Juniper Asthma Control Questionnaire (ACQ), which is three shortened versions of the ACQ,25 increased by 0.59 points with an increase in blood eosinophil count. This study concluded that the withdrawal of mepolizumab was associated with increased asthma exacerbations due to the recurrence of eosinophilic airway inflammation. However, in this study, the baseline mean modified Juniper ACQ score upon the discontinuation of mepolizumab was 2.1 points, which is considered a “not well-controlled” condition.26 Thus, the deterioration of asthma control after the discontinuation of mepolizumab might be due to residual asthma symptoms.
Ortega et al reported the outcomes following the discontinuation of mepolizumab of 592 patients who participated in the COSMOS trial.22 This study evaluated the changes in ACQ-5 score and blood eosinophil counts 12 weeks after the cessation of mepolizumab. At the discontinuation of mepolizumab, the mean ACQ-5 score was 1.31 points. Twelve weeks after the last administration of mepolizumab, the mean ACQ-5 score had increased to 1.66 points in parallel with increasing blood eosinophil counts. The mean ACQ-5 score increase after discontinuation was only 0.35 points, which is not considered clinically significant. Thus, this study indicated that the cessation of mepolizumab does not contribute to a significant deterioration in asthma symptoms over 12 weeks post-discontinuation.
The randomized double-blind placebo-controlled stopping versus continuing long-term mepolizumab treatment in severe eosinophilic asthma (COMET) study examined the impact of mepolizumab discontinuation on exacerbations defined as worsening of asthma requiring the use of systemic corticosteroids and/or hospitalization or an ED visit.23 The COMET study randomized 151 patients to the stopped group (placebo) and 144 patients to the continued group (mepolizumab) for 1 year. Patients who stopped mepolizumab experienced more exacerbations than those who continued therapy (61% versus 47%, respectively). In contrast, the exacerbations rate did not differ significantly between the two groups (5% versus 7%, respectively) in cases of severe exacerbations (ED visits or hospitalizations). Moreover, the difference in ACQ-5 scores and FEV1 values for patients stopping versus those continuing mepolizumab was 0.23 points and 56 mL, which was not statistically or clinically significant. Thus, in the COMET study, the increase in asthma exacerbations after the discontinuation of mepolizumab was small (the difference between the stopped and continued group was 14%), and severe exacerbations were not increased in stopped group. Asthma symptoms and pulmonary function did not deteriorate even 1 year post-discontinuation.
The remaining study on biologic cessation was an observational analysis of the United States insurance claims data of 4960 biologic users (omalizumab, dupilumab mepolizumab, benralizumab, and reslizumab).24 Among the cohort of biologics users, 1247 discontinued them (stoppers). The other 1247 biologics users who continued using them (continuers) were identified using propensity score matching with variables including age, sex, exacerbation count, comorbidities, and income. The rate of failure after the discontinuation of biologics, defined as an increase of 50% or more in exacerbations requiring the administration of systemic corticosteroids and/or hospitalization or an ED visit, was 10.2% among the stoppers and 9.5% among the continuers. This result supports the claim that the discontinuation of a biologic agent is a feasible option for patients with severe asthma, although this study had several limitations including its observational database research design using administrative data, including an unavailability of asthma symptom and pulmonary function data.
Considering the results of these studies, discontinuing biologics is a feasible strategy in suitable patients with severe asthma. These studies of discontinuing biologics verified that some patients could successfully discontinue biologics. Thus, here we discuss the characteristics of patients who successfully discontinued biologics. In the study by Vennera, patients who successfully discontinued biologics tended to have fewer asthma comorbidities (eg sinusitis, nasal polyp) than those who did not. Notably, in the XPORT study, patients who successfully discontinued omalizumab showed lower peripheral eosinophil counts during biologic treatment than those who failed to discontinue treatment, which indicates that suppressed T2 inflammation may be a predictor of successful discontinuation. Halder et al concluded that the cessation of mepolizumab led to the deterioration of asthma control, whereas the mean ACQ score at discontinuation was 2.1 points (“not well-controlled”).21 This may merely indicate that residual asthma symptoms are associated with worsening asthma outcomes after the discontinuation of biologics rather than denying the feasibility of discontinuation. In the post hoc analysis of the COSMOS trial, worsening of asthma symptoms after the discontinuation of mepolizumab was not clinically significant (a 0.35 increase in ACQ score). Thus, these studies indicate that fewer asthma symptoms, the suppression of T2 inflammation (lower blood eosinophil count and/or FeNO level), and control of asthma comorbidities may be associated with discontinuation success of biologics. Further research on predictors of sustained well-controlled conditions after the discontinuation of biologics is required to identify patients who are suitable candidates for discontinuation.
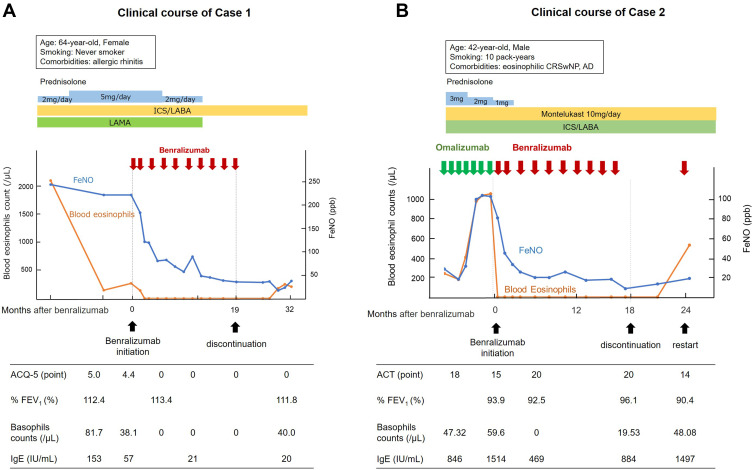
Next we showed the effects of the discontinuation of benralizumab in two cases of severe eosinophilic asthma in our hospital with extremely well-controlled conditions after treatment. The clinical courses of our two cases are shown in Figure 1A and B. Although both cases were OCS-dependent due to severe uncontrolled asthma, after long-term treatment with benralizumab, both patients achieved no exacerbations, no OCS use, an absence of asthma symptoms, normal lung function, and suppressed T2 inflammation assessed by blood eosinophil count and FeNO level. Notably, in both patients, FeNO levels and basophil counts were substantially decreased after treatment with benralizumab, a biologic agent specifically targeting IL-5 receptor alpha. In contrast, FeNO level was derived from the IL-4/IL-13/STAT-6 signaling pathway via the upregulation of inducible nitric oxide synthase.27,28 Interestingly, benralizumab decreases FeNO levels in asthma patients with high FeNO levels,29 and our results are consistent with this. In addition to the original target of the IL-5 pathway, benralizumab substantially suppresses counts of basophil and group 2 innate lymphoid cells,30,31 which are a major source of IL-4 and IL-13. Therefore, in our two patients, benralizumab might have indirectly reduced FeNO levels by suppressing innate immune cells, leading to fundamental suppression of airway and systemic T2 inflammation. After discontinuation, case 1 sustained a well-controlled status, whereas case 2 experienced deteriorated asthma symptom control. The differences between the two cases were a history of smoking and comorbidities. Case 2 had a history of smoking and comorbidity of eosinophilic chronic rhinosinusitis with nasal polyps (CRSwNP) and atopic dermatitis, while case 1 did not. Smoking and asthma comorbidities, including eosinophilic CRSwNP, adversely affect asthma control.32,33 Accordingly, the deterioration of asthma control after discontinuation in case 2 might have been due to the comorbid eosinophilic CRSwNP and atopic dermatitis. Thus, the difference of clinical course between our two cases indicates that control of comorbidities in addition to asthma control may be of importance for the maintenance of a well-controlled condition after long-term biologic therapy, even if asthma control dramatically improved (ie super-responders).
Figure 1.
The clinical courses of two cases of severe eosinophilic asthma following the discontinuation of benralizumab. (A) The clinical course of case 1. (B) The clinical course of case 2.
Abbreviations: ACQ-5, asthma control questionnaire 5; ACT, asthma control test; AD, atopic dermatitis; CRSwNP, chronic rhinosinusitis with nasal polyps; FeNO, fraction of exhaled nitric oxide; FEV1, forced exhaled volume in 1 second; ICS, inhaled corticosteroid; IgE, immunoglobulin E; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist.
Super-Responders as Candidates for Discontinuing Biologics
Recent real-world observational studies of biologics in cases of severe asthma verified that responses to biologics vary among patients.13–17 According to the degree of response to biologics, patients receiving biologics are usually classified into three categories: non-responders, partial responders, and super-responders. Non-responders are patients who show no improvement or in worsening status with biologics treatment. Partial responders are those who show some improvement but residual asthma manifestations after biologics treatment. Super-responders are patients who show a great response to biologics or complete asthma control. Here we reviewed current real-world evidence of biologic therapies focusing on super-responders as candidates for their discontinuation.
To date, the evaluation of super-responders was related to omalizumab, mepolizumab, benralizumab, and reslizumab.13–17 Fong et al reported that 33.7% of patients treated with omalizumab, an anti-IgE antibody, met the super-responders definition, which was the top quartile of the percentage reduction in OCS and no exacerbation at 16 weeks.13 They also showed that 19% of patients treated with mepolizumab, an anti-IL-5 antibody, were super-responders defined in the same way. In this study, the predictors of a super-response were no depression and no regular OCS in patients treated with omalizumab and a lower baseline ACQ-6 and lower annual exacerbation rate in patients treated with mepolizumab.13 Another study of super-response by Kavanagh et al showed that the rate of super-responders to mepolizumab, defined as no oral OCS and no asthma exacerbations at one year, was 28.3%.14 The predictors of super-responders in this report were a low BMI, nasal polyps, a lower OCS, and lower ACQ-6 scores. The same author also reported that benralizumab, an anti-IL-5 receptor antibody, led to a super-response defined as no OCS and no exacerbation at 48 weeks in 39% of patients, and the predictors were a lower OCS, adult-onset disease, nasal polyps, higher blood eosinophil count, and higher predicted FEV1.15 Another report showed that 24% of patients treated with mepolizumab met the super-responder criterion, which was the top quartile of ACQ-5 score improvement,16 while the predictors were higher blood eosinophil levels and later age at asthma onset. Notably, Eger et al reported that 14% of patients treated with anti-IL-5-targeting therapy (mepolizumab, benralizumab, and reslizumab) were super-responders defined as no OCS use, ACQ<1.5, a predicted FEV1 ≥80%, FeNO<50 ppb, and complete comorbidity control,17 which are “stricter” criteria than those of the other reports.
Thus, the definitions of super-responders vary among reports, which result in a range of prevalence (14–39%) and various features of super-responders, although asthma experts recently tried to develop an international consensus on the definition of super-responders using the Delphi process.34 When we consider discontinuing biologic therapies in patients with severe asthma receiving biologics, super-responders are candidates. Nonetheless, the various definitions of super-responders may make it complicated to identify suitable patients. Not all super-responders are eligible for discontinuing biologics because a portion of them may be at a risk of asthma deterioration after discontinuation according to the results of these studies. For example, super-responders defined as those with no exacerbations and no OCS use after biologics can present residual asthma symptoms or may be affected by comorbidities, which would lead to relapse of their asthma symptoms. The “stricter” definition of super-responder in the study by Eger et al (no regular or burst OCS use, an ACQ<1.5, a predicted FEV1 ≥80%, an FeNO <50 ppb, and complete comorbidity control) may be more suitable for use as criteria to discontinue biologics although the strictness of the definition led to a smaller prevalence of super-responders (14%).17 The reason why we prefer the “stricter” criteria is that super-responders defined by this “stricter” criteria are relevant to potential predictors for successful discontinuation (eg absence of significant asthma symptoms, suppressed T2 inflammation, control of asthma comorbidities), which may lead to a lower risk of deterioration of asthma after discontinuation.
Future Directions
When some of severe asthma has become a “controllable” condition with the advent of T2-targeting biologics, the question of their discontinuation arises due to cost and side effects. Although several studies of the discontinuation of biologics described that it was associated with the worsening of asthma outcomes in the whole population of these studies,19,21,23 a subset of patients in these studies did not worsen after biologics discontinuation, which indicated that the discontinuation of biologics could be a feasible strategy in a subset of patients. Here we discussed the feasibility of the discontinuation of biologics in patients with well-controlled conditions after treatment (ie super-responders).
We must define criteria for the discontinuation of biologics based on the measurement of disease activity in asthma. One approach incorporates established criteria for other chronic inflammatory diseases, such as rheumatoid arthritis (RA), into asthma. In RA patients, the withdrawal of biologics (eg, anti-tumor necrotizing factor-alpha antibodies, anti-IL-6 antibodies) is usually considered in patients who achieved RA remission.35 RA remission is defined by disease activity measures for RA, including disease activity score–28,36 the clinical disease activity index,37 and the simplified disease activity index.38 Importantly, these assessment tools consist of various aspects of RA, including arthritis symptoms, impaired joint count, and inflammation (C-reactive protein level and erythrocyte sedimentation rate).
Hence, the first step in discontinuing biologics is to create a disease activity measurement of asthma consisting of various aspects of asthma, including symptoms, exacerbation frequency, pulmonary function, and airway inflammation. There are currently few tools for the quantification of asthma severity. The Composite Asthma Severity Index (CASI) and Asthma Severity Scoring System (ASSESS) are comprehensive scoring systems that include subjective and objective measurements of asthma, specifically asthma symptoms, exacerbation frequency, pulmonary function, and medication use.39,40 However, the CASI was developed for childhood asthma; therefore, it lacks validation in adulthood asthma.40 The CASI and ASSESS include treatment intensity domain,39,40 which may raise the need to modify these scoring systems for use as a treatment goal. In addition to establishing criteria for discontinuing biologics, such an objective tool may also contribute to asthma management by attenuating the discordance between objective assessments and subjective judgments by physicians as described in our previous work.41 Thus, objective and comprehensive measures of asthma severity may help establish criteria for the discontinuation of biologics and reduce potential over- or undertreatment due to physician–patient discordance.
Next, it is important to cautiously determine an optimal cut-off point for the discontinuation of biologics using comprehensive assessment tools of disease activity. If the criteria are loose, the discontinuation of biologics may worsen asthma control in patients with insufficient asthma control and residual airway inflammation. As described above, real-world data of mepolizumab showed worsening of asthma symptoms after discontinuation among patients whose conditions were not completely controlled,21 while results from the discontinuation of omalizumab (XPORT study) indicate that a well-controlled status with a lack of airway inflammation characterized by eosinophil count and FeNO level was associated with no worsening of asthma control even after discontinuation.19 Sufficient control of asthma symptoms, airway inflammation, and comorbidities could be the rationale for the discontinuation of biologics. Similarly, the conditions of RA patients with residual inflammation worsened after the discontinuation of biologics, whereas those of RA patients in remission were less worsened.42–46
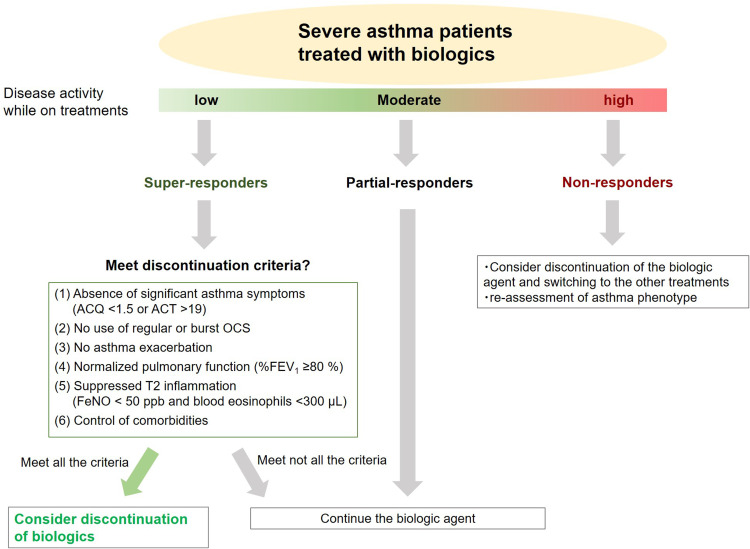
Considering these findings, we proposed a treatment algorithm for patients with severe asthma during treatment with biologics (Figure 2). Our discontinuation criteria are as follows: (1) absence of significant asthma symptoms (ACQ < 1.5 or asthma control test score >19); (2) no use of regular or burst OCS; (3) no exacerbations; (4) normalized pulmonary function (%FEV1 ≥ 80%); (5) suppressed T2 inflammation (blood eosinophil count < 300 cells/μL and FeNO level < 50 ppb); and (6) control of asthma comorbidities. Among super-responders, a candidate for discontinuation, physicians will assess whether they meet the criteria. If super-responders meet all criteria, physicians may consider discontinuing the biologics; otherwise, the patients may be recommended to continue the biologic agent. Of course, our proposed criteria for discontinuation require validation and refining to bring value to patients.
Figure 2.
The treatment algorithm for severe asthma patients during treatment with biologics. If super-responders meet all criteria for discontinuation, physicians may consider discontinuing the biologics.
Thus, although strict criteria for the discontinuation of biologics may reduce the number of patients who meet the criteria, the criteria must be rather strict to prevent worsening of asthma outcomes after discontinuation. In the process of determining a cut-off point of assessment tools for the criteria, the frequency of patients who meet the criteria also requires assessment. This is easier said than done, as an optimal “cut point” of severity measurements for criteria of the discontinuation should be determined with balancing of the strictness of criteria and the proportion of the population meeting the criteria. After establishing a treatment goal and a comprehensive assessment tool for asthma, the effects of stopping biologics should be carefully evaluated. A randomized controlled clinical trial of withdrawal based on criteria for discontinuation of biologics is required to verify the feasibility of discontinuing biologics and identifying the predictors of discontinuation success in patients with asthma.
Conclusion
In summary, evidence from studies of the discontinuation of biologics suggests that discontinuation of biologics is a feasible option in a subset of patients with severe asthma who attained a well-controlled condition such as super-responders. From these studies, the absence of asthma symptoms, suppressed T2 inflammation characterized by blood eosinophil count and FeNO level, and the control of allergic comorbidities may be associated with successful discontinuation. Incorporating the evidence of discontinuation and super-responders, we proposed criteria for the discontinuation of biologics. We hope that our proposed criteria support physicians’ decisions about their patients stopping or continuing biologics in cases of severe asthma. Our proposed discontinuation criteria require validation and refining through further studies.
Acknowledgments
We would like to thank for Editage for English language editing.
Disclosure
The authors report no conflicts of interest regarding this work.
References
- 1.Global initiative for asthma. Global strategy for asthma management and prevention; 2021. Available from: https://ginasthma.org/gina-reports/. Accessed July 1, 2021.
- 2.Hekking PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 3.Frøssing L, Silberbrandt A, Von Bülow A, Backer V, Porsbjerg C. The prevalence of subtypes of type 2 inflammation in an unselected population of patients with severe asthma. J Allergy Clin Immunol Pract. 2021;9(3):1267–1275. doi: 10.1016/j.jaip.2020.09.051 [DOI] [PubMed] [Google Scholar]
- 4.Matsusaka M, Fukunaga K, Kabata H, Izuhara K, Asano K, Betsuyaku T. Subphenotypes of type 2 severe asthma in adults. J Allergy Clin Immunol Pract. 2018;6(1):274–e276.e2. doi: 10.1016/j.jaip.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 5.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (Sirocco): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 6.Busse W, Corren J, Lanier BQ, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108(2):184–190. doi: 10.1067/mai.2001.117880 [DOI] [PubMed] [Google Scholar]
- 7.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 8.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 9.Khatri S, Moore W, Gibson PG, et al. Assessment of the long-term safety of mepolizumab and durability of clinical response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2019;143(5):1742–1751.e7. doi: 10.1016/j.jaci.2018.09.033 [DOI] [PubMed] [Google Scholar]
- 10.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 11.Akenroye A, McCormack M, Keet C. Severe asthma in the US population and eligibility for mAb therapy. J Allergy Clin Immunol. 2020;145(4):1295–1297.e6. doi: 10.1016/j.jaci.2019.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu AC, Busse WW. Biologic therapy in allergy practice: a new era in treatment has begun. J Allergy Clin Immunol Pract. 2021;9(3):1118–1120. doi: 10.1016/j.jaip.2020.12.036 [DOI] [PubMed] [Google Scholar]
- 13.Fong WCG, Azim A, Knight D, et al. Real-world omalizumab and mepolizumab treated difficult asthma phenotypes and their clinical outcomes. Clin Exp Allergy. 2021;51(8):1019–1032. doi: 10.1111/cea.13882 [DOI] [PubMed] [Google Scholar]
- 14.Kavanagh JE, d’Ancona G, Elstad M, et al. Real-world effectiveness and the characteristics of a “super-responder” to mepolizumab in severe eosinophilic asthma. Chest. 2020;158(2):491–500. doi: 10.1016/j.chest.2020.03.042 [DOI] [PubMed] [Google Scholar]
- 15.Kavanagh JE, Hearn AP, Dhariwal J, et al. Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest. 2021;159(2):496–506. doi: 10.1016/j.chest.2020.08.2083 [DOI] [PubMed] [Google Scholar]
- 16.Harvey ES, Langton D, Katelaris C, et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J. 2020;55(5):1902420. doi: 10.1183/13993003.02420-2019 [DOI] [PubMed] [Google Scholar]
- 17.Eger K, Kroes JA, Ten Brinke A, Bel EH. Long-term therapy response to anti-IL-5 biologics in severe asthma-a real-life evaluation. J Allergy Clin Immunol Pract. 2021;9(3):1194–1200. doi: 10.1016/j.jaip.2020.10.010 [DOI] [PubMed] [Google Scholar]
- 18.Anderson WC 3rd, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol. 2019;122(4):367–372. doi: 10.1016/j.anai.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 19.Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140(1):162–169.e2. doi: 10.1016/j.jaci.2016.08.054 [DOI] [PubMed] [Google Scholar]
- 20.Vennera MDC, Sabadell C, Picado C. Spanish omalizumab registry. duration of the efficacy of omalizumab after treatment discontinuation in “real life” severe asthma. Thorax. 2018;73(8):782–784. doi: 10.1136/thoraxjnl-2017-210017 [DOI] [PubMed] [Google Scholar]
- 21.Haldar P, Brightling CE, Singapuri A, et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysis. J Allergy Clin Immunol. 2014;133(3):921–923. doi: 10.1016/j.jaci.2013.11.026 [DOI] [PubMed] [Google Scholar]
- 22.Ortega H, Lemiere C, Llanos JP, et al. Outcomes following mepolizumab treatment discontinuation: real-world experience from an open-label trial. Allergy Asthma Clin Immunol. 2019;15:37. doi: 10.1186/s13223-019-0348-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore WC, Kornmann O, Humbert M, et al. Stopping versus continuing long-term mepolizumab treatment in severe eosinophilic asthma (COMET study). Eur Respir J. 2021:2100396. doi: 10.1183/13993003.00396-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffery MM, Inselman JW, Maddux JT, Lam RW, Shah ND, Rank MA. Asthma patients who stop asthma biologics have a similar risk of asthma exacerbations as those who continue asthma biologics. J Allergy Clin Immunol Pract. 2021;9(7):2742–2750.e1. doi: 10.1016/j.jaip.2021.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med. 2005;99(5):553–558. doi: 10.1016/j.rmed.2004.10.008 [DOI] [PubMed] [Google Scholar]
- 26.Juniper EF, Bousquet J, Abetz L, Bateman ED; GOAL Committee. Identifying “well-controlled” and “not well-controlled” asthma using the asthma control questionnaire. Respir Med. 2006;100(4):616–621. doi: 10.1016/j.rmed.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 27.Ludviksdottir D, Diamant Z, Alving K, Bjermer L, Malinovschi A. Clinical aspects of using exhaled NO in asthma diagnosis and management. Clin Respir J. 2012;6(4):193–207. doi: 10.1111/crj.12001 [DOI] [PubMed] [Google Scholar]
- 28.Matsunaga K, Kuwahira I, Hanaoka M, et al. An official JRS statement: the principles of fractional exhaled nitric oxide (FeNO) measurement and interpretation of the results in clinical practice. Respir Investig. 2021;59(1):34–52. doi: 10.1016/j.resinv.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 29.Hearn AP, Kavanagh J, d’Ancona G, et al. The relationship between feno and effectiveness of mepolizumab and benralizumab in severe eosinophilic asthma. J Allergy Clin Immunol Pract. 2021;9(5):2093–2096.e1. doi: 10.1016/j.jaip.2021.01.008 [DOI] [PubMed] [Google Scholar]
- 30.Lommatzsch M, Marchewski H, Schwefel G, Stoll P, Virchow JC, Bratke K. Benralizumab strongly reduces blood basophils in severe eosinophilic asthma. Clin Exp Allergy. 2020;50(11):1267–1269. doi: 10.1111/cea.13720 [DOI] [PubMed] [Google Scholar]
- 31.Sehmi R, Lim HF, Mukherjee M, et al. Benralizumab attenuates airway eosinophilia in prednisone-dependent asthma. J Allergy Clin Immunol. 2018;141(4):1529–1532.e8. doi: 10.1016/j.jaci.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 32.Boulet LP. Influence of comorbid conditions on asthma. Eur Respir J. 2009;33(4):897–906. doi: 10.1183/09031936.00121308 [DOI] [PubMed] [Google Scholar]
- 33.Hamada K, Oishi K, Chikumoto A, et al. Impact of sinus surgery on type 2 airway and systemic inflammation in asthma. J Asthma. 2021;58(6):750–758. doi: 10.1080/02770903.2020.1729380 [DOI] [PubMed] [Google Scholar]
- 34.Upham JW, Le Lievre C, Jackson DJ, et al. Defining a severe asthma super-responder: findings from a delphi process. J Allergy Clin Immunol Pract. 2021;9(11):3997–4004. doi: 10.1016/j.jaip.2021.06.041 [DOI] [PubMed] [Google Scholar]
- 35.Schett G, Emery P, Tanaka Y, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis. 2016;75(8):1428–1437. doi: 10.1136/annrheumdis-2016-209201 [DOI] [PubMed] [Google Scholar]
- 36.Fransen J, Creemers MC, Van Riel PL. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology. 2004;43(10):1252–1255. doi: 10.1093/rheumatology/keh297 [DOI] [PubMed] [Google Scholar]
- 37.Aletaha D, Nell VP, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7(4):R796–R806. doi: 10.1186/ar1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology. 2003;42(2):244–257. doi: 10.1093/rheumatology/keg072 [DOI] [PubMed] [Google Scholar]
- 39.Fitzpatrick AM, Szefler SJ, Mauger DT, et al. Development and initial validation of the Asthma Severity Scoring System (ASSESS). J Allergy Clin Immunol. 2020;145(1):127–139. doi: 10.1016/j.jaci.2019.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wildfire JJ, Gergen PJ, Sorkness CA, et al. Development and validation of the composite asthma severity index–an outcome measure for use in children and adolescents. J Allergy Clin Immunol. 2012;129(3):694–701. doi: 10.1016/j.jaci.2011.12.962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsunaga K, Hamada K, Oishi K, Yano M, Yamaji Y, Hirano T. Factors associated with physician–patient discordance in the perception of asthma control. J Allergy Clin Immunol Pract. 2019;7(8):2634–2641. doi: 10.1016/j.jaip.2019.04.046 [DOI] [PubMed] [Google Scholar]
- 42.Hirata S, Saito K, Kubo S, et al. Discontinuation of adalimumab after attaining disease activity score 28-erythrocyte sedimentation rate remission in patients with rheumatoid arthritis (HONOR study): an observational study. Arthritis Res Ther. 2013;15(5):R135. doi: 10.1186/ar4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smolen JS, Nash P, Durez P, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet. 2013;381(9870):918–929. doi: 10.1016/S0140-6736(12)61811-X [DOI] [PubMed] [Google Scholar]
- 44.Tanaka Y, Hirata S, Kubo S, et al. Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study. Ann Rheum Dis. 2015;74(2):389–395. doi: 10.1136/annrheumdis-2013-204016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka Y, Smolen JS, Jones H, Szumski A, Marshall L, Emery P. The effect of deep or sustained remission on maintenance of remission after dose reduction or withdrawal of etanercept in patients with rheumatoid arthritis. Arthritis Res Ther. 2019;21(1):164. doi: 10.1186/s13075-019-1937-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka Y, Takeuchi T, Mimori T, et al. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by remicade in RA) study. Ann Rheum Dis. 2010;69(7):1286–1291. doi: 10.1136/ard.2009.121491 [DOI] [PMC free article] [PubMed] [Google Scholar]