Abstract
Stewart, Glenn M., Troy J. Cross, Michael J. Joyner, Steven C. Chase, Timothy Curry, Josh Lehrer-Graiwer, Kobina Dufu, Nicholas E. Vlahakis, and Bruce D. Johnson. Impact of pharmacologically left shifting the oxygen–hemoglobin dissociation curve on arterial blood gases and pulmonary gas exchange during maximal exercise in hypoxia. High Alt Med Biol. 22:249–262, 2021.
Introduction: Physiological and pathological conditions, which reduce the loading of oxygen onto hemoglobin (Hb), can impair exercise capacity and cause debilitating symptoms. Accordingly, this study examined the impact of pharmacologically left shifting the oxygen–hemoglobin dissociation curve (ODC) on arterial oxygen saturation (SaO2) and exercise capacity.
Methods: Eight healthy subjects completed a maximal incremental exercise test in hypoxia (FIO2: 0.125) and normoxia (FIO2: 0.21) before (Day 1) and after (Day 15) daily ingestion of 900 mg of voxelotor (an oxygen/Hb affinity modulator). Pulmonary gas exchange and arterial blood gases were assessed throughout exercise and at peak. Data for a 1,500 mg daily drug dose are reported in a limited cohort (n = 3).
Results: Fourteen days of drug administration left shifted the ODC (p50 measured under standard conditions, Day 1: 28.0 ± 2.1 mmHg vs. Day 15: 26.1 ± 1.8 mmHg, p < 0.05). Throughout incremental exercise in hypoxia, SaO2 was systematically higher after drug (peak exercise SaO2 on Day 1: 71 ± 2 vs. Day 15: 81% ± 2%, p < 0.001), whereas oxygen extraction (Ca-vO2 diff) and consumption (VO2) were similar (peak exercise Ca-vO2 diff on Day 1: 11.5 ± 1.7 vs. Day 15: 11.0 ± 1.8 ml/100 ml blood, p = 0.417; peak VO2 on Day 1: 2.59 ± 0.39 vs. Day 15: 2.47 ± 0.43 l/min, p = 0.127). Throughout incremental exercise in normoxia, SaO2 was systematically higher after drug, whereas peak VO2 was reduced (peak exercise SaO2 on Day 1: 93.9 ± 1.8 vs. Day 15: 95.8% ± 1.0%, p = 0.008; peak VO2 on Day 1: 3.62 ± 0.55 vs. Day 15: 3.26 ± 52 l/min, p = 0.001).
Conclusion: Pharmacologically increasing the affinity of Hb for oxygen improved SaO2 during hypoxia without impacting exercise capacity; however, left shifting the ODC in healthy individuals appears detrimental to exercise capacity in normoxia. Left shifting the ODC to different magnitudes and under more chronic forms of hypoxia warrants further study.
Keywords: GBT440, hematology, hypoxia, maximal oxygen uptake, oxygen affinity of hemoglobin, voxelotor
Introduction
Humans generally respond to mild or moderate hypoxic stimuli by decreasing the oxygen affinity of hemoglobin (Hb) and “right shifting” the oxygen–hemoglobin dissociation curve (ODC) to facilitate the unloading of oxygen at the tissue (Macdonald, 1977; Samaja et al., 1986; Richardson et al., 1998; Storz and Moriyama, 2008). A rightward ODC shift occurs predominantly through metabolic acidosis (elimination of bicarbonate in the urine) and increased 2,3diphosphoglyerate to compensate for the opposing leftward shift prompted by respiratory alkalosis (hypoxia-induced hyperventilation) (Mairbaurl and Weber, 2012).
Previous studies have attempted to pharmacologically right shift the ODC (e.g., with methylpropionic acid, RSR-13) to facilitate the unloading of oxygen from Hb into the muscle and attenuate the diffusive limitations that determine maximal oxygen uptake (Richardson et al., 1998). However, reducing the oxygen affinity of Hb can exacerbate arterial hypoxemia, thus reducing mean capillary partial pressure of oxygen (PO2) during periods of diminished inspired oxygen content (e.g., high altitude), and in chronic diseases, where hypoxia is part of the pathophysiology. Under such conditions, a reduced capillary PO2 may negate the beneficial effects of right shifting the ODC and: (1) exacerbate hypoxemia-related symptoms such as dyspnea (Burki and Lee, 2010); (2) limit exercise capacity (Adams and Welch, 1980; Calbet et al., 2003); and (3) in extreme cases lead to death (Eaton et al., 1974).
Increasing the oxygen affinity of Hb during periods of hypoxia may therefore be beneficial by improving arterial oxygen saturation and content, and thereby enhancing the delivery of oxygen to the tissue. Indeed, numerous high-altitude birds and mammals have adapted to their hypoxic environments, at least in part, by increasing Hb affinity for oxygen (Jessen et al., 1991; Storz and Moriyama, 2008), and humans with naturally occurring high-affinity Hb preserve arterial oxygen content and maximal oxygen uptake during acute hypoxia (Hebbel et al., 1978a, 1978b; Dominelli et al., 2020). In animal studies, which utilized allosteric modulators, such as 5-HMF (Eaton et al., 1974) and GBT1118 to pharmacologically increase the oxygen affinity of Hb, improvements in hemodynamics and tissue oxygenation (Dufu et al., 2017) reportedly induce protective benefits during acute and severe hypoxia (Eaton, 1974; Eaton et al., 1974; Yalcin and Cabrales, 2012).
Voxelotor, an allosteric Hb-oxygen affinity modulator, reversibly binds with high affinity and specificity to the alpha chain of Hb; stabilizing it in the R-state (Oxy–Hb state) (Dufu et al., 2017). In human and animal studies, 10%–30% Hb occupancy with voxelotor has been shown to increase the overall affinity of Hb for oxygen and increase arterial oxygen saturation and content under hypoxic conditions (Dufu et al., 2017; Stewart et al., 2020). Moreover, in experimental models, voxelotor-mediated Hb retains the Bohr effect (i.e., releases more oxygen with increasing acidity) and responds to allosteric modulators such as 2,3diphosphoglyerate, although with a mildly reduced rate of oxygen unloading (Pochron et al., 2019).
At present, it is not clear whether pharmacologically increasing the oxygen affinity of Hb in humans will alter oxygen offloading from Hb and improve tissue utilization during high-intensity hypoxic exercise. This point is of particular interest considering that a high rate of blood flow (cardiac output) can attenuate the diffusive components of oxygen transport (lung and muscle O2 diffusion) (Saltin and Strange, 1992; Calbet et al., 2003). Moreover, while potentially beneficial during acute exposure to reduced inspired oxygen tensions, left shifting the ODC during normoxia (when Hb is near maximally saturated and the effects of increasing Hb oxygen affinity are likely negligible) may impede the offloading of oxygen at the muscle. However, and since mitochondrial oxygen supply is tightly regulated by numerous determinants (Winslow et al., 1981; Winslow, 2007), it is possible that an increase in arterial oxygen content would be commensurate with an increase in venous content, such that tissue oxygenation would be preserved.
Accordingly, this study tested the hypothesis that pharmacologically increasing the oxygen affinity of Hb in healthy individuals would augment arterial oxygen saturation and delivery without altering oxygen uptake during maximal hypoxic exercise, while decrements in peak oxygen uptake would be observed under normoxic conditions when arterial oxygen tension is near maximally saturated.
Methods
Subjects
Fourteen recreationally active individuals (7 Female, 24–48 years) were recruited for the study. Participants were considered recreationally active if they attained a peak oxygen uptake above 40 ml·kg−1·min−1 for males or 35 ml·kg−1·min−1 for females during an incremental cycling test, and were currently training >5 hour·week−1. Preparticipation health screening, in accordance with the American College of Sports Medicine, ensured subjects were apparently healthy nonsmokers, had no history of cardiopulmonary, metabolic, or neuromuscular disorders, and were not taking any medications. The experimental procedures were registered (NCT03051711) and approved by the Institutional Human Research Ethics Committee in accordance with the Declaration of Helsinki, and all subjects provided written and witnessed informed consent.
Study design
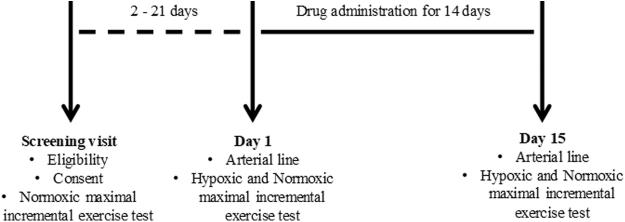
This project was an open-label study comparing exercise capacity under normoxic and hypoxic conditions before and following 14 days of either 900 or 1,500 mg daily oral administration of an allosteric Hb-oxygen affinity modulator (voxelotor). All subjects visited the laboratory on three occasions after abstaining from exercise for a minimum of 24 hours. During the first visit, subjects underwent preparticipation health screening and performed an incremental cycling test for the determination of peak exercise values (oxygen uptake and heart rate). The two subsequent visits were undertaken before (Day 1) and after (Day 15) daily oral administration of the study drug at 900 mg (n = 8) or 1,500 mg (n = 3, see details below for dropouts) for 14 days, and during each visit, subjects performed an incremental maximal exercise test while breathing hypoxic (FIO2 = 0.125; O2 partial pressure: ∼85 mmHg) or normoxic air (FIO2 = 0.21; O2 partial pressure: ∼144 mmHg), see Figure 1. The FIO2 for each condition was continuously delivered through a gas tank connected to a reservoir and inline humidifier with subjects breathing from the reservoir through tubing and a two-way nonrebreathing valve. Throughout the exercise test pulmonary gas exchange, electrocardiography, and cardiac output were measured noninvasively, whereas hemodynamic variables were measured invasively, and arterial and venous blood samples were drawn for the determination of hematological variables. Following enrollment, two subjects in the 1,500 mg group were discontinued before Day 15 testing due to adverse events. One subject withdrew following development of a rash and the other subject withdrew following symptoms of fever, fainting, rash, fatigue, and headache, reported after 11 days of drug administration, listed as possibly related to study drug or possible viral infection by the monitoring physician. No abnormal cardiac, respiratory, hematological, or neurological test results were reported, and the subject was released from the study without any medical treatment. Furthermore, a third subject was withdrawn before receiving any study drug due to premature termination of the entire study by the sponsor because an associated arm of the study in patients with interstitial lung disease did not show beneficial effects for that population. Accordingly, the 1,500 mg group was not fully enrolled, resulting in 11 subjects (5 Female, 24–48 years) completing the entire study.
FIG. 1.
Schematic of the study design and experimental procedures.
Incremental exercise tests
Incremental exercise tests were performed on an upright, electronically braked cycle ergometer (Lode BV, Groningen, Netherlands) and comprised 3 min of warm-up at 60 W, before the workload was increased by 20–40 W every 3 min until the subject reached volitional exhaustion. The increments were determined by estimating each individuals' peak power output and predicting a time to exhaustion between 15 and 18 min. Peak heart rate and oxygen uptake, measured through 12-lead ECG (Case v6.01, GE Health care, Canada) and pulmonary gas exchange (Ultima CardiO2; MGC Diagnostics, St Paul, MN), were calculated as the average of the highest two consecutive 30-s bin-averaged values attained during the test. During the initial maximal exercise test (normoxic screening visit), the experimental protocol and set-up was identical to Day 1 and 15, except that subjects breathed room air and there was no arterial catheter placed. On Day 1 and 15, subjects completed two maximal incremental exercise tests separated by 60 min, which were performed under (1) hypoxia (FIO2: 0.125; O2 partial pressure: ∼85 mmHg) and (2) normoxia (FIO2: 0.21; O2 partial pressure: ∼144 mmHg), with the inspired gas mixture humidified and delivered through a nonrebreathing valve. The order of the hypoxia/normoxia exercise tests was balanced on Day 1, such that six subjects performed normoxia, then hypoxia, and five subjects performed hypoxia, then normoxia. Subjects were instructed to lay supine for 1 hour between the two exercise tests, and each subject's test order was held constant on Day 1 and 15. During each stage of exercise, ratings of perceived exertion and dyspnea were determined through the Borg and modified Borg scales, respectively.
Pulmonary gas exchange and blood flow
Breath-by-breath pulmonary gas exchange was measured using a pneumotach (Medical Graphics Corporation, St. Paul, MN) and mass spectrometer (MGA 1100; Marquette Electronics, Milwaukee, WI) configured with a commercially available software package (BreezeSuite 6.4.1 SP5; Medical Graphics Corporation). Minute ventilation (VE), oxygen uptake (VO2), carbon dioxide production (VCO2), the respiratory exchange ratio (RER), and the ventilatory equivalents for VO2 (VE/VO2) and VCO2 (VE/VCO2) were quantified breath-by-breath before being averaged for analysis. The last 60 seconds of gas exchange data during each stage of exercise was reported. The partial pressure of oxygen in the alveoli (PAO2) was calculated from the alveolar gas equation (Whipp and Wasserman, 1969), and the alveolar-to-arterial oxygen difference (A-a O2 difference) was quantified (blood gas analysis detailed below).
In addition, pulmonary blood flow as an estimate of cardiac output was measured through the open circuit acetylene “wash-in” technique, as previously described and previously validated against direct Fick cardiac output measurements (Johnson et al., 2000; Bell et al., 2003). Briefly, subjects breathed on a nonrebreathing valve that enabled the inspired air to be switched between the normoxic/hypoxic gas mixture (described above) and a mixture of 0.9% He, 0.6% C2H2, with either 21% (normoxia) or 12.5% (hypoxia) O2 and N2 balance. Subjects were instructed to breathe at 32 breaths per minute, for 12 breaths, and cardiac output was estimated from the rate of disappearance of acetylene (Bell et al., 2003).
Hemodynamic and hematological measures
Whole blood, serum, and plasma samples were collected through venipuncture and centrifugation before exercise on Day 1 and 15 for hematological, blood chemistry, and liver function assessments. Hb concentration was determined from complete blood count measurements using fresh whole blood. The oxygen partial pressure at which Hb is 50% saturated (p50) was determined from oxygen association curves using fresh whole blood from an arterial blood sample and reported at standard conditions: 37°C, 40 mmHg carbon dioxide partial pressure, and a pH of 7.40 (Hemox Analyzer; TCS Scientific Corporation, Southampton). In our laboratory, the coefficient of variation for historical data in a large cohort of healthy control subjects was 3.7% with a mean p50 of 28.6 ± 1.05 mmHg. Erythropoietin (EPO) concentration, plasma lactate, blood chemistries, and liver function were assessed from serum and plasma samples according to guidelines (Kwo et al., 2017).
An arterial catheter (FA-04020; Arrow International, Inc., Reading, PA) was placed under local anesthesia (2% lidocaine) into either the right or left radial artery by an experienced anesthesiologist for arterial blood draws and continuous blood pressure recording (transducer positioned at the height of the right atrium). At rest and during each stage of exercise, an arterial blood sample was collected for blood gas analysis (IL-1620; Instrumentation Laboratories, Lexington), including arterial oxygen saturation (SaO2), partial pressure of arterial oxygen (PaO2) and carbon dioxide (PaCO2), pH, Hb, and carboxyhemoglobin (COHb). Arterial oxygen content (CaO2) was computed as CaO2 = (1.34 × Hb × SaO2)+(PaO2 × 0.0031). Venous oxygen content (CvO2) and the arterial–venous oxygen content difference (Ca-vO2) were inferred by rearranging the Fick equation, and fractional O2 extraction was calculated by dividing Ca-vO2 difference by CaO2. Oxygen delivery (DO2) was calculated as the product of Q and CaO2, and estimates of regional tissue oxygenation (r-SO2) were continuously recorded from a sensor (SensSmart Model 8004CA; Nonin Medical, Inc., Minnesota) placed on the midline of the Vastus Lateralis through near-infrared spectroscopy using a 3-wavelength 2-sensor system (Equanox Model 7600; Nonin Medical, Inc.).
Statistical analyses
Normality of the data was determined using the Shapiro–Wilk test and all significance testing was performed using SPSS software (v22.0; SPSS, Inc., Chicago, IL). A paired samples t-test was used to determine the effect of drug on clinical measures and baseline hematological parameters performed on Day 1 and 15. While subject numbers in the 1,500 mg group (n = 3) limited the available statistical power for dose–effect comparisons (i.e., 900 mg vs. 1,500 mg), Mann–Whitney U tests were used to determine whether key outcome variables were different between doses for the available data (despite the limited statistical power). Nonetheless, data for the 900 and 1,500 mg groups are reported separately for all subsequent analysis. As such, this article reports on the effect of left shifting the ODC using a 900 mg drug dose, whereas providing supplemental data for the 1,500 mg group and refraining from extrapolating the dose–effect, which requires additional study. Two-way analyses of variance with repeated measures were performed for the normoxia and hypoxia datasets for the 900 mg cohort (n = 8) to determine any differences in physiological parameters during exercise on Day 1 and 15. Where significant main effects or interactions were observed, Bonferroni post hoc adjustments were made to further examine differences during exercise and between time periods and an alpha level of 0.05 was used for statistical significance.
Results
Subject characteristics
Baseline demographics and hematological measures on Day 1 and 15 for the 11 subjects (900 mg dose: n = 8, 1,500 mg dose: n = 3) who completed the study are presented in Table 1. Consistent with subject diaries documenting 100% adherence to drug dosing, pharmacokinetic assessment at Day 15 confirmed pharmacologically active blood levels of 571 ± 82 and 809 ± 198 μM for the 900 and 1,500 mg dosed groups, respectively. Accordingly, 14 days of drug administration left shifted the ODC as evidenced by a decrease in p50 (Table 1). EPO levels were increased on Day 15 but remained within the normal range (Fisher, 2003), whereas Hb was unchanged on Day 1 and 15 and within the expected normal healthy ranges (Table 1). Laboratory assessments at rest, including liver function tests, creatinine, lactate, and creatine kinase were not different on Day 1 and 15, with no adverse findings observed for hematological variables in any subject at any time point.
Table 1.
Subject Characteristics and Hematological Measures
| Demographic and functional measures |
900 mg dose |
1,500 mg dose |
Combined doses |
|---|---|---|---|
| Gender | (n = 8, 3 female) | (n = 3, 2 female) | (n = 11, 5 female) |
| Age, years | 36 ± 7 | 26 ± 2 | 33 ± 7 |
| Height, cm | 176 ± 8 | 171 ± 15 | 175 ± 10 |
| Weight, kg | 73.8 ± 9.5 | 68.0 ± 7.8 | 72.2 ± 9.1 |
| Body mass index, kg·m2 | 23.7 ± 2.0 | 23.3 ± 1.3 | 23.6 ± 1.8 |
| Mean arterial pressure, mmHg | 88 ± 6 | 86 ± 9 | 88 ± 7 |
| Resting heart rate, beats·min−1 | 69 ± 14 | 57 ± 5 | 66 ± 13 |
| Peak heart rate, beats·min−1 | 176 ± 6 | 180 ± 3 | 177 ± 6 |
| Peak oxygen uptake, ml·kg·min−1 | 48.8 ± 6.7 | 43.0 ± 10.6 | 47.2 ± 7.8 |
| Baseline hematological measures | |||
| EPO, mU·ml−1 | 9.4 ± 3.8 | 11.9 ± 8.7 | 10.1 ± 5.2 |
| Hb, g·dl−1 | 14.2 ± 1.0 | 13.7 ± 2.1 | 14.0 ± 1.3 |
| p50, mmHg | 28.0 ± 2.1 | 27.0 ± 0.6 | 27.7 ± 1.8 |
| Day 15 hematological measures | |||
| EPO, mU·ml−1 | 13.8 ± 4.9* | 31.3 ± 22.8 | 18.6 ± 13.7* |
| Hb, g·dl−1 | 14.5 ± 1.4 | 13.8 ± 2.9 | 14.3 ± 1.8 |
| p50, mmHg | 26.1 ± 1.0* | 24.0 ± 1.0 | 25.5 ± 1.4* |
Data are mean ± SD. p50: Oxygen tension at which Hb is 50% saturated with oxygen.
Significantly different to baseline hematological value.
EPO, erythropoietin; Hb, hemoglobin; SD, standard deviation.
Maximal cycling tests
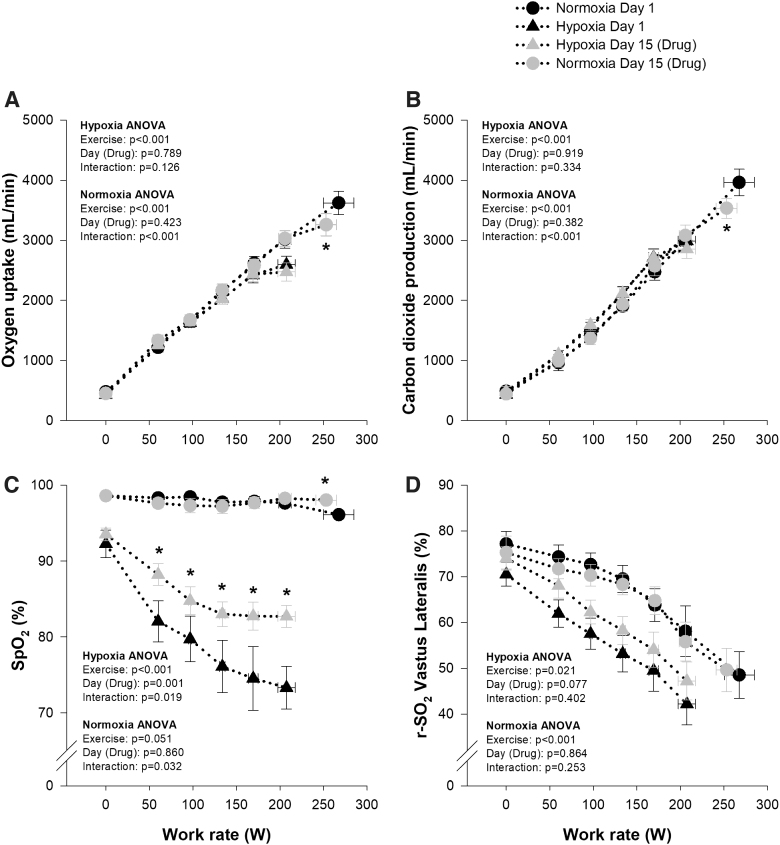
When comparing the normoxic and hypoxic maximal cycling tests on Day 1, all metrics changed as expected (Fig. 2). Briefly, maximal work rate and peak oxygen uptake were decreased during hypoxia, whereas SpO2 and r-SO2 were systematically lower at each stage of exercise (Fig. 2). When comparing maximal cycling tests between Day 1 and 15, maximal work rate was similar during hypoxia; however, time to exhaustion was slightly shorter on Day 15 (16:36 vs. 16:02 minutes:seconds, p < 0.05), whereas peak VO2 and VCO2 were unchanged (Fig. 2). During normoxia, maximal work rate, time to exhaustion (21:22 vs. 19:40 minutes:seconds, p < 0.01), and peak VO2 and VCO2 were reduced on Day 15 (Fig. 2). On Day 15, SpO2 was systematically higher throughout exercise and at peak during hypoxia and was marginally higher at peak (96.1 ± 0.5 vs. 98.0 ± 0.3, p = 0.04) during normoxia compared with Day 1, whereas r-SO2 over the Vastus Lateralis tended to be higher throughout hypoxic exercise on Day 15 compared with Day 1, and was not different during normoxia between Day 15 and 1 (Fig. 2). Ratings of perceived exertion and dyspnea at peak exercise were not different between Day 1 and 15 during hypoxia (RPE: 19 vs. 19, p = 0.732; dyspnea: 9 vs. 9, p = 0.763) or normoxia (RPE: 19 vs. 19, p = 0.685; dyspnea: 9 vs. 9, p = 0.685), nor were they different at any stage throughout the hypoxic and normoxic incremental tests on Day 1 and 15 (all p > 0.05).
FIG. 2.
Oxygen uptake (A), carbon dioxide production (B), peripheral oxygen saturation (SpO2, C), and regional tissue saturation at the site of the vastus lateralis (r-SO2, D) measured during the normoxic (FIO2 = 0.21, circles) and hypoxic (FIO2 = 0.125, triangles) maximal exercise tests before (Day 1, black symbols) and after (Day 15, gray symbols) 14 days of 900 mg daily oral voxelotor administration. *Significantly different to Day 1.
Pulmonary gas exchange
While stationary on the cycle ergometer and during the incremental exercise test VE, RER, and VE/VO2 were similar on Day 1 and 15 at all stages during both hypoxia and normoxia (Tables 2 and 3). Similarly, the VE/VCO2 ratio was not different during hypoxia (Table 2), but was higher on Day 15 compared with Day 1 during normoxia (Table 3). While stationary on the cycle ergometer and during exercise PAO2 was similar on Day 1 and 15 during hypoxia but was marginally higher on Day 15 compared with Day 1 during normoxia, whereas A-a difference was not different on Day 1 and 15 during both hypoxia and normoxia (Tables 2 and 3).
Table 2.
Hemodynamic, Gas Exchange, and Blood Gas Variables During Hypoxic Incremental Exercise on Day 1 and 15 (n = 8)
| Stationary (0 W) | Stage 2 (97 ± 2 W) | Stage 4 (171 ± 5 W) | Peak (208 ± 10 W) | ||
|---|---|---|---|---|---|
| Hemodynamic variables | |||||
| Heart rate, beats·min−1 (**) | D1 | 86 ± 6 | 137 ± 5 | 165 ± 3 | 171 ± 2 |
| D15 | 93 ± 4 | 141 ± 5 | 165 ± 4 | 172 ± 2 | |
| Mean arterial pressure, mmHg (**) | D1 | 95 ± 4 | 112 ± 4 | 121 ± 4 | 129 ± 4 |
| D15 | 95 ± 5 | 116 ± 6 | 123 ± 4 | 129 ± 3 | |
| Cardiac output, l·min−1 (**) | D1 | 8.8 ± 0.8 | 16.9 ± 0.5 | 21.8 ± 0.9 | 22.8 ± 1.2 |
| D15 | 9.5 ± 0.6 | 18.1 ± 1.1 | 22.4 ± 1.0 | 23.0 ± 1.9 | |
| Gas exchange variables | |||||
| Oxygen consumption, ml·min−1 (**) | D1 | 428 ± 26 | 1,628 ± 80 | 2,424 ± 131 | 2,597 ± 140 |
| D15 | 440 ± 52 | 1,654 ± 77 | 2,437 ± 121 | 2,474 ± 153 | |
| Carbon dioxide production, ml·min−1 (**) | D1 | 435 ± 37 | 1,550 ± 90 | 2,714 ± 143 | 2,987 ± 135 |
| D15 | 486 ± 88 | 1,593 ± 91 | 2,704 ± 146 | 2,856 ± 161 | |
| Ventilation, l·min−1 (**) | D1 | 21 ± 2 | 55 ± 3 | 105 ± 8 | 128 ± 9 |
| D15 | 29 ± 9 | 60 ± 3 | 114 ± 10 | 127 ± 9 | |
| RER (**) | D1 | 1.04 ± 0.05 | 0.95 ± 0.02 | 1.12 ± 0.01 | 1.17 ± 0.01 |
| D15 | 1.08 ± 0.08 | 0.97 ± 0.04 | 1.11 ± 0.02 | 1.16 ± 0.03 | |
| Ventilatory equivalent for oxygen uptake (**) | D1 | 52 ± 3 | 34 ± 1 | 43 ± 2 | 50 ± 2 |
| D15 | 61 ± 10 | 36 ± 2 | 47 ± 3 | 52 ± 3 | |
| Ventilatory equivalent for carbon dioxide production (**) | D1 | 50 ± 2 | 36 ± 1 | 39 ± 1 | 42 ± 2 |
| D15 | 54 ± 4 | 37 ± 1 | 42 ± 2 | 44 ± 2 | |
| Alveolar oxygen partial pressure, mmHg (**) | D1 | 57 ± 3 | 53 ± 1 | 60 ± 1 | 67 ± 3 |
| D15 | 60 ± 3 | 55 ± 1 | 61 ± 1 | 65 ± 2 | |
| Alveolar-to-arterial oxygen difference, mmHg (**) | D1 | 9 ± 1 | 16 ± 2 | 22 ± 2 | 23 ± 2 |
| D15 | 9 ± 1 | 15 ± 2 | 20 ± 2 | 20 ± 2 | |
| Blood gas variables | |||||
| Arterial oxygen saturation, % (**,††) | D1 | 82.9 ± 3.4 | 71.1 ± 2.4 | 70.4 ± 3.0 | 70.8 ± 2.6 |
| D15 | 90.9 ± 1.8† | 79.6 ± 1.5† | 79.3 ± 2.2† | 78.3 ± 1.7† | |
| Arterial oxygen partial pressure, mmHg (**) | D1 | 49 ± 4 | 37 ± 1 | 38 ± 2 | 41 ± 2 |
| D15 | 51 ± 3 | 39 ± 2 | 41 ± 2 | 45 ± 2 | |
| Arterial carbon dioxide partial pressure, mmHg (**,††) | D1 | 29 ± 2 | 31 ± 1 | 29 ± 1 | 25 ± 2 |
| D15 | 27 ± 2 | 30 ± 1† | 27 ± 1 | 24 ± 2 | |
| Arterial oxygen content, mLO2·100 ml−1 (**,††) | D1 | 17.3 ± 0.9 | 15.2 ± 0.8 | 15.2 ± 0.8 | 15.9 ± 0.8 |
| D15 | 18.5 ± 0.7 | 16.7 ± 0.6† | 17.2 ± 0.8† | 16.5 ± 0.5 | |
| Delivery of oxygen, ml·min−1 (**,††) | D1 | 1,543 ± 189 | 2,571 ± 146 | 3,313 ± 230 | 3,549 ± 318 |
| D15 | 1,792 ± 153† | 3,044 ± 224† | 3,813 ± 223† | 3,816 ± 341† | |
| Fractional oxygen extraction, % (**,††) | D1 | 31 ± 5 | 64 ± 3 | 74 ± 3 | 76 ± 4 |
| D15 | 25 ± 2 | 55 ± 2† | 63 ± 3† | 67 ± 4† | |
| Hb, g·dl−1 (**) | D1 | 14.9 ± 0.4 | 15.2 ± 0.3 | 15.3 ± 0.4 | 15.9 ± 0.4 |
| D15 | 14.4 ± 0.4 | 15.0 ± 0.3 | 15.4 ± 0.4 | 15.6 ± 0.3 | |
| COHb, % of total Hb (††) | D1 | 1.2 ± 0.1 | 1.1 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 |
| D15 | 1.5 ± 0.1† | 1.4 ± 0.1† | 1.4 ± 0.1† | 1.3 ± 0.1† | |
| Bicarbonate, mmol·l−1 (**,††) | D1 | 22 ± 0 | 22 ± 0 | 19 ± 0 | 13 ± 0 |
| D15 | 20 ± 1† | 21 ± 1 | 17 ± 1† | 12 ± 1† | |
| pH (**) | D1 | 7.50 ± 0.02 | 7.46 ± 0.01 | 7.41 ± 0.01 | 7.33 ± 0.03 |
| D15 | 7.49 ± 0.04 | 7.47 ± 0.01 | 7.41 ± 0.01 | 7.32 ± 0.02 | |
| Lactate, mmol·l−1 (**) | D1 | 2.0 ± 0.3 | — | — | 15.6 ± 1.7 |
| D15 | 2.2 ± 0.4 | — | — | 14.3 ± 1.3 | |
Data are mean ± SEM. D1: Day 1. D15: Day 15.
Significant main effect of exercise.
Significantly different to Day 1.
Significant main effect of Day (Drug).
COHb, carboxyhemoglobin; RER, respiratory exchange ratio; SEM, standard error of the mean.
Table 3.
Hemodynamic, Gas Exchange, and Blood Gas Variables During Normoxic Incremental Exercise on Day 1 and 15 (n = 8)
| Stationary (0 W) | Stage 2 (97 ± 2 W) | Stage 4 (171 ± 5 W) | Peak (D1/D15: 268 ± 17/253 ± 17 W) | ||
|---|---|---|---|---|---|
| Hemodynamic variables | |||||
| Heart rate, beats·min−1 (**) | D1 | 88 ± 5 | 120 ± 5 | 151 ± 5 | 176 ± 2 |
| D15 | 85 ± 6 | 125 ± 5 | 156 ± 5 | 174 ± 3 | |
| Mean arterial pressure, mmHg (**) | D1 | 95 ± 3 | 115 ± 10 | 118 ± 3 | 132 ± 3 |
| D15 | 95 ± 5 | 109 ± 5 | 122 ± 4 | 130 ± 3 | |
| Cardiac output, l·min−1 (**) | D1 | 8.7 ± 0.6 | 15.3 ± 0.6 | 19.4 ± 0.8 | 25.3 ± 1.5 |
| D15 | 9.2 ± 0.7 | 16.4 ± 0.8 | 19.8 ± 1.0 | 23.2 ± 1.8 | |
| Gas exchange variables | |||||
| Oxygen consumption, l·min−1 (**, *,†) | D1 | 481 ± 67 | 1,672 ± 90 | 2,619 ± 112 | 3,622 ± 194 |
| D15 | 452 ± 46 | 1,675 ± 86 | 2,583 ± 99 | 3,260 ± 185† | |
| Carbon dioxide production, l·min−1 (**, *,†) | D1 | 491 ± 68 | 1,419 ± 86 | 2,475 ± 137 | 3,965 ± 221 |
| D15 | 443 ± 50 | 1,366 ± 101 | 2,574 ± 163 | 3,534 ± 167† | |
| Ventilation, l·min−1 (**) | D1 | 23 ± 4 | 44 ± 3 | 71 ± 5 | 138 ± 12 |
| D15 | 23 ± 4 | 46 ± 4 | 84 ± 9 | 132 ± 8 | |
| RER (**) | D1 | 1.04 ± 0.07 | 0.85 ± 0.03 | 0.94 ± 0.02 | 1.10 ± 0.02 |
| D15 | 0.98 ± 0.07 | 0.82 ± 0.04 | 1.00 ± 0.04 | 1.10 ± 0.02 | |
| Ventilatory equivalent for oxygen uptake (**) | D1 | 52 ± 7 | 27 ± 2 | 27 ± 1 | 38 ± 3 |
| D15 | 51 ± 5 | 28 ± 2 | 32 ± 3 | 41 ± 3 | |
| Ventilatory equivalent for carbon dioxide production (**,††) | D1 | 48 ± 3 | 31 ± 1 | 28 ± 1 | 35 ± 2 |
| D15 | 52 ± 2† | 33 ± 1 | 33 ± 2† | 37 ± 2† | |
| Alveolar oxygen partial pressure, mmHg (**,††) | D1 | 112 ± 3 | 100 ± 2 | 105 ± 2 | 115 ± 2 |
| D15 | 113 ± 4 | 101 ± 3 | 109 ± 3† | 121 ± 2† | |
| Alveolar-to-arterial oxygen difference, mmHg (**) | D1 | 8 ± 2 | 10 ± 3 | 16 ± 2 | 27 ± 5 |
| D15 | 10 ± 2 | 10 ± 2 | 18 ± 2 | 25 ± 3 | |
| Blood gas variables | |||||
| Arterial oxygen saturation, % (**,††) | D1 | 96.8 ± 0.2 | 95.9 ± 0.4 | 95.5 ± 0.3 | 93.9 ± 0.7 |
| D15 | 97.2 ± 0.2 | 96.6 ± 0.3† | 96.1 ± 0.4† | 95.8 ± 0.3† | |
| Arterial oxygen partial pressure, mmHg (**,††) | D1 | 103 ± 4 | 90 ± 4 | 89 ± 2 | 88 ± 5 |
| D15 | 105 ± 5 | 94 ± 4 | 95 ± 5† | 96 ± 4† | |
| Arterial carbon dioxide partial pressure, mmHg (**,††) | D1 | 32 ± 2 | 37 ± 1 | 37 ± 1 | 32 ± 3 |
| D15 | 29 ± 2 | 35 ± 1 | 34 ± 2† | 25 ± 2† | |
| Arterial oxygen content, mLO2·100 ml−1 | D1 | 19.8 ± 0.6 | 19.8 ± 0.6 | 20.3 ± 0.7 | 20.7 ± 0.7 |
| D15 | 19.7 ± 0.5 | 20.0 ± 0.6 | 20.4 ± 0.5 | 19.9 ± 0.8 | |
| Delivery of oxygen, ml·min−1 (**) | D1 | 1,738 ± 134 | 3,041 ± 185 | 3,954 ± 266 | 5,199 ± 477 |
| D15 | 1,824 ± 163 | 3,306 ± 249 | 4,070 ± 304 | 4,586 ± 520 | |
| Fractional oxygen extraction, % (**) | D1 | 28 ± 4 | 56 ± 3 | 67 ± 3 | 69 ± 4 |
| D15 | 25 ± 1 | 52 ± 3 | 65 ± 4 | 74 ± 7 | |
| Hb, g·dl−1 (**) | D1 | 14.5 ± 0.5 | 14.6 ± 0.5 | 15.1 ± 0.5 | 15.6 ± 0.5 |
| D15 | 14.3 ± 0.4 | 14.7 ± 0.4 | 15.0 ± 0.3 | 15.6 ± 0.3 | |
| COHb, % of total Hb (**,††) | D1 | 1.1 ± 0.0 | 1.1 ± 0.0 | 1.1 ± 0.0 | 1.0 ± 0.0 |
| D15 | 1.6 ± 0.1† | 1.3 ± 0.1† | 1.3 ± 0.1† | 1.3 ± 0.0† | |
| Bicarbonate, mmol·l−1 (**,††) | D1 | 23 ± 1 | 23 ± 1 | 22 ± 1 | 15 ± 2 |
| D15 | 21 ± 1 | 23 ± 1 | 20 ± 1† | 12 ± 1† | |
| pH (**) | D1 | 7.46 ± 0.02 | 7.42 ± 0.01 | 7.40 ± 0.01 | 7.30 ± 0.03 |
| D15 | 7.49 ± 0.02 | 7.42 ± 0.00 | 7.39 ± 0.01 | 7.28 ± 0.02 | |
| Lactate, mmol·l−1 (**) | D1 | 2.0 ± 0.3 | − | − | 14.5 ± 1.4 |
| D15 | 1.9 ± 0.5 | − | − | 12.9 ± 1.8 | |
Data are mean ± SEM. D1: Day 1. D15: Day 15.
,†Significant interaction.
Significantly different to Day 1.
Significant main effect of exercise.
Significant main effect of Day (Drug).
Hemodynamic and hematological measures
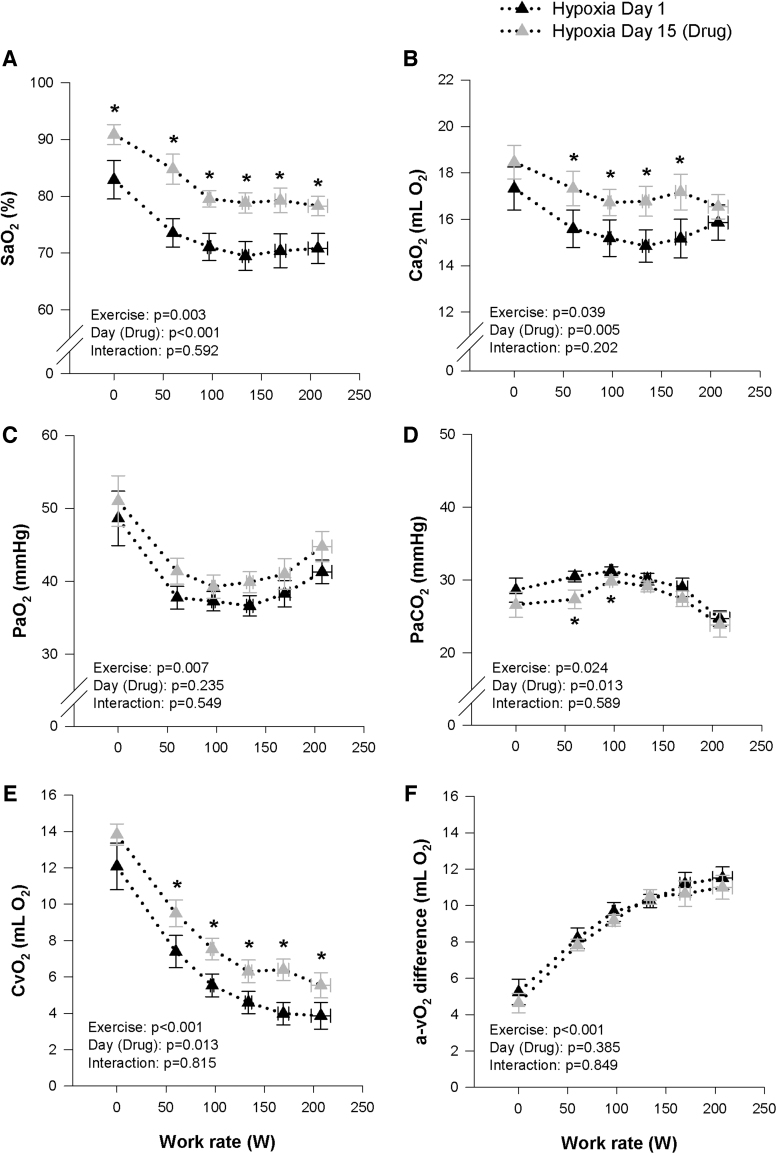
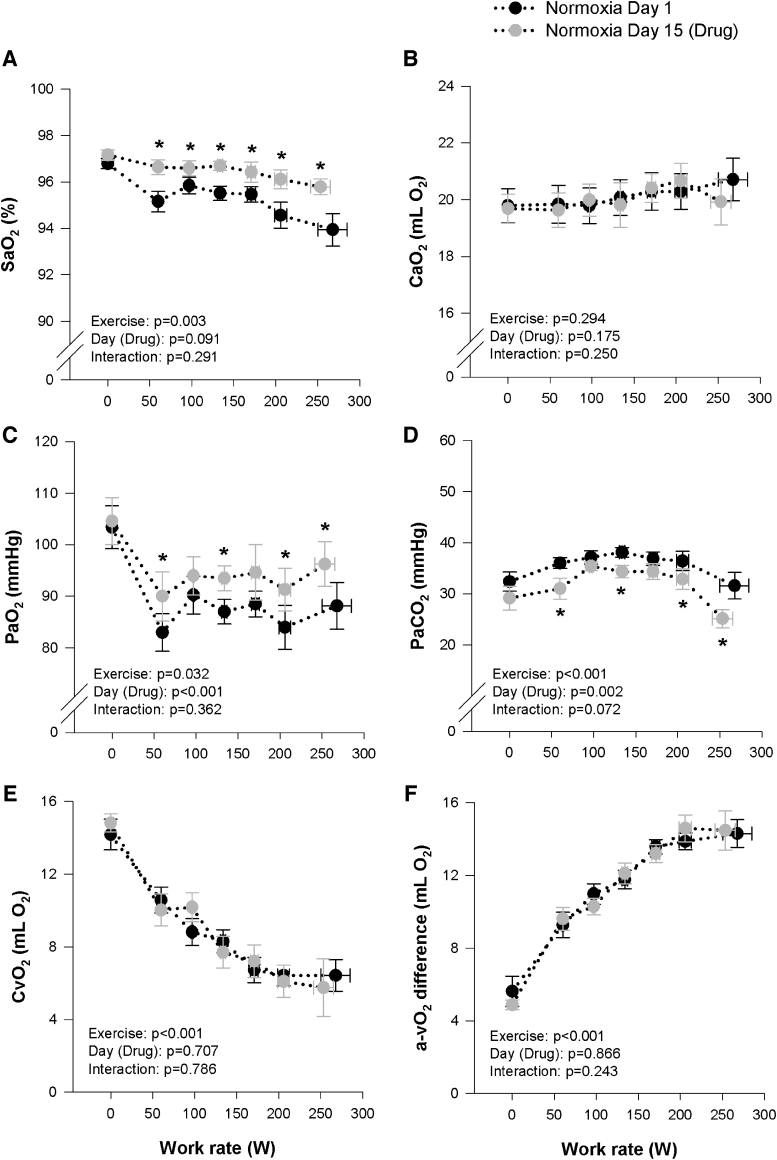
All hemodynamic variables (HR, Q, MAP) were similar during all stages of exercise on Day 1 and 15 under both normoxic and hypoxic conditions (Tables 2 and 3). However, HR did tend to be marginally higher on Day 15 during the hypoxic exercise (p = 0.064). The effects of the study drug on blood gases at rest and during incremental exercise under normoxic and hypoxic conditions are presented in Tables 2 and 3, and Figures 3 and 4. During hypoxia, SaO2, CaO2, CvO2, and PaO2 were increased on Day 15 at rest, whereas PaCO2 were unchanged. Throughout incremental hypoxic exercise and at peak, SaO2, CaO2, CvO2 were systematically higher on Day 15, compared with Day 1, while PaO2 was not different, PaCO2 was systematically lower and Ca-vO2 difference was unchanged (Fig. 3). During normoxia, the study drug had no effect on blood gases at rest. Throughout incremental normoxic exercise and at peak, SaO2 and PaO2 were systematically higher on Day 15 compared with Day 1, whereas PaCO2 was reduced and CaO2, CvO2, and Ca-vO2 difference were unchanged (Fig. 4). The increase in DO2 during hypoxia on Day 15, combined with no change in Ca-vO2 difference, lead to a decrease in fractional O2 extraction, while no differences were observed between Day 1 and 15 for DO2 or fractional O2 extraction during normoxia (Tables 2 and 3). COHb increased slightly on Day 15 compared with Day 1 during hypoxia and normoxia, but remained within normal range at all time points. Bicarbonate was systematically lower on Day 15 during normoxia and hypoxia, while lactate and pH were not different between Day 1 and 15 during both the hypoxic and normoxic trials (Tables 2 and 3).
FIG. 3.
Measures of arterial oxygen saturation (SaO2, A) and content (CaO2, B), arterial partial pressure of oxygen (PaO2, C) and carbon dioxide (PaCO2, D), and calculated venous oxygen content (CvO2, E) and arterial/venous oxygen difference (a-vO2 diff, F) during maximal hypoxic (FIO2 = 0.125) exercise before (Day 1, black triangles) and after (Day 15, gray triangles) 14 days of 900 mg daily oral voxelotor administration. *Significantly different to Day 1.
FIG. 4.
Measures of arterial oxygen saturation (SaO2, A) and content (CaO2, B), arterial partial pressure of oxygen (PaO2, C) and carbon dioxide (PaCO2, D), and calculated venous oxygen content (CvO2, E), and arterial/venous oxygen difference (a-vO2 diff, F) during maximal normoxic (FIO2 = 0.21) exercise before (Day 1, black circles) and after (Day 15, gray circles) 14 days of 900 mg daily oral voxelotor administration. *Significantly different to Day 1.
The relative contribution of VE (independent of p50) to the total change in saturation at a similar submaximal work rate (97 ± 2 W) and at peak hypoxic and normoxic exercise was estimated from the alveolar gas equation by imputing the measured PaCO2 and RER, while keeping all other parameters constant. Then, the expected change in SaO2 from a rise in PaO2 purely due to changes in VE was estimated from the relevant portion of the standard ODC curve. During submaximal hypoxic exercise (on the descending portion of the ODC), the rise in PaO2 solely from increased VE (∼2 mmHg) would account for an ∼2.8% increase in SaO2 (i.e., ∼35% of the observed increase in SaO2). During peak hypoxic exercise, the rise in PaO2 solely from increased VE (∼1 mmHg) would account for an ∼1.2% increase in SaO2 (i.e., ∼20% of the observed increase in SaO2). During submaximal normoxic exercise (on the flat portion of the ODC), the rise in PaO2 solely from increased VE (∼2 mmHg) would account for an ∼0.2% increase in SaO2 (i.e., ∼30% of the observed increase in SaO2). During peak normoxic exercise, the rise in PaO2 solely from increased VE (∼6 mmHg) would account for an ∼0.5% increase in SaO2 (i.e., around 25% of the observed increase in SaO2).
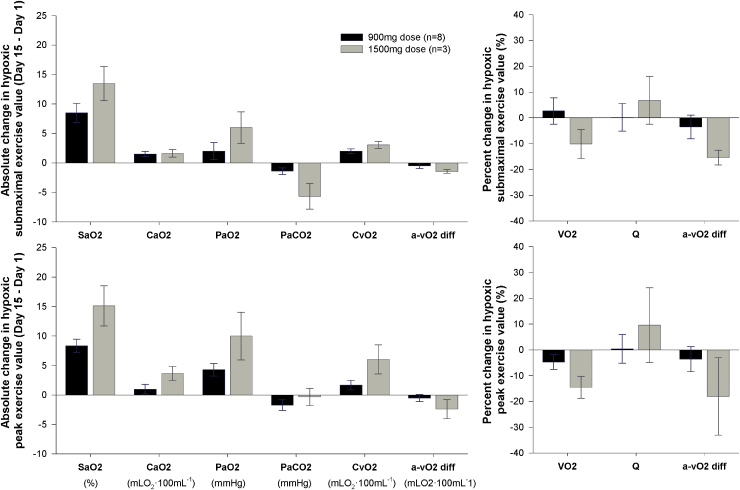
Supplemental data of dose effects
The absolute change in arterial blood gasses and oxygen transport variables during submaximal and peak hypoxic exercise from Day 1 to 15 are provided for the 1,500 mg (n = 3) and 900 mg (n = 8) groups in Figure 5. While limited subject numbers precluded any statistical comparisons, the mean data are presented in brief. During a similar submaximal work rate (97 ± 2 W) and at peak exercise, the 1,500 mg group tended to have a larger rise in SaO2 on Day 15 compared with the 900 mg group, but also tended to have a reduced VO2. The reduced peak VO2 for the 1,500 mg group on Day 15 appears to be related to an impaired Ca-vO2 difference, which was driven predominantly by a greater rise in CvO2.
FIG. 5.
Data comparing the change in arterial blood gas and oxygen transport variables at a similar submaximal exercise workload (97 ± 2 W) and at peak exercise from before (Day 1) to after (Day 15) 14 days of daily oral voxelotor administration at either 900 mg (low dose, n = 8) or 1,500 mg (high dose, n = 3).
Discussion
This study demonstrates that pharmacologically increasing the oxygen affinity of Hb left shifted the ODC and systematically increased SaO2 and content during maximal exercise in hypoxia. Oxygen uptake and cardiac output were largely unaffected by the altered Hb-oxygen affinity during incremental exercise in hypoxia. Accordingly, pharmacologically increasing the oxygen affinity of Hb preserved SaO2 and increased oxygen delivery during maximal exercise in hypoxia, but this did not improve VO2 peak. On the contrary, pharmacologically increasing the oxygen affinity of Hb during normoxia (when arterial Hb-oxygen levels are already saturated) had a minimal impact on SaO2 and content, and reduced VO2 peak. Preliminary data suggest a possible dose dependency and warrants further study.
Impact of pharmacologically left shifting the ODC during hypoxia
Similar to prior studies, shifting the ODC left (Eaton, 1974; Eaton et al., 1974) or right (Gersonde and Nicolau, 1980; Kunert et al., 1996; Wahr et al., 2001) during hypoxia, the increased SaO2 and content after drug administration accompanied a commensurate change (in this case an increase) in venous oxygen content, such that the absolute extraction at the tissue was predominantly unchanged (Eaton et al., 1974) throughout submaximal work rates and at peak exercise. Indeed, exercise-induced reductions in venous oxygen content and a widening of the arterial–venous oxygen difference (i.e., increased extraction of oxygen during exercise) were not altered by drug during low, moderate, and high-intensity hypoxic exercise, suggesting that oxygen delivery and utilization at the muscle was not limited by pharmacologically “locking” a portion of the available Hb in the R-state (high oxygen affinity) under these conditions. While the increased oxygen delivery, and thus capillary PO2, associated with voxelotor-mediated Hb should theoretically improve O2 flux into the muscle and reduce diffusion limitations, there may have been a subtle limit to oxygen extraction at peak exercise, when high flow rates through the microcirculation may evoke diffusive limitations to maximal oxygen uptake (Roca et al., 1989). However, this did not reduce the maximal work rate attained during exercise in hypoxia in this study.
Interestingly, oxygen delivery was increased (by ∼15% on average throughout exercise and ∼8% at peak) during hypoxia while on drug and this was solely driven by increased arterial oxygen content (i.e., Q did not change). In humans with naturally occurring high oxygen affinity Hb, and thus increased oxygen delivery during exercise, VO2 peak is preserved during acute hypoxic exposure (Hebbel et al., 1978a, 1978b). On the contrary, an increase in maximal oxygen uptake, which theoretically could occur with increased oxygen delivery, was not observed suggesting that diffusive O2 limitations at the muscle may have restricted any potential for an increase in maximal oxygen uptake (Hogan et al., 1991; Calbet et al., 2003). Alternatively, it cannot be ruled out that an increased blood flow and arterial oxygen supply to the tissue may have been in excess of a theoretical rate limit to oxygen offloading for the portion of Hb in the R-state, resulting in a null overall effect of drug on maximal muscle oxygen extraction and utilization during hypoxia. Moreover, the different fractions of Hb (i.e., voxelotor-mediated Hb and natural Hb) may have differing O2 unloading kinetics when passing through the peripheral capillaries. The lack of any change in regional tissue saturation of the exercising limb (although with a trend for increased tissue saturation), as well as metabolic markers, such as VO2, VCO2, and pH and lactate, during incremental exercise in hypoxia supports the notion that overall tissue oxygenation was not adversely diminished, and that oxygen diffusion out of the microcirculation sufficiently matched skeletal muscle metabolism (Koike et al., 1990).
Despite a mildly higher VE/VCO2 and reduced PaCO2 throughout incremental exercise in hypoxia after drug administration, the major determinants of oxygen transport and delivery aside from the oxygen affinity of Hb (e.g., cardiac output) were not different at peak exercise. It is possible that changes in lung diffusion and alveolar ventilation can also contribute to the increases in SaO2. Given that PAO2 and PA-a O2 difference were similar between testing days, gross changes in lung diffusion likely did not contribute to the observed changes in arterial oxygen content and saturation (Filley et al., 1954). However, the higher PaO2 and lower PaCO2 observed during exercise after drug administration suggest that hyperventilation, in part, contributed to the preserved arterial saturation during hypoxic submaximal exercise (Rotsztain et al., 1970). Using the alveolar gas equation to estimate the rise in PAO2 and thus PaO2 due purely to hyperventilation (i.e., independent from the effect of p50 or PA-a O2 difference) suggests that approximately one-third of the increase in SaO2 was due to a rise in PaO2 from hyperventilation. Thus, the observed increase in SaO2 on Day 15 does not solely reflect the impact of drug, per se, but also any other potential study effects (e.g., metabolic acidosis, placebo effect) on ventilation. Nonetheless, while in vivo p50 during exercise may differ from the pre-exercise p50 measured under standard conditions, the increase in Hb oxygen affinity observed after drug administration is likely the predominant factor which augmented SaO2 during maximal hypoxic exercise on Day 15.
Despite the trend toward a mildly increased heart rate during low-intensity exercise, there were no significant effects of left shifting the ODC on hemodynamic variables (e.g., cardiac output, mean arterial pressure) during hypoxic exercise, and despite the increased SaO2 and delivery, ratings of perceived exertions and dyspnea were not improved during maximal exercise in hypoxia. There are numerous similarities between high-altitude illness due to rapid hypoxic exposure in healthy individuals and the pathophysiological ails observed in chronic hypoxic diseases (Hackett and Roach, 2001). Notably, hypoxia-related symptoms, including dyspnea, lethargy, and nausea are commonly reported in chronic diseases where hypoxemia is a part of the pathophysiology and high-altitude sojourners alike (Honigman et al., 1993; Clark et al., 1996; Ghofrani et al., 2006). Normalization of arterial hypoxemia (either pharmacologically or through supplemental oxygen) is a common therapeutic intervention (Hackett and Roach, 2001). While the mechanisms and pathways which elicit the sensation of exertional dyspnea are incompletely understood, work rate, VO2, VCO2 and acid–base balance were mostly comparable across conditions in this study. As such, motor unit recruitment, muscle mechanoreceptors, reflex chemostimulation by CO2 and vagal C-fiber stimuli—all known to have some involvement in the genesis of dyspnea (Burki and Lee, 2010)—are unlikely to have been fundamentally altered by the ∼2–3 mmHg left shift in the ODC observed in this study. Whether pharmacologically increasing the oxygen affinity of Hb induces subtle localized changes to mitochondrial respiration, myoglobin O2 binding and acid–base balance is not clear and warrants further study.
Impact of pharmacologically left shifting the ODC during normoxia
During conditions where arterial Hb-oxygen levels are naturally saturated—for example, in healthy individuals at sea level—increasing the affinity of Hb for oxygen is anticipated to have little to no benefit while potentially simultaneously limiting the offloading of oxygen from Hb at the tissue (Turek et al., 2015). Indeed, the present study highlights minimal impact of pharmacologically left shifting the ODC on oxygen transport variables up until heavy-intensity exercise (i.e., near peak), where the physiological limits of compensability of convective and diffusive O2 transport may be reached. Interestingly, and somewhat in contrast to the studies by Lundby and Colleagues, which suggest convective oxygen delivery is the primary limiting factor for muscle VO2 during whole body incremental exercise (Calbet et al., 2015), we did not observe an increase in VO2 peak despite an increase in oxygen delivery. While direct measures of muscle p50 and kinetics of O2 offloading from Hb were not possible in the current study, local tissue hypoxia and acidosis may have invoked the mild hyperventilation and bicarbonate buffering that was observed in the current data; however, this was predominantly within a normal physiological range. As such, increasing arterial (and presumably capillary) PO2 had minimal impact until near peak exercise, where a reduced maximal VO2 and exercise capacity was observed.
The reduced mitochondrial oxidative capacity observed in normoxia, but not in hypoxia—where mitochondrial capacity is only partially recruited (Calbet et al., 2015), suggests that the O2 kinetics of voxelotor-mediated Hb may limit O2 flux into the muscle during periods of high capillary blood flow, effectively reducing the “functional” Hb available for O2 delivery into the muscle. Additional in vivo study is required to determine whether voxelotor-mediated Hb induces an absolute reduction in functional Hb (i.e., does not unload any O2 at the muscle), a partial or rate-limited reduction in functional Hb (i.e., reduced O2 unloading kinetics) or if other factors intrinsic to the muscle such as myoglobin and mitochondrial respiration are also implicated in the observed decrease in VO2 peak. Of note, in vitro studies suggest that voxelotor-mediated Hb retains the Bohr effect (i.e., releases more oxygen with increasing acidity) and responds to allosteric modulators, such as 2,3diphosphoglyerate, although with a mildly reduced rate of oxygen unloading (Pochron et al., 2019).
Supplemental data on the impact of dose–effect when pharmacologically left shifting the ODC
The preliminary data presented in Figure 5 contrast the blood gas and pulmonary gas exchange variables during hypoxic exercise for a small cohort of subjects who received a 1,500 mg drug dosage with the 900 mg group. Interestingly, the 1,500 mg group tended to have a slightly larger ODC shift, concomitant with a larger rise in SaO2, when compared with the 900 mg group; however, this group also tended to have a reduced peak VO2. The reduced VO2 peak in the 1,500 mg group after drug administration occurred concomitantly to an impaired Ca-vO2 difference, which was driven predominantly by a greater rise in venous oxygen content. While additional study is needed, these findings suggest a possible dose dependency, whereby an optimal effect may be possible by balancing the severity of the hypoxia (i.e., FIO2) and the net overall effect of altering the oxygen affinity of Hb at both the lungs and at the tissue. Indeed, if the different fractions of Hb (i.e., voxelotor-mediated Hb and natural Hb) have differing O2 unloading kinetics when passing through the peripheral capillaries, dose-dependent effects on VO2 peak would be anticipated.
Physiological and methodological considerations
Interestingly, pharmacologically left shifting the ODC resulted in a small increase in EPO (although within the normal range), suggesting a possible stimulus for EPO production by the drug. This is not surprising, given that under normoxic conditions with near 100% arterial saturation, left shifting the ODC is not expected to provide benefit and may decrease oxygen extraction fraction, thus stimulating compensatory mechanisms to increase erythropoiesis. While polycythemia was not evident during this 14-day study, increased Hb and hematocrit has been described with naturally occurring high-affinity Hb mutations (Dominelli et al., 2019, 2020). Given that oxygen consumption and extraction during exercise in hypoxia were not altered by the study drug, and only marginally at peak exercise in normoxia, there may have been overnight and/or diurnal differences that lead to the variable increase in EPO (Chapman et al., 1998; Friedmann et al., 2005). At present, it cannot be ruled out that regional tissue hypoxia or altered renal O2 transfer did not prelude the observed increase in EPO (Shirasawa et al., 2003). Indeed, the fall in PaCO2 without any change in pH in conjunction with lower bicarbonate levels in the blood suggests a possible metabolic stimulus for hyperventilation alongside bicarbonate buffering. Nonetheless, higher-intensity exertional efforts (outside of the study intervention) and the presence of responders and nonresponders may have contributed to the rise in EPO (Chapman et al., 1998; Friedmann et al., 2005). The participants were asked to maintain their habitual exercise regimes throughout the study and each reported a regular exercise training regime that included varying amounts of high-intensity exercise.
Given the complex biochemical regulation of Hb and the numerous physiological determinants of oxygen transport—each of which is both individually and collectively modulated by hypoxia—further integrative physiology studies are needed to determine the clinical and physiological utility of allosteric Hb modulators. The inability to acquire muscle and/or mixed venous blood samples and correct for temperature is a limitation of the current study. Direct skeletal muscle and/or capillary arterial and venous blood sampling, including temperature, pH, and CO2 measures, are needed to further model and examine the impact of voxelotor-mediated Hb on O2 off-loading kinetics from the peripheral capillaries into the muscle for gas exchange, whereas superimposing hyperoxia with voxelotor treatment would help determine whether mitochondrial capacity itself is altered. While there was not sufficient statistical power for dose comparisons in this study, defining the relationship between the magnitude of an ODC shift, as well as the impact of ODC convergence when natural and voxelotor-mediated blood fractions are mixed, and the severity of hypoxia exposure would be beneficial for determining if there is an optimal dose to enhance incremental exercise capacity under physiological and pathological conditions, which reduce the loading of oxygen onto Hb. In addition, the effects of increasing the oxygen affinity of Hb during hypobaric hypoxia, and the extent of overnight and diurnal oxygen desaturation events at sea level and altitude—an important factor in the pathogenesis of sleep apnea and acute mountain sickness—should be explored.
Conclusions
This study demonstrates that pharmacologically left shifting the ODC improved SaO2 and CaO2 throughout incremental and maximal exercise during acute hypoxia. Pulmonary gas exchange and hemodynamic responses to exercise were largely unaffected, such that the systematic rise in SaO2 and CaO2 during hypoxia was matched with a concomitant rise in CvO2 and a null overall effect on oxygen extraction and exercise capacity was observed. During normoxia, pharmacologically left shifting the ODC had a minimal impact on cardiopulmonary responses to exercise until near peak, where exercise capacity was reduced. This may be due, in part, to a possible effect of altered O2 unloading kinetics of the heterogeneous mix of natural- and voxelotor-mediated Hb passing through the peripheral capillaries. The effect of left shifting the ODC to different magnitudes and under more chronic forms of pathological or physiological hypoxia warrants further study.
Acknowledgments
The authors would like to acknowledge the valuable contributions from Briana Ziegler, Jennifer Isautier, and the Global Blood Therapeutics support team: Bella Oguno, Mary Poppenheimer, and Sandy Dixon.
Author Disclosure Statement
The study was funded by Global Blood Therapeutics, and authors J.L.-G., K.D., and N.E.V. are employees of Global Blood Therapeutics.
Funding Information
G.M.S. was supported by an American Heart Association Postdoctoral Fellowship (AHA#19POST34450022) and a Career Development Award in Cardiovascular Disease Research Honoring Dr. Earl H. Wood at Mayo Clinic.
References
- Adams RP, and Welch HG. (1980). Oxygen uptake, acid-base status, and performance with varied inspired oxygen fractions. J Appl Physiol Respir Environ Exerc Physiol 49:863–868. [DOI] [PubMed] [Google Scholar]
- Bell C, Monahan KD, Donato AJ, Hunt BE, Seals DR, and Beck KC. (2003). Use of acetylene breathing to determine cardiac output in young and older adults. Med Sci Sport Exer 35:58–64. [DOI] [PubMed] [Google Scholar]
- Burki NK, and Lee LY. (2010). Mechanisms of dyspnea. Chest 138:1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, and Saltin B. (2003). Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol 284:R291–303. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Losa-Reyna J, Torres-Peralta R, Rasmussen P, Ponce-Gonzalez JG, Sheel AW, de la Calle-Herrero J, Guadalupe-Grau A, Morales-Alamo D, Fuentes T, Rodriguez-Garcia L, Siebenmann C, Boushel R, and Lundby C. (2015). Limitations to oxygen transport and utilization during sprint exercise in humans: Evidence for a functional reserve in muscle O2 diffusing capacity. J Physiol 593:4649–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RF, Stray-Gundersen J, and Levine BD. (1998). Individual variation in response to altitude training. J Appl Physiol 85:1448–1456. [DOI] [PubMed] [Google Scholar]
- Clark AL, PooleWilson PA, and Coats AJS. (1996). Exercise limitation in chronic heart failure: Central role of the periphery. J Am Coll Cardiol 28:1092–1102. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Baker SE, Wiggins CC, Stewart GM, Sajgalik P, Shepherd JRA, Roberts SK, Roy TK, Curry TB, Hoyer JD, Oliveira JL, Foster GE, and Joyner MJ. (2019). Dissociating the effects of oxygen pressure and content on the control of breathing and acute hypoxic response. J Appl Physiol (1985) 127:1622–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominelli PB, Wiggins CC, Baker SE, Shepherd JRA, Roberts SK, Roy TK, Curry TB, Hoyer JD, Oliveira JL, and Joyner MJ. (2020). Influence of high affinity haemoglobin on the response to normoxic and hypoxic exercise. J Physiol 598:1475–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufu K, Yalcin O, Ao-Ieong ESY, Hutchaleelala A, Xu Q, Li Z, Vlahakis N, Oksenberg D, Lehrer-Graiwer J, and Cabrales P. (2017). GBT1118, a potent allosteric modifier of hemoglobin O2 affinity, increases tolerance to severe hypoxia in mice. Am J Physiol Heart Circ Physiol 313:H381–H391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JW. (1974). Oxygen affinity and environmental adaptation. Ann N Y Acad Sci 241:491–497. [DOI] [PubMed] [Google Scholar]
- Eaton JW, Skelton TD, and Berger E. (1974). Survival at extreme altitude: Protective effect of increased hemoglobin-oxygen affinity. Science 183:743–744. [DOI] [PubMed] [Google Scholar]
- Filley GF, Gregoire F, and Wright GW. (1954). Alveolar and arterial oxygen tensions and the significance of the alveolar-arterial oxygen tension difference in normal men. J Clin Invest 33:517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JW. (2003). Erythropoietin: Physiology and pharmacology update. Exp Biol Med (Maywood) 228:1–14. [DOI] [PubMed] [Google Scholar]
- Friedmann B, Frese F, Menold E, Kauper F, Jost J, and Bartsch J. (2005). Individual variation in the erythropoietic response to altitude training in elite junior swimmers. Brit J Sport Med 39:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersonde K, and Nicolau C. (1980). Modification of the oxygen affinity of intracellular haemoglobin by incorporation of polyphosphates into intact red blood cells and enhanced O2 release in the capillary system. Bibl Haematol 46:81–92. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Voswinckel R, Reichenberger F, Weissmann N, Schermuly RT, Seeger W, and Grimminger F. (2006). Hypoxia- and non-hypoxia-related pulmonary hypertension—Established and new therapies. Cardiovasc Res 72:30–40. [DOI] [PubMed] [Google Scholar]
- Hackett PH, and Roach RC. (2001). Current concepts: High-altitude illness. New Engl J Med 345:107–114. [DOI] [PubMed] [Google Scholar]
- Hebbel RP, Eaton JW, Berger EM, Kronenberg RS, Zanjani ED, and Moore LG. (1978a). Hemoglobin oxygen affinity and adaptation to altitude: Evidence for pre-adaptation to altitude in humans with left-shifted oxyhemoglobin dissociation curves. Trans Assoc Am Physicians 91:212–228. [PubMed] [Google Scholar]
- Hebbel RP, Eaton JW, Kronenberg RS, Zanjani ED, Moore LG, and Berger EM. (1978b). Human llamas: Adaptation to altitude in subjects with high hemoglobin oxygen affinity. J Clin Invest 62:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Bebout DE, and Wagner PD. (1991). Effect of increased Hb-O2 affinity on VO2max at constant O2 delivery in dog muscle in situ. J Appl Physiol (1985) 70:2656–2662. [DOI] [PubMed] [Google Scholar]
- Honigman B, Theis MK, Koziolmclain J, Roach R, Yip R, Houston C, and Moore LG. (1993). Acute mountain-sickness in a general tourist population at moderate altitudes. Ann Intern Med 118:587–592. [DOI] [PubMed] [Google Scholar]
- Jessen TH, Weber RE, Fermi G, Tame J, and Braunitzer G. (1991). Adaptation of bird hemoglobins to high-altitudes—Demonstration of molecular mechanism by protein engineering. Proc Natl Acad Sci U S A 88:6519–6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, and Joyner MJ. (2000). Cardiac output during exercise by the open circuit acetylene washin method: Comparison with direct Fick. J Appl Physiol (1985) 88:1650–1658. [DOI] [PubMed] [Google Scholar]
- Koike A, Wasserman K, Mckenzie DK, Zanconato S, and Weilerravell D. (1990). Evidence that diffusion limitation determines oxygen-uptake kinetics during exercise in humans. J Clin Invest 86:1698–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert MP, Liard JF, Abraham DJ, and Lombard JH. (1996). Low-affinity hemoglobin increases tissue PO2 and decreases arteriolar diameter and flow in the rat cremaster muscle. Microvasc Res 52:58–68. [DOI] [PubMed] [Google Scholar]
- Kwo PY, Cohen SM, and Lim JK. (2017). ACG Clinical Guideline: Evaluation of abnormal liver chemistries. Am J Gastroenterol 112:18–35. [DOI] [PubMed] [Google Scholar]
- Macdonald R. (1977). Red-cell 2,3-diphosphoglycerate and oxygen-affinity. Anaesthesia 32:544–553. [DOI] [PubMed] [Google Scholar]
- Mairbaurl H, and Weber RE. (2012). Oxygen transport by hemoglobin. Compr Physiol 2:1463–1489. [DOI] [PubMed] [Google Scholar]
- Pochron M, Siu V, Oksenberg D, and Dufu K. (2019). PS1522 central physiologic mechanisms which augment oxygen release (Bohr Effect and 2,3-Dpg binding) are preserved in the presence of voxelotor at the therapeutic target of 30% Hb modification. HemaSphere 3:701–702. [Google Scholar]
- Richardson RS, Tagore K, Haseler LJ, Jordan M, and Wagner PD. (1998). Increased VO2 max with right-shifted Hb-O2 dissociation curve at a constant O2 delivery in dog muscle in situ. J Appl Physiol (1985) 84:995–1002. [DOI] [PubMed] [Google Scholar]
- Roca J, Hogan MC, Story D, Bebout DE, Haab P, Gonzalez R, Ueno O, and Wagner PD. (1989). Evidence for tissue diffusion limitation of VO2max in normal humans. J Appl Physiol (1985) 67:291–299. [DOI] [PubMed] [Google Scholar]
- Rotsztain A, Haddad R, and Canter HG. (1970). Blood gas changes during voluntary hyperventilation in normal and disease states. Am Rev Respir Dis 102:205–212. [DOI] [PubMed] [Google Scholar]
- Saltin B, and Strange S. (1992). Maximal oxygen uptake: “old” and “new” arguments for a cardiovascular limitation. Med Sci Sports Exerc 24:30–37. [PubMed] [Google Scholar]
- Samaja M, Diprampero PE, and Cerretelli P. (1986). The role of 2,3-Dpg in the oxygen-transport at altitude. Resp Physiol 64:191–202. [DOI] [PubMed] [Google Scholar]
- Shirasawa T, Izumizaki M, Suzuki Y, Ishihara A, Shimizu T, Tamaki M, Huang F, Koizumi K, Iwase M, Sakai H, Tsuchida E, Ueshima K, Inoue H, Koseki H, Senda T, Kuriyama T, and Homma I. (2003). Oxygen affinity of hemoglobin regulates O2 consumption, metabolism, and physical activity. J Biol Chem 278:5035–5043. [DOI] [PubMed] [Google Scholar]
- Stewart GM, Chase SC, Cross TJ, Wheatley CM, Joyner MJ, Curry TB, Lehrer-Graiwer J, Dufu K, Vlahakis NE, and Johnson BD. (2020). Effects of an allosteric hemoglobin affinity modulator on arterial blood gases and cardiopulmonary responses during normoxic and hypoxic low-intensity exercise. J Appl Physiol (1985) 128(6):1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, and Moriyama H. (2008). Mechanisms of hemoglobin adaptation to high altitude hypoxia. High Alt Med Biol 9:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek Y, Maimaiti W, Shikano Y, Sun CP, and Al-Amri M. (2015). Advantages of nonclassical pointer states in postselected weak measurements. Phys Rev A 92:022109. [Google Scholar]
- Wahr JA, Gerber M, Venitz J, and Baliga N. (2001). Allosteric modification of oxygen delivery by hemoglobin. Anesth Analg 92:615–620. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, and Wasserman K. (1969). Alveolar-arterial gas tension differences during graded exercise. J Appl Physiol 27:361–365. [DOI] [PubMed] [Google Scholar]
- Winslow RM. (2007). The role of hemoglobin oxygen affinity in oxygen transport at high altitude. Respir Physiol Neurobiol 158:121–127. [DOI] [PubMed] [Google Scholar]
- Winslow RM, Monge CC, Statham NJ, Gibson CG, Charache S, Whittembury J, Moran O, and Berger RL. (1981). Variability of oxygen affinity of blood: Human subjects native to high altitude. J Appl Physiol Respir Environ Exerc Physiol 51:1411–1416. [DOI] [PubMed] [Google Scholar]
- Yalcin O, and Cabrales P. (2012). Increased hemoglobin O2 affinity protects during acute hypoxia. Am J Physiol Heart Circ Physiol 303:H271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]