Abstract
Objective
The current work aimed to examine the rates of and risk factors for mortality and readmission after heart failure (HF).
Setting
A systematic search was carried out in PubMed, the Cochrane Library, and EMBASE to identify eligible reports. The random-effects model was utilized to evaluate the pooled results.
Participants
A total of 27 studies with 515,238 participants were finally meta-analysed. The HF patients had an average age of 76.3 years, with 51% of the sample being male, in the pooled analysis.
Primary and Secondary Outcome Measures
The outcome measures were 30-day and 1-year readmission rates, mortality, and risk factors for readmission and mortality.
Results
The effect sizes for readmission and mortality were estimated as the mean and 95% confidence interval (CI). The estimated 30-day and 1-year all-cause readmission rates were 0.19 (95% CI 0.14–0.23) and 0.53 (95% CI 0.46–0.59), respectively, while the all-cause mortality rates were 0.14 (95% CI 0.10–0.18) and 0.29 (95% CI 0.25–0.33), respectively. Comorbidities were highly prevalent in individuals with HF.
Conclusion
Heart failure hospitalization is followed by high readmission and mortality rates.
Keywords: heart failure, meta-analysis, prevalence, readmission, mortality, hospitalization
Background
Heart failure (HF) represents a global public health threat. The effect of HF on the elderly population is disproportionate. Even assuming that the incidence for a specific age, sex, or ethnicity is stable, heart failure prevalence shows a steady elevation over the next 20 years,1 mainly in association with population ageing.2 Epidemiological changes in usual risk factors for heart failure may influence the above prediction. Even if the incidence for a specific age, sex, or race remains stable, the prevalence rates of hypertension and coronary heart disease would rise due to demographic changes.3 Meanwhile, the incidence rates of obesity4 and diabetes5 for specific ages, sexes, and races are also expected to increase, and the increased prevalence rates of these risk factors may further elevate the prevalence of HF. As a result, heart failure remains a substantial and growing public health burden. According to available data in Europe and the United States,2–6 the prevalence of heart failure ranges from 1% to 14%. When all adults are included, heart failure is considered a chronic debilitating disease regardless of age.2,5
In other developed countries, the one-year mortality rate after hospitalization for HF is 25%-30%,7 which is higher than those of many common cancers.8 The prevalence of heart failure increases with age.9,10 In addition, heart failure is the main cause of hospitalization in individuals over 64 years of age.11 The treatment of heart failure is important for improving the prognosis of patients and reducing health system expenditures.12 In addition, the in-hospital mortality rate of HF patients is relatively low; however, high rates of death and readmission are found after discharge.13
Readmission constitutes a common negative result for health care facilities and patients and a huge financial burden imposed on medical insurance beneficiaries and private payers.11 Decreased 30-day readmission and 30-day hospital mortality rates are weak but significantly correlated.14 From 2001–2003 to 2009–2011, the 30-day readmission rate following myocardial infarction declined from 20.5% to 15.8%, although the trend decreased slightly upon adjustment for patient features and treatments.15 Predicting the risk factors for and causes of 30-day rehospitalization would help optimize the allocation of meagre medical resources and design profitable and viable interventions.16,17
However, multiple previous trials have been conducted in single centres with few patients, with inconsistent readmission and mortality rates in heart failure patients. For instance, 30-day readmission rates for heart failure in previous reports ranged between 4.3% and 30.4%.18–28 Based on the above, further assessing the prevalence and potential causes of and risk factors for readmission is of prime importance. Therefore, this meta-analysis aimed to examine the prevalence of readmission after HF, as well as the potential risk factors for and causes of HF. In addition, we discussed potential intervention approaches for mitigating the risk of readmission after HF.
Methods
Search Strategy
Two independent medical librarians systematically searched three electronic databases, including PubMed, Web of Science, and the Cochrane Library. All articles published in English were obtained before May 26, 2019. The Keywords/terms were “Heart Failure”, “Patient Readmission” and “Mortality”.
Selection Criteria
Two investigators performed screening of all titles and abstracts in an independent fashion, retrieving and evaluating the retrieved studies based on full texts. In the final analysis, studies selected for inclusion must have provided data for 30-day and/or 1-year readmission or mortality in hospitalized individuals with HF. Studies were excluded for the following reasons: 1) Did not report risk factors or causes; 2) had already sub-grouped patients; 3) were cell culture or animal studies; 4) provided no available data; 5) had a small sample size (<100); and 6) was a systematic review, meta-analysis, case report or comments. In the case of patient cohort overlap, studies with the longest follow-up were included.
Data Extraction and Methodological Quality Assessment
To facilitate the data extraction process, two researchers generated a standardized form and independently extracted the data. From all eligible articles, the extracted information included the first author, year, country, study period, method of HF diagnosis and data source, study design, study population, sample size, demographic features, 30-day and 1-year readmission rates, mortality, and risk factors for readmission and mortality.
Study quality was evaluated based on the Critical Appraisal of the Health Research Literature29 taking into account the sample size, sample design, sampling frame, study and setting, measures, unbiased assessors, response rate and refusers, and prevalence rates. Each item was given a score. A study with a total score below 6 was considered to be of low quality; otherwise, it was considered to be of high quality (≥6).
Statistical Analysis
The estimated effect sizes for readmission and mortality are expressed as the mean and 95% confidence interval (CI). The random-effects model was used to pool 30-day and 1-year mortality or readmission rates across studies, as well as the mean age, sex, and comorbidities.30 Heterogeneity was assessed by I2 statistics. Subgroup analysis was carried out based on the region, study population, and study quality to determine the sources of heterogeneity. The median and interquartile range for age were converted to the mean and standard deviation (SD) as previously proposed.31 Inverse funnel plots were generated to visually assess publication bias. STATA/SE 15.1 was utilized for data analysis.
Patient and Public Involvement Statement
No patients were involved.
Results
Study Characteristics
In total, 2691 articles were reviewed for titles/abstracts, and 2609 articles were excluded. The remaining 27 reports were further assessed. The flow diagram of the study selection process is shown in Figure 1. We identified 27 studies included in this meta-analysis that reported 30-day and 1-year readmission data and mortality after HF,18–28,32–47 including 1 trial conducted in Japan,18 12 in the US,19,21,23–25,27,33,34,36,38,44,47 1 in Italy,20 2 in Spain,22,39 1 in India,40 1 in Singapore,41 1 in Romania,42 1 in France,26 1 in Australia,28 1 in Switzerland,32 1 in Korea,35 1 in Argentina,37 1 in South Africa,43 1 in Europe45 and 1 in England.46 The total number of participants was 515,238. Two articles reported both mortality and readmission rates, eleven reported mortality only, and fourteen assessed readmissions only. Ten and four studies were single centre and multicentre trials, respectively, and 13 assessed data from a large national database (Table 1). Twenty-one and 8 studies were of low and high quality, respectively (Tables S1 and S2).
Figure 1.
Flow diagram of the study.
Table 1.
Characteristics of Included Studies
| Author (Year) | Country | Study Period | Method of HF Diagnosis | Data Source | Study Type | Study Population |
|---|---|---|---|---|---|---|
| Aizawa H 201518 | Japan | 2012.4.1–2013.3.31 | ICD-10 | DPC database | Retrospective cohort study | ≥15 |
| Arenja N 201132 | Switzerland | 2001.5–2002.4, 2006.4–2007.3 | Two independent cardiologists | University Hospital of Basel | Prospective study | – |
| Babayan ZV 200333 | USA | 1996.1.1–1997.12.31 | Modified Framingham criteria | Johns Hopkins Hospital | Retrospective cohort | – |
| Bradford C 201619 | USA | 2008.10–2014.11 | ICD-9-CM | Sharp Memorial Hospital | Retrospective observational study | – |
| Chaudhry SI 201034 | USA | 1998.4–1999.3, 2000.7–2001.6 | ICD-9-CM | Medicare | – | – |
| Choi DJ 201135 | Korea | 2004.6–2009.4 | Framingham criteria | KorHF Registry database | – | – |
| Coles AH 201536 | USA | 1995, 2000, 2002, 2004, 2006 | Framingham criteria, ICD-9 | Massachusetts medical centers | – | – |
| Corrao G 201520 | Italy | 2011 | ICD-9 | HCU Databases | Retrospective cohort study | ≥50 |
| Costa D 201837 | Argentina | 2016.6.1–2017.5.31 | Framingham criteria | University Hospital in Buenos Aires | Prospective, observational study | – |
| Dai S 201621 | USA | – | – | Florida Hospital | Prospective study | 20~89 |
| Eapen ZJ 201338 | USA | 2005.1–2009.12 | ICD-9 | CMS | – | ≥65 |
| Fernandez-Gasso L 201722 | Spain | 2003–2013 | ICD-9 | Minimum Basic Set discharge registry | Retrospective observational study | – |
| Formiga F 201839 | Spain | 2012.1–2014.12 | Framingham criteria | Bellvitge University Hospital | – | >70 |
| Golas SB 201823 | USA | 2014.10~2015.9 | ICD-9-CM | PHS | Retrospective study | ≥18 |
| Harikrishnan S 201740 | India | 2013–2014 | European Society of HF | THFR | – | – |
| Leong KT 200741 | Singapore | 2003.11.10–2004.4.10 | Modified Framingham criteria | Changi General Hospital | Observational prospective study | – |
| Mavrea AM 201542 | Romania | 2013.1.1–2013.12.31 | LVEF | Timisoara City Hospital | Prospectively | – |
| McLaren DP 201624 | USA | 2007.1.1–2007.12.31 | ICD-9 | Rochester Medical Center | Retrospective | ≥18 |
| Mwita JC 201743 | South Africa | 2014.2–2015.2 | – | PMH | Observational study | ≥18 |
| Reynolds K 201544 | USA | 2008–2011 | ICD-9-CM | KPNW, Kaiser Permanente Georgia | Retrospective cohort | – |
| Rudiger A 200545 | European | 2001.12–2003.2 | Physicians | University Hospital of Zurich, Helsinki University Central Hospital | Prospective study | – |
| Siirila-Waris K 200646 | England | 2004.2.2–2004.5.30 | ESC AHF guideline criteria | Hospitals in Finland | Prospective multicenter study | – |
| Stampehl M 201947 | USA | 2010.1.1–2014.12.31 | ICD-9-CM | Medicare | Retrospective study | – |
| Sterling MR 201825 | USA | 2011–2015 | – | Vanderbilt University Medical Center | Prospective observational study | ≥18 |
| Tuppin P 201326 | France | 2009 | ICD-10 | SNIIRAM | – | – |
| Whittaker BD 201427 | USA | 2009.7.1–2010.6.30 | ICD-9 | Core Measures databases | Retrospective cohort study | ≥18 |
| Wiley JF 201728 | Australia | – | Cardiologist | Multicenter RCT | RCT | ≥18 |
Abbreviations: ICD-10, Codes of the 10th Revision of the International Statistical Classification of Diseases; DPC, Diagnosis Procedure Combination; ICD-9-CM, International Classification of Diseases-9th Revision-Clinical Modification codes; KorHF, Korean Heart Failure; ICD-9, International Classification of Diseases 9th Revision codes; HCU, Healthcare Utilization; CMS, Centers for Medicare and Medicaid Services; PHS, Partners Healthcare System; HF, Heart Failure; THFR, Trivandrum Heart Failure Registry; LVEF, Left Ventricular Ejection Fraction; PMH, Princess Marina Hospital; KPNW, Kaiser Permanente Northwest; ESC, European Society of Cardiology; AHF, Acute heart failure; SNIIRAM, National Health Insurance Information System; RCT, randomized controlled trial.
30-Day and 1-Year Readmission Rates
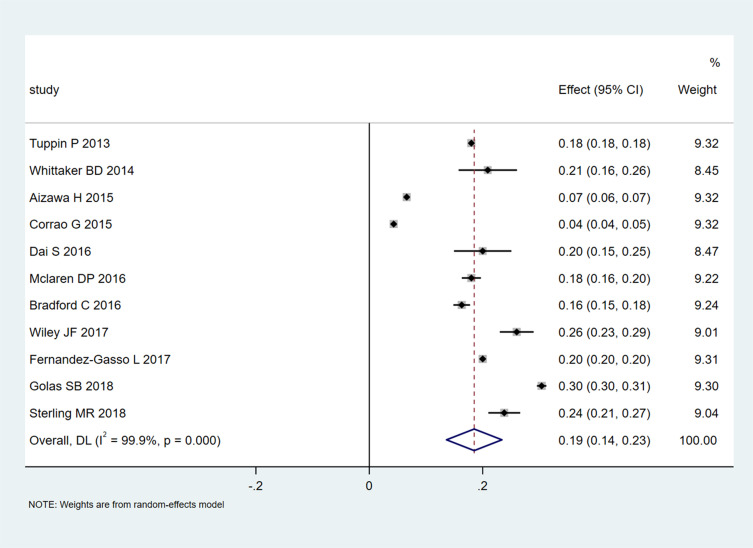
A pooled 30-day readmission rate of 0.19 (95% CI 0.14–0.23; Figure 2) was recorded in 11 studies that included 194,161 patients. Heterogeneity was extremely high (I2=99.9%, P<0.001), and the funnel plot displayed asymmetry. Then, the studies were grouped by region, sample size, and quality for the subgroup analysis (Table S2). The rate was reduced for the non-American region (0.15, 95% CI 0.08–0.21) compared with the American region (0.22, 95% CI, 0.15–0.28) at 30 days (Table S3). The 30-day readmission rates in studies with sample sizes<10,000 (0.21, 95% CI 0.18–0.24) and high quality (score ≥6; 0.22, 95% CI 0.16–0.27) were higher than those for trials with sample sizes>10,000 (0.16, 95% CI 0.09–0.23) and low quality (score<6; 0.17, 95% CI 0.11–0.23). For 1-year readmissions, the results were similar. The rate was lower for the non-American region (0.50, 95% CI 0.31–0.68) than for the American region (0.59, 95% CI 0.56–0.62, Table S4). However, the 1-year admission rate was lower in studies with sample sizes <10,000 (0.49, 95% CI 0.30–0.69) compared with those with sample sizes >10,000 (0.59, 95% CI 0.56–0.61). Only one study had a quality assessment score above 6.
Figure 2.
Meta-analysis of 30-day readmission rates.
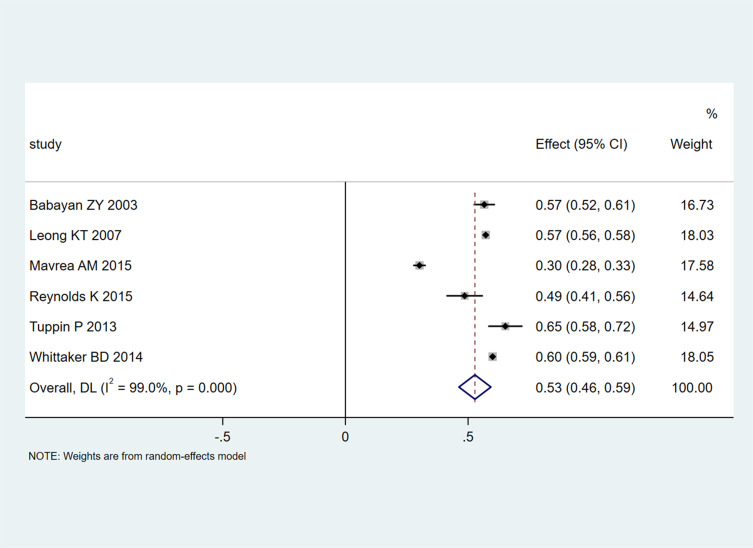
Six studies with 35,147 patients reported 1-year readmission rates. A pooled 1-year readmission rate of 0.53 (95% CI 0.46–0.59; Figure 3) was obtained, with significant heterogeneity among the trials (I2=99%, P<0.001).
Figure 3.
Meta-analysis of 1-year readmission rates.
Risk Factors for Readmission
Fourteen risk factors were revealed by ≥2 trials by multivariate analysis. Common comorbidities, including kidney disease, diabetes, chronic obstructive pulmonary disease (COPD), and cardiac arrhythmia, were tightly associated with elevated 30-day readmission rates. Table 2 depicts all the risk factors for readmission.
Table 2.
Risk Factors for Readmission
| Author (Year) | Age | Male | DM | HTN | IHD | CKD | AF | COPD | EF | HF Type | Beta- Blockers | ACEI/ARB | AA | Diuretics | Digoxin | LOS | 30-Day Readmission/ Total Patients | 1-Year Readmission/ Total Patients |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aizawa H 201518 | 36,313 (53.2) | 26,825 (39.3) | 38,292 (56.1) | 23,890 (35) | 53,309 (78.1) | 7850 (11.5) | 19 (Median) | 4479/68,257 (6.56) | ||||||||||
| Babayan ZY 200333 | 236 (47.87) | 199 (40.37) | 344 (69.78) | 96 (19.47) | 166 (33.67) | 279/493 (56.6) | ||||||||||||
| Bradford C 201619 | 72 | 1331 (55) | 721 (29.8) | 56 (2.3) | 1087 (44.9) | 1031 (42.6) | 394/2420 (16.28) | |||||||||||
| Corrao G 201520 | 79.3 (9.5) | 6103 (46.3) | CAD 2081 (15.8) | 886 (6.7) | 2441 (18.5) | RD 2459 (18.7) | 5537 (42) | 8739 (66.4) | 1163 (8.8) | 6334 (48.1) | 12.0 (10.3) | 566/13,171 (4.3) | 7534/13,171 (57.2) | |||||
| Dai S 201621 | 173 (72.1) | 129 (53.75) | 194 (80.83) | 165 (68.75) | 54 (22.5) | ≤40% | Decompensated HF | 233 (97.08) | 135 (56.25) | 121 (50.4) | 208 (86.6) | 48/240 (20) | ||||||
| Fernandez-Gasso L 201722 | 76.9 | 10,601 (43) | 814 (3.3) | 3156 (12.8) | 4938/24,654 (20) | |||||||||||||
| Golas SB 201823 | 75.7 | 6073 (52.8) | 2470 (21.46) | 4293 (37.3) | 3004 (26.1) | 4949 (43) | 4259 (37) | 6909 (60) | 3502/11,510 (30.4) | |||||||||
| Harikrishnan S 201740 | 61.2 (13.7) | 831 (69) | 662 (54.94) | 696 (57.76) | 866 (71.87) | 216 (17.93) | 177 (14.69) | 186 (15.44) | 333/1205 (30.2) | |||||||||
| Leong KT 200741 | 68.7 | 89 (51.4) | 87 (50.3) | 117 (67.6) | 81 (46.8) | 29 (16.5) | 72 (41.6) | 130 (75.1) | 63 (36.4) | 155 (89.6) | 33 (19.1) | 84/173 (48.55) | ||||||
| Mavrea AM 201542 | 64.6 | 98 (55) | 57 (32.02) | 136 (76.4) | CAD 108 (60.67) | CKD 87 (48.88) | 70 (39.33) | 44 (24.72) | HFpEF | 152 (85.39) | 129 (72.4) | 129 (72.4) | 116/178 (65.17) | |||||
| McLaren DP 201624 | 68.2 (15.6) | 1175 (59) | 714 (36) | 718 (36) | 784 (39) | 7.9 ± 15.2 | 366/1999 (18) | |||||||||||
| Reynolds K 201544 | 73.9 | 10,541 (52.9) | 9326 (46.8) | 17,077 (85.7) | CAD 9047 (45.4) | CKD 12415 (62.3) | 10,003 (50.2) | 9206 (46.2) | 9386 (47.1) | 2013 (10.1) | 11,956/19,927 (60) | |||||||
| Sterling MR 201825 | 60 | 477 (54) | 377 (44) | CAD 375 (43) | COPD 242 (27.4) | 40 (15, 60) | 210/883 (23.8) | |||||||||||
| Tuppin P 201326 | 78 | 33,580 (48) | 13,852 (19.8) | 6996 (1) | CAD 10704 (15.3) | 27,703 (39.6) | 39,176 (56) | 41,835(59.8) | 9 | 12,592/69,958 (18) | ||||||||
| Whittaker BD 201427 | 59 (17) | 148 (61.9) | 88 (36.8) | 117 (49) | CHD 90 (37.7) | 119 (49.8) | COPD 43 (18.0) | 119 (49.8) | 9.7 ± 14.9 | 50/239 (20.9) | ||||||||
| Wiley JF 201728 | 73 (13) | 540 (65) | 510 (61) | 590 (71) | CAD 494 (60) | RD 409 (49) | CHF | 216/830 (26) |
Abbreviations: DM, Diabetes Mellitus; HTN, Hypertension; IHD, Ischemic Heart Disease; CKD, Chronic Kidney Disease; AF, Atrial Fibrillation; COPD, Chronic Obstructive Pulmonary Disease; EF, Ejection Fraction; HF, Heart Failure; ACEI/ARB, Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers; AA, Aldosterone Antagonist; LOS, Length Of Stay; CAD, Coronary Artery Disease; RD, Respiratory Disease; HFpEF, Heart Failure with preserved Ejection Fraction; CHD, Coronary Heart Disease; CHF, Chronic Heart Failure.
30-Day and 1-Year Mortality Rates
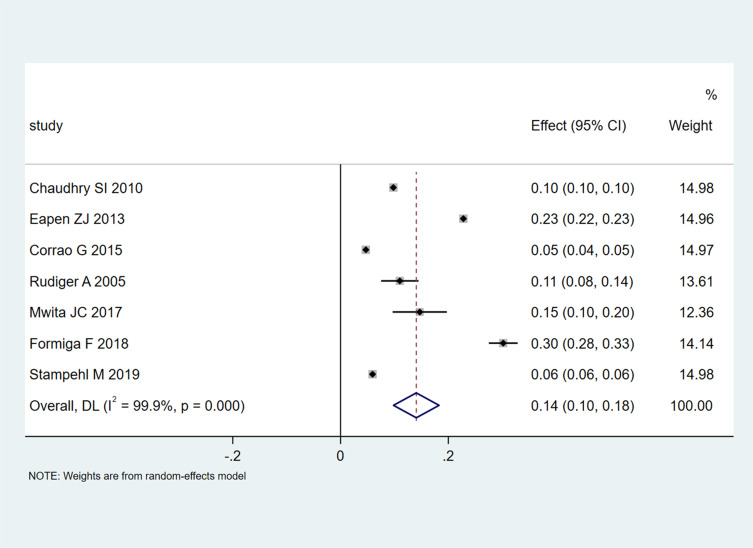
A pooled 30-day mortality rate of 0.14 (95% CI 0.10–0.18; Figure 4) in 7 studies that included 317,128 participants was found. Heterogeneity was extremely high (I2=99.9%, P<0.001), and the funnel plot showed asymmetry. The results of the subgroup analyses were not significantly different for 30-day mortality rates (Table S5). It is worth noting that heterogeneity for studies with a sample size<10,000 was low (I2=23%). The 1-year mortality rate for the non-American region (0.28, 95% CI 0.25–0.32) was reduced in comparison with the American rate (0.31, 95% CI 0.31–0.31; I2=0%) (Table S6).
Figure 4.
Meta-analysis of 30-day mortality rates.
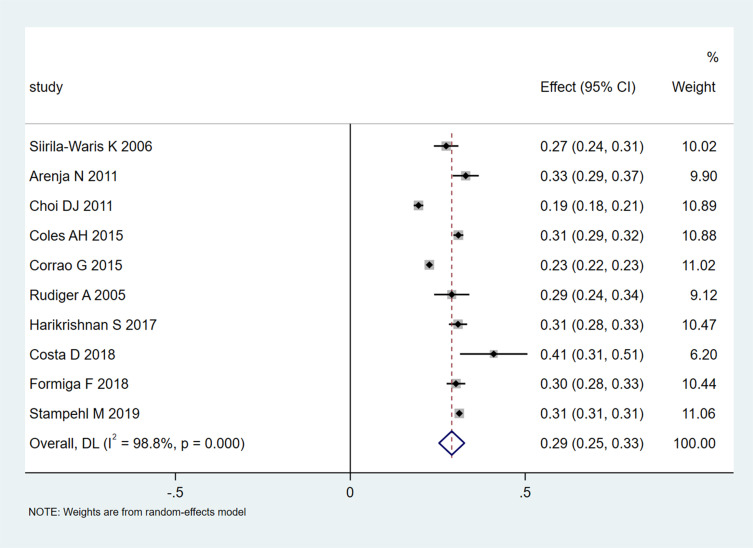
A pooled 1-year mortality rate of 0.29 (95% CI 0.25–0.33; Figure 5) was obtained in 10 studies that included 231,019 participants. Heterogeneity was extremely high (I2=98.8%, P<0.001), and the funnel plot showed asymmetry.
Figure 5.
Meta-analysis of 1-year mortality rates.
Risk Factors for Mortality
Fifteen risk factors were revealed by the multivariable analysis in 2 or more trials (Table 3). The risk factors for 30-day mortality included ischaemic heart disease (IHD) in 4 studies. The use of beta-blockers was positively correlated with elevated readmission rates in 3 trials. Meanwhile, a history of lung disease was negatively correlated with readmission in 3 trials.
Table 3.
Risk Factors for Mortality
| Author (Year) | Age | Male | DM | HTN | IHD | CKD | AF | CLRD | EF | HF Type | Beta- Blockers | ACEI/ARB | AA | Diuretics | Digoxin | LOS | COPD | 30-Day Mortality/ Total Patients | 1-Year Mortality/ Total Patients |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arenja N 201132 | 82 (median) | 330 (54) | 180 (30) | 430 (71) | – | 243 (39) | – | – | – | AHF | 357 (61) | 440 (76) | – | 475 (82) | Digitalis 51 (9) | – | 154 (25) | – | 201/610 (33) |
| Chaudhry SI 201034 | 79.6 (7.8) | 25,867 (41.5) | 24,745 (39.7) | 39,704 (63.7) | CAD35653 (57.2) | – | – | – | – | – | – | – | – | – | – | – | 21,379(34.3) | 6124/62,330 (9.8) | – |
| Choi DJ 201135 | 67.6 (14.3) | 1600 (50) | 975 (30.5) | 1486 (46.5) | 1544 (52.3) | 295 (9.2) | – | 104 (3.5) | 38.5±15.70 | – | 1109 (58.6) | 648 (53.7) | 913 (53.1) | 1982 (68.1) | Inotropic agents 711 (21.7) | – | 1289 (32.2) | – | 625/3200 (0.195) |
| Coles AH 201536 |
75 | 1771 (44) | 1493 (37.1) | 2874 (71.4) | CHD 2028 (50.4) | 1027 (25.5) | 1453 (36.1) | – | – | ADHF | 2290 (56.9) | 2228 (55.4) | 255 (6.34) | 3201 (79.5) | 1423 (35.4) | – | 403 (10) | – | 1245/4025 (30.9) |
| Corrao G 201520 | 79.3 (9.5) | 6103 (46.3) | – | – | CAD 2081 (15.8) | 886 (6.7) | Arrhythmia 2441 (18.5) | RD 2459(18.7) | – | – | 5537 (42) | 8739 (66.4) | 1163(8.8) | 6334 (48.1) | – | 12.0 (10.3) | – | 619/13,171 (4.7) | 2977/13,171 (22.6) |
| Costa 201837 | 77 (13.4) | 56 (56) | 36% | 78% | – | – | 33% | – | – | AHF | 60% | 63% | 24% | – | 3% | – | – | – | 41/100 (41) |
| Eapen ZJ 201338 | 80 (74, 86) | 15, 221 (45.6) | 13, 002 (39.7) | 24, 673 (75.3) | 20, 308 (60.9) | – | 11, 817 (36.1) | – | 43 (30, 55) | – | – | – | – | – | – | – | – | 7020/33,349 (22.8) | – |
| Formiga F 201839 | 81.6 | 484 (42.8) | 460 (40.6) | 978 (86.4) | CAD 267 (23.6) | 298 (26.3) | 444 (39.2) | – | – | AHF | 539 (47.6) | 586 (51.8) | 164 (14.5) | – | – | – | 267 (23.6) | 117/1132 (10.3) | 342/1132 (30.2) |
| Harikrishnan S 201740 | 61.2 (13.7) | 831 (69) | 662 | 696 | 866 | 216 | 177 | – | – | – | – | – | – | – | – | – | 186 | – | 371/1205 (0.308) |
| Mwita JC 201743 | 54.2 (17.1) | 104 (53.9) | 30 (15.5) | 106 (54.9) | 11 (5.7) | – | 19 (9.8) | – | 41.8 (20) | AHF | 124 (72.1) | 126 (73.2) | - | 148 (86) | 38 (22.1) | 9medium | - | 28/190 (14.7) | – |
| Rudiger A 200545 | 73 (12) | 176 (56.4) | 100 (32.1) | 78 (25) | – | 91 (29.2) | – | – | AHF | – | – | – | – | – | – | – | 34/312 (11) | 90/312 (29) | |
| Siirila- Waris K 200646 | 75.1 (10.4) | 312 (50.4) | 32.3 | 54.7 | CAD 55.2 | 9.4 | 29.4 | – | – | – | – | – | – | – | – | – | 12.6 | – | 170/620 (27.4) |
| Stampehl M 201947 | 80.5 (11.2) | 79, 076 (39.3) | 107, 540 (52.0) | 199, 439 (96.5) | 753 (0.4) | 102, 546 (49.6) | 113, 163 (54.8) | – | – | – | – | – | – | – | – | – | 92,688 (44.9) | 12,278/206,644 (5.94) | 64,363/206,644 (31.15) |
Abbreviations: DM, Diabetes Mellitus; HTN, Hypertension; IHD, Ischemic Heart Disease; CKD, Chronic Kidney Disease; AF, Atrial Fibrillation; CLRD, Chronic Lower Respiratory Disease; EF, Ejection Fraction; HF, Heart failure; ACEI/ARB, Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers; AA, Aldosterone Antagonist; LOS, Length of Stay; COPD, Chronic Obstructive Pulmonary Disease; AHF, Acute Heart Failure; CHD, Coronary Heart Disease; ADHF, Acute Decompensated Heart Failure; CAD, Coronary Artery Disease; RD, Respiratory Disease.
Heterogeneity Analysis
The results of this study may be more than expected based on chance alone, with a P ≤0.10 in the heterogeneity test. Given the potential statistical heterogeneity, we formed a hypothesis before conducting the above analysis, which may help explain the differences in the results: differences in intervention methods, such as telephone follow-up, home visits, and heart failure clinic visits, may explain the variability leading to the differences in the results.
Discussion
This was the first comprehensive systematic review and meta-analysis of 30-day and 1-year readmission and mortality rates following HF. Readmission and mortality mostly resulted from cardiac and noncardiac factors. Nonspecific chest pain was the top noncardiac cause of readmission, while cardiac factors encompassed angina and acute ischaemic heart disease, chest pain, etc. In addition, kidney disease, female sex, diabetes mellitus, chronic obstructive pulmonary disease (COPD), and HF were the major predictive factors of early readmission,48 which may provide a possible correct direction for reducing the readmission rate of HF patients. Among the 13,171 newly hospitalized HF patients, respiratory disease accounted for 18.7%, arrhythmia accounted for 18.5%, coronary/aortic disease accounted for 15.8%, and renal dysfunction accounted for 6.7%.20 The 3 most common reasons for readmission were HF (36.0%), renal disorders (8.4%), and other cardiac diseases (6.9%).19 In addition, comparing the baseline patients and clinical characteristics of the readmission and nonrehospitalization HF patient groups, COPD and renal disease accounted for the majority of readmissions.19
The results indicated that the 30-day readmission rate recorded in 11 studies was 0.19 (95% CI 0.14–0.23; Figure 2). Our findings corroborate the data from other nations. For instance, 30-day HF readmission rates after HF hospitalization in the USA and France are both 18%.24,26 Meanwhile, the 1-year HF readmission and 30-day mortality rates in South Africa are 14.7%.43 Most of the articles reported similar data for 1-year all-cause mortality, ie, 29%.32,34,36,38–40,43,45–47 The careful management of HF outpatients who are elderly, high disease severity, multiple comorbidities, or taking beta-blockers, loop diuretics, thiazide, or nitrates when discharged from the hospital may be critical to reducing the 30-day readmission.18
In some studies assessed in this meta-analysis, the authors identified multiple predictive factors of readmission, including age18,20,21 and clinical comorbidities.20,33,40–42 Some reports revealed multiple parameters that increased 30-day readmission rates, including elevated New York Heart Association functional class (NYHA) and Charlson Comorbidity Index (CCI) and treatment with beta-blockers, loop diuretics, thiazide, or nitrates.18 In addition, retired and/or disabled patients had one or more emergency room visits in the last 3 months, hospitalization durations above 5 days, and BUN levels >45 mg/dL at discharge.19 However, only one study reported that age, sex, race, marital status, payer type, and multiple patient features did not predict readmission in their model from Bradford et al.19
This study had limitations. First, due to limited data, the original causes or comorbidities of CHF patients are not yet clear. Second, multiple risk factors for and/or causes of readmission had no clear definitions, and various reports classified and grouped the causes and risk factors differently with variable definitions of the parameters, which were hardly combined for the meta-analysis. Finally, the studies were highly heterogeneous. Most reports had incomplete datasets, and subgroup analyses could not be performed for all variables.
Conclusions
We found that multiple diseases are very common in hospitalized chronic HF patients. In addition, the increase in recurrent diseases itself was shown to be parallel to an elevated all-cause 30-day readmission rate. This is a major problem for individuals and the health care system as a whole. Based on the present evidence of common comorbid disease clusters in chronic HF, the development and testing of new interventions tailored to patients in each cluster may be a key direction for future clinical trials.
Acknowledgments
Our work was funded by fundamental research funds for the National Center for Clinical Medicine of Digestive Diseases (No. 2015BAI13B07, to Dai-Ming Fan).
Data Sharing Statement
No additional data available.
Disclosure
The authors declare that they have no conflict of interest.
References
- 1.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circulation. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population 2014 to 2060. U.S. Department of Commerce: Economics and Statistics Administration: U.S. Census Bureau; 2014. [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5 [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein EA, Khavjou OA, Thompson H, et al. Medicine, obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42(6):563–570. doi: 10.1016/j.amepre.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 5.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8(1):29. doi: 10.1186/1478-7954-8-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265 [DOI] [PubMed] [Google Scholar]
- 8.Stewart S, Macintyre K, Hole DJ, Capewell S, McMurray JJV. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail. 2001;3(3):315–322. doi: 10.1016/S1388-9842(00)00141-0 [DOI] [PubMed] [Google Scholar]
- 9.Abhayaratna WP, Smith WT, Becker NG, Marwick TH, Jeffery IM, Mcgill DA. Prevalence of heart failure and systolic ventricular dysfunction in older Australians: the Canberra Heart Study. Med J Aust. 2006;184:151–154. [DOI] [PubMed] [Google Scholar]
- 10.Sahle BW, Owen AJ, Mutowo MP, Krum H, Reid CM. Prevalence of heart failure in Australia: a systematic review. BMC Cardiovasc Disord. 2016;16(1):32. doi: 10.1186/s12872-016-0208-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. J Vasc Surg. 2009;50(1):234. doi: 10.1016/j.jvs.2009.05.045 [DOI] [PubMed] [Google Scholar]
- 12.Giamouzis G, Kalogeropoulos A, Georgiopoulou V, et al. Hospitalization epidemic in patients with heart failure: risk factors, risk prediction, knowledge gaps, and future directions. J Card Fail. 2011;17(1):0–75. doi: 10.1016/j.cardfail.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 13.Ambrosy AP, Fonarow GC, Butler J, Chioncel O. The global health and economic burden of hospitalizations for heart failure lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 14.Dharmarajan K, Wang Y, Lin Zet al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. 2017;318:270–278. doi: 10.1001/jama.2017.8444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen HY, Tisminetzky M, Lapane KL, et al. Disease, decade‐long trends in 30‐day rehospitalization rates after acute myocardial infarction. J Am Heart Assoc. 2015;4:e002291. doi: 10.1161/JAHA.115.002291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amarasingham R, Patel PC, Toto K, et al. Allocating scarce resources in real-time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf. 2013;22(12):998–1005. doi: 10.1136/bmjqs-2013-001901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amarasingham R, Moore BJ, Tabak YP, et al. An automated model to identify heart failure patients at risk for 30-day readmission or death using electronic medical record data. Med Care. 2010;48:981–988. [DOI] [PubMed] [Google Scholar]
- 18.Aizawa H, Imai S, Fushimi K. Factors associated with 30-day readmission of patients with heart failure from a Japanese administrative database. BMC Cardiovasc Disord. 2015;15(1):134. doi: 10.1186/s12872-015-0127-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradford C, Shah BM, Shane P, Wachi N, Sahota K. Patient and clinical characteristics that heighten risk for heart failure readmission. Res Social Adm Pharm. 2017;13(6):1070–1081. doi: 10.1016/j.sapharm.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 20.Corrao G, Ghirardi A, Ibrahim B, Merlino L, Maggioni AP. Short- and long-term mortality and hospital readmissions among patients with new hospitalization for heart failure: a population-based investigation from Italy. Int J Cardiol. 2015;181:81–87. doi: 10.1016/j.ijcard.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 21.Dai S, Manoucheri M, Gui J, et al. Kansas City cardiomyopathy questionnaire utility in prediction of 30-day readmission rate in patients with chronic heart failure. Cardiol Res Pract. 2016;2016:4571201. doi: 10.1155/2016/4571201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Gasso L, Hernando-Arizaleta L, Palomar-Rodriguez JA, Abellan-Perez MV, Pascual-Figal DA. Trends, causes and timing of 30-day readmissions after hospitalization for heart failure: 11-year population-based analysis with linked data. Int J Cardiol. 2017;248:246–251. doi: 10.1016/j.ijcard.2017.07.094 [DOI] [PubMed] [Google Scholar]
- 23.Golas SB, Shibahara T, Agboola S, et al. A machine learning model to predict the risk of 30-day readmissions in patients with heart failure: a retrospective analysis of electronic medical records data. BMC Med Inform Decis Mak. 2018;18(1):44. doi: 10.1186/s12911-018-0620-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLaren DP, Jones R, Plotnik R, et al. Prior hospital admission predicts thirty-day hospital readmission for heart failure patients. Cardiol J. 2016;23(2):155–162. doi: 10.5603/CJ.a2016.0005 [DOI] [PubMed] [Google Scholar]
- 25.Sterling MR, Safford MM, Goggins K, et al. Numeracy, health literacy, cognition, and 30-day readmissions among patients with heart failure. J Hosp Med. 2018;13:145–151. doi: 10.12788/jhm.2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuppin P, Cuerq A, de Peretti C, et al. First hospitalization for heart failure in France in 2009: patient characteristics and 30-day follow-up. Arch Cardiovasc Dis. 2013;106:570–585. doi: 10.1016/j.acvd.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 27.Whittaker BD, Soine LA, Errico KM. Patient and process factors associated with all-cause 30-day readmission among patients with heart failure. J Am Assoc Nurse Pract. 2015;27:105–113. doi: 10.1002/2327-6924.12123 [DOI] [PubMed] [Google Scholar]
- 28.Wiley JF, Chan YK, Ahamed Y, et al. Multimorbidity and the risk of all-cause 30-day readmission in the setting of multidisciplinary management of chronic heart failure: a retrospective analysis of 830 hospitalized patients in Australia. J Cardiovasc Nurs. 2018;33:437–445. doi: 10.1097/JCN.0000000000000391 [DOI] [PubMed] [Google Scholar]
- 29.Loney PL, Chambers LW, Bennett KJ, Roberts JG. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. 1998;19:170–176. [PubMed] [Google Scholar]
- 30.Nyaga VN, Arbyn M. Metaprop: a stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):1–3. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arenja N, Breidthardt T, Socrates T, et al. Risk stratification for 1-year mortality in acute heart failure: classification and regression tree analysis. Swiss Med Wkly. 2011;141:w13259. doi: 10.4414/smw.2011.13259 [DOI] [PubMed] [Google Scholar]
- 33.Babayan ZV, McNamara RL, Nagajothi N, et al. Predictors of cause-specific hospital readmission in patients with heart failure. Clin Cardiol. 2003;26:411–418. doi: 10.1002/clc.4960260906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhry SI, Wang Y, Gill TM, Krumholz HM. Geriatric conditions and subsequent mortality in older patients with heart failure. J Am Coll Cardiol. 2010;55:309–316. doi: 10.1016/j.jacc.2009.07.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi DJ, Han S, Jeon ES, et al. Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: a report from the Korean heart failure registry. Korean Circ J. 2011;41:363–371. doi: 10.4070/kcj.2011.41.7.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coles AH, Tisminetzky M, Yarzebski J, et al. Magnitude of and prognostic factors associated with 1-year mortality after hospital discharge for acute decompensated heart failure based on ejection fraction findings. J Am Heart Assoc. 2015;4. doi: 10.1161/JAHA.115.002303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa D, Aladio M, Girado CA, de la Hoz RP, Berensztein CS. Frailty is independently associated with 1-year mortality after hospitalization for acute heart failure. Int J Cardiol Heart Vasc. 2018;21:103–106. doi: 10.1016/j.ijcha.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eapen ZJ, Liang L, Fonarow GC, et al. Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. JACC Heart Fail. 2013;1:245–251. doi: 10.1016/j.jchf.2013.01.008 [DOI] [PubMed] [Google Scholar]
- 39.Formiga F, Moreno-Gonzalez R, Chivite D, et al. Sex differences in 1-year mortality risks in older patients experiencing a first acute heart failure hospitalization. Geriatr Gerontol Int. 2019;19:184–188. [DOI] [PubMed] [Google Scholar]
- 40.Harikrishnan S, Sanjay G, Agarwal A, et al. One-year mortality outcomes and hospital readmissions of patients admitted with acute heart failure: data from the trivandrum heart failure registry in Kerala, India. Am Heart J. 2017;189:193–199. doi: 10.1016/j.ahj.2017.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leong KT, Goh PP, Chang BC, Lingamanaicker J. Heart failure cohort in Singapore with defined criteria: clinical characteristics and prognosis in a multi-ethnic hospital-based cohort in Singapore. Singapore Med J. 2007;48:408–414. [PubMed] [Google Scholar]
- 42.Mavrea AM, Dragomir T, Bordejevic DA, Tomescu MC, Ancusa O, Marincu I. Causes and predictors of hospital readmissions in patients older than 65 years hospitalized for heart failure with preserved left ventricular ejection fraction in western Romania. Clin Interv Aging. 2015;10:979–990. doi: 10.2147/CIA.S83750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mwita JC, Dewhurst MJ, Magafu MG, et al. Presentation and mortality of patients hospitalised with acute heart failure in Botswana. Cardiovasc J Afr. 2017;28:112–117. doi: 10.5830/CVJA-2016-067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds K, Butler MG, Kimes TM, Rosales AG, Chan W, Nichols GA. Relation of acute heart failure hospital length of stay to subsequent readmission and all-cause mortality. Am J Cardiol. 2015;116:400–405. doi: 10.1016/j.amjcard.2015.04.052 [DOI] [PubMed] [Google Scholar]
- 45.Rudiger A, Harjola VP, Muller A, et al. Acute heart failure: clinical presentation, one-year mortality and prognostic factors. Eur J Heart Fail. 2005;7:662–670. doi: 10.1016/j.ejheart.2005.01.014 [DOI] [PubMed] [Google Scholar]
- 46.Siirila-Waris K, Lassus J, Melin J, Peuhkurinen K, Nieminen MS, Harjola VP. Characteristics, outcomes, and predictors of 1-year mortality in patients hospitalized for acute heart failure. Eur Heart J. 2006;27:3011–3017. doi: 10.1093/eurheartj/ehl407 [DOI] [PubMed] [Google Scholar]
- 47.Stampehl M, Friedman HS, Navaratnam P, Russo P, Park S, Obi EN. Risk assessment of post-discharge mortality among recently hospitalized medicare heart failure patients with reduced or preserved ejection fraction. Curr Med Res Opin. 2019;36(2):179–188. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Zhao T, Wei X, Lu H, Lin X. The prevalence of 30-day readmission after acute myocardial infarction: a systematic review and meta-analysis. Clin Cardiol. 2019;42(10):889–898. doi: 10.1002/clc.23238 [DOI] [PMC free article] [PubMed] [Google Scholar]