Abstract
Background
Both trans-arterial radioembolization (TARE) and conventional trans-arterial chemoembolization (TACE) can effectively control hepatocellular carcinoma (HCC) in patients who are not suitable for curative resection. This study compared the effectiveness of TARE and conventional TACE as the initial trans-arterial treatment for hepatocellular carcinoma (HCC) assessed by tumor response and clinical outcomes.
Material and Methods
Data were retrospectively analyzed the propensity score-matched cohort for overall survival (OS), progression-free survival (PFS), and intrahepatic PFS in patients who have received TARE or TACE as the first HCC treatment from March 2012 to December 2017.
Results
A total of 138 patients initially treated with TARE (n = 54) or TACE (n = 84) was included in this study. Of 138 patients, median age was 59 years and the mean follow-up period was 27.6 months. TARE showed better OS (hazard ratio [HR] = 0.54, 95% confidence interval [CI] = 0.31–0.92, log-rank P = 0.02), better PFS (HR = 0.51, 95% CI = 0.36–0.97, log-rank P = 0.04), and better intrahepatic PFS (HR = 0.51, 95% CI = 0.30–0.88, log-rank P = 0.01) compared with TACE. TARE was an independent prognostic factor for OS (adjusted HR [aHR] = 0.52, 95% CI = 0.30–0.90, P = 0.02), PFS (aHR = 0.57, 95% CI = 0.35–0.94, P = 0.03), and intrahepatic PFS (aHR = 0.49, 95% CI = 0.28–0.84, P = 0.01).
Conclusion
TARE as initial trans-arterial treatment is associated with better clinical outcomes such as longer OS compared with TACE in patients with HCC.
Keywords: liver neoplasms, brachytherapy, yttrium radioisotopes, embolization
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers and is a major cause of death globally.1,2 HCC can be cured through hepatic resection, thermal ablation, and liver transplantation (LT). However, most patients already have advanced diseases such as multiple masses and portal vein invasion at the time of diagnosis. Furthermore, it is difficult to perform a resection due to the presence of portal hypertension, decreased liver function, proximity/invasion into adjacent vessels, and older age with comorbidities in these patients.3–6 Unfortunately, less than 30% of patients with HCC can receive curative surgery.7
Locoregional therapies are now used globally to help control disease in patients with advanced HCC.8–11 In randomized controlled trials (RCT), conventional trans-arterial chemoembolization (TACE) showed better survival rates compared with symptomatic treatment and has been widely used in patients with the intermediate stage (Barcelona Clinic Liver Cancer [BCLC]-B) not suitable for surgery.12,13 Recently, other trans-arterial treatments such as drug-eluting bead (DEB)-TACE and trans-arterial radioembolization (TARE) have been widely used.14,15 A retrospective study comparing TARE with TACE in HCC patients with T3 stage by the United Network for Organ Sharing (UNOS) found that patients in the TARE group demonstrated a higher partial response and a higher percentage of downstaging into the Milan criteria compared with TACE.5 Retrospective studies in patients with unresectable HCC found that TARE demonstrated better time to progression with less post-embolization syndrome and fewer complications versus TACE.16,17 Although TARE benefits better tolerability than TACE, recent guidelines only recommend TARE if HCC patients who are not eligible for TACE have PVTT or large tumor size invading two or more segments.8,9,11 Although TARE can strongly control the intrahepatic tumor, it is still not clear whether TARE is more effective than TACE in patients with HCC.5,18–22 Two meta-analyses including RCTs did not show a significant difference in OS between both treatments except the observational subgroup analysis.23,24 TARE is not recommended in the recent international guidelines for HCC despite its favorable tumor suppression and safety profile.8,9
In the present study, we aimed to compare clinical outcomes and safety between TARE and TACE as first-line locoregional therapy in propensity score (PS)-matched cohort patients with HCC. The primary outcome was OS, and secondary outcomes were progression-free survival (PFS) and intrahepatic PFS, and treatment-related toxicity.
Materials and Methods
Study Design
Data of the present retrospective cohort study were reviewed for consecutive patients diagnosed with HCC and initially treated with Y-90 TARE or TACE between March 2012 and December 2017 at Seoul National University Hospital (SNUH), and demographic and clinical data were collected. The study was supervised by the institutional review board of SNUH and the informed consent was waived due to the retrospective nature with maintained patient data confidentiality and compliance with the Declaration of Helsinki.
Patients
HCC diagnosis was based on two dynamic imaging studies (computed tomography [CT] or magnetic resonance imaging [MRI]) with malignant features according to the guidelines for HCC diagnosis.11 The index date was the closest imaging date (CT or MRI) from the date of the first treatment. Inclusion criteria were: (1) Age over 18 years old, (2) patients who were diagnosed HCC in SNUH, and (3) patients who were firstly treated with TARE or TACE. Exclusion criteria were: (1) patients who received sorafenib or other systemic treatment, (2) patients who had other malignancies within 2 years, (3) patients with decompensated liver cirrhosis who have hepatic encephalopathy, hepatorenal syndrome, jaundice, refractory ascites, and variceal bleeding, (4) patients with ECOG performance status of 3 or 4, and (5) patients with Child-Pugh classification C at the time of diagnosis. The decision for the treatment choice was determined according to physician preference after sufficient discussion with the patient and experienced interventional radiologists. All tumors including single and multifocal lesions were considered treatment. Adjuvant or following treatments were performed for remnant or recurred tumors. A patient who was received systemic treatment during follow-up period after TARE or TACE was censored from the systemic treatment start date.
Propensity Score Matching
PS matching was performed to reduce selection bias. Estimated PS was generated by including influencing factors such as age at diagnosis, sex, serum AFP, the diameter of index tumor ≥5cm, and Barcelona Clinic Liver Cancer (BCLC) stage. The nearest neighbor 1:2 matching method was used based on patients who received TARE. A matched cohort was created by matching patients treated with TARE and TACE using a caliper width of 0.2 of the standard deviation of the logit of the PS to directly compared the two groups.
Conventional Trans-Arterial Chemoembolization
Five fellowship-trained interventional radiologists with 2 to more than 20 years of experience performed the trans-arterial procedures. TACE was performed using a previously described method.25 Superselective TACE was routinely performed for all tumors. A mixture of 2–12 mL of iodized oil (Lipiodol; Andre Guerbet, Aulnay-sous-Bios, France) and 10–50 mg of doxorubicin hydrochloride (Adriamycin RDF; Ildong Pharmaceutical, Seoul, Korea) were selectively infused into the feeding vessels of the tumor depending on tumor size. 50 mg of doxorubicin hydrochloride was dissolved in 2.5 mL of non-ionic contrast media (Pamiray 250, DongKook Pharmaceutical, Seoul, Korea), and the volume ratio of iodized oil and doxorubicin solution was 4:1. Then, gelatin sponge particles (Gelfoam; Upjohn, Kalamazoo, Michigan, or Cutanplast; Mascia Brunelli, Milano, Italy) were administered into the feeding vessels, resulting in complete stasis of target vessels. Patients who received TACE treatment were hospitalized the day before the procedure and underwent basic laboratory tests and hydration without routine prophylactic antibiotics. After the procedure, patients rested overnight and were discharged the next day if there were no acute complications such as post-embolization syndrome. Further hospitalization and antibiotics were offered as needed. Lipiodol uptake was confirmed by non-contrast CT within 2 weeks after TACE.
Trans-Arterial Radioembolization
Two types of 90Y-loaded microspheres (20 μm to 60 μm resin-based [SIR-Sphere; Sirtex Medical Ltd, North Sydney, Australia] and 20 μm to 30 μm glass-based spheres [TheraSphere; BTG International, Ottawa, Ontario, Canada]) were used in the present study, and both were administered using dosimetry and infusion protocols as previously described.15,26 The target perfused tissue dose was ranged from 80 Gy to 360 Gy, and the boosted dose was defined as over 150 Gy. The boosted dose was considered when meeting the following criteria: (1) nodular tumor, (2) tumor size larger than 5 cm, and (3) BCLC stage A/B disease. Prior to the procedure, a lung shunt scan and simulation were performed. Lung shunt scan was evaluated by celiac-mesenteric angioscintigraphy with 99mTc macro aggregated albumin scanning. Ten of 54 patients (18.5%) had lung shunt fraction exciding 10% (range, 1.03–18.3%), but no radiation pneumonitis occurred. Thereafter, the simulation including right hepatic, celiac, and superior mesenteric arteriography was performed, and patients were treated within 1 to 2 weeks.
Response Evaluation, Outcomes, and Assessment of Toxicity
Patients were followed up at 3- to 6-month intervals with imaging such as CT or MRI. The response was assessed by the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria 2 to 3 months after the intervention.27 CT or MRI measurement of the relevant tumor was performed obtained in the arterial phase and did not include any major intervening areas of necrosis. Complete response (CR) was defined as the absence of any intra-tumoral arterial enhancement (viable target lesion) in all target lesions. The objective response rate (ORR) was defined as CR plus partial response (PR), and the disease control rate (DCR) was defined as the sum of CR, PR, and stable disease (SD). Death was confirmed using data from the Ministry of the Interior and Safety of South Korea. Toxicity was classified as clinical and laboratory and graded based on Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Subgroup analyses were performed regarding no extrahepatic disease, large tumor (≥5 cm), single tumor, and PVTT.
Statistical Analysis
Categorical variables were analyzed using the Fisher exact and the χ2 tests, and a continuous variable was analyzed using the Mann–Whitney U-test. PFS, intrahepatic PFS, and OS were calculated by the Kaplan–Meier survival analysis and evaluated by the Log rank test. Univariable and multivariable analyses were performed using the Cox regression method, significant prognostic factors from univariate analyses were analyzed using multivariable analyses to confirm their independence. All statistical analyses were performed using Stata/SE 14.0 (Statacorp LP, College Station, TX) and a statistically significant P value was accepted as less than 0.05.
Results
Patient Characteristics
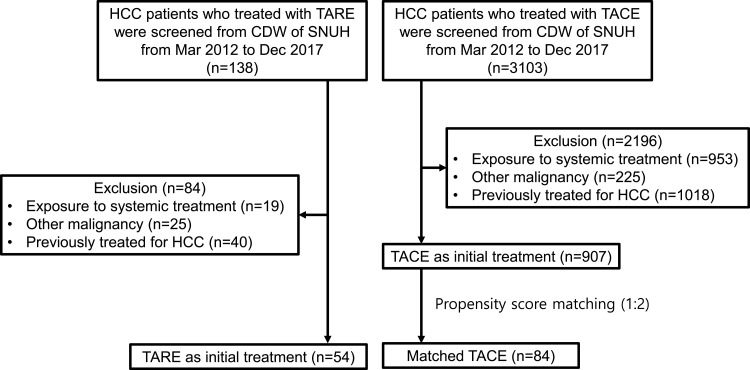
A total of 138 PS matched patients with HCC (TARE group n = 54; TACE group n = 84) were included in the present study (Figure 1). The median age was 59 years (range 33–87) with the majority of patients being male (83.3%) and all patients were Asians. There was no significant clinical difference in patient characteristics between both groups at the inclusion (Table 1). Hepatitis B virus (HBV) infection (70.3%) was the most frequent etiology in the entire cohort, but there was no significant difference in etiology between the two groups. An index tumor ≥5 cm occurred more frequently in the TARE group compared with the TACE group, but there was no significant difference between the two groups. The proportion of boosted dose in the TARE group was 22/54 (40.7%). The Eastern Cooperative Oncology Group (ECOG) performance status, Child-Pugh class, and tumor distribution were not significantly different between both groups. The TACE group had more PVTT than the TARE group, but there is no significant difference (27.8% in the TARE group vs 39.3% in the TACE group; P = 0.13). Various tumor stages were comparable and well balanced between the two groups. Barcelona Clinic Liver Cancer (BCLC) stages of patients in the present study were A, B, or C. Serum alpha-fetoprotein (AFP) and the number of patients who did not meet the Milan criteria at the time of diagnosis were not significantly different between both groups. Patient characteristics between the two groups in the present study were well balanced in all variables.
Figure 1.
CONSORT flow diagram of the study.
Abbreviations: CDW, clinical data warehouse; TACE, trans-arterial chemoembolization; TARE, trans-arterial radioembolization.
Table 1.
Patient Characteristics
| Total (n=138) | TARE (n=54) | TACE (n=84) | P value | SMD | |
|---|---|---|---|---|---|
| Age, median (range) | 59 (33–87) | 58 (33–83) | 60 (40–87) | 0.82 | −0.026 |
| Male, n (%) | 115 (83.3%) | 45 (83.3%) | 70 (83.3%) | 1.00 | 0.000 |
| Etiology, n (%) | 0.15 | −0.096 | |||
| HBV | 97 (70.3%) | 42 (77.8%) | 55 (65.5%) | ||
| HCV | 12 (8.7%) | 3 (5.6%) | 9 (10.7%) | ||
| Alcohol | 11 (8.0%) | 2 (3.7%) | 9 (10.7%) | ||
| HBV + HCV | 1 (0.7%) | 1 (1.8%) | 0 (0.0%) | ||
| HBV + Alcohol | 6 (4.4%) | 0 (0.0%) | 6 (7.1%) | ||
| HCV + Alcohol | 2 (1.4%) | 0 (0.0%) | 2 (2.4%) | ||
| HBV + HCV + Alcohol | 1 (0.7%) | 1 (1.8%) | 0 (0.0%) | ||
| Unknown | 8 (5.8%) | 5 (9.3%) | 3 (3.6%) | ||
| ECOG performance status, n (%) | 0.53 | −0.108 | |||
| 0 | 123 (89.1%) | 47 (87.0%) | 76 (90.5%) | ||
| 1 | 15 (10.9%) | 7 (13.0%) | 8 (9.5%) | ||
| Child-Pugh score, n (%) | 0.47 | 0.128 | |||
| A | 119 (86.2%) | 48 (88.9%) | 71 (84.5%) | ||
| B | 19 (13.8%) | 6 (11.1%) | 13 (15.5%) | ||
| Size ≥ 5cm*, n (%) | 110 (79.7%) | 45 (83.3%) | 65 (77.4%) | 0.40 | −0.149 |
| Tumor extent, n (%) | 0.53 | 0.108 | |||
| Unilobar | 85 (61.6%) | 35 (64.8%) | 50 (59.5%) | ||
| Bilobar | 53 (38.4%) | 19 (35.2%) | 34 (40.5%) | ||
| PVTT, n (%) | 48 (34.8%) | 15 (27.8%) | 33 (39.3%) | 0.13 | 0.270 |
| Vp1 | 1 (0.7%) | 0 (0%) | 1 (1.2%) | ||
| Vp2 | 11 (8.0%) | 5 (9.3%) | 6 (7.1%) | ||
| Vp3 | 24 (17.4%) | 7 (13.0%) | 17 (20.2%) | ||
| Vp4 | 12 (8.7%) | 3 (5.6%) | 9 (10.7%) | ||
| BCLC stage, n (%) | 0.64 | 0.070 | |||
| A | 35 (25.4%) | 13 (24.1%) | 22 (26.2%) | ||
| B | 48 (34.8%) | 22 (40.7%) | 26 (31.0%) | ||
| C | 55 (39.9%) | 19 (35.2%) | 36 (42.9%) | ||
| TNM stage, n (%) | 0.22 | 0.233 | |||
| IA | 2 (1.4%) | 1 (1.8%) | 1 (1.2%) | ||
| IB | 36 (26.1%) | 19 (35.2%) | 17 (20.2%) | ||
| II | 19 (13.8%) | 3 (5.6%) | 16 (19.0%) | ||
| IIIA | 39 (28.3%) | 16 (29.6%) | 23 (27.4%) | ||
| IIIB | 24 (17.4%) | 11 (20.4%) | 13 (15.5%) | ||
| IVA | 15 (10.9%) | 3 (5.6%) | 12 (14.3%) | ||
| IVB | 3 (2.2%) | 1 (1.8%) | 2 (2.4%) | ||
| HKLC stage, n (%) | 0.90 | −0.037 | |||
| I | 22 (15.9%) | 8 (14.8%) | 14 (16.7%) | ||
| IIa | 1 (0.7%) | 0 (0.0%) | 1 (1.2%) | ||
| IIb | 49 (35.5%) | 21 (38.9%) | 28 (33.3%) | ||
| IIIa | 5 (3.6%) | 3 (5.6%) | 2 (2.4%) | ||
| IIIb | 55 (39.9%) | 19 (35.2%) | 36 (42.9%) | ||
| IVa | 1 (0.7%) | 2 (3.7%) | 2 (2.4%) | ||
| Vb | 1 (0.7%) | 1 (1.8%) | 1 (1.2%) | ||
| Okuda stage, n (%) | 0.90 | 0.097 | |||
| I | 110 (79.7%) | 43 (79.6%) | 67 (79.8%) | ||
| II | 24 (17.4%) | 11 (20.4%) | 13 (15.5%) | ||
| III | 4 (2.9%) | 0 (0.0%) | 4 (4.8%) | ||
| AFP ≥ 200 ng/mL, n (%) | 46 (33.3%) | 17 (31.5%) | 29 (34.5%) | 0.71 | 0.065 |
| Beyond the Milan criteria, n (%) | 120 (87.0%) | 46 (85.2%) | 74 (88.1%) | 0.62 | 0.085 |
Note: *Size of primary index tumor.
Abbreviations: AFP, serum alpha-fetoprotein; ECOG, Eastern Cooperative Oncology Group; HKLC, Hong Kong Liver Cancer; PVTT, portal vein tumor thrombosis; TACE, trans-arterial chemoembolization; TARE, trans-arterial radioembolization; SMD, standardized mean difference; TNM, Tumor, nodes, metastasis.
Tumor Response
Follow-up imaging was performed within 1–3 months after the treatment. There was no significant difference between both groups (Supplementary Table S1). Of 137 patients, 75 (54.4%) and 42 (30.4%) patients experienced a favorable tumor CR (57.4% in TARE vs 52.4% in TACE; P = 0.61) and PR (29.6% in TARE vs 31.0% in TACE; P = 0.83), respectively (Table 2). The SD response was higher in the TARE group compared with the TACE group (9.3% vs 2.4%; P = 0.08) and the PD response was lower in the TARE group compared with the TACE group (3.7% vs 13.1%; P = 0.06) without statistical significance. There was no significant difference in CR and PR, and ORR was also not significantly different between the two groups. However, DCR was higher in the TARE group compared with the TACE group without statistical significance (96.3% vs 85.8%; P = 0.06). There was no significant difference for the proportion of adjuvant or following treatments between the two groups (P = 0.08; Supplementary Table S2).
Table 2.
Best Responses of Each Group
| Total (n=137) | TARE (n=54) | TACE (n=83) | P value | |
|---|---|---|---|---|
| Complete response, n (%) | 75 (54.4%) | 31 (57.4%) | 44 (52.4%) | 0.61 |
| Partial response, n (%) | 42 (30.4%) | 16 (29.6%) | 26 (31.0%) | 0.83 |
| Stable disease, n (%) | 7 (5.1%) | 5 (9.3%) | 2 (2.4%) | 0.08 |
| Progressive disease, n (%) | 13 (9.4%) | 2 (3.7%) | 11 (13.1%) | 0.06 |
| Objective response rate, % | 84.8% | 87.0% | 83.4% | 0.66 |
| Disease control rate, % | 89.9% | 96.3% | 85.8% | 0.06 |
Abbreviations: TACE, conventional trans-arterial chemoembolization; TARE, trans-arterial radioembolization.
Overall Survival
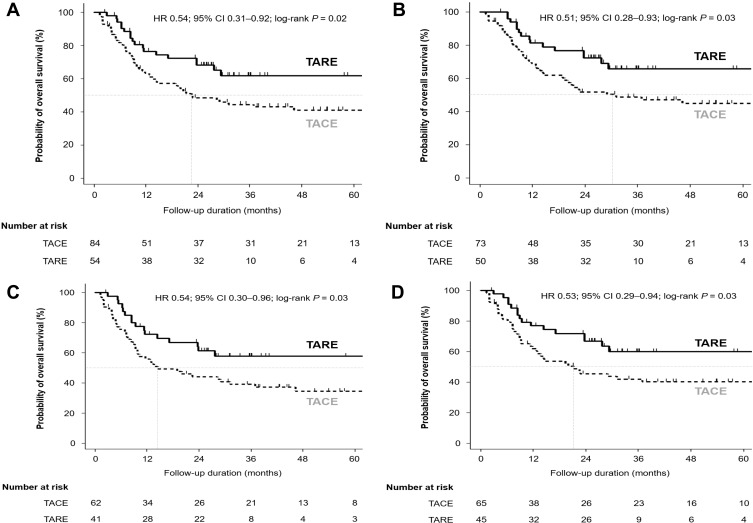
With a median follow-up of 27.6 months, 66 patients (TARE group n = 18 [33.3%]; TACE group n = 48 [57.1%]) died. The median overall survival in the TARE group was not reached and the median overall survival in the TACE group was 20.8 months. Patients in the TARE group (full population) showed significantly longer overall survival compared with the TACE group (hazard ratio [HR] = 0.54; 95% confidence interval [CI] = 0.31–0.92; log-rank P = 0.02; Figure 2A). In subgroup analyses, patients in the TARE group showed significantly better overall survival in patients without lymph node or distant metastases (n = 123 including 65 patients with T3 disease) than those in the TACE group (HR 0.51; 95% CI 0.28–0.93; log-rank P = 0.03; Figure 2B). In patients with BCLC stage B or C (n = 103), OS in the TARE group was significantly longer than those in the TACE group (HR = 0.54; 95% CI = 0.30–0.96; log-rank P = 0.03; Figure 2C). Patients in the TARE group whose largest tumor was ≥5 cm (n = 110) showed significantly longer OS compared with patients in the TACE group (HR = 0.53; 95% CI = 0.29–0.94; log-rank P = 0.03; Figure 2D). However, there was no statistical significance in OS between the two groups in patients with single HCC (n = 60) than TACE (HR = 0.47; 95% CI = 0.20–1.10; log-rank P = 0.08; Supplementary Figure S1A). In patients diagnosed with HCC with Vp3 or Vp4 PVTT (n = 36), there is no significant difference in OS between the two groups (HR = 0.44; 95% CI = 0.18–1.10; log-rank P = 0.07; Supplementary Figure S1B). After excluding 10 patients (6 TARE and 4 TACE) who were received any systemic treatment, OS in the TARE group was significantly longer than the TACE group (log-rank P = 0.02, Supplementary Figure S1C). In multivariable analyses, TARE was an independent prognostic factor for OS (adjusted HR [aHR] = 0.51; 95% CI = 0.30–0.89; P = 0.02; Table 3).
Figure 2.
Comparisons of OS between patients initially treated with TARE and TACE in (A) the entire study population (n = 138), (B) patients without lymph node and distant metastases (n = 123), (C) patients whose BCLC stage at diagnosis was stage B or C (n = 103), and (D) patients with tumor diameter ≥5 cm before the first treatment (n = 110).
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; TACE, trans-arterial chemoembolization; OS, overall survival; TARE, trans-arterial radioembolization.
Table 3.
Univariable and Multivariable Analyses for Overall Survival
| Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | aHR | 95% CI | P value | |
| Age ≥60 | 1.37 | 0.84–2.23 | 0.20 | |||
| Male | 0.44 | 0.25–0.78 | 0.005 | 0.40 | 0.23–0.72 | 0.002 |
| Cirrhosis | 1.50 | 0.78–2.86 | 0.22 | |||
| ECOG 0 | 0.64 | 0.31–1.35 | 0.25 | |||
| BCLC stage B or C | 2.32 | 1.18–4.54 | 0.02 | 1.37 | 0.66–2.83 | 0.30 |
| Largest tumor diameter ≥5cm | 1.22 | 0.65–2.29 | 0.53 | |||
| PVTT | 3.38 | 2.34–6.25 | <0.001 | 3.46 | 2.04–5.90 | <0.001 |
| TARE | 0.54 | 0.31–0.92 | 0.02 | 0.52 | 0.30–0.90 | 0.02 |
Note: *Adjusted for male, BCLC stage B or C and PVTT.
Abbreviations: aHR, adjusted HR; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; HR, hazard ratio; PVTT, portal vein tumor thrombosis; TARE, trans-arterial radioembolization.
Progression-Free Survival
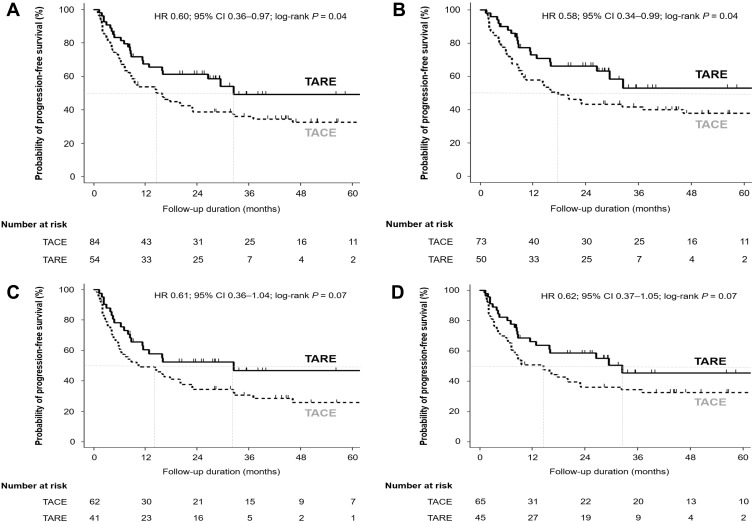
Patients in the TARE group showed significantly better PFS than those in the TACE group (HR = 0.60; 95% CI = 0.36–0.97; log-rank P = 0.04; Figure 3A). In subgroup analyses, patients in the TARE group without lymph node or distant metastases showed significantly better PFS compared with the TACE group (HR = 0.58; 95% CI = 0.34–0.99; log-rank P = 0.04; Figure 3B). However, there was no significant difference in PFS between the two groups in patients with BCLC stage B or C (HR = 0.61; 95% CI = 0.36–1.04; log-rank P = 0.07; Figure 3C). Patients in the TARE group whose largest tumor was ≥5 cm did not show significant difference in PFS compared with the TACE group (HR = 0.62; 95% CI = 0.37–1.05; log-rank P = 0.07; Figure 3D). In addition, patients with a single HCC in the TARE group did not show significant difference in PFS compared with the TACE group (HR = 0.46; 95% CI = 0.20–1.05; log-rank P = 0.06; Supplementary Figure S2A). However, in patients diagnosed with HCC with Vp3 or Vp4 PVTT (n=36), TARE showed significantly better PFS than TACE (HR = 0.39; 95% CI = 0.16–0.96; log-rank P = 0.03; Supplementary Figure S2B). In multivariable analyses, TARE was an independent prognostic factor for PFS (aHR = 0.58; 95% CI = 0.35–0.95; P = 0.03; Table 4).
Figure 3.
Comparisons of PFS between patients initially treated with TARE and TACE in (A) the entire study population (n = 138), (B) patients without lymph node and distant metastases (n = 123), (C) patients whose BCLC stage at diagnosis was stage B or C (n = 103), and (D) patients with tumor diameter ≥ 5 cm before the first treatment (n = 110).
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; TACE, trans-arterial chemoembolization; PFS, progression-free survival; TARE, trans-arterial radioembolization.
Table 4.
Univariable and Multivariable Analyses for Progression-Free Survival
| Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | aHR | 95% CI | P value | |
| Age ≥60 | 1.15 | 0.74–1.80 | 0.53 | |||
| Male | 0.57 | 0.33–0.99 | 0.05 | 0.52 | 0.30–0.92 | 0.02 |
| Cirrhosis | 1.07 | 0.62–1.85 | 0.81 | |||
| ECOG 0 | 0.64 | 0.29–1.42 | 0.27 | |||
| BCLC stage B or C | 0.66 | 0.33–1.32 | 0.24 | |||
| Largest tumor diameter ≥5cm | 2.16 | 1.19–3.93 | 0.01 | 1.57 | 0.88–2.82 | 0.13 |
| PVTT | 2.70 | 1.72–4.24 | <0.001 | 2.73 | 1.74–4.29 | <0.001 |
| TARE | 0.60 | 0.36–0.97 | 0.04 | 0.57 | 0.35–0.94 | 0.03 |
Note: *Adjusted for male, largest tumor diameter ≥5cm, and PVTT.
Abbreviations: aHR, adjusted HR; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; HR, hazard ratio; PVTT, portal vein tumor thrombosis; TARE, trans-arterial radioembolization.
Intrahepatic Progression-Free Survival
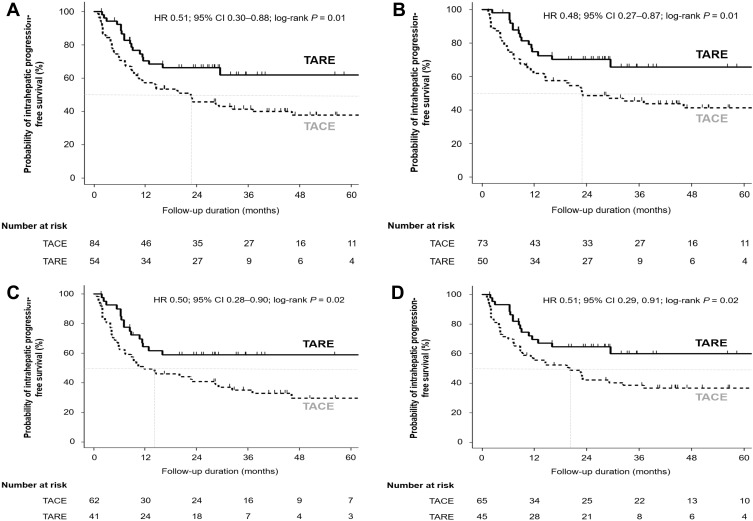
Patients in the TARE group showed significantly better intrahepatic PFS than those in the TACE group (HR = 0.51; 95% CI = 0.30–0.88; log-rank P = 0.01; Figure 4A). In subgroup analyses, patients in the TARE group showed significantly better intrahepatic PFS in patients without lymph node or distant metastases than those in the TACE group (HR = 0.48; 95% CI = 0.27–0.87; log-rank P = 0.01; Figure 4B). The TARE group showed significantly better intrahepatic PFS in patients with BCLC stage B or C than the TACE group (HR = 0.50; 95% CI = 0.28–0.90; log-rank P = 0.02; Figure 4C). In addition, TARE showed significantly better intrahepatic PFS in patients whose largest tumor was ≥5 cm compared with TACE (HR = 0.51; 95% CI = 0.29–0.91; log-rank P = 0.02; Figure 4D). In patients with a single HCC, there was no significant difference in intrahepatic PFS between the two groups (HR = 0.46; 95% CI = 0.20–1.08; log-rank P = 0.07; Supplementary Figure S3A). However, in patients who diagnosed HCC with Vp3 or Vp4 PVTT (n = 36), TARE showed significantly better intrahepatic PFS than TACE (HR = 0.40; 95% CI = 0.16–0.995; log-rank P = 0.04; Supplementary Figure S3B). In multivariable analyses, TARE was an independent prognostic factor for intrahepatic PFS (aHR = 0.49; 95% CI = 0.28–0.85; P = 0.01; Table 5).
Figure 4.
Comparisons of intrahepatic PFS between patients initially treated with TARE and TACE in (A) the entire study population (n = 138), (B) patients without lymph node and distant metastases (n = 123), (C) patients whose BCLC stage at diagnosis was stage B or C (n = 103), and (D) patients with tumor diameter ≥5 cm before the first treatment (n = 110).
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; TACE, trans-arterial chemoembolization; PFS, progression-free survival; TARE, trans-arterial radioembolization.
Table 5.
Univariable and Multivariable Analyses for Intrahepatic Progression-Free Survival
| Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | aHR | 95% CI | P value | |
| Age ≥60 | 1.33 | 0.82–2.14 | 0.24 | |||
| Male | 0.49 | 0.28–0.86 | 0.01 | 0.44 | 0.25–0.79 | 0.006 |
| Cirrhosis | 1.38 | 0.74–2.57 | 0.31 | |||
| ECOG 0 | 0.68 | 0.32–1.43 | 0.31 | |||
| BCLC stage B or C | 2.49 | 1.27–4.88 | 0.008 | 1.62 | 0.79–3.33 | 0.18 |
| Largest tumor diameter ≥5cm | 1.26 | 0.68–2.36 | 0.46 | |||
| PVTT | 3.44 | 2.13–5.59 | <0.001 | 2.96 | 1.77–4.96 | <0.001 |
| TARE | 0.51 | 0.30–0.88 | 0.02 | 0.49 | 0.28–0.84 | 0.01 |
Note: *Adjusted for male, BCLC stage B or C, and PVTT.
Abbreviations: aHR, adjusted HR; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; HR, hazard ratio; PVTT, portal vein tumor thrombosis; TARE, trans-arterial radioembolization.
Toxicity
Data for toxicities are presented in Supplementary Table 3. Fever/chill (60.7%) was the most frequently reported immediate symptom in the TACE group, and abdominal pain (16.7%) was the most frequently reported immediate symptom in the TARE group. Immediate low-grade (1 or 2) clinical toxicity was significantly more frequent in the TACE group than the TARE group (P < 0.001). However, high-grade (3 or 4) clinical toxicity was not significantly different between the two groups (P = 0.24). High grade (3 or 4) elevated transaminase and hyperbilirubinemia were more frequently observed in the TACE group than the TARE group without statistical significance (P = 0.08). Patients who had the MELD score of 10 or more were significantly frequent in the TACE group than those in the TARE group (P = 0.02).
Discussion
Results from the present PS-matched study in 138 HCC patients initially treated with TARE or TACE showed that initial TARE treatment achieved better median OS (not reached vs 20.8 months; P = 0.02) through a favorable intrahepatic tumor control (HR = 0.60; P = 0.01) than initial TACE treatment. TARE as an initial treatment for HCC was an independent prognostic factor for OS, PFS, and intrahepatic PFS (aHR = 0.51, 0.58, and 0.49, respectively). Furthermore, TARE showed significantly less low-grade (1 or 2) clinical toxicity (P < 0.001) and better hepatic function (P = 0.02) after the treatment than TACE in treatment-related adverse events. Although tumor response did not show a statistically significant difference between two groups, it should be considered that the tumor response can be underestimated considering the characteristics of persistent enhancement on CT in early period after TARE. The present study showed that TARE has an association with better clinical outcomes than TACE in patients newly diagnosed with HCC.
Treatment selection at the start of treatment is critical for tumor control. In addition, patients in the TARE group with a boosted dose were included in the present study. A recent retrospective study reported that TARE with a boosted dose in patients with large HCC showed a favorable tumor response.28 A recent subgroup analysis of the SARAH trial reported that high tumor absorbed dose was associated with better overall survival (14.1 months vs 6.1 months; P < 0.001) and tumor absorbed dose was significantly higher in patients with disease control than those with progressive disease (121 Gy vs 85 Gy; P = 0.02) in patients with inoperable locally advanced HCC. Future studies are warranted to elucidate the optimal radiation dose and indication of TARE to maximize the therapeutic effect.29 Therefore, TARE can be more suitable to effectively control HCC compared with TACE at the start of treatment, especially when treated with a boosted dose.
Although the current study includes patients with extrahepatic HCC lesions and/or PVTT, the TARE group demonstrated better OS and PFS, which can be explained by powerful intrahepatic tumor control. Particularly, the intrahepatic progression of HCC is a common cause of HCC-related death in Asia, trans-arterial therapies such as TACE or TARE are widely used in practice even if patients were initially diagnosed with HCC and PVTT or extrahepatic metastasis.30–33 Moreover, several Asian guidelines recommend TACE or TARE when patients have HCC with vascular invasion,11,34–36 and some studies supported that both TACE and TARE can be safely conducted in patients with PVTT.37–40 Therefore, TARE as initial treatment might affect the better clinical outcome when patients have more advanced disease at diagnosis.
Since TARE is a type of local radiotherapy, it can have systemic effects as conventional radiation therapy. Irradiated cells transfer signals to non-irradiated cells, which affects induction of p53 from non-irradiated cells or expression of various cytokines.41 These immune-modulating effects outside the field of radiotherapy are affected by the dose of radiation, the types of signals, and the activation of various innate immune cells. In addition, the irradiated tumor cells act as an immunogenic hub to affect the tumor microenvironment. Anti-tumor immune responses from the irradiated tumor cells can mediate an abscopal effect outside of the treatment field. TARE can trigger complex tissue responses that can systemically suppress tumor growth. However, these mechanisms are potential explanations. More studies are needed to prove the systemic effects of TARE in the future.
In the present study, TARE showed better intrahepatic PFS than TACE as initial treatment. A prospective randomized study also confirmed that the TARE group had a longer time to progression (TTP) than the TACE group.42 However, OS was comparable between the two groups in the prospective study, unlike our study. That study had an insufficient number of patients to analyze (TARE group n = 24; TACE group n = 21) and patients who received LT were censored in the study. In addition, patients in the TARE group had worse liver function (more Child-Pugh class B or C) before the treatment than those in the TACE group (58% in the TARE group vs 24% in the TACE group) and the proportion of preserved liver function evaluated by MELD score after the treatment was higher in the TARE group than the TACE group in the present study. It is well known that TARE has superior tolerability and toxicity compared with TACE.43 A systematic review for the cost-effectiveness of TARE reported that treatment is more cost-effective in patients with higher stages of HCC.44 Thus, the present study suggests that TARE may outperform TACE, and a randomized controlled study with large sample size is warranted to clarify this issue.
Previous systematic reviews comparing TARE to TACE failed to prove the superiority of TARE in OS. A meta-analysis of 3 randomized trials reported that TARE and TACE showed similar outcomes in unresectable HCC.23 Limitations of the study were that OS was not the main endpoint of all RCT, sample size was small with the high heterogeneity (45, 25, and 28 patients), and there was no data for adjuvant or following treatment. The other meta-analysis which included 9 observational studies and 2 RCTs reported that 2-year OS in TARE was significantly longer than TACE only in the observational subgroup.24 Limitations of that study were heterogeneity, a few RCTs, technical diversity among different centers, and the diversity of human ethnicity. On the other hand, the present study has strengths despite a retrospective study. 1) SNUH is the center performing large amounts of TARE and TACE in South Korea, which reduces the technical diversity and technicians were well experienced. 2) Superselective TACE and TARE were routinely performed to enhance therapeutic effect and reduce complications. 3) This study included patients treated with boosted dose TARE. It might be effective in large HCCs.28 4) Patients included in the study were all Asians. It can reduce heterogeneity from the diversity of human ethnicity. Thus, a randomized controlled study focused on OS with large sample size is warranted to clarify this issue.
Our study has several limitations. First, the current study is a retrospective study; however, we performed PS-matching to overcome the limitations of the retrospective design. No significant differences were observed in patient characteristics between the two groups. Second, standardized mean differences of some variables were over 0.1 in the patient characteristics (Table 1). Although it is generally accepted that a standardized difference of <0.1 is negligible, the cut-off is not universally established.45 Moreover, since the present study was focused on age, sex, serum AFP, the largest index tumor, and BCLC stage, differences in those variables were negligible. Therefore, patient characteristics between the two groups were considered as well matched.
In conclusion, patients initially diagnosed with HCC and underwent TARE as their first treatment had better OS, PFS, and intrahepatic PFS with less toxicity compared with TACE. In addition, TARE as an initial treatment for HCC was an independent prognostic factor for OS, PFS, and intrahepatic PFS. Thus, TARE is associated with better clinical outcomes as initial trans-arterial treatment compared with TACE in patients with HCC. To confirm these findings, a randomized controlled study with a large sample size is warranted.
Acknowledgments
A version of this study was presented as a poster presentation at the virtual meeting of the 30th Asian Pacific for The Study of Liver (APASL) 2021 (Bangkok, Thailand) held by APASL, of which abstract was published in Hepatology International (Hepatology International. 2021;15:J41).
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
AFP, serum alpha-fetoprotein; aHR, adjusted HR; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; HKLC, Hong Kong Liver Cancer; HR, hazard ratio; mUICC, modified the Union for International Cancer Control; OS, overall survival; PVTT, portal vein tumor thrombosis; TACE, chemoembolization; TARE, radioembolization; SMD, standardized mean difference; TNM, Tumor, nodes, metastasis.
Ethics Approval
This study was approved by the institutional review board of Seoul National University Hospital (H-1810-119-982).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors have no conflicts of interest to disclose.
References
- 1.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi: 10.1055/s-2007-1007122 [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. doi: 10.1055/s-0030-1247133 [DOI] [PubMed] [Google Scholar]
- 3.Kulik LM, Atassi B, van Holsbeeck L, et al. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94(7):572–586. doi: 10.1002/jso.20609 [DOI] [PubMed] [Google Scholar]
- 4.Lesurtel M, Mullhaupt B, Pestalozzi BC, Pfammatter T, Clavien PA. Transarterial chemoembolization as a bridge to liver transplantation for hepatocellular carcinoma: an evidence-based analysis. Am J Transplant. 2006;6(11):2644–2650. doi: 10.1111/j.1600-6143.2006.01509.x [DOI] [PubMed] [Google Scholar]
- 5.Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9(8):1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x [DOI] [PubMed] [Google Scholar]
- 6.Pung L, Ahmad M, Mueller K, et al. The role of cone-beam CT in transcatheter arterial chemoembolization for hepatocellular carcinoma: a systematic review and meta-analysis. J Vasc Interv Radiol. 2017;28(3):334–341. doi: 10.1016/j.jvir.2016.11.037 [DOI] [PubMed] [Google Scholar]
- 7.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2–S6. doi: 10.1097/MCG.0b013e3182872f29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 9.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 10.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi: 10.1007/s12072-017-9799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korean Liver Cancer Association, National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2019;20(7):1042–1113. doi: 10.3348/kjr.2019.0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X [DOI] [PubMed] [Google Scholar]
- 13.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi: 10.1053/jhep.2002.33156 [DOI] [PubMed] [Google Scholar]
- 14.Woo HY, Heo J. New perspectives on the management of hepatocellular carcinoma with portal vein thrombosis. Clin Mol Hepatol. 2015;21(2):115–121. doi: 10.3350/cmh.2015.21.2.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HC. Radioembolization for the treatment of hepatocellular carcinoma. Clin Mol Hepatol. 2017;23(2):109–114. doi: 10.3350/cmh.2017.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kooby DA, Egnatashvili V, Srinivasan S, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21(2):224–230. doi: 10.1016/j.jvir.2009.10.013 [DOI] [PubMed] [Google Scholar]
- 17.Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(2):497–507 e492. doi: 10.1053/j.gastro.2010.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(1):52–64. doi: 10.1053/j.gastro.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 19.Akinwande O, Philips P, Scoggins C, Martin RC. Radioembolization versus chemoembolization (DEBDOX) for the treatment of unresectable hepatocellular carcinoma: a propensity matched study. Anticancer Res. 2016;36(1):239–246. [PubMed] [Google Scholar]
- 20.Kim DY, Han KH. Transarterial chemoembolization versus transarterial radioembolization in hepatocellular carcinoma: optimization of selecting treatment modality. Hepatol Int. 2016;10(6):883–892. doi: 10.1007/s12072-016-9722-9 [DOI] [PubMed] [Google Scholar]
- 21.McDevitt JL, Alian A, Kapoor B, et al. Single-center comparison of overall survival and toxicities in patients with infiltrative hepatocellular carcinoma treated with yttrium-90 radioembolization or drug-eluting embolic transarterial chemoembolization. J Vasc Interv Radiol. 2017;28(10):1371–1377. doi: 10.1016/j.jvir.2017.05.017 [DOI] [PubMed] [Google Scholar]
- 22.Lobo L, Yakoub D, Picado O, et al. Unresectable hepatocellular carcinoma: radioembolization versus chemoembolization: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2016;39(11):1580–1588. doi: 10.1007/s00270-016-1426-y [DOI] [PubMed] [Google Scholar]
- 23.Casadei Gardini A, Tamburini E, Inarrairaegui M, Frassineti GL, Sangro B. Radioembolization versus chemoembolization for unresectable hepatocellular carcinoma: a meta-analysis of randomized trials. Onco Targets Ther. 2018;11:7315–7321. doi: 10.2147/OTT.S175715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Si T. Yttrium-90 transarterial radioembolization versus conventional transarterial chemoembolization for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Cancer Biol Med. 2018;15(3):299–310. doi: 10.20892/j.issn.2095-3941.2017.0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi JW, Kim HC, Lee JH, et al. Transarterial chemoembolization of hepatocellular carcinoma with segmental portal vein tumour thrombus. Eur Radiol. 2017;27(4):1448–1458. doi: 10.1007/s00330-016-4511-3 [DOI] [PubMed] [Google Scholar]
- 26.Kim HC, Kim YJ, Paeng JC, Chung JW. Yttrium-90 radioembolization of the right inferior phrenic artery in 20 patients with hepatocellular carcinoma. J Vasc Interv Radiol. 2018;29(4):556–563. doi: 10.1016/j.jvir.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 27.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 28.Kim HC, Kim YJ, Lee JH, Suh KS, Chung JW. Feasibility of boosted radioembolization for hepatocellular carcinoma larger than 5 cm. J Vasc Interv Radiol. 2019;30(1):1–8. doi: 10.1016/j.jvir.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 29.Hermann AL, Dieudonne A, Ronot M, et al. Relationship of tumor radiation-absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial radioembolization with (90)Y in the SARAH study. Radiology. 2020;296(3):673–684. doi: 10.1148/radiol.2020191606 [DOI] [PubMed] [Google Scholar]
- 30.Yoo DJ, Kim KM, Jin YJ, et al. Clinical outcome of 251 patients with extrahepatic metastasis at initial diagnosis of hepatocellular carcinoma: does transarterial chemoembolization improve survival in these patients? J Gastroenterol Hepatol. 2011;26(1):145–154. doi: 10.1111/j.1440-1746.2010.06341.x [DOI] [PubMed] [Google Scholar]
- 31.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HS. Management of patients with hepatocellular carcinoma and extrahepatic metastasis. Dig Dis. 2011;29(3):333–338. doi: 10.1159/000327572 [DOI] [PubMed] [Google Scholar]
- 33.Chan KM, Yu MC, Wu TJ, et al. Efficacy of surgical resection in management of isolated extrahepatic metastases of hepatocellular carcinoma. World J Gastroenterol. 2009;15(43):5481–5488. doi: 10.3748/wjg.15.5481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han KH, Kudo M, Ye SL, et al. Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology. 2011;81(Suppl 1):158–164. doi: 10.1159/000333280 [DOI] [PubMed] [Google Scholar]
- 35.Kokudo N, Takemura N, Hasegawa K, et al. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49(10):1109–1113. doi: 10.1111/hepr.13411 [DOI] [PubMed] [Google Scholar]
- 36.Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691–1700 e1693. doi: 10.1053/j.gastro.2014.02.032 [DOI] [PubMed] [Google Scholar]
- 37.Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology. 2011;258(2):627–634. doi: 10.1148/radiol.10101058 [DOI] [PubMed] [Google Scholar]
- 38.Cho YY, Lee M, Kim HC, et al. Radioembolization is a safe and effective treatment for hepatocellular carcinoma with portal vein thrombosis: a propensity score analysis. PLoS One. 2016;11(5):e0154986. doi: 10.1371/journal.pone.0154986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leng JJ, Xu YZ, Dong JH. Efficacy of transarterial chemoembolization for hepatocellular carcinoma with portal vein thrombosis: a meta-analysis. ANZ J Surg. 2016;86(10):816–820. doi: 10.1111/ans.12803 [DOI] [PubMed] [Google Scholar]
- 40.Xue TC, Xie XY, Zhang L, Yin X, Zhang BH, Ren ZG. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: a meta-analysis. BMC Gastroenterol. 2013;13:60. doi: 10.1186/1471-230X-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7):718–726. doi: 10.1016/S1470-2045(09)70082-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(6):1155–1163 e1152. doi: 10.1053/j.gastro.2016.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golfieri R, Bilbao JI, Carpanese L, et al. Comparison of the survival and tolerability of radioembolization in elderly vs. younger patients with unresectable hepatocellular carcinoma. J Hepatol. 2013;59(4):753–761. doi: 10.1016/j.jhep.2013.05.025 [DOI] [PubMed] [Google Scholar]
- 44.Rostambeigi N, Dekarske AS, Austin EE, Golzarian J, Cressman EN. Cost effectiveness of radioembolization compared with conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Vasc Interv Radiol. 2014;25(7):1075–1084. doi: 10.1016/j.jvir.2014.04.014 [DOI] [PubMed] [Google Scholar]
- 45.Deb S, Austin PC, Tu JV, et al. A review of propensity-score methods and their use in cardiovascular research. Can J Cardiol. 2016;32(2):259–265. doi: 10.1016/j.cjca.2015.05.015 [DOI] [PubMed] [Google Scholar]