Abstract
While extensive literature exists on barriers and strategies to increase minority participation in clinical trials, progress is limited. Few strategies were evaluated in randomized trials. We studied the impact of RECRUIT, a trust-based, cluster randomized minority recruitment trial layered on top of four traditional NIH-funded parent trials (BMT CTN, CABANA, PACES, STEADY-PD III; fifty specialty sites).
RECRUIT was conducted from July 2013 through April 2017. Intervention sites implemented trust-based approaches customized to individual sites, promoting relationships between physician-investigators and minority-serving physicians and their minority patients. Control sites implemented only parent trials’ recruitment procedures. Adjusting for within-site clustering, we detected no overall intervention effect, odds ratio 1.3 (95% confidence limits 0.7,2.4). Heterogeneity among parent trials may have obscured the effect. Of the four parent trials, three enrolled more minorities in intervention versus control sites. CABANA odds ratio = 4.2 (adjusted 95%CL 1.5,11.3). PACES intervention sites enrolled 63% (10/16) minorities; control sites enrolled one participant in total, a minority, yielding an incalculable odds ratio. STEADY-PD III odds ratio = 2.2 (adjusted 95%CL 0.6,8.5). BMT CTN odds ratio <1, 0.8 (adjusted 95%CL 0.4,1.8).
In conclusion, RECRUIT findings suggest the unique trust-based intervention increased minority recruitment to intervention trials in ¾ of studied trials. Physician-investigators’ participation was critical to recruitment success. Lack of commitment to minority recruitment remained a barrier for some physician-investigators, especially in control sites. We recommend prospective physician investigators commit to minority recruitment activities prior to selection as site investigators and trial funding include some compensation for minority recruitment efforts.
Keywords: Minority recruitment, cluster-randomized trial, intervention mapping, continuous quality improvement, specialty clinics
INTRODUCTION
Clinical trials of health interventions often lack racial and ethnic diversity [1–3],although prior research suggests different races/ethnicities have varying responses to some interventions [4–6]. Consequently, research extrapolated from largely non-Latinx White populations informs clinicians and scientists, A growing proportion of Americans may receive less than full benefit from clinical and biomedical advances and may be exposed to treatments untested in their populations [4, 7].
Extensive observational literaturedocuments barriers and strategies to increase minority participation in trials [7–11]. Few strategies have focused on specialty clinics and few have been evaluated in randomized trials [12–14]. One review of approaches to effective recruitment and retention of minority research participants identified few randomized assessments. The authors recommended that federal and state public health agencies support analytical or hypothesis-testing of recruitment and retention research. [15]
Observational and anecdotal community-based participatory research (CBPR) led to the assumption that CBPR approaches are helpful in minority recruitment. An in depth review of over 2800 studies by authors from Mayo Clinic resulted in 66 studies in the United States that met the authors’ criteria as using approaches to engage the community with a focus on minority enrollment. Of the 66, 62 were observational documenting lessons learned in minority recruitment across a spectrum of community-related activities. Four studies were observational and analytic or used mixed methods. A primary theme was the role of community engagement in promoting community trust. In general, CBPR effectively identified potential participants with conditions of high prevalence easily observed in the community (e.g. hypertension, diabetes, obesity, etc.). However, there was a paucity of rigorous randomized comparisons of CBPR approaches to recruitment . [16]
Among few randomized trials of approaches to minority recruitment in the community one successful randomized trial showed the use of church settings for minority recruitment to be more effective than other settings or approaches in minority recruitment to colon cancer screening trial. [12] Another successful randomized trial compared four approaches to direct mailing in the minority community with the primary outcome being increased minority completion of a screening questionnaire for a weight management study. [17] There was no randomized comparison of approaches to increase the percent of minorities enrolled in the trial itself.
Community-based approaches can be less helpful for the lower prevalence conditions typically treated in specialty practices (e.g. oncologists, cardiologists, rheumatologists) as the yield of potential participants from broad-based community approaches is likely to be small. Thus specialty clinics generally rely their own patients or physician referrals. [8, 9] It is in specialty clinics where many treatments for complex diseases are assessed, and as noted, these trials often lack minority participation, partly attributable to the lack of access for minority patients to specialty clinics. [18–20] Only a few randomized minority-targeted recruitment trials focused on specialty clinics. A randomized trial of an approach to minority recruitment in Parkinson’s disease specialty clinics was not successful. [13] A randomized trial of coaching to increase potential trust in cancer clinical trials was also not successful and did not assess the role of physicians in recruitment. [14]
Minority-serving community physicians may be reluctant to refer their patients to specialists’ clinics because of issues of trust in physician-investigators. [21–25] Findings suggest that physician-investigators need to develop trusting relationships with minority-serving physicians if they expect these physicians to refer patients to clinical trials. The trust-related barriers for minority-serving physicians, regardless of their own race/ethnicity, mirror trust-related issues that exist for their minority patients [24]. In a detailed review of issues in minority recruitment to cancer treatment trials, lack of trust of community physicians in physician-investigators was identified as a barrier, as well as lack of trust among potential participants. [25]
The Parkinson’s recruitment trial informed the design of The Randomized Recruitment Intervention Trial (RECRUIT). [13] A primary difficulty with the Parkinson’s recruitment trial was its “one-size-fits-all” design. RECRUIT was conducted in specialty clinics in four NIH-funded intervention trials where all four trials desired to increase minority recruitment. RECRUIT investigators encouraged each specialty clinic to tailor their recruitment intervention to the needs of their site emphasizing participation by the physician-investigators at each site. RECRUIT was the largest randomized trial ever conducted in specialty clinics to assess approaches to minority recruitment. We present results of RECRUIT and lessons learned to provide guidance to future clinical trials in specialty clinics that wish to increase minority participation.
METHODS
Study Design, Setting, and Hypothesis
RECRUIT was an un-blinded, cluster-randomized, minority recruitment trial, layered on top of four separately funded NIH-funded multi-site trials (parent trials) treating four different conditions. To be eligible for RECRUIT a parent trial had a randomized design and studied a treatment for a condition requiring a specialist such as an oncologist, cardiologist, or rheumatologist. Each parent trial included at least six clinics (sites). Each site was to randomize at least 10 participants to the parent trial. Each parent trial had a commitment to increasing minority enrollment. The sponsor of each parent trial, whether NIH or industry, and the parent trial Coordinating Center principal investigator approved participation in RECRUIT. Our goal was to enroll enough parent trials to obtain 60 sites. Sites were not consented until after the enrollment of a parent trial. Not all sites within a parent trial were eligible for RECRUIT. Each eligible site within an individual parent trial had to have at least 20% minorities in the age group of interest to the specific parent trial living within 30 miles of the site. Also, to participate in RECRUIT, each site Principal Investigator and Coordinator consented to be randomized to intervention or control without knowing the specifics of the recruitment intervention (since the RECRUIT trial was un-blinded).All sites agreed to attend a six-hour off-site kick-off meeting if randomized to the intervention. Once identified, all consenting eligible sites from an enrolled parent trial were randomized to the RECRUIT intervention or control. The cluster-randomized design was chosen so that all staff within a specialty site would have exposure to the RECRUIT intervention or control, depending on the site’s randomization assignment. Within site contamination would be limited. Sites, the units of analyses, were matched within parent trial, where possible, on total minorities living within a 30-mile radius of the site, past minority recruitment or projected minority enrollment at the site, and geographic region. Within each pair, sites were randomly assigned to intervention or control using simple randomization. Simple randomization for each pair was based on a single sequence of random assignments, in this case a random numbers table. Three sites were randomized in PACES after site matching and site randomization for the parent trial had been completed. We used simple randomization to assign late enrolling sites to intervention or control, and by chance all three sites were assigned to intervention. Other specifics of the trial design were published elsewhere [26, 27]. The primary outcome of RECRUIT was the proportion of minorities enrolled in intervention and control group sites. We tested the alternative hypothesis that the proportion of minorities recruited in sites receiving the trust-based educational program, coached in continuous quality improvement approaches, and coached in approaches for active listening would exceed the proportion of minorities recruited in control sites. We followed all enrolled sites for two years after their RECRUIT enrollment or until parent trial recruitment ended, whichever came first. All site follow-up concluded by April 2017. RECRUIT was not powered to detect parent-trial specific differences in overall recruitment or minority recruitment. Because the four parent trials had many design differences (see Discussion), we planned additional subgroup analyses by parent trial. RECRUIT was not designed to track participants’ retention in the parent trials.
In RECRUIT we defined minorities as racial/ethnic groups traditionally underrepresented in clinical trials (i.e., all groups except non-Latinx Whites). By this definition Asians were considered minorities in addition to other persons of color. Selected sites had populations in the age group being studied in their trial of least 20% minority, living within 30 miles of the site. We defined minority-serving physicians as physicians serving minorities in this area.
RECRUIT Trust-Based and Continuous Quality Improvement Intervention
Control and intervention sites both followed recruitment procedures prescribed by the parent trial. The intervention for RECRUIT was developed using Intervention Mapping, based on theory, and extensive preliminary research [27]. Intervention site physician-investigators (specialists) and coordinators attended a six-hour off-site kick-off meeting. We provided information on the minority recruitment literature, on the importance of minority recruitment for the condition studied in the parent trial, and on strategies to increase trust between physician-investigators and minority-serving physicians [24, 28]. Minority patients referred by their own physicians were expected to be more likely to participate in a clinical trial [29, 30]. The trust triangle (Figure 2) was a key aspect of the trust-based intervention [26]. During the kick-off meeting intervention site physician-investigators and coordinators were guided in continuous quality improvement approaches to identify site-specific barriers for increasing referrals from local minority serving physicians, and for increasing minority recruitment in general [31, 32]. To facilitate identification of minority-serving physicians we provided intervention sites at the kick-off meeting with census maps of areas within 30 miles of their site showing locations of minorities in the age group of interest to the trial. Sites were encouraged to recruit as many minorities as possible without consideration of the incidence or prevalence of the condition under study.
Figure 2:
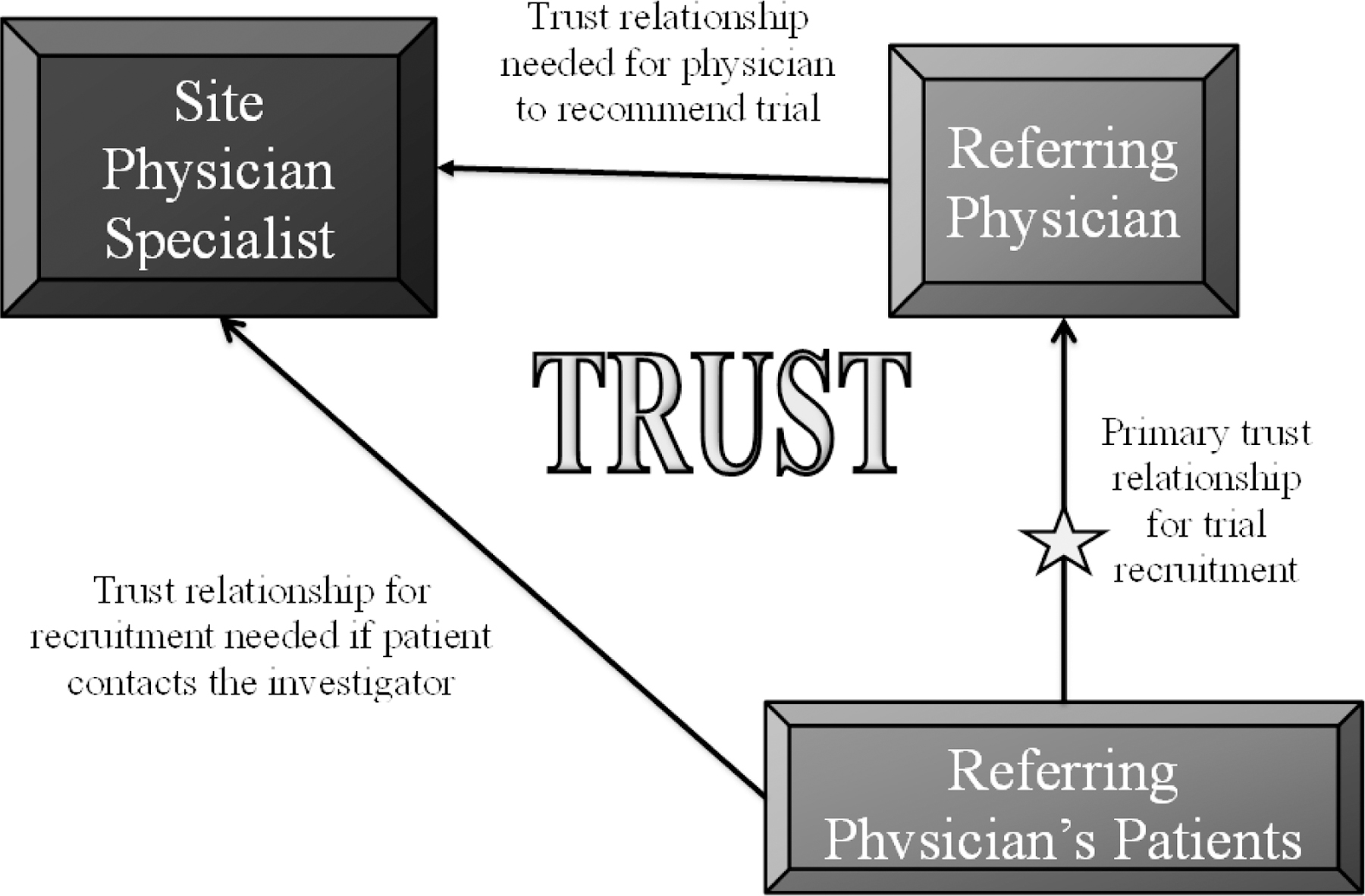
RECRUIT Trust Triangle [26]
Full details on the recruitment intervention were published in the RECRUIT design article [26] and in the qualitative article on intervention development and intervention mapping [27]. The RECRUIT intervention was based on several years of preliminary research with some sources of past funding identified in the Acknowledgements Section of this manuscript. The former article gave specifics on intervention delivery during each of the four webinars. The latter article on development of the intervention,provided the intervention social cognitive theory (SCT) determinants, methods, and practical applications. Additionally the latter article provided the title, content activity, and take home activity for the kick-off and each of the four subsequent webinars (modules).For five to six months after the kick-off, during three one-hour webinars with intervention site physician-investigators and coordinators, we reinforced the themes as defined by intervention mapping and reviewed sites’ updated continuous quality improvement plans. To facilitate minority recruitment we coached physician-investigators and coordinators in techniques of active listening to minority-serving physicians and to potential minority participants as a trust-building approach. The final coordinators’ webinar focused on using participant navigation [33] for minority recruitment. Intervention site coordinators received monthly calls from RECRUIT team members to problem solve and reinforce trust-based strategies and continuous quality improvement plans [26, 27].
Data Collection
Monthly, all site coordinators sent de-identified screening and recruitment logs with age group, race/ethnicity, and gender to the RECRUIT team that coordinated the RECRUIT trial. We collected no information on contacts with minority-serving physicians to avoid influencing control sites and we did not have site permission to contact trial patient participants directly. Parent trial coordinating centers provided site screening and recruitment data as of trial drop-out dates for any sites dropped from the parent trial or for RECRUIT drop-outs as of the end of RECRUIT. There were no missing values for the primary outcome (proportion of minorities enrolled) or for counts of participants enrolled or minorities enrolled.
Sample Size and Statistical Analysis
Sample size (number of sites per intervention and control) required estimation of across site correlation and variability. RECRUIT was powered to detect a 0.10 absolute difference from a range of control proportions of minorities recruited (0.05 to 0.15), assuming a 2-sample test and an intra-cluster (specialty clinic) correlation, ICC, of 0.10. An ICC of 0.1 has been a commonly used benchmark in medical studies. [34, 35] With a two-sided alpha of 0.05, 30 specialty clinics per group, and an average cluster size of 10 participants per specialty clinic, a power of 83% or greater could be achieved to detect a difference if one exists. RECRUIT was not powered to detect differences in secondary outcomes or sub-groups. [26]
Initially we planned to enroll two large parent trials and a total of 60 sites. We implemented a planned generalized estimating equations logit-link model to analyze participants’ minority status (yes, no) with independence working correlation structure to take the clustered design into account, and we included parent trial as a covariate. This approach allowed for comparison of the primary outcome, the proportion of minority participants enrolled in intervention and control sites, using an odds ratio. Two intervention sites from PACES and six control sites (2 from CABANA, four from PACES), enrolled no participants yielding unidentifiable proportions (0/0) for the logit-link analysis [26]. The imbalance in unidentifiable values did not appear random since more control than intervention sites reported 0/0 and six PACES sites reported 0/0, more than any other trial. These unidentifiable values differ from missing values as the numerator and denominators of the proportions were observed, but were zero. We omitted those sites with unidentifiable proportions from the primary overall analysis and from the sub-group analyses by parent trial where applicable (CABANA and PACES only).
To perform an intention-to-treat sensitivity analysis including all enrolled sites, we developed a novel re-parameterization of the GEE model [36]. The re-parameterization allowed a test of the primary hypothesis accommodating the eight sites with no participants enrolled.
As secondary descriptive analyses, using negative binomial regressions, we compared counts of potential participants screened and potential minority participants screened between intervention and control sites. We compared counts of participants enrolled and minority participants enrolled again using negative binomial regressions. We presented proportions of participants enrolled by racial/ethnic sub-groups within parent trials without formal statistical testing (Table 2). In keeping with NIH reporting guidelines we also presented the proportion of female participants enrolled by trial.
Although RECRUIT was not powered to detect parent-trial specific differences in overall recruitment or minority recruitment, we planned additional descriptive GEE logit link subgroup analyses by parent trial. RECRUIT was not designed to track participants’ retention in the parent trials. Only the principal investigator, program manager, and biostatisticians saw outcome data before the trial intervention ended. All analyses were performed in SAS Version 9.4.
HUMAN PARTICIPANT PROTECTION
All participating physician-investigators and coordinators provided written informed consent according to The University of Texas Health Science Center at Houston Institutional Human Subjects Review Board (IRB) procedures. Parent trial Coordinating Centers and participating parent trial sites received local IRB approval to send de-identified screening and recruitment data to the RECRUIT team. All intervention and control sites obtained local IRB approval to participate in their respective parent trials.
RESULTS
Figure 1, the CONSORT Diagram, previously published [26] now includes data on sites lost-to-follow up. The search for parent trials began in late 2011 and enrollment occurred between August 2012 and November 2014. Although we extended the search for parent trials, parent trial enrollment, and site recruitment an additional two years beyond the planned two-year recruitment time frame, we could enroll only 50 sites. The impact of the decrease in number of sites on the power of the trial is described in the Appendix. Enrolled parent trials included: CABANA [37], PACES [38], STEADY-PD III [39], and an aggregate of four small trials from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) [40]. The Consort Diagram, Figure 1, indicates the conditions and treatments studied in each parent trial and the clincaltrials.gov links for the trials. The CABANA began in November 2009 and did not join RECRUIT until October 2013 in hopes of increasing recruitment in general as well as recruitment of minorities. The PACES trial, a secondary cancer prevention trial had just started and was having recruitment difficulties when the investigators signed up for RECRUIT. STEADY-PD III was also just beginning. STEADY-PD III recruited rapidly using a unique broad-based community outreach to the target a population of early, untreated PD participants and successfully engaged Parkinson’s disease organizations to increase overall recruitment. Multiple BMT CTN trials were included to determine if minority recruitment could be increased across the BMT CTN trials networks and were beginning participant recruitment when enrolled in RECRUIT. More specific details for the individual parent trials can be found through the clinicaltrials.gov links. A total of 50 sites from the four trials (Appendix B) were consented and randomized between July 2013 and February 2015. Follow-up of the last sites concluded in April 2017. Eight more months were required to transcribe qualitative interviews of site investigators. Nineteen additional months were required to develop and publish on our new intent-to-treat statistical methods [36].
Figure 1:
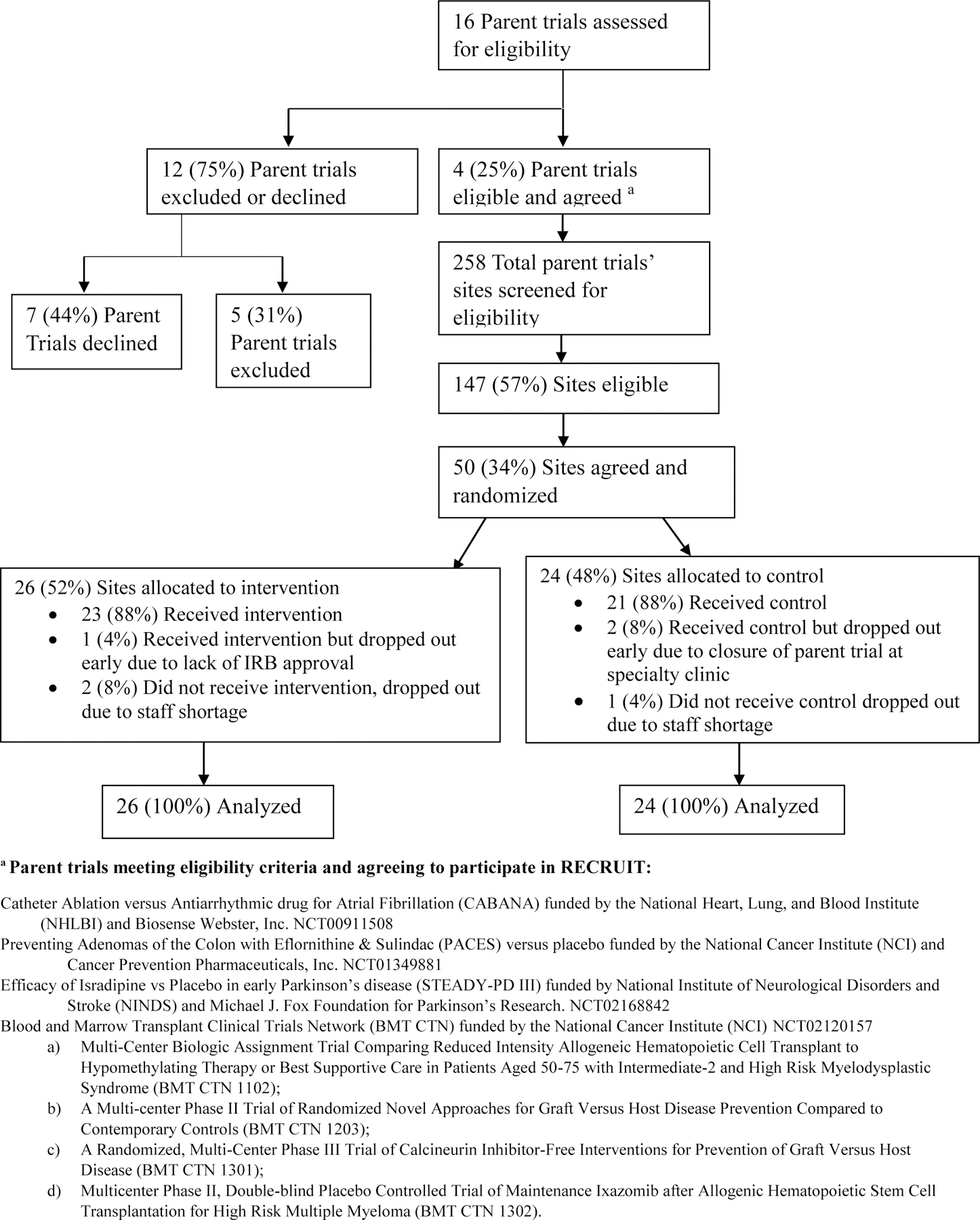
RECRUIT CONSORT DIAGRAM
The CONSORT Diagram highlights the difficulties of parent trial and site recruitment. Only 4/7 eligible parent trials chose to participate in RECRUIT. Of 147 eligible sites in the four participating parent trials, only 50 sites consented to participate. Eligible parent trial refusals and eligible site refusals from participating parent trials gave similar primary reasons: concern about potential staff time and resources for minority recruitment activities. More details on refusals were provided in the RECRUIT design paper [26]. The participating sites’ principal investigators and coordinators were generally more motivated to recruit minorities than refusing sites; however, randomization provided some balance in motivation between intervention and control arms.
We did not detect differences between intervention and control sites in baseline site characteristics (Appendix C). The largest baseline difference was in numbers of participants enrolled in any type of previous trial conducted by the sites, with more participants enrolled in control site trials. We detected minimal demographic differences between intervention and control physician-investigators or coordinators (all p-values > 0.20). Of the physician-investigators, 60% were non-Latinx White as were 71% of coordinators. Because physician-investigators and coordinators in the four parent trials were predominantly non-Latinx White [26], similar to most specialist practices, racial/ethnic participant-provider concordance [41] was unlikely.
Primary Analysis
Results are presented in Table 1. After excluding the eight sites with unidentifiable values we detected no overall intervention effect on the proportion of minority enrolled, odds ratio 1.3 (95% confidence limits 0.7, 2.4). Although RECRUIT was not powered to detect intervention effects by parent trial, the pre-planned analysis by trial (strata) was conducted. Three of four parent trials trended in the direction of enrolling more minorities in the intervention versus control group. The 95% confidence limits for CABANA excluded 1.0. The PACES model did not converge because Paces control sites enrolled only enroll one participant, a minority, among combined control sites. Paces intervention sites enrolled 63% (10/16) minority participants. The STEADY-PD III odds ratio was 2.2 (95% confidence limits 0.6, 8.5). The intention-to-treat sensitivity analysis including all sites gave results similar to the primary analysis, rate ratio 1.3 (95% confidence limits 0.8, 2.2).
TABLE 1:
PRIMARY ANALYSIS FOR THE PROPORTION OF MINORITY ENROLLED.
| Odds Ratiob | 95% confidence limitsb | # of Sites (# of Observations) | |
|---|---|---|---|
| All Parent Trials Combined a | 1.3 | 0.7, 2.4 | 42 (387) |
| By Parent Trial c | |||
| BMT CTN | 0.8 | 0.4, 1.6 | 13 (196) |
| CABANA | 4.2 | 1.6, 11.3 | 8 (86) |
| PACES | Model did not converged | -- | 7 (17) |
| STEADY-PD III | 2.2 | 0.6, 8.5 | 14 (88) |
Eight sites enrolling no participants, resulting in unidentifiable outcome proportions (0/0), were excluded from this analysis (2 control sites from CABANA, 2 intervention sites and 4 control sites from PACES).
Generalized estimating equations, logit link model, independence working correlation structure. All confidence intervals adjusted for clustering.
While analyses by parent trial were planned when the trial was designed, the trial was not powered to detect individual trial effects.
Intervention sites enrolled 10 minorities (63%), all control sites combined enrolled only 1 participant, a minority.
Count Data
We detected parent trial-intervention interactions in analyses of all counts so we reported count ratios by parent trial (Table 2a). The count ratios for minority participants screened were greater than 1.0 for three of the four trials. The count ratio for PACES was 20.9, and confidence intervals were wide (4.5, 97.5) due to the small number screened in the control group (Table 2a).
TABLE 2:
SCREENING LOG COUNTS, ENROLLMENT COUNTS, AND MODEL-BASED EFFECT SIZE ESTIMATES a
| Table 2a: Screening Log Counts | |||||||
|---|---|---|---|---|---|---|---|
| Parent Trial | # Sites | Count of Screening Logs Receivedc | Count of Potential Participants Screened | Count Ratio for Potential Participants Screened (95% CI) | Count of Potential Minority Participants Screened | Count Ratio for Potential Minority Participants Screened (95% CI) | |
| CABANA | Intervention | 5 | 97 | 443 | 0.7 (0.2, 2.9) | 140 | 1.5 (0.3, 7.2) |
| Control | 5 | 117 | 592 | 91 | |||
| PACES | Intervention | 8 | 203 | 457 | 15.9 (4.3, 58.9) | 267 | 20.9 (4.5, 97.5) |
| Control | 5 | 84 | 18 | 8 | |||
| STEADY-PD III | Intervention | 7 | 33 | 55 | 1.1 (0.3, 3.8) | 11 | 1.8 (0.4, 9.3) |
| Control | 7 | 30 | 49 | 6 | |||
| BMT CTN | Intervention | 6 | 99 | 276 | 1.5 (0.4, 5.0) | 53 | 0.9 (0.2, 3.6) |
| Control | 7 | 113 | 217 | 68 | |||
| Overallb | Intervention | 26 | 432 | 1231 | -- | 471 | -- |
| Control | 24 | 344 | 876 | 173 | |||
| Table 2b: Enrollment Counts. | |||||||
|---|---|---|---|---|---|---|---|
| Parent Trial | # Sites | Count of Total Participants Enrolled | Count Ratio for Total Participants Enrolled (95% CI) | Count of Minority Participants Enrolled | Count Ratio for Minority Participants Enrolled (95% CI) | ||
| CABANA | Intervention | 5 | 64 | 2.9 (1.1 ,7.4) | 19 | 9.5 (2.0, 45.0) | |
| Control | 5 | 22 | 2 | ||||
| PACES | Intervention | 8 | 16 | 10.0 (1.2 ,85.2) | 10 | 6.3 (0.8 ,51.7) | |
| Control | 5 | 1 | 1 | ||||
| STEADY-PD III | Intervention | 7 | 47 | 1.1 (0.5 ,2.5) | 9 | 2.2 (0.6 ,8.0) | |
| Control | 7 | 41 | 4 | ||||
| BMT CTN | Intervention | 6 | 98 | 1.2 (0.6 ,2.5) | 15 | 0.9 (0.4 ,2.1) | |
| Control | 7 | 98 | 19 | ||||
| Overallb | Intervention | 26 | 225 | -- | 53 | -- | |
| Control | 24 | 162 | 26 | ||||
A negative binomial distribution was assumed for each of the count outcomes based on the over dispersion of the data. Covariates included intervention, parent trial, and intervention by trial interaction.
Intervention by trial interaction was detected, p ≤ 0.03 for number of potential subjects screened, and p <0.05 for potential minority subjects screened, p <0.1 for total participants enrolled, and p ≤ 0.03 for number of minority participants enrolled. P-values <0.1 suggest an interaction is present so effect sizes are presented separately by trial.
Sites sent blank screening logs when they did not find anyone to screen.
Count ratios of minority participants enrolled were numerically higher than 2.0 for three of the four parent trials. Confidence intervals for these three parent trials were wide, particularly for PACES (0.8, 51.7) again because only a single participant was enrolled in the control group. The confidence limits excluded 1.0 for CABANA.
Minority enrollment by race/ethnicity by parent trial
In minority sub-groups (Table 3) the largest intervention-control difference was observed for Blacks, followed by Asians. Slightly more Latinx participants were enrolled by control sites. Both CABANA and STEADY-PD III completed overall trial enrollment shortly after the end of RECRUIT. These data allowed comparison of minority recruitment in the complete parent trials to minority recruitment in the RECRUIT sites enrolled from those trials. CABANA activated a total of 173 sites worldwide. Only 126 sites (73%) enrolled participants. Of the 2,198 CABANA participants with known minority status, 10.2% were minorities [37]. In contrast Table 3 shows all five CABANA intervention sites in RECRUIT enrolled participants and 30% were minorities. Only three of the five CABANA control sites in RECRUIT enrolled participants and 9% were minorities. STEADY-PD III parent trial activated 57 sites in the US and Canada, and 55 sites (96%) enrolled 34 minorities (10.1%) [42]. Table 3 shows that seven STEADY-PD III intervention sites in RECRUIT enrolled 19% minorities constituting 27% of total minorities enrolled in the entire STEADY-PD III parent trial. The seven control sites in RECRUIT enrolled 10% minorities.
TABLE 3:
ENROLLMENT BY RACE/ETHNICITY AND BY GENDER, BY PARENT TRIAL AND OVERALL
| Parent Trials | CABANA n (proportion) |
PACES n (proportion) |
STEADY-PD III n (proportion) |
BMT CTN n (proportion) |
Total n (proportion) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sites | 5 | 5 | 8 | 5 | 7 | 7 | 6 | 7 | 26 | 24 |
| Participants by Group | Intervention n = 64 | Control n = 22 | Intervention n = 16 | Control n = 1 | Intervention n = 47 | Control n = 41 | Intervention n = 98 | Control n = 98 | Intervention n = 225 | Control n = 162 |
| Total Minority | 19 (0.30) | 2 (0.09) | 10 (0.63) | 1 (1.00) | 9 (0.19) | 4 (0.10) | 15 (0.15) | 19 (0.19) | 53 (0.24) | 26 (0.16) |
| American Indian/Alaskan Native | 1 (0.02) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.02) | 1 (0.02) | 0 (0.00) | 0 (0.00) | 2 (0.01) | 1 (0.01) |
| Asian | 3 (0.05) | 0 (0.00) | 5 (0.31) | 0 (0.00) | 4 (0.09) | 0 (0.00) | 5 (0.05) | 5 (0.05) | 17 (0.08) | 5 (0.03) |
| Black | 13 (0.20) | 2 (0.09) | 0 (0.00) | 0 (0.00) | 2 (0.04) | 0 (0.00) | 5 (0.05) | 4 (0.04) | 20 (0.09) | 6 (0.04) |
| Latinx | 1 (0.02) | 0 (0.00) | 4 (0.25) | 1 (1.00) | 2 (0.04) | 3 (0.07) | 5 (0.05) | 9 (0.09) | 12 (0.05) | 13 (0.08) |
| Native Hawaiian/Pacific Islander | 0 (0.00) | 0 (0.00) | 1 (0.06) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.00) | 0 (0.00) |
| More than one race | 1 (0.02) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.01) | 1 (0.00) | 1 (0.01) |
| Total Latinx White | 45 (0.70) | 20 (0.91) | 6 (0.38) | 0 (0) | 38 (0.81) | 37 (0.90) | 83 (0.85) | 79 (0.81) | 172 (0.76) | 136 (0.84) |
| Total Female | 21 (0.33) | 6 (0.27) | 12 (0.75) | 1 (1.0) | 17 (0.36) | 12 (0.29) | 15 (0.15) | 41 (0.42) | 101 (0.45) | 60 (0.37) |
Intervention Adherence
All intervention group physician-investigators and coordinators attended a kick-off meeting where they received training and participated in continuous quality improvement activities. Overall, 88% of enrolled RECRUIT intervention sites participated (Fig. 1) in the complete set of RECRUIT intervention webinars. We arranged make-up sessions as needed to maintain 88% intervention adherence. The make-up sessions duplicated the webinar the investigator or coordinator missed.
Some site physician-investigators had difficulty or were reluctant to make minority-serving physician contacts and sent site coordinators as their representatives. During monthly coordinator calls, some intervention site coordinators reported directly approaching community practices, or sending pamphlets, brochures, or web blasts to the minority-serving physicians in place of physician-investigator contact. These activities had not been encouraged by RECRUIT. Coordinators reported little minority recruitment success with these activities. The marketing group at one BMT CTN intervention site organized a meeting for community minority-serving physicians. Only one physician attended, similar to the experience of NET-PD [13]. Coordinators were reminded to encourage direct physician-investigator contact with minority-serving physicians. Table 4, based on qualitative interviews of some participating physicians gives some information by trial on contact with minority serving physicians for both intervention and control specialist. BMT CTN showed less minority serving physician contact than the three other parent trials.
Table 4:
SITE PHYSICIAN-INVESTIGATOR CONTACT WITH MINORITY SERVING PHYSICIANS BY TRIAL
| Parent Trial | Group | Interviewed Post-trial | Contact with Minority Serving Physicians | Number of Sitesb Where Physician Investigators Made Contact | Average # of Participants per Site | Proportion of Minorities Enrolled |
|---|---|---|---|---|---|---|
| Yes | Yes a | |||||
| CABANA | Intervention | 3 | 20.3 | 0.31 | ||
| PACES | Intervention | 4 | 2.8 | 0.64 | ||
| STEADY | Intervention | 5 | 8.2 | 0.17 | ||
| BMT | Intervention | 2 | 16.5 | 0.18 | ||
| Total | Intervention | 14 | 10.4 | 0.27 | ||
| Yes | No | |||||
| CABANA | Intervention | 1 | 1 | 0 | ||
| PACES | Intervention | 3 | 1.7 | 0.60 | ||
| STEADY | Intervention | 2 | 3 | 0.33 | ||
| BMT | Intervention | 3 | 19.7 | 0.12 | ||
| Total | Intervention | 9 | 7.9 | 0.17 | ||
| No | N/A c | |||||
| CABANA | Intervention | 1 | 2 | 0 | ||
| PACES | Intervention | 1 | 0 | - | ||
| STEADY | Intervention | 0 | - | - | ||
| BMT | Intervention | 1 | 6 | 0.33 | ||
| Total | Intervention | 3 | 2.7 | 0.25 | ||
| Yes | Yes a | |||||
| CABANA | Control | 0 | - | - | ||
| PACES | Control | 0 | - | - | ||
| STEADY | Control | 0 | - | - | ||
| BMT | Control | 1 | 8 | 0.25 | ||
| Total | Control | 1 | 8 | 0.25 | ||
| Yes | No | |||||
| CABANA | Control | 4 | 5.5 | 0.09 | ||
| PACES | Control | 3 | 0 | - | ||
| STEADY | Control | 7 | 5.9 | 0.10 | ||
| BMT | Control | 5 | 16.2 | 0.20 | ||
| Total | Control | 19 | 7.6 | 0.15 | ||
| No | N/A c | |||||
| CABANA | Control | 1 | 0 | - | ||
| PACES | Control | 2 | 0.5 | 1.0 | ||
| STEADY | Control | 0 | - | - | ||
| BMT | Control | 1 | 9 | 0.11 | ||
| Total | Control | 4 | 2.5 | 0.20 |
Minority-serving physician contact reported by site physician-investigators in response to open-ended question regarding approaches to minority recruitment. Minority-serving physicians either had practices in communities near the site or in specialty clinics with a high proportion of minorities.
No minority-serving physician contact reported by site physician-investigators in response to open-ended question regarding approaches to minority recruitment.
Not interviewed
Incentives
Parent trial coordinating centers and consenting intervention specialty clinics each received $22,000 as partial reimbursement for time and effort. Consenting control specialty clinics each received $5,000. Funds supported recruitment or other institutionally allowed expense except tuition or equipment costing $5,000 or more. RECRUIT specialist investigators and coordinators completing qualitative key informant interviews at the end of RECRUIT received a $250 Amazon gift card if permitted by their institution. [26]
DISCUSSION
RECRUIT’s multi-level intervention was similar to recommendations by others [11] but unique in emphasizing trust-building between physician-investigators and minority-serving-physicians. Previous studies have pointed to the essential role of participant trust in recruiting minorities [24, 25]. Developing participant trust in specialists was especially challenging because lack of specialist diversity in RECRUIT but was addressed using contacts with participants’ physicians and active listening techniques [26, 27]. Specialist diversity remained a general problem in the types of specialist practices enrolled in the parent trials [43–45].
A previous, unsuccessful study employed a randomized trial design to test a minority recruitment intervention in Parkinson’s disease specialty clinics, but did not use an intervention mapping framework to develop the intervention. Because the trial was not successful investigators conducted in-depth post-trial interviews with specialist investigators (n=24) and coordinators (n=24). Reported challenges to recruitment included lack of flexibility in recruitment methodology, insufficient understanding of the definition of ethnic and racial groups, the use of inappropriate outreach strategies (e.g., recruiting through support groups that had low minority membership), and lack of bilingual resources within the specialty clinics. [23] In a second qualitative study, six focus groups were conducted to elicit potential solutions to commonly reported barriers to clinical trial participation from Black (n=32) and Latinx (n=25) participants. Findings suggested that increasing physician-patient trust by training physicians on how to communicate about clinical trials to diverse audiences, providing participant incentives, prioritizing participant convenience, and utilizing patient navigation interventions could improve minority participation. [46] Findings from these two formative studies guided the planning group’s decisions for the development of the RECRUIT intervention.
Throughout our needs assessment, trust continually emerged as an important component in developing relationships between specialty investigators and minority-serving physicians and between minority patients and their providers. The planning group conceptualized these findings as a trust triangle dynamic whereby enrollment would be facilitated by increasing trust between patients, referring physicians, and specialist investigators (Figure 2). The planning group used information gathered from the literature and prior research to develop a logic model that described the problem of low enrollment of minorities in trials. [47] The model outlined predisposing, enabling, and reinforcing determinants of the target behavior, i.e., recruitment of minority participants . [26]
RECRUIT also used physician-investigator/coordinator driven continuous quality improvement to tailor the intervention in each site to site-specific barriers, local racial/ethnic populations, and parent trial differences in clinical outcomes. Based on the literature and our own experience we believe neither the trust-based intervention nor continuous quality improvement alone would have been sufficient [13, 48]. While we could not demonstrate multi-level intervention effectiveness across all four parent trials, we did have more minorities enrolled in the intervention group as compared to the control groups in three of the four parent trials (Table 3). In PACES, the control group enrolled only 1 participant, a minority (100% minority), although the intervention group enrolled 10 minorities corresponding to 63%.
Parent Trial Effects
We believe the heterogeneity among parent trials strongly influenced the results. BMT CTN, with the most participants and sites was the least successful in minority recruitment of the four parent trials. BMT CTN used multiple small trials as listed in the CONSORT Diagram (Figure 1) with multiple investigators and coordinators and diverse entry criteria making consistent training of BMT CTN investigators and coordinators difficult. Intervention and control sites relied heavily on existing institutional marketing programs often directed to general community physicians, rather than minority-serving physicians. Motivation to increase minority recruitment was low in BMT CTN intervention sites. Some BMT CTN physician-investigators were discouraged by past experience in obtaining minority blood and marrow donors [49], similar to donor shortages of Black kidney donors [50]. However, a donor shortage is not the full explanation for lack of an intervention effect in BMT CTN. (M. Horowitz, MD, Steering Committee Chair, written communication, 2018).
During the time prior to CABANA joining RECRUIT cardiac ablation became more accepted and some insured participants were reluctant to accept possible randomization to long-term medication. Additionally, some CABANA sites would not accept possible randomization of uninsured participants to expensive ablation procedures. Never the less, the RECRUIT intervention group overcame these recruitment barriers and overcame the expected lower prevalence of atrial fibrillation in minorities [51, 52]. As a result, CABANA intervention sites enrolled 30% minorities compared to 9% in control sites (Table 3).
In PACES, due to trial admission criteria, eligible participants were not always available in the RECRUIT sites. On PACES’ monthly calls, we encouraged intervention site coordinators to conduct physician-investigator outreach to local minority-serving physicians in order to identify eligible early-stage cancer patients outside of the specialty clinics. Site focusing mainly on participants from specialty clinics (two of eight intervention sites and four of five control sites) failed to enroll any participants. PACES was unique among the four parent trials as potential participant screening and participant enrollment in intervention sites far exceeded control sites (457 versus 18, and 16 versus 1 respectively). Given the lack of detectable baseline differences in site characteristics (See Appendix C) these PACES site differences were unlikely to be attributable to imbalances in site characteristics.
While STEADY-PD III recruited rapidly due to the use of a broad-based community outreach, and the use of Parkinson’s disease organizations, this approach had little effect on minority recruitment. The overall 10.1% minority recruitment [53] was similar to the overall 9.8% minorities observed in the unsuccessful NET-PD recruitment trial [13], while RECRUIT intervention sites recruited 19% minorities. STEADY-PD III was unique among the four trials as the funding institute required that the trial enroll at least 10% minorities to continue funding. This enrollment policy could be considered by funders of future trials. Although the policy had a modest effect on the control sites enrollment of minorities, the policy possibly provided encouragement to the intervention sites to conduct the more intensive and time-consuming minority recruitment activities and kept a focus on minority recruitment for all sites.
Importance of Leadership Efforts
The NHLBI Science Officer from CABANA advocated RECRUIT participation in letters to sites. STEADY-PD III and PACES clinical principal investigators strongly encouraged site participation. NHLBI brought RECRUIT to the attention of the BMT CTN Steering Committee and encouraged site participation. BMT CTN also had a task force focusing on minority recruitment. In general, parent trial leadership provided strong motivation for RECRUIT participation. Within participating parent trials when local sites refused to participate in RECRUIT, there was often a lack of local leadership encouragement. In potential parent trials outside of RECRUIT that refused to participate, there was often lack of overall leadership support. These findings highlight the importance of obtaining leadership buy-in prior to implementing a minority recruitment programs at the site level.
Limitations
No test of specific components of the intervention
RECRUIT was a large, complex trial to address the complex problem of minority recruitment. An innovation of RECRUIT was acknowledging that each site and local patient population has differences structurally, culturally and attitudinally. Past research has shown that some interventions designed and tested in urban environments may not be successful in rural areas and rural populations. Consequently, the QI aspect of the trial allowed the sites to take standardized training and modify the intervention within parameters to their local environment. We provided a roadmap and training to the sites but we felt that it was important to acknowledge that recruiting patients for one disease to a site in New York City may require some local modifications to the strategy that may not be useful for recruiting patients for a different disease in New Mexico, Hawaii or rural North Carolina. Consequently, there were slight variations in the intervention across sites. This may be seen as a limitation, but the ability of sites to use our roadmap and training to mold the intervention to the local environment can also be seen as a strength.
Lack of universal buy-in from physician-investigators
As observed previously [13], what was embraced as a concept was not always embraced as an outreach strategy. Across parent trials motivated physician-investigators who adhered to the trust-based aspect of the intervention found it time consuming but actively complied with the intervention. Those who did not adhere cited lack of available time as the primary constraint. In successful sites, adherence to the trust-based aspect of the intervention and recognition that minority-serving physician contact could not be delegated to staff, despite the time and effort, were major contributors to minority recruitment. Physician-investigators found that once they established local physician contacts, coordinators could effectively do some of the follow-up. Achieving skills-training to enhance trust in a cost-efficient and not overly burdensome manner should be a significant target for future researchers interested in trust-based recruitment methods.
Issues with Language and IRBs
A low number of Latinx participants was recruited considering the proportion of Latinx served by some site locations. Challenges to inclusion of non-English speakers included lack of central support for rapid translation of consent forms, and slowness in translation of study materials by some trials. While many sites lacked coordinators who spoke Spanish languages, most specialty sites had interpreter services for their clinical patients. While these services were accessible to the parent trials, consent procedures were typically lengthy, especially when consent was obtained in a language other than English. This may have stretched limited interpreter and investigator time. A few IRBs were concerned about using interpreters, although the Office for Human Research Protection’s (OHRP) guidelines allow interpreters for federally funded research [54]. During the time period of RECRUIT, one large intervention site with a high Latinx population had a policy that non-English speakers would not be recruited for trials. A few local IRBs prohibited transportation assistance as too coercive. Based on comments from site coordinators, these local IRB policies hindered minority recruitment in their sites.
Adherence to active listening
We did not measure adherence to the parts of the intervention focused on active listening and communicating effectively with minority-serving physicians and potential minority participants. It is possible that our webinar-based educational method was not an optimal method for skill development. Future studies may want to build in more supervised/monitored practice of these skills in physician-investigators and coordinators, although such methods could be perceived as burdensome and increase the time required for participation. An in-depth qualitative analysis of post-trial interviews is in progress to obtain more insights.
Reduced power
Reduced power was a primary limitation. RECRUIT enrolled ten fewer sites than planned due to the high number of parent trial and site refusals, generally due to lack of physician-investigator motivation to spend time on minority recruitment. There was greater variability than expected in numbers of participants per site (cluster) partially due to the 8 of 50 sites with zero enrollment. See Appendix A, recalculation of overall power.
CONCLUSIONS
RECRUIT was an ambitious effort to fill the large gap in evidence-based data testing approaches for minority recruitment in specialty clinics. Most reported assessments of minority recruitment approaches are not evidence-based. Many reports come from observational studies or studies with controls but without rigorous randomization. RECRUIT was a large trial for cluster-randomized designs in general and the largest randomized recruitment trial ever conducted. Personal participation by the RECRUIT physician-investigators in interactions with minority-serving physicians was essential to participant recruitment. These initial personal interactions could not be replaced by coordinators or by flyers or blast messages. Also, the use of respectful active listening techniques by physician-investigators encouraged trust and trial enrollment by prospective participants. The trust-based approaches developed in RECRUIT may help to increase trial diversity where studies are conducted in specialty settings, but additional approaches are required to overcome the reluctance of some physician-investigators (specialists) to make minority recruitment a priority. Problems with minority recruitment are appearing in research related to COVID-19 [55]. RECRUIT methods may enhance minority recruitment when COVID-19 research is being conducted in specialty settings. Also minority-serving physicians may help their minority patients to overcome vaccine reluctance.
Supplementary Material
ACKNOWLEDGEMENTS
Role of the Sponsor: NIMHD through the RECRUIT Steering Committee reviewed the design and conduct of the study, and reviewed RECRUIT results and the manuscript after the trial was completed. The sponsor had no role in approval of the manuscript. The sponsor had no role in the collection and analysis of the data. RECRUIT Steering Committee: Anna Napoles, PhD with University of California at San Francisco; Jennifer Alvidrez, PhD, Dorothy Castille, PhD, Derrick Tabor, PhD, Anna Napoles as of 2017 are with the National Institute on Minority Health and Health Disparities; Sue Levkoff, PhD is with University of South Carolina, Columbia, SC.
Additional Contributions: We thank the parent trials that helped to test the RECRUIT intervention. Their names and NIH funding institutes are listed in Fig. 1 (CONSORT Diagram). We also thank the funding agencies that supported collection of background evidence used in intervention mapping to develop the RECRUIT intervention: NIH-NIA 5 P30 AG021677 SC Cooperative for Healthy Aging in Minority Populations – Resource Center for Minority Aging Research; NIH NINDS 9U01NS043127 Parkinson’s Disease Clinical Trial: Statistical Coordination Center for NET-PD; and the Duke Endowment 6098-SP. We thank Michael Gonzalez for programming support and Rodney Ball and Marice Barahona for assistance in grants management from the Department of Biostatistics and Data Science, The University of Texas Health Science Center School of Public Health at Houston.
FUNDING: This work was supported by the National Institute on Minority Health and Health Disparities [U24MD006941]; and the Susan G. Komen Traineeship in Breast Cancer Disparities [GTDR14300827].
APPENDIX
Appendix A: UPDATED POWER CALCULATIONS
We designed RECRUIT assuming we would recruit 30 intervention and 30 control sites (clusters) with at least 10 participants per site with an ICC of 0.1 [1]. Power was initially calculated for a range of differences (0.05, 0.10, 0.15) from a control proportion of 0.10, with a target difference of 0.10. Although we extended parent trial and site recruitment an additional two years beyond the planned two-year recruitment time frame, we could enroll only 50 sites with an average of 8.65 participants per intervention cluster (range 0, 38) and 6.75 per control cluster (range 0,28). There was greater variability than expected in numbers of participants per site (cluster) [2] partially due to the 8/50 sites with zero enrollment. We recalculated the power we achieved using PASS [3] to allow differing numbers of intervention and control sites (clusters). We used the observed ICC [4], calculated by imputing 1 for numbers of participants enrolled and 0 for numbers of minority participants enrolled for the eight sites with no participants enrolled. Under the original design using an ICC of 0.04 we had power of 0.35, 0.84, and 0.99 to detect the pre-specified differences of 0.05, 0.10 (our target difference), 0.15, respectively, from a control value of 0.10, if differences existed. Under the achieved sample size, our recalculated power was 0.30, 0.77 (for our target difference), and 0.97, respectively. Using the bivariate sensitivity model with interactions we had to account for additional degrees of freedom that could have further reduced our power.
Appendix B: RECRUIT SITE LOCATIONS
RECRUIT Site List.
| Blood and Marrow Transplant Clinical Trials Network (BMT CTN) |
|
|
| City of Hope National Medical Center, Duarte, CA Duke University, Durham, NC Emory University, Atlanta, GA Johns Hopkins University, Baltimore, MD Memorial Sloan Kettering Cancer Center, New York, NY Moffitt Cancer Center, Tampa, FL Northside Hospital, Atlanta, GA Oregon Health Sciences University, Portland, OR University of Florida, Gainesville, FL University of Kansas Medical Center, Westwood, KS University of Pennsylvania, Philadelphia, PA University of Texas MD Anderson Cancer Center, Houston, TX Washington University School of Medicine in St. Louis, St. Louis, MO |
|
|
| Catheter Ablation versus Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA) |
|
|
| Augusta University, previously Georgia Regents University, Augusta, GA Cooper University Hospital, Camden, NJ Duke University Medical Center, Durham, NC Fort Worth Heart PA/Baylor All Saints Medical Center, Fort Worth, TX Georgia Arrhythmia Consultants, Macon, GA Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ Scott and White Memorial Hospital, Temple, TX Swedish Medical Center- Providence Campus, Seattle, WA The University of California at San Francisco Medical Center, San Francisco, CA University of North Carolina at Chapel Hill, Chapel Hill, NC |
|
|
| Preventing Adenomas of the Colon with Eflornithine and Sulindac (PACES) |
|
|
| Columbia University Medical Center, New York, NY Dwight D. Eisenhower Army Medical Center, Augusta, GA Gibbs Cancer Center, Spartanburg Regional Healthcare System, Spartenburg, SC Greenville Health System, Greenville, SC Lewis Cancer & Research Pavilion at St. Joseph’s/Candler Health System, Inc, Savannah, GA Loyola University Medical Center, Maywood, IL Nevada Cancer Research Foundation, Las Vegas, NV Sinai Hospital of Baltimore, Baltimore, MD University of California Irvine, Orange, CA University of Hawaii, Honolulu, HI/ and The Queen’s Medical Center, Honolulu, HI* |
|
|
| Preventing Adenomas of the Colon with Eflornithine and Sulindac (PACES) Continued |
|
|
| University of Mississippi Medical Center, Jackson, MS University of Texas Health Science Center at San Antonio, San Antonio, TX Weiss Hospital, Chicago, IL/ and West Suburban Hospital, Berwyn, IL* |
|
|
| Efficacy of Isradipine in Early Parkinson Disease (STEADY-PD III) |
|
|
| Atlantic Health System, Summit, NJ Banner Sun Health Research Institute, Sun City, AZ John Hopkins, Baltimore, MD North Shore LIJ Health System/Feinstein Institute, Manhasset NY Northwestern University Feinberg School of Medicine, Chicago, IL Rush University, Chicago, IL The Parkinson’s & Movement Disorder Institute, Fountain Valley, CA University of California San Diego, La Jolla, CA University of Alabama at Birmingham, Birmingham, AL University of California Irvine, Irvine, CA University of Maryland, Baltimore, MD University of Nevada Las Vegas School of Medicine, Las Vegas, NV University of Pennsylvania, Philadelphia, PA University of Texas Health Science Center-Medical School, Houston, Texas |
At kickoff meeting, after randomization we discovered these two set of sites had two geographically separated facilities so they were split into two sub-sites. The sub-sites each submitted a screening log and recruitment activities check list but were treated as one site during the analysis.
Appendix C:
The table below was published in the RECRUIT design paper [1]. Standard deviations reported below differ from the design paper as these analyses employed more rigorous analyses. Conclusions remained unchanged from the design paper.
BASELINE SPECIALTY CLINIC CHARACTERISTICS.
| Intervention (N = 26) |
Control (N = 24) |
|||
|---|---|---|---|---|
| n (%) | n (%) | p-value a | ||
| Site geographic location b | Northeast | 6 (23.1) | 6 (25.0) | 0.58 a |
| Southeast | 7 (26.9) | 8 (33.3) | ||
| Midwest and Northwest | 3 (11.5) | 5 (20.8) | ||
| Southwest and West | 10 (38.5) | 5 (20.8) | ||
| Percent minorities enrolled in previous trials at the site b,c | 0%-<10% | 7 (26.9) | 7 (29.2) | 0.63 a |
| 10%-<20% | 6 (23.1) | 3 (12.5) | ||
| ≥ 20% | 9 (34.6) | 11 (45.8) | ||
| data N/Ad | 4 (15.4) | 3 (12.5) | ||
| % minorities ≤ 30 miles of siteb,e | 17%-<20% | 1 (3.8) | 1 (4.2) | 0.59 a |
| 20%-<40% | 14 (53.8) | 13 (54.2) | ||
| 40%-<60% | 7 (26.9) | 9 (37.5) | ||
| 60% or more | 4 (15.4) | 1 (4.2) | ||
| % Foreign born ≤ 30 miles of sitee | 0%-<10% | 7 (26.9) | 6 (25.0) | 0.60 a |
| 10%-<20% | 9 (34.6) | 11 (45.8) | ||
| 20%-<30% | 6 (23.1) | 2 (8.3) | ||
| 30% or more | 4 (15.4) | 5 (20.8) | ||
| % Non-English speaking ≤ 30 miles of sitee,f | 0%-<10% | 7 (26.9) | 8 (33.3) | 0.89 a |
| 10%-<20% | 7 (26.9) | 7 (29.2) | ||
| 20%-<30% | 5 (19.2) | 3 (12.5) | ||
| 30% or more | 7 (26.9) | 6 (25.0) | ||
| p-value | ||||
| Site patients seen per | Mean (Standard Deviation) | 41.2 (6.3) | 78.9 (16.0) | 0.10 g |
| week for any trial c | Median | 32.5 | 46 |
P-values from logistic regressions are reported because the analysis had to include parent trial as strata in order to interpret the comparison. Each site contributes one data record.
Baseline matching criteria used in randomization
Information from investigator demographics form
Site not in a previous trial or data not available.
Information from US Census
Speak a language other than English at home
A p-value is reported because the p-value had to be adjusted for clustering within site and stratification by parent trial in order to interpret the comparison
Additional RECRUIT Trial Center Investigators Include:
Joy M. De Los Reyes, MPH, Yefei Zhang, MS, Derrick Tabor, PhD, Sheryl A. McCurdy, PhD, Yue Xu, MPH, Mariana Arevalo, MPH, Elvan Daniels, MD, MPH, Carlos Singer, MD, and Robert A. Hauser, MD.
Additional RECRUIT Trial Center Affiliations: The University of Texas Health Science Center, School of Public Health at Houston (De Los Reyes, Zhang, McCurdy, Xu, Arevalo); National Institute on Minority Health and Health Disparities (NIMHD) (Tabor); American Cancer Society (Daniels); University of Miami (Singer); University of South Florida (Hauser).
Parent Trial Investigators
Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Investigators: Coordinating Center: Mary Horowitz MD, MS; BMT CTN Sites: Amandeep Salhotra, MD, Ryotaro Nakamura, MD, Mitchell E. Horwitz, MD, Edmund K. Waller, MD, PhD, Neera Jagirdar MD, MPH, Javier Bolaños-Meade, MD, Judy Baker, AA, CNA, Sergio Giralt, MD, Miguel-Angel Perales, MD, Beth Hoover, Asmita Mishra, MD, Lia Elena Perez MD, Tatiana Restrepo, Melhem Sohl, MD, Siddhartha Ganguly, MD, FACP, Joseph McGuirk, DO, FACP, Leyla O. Shune, MD, Amy Haun, RN, Hilary M.D. Kitson, RN, John R. Wingard, MD, Edward A. Stadtmauer, MD, Iskra Pusic, MD, MSCI, Bettina F. Drake, PhD.
BMT CTN Affiliations: Coordinating Center: Medical College of Wisconsin (Horowitz); BMT Sites: City of Hope National Medical Center (Salhotra, Nakamura); Duke University (ME Horwitz); Emory University (Waller, Jagirdar); Johns Hopkins University (Bolaños-Meade, Baker); Memorial Sloan Kettering Cancer Center (Giralt, Perales, Hoover); Moffitt Cancer Center (Mishra, Perez, Restrepo); Northside Hospital (Sohl); The University of Kansas Health System (Ganguly, McGuirk, Shune, Haun, Kitson); Children’s Mercy as of 2018 (Kitson); University of Florida (Wingard); University of Pennsylvania (Stadtmauer); Washington University School of Medicine in St. Louis (Pusic, Drake).
Catheter Ablation Versus Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA) Investigators: Coordinating Center: Adam Silverstein, MS; CABANA Site Investigators: Adam E. Berman, MD, MPH, Andrea M. Russo, MD, FACC, FHRS, Julie W. Field, RCVT, CEPS, Kevin L. Thomas, MD, J. Vijay Jayachandran, MD, FACC, FHRS, Felix Sogade, MD, FACC, FHRS, Angie Buice, RN, James Coromilas, MD, Darryl Wells, MD, Elizabeth A. Vogt, PhD, CCRC, Nitish Badhwar, MD, J. Paul Mounsey, MD, PhD, FACC, Anil K. Gehi, MD and Tyrone Wade Jr., MS, CCRC.
CABANA Affiliations: Duke Clinical Research Institute (Silverstein), CABANA Sites: Augusta University (Berman); Cooper University Hospital (Russo, Field); Duke University (Thomas); Fort Worth Heart PA/Baylor All Saints (Jayachandran); Georgia Arrhythmia Consultants and Research Institute: (Sogade, Buice); Rutgers Robert Wood Johnson Medical School (Coromilas); Swedish Medical Center (Wells, Vogt); University of California at San Francisco (Badhwar), Stanford University as of 2018 (Badhwar); University of North Carolina (Mounsey, Gehi, Wade Jr.).
Preventing Adenomas of the Colon with Eflornithine and Sulindac (PACES) Investigators: Coordinating Center: Monica Yee, CCRP; SWOG Statistical Center: Joseph Unger, PhD; PACES Site Investigators: LTC Andrew S. Delmas, Susan D. Rogers, CCRC, Joe J. Stephenson, MD, Karen Pilman, RN, Howard Zaren, MD, FACS, Elizabeth Bruce MEd, MSN, Cheryl Farlow, RN, John A. Ellerton, MD, CM, Karen Sartell, MA, CCRP, Jason Zell, DO, MPH, Jeffrey L. Berenberg, MD, MACP, Scott K. Kuwada, MD, Virginia McMahon, Barbara S. Craft, MD, Takila J. Keys, MHS, RHIA, Anand B. Karnad, MD, Keith Shulman, MD, Isoken Koko, MD, Julie Koch RN, CCRP.
PACES Affiliations: Coordinating Center: Cancer Research and Biostatistics (CRAB) (Yee); Southwest Oncology Group (SWOG) Statistical Center (Unger); PACES Sites: Dwight D. Eisenhower Army Medical Center (Delmas, Rogers); Greenville Health System (Stephenson); Loyola University Medical Center (Pilman); Nancy N. and J.C. Lewis Cancer and Research Pavilion at St. Joseph’s/Candler Hospital System (Zaren, Bruce, Farlow); Nevada Cancer Research Foundation (Ellerton, Sartell); University of California Irvine (Zell); University of Hawaii (Berenberg, Kuwada, McMahon); University of Mississippi Medical Center (Craft, Keys); The University of Texas Health Science Center at San Antonio (Karnad); Weiss Memorial Hospital (K Shulman), Oncology of North Shore as of 2018 (K Shulman); West Suburban Medical Center (Koko, J Koch).
Efficacy of Isradipine in Early Parkinson Disease (STEADY-PD III) Investigators: Coordinating Center: Tanya Simuni, MD; Elise Kayson MS, Brittany L.Greco, CCRA; STEADY-PD III Site Investigators: Diane Babek, RN, MSN, Holly A. Shill, MD, Kelly Mills, MD, Andrew Feigin, MD, Jean Ayan, Cindy Zadikoff, MD, Karen Williams, CCRP, Daniel Truong, MD, FAAN, Deborah Hall, MD, PhD, Natividad Stover, MD, Nicolas Phielipp, MD, Irene Litvan, MD, Melissa J. Armstrong, MD, Lisa M. Shulman, MD, Eric S. Farbman, MD, Shamine K. Poynor, MA, CRC, Andres Deik, MD, Mya C. Schiess, MD, Jessika I. Suescun Ocampo, MD. STEADY-PD III Affiliations: Coordinating Center: Northwestern University Feinberg School of Medicine (Simuni), University of Rochester (Kayson, Greco); STEADY-PD III Site Investigators: Atlantic Health System (Babek); Banner Sun Health Research Institute (Shill); Hopkins University (Mills); North Shore LIJ Health System (Feigin, Ayan); Northwestern University (Zadikoff, Williams); Parkinson’s & Movement Disorders Institute (Truong); Rush University (Hall); University of Alabama at Birmingham (Stover); University of California Irvine (Phielipp); University of California San Diego (Litvan); University of Maryland (Armstrong, L Shulman); University of Nevada Las Vegas School of Medicine (Farbman, Poynor); University of Pennsylvania (Deik); The University of Texas Health Science Center at Houston McGovern Medical School (Schiess, Suescun Ocampo); Baylor College of Medicine as of 2018 (Schiess).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
TRIAL REGISTRATION: ClinicalTrials.gov NCT01911208
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the official policy of the Department of Defense, Department of the Army, US Army Medical Department, or the US Government.
CONFLICTS OF INTEREST
We do not believe there were any conflicts of interest for any of the authors for this manuscript given its topic of minority recruitment and the fact that the work was completed with NIH grant funding. This work has not been previously published.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- [1].Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004; 291: 2720–6. [DOI] [PubMed] [Google Scholar]
- [2].Sardar MR, Badri M, Prince CT, Seltzer J, Kowey PR. Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern Med 2014; 174: 1868–70. [DOI] [PubMed] [Google Scholar]
- [3].Schneider MG, Swearingen CJ, Shulman LM, Ye J, Baumgarten M, Tilley BC. Minority enrollment in Parkinson’s disease clinical trials. Parkinsonism Relat Disord 2009; 15: 258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, Mountain JL, Perez-Stable EJ, Sheppard D, Risch N. The importance of race and ethnic background in biomedical research and clinical practice. New England Journal of Medicine 2003; 348: 1170–5. [DOI] [PubMed] [Google Scholar]
- [5].Taylor JS, Ellis GR. Racial differences in responses to drug treatment: implications for pharmacotherapy of heart failure. Am J Cardiovasc Drugs 2002; 2: 389–99. [DOI] [PubMed] [Google Scholar]
- [6].Bjornsson TD, Wagner JA, Donahue SR, Harper D, Karim A, Khouri MS, et al. A review and assessment of potential sources of ethnic differences in drug responsiveness. J Clin Pharmacol 2003; 43: 943–67. [DOI] [PubMed] [Google Scholar]
- [7].Oh SS, Galanter J, Thakur N, Pino-Yanes M, Barcelo NE, White MJ, et al. Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled. PLoS Med 2015; 12: e1001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Heller C, Balls-Berry JE, Nery JD, Erwin PJ, Littleton D, Kim M, et al. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: a systematic review. Contemp Clin Trials 2014; 39: 169–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].UyBico SJ, Pavel S, Gross CP. Recruiting vulnerable populations into research: a systematic review of recruitment interventions. J Gen Intern Med 2007; 22: 852–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health 2014; 104: e16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, Eggly S. Barriers to Clinical Trial Enrollment in Racial and Ethnic Minority Patients With Cancer. Cancer Control 2016; 23: 327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ford ME, Havstad SL, Tilley BC. Recruiting older African American Men to a cancer screening trial (the AAMEN Project). Gerontologist 2003; 43: 27–35. [DOI] [PubMed] [Google Scholar]
- [13].Tilley BC, Mainous AG 3rd, Elm JJ, Pickelsimer E, Soderstrom LH, Ford ME, et al. A randomized recruitment intervention trial in Parkinson’s disease to increase participant diversity: early stopping for lack of efficacy. Clin Trials 2012; 9: 188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fracasso PM, Goodner SA, Creekmore AN, Morgan HP, Foster DM, Hardmon AA, et al. Coaching intervention as a strategy for minority recruitment to cancer clinical trials. J Oncol Pract 2013; 9: 294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health 2006; 27: 1–28. [DOI] [PubMed] [Google Scholar]
- [16].Wieland ML, Njeru JW, Alahdab F, Doubeni CA, Sia IG. Community-Engaged Approaches for Minority Recruitment Into Clinical Research: A Scoping Review of the Literature. Mayo Clin Proc 2021; 96: 733–43. [DOI] [PubMed] [Google Scholar]
- [17].Brown SD, Lee K, Schoffman DE, King AC, Crawley LM, Kiernan M. Minority recruitment into clinical trials: experimental findings and practical implications. Contemp Clin Trials 2012; 33: 620–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Blustein J, Weiss LJ. Visits to specialists under Medicare: socioeconomic advantage and access to care. J Health Care Poor Underserved 1998; 9: 153–69. [DOI] [PubMed] [Google Scholar]
- [19].Cook NL, Hicks LS, O’Malley AJ, Keegan T, Guadagnoli E, Landon BE. Access to specialty care and medical services in community health centers. Health Aff 2007; 26: 1459–68. [DOI] [PubMed] [Google Scholar]
- [20].Durant RW, Wenzel JA, Scarinci IC, Paterniti DA, Fouad MN, Hurd TC, et al. Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: enhancing minority participation in clinical trials (EMPaCT). Cancer 2014; 120 Suppl 7: 1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McCaskill-Stevens W, Pinto H, Marcus AC, Comis R, Morgan R, Plomer K, et al. Recruiting minority cancer patients into cancer clinical trials: a pilot project involving the Eastern Cooperative Oncology Group and the National Medical Association. J Clin Oncol 1999; 17: 1029–39. [DOI] [PubMed] [Google Scholar]
- [22].Siminoff LA, Step MM. A communication model of shared decision making: accounting for cancer treatment decisions. Health Psychol 2005; 24: S99–S105. [DOI] [PubMed] [Google Scholar]
- [23].Royal C, Baffoe-Bonnie A, Kittles R, Powell I, Bennett J, Hoke G, et al. Recruitment experience in the first phase of the African American Hereditary Prostate Cancer (AAHPC) Study. Ann Epidemiol 2000; 10: S68–77. [DOI] [PubMed] [Google Scholar]
- [24].Mainous AG 3rd, Smith DW, Geesey ME, Tilley BC. Factors influencing physician referrals of patients to clinical trials. J Natl Med Assoc 2008; 100: 1298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer 2008; 112: 228–42. [DOI] [PubMed] [Google Scholar]
- [26].Tilley BC, Mainous AG, 3rd, Smith DW, McKee MD, Amorrortu RP, Alvidrez J, et al. Design of a cluster-randomized minority recruitment trial: RECRUIT. Clin Trials 2017; 14: 286–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Amorrortu RP, Arevalo M, Vernon SW, Mainous AG 3rd, Diaz V, McKee MD, et al. Recruitment of racial and ethnic minorities to clinical trials conducted within specialty clinics: an intervention mapping approach. Trials 2018; 19: 115,018–2507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gorelick PB, Harris Y, Burnett B, Bonecutter FJ. The recruitment triangle: reasons why African Americans enroll, refuse to enroll, or voluntarily withdraw from a clinical trial. An interim report from the African-American Antiplatelet Stroke Prevention Study (AAASPS). J Natl Med Assoc 1998; 90: 141–5. [PMC free article] [PubMed] [Google Scholar]
- [29].Levkoff S, Sanchez H. Lessons learned about minority recruitment and retention from the Centers on Minority Aging and Health Promotion. Gerontologist 2003; 43: 18–26. [DOI] [PubMed] [Google Scholar]
- [30].Anonymous. A Research!America poll of U.S. adults conducted in partnership with Zogby Analytics. Clinical Trials: Poll http://www.researchamerica.org/sites/default/files/uploads/June2013clinicaltrials.pdf. Accessed 2018. [Google Scholar]
- [31].McLaughlin CP and Kaluzny AD. Continuous quality improvement in health care: Theory, implementations and applications 3rd ed: Jones & Bartlett Learning; 2005. [Google Scholar]
- [32].Tilley BC, Lyden PD, Brott TG, Lu M, Levine SR, Welch KM. Total quality improvement method for reduction of delays between emergency department admission and treatment of acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Arch Neurol 1997; 54: 1466–74. [DOI] [PubMed] [Google Scholar]
- [33].Vargas RB, Ryan GW, Jackson CA, Rodriguez R, Freeman HP. Characteristics of the original patient navigation programs to reduce disparities in the diagnosis and treatment of breast cancer. Cancer 2008; 113: 426–33. [DOI] [PubMed] [Google Scholar]
- [34].Donner A Sample size requirements for stratified cluster randomization designs. Stat Med 1992; 11: 743–50. [DOI] [PubMed] [Google Scholar]
- [35].Donner A, Klar N. Design and analysis of cluster randomization trials in health research London, UK: Arnold Publishing; 2000. [Google Scholar]
- [36].DeSantis SM, Li R, Wang X, Tilley BC, Vernon S, Koch G. Intent-to-treat analysis of cluster randomized trials when clusters report unidentifiably outcome proportions. Clinical Trials 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zell J, You YN, Boughey JC. PACES Trial: Evaluating the effectiveness of eflornithine and sulindac in preventing colon adenomas. Bull Am Coll Surg 2015; 100: 70–1. [PubMed] [Google Scholar]
- [39].Anonymous. Efficacy of Isradipine in Early Parkinson Disease; https://clinicaltrials.gov/ct2/show/NCT02168842. Accessed 2018.
- [40].Anonymous. Blood and Marrow Transplant Clinical Trials Network https://web.emmes.com/study/bmt2. Accessed 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fryer CS, Passmore SR, Maietta RC, Petruzzelli J, Casper E, Brown NA, et al. The Symbolic Value and Limitations of Racial Concordance in Minority Research Engagement. Qual Health Res 2016; 26: 830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fiore K Quick Enrollment for STEADY-PD III Trial 2016; 2018. [Google Scholar]
- [43].Peckham C Medscape Oncologist Lifestyle Report 2017: Race and Ethnicity, Bias and Burnout 2017; Accessed 2019. [Google Scholar]
- [44].Peckham C Medscape Rheumatologist Lifestyle Report 2017: Race and Ethnicity, Bias and Burnout 2017; Accessed 2019. [Google Scholar]
- [45].Peckham C Medscape Cardiologist Lifestyle Report 2017: Race and Ethnicity, Bias and Burnout 2017; Accessed 2019. [Google Scholar]
- [46].Ford ME, Siminoff LA, Pickelsimer E, Mainous AG, Smith DW, Diaz VA, et al. Unequal burden of disease, unequal participation in clinical trials: solutions from African American and Latino community members. Health Soc Work 2013; 38: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Green L, Kreuter M. The precede-proceed model. Health promotion planning: an educational approach In: , Mountain View (CA): Mayfield Publishing Company; 1999, p. 32–43. [Google Scholar]
- [48].Huffman MD, Mohanan PP, Devarajan R, Baldridge AS, Kondal D, Zhao L, et al. Effect of a Quality Improvement Intervention on Clinical Outcomes in Patients in India With Acute Myocardial Infarction: The ACS QUIK Randomized Clinical Trial. JAMA 2018; 319: 567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Majhail NS, Nayyar S, Santibanez ME, Murphy EA, Denzen EM. Racial disparities in hematopoietic cell transplantation in the United States. Bone Marrow Transplant 2012; 47: 1385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rodrigue JR, Kazley AS, Mandelbrot DA, Hays R, LaPointe Rudow D, Baliga P, et al. Living Donor Kidney Transplantation: Overcoming Disparities in Live Kidney Donation in the US--Recommendations from a Consensus Conference. Clin J Am Soc Nephrol 2015; 10: 1687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chow GV, Marine JE, Fleg JL. Epidemiology of arrhythmias and conduction disorders in older adults. Clin Geriatr Med 2012; 28: 539–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Amponsah MK, Benjamin EJ, Magnani JW. Atrial Fibrillation and Race - A Contemporary Review. Curr Cardiovasc Risk Rep 2013; 7: 10.1007/s12170,013–0327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Berk S, Greco BL, Biglan K, Kopil CM, Holloway RG, Meunier C, et al. Increasing Efficiency of Recruitment in Early Parkinson’s Disease Trials: A Case Study Examination of the STEADY-PD III Trial. J Parkinsons Dis 2017; 7: 685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Office for Human Research Protections. Informed Consent of Subjects Who Do Not Speak English https://www.hhs.gov/ohrp/regulations-and-policy/guidance/obtaining-and-documenting-infomed-consent-non-english-speakers/index.html. Updated 1995; Accessed 2018.
- [55].Chastain DB, Osae SP, Henao-Martinez AF, Franco-Paredes C, Chastain JS, Young HN. Racial Disproportionality in Covid Clinical Trials. N Engl J Med 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


