Abstract
Aim
Although an increased risk of gestational diabetes mellitus (GDM) has been noted in women exposed to stressful conditions and traumatic events, limited information is available about such risk in the context of the COVID-19 pandemic.
Methods
The study was designed as a non-concurrent case-control study on the prevalence of GDM, defined according to IADPSG 2010, in women giving birth during the COVID-19 pandemic in the hot spot of Northeast Italy from March 9th to May 18th, 2020, with an antecedent puerperae‐matched group whose women had given birth in 2019.
Results
Analysis revealed that during the COVID-19 pandemic in 2020, GDM prevalence was significantly higher than in 2019 (GDM, 48/533, 9 vs 86/637, 13.5%, p = 0.01), as illustrated by a higher GDM prevalence in 5/6 months of the final semester of 2020. In addition, logistic regression analysis confirmed a statistically significant temporal relationship between experiencing the lockdown during the first trimester of gestation and later GDM incidence (t = 2.765, P = 0.012), with an 34% increase in mean number of GDM diagnoses per month (antilog of the parameter = 1.34).
Conclusion
The COVID-19 pandemic negatively impacted GDM prevalence in 2020 compared to 2019, especially for pregnant women in the 1st trimester of gestation.
Keywords: Covid-19 pandemic, Lockdown, Gestational Diabetes Mellitus
1. Introduction
Gestational diabetes mellitus (GDM) is one of the most common pregnancy complications with a rising prevalence worldwide. GDM carries with it both short- and long-term adverse effects on women as well as their offspring, including preeclampsia, primary caesarean section, excessive fetal growth, shoulder dystocia or birth injury, neonatal hypoglycemia, RDS, and admission to the NICU [1]. While the high blood glucose of GDM usually resolves after delivery, women with GDM have an increased risk of further episodes of GDM and are seven times more likely to develop type 2 diabetes mellitus (T2DM) than women with normoglycemic pregnancies [2]. In addition, there is growing evidence that hyperglycemia in pregnancy has a programming effect on the long-term metabolic health of the offspring, increasing their risk of developing T2DM later in life [3].
Recent evidence has shown the importance of ensuring that all pregnancies with GDM are identified and managed appropriately [4]. However, there remains a lack of consensus as to how to best identify the GDM risk factors to inform prevention and treatment practices in maternal and infant health care. In humans, prospective studies have documented that exposure to stressful life events may predispose one to disturbances of glucose metabolism, metabolic syndrome, and undetected T2DM [5], [6]. Nevertheless, the literature about the relationship between stressful life events and GDM remains limited and there are few studies that look into the specific types of stressors experienced in gestation [7].
These kinds of research gaps pose uncertainty for GDM stakeholders who might plan to conduct interventions to address potentially harmful life stressors in pregnancy. [On the other hand, as stress has been shown to be linked with T2DM [8], we hypothesized that there might a link between COVID-pandemic during pregnancy and GDM. After the SARS outbreak in 2003, both healthcare workers and people who were self-quarantined exhibited symptoms of post-traumatic stress disorder [9]. ]Hence, the effect of COVID-19 calamity‐induced stress on pregnant women cannot be ignored, considered a population at risk of viral respiratory infections for possible consequences on the mother and foetus [10].
Therefore, this study verified that there might be a link between the COVID-19 pandemic and GDM occurrence among quarantined women giving birth in a Covid-19 hot spot in Northeast Italy. The objective of the study was twofold: first, to examine if the 2020 COVID-19 pandemic increased the prevalence of GDM in comparison to women who gave birth the year before and second, to investigate whether the COVID-19 lockdown (March 9th to May 18th, 2020) differentially affected GDM prevalence depending on whether it occurred in the first trimester of gestation or the second.
2. Materials and Methods
The study was designed as a non-concurrent case–control analysis on GDM prevalence in women who gave birth at Policlinico Abano Terme in the 2020 COVID-19 pandemic (study group) and in an antecedent group of matched women (control group), who gave birth in 2019. The hospital where this study took place is located in an industrialized area that borders the COVID-19 hot spot in Northeast Italy where, on 21st February 2020, the first death from pneumonia due to COVID-19 infection was recorded [11] since the emergence of SARS-CoV-2 in the Chinese city of Wuhan, Hubei Province [12]. The maternity ward supports about 1000 births per year, 75 % of them pertaining to native Italian women with high socioeconomic status and low and late fertility [10], and the rest pertaining to migrant women, mostly from Eastern European Countries and, in descending order, North Africa, India, and China.
The study was performed in accordance with the principles of the Helsinki Declaration of 1964, as revised in 2013. Data collection was approved by the Institutional Review Board (IRB) of Policlinico Abano Terme. Maternal demographic information, medical history, obstetric and gynecological history, prenatal history, labor and delivery information with neonatal anthropometrical and clinical data were abstracted from patients’ charts by trained research nurses.
Inclusion criteria were singleton low-risk pregnancies delivered at term in Italian puerperae. Multiple gestations and pregnancies without OGTT (at weeks 16–18 and/or at week 24–28 of pregnancy, according to pre-defined risk factors including age, body mass index (BMI), family history of T2DM, previous history of GDM, and ethnicity [13] or with fetal anomalies, severe acidosis (pH < 7.0 and base deficit > 12 mmol/L) or other diseases that could affect the physiological puerperium or postnatal adaptation were excluded. Term pregnancy was defined as gestational age ≥ 37 weeks, dated by a woman’s last menstrual period and confirmed with first or second trimester ultrasound.
As a public health response to limit viral transmission, on March 9th, 2020, Italy imposed a nationwide lockdown until May 18th, 2020, which was planned to be followed by Italy's “phase two,” the next stage in which the country would learn to coexist with the virus [11]. The Italian Central Government implemented primary prevention measures and instituted several containment procedures to limit the spread of the infection, including case isolation, contact tracing, and quarantine.14 Hospitals changed their policies concerning prenatal care, labor and delivery, and postnatal care, replacing office visits with remote checkups, sending patients to an offsite laboratory for blood draws, cancelling birth center tours and other nonessential visits, and barring extra people (fathers, doulas, and visitors…) from in-person contact with the laboring mom, the delivery room, and the postpartum units in an effort to keep moms and babies safe [10].
SPSS version 26 for MAC (IBM, Armonk, NY, USA) was used for statistical analysis. Data are expressed as mean ± SD or frequency (percentage). Continuous variables were analyzed by independent sample t-test, while the chi-squared test was used to analyze qualitative variables. In more detail, GDM prevalence was compared between the study group women during the COVID-19 lockdown and the non-concurrent 2019 control group women using the chi-squared test. The odds ratio was also calculated to estimate the risk of GDM in both groups. Moreover, to estimate the impact of the lockdown on the prevalence of GDM, two separate binary logistic analyses were performed. We first tested the association between GDM occurrence and the month of delivery in both the study and control groups considering the occurrence of GDM as a binary dependent variable and the month of delivery as an independent factor. Then, we tested the association between the GDM (dependent variable) and the period of gestation in which lockdown occurred (independent variable) in the study group. For this second regression logistic analysis, we classified women into four subgroups: i) pregnancy completed before lockdown; ii) lockdown occurred during the first trimester of pregnancy; iii) lockdown occurred during the second or third trimester of pregnancy; iv) pregnancy completed after the lockdown period. Maternal age, pre-pregnancy BMI, maternal height, and gestational weight gain (GWG) were considered as covariates. Preliminary checks were conducted to ensure independence of observations, linearity, absence of multicollinearity, homogeneity of error variances, absence of outliers, and approximate normal distribution of residuals. To estimate the effect of lockdown on the incidence of GDM in 2020, we also performed an interrupted time-series analysis using an Auto-Regressive, Integrated, Moving Average (ARIMA) model. More specifically, this analysis began with identification, estimation, and diagnosis of GDM before the lockdown. The model was then re-estimated for the entire series before and after the lockdown period, and the effect of lockdown on GDM incidence was subsequently assessed by interpreting the coefficients for the indicator variable. The model was adjusted for autocorrelation which was checked by plotting autocorrelation function (ACF) and partial autocorrelation function (PACF) demonstrating the Autoregressive (AR) and Moving Average (MA) process [14]. Statistical significance was set at P < 0.05.
3. Results
In 2019, of eligible Italian women 3 without OGTT and 14 with multiple gestation were excluded. In 2020, of eligible Italian women, 2 without OGTT and 11 with multiple gestation were excluded. Thus, data from 637 GDM eligible Italian women in the 2020 study group and 533 in the control group were analyzed. Table 1
Table 1.
Anthropometrical and clinical features of 2020 study and 2019 control group GDM women.
| Puerperae Number, % or Mean ± SD |
2019 Control group 533 |
2020 Study group 637 |
p |
|---|---|---|---|
| GDM | 48 (9) | 86 (13.5) | 0.01 |
| Age, years | 34.22 ± 4.78 | 35.65 ± 4.86 | 0.137 |
| Nulliparous | 20(41.67) | 43(50.00) | 0.372 |
| Gestational age, weeks | 38.92 ± 0.87 | 38.99 ± 0.92 | 0.665 |
| Prepre-gnancy weight, kg | 61.54 ± 11.34 | 61.93 ± 1072 | 0.639 |
| Pre-pregnancy BMI, kg/m2 | 25.05 ± 4.89 | 24.26 ± 5.09 | 0.362 |
| Gestational BMI, kg/m2 | 29.27 ± 4.92 | 28.29 ± 5.12 | 0.277 |
| GWG, Kg | 11.21 ± 4.90 | 10.88 ± 4.22 | 0.707 |
| Cesarean delivery: -Elective -Emergency |
8(16.67) 3 (6.25) |
18(20.93) 9(10.47) |
0.651 0.536 |
| Neonatal birth weight, g | 3,401.87 ± 412.06 | 3,287.44 ± 404.64 | 0.133 |
BMI, body mass index; GWG, gestational weight gain.
Data expressed as Mean ± SD or n number (%): p, statistical significance at p < 0.05.
There were no significant differences in sociodemographic characteristics, maternal age, parity, gestational age or delivery mode nor in pre- pregnancy- and gestational- BMI, GWG or insulin therapy between the 2020 and 2019 puerperae populations. However, the chi-squared analysis revealed that during the 2020 COVID-19 pandemic, GDM prevalence was significantly higher compared to 2019 (GDM, 86/637, 13.5% vs 48/533, 9% p = 0.01). Table 1.
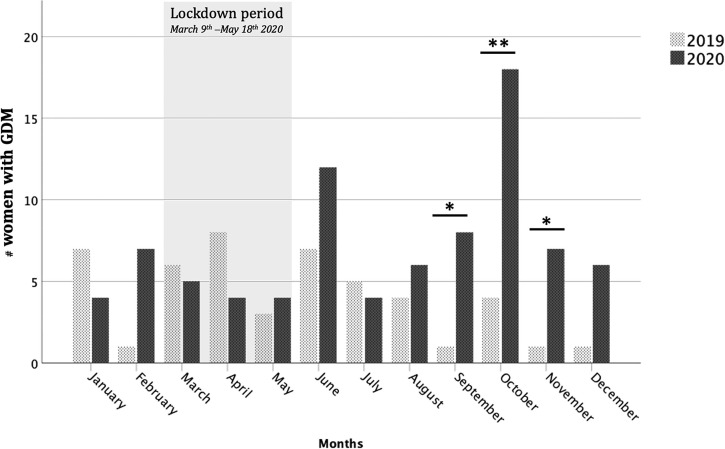
In particular, a higher prevalence of GDM was observed during the last 5/6 months of 2020 when compared to the same period in 2019. Fig. 1
Fig. 1.
Monthly GDM prevalence during the 2020 COVID-19 pandemic compared with 2019. A higher prevalence of GDM was observed during the last 5/6 months of 2020 when compared to the same period in 2019.
In addition, logistic regression analysis revealed a statistically significant temporal relationship between the prevalence of GDM and the phase of gestation in which the lockdown occurred, after adjusting for the effect of maternal age and height, pre-pregnancy BMI, and GWG. Of note, experiencing lockdown during the first trimester of gestation (these women gave birth in September and October of 2020) increased the risk of GDM in these women by a factor of 2.29 (P = 0.002) compared to women whose pregnancies occurred before and after lockdown. Table 2
Table 2.
Multivariate logistic regression analysis testing the association between clinical factors and GDM.
| Factors | B | S.E. (B) | Exp(B) | 95% C.I.- Exp(B) |
P | |
|---|---|---|---|---|---|---|
| Inferior | Superior | |||||
| Pregnancy before lockdown | −0.105 | 0.326 | 0.901 | 0.343 | 2.431 | 0.724 |
| Lockdown - I trimester | 0.819 | 0.259 | 2.269 | 1.365 | 3.772 | 0.002 |
| Lockdown - II & III trimester | 0.445 | 0.229 | 1.561 | 0.997 | 2.444 | 0.051 |
| Pregnancy after lockdown | −0.066 | 0.456 | 0.936 | 0.383 | 2.286 | 0.884 |
| Maternal Age | 0.079 | 0.02 | 1.082 | 1.04 | 1.127 | <0.001 |
| Maternal Height | −0.028 | 0.016 | 0.972 | 0.942 | 1.003 | 0.078 |
| Maternal BMI | 0.095 | 0.023 | 1.1 | 1.051 | 1.151 | <0.001 |
| GWG | 0.107 | 0.023 | 0.899 | 0.859 | 0.941 | <0.001 |
BMI, body mass index; GWG, gestational weight gain.
B, unstandardized regression coefficient; S.E.(B) Standard error of (B); Exp(B) standardized regression coefficient; 95% C.I.-Exp(B) 95% confidence intervals of Exp(B); P statistical significance.
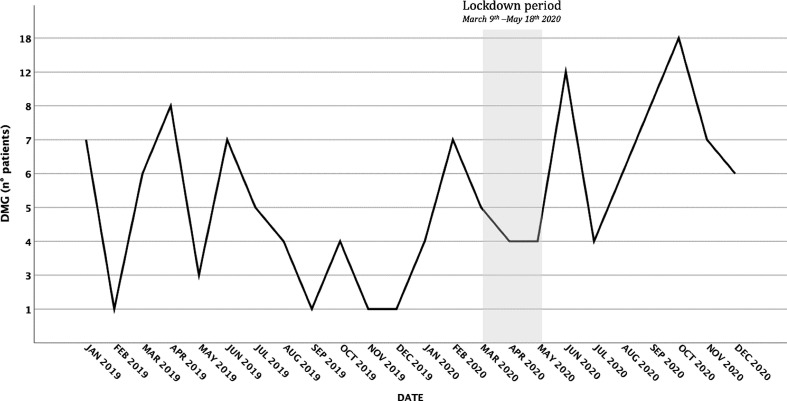
Moreover, the interrupted time-series model found a trend of increased GDM incidence after the lockdown period. In particular, the intervention parameter (lockdown period) determined that there was a statistically significant increase in GDM incidence (t = 2.765, P = 0.012), with a 34% increase in mean number of GDM diagnoses per month (antilog of the parameter = 1.34). Fig. 2 )
Fig. 2.
Interrupted time-series analysis testing the effect of the lockdown period on the prevalence of GMD. The intervention parameter (lockdown period) determined statistically significant increase of GDM rate (t = 2.765, P = 0.012), with an 34% increase in mean number of GDM per month (antilog of the parameter = 1.34).
In the control group, no significant associations were observed between the occurrence of GDM and the month of delivery (P = 0.459). Table 3
Table 3.
Monthly GDM prevalence, comparison between 2020 and 2019.
| Puerperae |
2019 |
2020 |
||||
|---|---|---|---|---|---|---|
| Month | Groups | Number | % | Number | % | p |
| January | Healthy | 56 | 88.90 | 43 | 91.50 | 0.454 |
| GDM | 7 | 11.10 | 4 | 8.50 | ||
| February | Healthy | 43 | 97.70 | 55 | 88.70 | 0.083 |
| GDM | 1 | 2.30 | 7 | 11.30 | ||
| March | Healthy | 40 | 87.00 | 40 | 88.90 | 0.516 |
| GDM | 6 | 13.00 | 5 | 11.10 | ||
| April | Healthy | 48 | 85.70 | 51 | 92.70 | 0.189 |
| GDM | 8 | 14.30 | 4 | 7.30 | ||
| May | Healthy | 53 | 94.60 | 51 | 92.70 | 0.490 |
| GDM | 3 | 5.40 | 4 | 7.30 | ||
| June | Healthy | 47 | 87.00 | 35 | 74.50 | 0.087 |
| GDM | 7 | 13.00 | 12 | 25.50 | ||
| July | Healthy | 39 | 88.60 | 44 | 91.70 | 0.444 |
| GDM | 5 | 11.40 | 4 | 8.30 | ||
| August | Healthy | 31 | 88.60 | 23 | 79.30 | 0.251 |
| GDM | 4 | 11.40 | 6 | 20.70 | ||
| September | Healthy | 39 | 97.50 | 52 | 86.70 | 0.042 |
| GDM | 1 | 2.50 | 8 | 13.30 | ||
| October | Healthy | 31 | 88.60 | 55 | 75.30 | 0.007 |
| GDM | 4 | 11.40 | 18 | 24.70 | ||
| November | Healthy | 36 | 97.30 | 44 | 86.30 | 0.047 |
| GDM | 1 | 2.70 | 7 | 13.70 | ||
| December | Healthy | 22 | 95.70 | 58 | 90.60 | 0.401 |
| GDM | 1 | 4.30 | 6 | 9.40 | ||
P, statistical significance at Chi-squared analysis.
4. Discussion
Using a non-concurrent case–control study in women who gave birth in an industrialized area of Northeast Italy, we found a significantly higher prevalence of GDM during the 2020 COVID-19 pandemic in comparison to the matched group of women who gave birth in 2019, independent of maternal age, parity, socio-educational attainment, maternal BMI prior to and at the time of pregnancy or GWG. In addition, experiencing lockdown in the first trimester of gestation was found to be a statistically significant contributing factor in increasing the prevalence of GDM at a later point in time.
These data may have some clinical relevance. Firstly, the significantly higher prevalence of GDM during the COVID-19 pandemic may have resulted from exposure to harmful life stressors that predisposed women to disturbances in their glucose metabolism. In humans, prospective studies have shown that increased levels of inflammatory markers predict the risk of developing T2DM [15], [16]. Given that GDM has a similar etiology as T2DM [17], [18], it is plausible that exposure to stressful events during pregnancy causes chronic inflammation and, therefore, results in a higher risk of GDM. This hypothesis is supported by the fact that pregnancy is a highly stressful time period in a woman’s life and is considered one of the most stressful conditions during normal circumstances [18]. The severity of COVID-19, little evidence on the effectiveness of potential therapeutic agents against it, the unavailability of a vaccine, and the presumable lack of pre-existing immunity to it in the population make everyone potentially susceptible and fearful about catching the infection [19]. However, stressors for GDM are generally not well understood and any relationship between stressors and GDM is still inconclusive. Secondly, it seems relevant that anthropometric and peripartal features did not appear to be modified in women who were quarantined in the first trimester of gestation despite the fact that they suffered lockdown with potentially stressful consequences resulting in a detrimental metabolic impact that was 3.5 times greater. Although the reported causal relationship between stress in early gestation and GDM cannot be ascertained in this study, the large number of first-trimester biochemical glycemic, inflammatory, insulin resistance, adipocyte-derived, and placenta-derived markers [20], [21], [22] that can help predict GDM might help us interpret the link between experiencing ‘lockdown’ in the first trimester of gestation in the COVID-19 pandemic and enhanced GDM occurrence later on. In humans, prospective studies have also shown that increased levels of inflammatory markers predict one’s risk of developing T2DM [20], [21], [22] and, given that GDM and T2D have similar aetiologies, it is plausible that exposure to stressful events during pregnancy causes chronic inflammation and therefore result in a higher risk of GDM.
GDM is the result of a complex and variable interaction of genetic, environmental, maternal, and fetoplacental factors in an integrated manner with onset or first recognition during the second half of pregnancy [23]. During early gestation, insulin sensitivity increases, promoting the uptake of glucose into adipose stores in preparation for the energy demands of later pregnancy [7]. Conversely, as pregnancy progresses, a surge of local and placental hormones promotes a state of insulin resistance [24]. As a result, blood glucose becomes slightly elevated, and this glucose is readily transported across the placenta to fuel the growth of the fetus [25]. Evidence also suggests that, in order to maintain glucose homeostasis, pregnant women compensate for these changes through hypertrophy and hyperplasia of pancreatic β-cells, as well as increased glucose-stimulated insulin secretion [26].
The incidence of GDM has increased significantly in the last few decades worldwide [1], [2]. A further understanding of the pathophysiology and risk factors of GDM will enhance the possibility of effective screening, early intervention, and even prevention [6], [8]. Recent evidence has shown that advanced maternal age, overweightness, obesity, westernized diet, ethnicity, intrauterine environment, hypertension, family history of GDM or T2DM, and personal history of GDM or polycystic ovarian syndrome may participate in the pathophysiology of GDM [6], [8]. Although the cellular mechanisms involved are not yet completely understood, there is a growing body of evidence suggesting the importance of identifying risk factors for the prevention of GDM and its sequelae.
A major strength of our study is that our 2020 COVID-19 pandemic analysis focused on the differential effects of lockdown on GBM prevalence depending on what stage in pregnancy women experienced the lockdown – the first or second trimester of gestation, prior to OGTT screening. Because checking pregnant women's blood sugar is a routine process of prenatal care regardless of the woman's mental health status, it is possible that holding GDM diagnosis and care in the COVID-19 hotspot affected the frequency with which participants were exposed to pandemic-related stressful events together with the number of specific events occurring during pregnancy [14]. It should be noted that our participants comprised a group of women attending one hospital in Northeast Italy, supporting childbearing women characterized by a high socio-cultural level and low and late fertility. The fact that there were more nulliparous women in the study group than the control group is a source of potential bias in this study as there is evidence that nulliparity influences risk of developing GDM in a subsequent pregnancy. Even if the findings of this study may not be directly applicable to other population groups using different models of care, it should be noted that many of the themes elicited articulate social, cultural, and psychological situations that are likely to be applicable in contexts outside of the Northeast Italy, indeed reflecting findings from previous studies conducted in a variety of countries. This indicates that the issues discussed may be widespread in contexts outside of Italy. If this is indeed the case, we may expect women with GDM to report a higher number of stressful events. It remains, however, inconclusive whether the observed link between stressful life events and GDM is due to a mechanism whereby repeated episodes of acute or chronic physiological responses to stress induce a chronic inflammatory process that increases the risk of GDM [27].
Conclusions. Using a non-concurrent case–control study in women who gave birth in an industrialized area of Northeast Italy, we found a significantly increased prevalence of GDM in women who were pregnant during the 2020 COVID-19 pandemic in comparison to the matched group of women who gave birth in 2019. Experiencing lockdown in the first trimester of gestation played a significant contributing role in enhancing the prevalence of GDM in the women who were pregnant during the lockdown. It is possible that exposure to the stress of the COVID-19 pandemic before GDM screenings caused chronic inflammation and therefore resulted in a higher risk of GDM. Further studies to confirm our findings and to investigate the underlying pathway between stress and GDM in early gestation are warranted to alleviate the stress of pregnant women so as to reduce the consequent health risks for women and children.
Author contributions
VZ carried out the study. DT and AS participated in study design. LS participated in study design and coordination. PM and GS helped draft the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Chen L, Mayo R, Chatry A, Hu G. Gestational Diabetes Mellitus: Its Epidemiology and Implication beyond Pregnancy. Current Epidemiology Reports 2016; s1–11.
- 2.Buchanan T.A., Xiang A.H., Page K.A. Gestational Diabetes Mellitus: Risks and Management during and after Pregnancy. Nat Rev Endocrinol. 2012;8(11):639–649. doi: 10.1038/nrendo.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nijs H., Benhalima K. Gestational Diabetes Mellitus and the Long-Term Risk for Glucose Intolerance and Overweight in the Offspring: A Narrative Review. J Clin Med. 2020;9(2):599. doi: 10.3390/jcm9020599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damm P., Houshmand-Oeregaard A., Kelstrup L., Lauenborg J., Mathiesen E.R., Clausen T.D. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. 2016;59(7):1396–1399. doi: 10.1007/s00125-016-3985-5. [DOI] [PubMed] [Google Scholar]
- 5.Horri N., Haghighi S., Hosseini S.M., Zare M., Parvaresh E., Amini M. Stressful life events, education, and metabolic syndrome in women: are they related? A study in first-degree relatives of type 2 diabetics. Metab Syndr Relat Disord. 2010;8(6):483–487. doi: 10.1089/met.2010.0015. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S., Murphy M.L.M., Prather A.A. PratherAA. Ten Surprising Facts About Stressful Life Events and Disease Risk, Annu Rev Psychol. 2019;70(1):577–597. doi: 10.1146/annurev-psych-010418-102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH.. The Pathophysiology of Gestational Diabetes Mellitus. Int J Mol Sci 2018; 19: 3342. [DOI] [PMC free article] [PubMed]
- 8.Kelly S.J., Ismail M. Stress and type 2 diabetes: a review of how stress contributes to the development of type 2 diabetes. Annu Rev Public Health. 2015;36(1):441–462. doi: 10.1146/annurev-publhealth-031914-122921. [DOI] [PubMed] [Google Scholar]
- 9.Wu P., Fang Y., Guan Z., Fan B., Kong J., Yao Z., et al. The Psychological Impact of the SARS Epidemic on Hospital Employees in China: Exposure, Risk Perception, and Altruistic Acceptance of Risk. Can J Psychiatry. 2009;54(5):302–311. doi: 10.1177/070674370905400504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanardo V, Manghina V, Giliberti L, Vettore M. Severino L, Straface G. Psychological impact of COVID‐19 quarantine measures in northeastern Italy on mothers in the immediate postpartum period. 2020. Int J Gynecol Obstet 31 May 2020 2020;150:184-8. [DOI] [PMC free article] [PubMed]
- 11.Veronesi L, Colucci1ME, Pasquarella C, , Caruso L, Mahgoub Ibrahim MM , Zoni R , et al. Virological surveillance of SARS-CoV-2 in an Italian northern area: comparison of Real Time RT PCR cycle threshold (Ct) values in three epidemic periods, Acta Biomed 2020; 9: 19-21. [DOI] [PMC free article] [PubMed]
- 12.Lavezzo E, Franchin E, […] Imperial College COVID-19 Response Team. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 2020; 584; 25–29. [DOI] [PubMed]
- 13.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care 2010; 33: 676–82. [DOI] [PMC free article] [PubMed]
- 14.Bhaskaran K, Gasparrini A, Hajat S, Smeeth L, Armstrong B. Time series regression studies in environmental epidemiology. Int J Epidemiol 2013;42:1187–95. [DOI] [PMC free article] [PubMed]
- 15.Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 2003;52:1799-805. [DOI] [PubMed]
- 16.Dandona P. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Baz B., Riveline J.P., Gautier J. Gestational diabetes mellitus: definition, aetiological and clinical aspects. Eur J Endocrinol. 2016;174:R43–R51. doi: 10.1530/EJE-15-0378. [DOI] [PubMed] [Google Scholar]
- 18.Dunkel Schetter C., Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Curr Opin Psychiatry. 2012;25:141–148. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hawryluck L., Gold W.L., Robinson S., Wessely S., Greenberg N., Rubin G.J. ARS Control and Psychological Effects of Quarantine, Toronto. Canada. Emerg Infect Di. 2004;10:1206–1212. doi: 10.3201/eid1007.030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Syngelaki Argyro, Visser Gerard H.A., Krithinakis Konstantinos, Wright Alan, Nicolaides Kypros H. First trimester screening for gestational diabetes mellitus by maternal factors and markers of inflammation. Metabolism. 2016;65(3):131–137. doi: 10.1016/j.metabol.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Syngelaki A., Kotecha R., Pastides A., Kapur A., Hadar E., Divakar H., et al. First-trimester biochemical markers of placentation in screening for gestational diabetes mellitus. Metabolism. 2015;64:1485–1489. doi: 10.1016/j.metabol.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Kansu-Celik H, Ozgu-Erdinc AS, Kisa B, Findik RB, Yilmaz C, Tasci Y. Prediction of gestational diabetes mellitus in the first trimester: comparison of maternal fetuin-A, N-terminal proatrial natriuretic peptide, high-sensitivity C-reactive protein, and fasting glucose levels. Arch Endocrinol Metab 2019;63:121-127. [DOI] [PMC free article] [PubMed]
- 23.Sweeting AN, Wong J, Appelblom H,, Ross GP, Kouru H, Williams PF. A Novel Early Pregnancy Risk Prediction Model for Gestational Diabetes Mellitus. Fetal Diagn Ther 2019;45:76-84. [DOI] [PubMed]
- 24.Catalano Patrick M., Tyzbir Elaine D., Roman Noreen M., Amini Saeid B., Sims Ethan A.H. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet. Gynecol. 1991;165(6):1667–1672. doi: 10.1016/0002-9378(91)90012-g. [DOI] [PubMed] [Google Scholar]
- 25.Powe C.E. Early Pregnancy Biochemical Predictors of Gestational Diabetes Mellitus. Curr Diab Rep. 2017;17:12. doi: 10.1007/s11892-017-0834-y. [DOI] [PubMed] [Google Scholar]
- 26.Parsons J.A., Brelje T.C., Sorenson R.L. Adaptation of islets of Langerhans to pregnancy: Increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–1466. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- 27.Chen Liwei, Shi Lu, Chao Margaret Shin, Tong Xia, Wang Fan. Stressful life events, hypertensive disorders, and high blood sugar during pregnancy. Stress Health. 2020;36(2):160–165. doi: 10.1002/smi.2911. [DOI] [PubMed] [Google Scholar]