Abstract
Background and aims:
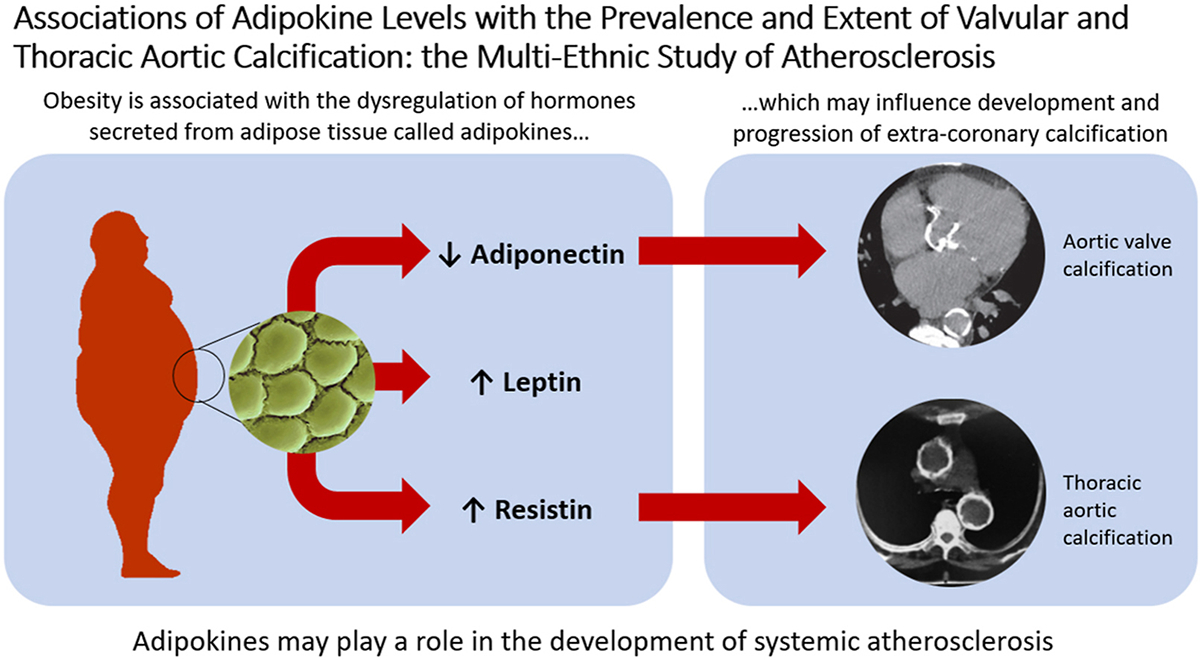
Extra-coronary calcification (ECC) is a marker of atherosclerosis and independently associated with cardiovascular disease (CVD). Adipokines may mediate the effect of obesity on atherosclerosis. However, the relationship of adipokines with ECC is not well-established. We examined the associations of leptin, resistin and adiponectin with ECC in a diverse community-based cohort.
Methods:
We performed a cross-sectional analysis of 1,897 adults without clinical CVD in the MESA cohort. Serum adipokine levels and non-contrast cardiac CT scans were obtained at Exam 2 or 3 (randomly assigned). ECC was quantified by Agatston score and included calcification of the mitral annulus (MAC), aortic valve (AVC), ascending thoracic aorta (ATAC) and descending thoracic aorta (DTAC). We used multivariable regression to evaluate the associations between leptin, resistin and adiponectin [per 1 SD ln(adipokine] with ECC prevalence (score >0) and extent [ln(score+1)].
Results:
The mean age of participants was 65±10 years; 49% women. After adjusting for demographic factors, adiponectin was inversely associated with AVC prevalence and extent; leptin positively associated with MAC prevalence and extent; and resistin positively associated with ATAC prevalence and extent and DTAC extent. After adjustment for BMI and other CVD risk factors, adiponectin remained inversely associated with AVC prevalence, and resistin remained associated with greater ATAC prevalence and extent. Leptin was not associated with measures of ECC after full adjustment. No adipokine was associated with MAC after full adjustment.
Conclusions:
We identified significant associations between select adipokines and specific markers of ECC. Adipokines may play a role in the development of systemic atherosclerosis.
1. INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in the United States and around the world.[1] As such, increased focus has been directed towards preventing the development and progression of CVD through the modification of risk factors.[1, 2] Obesity has been clearly implicated as one such risk factor,[3–5] though the mechanisms by which risk is conferred are complex and not fully understood. One potential contribution comes from the dysregulation of endogenous hormones secreted by adipose tissue—called adipokines.[6]
Leptin, adiponectin, and resistin are adipokines that influence various metabolic processes, including insulin sensitivity, endothelial function, and appetite regulation.[7–11] In general, increasing fat mass is associated with unfavorable changes in these adipokine levels. Leptin, involved in processes such as satiety and lowering blood glucose, is higher among individuals who are obese, secondary to a leptin-resistant phenotype.[12] Adiponectin, which reduces inflammation and improves insulin sensitivity, is lower in patients who are obese.[11] Resistin, which promotes endothelial dysfunction and foam cell formation, is greater in states of chronic inflammation such as obesity.[13] Given the variety of cardiometabolic pathways involving adipokines, it is plausible that dysregulation of these hormones can lead to negative cardiovascular health.[14] However, the association of adipokines with incident CVD events has been inconsistent, with some studies showing strong associations[15, 16] and others suggesting these relationships are attenuated after accounting for adiposity and other CVD risk factors.[17, 18]
A putative mechanism by which adipokine dysregulation would influence CVD is via atherosclerosis. For example, leptin is known to promote calcification of vascular cells in vitro—an important marker of atherosclerotic cardiovascular disease (ASCVD).[19] In this regard, coronary artery calcification (CAC) and extra-coronary calcification (ECC) are useful tools for cardiovascular prognostication as they have both been shown to predict incident CVD events independently of other CVD risk factors.[20–25] While CAC and ECC share many overlapping risk factors, they differ somewhat in terms of associated CVD risk and each provide added value for risk stratification.[26–29] ECCs (which include calcification of the ascending thoracic aorta [ATAC], descending thoracic aorta [DTAC], the mitral annulus [MAC], and the aortic valve [AVC]) are independent predictors of major adverse cardiovascular events, including myocardial infarction, stroke, and all-cause mortality.[20, 22, 23, 30, 24, 31] Therefore, ECC represents an important indicator of incipient CVD. Nevertheless, few studies have assessed the association of various adipokines with vascular calcification of different arterial beds, including ECC.
To explore the relationship of adipokines with calcified subclinical atherosclerosis, we performed a cross-sectional analysis of the Multi-Ethnic Study of Atherosclerosis (MESA) cohort examining the association of circulating adipokine levels with ECC. We also examined adipokine levels with history of ECC progression, which was examined retrospectively. Given the association with the relatively pro-inflammatory effects of leptin and resistin, as well as the purported anti-inflammatory effects of adiponectin, we hypothesized that higher serum levels of leptin and resistin, but lower levels of adiponectin, would be associated with a greater prevalence and burden of ECC. Given the dynamic nature of atherosclerosis, we also predicted that these same patterns of adipokine levels would predict a greater odds of having had ECC progression over time.
2. PATIENTS AND METHODS
2.1. Study population
The MESA is a prospective cohort study examining subclinical atherosclerosis in community-dwelling men and women who self-reported Black, Hispanic, Chinese, and non-Hispanic White race or ethnicity. The study enrolled 6,814 individuals aged 45–84 years without clinically apparent CVD from six field sites in the United States between July 2000 and August 2002. Further details regarding the MESA design are published elsewhere.[32]
In an ancillary study of MESA to study abdominal aortic calcification, a random subset of 1,970 participants underwent a non-contrast cardiac computed tomography (CT) scan at either Exam 2 (2002–2004) or Exam 3 (2004–2005), by random assignment.[33] In a subsequent ancillary study related to body composition, adipokine levels were measured among these same participants from stored frozen serum samples obtained at the respective visits (2 or 3) of their CT scan.[5] The present study focuses on this same subset of participants, and thus Exam 2 or 3 (the time of the adipokine assessment) is the baseline visit for the reported analyses.
In the parent MESA study, individuals with clinically significant CVD, defined as prior myocardial infarction, angina, stroke, transient ischemic attack, or heart failure; use of nitroglycerin; current atrial fibrillation; or those who had undergone a procedure related to CVD were excluded from enrollment. For the present analysis, participants were also excluded if there were missing data on adipokine levels, ECC measurements, or covariates relevant to our analyses. After exclusions, this gave a final analytical sample of 1,897 (Figure 1).
Figure 1.
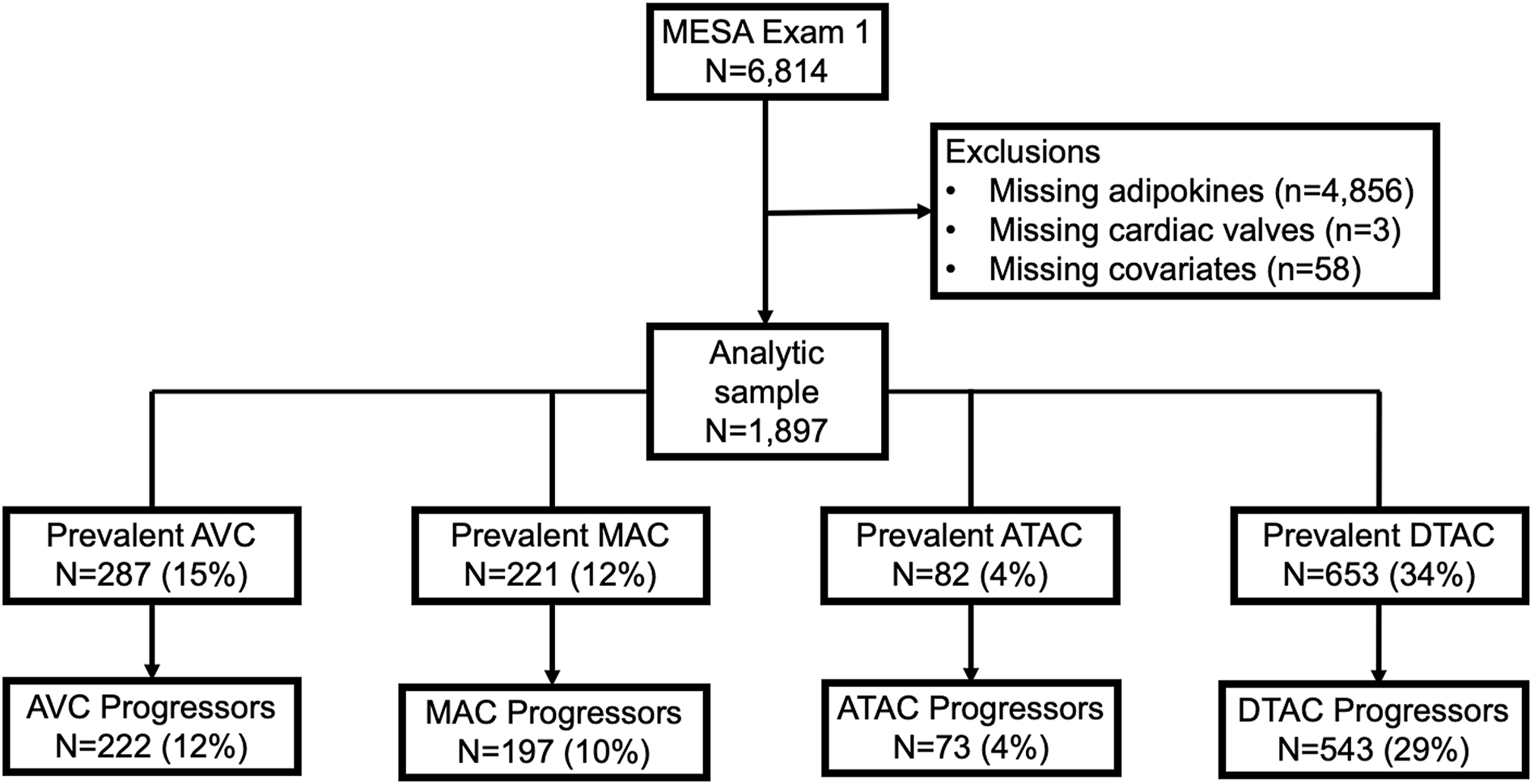
Flow chart of study inclusion and exclusion criteria, as well as the proportion of participants who had prevalent extra-coronary calcification (ECC) at baseline and the proportion with ECC progression.
The MESA study protocols were approved by the institutional review boards at all study sites, and participants gave written informed consent.
2.2. Measurement of endogenous adipokines
The primary independent variables of interest were the levels of the endogenous adipokines leptin, adiponectin, and resistin. Adiponectin, leptin, and resistin levels were obtained from serum at Exam 2 or 3 by random assignment and analyzed using Bio-Rad Luminex flow cytometry (Millepore, Billerica, MA) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT), as previously reported.[5] The coefficients of variation for these assays ranged from 6 to 13%.
2.3. Measurement of extra-coronary calcification
Four measures of ECC were assessed including ascending thoracic aortic calcification (ATAC), descending thoracic aortic calcification (DTAC), mitral annular calcification (MAC), and aortic valvular calcification (AVC). All ECC measures were assessed by Agatston scoring from ECG-gated non-contrast cardiac CT scans with density and area cutoffs of 130 Hounsfield units and 1 mm2, respectively. All MESA participants had a cardiac CT scan by either electron-beam CT or a four-slice multi-detector row helical CT at Exam 1 (2000–2002). Participants were then randomly assigned for a follow-up CT scan at either Exam 2 (2002–2004) or Exam 3 (2004–2005), as described above. Scanning parameters were kept identical between Exams 1, 2, and 3. CT scans were originally performed for CAC assessment but were later over-read by experienced readers at the core lab at the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. ECC was measured using a 3-dimensional reconstruction program (Rapidia, Infinite Co., Ltd, Seoul, Korea). Each scan was assessed by two readers who were blinded to clinical information and to the other reader’s evaluation. ECC measures are not available at subsequent MESA Exams 4, 5, or 6. Prevalent ECC was defined as ECC scores > 0. ECC progression was defined as > 0 Agatston units of change per year as has been previously defined in MESA.[34] Complete MESA CT scanning protocols are described elsewhere.[35]
2.4. Measurement of body composition assessment
As part of the aforementioned body composition ancillary study utilizing data from Exam 2 or 3, abdominal CT scans were used to measure visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT). VAT was measured as the total adipose tissue in the abdominal cavity and subcutaneous adipose tissue (SAT) measured as the total adipose tissue outside of the abdominal cavity but not within muscle tissue. VAT and SAT were measured as area values (in cm2) from the average of two CT slices obtained at L2-L3 and adjusted for height, as previously performed.[5]
2.5. Covariates
The primary covariates included in this analysis were obtained from Exam 2 or 3 (whichever visit was concordant with a given participant’s adipokine measurement) except for education and estimated glomerular filtration rate (eGFR), which were carried forward from Exam 1. These variables included the following demographic, behavioral and ASCVD risk factors measured at the baseline exam: age, sex, race/ethnicity, study site, level of education (less than a bachelor’s degree vs greater than or equal to a bachelor’s degree), body mass index (BMI; as a continuous variable), smoking status, pack-years of smoking, physical activity level (MET hours/week of moderate or vigorous activity, continuous), systolic blood pressure, use of antihypertensive medications, total cholesterol, high density lipoprotein cholesterol (HDL-C), use of lipid lowering therapy, history of diabetes (defined as fasting blood sugar ≥ 126 mg/dL, non-fasting glucose ≥ 200 mg/dl, or diabetic medication use), eGFR, and visceral and subcutaneous adipose tissue cross-sectional area (VAT, SAT) measured from abdominal CTs from Exam 2 or 3.
2.6. Statistical analysis
Demographic covariates were compared and stratified by BMI groups (≥30 vs <30 kg/m2) and by sex. Given their non-normal distributions, adipokine levels and their ratios were log transformed and modeled per one standard deviation (SD) increment in natural log-transformed adipokine level. Agatston scores were also subjected to ln(Agatston score+1) transformation as it has been done in previous work to address issues with skewing of data stemming from multiple measures equal to zero.[36]
Multivariable, progressively adjusted, robust Poisson regression models were used to estimate the adjusted prevalence ratios (PR) of ECC (AVC, MAC, ATAC, DTAC) per 1 SD increment in ln(adipokine) level at visit 2/3. Similar models were used to estimate the associations of adipokine level with ECC extent (handled as ln[ECC+1]) at visit 2/3 using linear regression. Beta coefficients from linear regression models were exponentiated and are reported as ratios of the geometric mean of the model coefficients (extent ratio, ER).
For secondary analysis, logistic regression was used to estimate the association of adipokine levels at Exams 2/3 with the odds of having met criteria for ECC progression between Exam 1 and 2/3, retrospectively assessed. ECC progressors between first and second CT scans (median 3 years) were defined as participants with >0 Agatston units of change per year and compared to non-progressor (i.e., participants with ≤0 Agatston units of change per year between CT scans).
For all analyses, we used progressively adjusted regression models. Model 1 adjusted for the demographics of age, sex, race/ethnicity and study site. Model 2 included Model 1 variables and added behavioral/lifestyle factors of education, BMI, smoking, pack years of smoking, and physical activity. Model 2 was our primary analytical model. Model 2b included Model 2 variables plus additionally VAT and SAT. Model 3 included Model 2 variables plus additionally ASCVD risk factors that may be intermediate variables in the association of adipokines with atherosclerosis (systolic blood pressure, anti-hypertensive medication, total cholesterol, HDL-C, lipid-lowering medication, diabetes mellitus and eGFR). Model 3b included Model 3 variables plus VAT and SAT.
The analyses were performed using the STATA 15.0 version (StataCorp LP, College Station, TX). p values were two-sided, and significance level was set at 0.05.
3. RESULTS
3.1. Baseline characteristics
The baseline characteristics of the study population are presented stratified by BMI and sex in Table 1 and Supplementary Table 1, respectively. The mean age of the 1,897 participants was 65±10 years; 49% were women. The cohort included 40% White, 13% Chinese, 20% Black and 26% Hispanic adults. When compared with participants with BMI <30, those with a BMI ≥30 kg/m2 were more likely to be female, to have higher blood pressure, or to have diabetes. These participants were also less likely to have a bachelor degree and, on average, had lower HDL-C. Women were also more likely to have higher leptin levels and higher adiponectin levels than men (Supplementary Table 1).
Table 1.
Characteristics of study participants by body mass index (BMI) categories.
| Total |
BMI <30 |
BMI ≥30 |
p value | |
|---|---|---|---|---|
| N = 1897 | n = 1304 | n = 593 | ||
|
| ||||
| Age | 65 (10) | 65 (10) | 64 (9) | 0.002 |
| Sex | ||||
| Men | 959 (51%) | 688 (53%) | 271 (46%) | 0.004 |
| Women | 938 (49%) | 616 (47%) | 322 (54%) | |
| Race/ethnicity | ||||
| White | 766 (40%) | 551 (42%) | 215 (36%) | |
| Chinese-American | 253 (13%) | 240 (18%) | 13 (2%) | <0.001 |
| Black | 383 (20%) | 227 (17%) | 156 (26%) | |
| Hispanic | 495 (26%) | 286 (22%) | 209 (35%) | |
| Education | ||||
| ≥ bachelor’s degree | 683 (36%) | 509 (39%) | 174 (29%) | <0.001 |
| < bachelor’s degree | 1212 (64%) | 793 (61%) | 419 (71%) | |
| BMI, kg/m2 | 28 (5) | 25 (3) | 34 (4) | <0.001 |
| Smoking status | ||||
| Never | 864 (46%) | 632 (49%) | 232 (39%) | |
| Former | 804 (43%) | 521 (40%) | 283 (48%) | 0.001 |
| Current | 216 (11%) | 143 (11%) | 73 (12%) | |
| Pack-years of smoking if > 0 | 17 (7–35) | 18 (8–35) | 15 (7–35) | 0.72 |
| Physical activity, MET-min/wk | 3540 (1815–6390) | 3600 (1928–6420) | 3458 (1624–6305) | 0.88 |
| Systolic blood pressure, mmHg | 124 (21) | 122 (21) | 128 (21) | <0.001 |
| Antihypertensive medication | 834 (44%) | 518 (40%) | 316 (53%) | <0.001 |
| Total cholesterol, mg/dLb | 190 (35) | 190 (34) | 189 (38) | 0.59 |
| HDL-C, mg/dLb | 51 (15) | 53 (16) | 47 (12) | <0.001 |
| Lipid-lowering medication | 477 (25%) | 315 (24%) | 162 (27%) | 0.14 |
| Diabetes | 276 (15%) | 141 (11%) | 134 (23%) | <0.001 |
| eGFR, ml/min per 1.73 m2 | 73 (15) | 73 (15) | 74 (16) | 0.29 |
| SAT, cm2 | 146 (102–202) | 128 (95–171) | 261 (202–328) | <0.001 |
| VAT, cm2 | 151 (93–226) | 128 (79–194) | 219 (158–281) | <0.001 |
| AVC | 287 (15%) | 189 (66%) | 98 (34%) | 0.25 |
| MVC | 221 (12%) | 134 (61%) | 87 (39%) | 0.01 |
| ATAC | 82 (4%) | 56 (68%) | 26 (32%) | 0.93 |
| DTAC | 653 (34%) | 465 (71%) | 188 (29%) | 0.09 |
| Leptin, ng/mLc | 13.4 (5.6–28.2) | 8.7 (4.0–17.8) | 30.0 (16.6–50.6) | <0.001 |
| Resistin, ng/mLc | 15.1 (11.9–19.1) | 14.8 (11.5–18.7) | 15.4 (12.5–19.7) | 0.03 |
| Adiponectin, mcg/mLc | 17.3 (11.8–26.1) | 18.6 (12.5–27.6) | 14.7 (10.4–21.5) | <0.001 |
ATAC, ascending thoracic aortic calcium; AVC, aortic valve calcium; BMI, body mass index; DTAC, descending thoracic aortic calcium; eGFR, estimated glomerular filtration rate; HDL-C, high density lipoprotein-cholesterol; MET, metabolic equivalent of task; MVC, mitral annular calcium; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Data were presented as mean (SD) or number (percentage) or median (interquartile range).
To convert total and HDL-C cholesterol from mg/dL to mmol/L, divide by 38.67.
Conversion to SI units not available.
Participants with higher BMI had higher median levels of leptin (30.0 ng/mL [interquartile range (IQR): 16.6–50.6] vs 8.7 ng/mL [4.0–17.8], p<0.001) and resistin (15.4 ng/mL [12.5–19.7] vs 14.8 ng/mL [11.5–18.17], p=0.03), and lower levels of adiponectin (14.7 mcg/mL [10.4–21.5] vs 18.6 mcg/mL [12.5–27.6], p<0.001) than non-obese counterparts (Table 1). The associations of adipokine levels with BMI, VAT, and SAT are shown in Supplementary Table 2. BMI, VAT, and SAT were all positively associated with leptin levels and inversely associated with adiponectin levels, whereas resistin levels were not significantly associated with these adiposity measures.
Across all participants, the unadjusted prevalence of AVC, MAC, ATAC, and DTAC was 15%, 12%, 4%, and 34%, respectively (Table 1 and Figure 1). ECC prevalence was not significantly different between obese and non-obese participants with the exception of MVC, which was more prevalent in non-obese participants (61% vs 39%, p=0.01, Table 1). AVC was more prevalent in male participants than female participants (63% vs 37%, p<0.001, Supplementary Table 1).
There was a median (IQR) of 3 (1.5, 3.3) years between baseline Exam 1 CT scan and subsequent scan at Exam 2 or 3. The proportion of study participants who showed progression of AVC, MAC, ATAC, or DTAC between these 2 scans was 12%, 10%, 4%, and 29%, respectively (Figure 1). The percent of population with prevalent ECC and history of ECC progression by tertiles of adipokine levels is shown in Supplementary Figures S1 and S2. The multivariable-adjusted associations of adipokines with ECC measures are described below.
3.2. Leptin
After adjusting for demographics (Model 1), higher serum leptin levels at Exam 2/3 were significantly associated with greater odds of having prevalent MAC (Table 2), greater MAC extent (Table 3), and greater odds of having had MAC progression between Exam 1 and Exam 2/3 (Table 4). However, the magnitude of these positive relationships was attenuated after adjustment for education, BMI, smoking, and physical activity and were no longer significant. There were no significant associations between leptin levels and other measures of ECC extent, prevalence, or progression.
Table 2.
Multivariable-adjusted association between adipokines and prevalent ECC
| Model 1 | Model 2 | Model 2b | Model 3 | Model 3b | |
|---|---|---|---|---|---|
| Aortic valve calcium (AVC) | |||||
| Leptin | 1.08 (0.95, 1.23) | 0.97 (0.83, 1.13) | 0.89 (0.72, 1.11) | 0.89 (0.77, 1.04) | 0.83 (0.67, 1.02) |
| Resistin | 0.99 (0.89, 1.11) | 0.99 (0.88, 1.10) | 0.97 (0.84, 1.11) | 0.93 (0.83, 1.04) | 0.91 (0.79, 1.05) |
| Adiponectin | 0.82 (0.73, 0.93) | 0.83 (0.73, 0.94) | 0.86 (0.73, 1.00) | 0.86 (0.76, 0.97) | 0.89 (0.76, 1.03) |
| Leptin/Adiponectin | 1.16 (1.03, 1.30) | 1.11 (0.96, 1.29) | 1.05 (0.87, 1.27) | 1.02 (0.88, 1.19) | 0.96 (0.79, 1.17) |
| Resistin/Adiponectin | 1.16 (1.04, 1.30) | 1.15 (1.02, 1.29) | 1.11 (0.96, 1.28) | 1.09 (0.96, 1.23) | 1.04 (0.90, 1.21) |
| Mitral annular calcium (MVC) | |||||
| Leptin | 1.20 (1.02, 1.41) | 1.04 (0.85, 1.27) | 0.96 (0.73, 1.27) | 1.03 (0.84, 1.28) | 0.97 (0.73, 1.29) |
| Resistin | 1.03 (0.92, 1.16) | 1.02 (0.91, 1.15) | 0.96 (0.82, 1.13) | 1.01 (0.88, 1.15) | 0.96 (0.81, 1.14) |
| Adiponectin | 0.99 (0.86, 1.14) | 1.05 (0.91, 1.22) | 0.97 (0.79, 1.18) | 1.04 (0.90, 1.20) | 0.96 (0.79, 1.18) |
| Leptin/Adiponectin | 1.14 (0.99, 1.31) | 0.99 (0.83, 1.17) | 1.00 (0.79, 1.27) | 1.00 (0.84, 1.19) | 1.01 (0.79, 1.28) |
| Resistin/Adiponectin | 1.03 (0.90, 1.17) | 0.97 (0.85, 1.12) | 1.00 (0.84, 1.19) | 0.97 (0.85, 1.12) | 1.01 (0.84, 1.21) |
| Ascending thoracic aortic calcium (ATAC) | |||||
| Leptin | 1.11 (0.85, 1.45) | 1.20 (0.83, 1.73) | 0.97 (0.58, 1.61) | 1.08 (0.76, 1.54) | 0.90 (0.56, 1.45) |
| Resistin | 1.29 (1.08, 1.55) | 1.37 (1.12, 1.68) | 1.15 (0.92, 1.44) | 1.32 (1.06, 1.64) | 1.10 (0.85, 1.41) |
| Adiponectin | 0.86 (0.69, 1.07) | 0.87 (0.69, 1.09) | 0.94 (0.70, 1.28) | 1.00 (0.80, 1.25) | 1.09 (0.81, 1.47) |
| Leptin/Adiponectin | 1.16 (0.92, 1.45) | 1.25 (0.92, 1.69) | 1.02 (0.70, 1.50) | 1.06 (0.79, 1.41) | 0.87 (0.62, 1.24) |
| Resistin/Adiponectin | 1.33 (1.08, 1.63) | 1.38 (1.11, 1.73) | 1.15 (0.89, 1.49) | 1.21 (0.95, 1.53) | 0.99 (0.74, 1.32) |
| Descending thoracic aortic calcium (DTAC) | |||||
| Leptin | 1.06 (0.99, 1.14) | 1.05 (0.96, 1.15) | 1.04 (0.93, 1.17) | 1.02 (0.93, 1.12) | 1.02 (0.91, 1.14) |
| Resistin | 1.04 (0.99, 1.10) | 1.04 (0.98, 1.10) | 1.04 (0.97, 1.12) | 1.02 (0.96, 1.09) | 1.04 (0.97, 1.12) |
| Adiponectin | 0.96 (0.91, 1.02) | 0.97 (0.91, 1.03) | 0.98 (0.91, 1.05) | 1.00 (0.94, 1.06) | 1.02 (0.95, 1.09) |
| Leptin/Adiponectin | 1.06 (0.99, 1.13) | 1.06 (0.98, 1.14) | 1.04 (0.95, 1.15) | 1.01 (0.94, 1.10) | 1.00 (0.90, 1.10) |
| Resistin/Adiponectin | 1.06 (1.00, 1.12) | 1.05 (0.99, 1.11) | 1.05 (0.98, 1.12) | 1.01 (0.95, 1.08) | 1.01 (0.94, 1.09) |
Results were derived from multivariable Poisson regression models with robust variance estimation and presented as prevalence ratios.
Adipokines were log-transformed for analysis and compared per 1 standard deviation (SD).
Prevalent extra-coronary calcium (ECC) was defined as Agatston score >0.
Model 1: adjusted for age, sex, race/ethnicity and study site
Model 2: Model 1 + education, body mass index, smoking, pack years of smoking, and physical activity.
Model 2b: Model 2 + visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT).
Model 3: Model 2 + systolic blood pressure, anti-hypertensive medication, total cholesterol, HDL-cholesterol, lipid-lowering medication, diabetes mellitus and eGFR.
Model 3b: Model 3: VAT and SAT.
Statistically significant results are in bold font. p value = 0.049 for result in italics.
Table 3.
Multivariable-adjusted association between adipokines and ECC extent.
| Model 1 | Model 2 | Model 2b | Model 3 | Model 3b | |
|---|---|---|---|---|---|
| Aortic Valve Calcium (AVC) | |||||
| Leptin | 1.07 (0.98, 1.16) | 0.99 (0.89, 1.11) | 0.94 (0.82, 1.07) | 0.95 (0.85, 1.07) | 0.91 (0.79, 1.04) |
| Resistin | 1.03 (0.96, 1.10) | 1.01 (0.94, 1.09) | 1.01 (0.93, 1.09) | 0.99 (0.92, 1.06) | 0.99 (0.91, 1.07) |
| Adiponectin | 0.89 (0.83, 0.96) | 0.90 (0.83, 0.97) | 0.93 (0.85, 1.02) | 0.92 (0.84, 1.00) | 0.95 (0.86, 1.05) |
| Leptin/adiponectin | 1.10 (1.02, 1.19) | 1.07 (0.97, 1.18) | 1.01 (0.89, 1.14) | 1.02 (0.92, 1.13) | 0.97 (0.86, 1.09) |
| Resistin/adiponectin | 1.11 (1.03, 1.19) | 1.10 (1.02, 1.19) | 1.06 (0.97, 1.16) | 1.06 (0.98, 1.15) | 1.03 (0.94, 1.13) |
| Mitral annular calcium (MVC) | |||||
| Leptin | 1.11 (1.02, 1.21) | 1.00 (0.89, 1.13) | 0.99 (0.86, 1.13) | 1.01 (0.90, 1.14) | 1.00 (0.87, 1.14) |
| Resistin | 1.06 (0.98, 1.13) | 1.04 (0.97, 1.12) | 1.01 (0.93, 1.09) | 1.04 (0.96, 1.12) | 1.01 (0.93, 1.10) |
| Adiponectin | 1.00 (0.92, 1.08) | 1.04 (0.96, 1.13) | 0.99 (0.90, 1.09) | 1.03 (0.95, 1.13) | 0.98 (0.89, 1.08) |
| Leptin/Adiponectin | 1.08 (1.00, 1.17) | 0.98 (0.88, 1.08) | 1.00 (0.88,1.13) | 0.99 (0.89,1.10) | 1.01 (0.90, 1.15) |
| Resistin/adiponectin | 1.04 (0.96, 1.12) | 1.00 (0.92, 1.08) | 1.01 (0.93, 1.11) | 1.00 (0.92, 1.09) | 1.03 (0.93, 1.13) |
| Ascending thoracic aortic calcium (ATAC) | |||||
| Leptin | 1.01 (0.96, 1.07) | 1.03 (0.96, 1.10) | 0.99 (0.91, 1.08) | 1.01 (0.94, 1.08) | 0.98 (0.90, 1.07) |
| Resistin | 1.06 (1.02, 1.11) | 1.07 (1.02, 1.11) | 1.03 (0.98, 1.09) | 1.06 (1.02, 1.11) | 1.03 (0.98, 1.08) |
| Adiponectin | 0.98 (0.93, 1.02) | 0.98 (0.93, 1.03) | 1.00 (0.94, 1.05) | 1.00 (0.95, 1.05) | 1.01 (0.95, 1.07) |
| Leptin/Adiponectin | 1.02 (0.97, 1.07) | 1.03 (0.97, 1.10) | 1.00 (0.93, 1.07) | 1.01 (0.95, 1.07) | 0.98 (0.91, 1.06) |
| Resistin/Adiponectin | 1.06 (1.01, 1.10) | 1.06 (1.01, 1.11) | 1.03 (0.97, 1.08) | 1.05 (1.00, 1.10) | 1.02 (0.96, 1.08) |
| Descending thoracic aortic calcium (DTAC) | |||||
| Leptin | 1.09 (0.96, 1.23) | 1.11 (0.94, 1.32) | 1.14 (0.93, 1.40) | 1.04 (0.88, 1.23) | 1.09 (0.89, 1.34) |
| Resistin | 1.13 (1.01, 1.26) | 1.11 (1.00, 1.24) | 1.12 (0.99, 1.26) | 1.07 (0.96, 1.19) | 1.08 (0.96, 1.22) |
| Adiponectin | 0.93 (0.83, 1.04) | 0.93 (0.82, 1.05) | 0.93 (0.81, 1.08) | 0.98 (0.87, 1.12) | 0.99 (0.86, 1.15) |
| Leptin/Adiponectin | 1.10 (0.98, 1.23) | 1.13 (0.97, 1.31) | 1.15 (0.96, 1.38) | 1.04 (0.90, 1.21) | 1.07 (0.89, 1.28) |
| Resistin/Adiponectin | 1.14 (1.02, 1.28) | 1.14 (1.02, 1.28) | 1.14 (1.00, 1.31) | 1.07 (0.95, 1.21) | 1.07 (0.93, 1.23) |
Results were derived from multivariable linear regression models and presented as exponentiated beta coefficients.
Adipokines were log-transformed for analysis and compared per 1 standard deviation (SD).
Extra-coronary calcium (ECC) was log-transformed and evaluated continuously as Log (ECC +1).
Model 1: adjusted for age, sex, race/ethnicity and study site
Model 2: Model 1 + education, body mass index, smoking, pack years of smoking, and physical activity.
Model 2b: Model 2 + visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT).
Model 3: Model 2 + systolic blood pressure, anti-hypertensive medication, total cholesterol, HDL-cholesterol, lipid-lowering medication, diabetes mellitus and eGFR.
Model 3b: Model 3: VAT and SAT.
Statistically significant results are in bold font. p value = 0.047 for result in italics.
Table 4.
Multivariable-adjusted association between adipokines and ECC progression (retrospectively assessed)
| Model 1 | Model 2 | Model 2b | Model 3 | Model 3b | |
|---|---|---|---|---|---|
| Aortic valve calcium (AVC) | |||||
| Leptin | 1.13 (0.95, 1.36) | 0.96 (0.75, 1.22) | 0.83 (0.60, 1.15) | 0.86 (0.67, 1.10) | 0.75 (0.54, 1.05) |
| Resistin | 1.00 (0.86, 1.17) | 0.99 (0.85, 1.16) | 0.93 (0.77, 1.13) | 0.93 (0.79, 1.10) | 0.88 (0.71, 1.07) |
| Adiponectin | 0.76 (0.65, 0.90) | 0.77 (0.65, 0.92) | 0.76 (0.62, 0.94) | 0.79 (0.65, 0.95) | 0.77 (0.61, 0.96) |
| Leptin/Adiponectin | 1.24 (1.05, 1.46) | 1.16 (0.93, 1.43) | 1.10 (0.82, 1.46) | 1.05 (0.84, 1.32) | 1.01 (0.75, 1.35) |
| Resistin/Adiponectin | 1.24 (1.06, 1.45) | 1.22 (1.03, 1.44) | 1.18 (0.96, 1.45) | 1.16 (0.97, 1.39) | 1.13 (0.91, 1.42) |
| Mitral annular calcium (MVC) | |||||
| Leptin | 1.26 (1.03, 1.54) | 1.06 (0.81, 1.39) | 0.94 (0.66, 1.32) | 1.08 (0.82, 1.42) | 0.95 (0.67, 1.36) |
| Resistin | 1.01 (0.86, 1.19) | 1.00 (0.84, 1.18) | 0.94 (0.77, 1.16) | 0.97 (0.80, 1.16) | 0.94 (0.75, 1.17) |
| Adiponectin | 0.96 (0.81, 1.15) | 1.04 (0.87, 1.25) | 0.94 (0.75, 1.18) | 1.01 (0.83, 1.23) | 0.94 (0.74, 1.19) |
| Leptin/Adiponectin | 1.19 (1.00, 1.43) | 1.01 (0.80, 1.28) | 1.01 (0.74, 1.36) | 1.04 (0.82, 1.33) | 1.02 (0.74, 1.39) |
| Resistin/Adiponectin | 1.04 (0.88, 1.23) | 0.96 (0.81, 1.15) | 1.01 (0.81, 1.26) | 0.97 (0.79, 1.17) | 1.01 (0.80, 1.28) |
| Ascending thoracic aortic calcium (ATAC) | |||||
| Leptin | 1.12 (0.83, 1.51) | 1.12 (0.75, 1.69) | 0.82 (0.49, 1.38) | 1.00 (0.66, 1.51) | 0.76 (0.45, 1.28) |
| Resistin | 1.29 (1.02, 1.64) | 1.33 (1.04, 1.70) | 1.13 (0.84, 1.52) | 1.27 (0.97, 1.66) | 1.06 (0.77, 1.47) |
| Adiponectin | 0.83 (0.63, 1.07) | 0.86 (0.65, 1.13) | 0.83 (0.59, 1.17) | 0.98 (0.73, 1.32) | 0.96 (0.67, 1.38) |
| Leptin/Adiponectin | 1.18 (0.90, 1.54) | 1.21 (0.85, 1.73) | 1.01 (0.64, 1.60) | 1.01 (0.70, 1.46) | 0.85 (0.53, 1.35) |
| Resistin/Adiponectin | 1.36 (1.06, 1.75) | 1.36 (1.05, 1.78) | 1.25 (0.90, 1.72) | 1.20 (0.89, 1.60) | 1.07 (0.75, 1.53) |
| Descending thoracic aortic calcium (DTAC) | |||||
| Leptin | 1.13 (0.98, 1.30) | 1.11 (0.92, 1.34) | 1.07 (0.84, 1.37) | 1.03 (0.85, 1.26) | 1.03 (0.80, 1.32) |
| Resistin | 1.15 (1.02, 1.29) | 1.13 (1.00,1.27) | 1.17 (1.01, 1.35) | 1.12 (0.99, 1.28) | 1.17 (1.00, 1.36) |
| Adiponectin | 0.90 (0.79, 1.02) | 0.91 (0.79, 1.04) | 0.94 (0.80, 1.10) | 0.97 (0.84, 1.13) | 1.03 (0.86, 1.23) |
| Leptin/Adiponectin | 1.14 (1.01, 1.30) | 1.15 (0.97, 1.36) | 1.11 (0.89, 1.37) | 1.04 (0.87, 1.25) | 1.00 (0.79, 1.26) |
| Resistin/Adiponectin | 1.18 (1.05, 1.34) | 1.17 (1.03, 1.33) | 1.18 (1.01, 1.37) | 1.11 (0.96, 1.28) | 1.10 (0.93, 1.31) |
Results were derived from multivariable logistic regression models and presented as odds ratios comparing extra-coronary calcium (ECC) progressors to non-progressors.
Adipokines were log-transformed for analysis and compared per 1 standard deviation (SD).
Model 1: adjusted for age, sex, race/ethnicity and study site.
Model 2: Model 1 + education, body mass index, smoking, pack years of smoking, and physical activity.
Model 2b: Model 2 + visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT).
Model 3: Model 2 + systolic blood pressure, anti-hypertensive medication, total cholesterol, HDL-cholesterol, lipid-lowering medication, diabetes mellitus and eGFR.
Model 3b: Model 3: VAT and SAT.
Statistically significant results are in bold font. p value = 0.047 and 0.045 for results in italics [1.13 (1.00, 1.27)] and [1.17 (1.00, 1.36)], respectively.
3.3. Resistin
With adjustment for the variables in Model 2, higher serum resistin levels were significantly associated with greater odds of having prevalent ATAC (Table 2), greater ATAC extent (Table 3), and greater odds of having had ATAC progression between Exam 1 and Exam 2/3 (Table 4). These associations for ATAC prevalence and extent remained significant after adjustment for ASCVD risk factors (model 3), but were attenuated after adjustment for VAT and SAT (models 2b and 3b).
There was also a significant association of higher serum resistin levels with extent and odds of progression of DTAC (Tables 3 and 4, model 2). Even in our most fully adjusted model including demographics, ASCVD risk factors, VAT and SAT (Model 3b, Table 4), greater resistin remained associated with a greater odds of having had DTAC progression between the 2 CT scans, retrospectively assessed (OR 1.17; 95% CI: 1.00–1.36, p = 0.045).
There were no significant interactions between resistin levels and other measures of ECC extent, prevalence, or progression.
3.4. Adiponectin
After adjustment for the variables in Model 2, higher serum adiponectin levels were significantly associated with a lower odds of prevalent AVC (Table 2), lower odds of extensive AVC extent (Table 3), and a higher odds of AVC progression between Exam 1 and Exam 2/3 (Table 4). The inverse association of adiponectin with AVC prevalence and history of progression remained significant after adjustment for ASCVD risk factors (model 3). Even in our most fully adjusted model that included demographics, ASCVD risk factors, VAT and SAT (Model 3b, Table 4), greater adiponectin remained associated with a lower odds of having had AVC progression between the 2 CT scans, retrospectively assessed (OR 0.77 95% CI: 0.61–0.96). There were no significant interactions between adiponectin levels and other measures of ECC extent, prevalence, or progression.
3.5. Adipokine ratios
The ratios of leptin to adiponectin and resistin to adiponectin were also assessed for associations with ECC. The ratio of leptin to adiponectin was directly proportional to the extent, prevalence, and likelihood of progression of AVC in our primary model, but these relationships were attenuated to non-significance after controlling for education, BMI, smoking, pack years of smoking, and physical activity. Somewhat similarly, the ratio of resistin to adiponectin was directly associated with AVC extent, prevalence, and likelihood of progression; ATAC extent, prevalence, and progression; and DTAC extent and likelihood of progression (but not prevalence). These relationships were robust after adjustment for age, sex, race/ethnicity, study site, education, BMI, smoking, pack years of smoking, and physical activity, but lost significance after further adjustment.
4. DISCUSSION:
In this cross-sectional analysis of data from the MESA cohort, we found some significant associations between specific circulating adipokine levels and the prevalence, extent, and likelihood of progression of ECC. Findings varied by adipokine and across vascular beds. Not surprisingly, given the established relationship of adiposity with circulating adipokines, many of these associations were attenuated and became not significant after adjustment for visceral fat measures. This suggests that visceral fat burden may, in part, explain the relationship between adipokines with atherosclerosis. For example, adiponectin was inversely associated with AVC prevalence and resistin positively associated with ATAC prevalence in models that included BMI and CVD risk factors (model 3) but not when further adjusted for VAT (model 3b). It should be noted that BMI is more strongly correlated with SAT than with VAT (Supplementary Table 2). However, we did find, even in our most adjusted models that included ASCVD risk factors and measures of visceral adiposity (VAT), that (1) adiponectin remained inversely associated with history of AVC progression and (2) resistin remained positively associated with history of DTAC progression. Leptin was not associated with any measures of ECC after adjustment. This suggests that not all of the relationship between adipokines and atherosclerosis is accounted for by visceral fat measures and vascular risk factors. In sum, our findings indicate that the pathophysiology of obesity associated inflammation and atherosclerosis is complex.
While a link between obesity and vascular calcification has been established,[37, 38] prior explorations into the associations of adipokines and ECC have been limited. A prior study from MESA did not find any independent association of adipokines with prevalence or severity of abdominal aortic calcification;[39] while another MESA analysis found higher leptin was associated with a history of CAC progression.[40] Other vascular beds and valvular calcium had not been explored before this present analysis. In some studies that did show a relationship between these hormones and CVD events, the effects were attenuated after adjustment for adiposity and other CVD risk factors.[17, 18] The results of our study suggest that adipokines, in particular resistin and adiponectin, are related to measures of ECC and that these relationships are preserved after adjustment for other CVD risk factors.
The mechanisms by which these various molecules influence atherosclerosis have been postulated. Adiponectin inhibits endothelial NF-kB, tumor necrosis factor-alpha (TNF-α)–induced monocyte adhesion, and the expression of other adhesion molecules that are involved in atherogenesis.[41, 42] TNF-α in particular has been implicated in the development and exacerbation of AVC,[43] which is consistent with our findings. The adverse effects of obesity on ASCVD may be mediated, in part, by reductions in adiponectin leading to activation of these atherogenic pathways. These putative pathways are not exclusive to the aortic valve tissue, though, and so further investigation is required to explain why the relationship between adiponectin and ECC was not seen in other arterial beds.
Leptin and adiponectin are thought of as having antagonistic effects with respect to endothelial function and inflammation. Indeed, we found the ratio of leptin to adiponectin was positively associated with calcification of the aortic valve and descending thoracic aorta, though this association did not remain significant after adjustment. Similarly, leptin alone was not associated with ECC in our models after adjustment for covariates. The relationship of leptin with CVD risk has been conflicting across studies. A prior analysis from MESA did find that higher leptin levels were associated with increased odds of having had CAC progression, retrospectively assessed.[40] Yet, a previous MESA study by Martin et al. failed to identify significant associations between leptin levels and incident CVD events.[17] A subsequent meta-analysis of 13 studies investigating leptin and risk of CVD and stroke also showed no relationship after adjustment for established cardiovascular risk factors.[44] Our study results are in line with these prior findings and suggest that changes in leptin associated with obesity may not play a significant role in development of atherosclerosis.
Resistin induces insulin resistance which is independently associated with vascular calcification.[13, 15] In our models, resistin was significantly associated with aortic calcification even after adjustment for diabetes history suggesting it is involved in mechanisms of ASCVD independent of insulin resistance. Resistin has been shown to potentiate the release of endothelin-1, increase chemokine expression, and upregulate cell surface adhesion molecules ICAM-1 and VCAM-1—all important steps in atherogenesis.[45–47] These effects have been demonstrated in human aortic endothelial cells, specifically, which supports the relationship identified in our models.[48]
4.1. Strengths/limitations
Our study has many strengths including evaluation of three key adipokine measures with multiple markers of calcification outside of the coronary territory to give further insight into mechanisms linking adiposity with atherosclerosis. We utilized a well-characterized, diverse cohort and were able to account for a number of potential confounding as well as intermediary variables such as the measurement of visceral fat.
On the other hand, our findings should be considered in the context of several limitations. First, adipokine levels and VAT/SAT were only in a subset of individuals as part of an ancillary study. This reduced sample size and limited the statistical power for subgroup analysis. Second, adipokine levels and VAT/SAT were only measured at Exams 2/3 and as such we were unable to examine the association of adipokines concurrently with Exam 1 ECC measures. We could only retrospectively examine for ECC progression between Exam 1 and Exams 2/3 in order to look at differences in adipokine levels between past “progressors” and “non-progressors” in secondary analysis. This introduces uncertainty by assuming adipokine levels were constant in the interval between CT scans when we know these levels are dynamic, especially in the setting of weight change and other lifestyle modifications.[49, 50] However participants’ mean BMI measured at Exam 1 was strongly correlated with BMI at Exams 2 and 3 (correlation coefficients 0.97–0.99, Supplementary Table 3), suggesting weight was generally very stable over this ~3year period in this cohort. Third, given the lack of currently available data on ECC values after Exams 2/3, we are unable to evaluate for incidence of ECC or future progression beyond Exam 2/3. Future studies should include CT scans obtained after adipokine measurement as well as repeat adipokine measurements to allow prospective analysis of the relationships identified here. Fourth, recent studies suggest an inverse relationship between arterial calcification density and incident CVD events.[51] Our study does not include ECC density measurements; however future studies examining the relationship of adipokines with calcification density and volume as distinct variables may provide additional and interesting insights. Finally, we performed a number of statistical tests and some significant findings may have been by chance. However, our analyses were driven by a priori hypotheses, our findings were generally consistent across models, and our analyses were meant only to be exploratory and hypothesis generating, warranting confirmation in other studies and populations
4.2. Conclusion
In summary, we have shown through a cross-sectional analysis of a large, diverse cohort of community-dwelling men and women free from CVD at baseline that serum levels of adipokines, particularly adiponectin and resistin, are associated with extra-coronary calcification. Moreover, serum leptin levels are not associated with the presence or severity of subclinical atherosclerosis. Our study was an exploratory effort to further characterize the role of adipokines in obesity-related CVD risk. Further work, including prospective studies and therapeutic trials targeting circulating adiponectin and resistin, is required to better understand these relationships.
Supplementary Material
Highlights.
Adiponectin, resistin, and leptin levels are dysregulated in obese individuals
Low adiponectin levels were associated with aortic valve calcification progression
Higher resistin levels were associated with thoracic aortic calcification progression
Leptin does not appear to be associated with extra-coronary calcification
There is complex interplay between obesity, adipokine levels, and atherosclerosis
Acknowledgements
The authors thank the other investigators, the staff, and the MESA participants for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Declaration of competing interests
Dr. Budoff receives grant support from General Electric. Unrelated to this work, Dr. Michos served on a Medical Advisory Board for Novartis, Esperion, Amarin, and Astra Zeneca. None of the other authors report any conflicts of interest.
Financial support
Drs. Michos is supported by the Amato Fund for Women’s Cardiovascular Health at Johns Hopkins. The MESA study is supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the NIH/NHLBI, by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. The ancillary data used in this analysis were funded by R01- HL088451 and R01 HL071739. Drs. Ndumele and Michos are additionally supported for this work by an American Heart Association Strategic Focused Research Network Grant 20SFRN35120152.
Footnotes
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Dr. Budoff receives grant support from General Electric. Unrelated to this work, Dr. Michos served on a Medical Advisory Board for Novartis, Esperion, Amarin, and Astra Zeneca. The other authors have no disclosures to make.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):1376–414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G et al. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc. 2016;5(8):e003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fliotsos M, Zhao D, Rao VN, Ndumele CE, Guallar E, Burke GL et al. Body Mass Index From Early-, Mid-, and Older-Adulthood and Risk of Heart Failure and Atherosclerotic Cardiovascular Disease: MESA. J Am Heart Assoc. 2018;7(22):e009599. doi: 10.1161/jaha.118.009599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH et al. Adiposity and Incident Heart Failure and its Subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC Heart Fail. 2018;6(12):999–1007. doi: 10.1016/j.jchf.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unamuno X, Gómez- Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Euro J Clin Invest. 2018;48(9):e12997. [DOI] [PubMed] [Google Scholar]
- 7.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB--binding protein and regulates growth factor--induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105(24):2893–8. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278(45):45021–6. [DOI] [PubMed] [Google Scholar]
- 9.D’Souza AM, Neumann UH, Glavas MM, Kieffer TJ. The glucoregulatory actions of leptin. Mol Metab. 2017;6(9):1052–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu W-Y, Chao Y-W, Tsai Y-L, Lien C-C, Chang C-F, Deng M-C et al. Resistin induces monocyte--endothelial cell adhesion by increasing ICAM-1 and VCAM-1 expression in endothelial cells via p38MAPK-dependent pathway. J Cell Physiol. 2011;226(8):2181–8. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302(2):179–88. [DOI] [PubMed] [Google Scholar]
- 12.Landecho MF, Tuero C, Valenti V, Bilbao I, de la Higuera M, Frühbeck G. Relevance of leptin and other adipokines in obesity-associated cardiovascular risk. Nutrients. 2019;11(11):2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acquarone E, Monacelli F, Borghi R, Nencioni A, Odetti P. Resistin: a reappraisal. Mech Ageing Dev. 2019;178:46–63. [DOI] [PubMed] [Google Scholar]
- 14.Sweeney T, Quispe R, Das T, Juraschek SP, Martin SS, Michos ED. The Use of Blood Biomarkers in Precision Medicine for the Primary Prevention of Atherosclerotic Cardiovascular Disease: a Review. Expert Rev Precis Med Drug Dev. 2021;6(4):247–58. doi: 10.1080/23808993.2021.1930531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muse ED, Feldman DI, Blaha MJ, Dardari ZA, Blumenthal RS, Budoff MJ et al. The association of resistin with cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;239(1):101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291(14):1730–7. [DOI] [PubMed] [Google Scholar]
- 17.Martin SS, Blaha MJ, Muse ED, Qasim AN, Reilly MP, Blumenthal RS et al. Leptin and incident cardiovascular disease: the Multi-ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2015;239(1):67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114(7):623–9. [DOI] [PubMed] [Google Scholar]
- 19.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88(9):954–60. [DOI] [PubMed] [Google Scholar]
- 20.Budoff MJ, Nasir K, Katz R, Takasu J, Carr JJ, Wong ND et al. Thoracic aortic calcification and coronary heart disease events: the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. 2011;215(1):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox CS, Vasan RS, Parise H, Levy D, O’Donnell CJ, D’Agostino RB et al. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107(11):1492–6. [DOI] [PubMed] [Google Scholar]
- 23.Kizer JR, Wiebers DO, Whisnant JP, Galloway JM, Welty TK, Lee ET et al. Mitral annular calcification, aortic valve sclerosis, and incident stroke in adults free of clinical cardiovascular disease: the Strong Heart Study. Stroke. 2005;36(12):2533–7. [DOI] [PubMed] [Google Scholar]
- 24.Owens DS, Budoff MJ, Katz R, Takasu J, Shavelle DM, Carr JJ et al. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging. 2012;5(6):619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tison GH, Guo M, Blaha MJ, McClelland RL, Allison MA, Szklo M et al. Multisite extracoronary calcification indicates increased risk of coronary heart disease and all-cause mortality: The Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2015;9(5):406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM et al. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32(1):140–6. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs PC, Prokop M, van der Graaf Y, Gondrie MJ, Janssen KJ, de Koning HJ et al. Comparing coronary artery calcium and thoracic aorta calcium for prediction of all-cause mortality and cardiovascular events on low-dose non-gated computed tomography in a high-risk population of heavy smokers. Atherosclerosis. 2010;209(2):455–62. [DOI] [PubMed] [Google Scholar]
- 28.Bos D, Leening MJG, Kavousi M, Hofman A, Franco OH, Avd Lugt et al. Comparison of atherosclerotic calcification in major vessel beds on the risk of all-cause and cause-specific mortality: the Rotterdam study. Circ Cardiovasc Imaging. 2015;8(12):e003843. [DOI] [PubMed] [Google Scholar]
- 29.Hamirani YS, Nasir K, Blumenthal RS, Takasu J, Shavelle D, Kronmal R et al. Relation of mitral annular calcium and coronary calcium (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2011;107(9):1291–4. [DOI] [PubMed] [Google Scholar]
- 30.O’Neal WT, Efird JT, Nazarian S, Alonso A, Michos ED, Szklo M et al. Mitral annular calcification progression and the risk of atrial fibrillation: results from MESA. Eur Heart J Cardiovasc Imaging. 2018;19(3):279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos RD, Rumberger JA, Budoff MJ, Shaw LJ, Orakzai SH, Berman D et al. Thoracic aorta calcification detected by electron beam tomography predicts all-cause mortality. Atherosclerosis. 2010;209(1):131–5. [DOI] [PubMed] [Google Scholar]
- 32.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 33.Forbang NI, McClelland RL, Remigio-Baker RA, Allison MA, Sandfort V, Michos ED et al. Associations of cardiovascular disease risk factors with abdominal aortic calcium volume and density: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2016;255:54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fashanu OE, Bizanti A, Al-Abdouh A, Zhao D, Budoff MJ, Thomas IC et al. Progression of valvular calcification and risk of incident stroke: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2020;307:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budoff MJ, Takasu J, Katz R, Mao S, Shavelle DM, O’Brien KD et al. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006;13(2):166–72. [DOI] [PubMed] [Google Scholar]
- 36.Ezeigwe A, Fashanu OE, Zhao D, Budoff MJ, Otvos JD, Thomas IC et al. The novel inflammatory marker GlycA and the prevalence and progression of valvular and thoracic aortic calcification: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2019;282:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox CS, Hwang S-J, Massaro JM, Lieb K, Vasan RS, O’Donnell CJ et al. Relation of subcutaneous and visceral adipose tissue to coronary and abdominal aortic calcium (from the Framingham Heart Study). Am J Cardiol. 2009;104(4):543–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensky NE, Criqui MH, Wright CM, Wassel CL, Alcaraz JE, Allison MA. The association between abdominal body composition and vascular calcification. Obesity. 2011;19(12):2418–24. doi: 10.1038/oby.2011.70. [DOI] [PubMed] [Google Scholar]
- 39.Hughes-Austin JM, Wassel CL, Jiménez J, Criqui MH, Ix JH, Rasmussen-Torvik LJ et al. The relationship between adiposity-associated inflammation and coronary artery and abdominal aortic calcium differs by strata of central adiposity: The Multi-Ethnic Study of Atherosclerosis (MESA). Vasc Med. 2014;19(4):264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varma B, Ogunmoroti O, Ndumele CE, Zhao D, Szklo M, Sweeney T et al. Abstract P104: Higher Leptin Levels Are Associated With Increased Odds Of Coronary Artery Calcium Progression: The Multi-ethnic Study Of Atherosclerosis. Circulation. 2021;143(Suppl_1):AP104–AP. doi:doi: 10.1161/circ.143.suppl_1.P104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100(25):2473–6. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 42.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation. 2000;102(11):1296–301. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez Rodriguez A, Schroeder ME, Grim JC, Walker CJ, Speckl KF, Weiss RM et al. Tumor necrosis factor-α promotes and exacerbates calcification in heart valve myofibroblast populations. FASEB J. 2021;35(3):e21382. doi: 10.1096/fj.202002013RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang H, Guo W, Li J, Cao S, Zhang J, Pan J et al. Leptin concentration and risk of coronary heart disease and stroke: A systematic review and meta-analysis. PLoS ONE. 2017;12(3). doi: 10.1371/journal.pone.0166360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cybulsky MI, Gimbrone MA. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251(4995):788–91. [DOI] [PubMed] [Google Scholar]
- 46.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor–deficient mice. Mol Cell. 1998;2(2):275–81. [DOI] [PubMed] [Google Scholar]
- 47.Verma S, Li S-H, Wang C-H, Fedak PWM, Li R-K, Weisel RD et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108(6):736–40. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 48.Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine–endothelial cell interactions. Biochem Biophys Res Commun. 2004;314(2):415–9. [DOI] [PubMed] [Google Scholar]
- 49.Miller GD, Jenks MZ, Vendela M, Norris JL, Muday GK. Influence of Weight Loss, Body Composition, and Lifestyle Behaviors on Plasma Adipokines: A Randomized Weight Loss Trial in Older Men and Women with Symptomatic Knee Osteoarthritis. J Obes. 2012;2012. doi: 10.1155/2012/708505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, You T, Murphy K, Lyles MF, Nicklas BJ. Addition of Exercise Increases Plasma Adiponectin and Release from Adipose Tissue. Med Sci Sports Exerc. 2015;47(11):2450–5. doi: 10.1249/MSS.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas IC, McClelland RL, Michos ED, Allison MA, Forbang NI, Longstreth WT, Jr. et al. Density of calcium in the ascending thoracic aorta and risk of incident cardiovascular disease events. Atherosclerosis. 2017;265:190–6. doi: 10.1016/j.atherosclerosis.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


