Abstract
Background
Postpartum haemorrhage (PPH) is the single leading cause of maternal mortality worldwide. Most of the deaths associated with PPH occur in resource‐poor settings where effective methods of prevention and treatment ‐ such as oxytocin ‐ are not accessible because many births still occur at home, or in community settings, far from a health facility. Likewise, most of the evidence supporting oxytocin effectiveness comes from hospital settings in high‐income countries, mainly because of the need of well‐organised care for its administration and monitoring. Easier methods for oxytocin administration have been developed for use in resource‐poor settings, but as far as we know, its effectiveness has not been assessed in a systematic review.
Objectives
To assess the effectiveness and safety of oxytocin provided in non‐facility birth settings by any way in the third stage of labour to prevent PPH.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register, the WHO International Clinical Trials Registry Platform (ICTRP), ClinicalTrials.gov (12 November 2015), and reference lists of retrieved reports.
Selection criteria
All published, unpublished or ongoing randomised or quasi‐randomised controlled trials comparing the administration of oxytocin with no intervention, or usual/standard care for the management of the third stage of labour in non‐facility birth settings were considered for inclusion.
Quasi‐randomised controlled trials and randomised controlled trials published in abstract form only were eligible for inclusion but none were identified. Cross‐over trials were not eligible for inclusion in this review.
Data collection and analysis
Two review authors independently assessed studies for eligibility, assessed risk of bias and extracted the data using an agreed data extraction form. Data were checked for accuracy.
Main results
We included one cluster‐randomised trial conducted in four rural districts in Ghana that randomised 28 community health officers (CHOs) (serving 2404 potentially eligible pregnant women) to the intervention group and 26 CHOs (serving 3515 potentially eligible pregnant women) to the control group. Overall, the trial had a high risk of bias. CHOs delivered the intervention in the experimental group (injection of 10 IU (international units) of oxytocin in the thigh one minute following birth using a prefilled, auto‐disposable syringe). In the control group, CHOs did not provide this prophylactic injection to the women they observed. CHOs had no midwifery skills and did not in any way manage the birth. All other CHO activities (outcome measurement, data collection, and early treatment and referral when necessary) were identical across the control and oxytocin CHOs.
Although only one of the nine cases of severe PPH (blood loss greater or equal to 1000 mL) occurred in the oxytocin group, the effect estimate for this outcome was very imprecise and it is uncertain whether the intervention prevents severe PPH (risk ratio (RR) 0.16, 95% confidence interval (CI) 0.02 to 1.30; 1570 women (very low‐quality evidence)). Similarly, because of the lack of cases of severe maternal morbidity (e.g. uterine rupture) and maternal deaths, it was not possible to obtain effect estimates for those outcomes (both very low‐quality evidence).
Oxytocin compared with the control group decreased the incidence of PPH (> 500 mL) in both our unadjusted (RR 0.48, 95% CI 0.28 to 0.81; 1569 women) and adjusted (RR 0.49, 95% CI 0.27 to 0.90; 1174 women (both low‐quality evidence)) analyses. There was little or no difference between the oxytocin and control groups on the rates of transfer or referral of the mother to a healthcare facility (RR 0.72, 95% CI 0.34 to 1.56; 1586 women (low‐quality evidence)), stillbirths (RR 1.27, 95% CI 0.67 to 2.40; 2006 infants (low‐quality evidence)); andearly infant deaths (0 to three days) (RR 1.03, 95% CI 0.35 to 3.07; 1969 infants (low‐quality evidence)). There were no cases of needle‐stick injury or any other maternal major or minor adverse event or unanticipated harmful event. There were no cases of oxytocin use during labour.
There were no data reported for some of this review's secondary outcomes: manual removal of placenta, maternal anaemia, neonatal death within 28 days, neonatal transfer to health facility for advanced care, breastfeeding rates. Similarly, the women's or the provider's satisfaction with the intervention was not reported.
Authors' conclusions
It is uncertain if oxytocin administered by CHO in non‐facility settings compared with a control group reduces the incidence of severe PPH (>1000 mL), severe maternal morbidity or maternal deaths. However, the intervention probably decreases the incidence of PPH (> 500 mL).
The quality of the one trial included in this review was limited because of the risk of attrition and recruitment biases related to limitations in the follow‐up of pregnant women in both arms of the trials and some baseline imbalance on the size of babies at birth. Additionally, there was serious imprecision of the effect estimates for most of the primary outcomes mainly because of the size of the trial, very few or no events and CIs around both relative and absolute estimates of effect that include both appreciable benefit and appreciable harm.
Although the trial presented data both for primary and secondary outcomes, it seemed to be underpowered to detect differences in the primary outcomes that are the ones more relevant for making judgments about the potential applicability of the intervention in other settings (especially severe PPH).
Therefore, taking into account the extreme setting where the intervention was implemented, the limited role of the CHO in the trial and the lack of power for detecting effects on primary (relevant) outcomes, the applicability of the evidence found seems to be rather limited.
Further well‐executed and adequately‐powered randomised controlled trials assessing the effects of using oxytocin in pre‐filled injection devices or other new delivery systems (spray‐dried ultrafine formulation of oxytocin) on severe PPH are urgently needed. Likewise, other important outcomes like possible adverse events and acceptability of the intervention by mothers and other community stakeholders should also be assessed.
Keywords: Adult, Female, Humans, Pregnancy, Rural Health, Community Health Workers, Community Health Workers/statistics & numerical data, Ghana, Oxytocics, Oxytocics/administration & dosage, Oxytocin, Oxytocin/administration & dosage, Postpartum Hemorrhage, Postpartum Hemorrhage/prevention & control, Randomized Controlled Trials as Topic
Plain language summary
Oxytocin for preventing postpartum haemorrhage in non‐facility birth settings
What is the issue?
Postpartum haemorrhage (PPH) ‐ excessive blood loss (of more than half a litre) following a vaginal birth ‐ is the single leading cause of maternal mortality worldwide. Most of the deaths associated with PPH occur in low‐income settings where effective methods of prevention and treatment are not easily accessible.
Why is this important?
Oxytocin is a drug widely used for preventing and treating PPH, but most of the evidence supporting its effectiveness comes from hospital settings in high‐income countries. Easier methods of oxytocin administration have been developed for use in low‐income settings, such as pre‐filled auto‐disposable intramuscular injection syringes or a spray‐dried ultrafine formulation of oxytocin. The effectiveness of these methods has not been assessed in a systematic review.
What evidence did we find?
On 12 November 2015 we searched for evidence from randomised controlled trials and found a single trial conducted across four rural districts in Ghana. The trial randomised 28 community health officers (serving 2404 potentially eligible pregnant women) to the intervention group and 26 community health officers (3515 potentially eligible pregnant women) to the control group.
It was uncertain from this trial whether the intervention prevented loss of more than one litre of blood (severe PPH) as the results were variable and suggested anything between a 98% decrease to a 30% increase in blood loss (very low‐quality evidence). Because there were no cases of severe maternal illness (for example, because of uterine rupture), or maternal deaths, it was not possible to fully assess the effect of the intervention on those outcomes (thequality of the evidence was very low).
The women receiving oxytocin had half the incidence of PPH (> 500 mL) compared with the control group (low‐quality evidence). There was little or no difference between the oxytocin and control groups on the rates for transfer or referral of women to a healthcare facility (low‐quality evidence), stillbirths (low‐quality evidence), or the numbers of babies that died within three days of being born (low‐quality evidence).
There were no cases of oxytocin use during labour, needle‐stick injury or any other major or minor adverse events or unanticipated harmful event.
Overall, the quality of the evidence was low/very low because of methodological limitations in the trial and imprecision in the effect estimates for all the important outcomes.
What does this mean?
It is uncertain if the administration of oxytocin by community health officers without midwifery skills administered in non‐health facility settings compared with a control group reduces the incidence of severe PPH, severe maternal illness or maternal deaths when compared with a control group. However, oxytocin probably decreases the incidence of PPH (> 500 mL).
Considering the very specific setting where the trial was conducted and the limited role played by the community health officer in the trial, the applicability of the evidence is rather limited.
Further high‐quality randomised controlled trials are urgently needed to assess the effects of using oxytocin in pre‐filled injection devices or other new delivery systems on severe PPH. Similarly, future studies should consider other important outcomes like possible adverse events and the acceptability of the intervention for mothers and other community stakeholders.
Summary of findings
Summary of findings for the main comparison. Prophylactic oxytocin injection (delivered by community health officer) versus control (no injection) for preventing postpartum haemorrhage.
| Prophylactic oxytocin injection (delivered by community health officer (CHO)) versus control (no injection) for preventing postpartum haemorrhage | ||||||
|
Patient or population: pregnant women giving birth in non‐facility settings Settings: rural districts in a low‐income country (Ghana) Intervention: one injection of oxytocin [10 IU] administered by a CHO) one minute after birth Comparison: no provision of prophylactic oxytocin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| control | oxytocin | |||||
|
Severe postpartum haemorrhage (> 1000 mL) [follow‐up] |
9.02 per 1000 | 1.46 per 1000 (0.18 to 11.73) | RR 0.16 (0.02 to 1.30) | 1569 (1) | ⊕⊝⊝⊝ very low1 | |
| Severe maternal morbidity | It was not possible to obtain effect estimates as there were no events reported | 1586 (1) |
⊕⊝⊝⊝ very low1 | |||
| Maternal deaths | It was not possible to obtain effect estimates as there were no events reported | 1586 (1) |
⊕⊝⊝⊝ very low1 | |||
|
Postpartum haemorrhage (PPH greater or equal to 500 mL) [follow‐up] |
55.2 per 1000 | 26.4 per 1000 (15.5 to 44.7) | RR 0.48 (0.28 to 0.81) | 1574 (1) | ⊕⊕⊝⊝ low2 | |
|
Postpartum haemorrhage (adjusted by design effect) [follow‐up] |
55.7 per 1000 | 27.5 per 1000 (15.0 to 50.1) | RR 0.49 (0.27 to 0.90) | 1574 (1) | ⊕⊕⊝⊝ low2 | |
|
Transfer or referral to health facility [follow‐up] |
14.5 per 1000 | 10.5 per 1000 (4.93 to 22.6) | RR 0.72 (0.34 to 1.56) | 1586 (1) | ⊕⊕⊝⊝ low2 | |
|
Stillbirths [follow‐up] |
16.4 per 1000 | 20.9 per 1000 (10.9 to 39.4) | RR 1.27 (0.67 to 2.40) | 2006 (1) | ⊕⊕⊝⊝ low2 | |
|
Early infant death (0‐3 days) [follow‐up] |
6.50 per 1000 | 6.72 per 1000 (2.28 to 20.0) | RR 1.03 (0.35 to 3.07) | 1969 (1) | ⊕⊕⊝⊝ low2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded because of serious methodological limitations (risk of attrition and recruitment bias) and very serious imprecision
2 Downgraded because of serious methodological limitations (risk of attrition and recruitment bias) and serious imprecision
Background
Global efforts on maternal health have decreased maternal mortality. The Millennium Development Goals (MDG) set by the United Nations proposed to reduce the 1990 maternal mortality ratio by three‐quarters by year 2015 (UN 2000). Global maternal mortality has decreased at an estimated annual rate of 1.3% per year between 1990 and 2013, with 83,052 fewer maternal deaths estimated for year 2013 compared to 1990 (Kassebaum 2014). However this reduction is still far from the milestone proposed in MDG 5. An absolute reduction of about 200,000 maternal deaths is still required to achieve the 75% reduction goal, considering maternal deaths and livebirth estimates for 2013 (Kassebaum 2014).
Description of the condition
Postpartum haemorrhage (PPH) ‐ defined as blood loss greater than 500 mL following a vaginal birth (WHO 2012) ‐ is the single leading cause of maternal mortality worldwide, accounting for nearly a fifth of all maternal deaths (Say 2014). PPH occurring during the first 24 hours after birth (primary PPH) is more likely to end in severe maternal morbidity and mortality (Ronsmans 2006), and will be the focus of this review. Severe primary PPH is defined as blood loss from the genital tract of 1000 mL or more in the first 24 hours after birth. A systematic review, aimed to estimate the prevalence of primary PPH and severe PPH that included close to four million women, found that the overall prevalence was 6.09% for PPH and 1.86% for severe PPH (Carroli 2008). Although PPH deaths have shown an important absolute reduction between 1990 and 2013, this decline has been observed mainly in high‐income countries, with few changes in low‐income countries (Kassebaum 2014). Most of the deaths associated with PPH occur in resource‐poor settings where effective methods of prevention and treatment are not accessible because many births still occur at home, or in community settings, far from a health facility. Every year about 60 million women worldwide give birth outside health facilities, and up to 52 million births occur without the supervision of a skilled birth attendant (Darmstadt 2009). Conservative estimates, based on current trends, indicate that 380 million births in South Asia and sub‐Saharan Africa during the 2010 to 2015 period would not have had a skilled attendant present (Crowe 2012).
Description of the intervention
A number of interventions for PPH prevention have been evaluated and recommended by several organisations worldwide (ICM‐FIGO 2003; NICE 2007; WHO 2012; Mousa 2014). One of the interventions universally recommended for PPH prevention is the active management of the third stage of labour (AMTSL). Although the definition of AMTSL varies, it usually includes giving a prophylactic uterotonic, and using controlled cord traction to deliver the placenta (Begley 2015). The recommended uterotonic drugs are oxytocin, ergometrine or a combination of the two. Even with a more expectant management of the third stage of labour, the World Health Organization (WHO) recommends that all women giving birth should be offered uterotonics during the third stage of labour (WHO 2012; Westhoff 2013). Oxytocin is a 9‐amino‐acid peptide that is secreted in vivo by the posterior pituitary gland; primarily it promotes smooth muscle contraction. Its postpartum release stimulates both uterine contractions ‐ for stopping blood loss, and the breast‐milk reflex. The dose used for PPH‐prophylaxis varies widely between practitioners and obstetric units, ranging from 2 IU to 10 IU (international units) for both intravenous bolus and intramuscular injections (Breathnach 2006).
Although there is a general consensus that oxytocin is an effective intervention to prevent PPH, its potential benefits can only be achieved if it is accessible to women who need it at the point of birth. Most of the evidence supporting the use of oxytocin comes from hospital settings in high‐income countries, mainly because of the need for well‐organised care in order for it to be administered and monitored (Begley 2015; Westhoff 2013). Whilst expanding actions for preventing PPH in rural and underserved settings are desirable in order to decrease maternal morbidity and mortality, this strategy requires the active participation of pregnant women and the co‐ordination of communities with their health systems (Miller 2004; Karoshi 2009). This complex set of interactions is not always implementable and sustainable in such resource‐constrained settings. Therefore, in these settings and in the absence of a trained health professional, interventions delivered by other individuals could be more appropriate. Two of these interventions (misoprostol and uterine massage) have already been assessed by Cochrane systematic reviews (Oladapo 2012a; Hofmeyr 2013). In the first review, no trials met the inclusion criteria for assessing the benefit of advance misoprostol distribution for preventing or treating postpartum haemorrhage (Oladapo 2012a). In the second review, the inconclusive evidence for uterine massage highlighted the need for trials in settings where uterotonics are not available (Hofmeyr 2013). Although misoprostol is currently included in the WHO essential medicine list for the prevention of PPH in non‐facility settings, there has been some controversy around its inclusion based on differences in the interpretation of the quality of evidence supporting its use (Chu 2012; Hundley 2013; Millard 2014). Therefore, efforts have been also directed towards developing methods that make oxytocin accessible at the community level.
Oxytocin is stable at temperatures up to 25°C but requires refrigeration to prolong its shelf‐life. This requirement constitutes a major challenge to ensure its potency in resource‐poor settings, where prolonged storage is common and the necessary facilities are either not available or in short supply. This concern has been addressed with a time‐temperature indicator that allows the provider to assess whether the injection system has had sufficient cumulative heat exposure to decrease its biological effect, and, if so, to discard the injection system (Stanton 2012). Oxytocin stability during long‐term storage has previously been tested; it retains 86% of its chemically active ingredient after being stored at 30°C for one year (WHO 1993). An 'all‐in‐one' auto‐disable injection system prefilled with a single dose of 10 IU of oxytocin has been developed to meet the requirements of community‐level implementation (Tsu 2003; Jangsten 2005; Strand 2005; Althabe 2011). Likewise, a spray‐dried ultrafine formulation of oxytocin has been recently developed to facilitate its aerosolised delivery via the lungs (Prankerd 2013). Although this formulation is still at an experimental phase, it highlights the potential for the development of new delivery systems that could be available for clinical use in the foreseeable future.
How the intervention might work
Oxytocin binds to receptors in the smooth muscles of the uterus and causes rhythmic contractions of the upper uterine segment; these are more powerful towards the end of pregnancy, during labour and immediately postpartum. It is deactivated in the gastrointestinal tract, and thus its main route of administration is parenteral (Breathnach 2006). When given by the intravenous route, oxytocin causes an almost immediate action and reaches a steady state after 40 minutes (Seitchik 1984; Gonser 1995), whereas intramuscular administration results in a slower onset of action, that takes between three and seven minutes, but produces an uterotonic measurable effect up to one hour postpartum (Amsalem 2014). Its elimination from the plasma is mainly through the liver and kidneys, with less than 1% excreted unchanged in the urine (Amico 1987).
Despite its beneficial effects preventing PPH, oxytocin is a vasoactive peptide with a complex hormonal activity. Apart from the uterine smooth muscles, specific receptors of oxytocin have been described in all kinds of tissues including the myocardium (heart muscle), blood vessels, central nervous system and the lactating glands (Evans 1997). Oxytocin shares about 5% of the antidiuretic properties of vasopressin as a result of certain similarities in their structure. This antidiuretic effect is responsible for water intoxication, which can result from repeated administration of oxytocin in large volumes of electrolyte‐free solutions. Depending on the degree of water overload, a woman could present with headaches, vomiting, drowsiness, confusion, lethargy, convulsions or coma (In 2011). It also has a direct relaxing effect on vascular smooth muscle leading to a decreased systemic vascular resistance, hypotension and tachycardia. These haemodynamic responses have been mainly associated with the intravenous route of administration, particularly when given by rapid bolus injection, and often in women under anaesthesia for caesarean section or other pregnancy‐related indications (Dyer 2011; Bhattacharya 2013). Oxytocin administered as an intravenous bolus of 10 IU has been reported to induce chest pain, transient profound tachycardia, hypotension, and electrocardiographic changes suggestive of myocardial ischaemia (Svanström 2008). These concerns have led to a call for caution in using intravenous oxytocin in women with unstable cardiovascular conditions such as hypovolaemia, shock or cardiac disease. Unlike intravenous oxytocin, there is a paucity of data regarding the side‐effects of intramuscular oxytocin, probably because they could be of low clinical importance, and the general impression, as evidenced by the recommendations of national and international organisations (ICM‐FIGO 2003; NICE 2007; WHO 2007), is that it is relative safe.
Expanding the provision of oxytocin at the community level poses important challenges: 1) it has to be easily administered by non‐skilled providers; 2) it has to be easily distributed and stored; 3) there is a need to ensure that the availability of the medication will not increase the risk for the mother and baby, avoiding incorrect (administration before the birth of the infant) or inappropriate use (use for any purpose other than PPH prevention); and 4) there needs to be assurance that availability of interventions at community level does not decrease the importance of giving birth at health facilities (Oladapo 2012a; Hundley 2013). Therefore, the development of any intervention at the community level should consider these interactions among the drug effects (beneficial and adverse), the skills and knowledge of the person administering the drug, and the birth and monitoring arrangements of the health system within which the intervention will be implemented.
Why it is important to do this review
Oxytocin is a drug used for preventing and treating PPH as part of the management of the third stage of labour, but most of the evidence supporting its effectiveness comes from hospital settings in high‐income countries, mainly because of the need of well‐organised care for its administration and monitoring. Easier methods for oxytocin administration have been developed for use in resource‐poor settings (single‐dose prefilled auto‐disable injection, spray‐dried ultrafine formulation), but as far as we know, its effectiveness has not been assessed in a systematic review.
Objectives
To assess the effectiveness and safety of oxytocin provided in non‐facility birth settings by any way in the third stage of labour to prevent postpartum haemorrhage.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised controlled trials (including those using a cluster‐randomised design and those published only as abstracts) comparing the administration of oxytocin with no intervention, or usual/standard care for the management of the third stage of labour in non‐facility birth settings were considered for inclusion.
Cross‐over trials were not eligible for inclusion.
Types of participants
All trials including pregnant woman with anticipated vaginal birth in non‐facility birth settings were considered for inclusion.
Types of interventions
Any strategy that includes the administration of oxytocin for postpartum haemorrhage (PPH) prevention in non‐facility deliveries. This included:
administration by non‐skilled attendants: any kind of volunteers or workers with no formal obstetric training in charge of the management of women in non‐facility settings;
administration by pregnant women themselves for postpartum self‐administration in home births.
We planned to include the use of oxytocin in emergency care for PPH in non‐facility births. We considered studies regardless of the dose, or the extent of education or training of end‐users.
We considered the following relevant comparators.
No intervention.
Usual/standard care as defined for the specific setting.
Administration of other uterotonics (e.g. misoprostol).
Types of outcome measures
We included studies reporting any of the following outcomes.
Primary outcomes
Severe PPH (blood loss greater than or equal to 1000 mL, or as defined by authors).
Severe maternal morbidity (hysterectomy, intensive care admission, massive blood products transfusion, organ failure, or as defined by authors).
Maternal deaths.
Secondary outcomes
Maternal
PPH (blood loss greater than or equal to 500 mL or as defined by authors).
Transfer or referral to a healthcare facility.
Manual removal of placenta or subsequent surgical evacuation of retained products of gestation.
Maternal anaemia within 24 to 48 hours after birth (as defined by authors).
Maternal minor adverse events (as defined by authors).
Maternal major adverse events (as defined by authors).
Blood loss (as measured by the authors) was included as a non pre‐specified outcome (see Differences between protocol and review)
Oxytocin use during labour was included as a non pre‐specified outcome (see Differences between protocol and review)
Fetal and neonatal
Although this is a postpartum intervention, it could affect this group of outcomes when the intervention is used inappropriately, i.e. given antenatally for other purposes: before the complete expulsion of the baby, before the birth of the second twin, or before cord clamping.
Stillbirth.
Early neonatal death (within the first seven days).
Neonatal death (within the first 28 days).
Neonatal transfer to health facility for advanced care.
Breastfeeding rates as reported by the authors.
Acceptability of the intervention
Woman's satisfaction with the intervention (as defined by authors).
Provider's satisfaction with the intervention (as defined by authors).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (12 November 2015).
The Register is a database containing over 20,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Trials Search Co‐ordinator searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included, Excluded or Awaiting Classification).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (12 November 2015).The search terms we used are given in Appendix 1.
Searching other resources
We searched reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed all the potential studies we identified as a result of the search strategy for inclusion. We resolved any disagreement through discussion or, if required, we consulted a third review author.
We created a study flow diagram to map out the number of records identified, included and excluded.
Data extraction and management
We designed a form to extract data based on template provided by the Cochrane Pregnancy and Childbirth Group. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2014), and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to request further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third review author.
1) Random sequence generation (checking for possible selection bias)
For the one included study we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as being at:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
2) Allocation concealment (checking for possible selection bias)
For the one included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
3.1) Blinding of participants and personnel (checking for possible performance bias)
For the one included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
3.2) Blinding of outcome assessment (checking for possible detection bias)
For the one included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as being at:
low, high or unclear risk of bias.
4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For the one included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as being at:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
5) Selective reporting (checking for reporting bias)
For the one included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For the one included study we described any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
7) Overall risk of bias
We made explicit judgements about whether the included study was at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to items 1) to 6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to have an impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Cluster‐randomised trials
As the included trial was a cluster‐randomised trial, we additionally assessed the following factors as potential sources of bias (as suggested in the section 16.3.2 of the Cochrane Handbook for Systematic Reviews of Interventions).
Recruitment bias: occurring when individuals are recruited to the trial after the clusters have been randomised, as the knowledge of whether each cluster is an 'intervention' or 'control' cluster could affect the types of participants recruited.
Baseline imbalance: because the numbers of clusters randomised in a cluster‐randomised trial are often small, there is a possibility of chance baseline imbalance between the randomised groups, in terms of either the clusters or the individuals.
Loss of clusters: just as with missing outcome data in individually‐randomised trials, the loss of information from complete clusters from cluster‐randomised trials may lead to bias. Additionally, missing outcomes for individuals within clusters may also lead to a risk of bias.
Incorrect analysis: occurs when cluster‐randomised trials are analysed by incorrect statistical methods, not taking the clustering into account and creating a 'unit of analysis error' that produces over‐precise results and P values that are too small.
Assessment of the quality of the evidence using the GRADE approach
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence in relation to the following outcomes for the main comparison.
Severe PPH (blood loss > 1000 mL)
Severe maternal morbidity
Maternal deaths
PPH (blood loss greater than or equal to 500 mL)
Transfer or referral to health facility
Stillbirths
Early infant death (0 to three days)
We used the GRADEpro Guideline Development Tool to create a 'Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary risk ratios with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials and the standardised mean difference to combine trials that measured the same outcome, but used different methods. As only one trial was included, we reported the results using the mean difference with 95% confidence intervals.
Unit of analysis issues
Cluster‐randomised trials
We included one cluster‐randomised trial in our analyses and adjusted the effect estimates when clustering was not considered using the "effective sample size" method as described in the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.3.5) using an estimate of the intracluster correlation coefficient (ICC) derived from the trial for each outcome (Higgins 2011). Therefore, we reported adjusted and unadjusted (as presented in the trial report) effect estimates. For those outcomes where an estimate of the ICC was not available we only presented the unadjusted effect estimates.
We planned to acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit, but this was not possible because only one study was identified.
Dealing with missing data
We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis, but this was not possible because only one study was identified.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in the trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We planned to assess statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We would have considered heterogeneity as substantial if an I² was greater than 30% and either a T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there were 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014), but were unable to combine data in a meta‐analysis as there was only one included study. In future updates of this review, we will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average of the range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We planned to use primary outcomes to carry out subgroup analyses based on the dose of oxytocin used, the management of the third stage of labour (active versus expectant), the type of administration (self‐administration versus administration by another person), the different methods for estimating blood loss, and the extent of education/training of the final user (person administrating oxytocin).
In future updates, if more studies are included, we will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates we will carry sensitivity analyses (if required) based on the primary outcomes in order to explore the effects of risk of bias associated with the quality of included trials (for instance, excluding those studies at high risk of bias in the overall assessment from the analysis ‐ see Assessment of risk of bias in included studies), the effects of fixed‐ or random‐effects model analyses for outcomes with statistical heterogeneity, and the effect of assumptions on the value of ICC used for cluster‐randomised trials.
Results
Description of studies
Results of the search
See: Figure 1. The search retrieved 14 reports in total. We identified six potential articles for the review after the initial screening of titles and abstracts.
1.

Study flow diagram.
We included one trial (Stanton 2013) published in two trial reports. We excluded two trials (NCT01487278; Stanton 2010) and two trials are awaiting classification (NCT01710566; NCT01713153).
Included studies
A single trial (reported in two trial reports) was included in this review (Stanton 2013). It was a cluster‐randomised trial conducted in four rural districts in Ghana. The unit of randomisation was the community health officer (CHO), and births attended by a CHO constituted a cluster.
CHOs delivered the intervention in the experimental group (injection of 10 IU of oxytocin in the thigh one minute following a birth using a prefilled, auto‐disposable syringe). In the control group CHOs did not provide this prophylactic injection to women they observed. CHOs had no midwifery skills and did not in any way manage the birth. All other CHO activities (outcome measurement, data collection, and early treatment and referral when necessary) were identical across the control and oxytocin group CHOs. Thus, the control condition could be deemed as usual/standard care.
The trial randomised 28 CHOs (serving 2404 potentially eligible pregnant women) to the intervention group and 26 CHOs (serving 3515 potentially eligible pregnant women) to the control group. For further details of this trial, see Characteristics of included studies.
The trial's authors used multiple definitions of postpartum haemorrhage (PPH) as the primary outcome to assess the effect of the intervention, but we reported only those previously defined in our protocol (Types of outcome measures).
Excluded studies
The two excluded studies were trials protocols published in ClinicalTrials.gov and were withdrawn prior to enrolment (NCT01487278; Stanton 2010).
Studies awaiting classification
For two reports (NCT01710566; NCT01713153), the recruitment status was unknown because the information has not been updated in ClinicalTrials.gov at the time of submitting the review and these two remain in Studies awaiting classification.
Risk of bias in included studies
See table of Characteristics of included studies.
Allocation
The sequence generation and allocation concealment seem to be appropriate (low risk of bias).
Blinding
Although it was an open trial and the intervention could not be masked, it was not clear from the report that the clusters (CHOs) had the same probability of being randomised to intervention or control arms. Even when there were clear instructions in the protocol on how to measure the primary outcomes, it was possible that the knowledge of the allocated group could affect outcome measurement (high risk of detection bias).
We were not sure about the level of bias introduced by the lack of blinding of the personnel caring for the participants (unclear risk of performance bias).
Incomplete outcome data
Although the risk of attrition bias at the cluster level was low (all the clusters provided data for the outcomes assessed in the trial at the follow‐up, with only 16 cases with missing or incomplete data, seven in the intervention and nine in the control arms), primary outcome data were available from only 682 out of 2404 potentially eligible women (28.3%) in the intervention group, and from 888 out of 3515 potentially eligible women (25.2%) in the control group. Consequently, we assessed the study as being at a high risk of attrition bias.
Selective reporting
There did not appear to be any selective reporting of outcomes; those outcomes listed on the available trial protocol are similar to those reported in the final publication (low risk of reporting bias).
Other potential sources of bias
Because recruitment was done after the CHOs were randomised, it is uncertain how the knowledge of the allocation arm influenced the effect estimates considering that recruitment in each cluster could be affected by pregnant women decisions (e.g. choosing to deliver in a health facility or at home but without a CHO), or CHOs attitudes and behaviours (e.g. referring women before birth), that could be associated with the outcomes assessed (recruitment bias). However, this did not produce relevant baseline imbalance between oxytocin and control clusters on a range of women’s characteristics or on indicators of recruitment, enrolment, and measurement procedures. There were only differences between the proportions of babies of smaller than normal size at birth with a higher proportion in the control group compared with the oxytocin group (3.9% versus 1.9%, respectively). We assessed the study as being at an unclear risk of other bias.
Effects of interventions
See: Table 1
Oxytocin delivered by CHO versus control group
Primary outcomes
Severe PPH (blood loss greater or equal to 1000 mL, or as defined by authors)
Although only one of the nine cases of severe PPH (> 1000 mL) occurred in the oxytocin group, the effect estimate for this outcome was very imprecise and it is uncertain whether the intervention prevents severe PPH (risk ratio (RR) 0.16, 95% confidence interval (CI) 0.02 to 1.30; 1570 women, very low‐quality of evidence) (Analysis 1.1).
1.1. Analysis.

Comparison 1 Prophylactic oxytocin injection (delivered by CHO) versus control (no injection), Outcome 1 Severe postpartum haemorrhage 1 (> 1000 mL).
Severe maternal morbidity and maternal deaths
Because there were no cases of severe maternal morbidity (e.g. uterine rupture) (Analysis 1.2) or maternal deaths (Analysis 1.3), it was not possible to obtain effect estimates for those outcomes. The effect of oxytocin on these outcomes is uncertain (very low‐quality of evidence).
1.2. Analysis.
Comparison 1 Prophylactic oxytocin injection (delivered by CHO) versus control (no injection), Outcome 2 Severe maternal morbidity.
1.3. Analysis.

Comparison 1 Prophylactic oxytocin injection (delivered by CHO) versus control (no injection), Outcome 3 Maternal deaths.
Secondary maternal outcomes
PPH (blood loss greater than or equal to 500 mL or as defined by authors)
Oxytocin administered by CHO in non‐facility settings compared with the control group decreased the incidence of PPH (> 500 mL) in both the unadjusted and adjusted (by clustering) analysis (low‐quality evidence). The unadjusted PPH rate among women in the oxytocin group was 2.64% versus 5.52% among women in the control group (RR 0.48, 95% CI 0.28 to 0.81; 1569 women) (Analysis 1.4). The adjusted PPH rate in the oxytocin group was 2.75% versus 5.57% in the control group (RR 0.49, 95% CI 0.27 to 0.90; 1174 women) (Analysis 1.5).
1.4. Analysis.
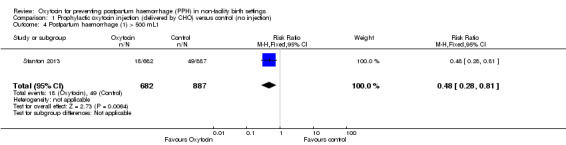
Comparison 1 Prophylactic oxytocin injection (delivered by CHO) versus control (no injection), Outcome 4 Postpartum haemorrhage (1) > 500 mL1.
1.5. Analysis.

Comparison 1 Prophylactic oxytocin injection (delivered by CHO) versus control (no injection), Outcome 5 Postpartum haemorrhage (1) >500 mL (adjusted by design effect).
The authors used additional definitions of PPH (PPH‐2 included PPH plus any woman receiving early treatment for PPH regardless of cumulative blood loss; and PPH‐3 included any woman without a quantitative blood loss measure who was referred to a healthcare facility care for postpartum bleeding, in addition to all women included in PPH and PPH‐2), for which they presented separate analysis. For PPH‐2, the unadjusted rates were 3.81% and 10.82% in the oxytocin and control groups, respectively (RR 0.35, 95% CI 0.23 to 0.54), while the adjusted rates were 3.91% and 10.96%, respectively (RR 0.36, 95% CI 0.19 to 0.68). The unadjusted PPH‐3 rates were 4.11% and 11.15% in the oxytocin and control groups, respectively (RR 0.37, 95% CI 0.25 to 0.55); and the adjusted PPH‐3 rates were 4.23% and 11.08% for the intervention and control groups, respectively (RR 0.38, 95% CI 0.20 to 0.71).
Transfer or referral to a healthcare facility
There was little or no difference between the oxytocin and control groups on the rates of transfer or referral to a healthcare facility (RR 0.72, 95% CI 0.34 to 1.56; 1586 women, low‐quality evidence) (Analysis 1.6).
1.6. Analysis.
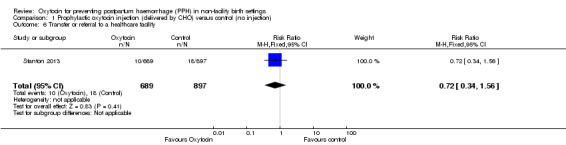
Comparison 1 Prophylactic oxytocin injection (delivered by CHO) versus control (no injection), Outcome 6 Transfer or referral to a healthcare facility.
Manual removal of placenta or subsequent surgical evacuation of retained products of gestation
This outcome was not measured/reported in the trial.
Maternal anaemia within 24 to 48 hours after birth (as defined by authors)
This outcome was not measured/reported in the trial.
Maternal minor and major adverse events
There were no cases of needle‐stick injury or any other major adverse event or unanticipated harmful event (Analysis 1.7).
1.7. Analysis.

Comparison 1 Prophylactic oxytocin injection (delivered by CHO) versus control (no injection), Outcome 7 Maternal major/minor adverse events.
Blood loss (outcome not pre‐specified in our protocol)
The mean blood loss by birth was 185.5 mL and 229.2 mL in the oxytocin and control groups, respectively but standard deviations were not reported by the trialist, thus preventing further analysis. The trial authors reported a mean reduction of blood loss between groups of 45.1 mL (95% CI 17.7 to 72.6 mL).
Oxytocin use during labour (outcome not pre‐specified in our protocol)
There were no cases of oxytocin use during labour in the intervention or control group (Analysis 1.8).
1.8. Analysis.
Comparison 1 Prophylactic oxytocin injection (delivered by CHO) versus control (no injection), Outcome 8 Oxytocin use during labour.
Secondary fetal and neonatal outcomes
Stillbirths
There was little or no difference between the oxytocin and control groups on the rates of stillbirths (RR 1.27, 95% CI 0.67 to 2.40; 2006 infants, low‐quality evidence) (Analysis 1.9).
1.9. Analysis.
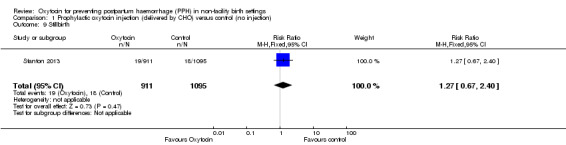
Comparison 1 Prophylactic oxytocin injection (delivered by CHO) versus control (no injection), Outcome 9 Stillbirth.
Early neonatal death (within the first three days)
There was little or no difference between the oxytocin and control groups on the rates of early neonatal deaths (RR 1.03, 95% CI 0.35 to 3.07; 1969 infants, low‐quality evidence) (Analysis 1.10).
1.10. Analysis.
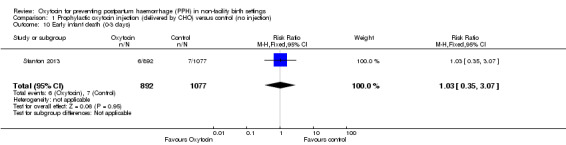
Comparison 1 Prophylactic oxytocin injection (delivered by CHO) versus control (no injection), Outcome 10 Early infant death (0‐3 days).
Neonatal death (within the first 28 days)
This outcome was not measured/reported in the trial.
Neonatal transfer to health facility for advanced care
This outcome was not measured/reported in the trial.
Breastfeeding rates as reported by the authors
This outcome was not measured/reported in the trial.
Acceptability of the intervention
Woman's satisfaction with the intervention (as defined by authors)
This outcome was not measured/reported in the trial.
Provider's satisfaction with the intervention (as defined by authors)
This outcome was not measured/reported in the trial.
Discussion
Summary of main results
This review included a single cluster‐randomised trial conducted in four rural districts in Ghana that randomised 28 community health officers (CHOs) (serving 2404 potentially eligible pregnant women) to the intervention group and 26 CHOs (3515 potentially eligible pregnant women) to the control group. It is uncertain if oxytocin administered by a CHO in non‐facility settings compared with a control group reduces the incidence of severe postpartum haemorrhage (PPH), severe maternal morbidity or maternal deaths. However, it probably decreases the incidence of PPH (independent of how it is defined). There was little or no difference between the oxytocin and control groups on a number of secondary outcomes such as the rates of: transfer or referrals to healthcare facilities, stillbirths, difficulty breathing at birth, early neonatal deaths, and infant resuscitation. There were no cases of oxytocin use during labour, needle‐stick injury, or any other major adverse event or unanticipated harmful event.
Overall completeness and applicability of evidence
This review found evidence from only one cluster‐randomised trial conducted in rural districts in Ghana assessing the effectiveness and safety of a strategy of administration of oxytocin for preventing PPH in non‐facility births. The trial's authors probably overcame a number of logistical barriers in order to implement a trial in an area with high maternal mortality ratio (363/100,000 live births), with a non‐facility birth rate of 36% before the study, and a lack of skilled birth attendants at home according to local surveys.
The CHOs in this trial did not have midwifery skills nor manage the deliveries. They were trained to provide outreach health services to the rural population, including childhood immunisation, antenatal and postnatal care, and family planning. Their role in this trial was to observe, provide the intervention (according to random allocation), measure the outcomes, and provide early treatment for PPH and facilitate referral, when needed.
Although the trial presented data both for primary and secondary outcomes, it seemed to be underpowered to detect differences in the primary outcomes that are the ones more relevant for making judgments about the potential applicability of the intervention in other settings (especially severe PPH).
Therefore, taking into account the extreme setting where the intervention was implemented, the limited role of the CHOs in the trial and the lack of power for detecting effects on primary (relevant) outcomes, the applicability of the evidence found seems to be rather limited.
Quality of the evidence
The quality of the only study included in this review was limited because of the risk of attrition and recruitment biases related to limitations in the follow‐up of pregnant women in both arms of the trials and some baseline imbalance on the size of babies at birth. Additionally, there was serious imprecision of the effect estimates for most of the primary outcomes mainly because of the size of the trial, very few or no events and confidence intervals (CI) around both relative and absolute estimates of effect that include both appreciable benefit and appreciable harm.
Potential biases in the review process
We tried to minimise potential biases by the use of a comprehensive search strategy and restriction of the study design to individual‐ or cluster‐randomised trials. Taking into account that 'new' delivery systems for oxytocin have been developed only recently (Tsu 2003; Prankerd 2013), we think that it is unlikely that our search strategy failed to identify other relevant studies for inclusion in the review.
Agreements and disagreements with other studies or reviews
More than a decade ago, Uniject was proposed as a device for administering intramuscular oxytocin in emergencies or in non‐health facilities (Tsu 2003). In agreement with our findings, a systematic review comparing intravenous versus intramuscular route for oxytocin administration found that there was no reliable evidence to advise as to whether one choice is better than the other in terms of effectiveness and safety when used for prophylactic management of the third stage of labour after vaginal birth (Oladapo 2012b). On the other hand, a secondary observational analysis of 39,202 hospital‐based births in four countries found that, in the context of active management of the third stage of labour, when oxytocin is the only intervention, intravenous administration reduced haemorrhage risk by 76% as compared with intramuscular administration (odds ratio (OR) 0.24, 95% CI 0.12 to 0.50). However, the route of administration had no effect when oxytocin was combined with other active management interventions (Sheldon 2013).
Misoprostol has also been proposed as an alternative for PPH prevention or treatment in rural or underserved areas. However, a systematic review of advanced misoprostol distribution to health workers or the community for prevention of PPH found no randomised or quasi‐randomised trials evaluating these options for non‐facility births (Oladapo 2012a).
A recently published study estimated the cost‐effectiveness of replacing the use of the 'classic' oxytocin injection by Uniject in the Latin America and Caribbean (LAC) setting (Pichon‐Riviere 2015). The authors used an epidemiological model and consultation with a panel of experts to estimate the quality‐adjusted life years and costs from a health system perspective. While recognising the limited reliable evidence of effectiveness available for Uniject, they found that the replacement of oxytocin in ampoules by Uniject is a cost‐saving intervention and very cost‐effective in most countries claiming that Uniject use could prevent more than 40,000 PPH events annually in LAC.
Authors' conclusions
Implications for practice.
Although oxytocin administered in non‐facility settings probably decreases the incidence of postpartum haemorrhage (PPH), its effect on severe PPH and a number of other maternal and neonatal outcomes is still uncertain and their applicability is still limited. Consequently, there is insufficient evidence to support or refute the policy of using prefilled injections of oxytocin in non‐facility settings for preventing PPH. Further well‐conducted and sufficiently‐powered clinical trials are urgently required.
Implications for research.
Further well‐executed and adequately‐powered randomised controlled trials assessing the effects of using oxytocin in pre‐filled injection devices or other new birth systems (spray‐dried ultrafine formulation of oxytocin) on PPH are urgently needed. Likewise, other important outcomes such as possible adverse events and acceptability of the intervention by mothers and other community stakeholders should be assessed.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by five peers (an editor and four referees who are external to the editorial team) and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms
WHO International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov.
prefill AND oxytocin
prefilled AND oxytocin
uniject AND oxytocin
penfill AND oxytocin
advance* AND distribution AND oxytocin
community AND oxytocin
home AND oxytocin AND postpartum
Data and analyses
Comparison 1. Prophylactic oxytocin injection (delivered by CHO) versus control (no injection).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe postpartum haemorrhage 1 (> 1000 mL) | 1 | 1570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.30] |
| 2 Severe maternal morbidity | 1 | 1570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Maternal deaths | 1 | 1570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Postpartum haemorrhage (1) > 500 mL1 | 1 | 1569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.81] |
| 5 Postpartum haemorrhage (1) >500 mL (adjusted by design effect) | 1 | 1174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.27, 0.90] |
| 6 Transfer or referral to a healthcare facility | 1 | 1586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.34, 1.56] |
| 7 Maternal major/minor adverse events | 1 | 1570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Oxytocin use during labour | 1 | 1570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Stillbirth | 1 | 2006 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.67, 2.40] |
| 10 Early infant death (0‐3 days) | 1 | 1969 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.35, 3.07] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Stanton 2013.
| Methods | Cluster‐randomised trial (randomisation at the CHO level). | |
| Participants | All pregnant women in 4 rural districts in Ghana (in Brong‐Ahafo region) who agreed to participate (through written informed consent). Enrolled women were those who provided final consent and whose birth was observed by a CHO. The trial randomised 28 CHO (serving 2404 potentially eligible pregnant women) to the intervention group and 26 CHO (3515 potentially eligible pregnant women) to the control group. |
|
| Interventions | An injection of oxytocin (10 IU) in the thigh 1 minute following birth using an Uniject device (an auto‐disposable prefilled syringe) administered by a CHO. In the control group CHOs did not provide this prophylactic injection to women they observed. | |
| Outcomes |
Primary
Secondary
Feasibility outcomes: percentage of women who call for a CHO during labour, the percentage of deliveries for which the CHO arrived prior to birth, mean duration of time spent (a) travelling to the household and (b) at the household, mean number of household visits per CHO, and mean response rate per CHO. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 52 CHOs were randomly allocated equally to either the intervention (oxytocin) or the control group; this allocation was stratified by both district and distance (< 10 km or > 10 km) to emergency obstetric care. The randomisation sequence was determined using Stata (version 12). |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence was generated by LCM, while enrolment of the clusters was done by SN and KHRC in collaboration with the GHS. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | We were not sure about the level of bias introduced by the lack of blinding of the personnel caring for the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although there was a clear protocol to measure the primary outcomes, it was possible that the knowledge of the allocated group could affect outcome measurement (being done by the CHO delivering/or not the intervention). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Primary outcome data available from 682 out of 2404 (28.3%) randomised to the intervention group, and from 888 out of 3515 (25.2%) randomised to the control group. |
| Selective reporting (reporting bias) | Low risk | There was no difference between the outcomes described in the published protocol and the publication of the final results in the primary reference. |
| Other bias | Unclear risk | Because recruitment was done after the CHOs were randomised, it is uncertain if the knowledge of the allocation arm could influence the recruitment of women in each cluster (recruitment bias). |
CHO: community health officer GHS: Ghana Health Service IU: international unit KHRC: Kintampo Health Research Center LCM: Luke C Munally (one of the authors based at Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, United States of America) PPH: postpartum haemorrhage SN: Samuel Newton (one of the authors based at Kintampo Health Research Centre, Ghana Health Service, Kintampo, Ghana)
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| NCT01487278 | This study was withdrawn prior to enrolment. |
| Stanton 2010 | This study was withdrawn prior to enrolment. |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT01710566.
| Methods | RCT. |
| Participants | Pregnant women planning vaginal birth with a trained study provider at a PHC who are eligible to participate in research according to national guidelines and able to provide informed consent. |
| Interventions | Misoprostol 600 mcg oral misoprostol administered after birth of baby and before placenta is expelled versus oxytocin 10 IU oxytocin in Uniject administered after birth of baby and before placenta is expelled. |
| Outcomes | Mean change in pre‐ and post‐birth haemoglobin. |
| Notes | The recruitment status of this study is unknown because the information has not been verified recently in ClinicalTrials.gov. |
NCT01713153.
| Methods | RCT. |
| Participants | Women delivering in community health centres (case de sante) with a trained study provider (matrone) who are able to provide informed consent. |
| Interventions | Misoprostol 600 mcg misoprostol orally versus UnijectTM 10 IU oxytocin delivered intramuscularly with UnijectTM. |
| Outcomes | Mean change in haemoglobin. |
| Notes | The recruitment status of this study is unknown because the information has not been verified recently in ClinicalTrials.gov. |
IU: international unit mcg: microgram PHC: primary health centre RCT: randomised controlled trial
Differences between protocol and review
There are some differences between our published protocol (Pantoja 2015) and the full review.
Methods/assessment of risk of bias in included studies: we have added further methods for assessing the potential for bias in cluster‐randomised trials (as recommended in the Cochrane Handbook for Systematic Reviews of Interventions).
We have used the GRADE approach to assess the quality of the body of evidence and have included a 'Summary of findings' table.
We have included two additional non pre‐specified outcomes: blood loss (measured in mL) as a measure of the amount of haemorrhage, and oxytocin use during labour as a measure of intervention safety.
One of our secondary outcomes was 'Early infant death within the first seven days' but the included trial only reported data in relation to 'Early infant death within the first three days'.
Methods/types of studies: we have clarified that the study setting relates to non‐facility birth settings.
Contributions of authors
Review authors TP and EA conceived the review. TP led the writing of the review. All review authors screened references and assessed full text articles. TP and VS extracted data from the selected study. TP and CV assessed the risk of bias of the selected study. CV, EA, EC and VS provided comments to draft versions of the review. CV, EA and EC provided a clinical perspective and TP, VS and CV provided a methodological perspective to the final version of the review.
Sources of support
Internal sources
Evidence‐Based Health Care Programme, School of Medicine, Pontificia Universidad Católica de Chile, Chile.
Centro Rosarino de Estudios Perinatales (CREP), Rosario, Argentina.
External sources
-
Cochrane Global Evidence Synthesis Initiative, UK.
This funding is supporting the work of Tomas Pantoja and Claudio Vera on this review
Declarations of interest
TP: My institution has received funding from the Cochrane Global Evidence Synthesis Initiative to perform the work related with this review. I am also an editor with the Cochrane Effective Practice and Organisation of Care (EPOC) group. EA: none known EC: none known CV: My institution has received funding from the Cochrane Global Evidence Synthesis Initiative to perform the work related with this review. VS: My institution has received funding from the Cochrane Global Evidence Synthesis Initiative to perform the work related with this review.
New
References
References to studies included in this review
Stanton 2013 {published data only}
- Stanton CK, Newton S, Mullany LC, Cofie P, Agyemang CT, Adiibokah E, et al. Impact on postpartum hemorrhage of prophylactic administration of oxytocin 10 IU via Uniject by peripheral health care providers at home births: Design of a community‐based cluster‐randomized trial. BMC Pregnancy and Childbirth 2012;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton CK, Newton S, Mullany LC, Cofie P, Tawiah Agyemang C, Adiibokah E, et al. Effect on postpartum hemorrhage of prophylactic oxytocin (10 IU) by injection by community health officers in Ghana: a community‐based, cluster‐randomized trial. PLoS Medicine 2013;10(10):e1001524. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
NCT01487278 {published data only}
- NCT01487278. Comparing misoprostol and oxytocin in UnijectTM postpartum hemorrhage (PPH) prevention in Mali. ClinicalTrials.gov (https://clinicaltrials.gov/) [accessed 7 April 2015] 2011.
Stanton 2010 {published data only}
- Stanton C. Effectiveness, safety and feasibility of auxiliary nurse midwives' (ANM) use of Oxytocin in uniject™ to prevent postpartum hemorrhage in India. ClinicalTrials.gov (https://clinicaltrials.gov/) [accessed 7 April 2015] 2010.
References to studies awaiting assessment
NCT01710566 {published data only}
- NCT01710566. Misoprostol and oxytocin in Uniject® for postpartum hemorrhage prevention in communities. ClinicalTrials.gov (https://clinicaltrials.gov/) [accessed 7 April 2015] 2012.
NCT01713153 {published data only}
- NCT01713153. Comparing misoprostol and oxytocin in uniject for postpartum hemorrhage (PPH) prevention in Senegal. ClinicalTrials.gov (https://clinicaltrials.gov/) [accessed 7 April 2015] 2012.
Additional references
Althabe 2011
- Althabe F, Mazzoni A, Cafferata ML, Gibbons L, Karolinski A, Armbruster D, et al. Using Uniject to increase the use of prophylactic oxytocin for management of the third stage of labor in Latin America. International Journal of Gynecology & Obstetrics 2011;114(2):184‐9. [DOI] [PubMed] [Google Scholar]
Amico 1987
- Amico JA, Ulbrecht JS, Robinson AG. Clearance studies of oxytocin in humans using radioimmunoassay measurements of the hormone in plasma and urine. Journal of Clinical Endocrinology and Metabolism 1987;64(2):340‐5. [DOI] [PubMed] [Google Scholar]
Amsalem 2014
- Amsalem H, Aldrich CJ, Oskamp M, Windrim R, Farine D. Postpartum uterine response to oxytocin and carbetocin. Journal of Reproductive Medicine 2014;59(3‐4):167‐73. [PubMed] [Google Scholar]
Begley 2015
- Begley CM, Gyte GML, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD007412.pub4] [DOI] [PubMed] [Google Scholar]
Bhattacharya 2013
- Bhattacharya S, Ghosh S, Ray D, Mallik S, Laha A. Oxytocin administration during cesarean delivery: Randomized controlled trial to compare intravenous bolus with intravenous infusion regimen. Journal of Anaesthesiology and Clinical Pharmacology 2013;29(1):32‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Breathnach 2006
- Breathnach F, Geary M. Standard medical therapy. In: B‐Lynch C, Keith LG, Lalonde AB, Karoshi M editor(s). A Textbook of Postpartum Haemorrhage. Sapiens Publishing, 2006. [Google Scholar]
Carroli 2008
- Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Practice & Research Clinical Obstetrics & Gynaecology 2008;22:999‐1012. [DOI] [PubMed] [Google Scholar]
Chu 2012
- Chu CS, Brhlikova P, Pollock AM. Rethinking WHO guidance: review of evidence for misoprostol use in the prevention of postpartum haemorrhage. Journal of the Royal Society of Medicine 2012;105:336‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowe 2012
- Crowe S, Utley M, Costello A, Pagel C. How many births in sub‐Saharan Africa and South Asia will not be attended by a skilled birth attendant between 2011 and 2015?. BMC Pregnancy and Childbirth 2012;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Darmstadt 2009
- Darmstadt GL, Lee AC, Cousens S, Sibley L, Bhutta Z, Donnay F, et al. 60 million non‐facility births: who can deliver in community settings to reduce intrapartum‐related deaths?. International Journal of Gynecology Obstetrics 2009;107 Suppl 1:S89‐S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dyer 2011
- Dyer RA, Butwick AJ, Carvalho B. Oxytocin for labour and caesarean delivery: implications for the anaesthesiologist. Current Opinion in Anesthesiology 2011;24(3):255‐61. [DOI] [PubMed] [Google Scholar]
Evans 1997
- Evans JJ. Oxytocin in the human‐‐regulation of derivations and destinations. European Journal of Endocrinology 1997;137(6):559‐71. [DOI] [PubMed] [Google Scholar]
Gonser 1995
- Gonser M. Labor induction and augmentation with oxytocin: pharmacokinetic considerations. Archives of Gynecology and Obstetrics 1995;256(2):63‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2013
- Hofmeyr GJ, Abdel‐Aleem H, Abdel‐Aleem MA. Uterine massage for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD006431.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hundley 2013
- Hundley VA, Avan BI, Sullivan CJ, Graham WJ. Should oral misoprostol be used to prevent postpartum haemorrhage in home‐birth settings in low‐resource countries? A systematic review of the evidence. BJOG: an international journal of obstetrics and gynaecology 2013;120:277‐85; discussion 86‐7. [DOI] [PubMed] [Google Scholar]
ICM‐FIGO 2003
- ICM/FIGO Joint Statement. Management of the Third Stage of Labour to Prevent Post‐Partum Haemorrhage. http://www.figo.org/files/figo‐corp/docs/PPH%20Joint%20Statement.pdf [accessed 4 August 2014] 2003.
In 2011
- In JH, Choi JW, Jung HS, Lee J, Joo J, Kim D, et al. Severe hypotension and water intoxication developed after an accidental oxytocin overdose in a morbidly obese patient undergoing cesarean section ‐ a case report. Korean Journal of Anesthesiology 2011;60(4):290‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jangsten 2005
- Jangsten E, Strand R, Gomez de Freitas EG, Hellstrom AL, Johansson A, Bergstrom S. Women's perceptions of pain and discomfort after childbirth in Angola. African Journal of Reproductive Health 2005;9(3):148‐58. [PubMed] [Google Scholar]
Karoshi 2009
- Karoshi M, Keith L. Challenges in managing postpartum hemorrhage in resource‐poor countries. Clinical Obstetrics and Gynecology 2009;52(2):285‐98. [DOI] [PubMed] [Google Scholar]
Kassebaum 2014
- Kassebaum NJ, Bertozzi‐Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, et al. Global, regional, and national levels and causes of maternal mortality during 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384(9947):980‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Millard 2014
- Millard C, Bhrlikova P, Pollock A. Commentary: Evidence versus influence in the WHO procedure for approving essential medicines: misoprostol for maternal health. BMJ 2014;349:g4823. [DOI] [PubMed] [Google Scholar]
Miller 2004
- Miller S, Lester F, Hensleigh P. Prevention and treatment of postpartum hemorrhage: new advances for low‐resource settings. Journal of Midwifery & Women's Health 2004;49(4):283‐92. [DOI] [PubMed] [Google Scholar]
Mousa 2014
- Mousa HA, Blum J, Abou El Senoun G, Shakur H, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD003249.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2007
- National Institute for Health and Clinical Excellence. Normal labour: third stage. Intrapartum Care: Care of Healthy Women and Their Babies During Childbirth, NICE Guideline 55. London: National Institute for Health and Clinical Excellence, 2007. [PubMed] [Google Scholar]
Oladapo 2012a
- Oladapo OT, Fawole B, Blum J, Abalos E. Advance misoprostol distribution for preventing and treating postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009336.pub2] [DOI] [PubMed] [Google Scholar]
Oladapo 2012b
- Oladapo OT, Okusanya BO, Abalos E. Intramuscular versus intravenous prophylactic oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009332.pub2] [DOI] [PubMed] [Google Scholar]
Pichon‐Riviere 2015
- Pichon‐Riviere A, Glujovsky D, Garay OU, Augustovski F, Ciapponi A, Serpa M, et al. Oxytocin in Uniject disposable auto‐disable injection system versus standard use for the prevention of postpartum hemorrhage in Latin America and the Caribbean: a cost‐effectiveness analysis. PLoS One 2015;10(6):e0129044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prankerd 2013
- Prankerd RJ, Nguyen TH, Ibrahim JP, Bischof RJ, Nassta GC, Olerile LD, et al. Pulmonary delivery of an ultra‐fine oxytocin dry powder formulation: potential for treatment of postpartum haemorrhage in developing countries. PLoS ONE 2013;8(12):e82965. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ronsmans 2006
- Ronsmans C, Graham WJ, Lancet Maternal Survival Series Steering Group. Maternal mortality: who, when, where, and why. Lancet 2006;368:1189‐200. [DOI] [PubMed] [Google Scholar]
Say 2014
- Say L, Chou D, Gemmill A, Tuncalp O, Moller A, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Golbal Health 2014;2(6):e323‐e333. [DOI] [PubMed] [Google Scholar]
Seitchik 1984
- Seitchik J, Amico J, Robinson AG, Castillo M. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics. American Journal of Obstetrics and Gynecology 1984;150(3):225‐8. [DOI] [PubMed] [Google Scholar]
Sheldon 2013
- Sheldon WR, Durocher J, Winikoff B, Blum J, Trussell J. How effective are the components of active management of the third stage of labor?. BMC Pregnancy and Childbirth 2013;13:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanton 2012
- Stanton CK, Newton S, Mullany LC, Cofie P, Agyemang CT, Adiibokah E, et al. Impact on postpartum hemorrhage of prophylactic administration of oxytocin 10 IU via Uniject by peripheral health care providers at home births: design of a community‐based cluster‐randomized trial. BMC Pregnancy and Childbirth 2012;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strand 2005
- Strand RT, Silva F, Jangsten E, Bergstrom S. Postpartum hemorrhage: a prospective, comparative study in Angola using a new disposable device for oxytocin administration. Acta Obstetricia et Gynecologica Scandinavica 2005;84(3):260‐5. [DOI] [PubMed] [Google Scholar]
Svanström 2008
- Svanström MC, Biber B, Hanes M, Johansson G, Näslund U, Bålfors EM. Signs of myocardial ischaemia after injection of oxytocin: a randomized double‐blind comparison of oxytocin and methylergometrine during caesarean section. British Journal of Anaesthesia 2008;100(5):683‐9. [DOI] [PubMed] [Google Scholar]
Tsu 2003
- Tsu VD, Sutanto A, Vaidya K, Coffey P, Widjaya A. Oxytocin in prefilled Uniject injection devices for managing third‐stage labor in Indonesia. International Journal of Gynecology & Obstetrics 2003;83(1):103‐11. [DOI] [PubMed] [Google Scholar]
UN 2000
- United Nations. United Nations Millennium Declaration. U.N. document A/RES/55/2, adopted September 8. http://www.un.org/millennium/declaration/ares552e.htm [accessed September 2014] 2000.
Westhoff 2013
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001808.pub2] [DOI] [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. Stability of Injectable Oxytocics in Tropical Climates: Results of Field Surveys and Simulation Studies on Ergometrine, Methylergometrine and Oxytocin. EDM Research Series No 008. Geneva: WHO, 1993. [Google Scholar]
WHO 2007
- World Health Organization. Guidelines for the Prevention of Postpartum Haemorrhage. Geneva: WHO, 2007. [Google Scholar]
WHO 2012
- World Health Organization, Department of Reproductive Health and Research. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: WHO, 2012. [PubMed] [Google Scholar]
References to other published versions of this review
Pantoja 2015
- Pantoja T, Abalos E, Chapman E, Vera C, Serrano VP. Oxytocin for preventing postpartum haemorrhage (PPH) in non‐facility birth settings. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011491] [DOI] [PMC free article] [PubMed] [Google Scholar]


