Abstract
Purpose:
To create a viable, mechanically expanded autograft long head biceps tendon (LHBT) scaffold for biologically augmenting the repair of torn rotator cuffs.
Methods:
The proximal aspect of the tenotomized LHBTs were harvested from patients during rotator cuff repair surgery and were mechanically formed into porous scaffolds using a surgical graft expander. LHBT scaffolds were evaluated for change in area, tensile properties, and tenocyte viability before and after expansion. The ability of endogenous tenocytes derived from the LHBT scaffold to promote tenogenic differentiation of human adipose derived mesenchymal stromal cells (ADMSCs) was also determined.
Results:
Autograft LHBTs were successfully expanded using a modified surgical graft expander to create a porous scaffold containing viable resident tenoctyes from patients undergoing rotator cuff repair. LHBT scaffolds had significantly increased area (length: 24.91mm [13.91, 35.90] x width: 22.69mm [10.87, 34.50]; p=0.011) compared to the native LHBT tendon (length: 27.16mm [20.70, 33.62] x width: 6.68mm [5.62, 7.74]). The structural properties of the autograft were altered including the ultimate tensile strength (LHBT scaffold: 0.56MPa [0.06, 1.06] vs. native LHBT: 2.35MPa [1.36, 3.33]; p=0.002) and tensile modulus (LHBT scaffold: 4.72MPa [−0.80, 10.24] vs. native LHBT: 37.17MPa [24.56, 49.78]; p=0.001). There was also a reduction in resident tenocyte percent viability (LHBT scaffold: 38.52% [17.94, 59.09] vs. native LHBT: 68.87% [63.67, 74.37]; p=0.004). Tenocytes derived from the LHBT scaffold produced soluble signals that initiated ADMSC differentiation into an immature tenocyte-like phenotype, as indicated by an 8.7x increase in scleraxis (p=0.040) and a 3.6x increase in collagen type III mRNA expression (p=0.050) compared to undifferentiated ADMSC controls.
Conclusions:
The ability to produce a viable autologous scaffold from the proximal biceps tendon having dimensions, porosity, mechanical characteristics, native ECM components, and viable tenocytes that produce bioactive signals conducive to supporting the biologic augmentation of rotator cuff repair surgery has been demonstrated.
Keywords: Biceps Tendon, Autograft, Rotator Cuff Repair, Biologic Augmentation, Tissue Engineering, Scaffolds, Mesenchymal Stromal Cells
INTRODUCTION
It is estimated that two million people seek medical attention each year for rotator cuff pathology in the United States and approximately 460,000 undergo surgery.1 Over the last 20 years, there have been numerous strides in surgical fixation techniques with introduction of the double row and suture bridge techniques that give greater load to failure and higher footprint contact pressure.2–5 Despite these advances, Galatz et al. found a 94% retear rate after surgery for large and massive rotator cuff tendon tears in their series of patients between 1997 and 2000.6 Current studies still demonstrate re-tear rates of approximately 20% for all tears and over 40% in massive tears.7–9 The retear rate remains unacceptably high despite the advances in mechanical stability at the time of repair.
Rather than insufficient mechanical stability, the damaged tendon may be biologically insufficient for healing and biologic augmentation may be necessary.10,11 Biologic augmentation during rotator cuff repair surgery has been recently popular, and various products have been proposed. Extracellular matrix (ECM)-based acellular grafts have been proposed to provide structural support while allowing tissue in-growth.12 Autologous protein solutions, such as platelet rich plasma, have been proposed to promote cell-mediated regeneration.13–17 However, limitations remain with these approaches. The grafts do not provide the microarchitecture of the native tendon, may elicit immune reactions, and do not contain viable cells which could contribute to tissue regeneration.18,19 Protein solutions often contain anabolic and catabolic growth factors that have short half-lives, providing ‘mixed signals’ to local cells and thus may not significantly improve clinical outcomes.17 Engineered tendon constructs composed of scaffolds,14,20–22 alternative cell sources (e.g. mesenchymal stromal cells; MSCs),23–25 and specific growth factors have demonstrated more promise. However, the clinical benefits of these expensive modalities remains equivocal in the current literature.26–29
Ideal biologic augmentation for rotator cuff tendon repair would provide favorable micro-architectural cues and biologic signals while not eliciting a severe immune response. While commercially available scaffolds may meet these criteria, their cost remains a limitation. Biceps tendonitis is often concurrently treated at the time of rotator cuff repair. With its long length, the proximal aspect of the LHBT can be harvested as a free graft with an arthroscopic or open technique. The distal aspect of the tendon can be used for tenodesis per surgeon discretion. The harvested proximal LHBT can provide a “no-cost”, technically straightforward, readily available graft that does not trigger an immune response and possesses the desired highly organized parallel-oriented collagen architecture. Depending on the clinical needs, the graft could also serve as a carrier for supplemented MSCs for use in tears with a higher risk of post-operative retear.
Ideally, grafts or scaffolds used to augment rotator cuff repair would cover the entirety of the diseased tendon. The LHBT graft at its original size would only cover smaller tears. Since many of the tears at high risk are larger, the LHBT graft needs to be enlarged to fit the size of the defect. The purpose of this study was to create a viable, mechanically expanded autograft long head biceps tendon (LHBT) scaffold for biologically augmenting the repair of torn rotator cuffs. A skin graft mesher was used to mechanically expand the LHBT similarly to how it would be used during a skin grafting procedure. The specific objectives of the studies herein were to determine: (1) the ability to form an expanded porous scaffold using LHBT, (2) the effect of the meshing process on the mechanical properties and tenocyte viability of the LHBT scaffold, (3) the ability of soluble mediators released by tenocytes derived from LHBT scaffolds to induce tenogenic differentiation of adipose derived MSCs (ADMSCs), and (4) the ability of scaffolds to support ADMSCs alignment and acquisition of a tenocyte-like morphology. It was hypothesized that a novel low morbidity, reproducible autograft scaffold that supports MSC differentiation could be created by mechanically expanding LHBT from patients undergoing rotator cuff repair.
METHODS
LHBT SCAFFOLD FORMATION
To complete objective 1, a single human cadaveric LHBT specimen was harvested and cut perpendicular to the long axis of the tendon yielding 25mm segments (n=4) (Figure 1). The LHBT segments were longitudinally bisected with a scalpel to near full diameter to “butterfly” the graft (Figure 1). The butterflied segments were then placed on a 1.1mm thick polymer carrier tray and perforated using a skin graft mesher (Zimmer Biomet, Warsaw, Indiana) with a 2:1 expansion ratio cutter. Average length, width, and thickness of each graft was determined by measuring at three different locations along the grafts using a digital caliper having a resolution of a one-hundredth of a millimeter. Measurements were taken prior to butterflying, after butterflying, and after meshing the tendon to determine how dimensions changed with each step. Area was calculated as length x width. Percent change in area was calculated using the following equation: (final area - initial area) / initial dimensions x 100.
Figure 1. Long head biceps tendons can be formed into porous scaffolds using a surgical graft expander.
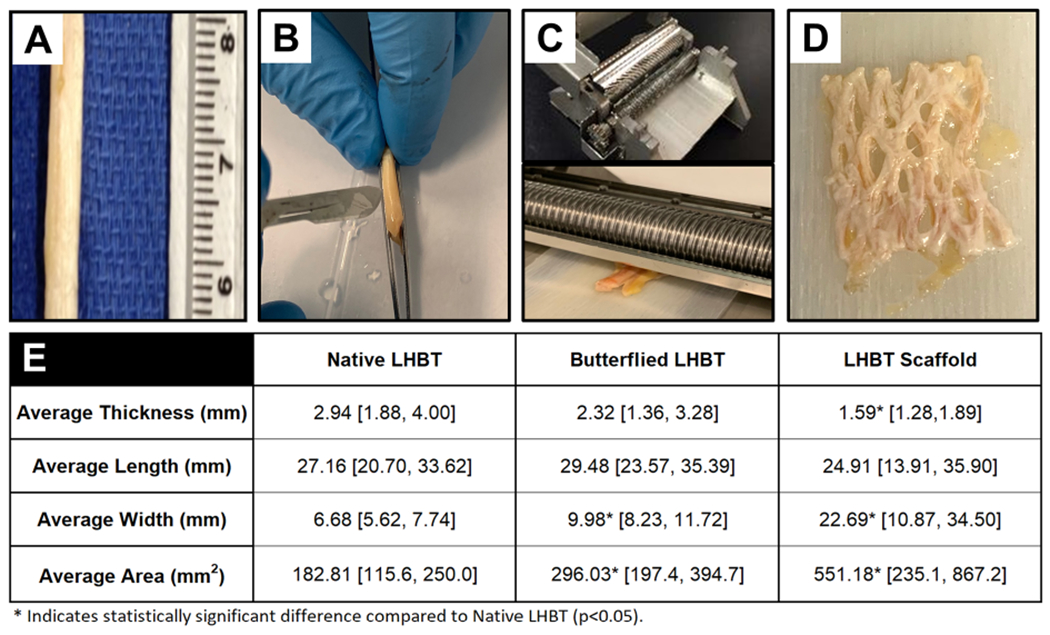
A) Native human LHBT segments (25mm in length) were isolated, B) held between forceps and butterflied using a #10 scalpel prior to C) placing them on a 1.1mm thick polymer carrier (lower panel) and processing through a graft expander (upper panel) which resulted in a D) porous LHBT scaffold with E) significantly increased width and area and reduced thickness compared to the native LHBT. * Indicates statistical difference (p<0.05) compared to native LHBT. Data represented as a mean and 95% confidence intervals [lower limit, upper limit].
LHBT SCAFFOLD TENSILE PROPERTIES
To complete objective 2, human cadaveric LHBT specimens were harvested and cut perpendicular to the long axis of the tendon yielding 20mm segments (n=10). LHBT scaffolds (n=5) and native LHBT segments (n=5) were clamped having their long axis aligned with the actuator of an MTS mechanical testing machine fitted with a 1000N load cell. Sample hydration was maintained throughout testing by using a room temperature physiological saline bath. Prior to testing, the distance between the tissue clamps was measured with digital calipers and was input as the gauge length into the test software for use in subsequent strain calculations. Additionally, segment width (W) and thickness (T) was measured using calibrated calipers and original cross-sectional area was calculated by W x T and expressed in square millimeters. Samples were pre-conditioned to 10% strain (i.e. segments were stretched to 10% of their original gauge length) for five cycles prior to undergoing uniaxial tensile testing to failure at a rate of 10mm/min. Load and displacement data were recorded in the units of Newtons and millimeters, respectively. Engineering stress and strain were calculated with TestWorks 4 software (City, State) using the original cross-sectional area and gauge length of the segments, respectively. The ultimate tensile strength (UTS) was calculated by dividing the maximum applied force by the original cross-sectional area of the segment (N/mm2 = Megapascal (MPa)). Tensile modulus was calculated as the slope of the linear region of the engineering stress versus strain graph and is expressed in the units of Megapascal. Peak tensile strain was calculated as the change in gauge length at failure divided by the original gauge length of the segment and is expressed in the units of millimeters / millimeters.
VIABLE HUMAN LONG HEAD BICEPS TENDON PROCUREMENT
Human LHBT samples were obtained via tenotomy from 6 consenting patients (68 ± 3.4 years: 4 males, 2 females) undergoing elective shoulder surgery to repair large (n = 4) or massive (n = 2) rotator cuff tears (Table 1). Tenotomy was performed based on the surgeon’s assessment that pathology was present. The gross appearance of the LHBTs ranged from normal to hour-glass shape with evidence of fraying and synovitis. Thus, the isolated tendons were not considered to be normal healthy tendons. LHBT samples were transported to the lab within two hours of harvest in sterile specimen vials containing 50ml of pre-warmed Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS) and 2% Antibiotic/Antimycotic (Ab/Am) solution. Samples were placed in 6-well plates in standard culture conditions (37°C, 5%CO2, 20% O2) for 48 hours prior to use in experiments to confirm the absence of microbial contamination. These samples were used to complete objectives 2 and 3.
Table 1.
Summary of patient demographics.
| Patient # | Male / Female | Age | Diagnosis |
|---|---|---|---|
| 1 | M | 74 | Rotator cuff tear |
| 2 | M | 75 | Rotator cuff tear |
| 3 | F | 75 | Rotator cuff tear |
| 4 | M | 54 | Rotator cuff tear |
| 5 | F | 66 | Rotator cuff tear |
| 6 | M | 64 | Rotator cuff tear |
TENOCYTE VIABILITY WITHIN LHBT SAMPLES AND SCAFFOLDS
To complete objective 2, viable LHBT samples (n=3) were bisected perpendicular to the long axis of the tendon yielding a total of six segments. One segment from each sample was formed into a scaffold while the other remained intact. Each segment was immediately rinsed in sterile phosphate buffered saline and incubated in Live/Dead staining solution in independent wells in a 6-well culture plate according to manufacturer’s instructions. Subsequently, confocal microscopy (Leica SPE; laser wavelengths of 488nm and 635nm) was used to capture (600Hz capture speed) three-dimensional images throughout the depth of a 50μm z-stack of the tissues. Three representative images were obtained at 400X total magnification from each segment and percent tenocyte viability was calculated as the number of live tenocytes divided by the total number of tenocytes (both live and dead) multiplied by 100.
TENOGENIC DIFFERENTIATION OF ADMSCs VIA LHBT SCAFFOLD TENOCYTE CONDITIONED MEDIA
To complete objective 3, viable LHBT samples (n=3) were bisected perpendicular to the long axis of the tendon under aseptic conditions yielding a total of six segments. One segment from each patient was formed into a scaffold while the other remained intact. Each segment was placed in 6-well plates containing 3ml of DMEM with 10% FBS and 1% Ab/Am and cultured for up to 28 days using aseptic technique in standard culture conditions (37°C and 5% CO2). Media was refreshed every three days. After 14, 21, and 28 days, LHBT samples were moved to new wells and tenocytes that had migrated out of the samples, presumably due to enhanced nutrient availability in the culture media, and attached to the adjacent well-plate were collected. Subsequently, the tenocytes obtained from the native LHBT or LHBT scaffold segments were pooled by their respective sources (e.g. native LHBT or scaffold) and cryopreserved in DMEM containing 20% FBS, 1% Ab/AM and 10% dimethyl sulfoxide at a density of 2x106 cells/ml.
Cryopreserved tenocytes were culture expanded to passage 4. Tenocytes (2x105) from LBHT scaffolds were seeded into each of three T-25 flasks and cultured for up to 21 days. Every three days, the culture media conditioned by the tenocytes were collected, pooled and sterile filtered through at 0.22μm filter. Subsequently, this conditioned media was mixed in a 1:1 volumetric ratio with fresh DMEM containing 10% FBS and 1% Ab/Am. This media was used to feed passage 4 human ADMSCs (Invitrogen) that were seeded into six T-25 flasks (2x105 cells / flask) and cultured for up to 21 days. Every three days, culture media was refreshed with conditioned media generated by tenocytes derived from LHBT scaffolds. Subsequently, RNA was isolated from ADMSCs (n=3 flasks / time-point) after day 14 and 21 of culture in conditioned media using an RNEasy Mini Kit (Qiagen) in accordance with the manufacturer’s protocol. For comparison, RNA was also isolated from undifferentiated ADMSCs (negative control) and tenocytes isolated from intact LHBT (positive control). RNA quantity and quality from each sample was evaluated using a Nanodrop spectrophotometer (ThermoFisher). A total of 500μg of RNA was reverse transcribed using a RETROscript kit (Ambion) according to manufacturer’s instructions. Resulting cDNA was amplified using a Rotogene 3000 thermocycler. Reaction products were detected using validated human primer sequences (Integrated DNA Technologies: Table 2) in conjunction with a QuantiTect SYBRgreen (Qiagen) polymerase chain reaction kit. Relative change in gene transcript (mRNA) expression was Calculated as. 2−ΔCt, where ΔCt = (Ct Gene of interest - Ct Gapdh internal housekeeping gene). Expression of a putative gene transcript marker profile indicating a tenocyte phenotype was used to evaluate differentiation of ADMSCs cultured in conditioned media compared to positive and negative controls. These markers included those expressed during early tendon development and repair, including the transcription factor scleraxis and the ECM protein collagen type 3, respectively.30,31 Additionally, collagen type 1 and tenascin-c expression were evaluated as they are ECM components found in the mature tendon.32
Table 2.
Summary of target genes, primer sequences, and polymerase chain reaction amplification protocol for gene transcript analysis.
| Target Gene Name | Primer Sequences (F: Forward, R: Reverse) | PCR Amplification Protocol |
|---|---|---|
| Collagen Type 1 | F: 5′-GGC GAG AGA GGT GAA CAA GG-3′ |
Activation: 95°C for 15 minutes Cycling (45 cycles): 1) Denaturation: 94°C for 15 seconds. 2) Anneal: 51°C for 30 seconds. 3) Extension: 72°C for 30 seconds. |
| R: 5′-GGC GAG AGA GGT GAA CAA GG-3′ | ||
| Collagen Type 3 | F: 5′-AGA AGG CCC TGA AGC TGA TG-3′ | |
| R: 5′-TGT TTC GTG CAA CCA TCC TC-3′ | ||
| Tenascin C | F: 5′-TTC AGG AAC CCA GAG GAA GC-3′ | |
| R: 5′-TCC GGT TCG GCT TCT GTA AC-3′ | ||
| Scleraxis B | F: 5′-CTG GCC TCC AGC TAC ATC TC-3′ | |
| R: 5′-CTT TCT CTG GTT GCT GAG GC-3′ | ||
| GAPDH | F: 5′-CCC ACT CCT CCA CCT TTG AC-3′ | |
| R: 5′-CCA CCA CCC TGT TGC TGT AG-3′ |
ADMSC ALIGNMENT AND MORPHOLOGY ON LHBT SCAFFOLDS
To complete objective 4, the ADMSC alignment on LHBT scaffolds was assessed by fluorescently labeling ADMSCs with PKH26 (Sigma) according to manufacturer’s instructions. Cells were then seeded at a density of 2x106 cells / scaffold (n=3/time-point) for up to 7 days. Culture was performed in DMEM containing 10% FBS and 1% Ab/Am under standard conditions (37°C and 5% CO2). Two hours and 7 days post-seeding, fluorescent imaging was performed using a Zeiss AxioVert microscope was used to visualize PKH26 labeled ADMSC alignment in relation to the scaffold fiber direction (visually confirmed via polarized light microscopy) and cell morphology. Three representative images were obtained for each scaffold captured at 400x total magnification.
STATISTICAL ANALYSIS
Statistical analysis of the data was performed using GraphPad Prism 7 software. Results are represented as means and 95% Confidence Intervals [lower interval, upper interval]. Statistical comparisons between study groups were performed using a Students t-test of equal variance. Significance was defined as p ≤ 0.05.
RESULTS
LHBT SCAFFOLDS CAN BE RELIABLY FORMED USING A SURGICAL GRAFT EXPANDER
Completion of objective 1 demonstrated that cadaveric human LHBT scaffolds had significant increases in width (p=0.027) and area (p=0.011) compared to native LHBT (Figure 1A thru D). There was a concomitant decrease in thickness (p=.008) compared to native LHBT (Figure 1E). Average percent change in LHBT tendon area after butterflying and meshing was 62.53% and 196.28%, respectively, resulting in average areas of 296.03mm2 [197.4, 394.7] (L: 29.48mm [23.57, 35.39] x W: 9.98mm [8.23, 11.72]) and 551.18mm2 [235.10, 867.20] (L: 24.91mm [13.91, 35.90] x W: 22.69mm [10.87, 34.50]), respectively.
LHBT SCAFFOLD TENSILE PROPERTIES
Completion of objective 2 demonstrated that the LHBT scaffold tensile properties were significantly different compared to native LHBT. The uniaxial tensile testing performed on native LHBT and LHBT scaffolds in the longitudinal axis (e.g. along the fiber direction) (Figure 2A) demonstrated that scaffold formation resulted in a 66.4% reduction in ultimate tensile strength and an 87.3% reduction in tensile modulus compared to native LHBT (Figure 2B&C). Conversely, scaffold formation resulted in a 113.5% increase in peak tensile strain compared to native LHBT (Figure 2D).
Figure 2. Scaffold formation results in altered tensile mechanical properties compared to native long head biceps tendon.
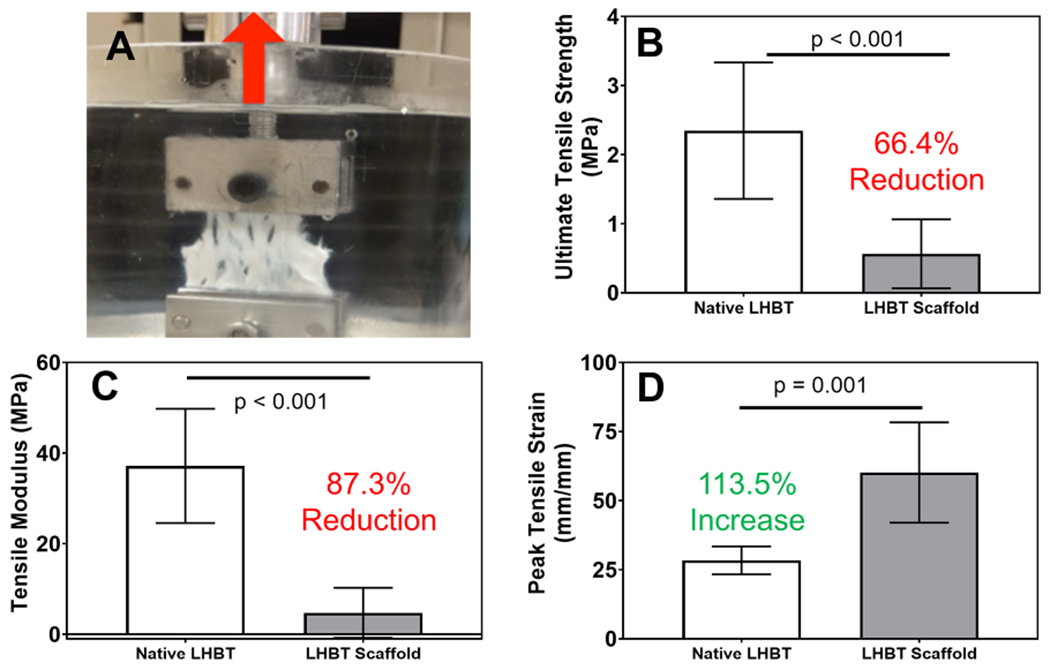
A) Uniaxial tensile testing set-up for evaluating the mechanical properties of human LHBT prior to and following scaffold formation (red arrow indicates longitudinal direction). Resultant tensile mechanical properties of LHBT scaffolds and relative percent change in B) ultimate tensile strength, C) tensile modulus and D) peak tensile strain compared to native LHBT. Horizontal lines indicate a statistical difference (p<0.05) compared to native LHBT. Graphed data represent means and 95% confidence intervals.
VIABILITY OF ENDOGENOUS TENOCYTES WITHIN LHBT SCAFFOLDS
Completion of objective 2 also illustrated that viable tenocytes were found throughout the native LHBT and LHBT scaffold samples via LIVE/DEAD staining (Figures 3A&B, respectively). Negative controls contained no viable cells (Figure 3C). Percent viability was significantly different (p=0.004) comparing tenocytes in native LHBT (68.87% [63.37, 74.37]) compared to tenocytes in LHBT scaffolds (38.52% [17.95, 59.09]) (Figure 3D).
Figure 3. Scaffold formation resulted in decreased viability of resident long head biceps tendon tenocytes.
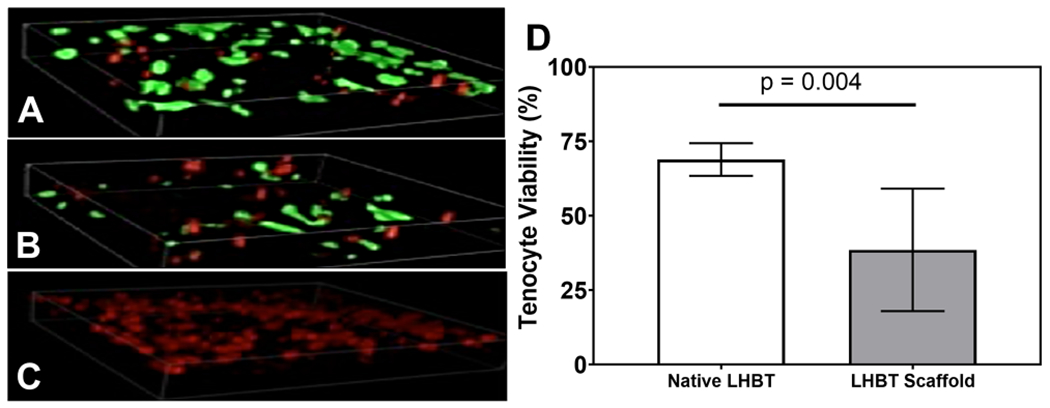
Representative 3-D confocal z-stack LIVE/DEAD images (400x total mag.) of A) native, B) meshed, and C) ethanol treated (negative control) LHBT samples depicting viable (green) and dead (red) tenocytes within two hours of harvest or expansion. D) Quantitative analysis of LHBT tenocyte viability from LIVE/DEAD images illustrating a significant reduction in resident LHBT tenocyte viability within two hours after LHBT scaffold formation. Horizontal lines indicate a statistical difference (p<0.05) compared to native LHBT. Graphed data represent means and 95% confidence intervals.
TENOGENIC DIFFERENTIATION OF ADMSCS CULTURED IN MEDIA CONDITIONED BY LHBT SCAFFOLD TENOCYTES
Completion of objective 3 demonstrated that tenogenic differentiation of ADMSCs was initiated by culturing them in the presence of media conditioned from tenocytes originating from the LHBT scaffolds. Scleraxis expression was 8.67-times higher (p=0.040) in ADMSCs cultured in conditioned media at Day 21 compared to undifferentiated ADMSC controls (Figure 4A). Additionally, collagen type 3 expression was 3.63- (p=0.042) and 4.25-times (p=0.034) higher at Days 14 and 21 in ADMSCs cultured in condition media compared to undifferentiated ADMSCs and 3.08- (p=0.026) and 3.61-times (p=0.048) higher at Days 14 and 21 in ADMSCs cultured in conditioned media compared to LHBT tenocyte controls (Figure 4B). The expression of collagen type I increased in ADMSCs cultured in conditioned media as time progressed but did not reach levels observed in tenocyte positive controls (Figure 4C). Tenascin C expression in ADMSCs cultured in conditioned media was comparable to tenocyte controls at 14 and 21 days (Figure 4D).
Figure 4. Soluble factors produced by tenocytes derived from long head bicep tendon scaffolds promote tenogenic differentiation of ADMSCs and scaffolds themselves support ADMSC alignment and a tenocyte-like morphology.
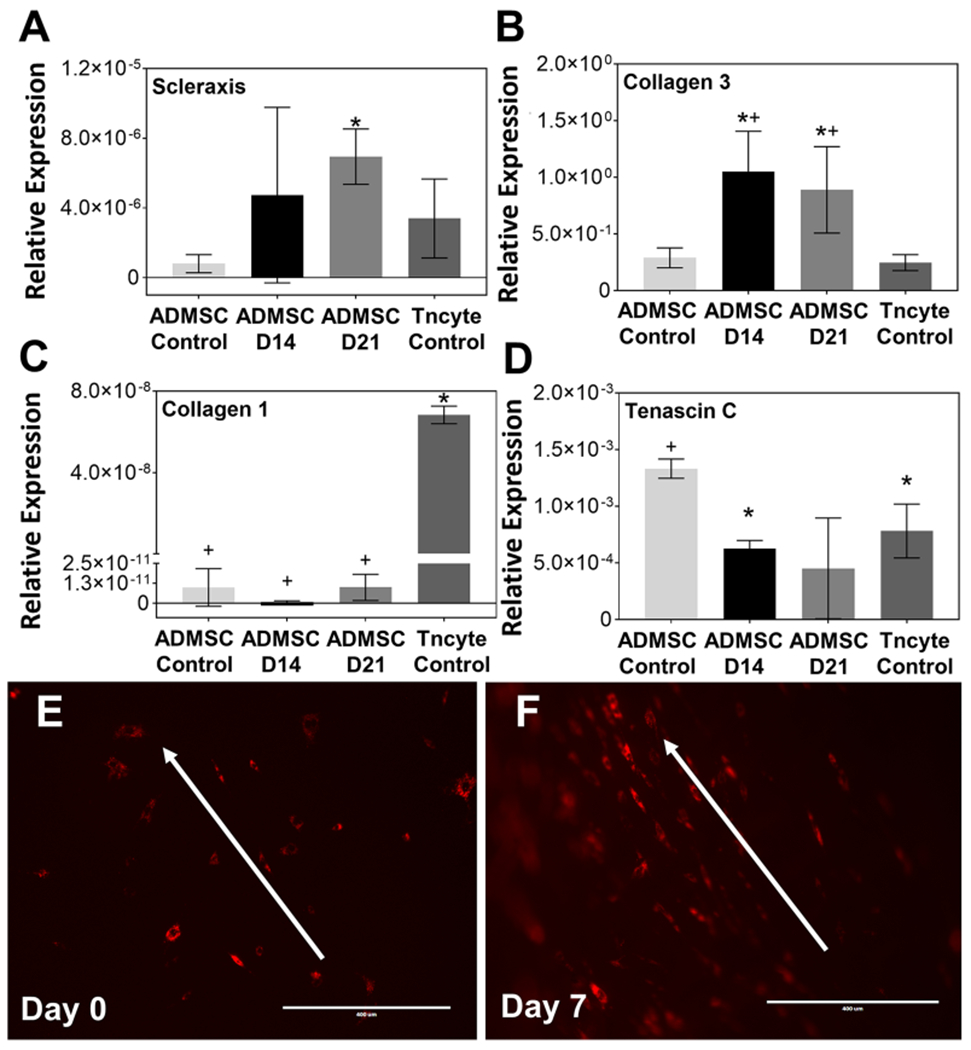
Gene transcript (mRNA) expression of A) scleraxis, B) collagen type 3, C) collagen type 1 and D) tenascin c relative to GAPDH for ADMSCs cultured in media conditioned by tenocytes from LHBT scaffolds for 14 (“ADMSC D14) and 21 (“AMSC D21”) days illustrating up-regulation of tenogenic markers Scleraxis and Collagen Type III compared to undifferentiated ADMSC negative controls (“ADMSC Control”). + and * indicate statistical differences (p<0.05) compared to LHBT tenocyte positive controls (“Tncyte Control”) and undifferentiated ADMSC negative controls, respectively. Graphed data represent means and 95% confidence intervals. Fluorescent imaging of ADMSCs cultured on LHBT scaffolds at E) Day 0 (i.e. 2 hours post-seeding) and F) Day 7 illustrating progressive cell alignment and development of spindle-shape morphology along the direction of collagen fibrils (white arrows) in scaffolds. Scale bar = 400μm.
LHBT SCAFFOLDS SUPPORT ADMSC ALIGNMENT AND ACQUISITION OF A TENOCYTE-LIKE MORPHOLOGY
Completion of objective 4 demonstrated that ADMSCS were aligned along collagen fibers in LHBT scaffolds. Two hours after seeding (e.g. Day 0) on LHBT scaffolds, ADMSCs were found on the scaffold and exhibited a rhombohedral morphology (Figure 4E). This indicated that the cells were able to attach to the ECM of the LHBT scaffold but had yet to align. Following seven days of culture on LHBT scaffolds, ADMSC morphology was elongated, and the cell bodies were aligned parallel to the direction of collagen fibers found within the LHBT (Figure 4F). Together, imaging illustrated a change in ADMSC morphology indicative of cell attachment and alignment over time.
DISCUSSION
This study has established that: 1) LHBT from patients undergoing rotator cuff repair can be formed into porous autograft scaffolds, 2) scaffolds exhibited reduced mechanical properties and viable autologous tenocytes compared to native LHBT, 3) tenocytes from LHBT scaffolds produced cytokines that promoted the initiation of tenogenic differentiation in ADMSCs, and 4) LHBT scaffolds supported ADMSC alignment and acquisition of a tenocyte-like morphology.
The LHBT is often released from the labrum during rotator cuff repair surgery,33 as the proximal portion of the tendon is thought to be a source of pain.34 The proximal segment of LHBT can potentially be harvested, processed, and used as an autograft. Autologous LHBT grafting has been described for the primary repair of massive rotator cuff tears.35 Various techniques including interpositional grafting for retracted cuff tears,36 and incorporation via weaving or overlay for large and massive cuff tears have demonstrated clinical utility.36–39 However, due to its narrow dimensions (5-6mm diameter)40 the LHBT must often be split in half length-wise (e.g. “butterflied”) to create a graft width that could adequately cover the surface area of large tendon tears.41,42 Herein, the current technique uses the LHBT to create autologous porous scaffold for biologically augmenting rotator cuff repair. The first major finding was that a surgical skin graft meshing device could be used to create a LHBT scaffold with a uniform thickness and a footprint that will adequately cover the insertion of the supraspinatus tendon which has a mean anterior-posterior dimension of 25mm.43 In addition to its dimensional characteristics, the LHBT was found to have favorable biological and mechanical properties. It contained intact collagen fibers at the microscopic level, similar to the native tendon, while having full-thickness porosity to allow cell infiltration and tissue integration as well as a low tensile modulus. These characteristics have been proven to promote tendon tissue repair and regeneration.44,45 More specifically, a similar approach has been taken to promote tendon regeneration and integration using a 2mm thick sheet of bovine collagen having a defined fiber alignment, high (85-90%) porosity and low tensile modulus. These characteristics are proposed to allow for improved cell alignment, infiltration, tissue integration and handling properties.46 Indeed, Bokor et al. showed the generation of 2-3mm of new tendon-like tissue over repaired rotator cuff tendons and promising early clinical results using this construct as a biological adjunct for rotator cuff repair.46 Similar results were also confirmed histologically in an ovine model of rotator cuff repair.47 However, the LHBT scaffold described herein has advantages compared to the bovine scaffolds studied previously, namely its autologous source, lower cost, and lack of immunogenicity.
Another significant finding from this study was the viability of human tenocytes within the LHBT scaffold following mechanical expansion. Initial viability of the LHBT tenocytes was approximately 70% and this viability decreased following tissue manipulation and expansion. This could have been the result of many factors including the pathological state of the tendon and the older patient population. Additionally, the mechanical damage induced during the graft expansion process and thus new methods of expansion could be explored which reduce damage to cells and increase their viability. Regardless, the viable cells may contribute to tendon healing and regeneration when the LHBT scaffold is incorporated at the repair site. Additionally, this study suggests that tenocytes originating from LHBT which reside within a shoulder joint having pathological changes,48–50 maintain an ability to respond to local biochemical cues as evidenced by their ability to migrate out of the meshed LHBT tissue during media conditioning (described in the methods section for objective 3). Thus, therapeutic strategies targeting migration and/or ECM anabolism by resident tenocytes at the repair site may also represent a feasible approach to improving tendon healing. Further study of this phenomenon is warranted.
It was also demonstrated that tenocytes from within LHBT scaffolds derived from patients undergoing rotator cuff repair were able to promote the initiation of tenogenic differentiation of ADMSCs via paracrine (e.g. growth factor / cytokine) signaling. Progressive tenogenic differentiation of ADMSCs following culture with LHBT scaffold tenocyte conditioned media was illustrated via increased expression levels of tenogenic markers. These markers included increased expression of scleraxis and collagen type 3 compared to undifferentiated ADMSCs. Scleraxis is the master transcription factor controlling tenogenesis,51,52 and collagen type 3 is a primary ECM component initially deposited during tendon repair prior to deposition of mature collagen type 1.53 Tenascin-c is a matricellular glycoprotein that modulate cell-ECM interaction and is expressed in tendon areas that undergo high force transmission (e.g. osteotendinous and myotendinous regions).54,55 However, it does appear at the time-points investigated that ADMSCs were not fully differentiated (i.e. did not achieve a mature tenocyte phenotype) as their collagen type 1 expression was significantly lower compared to positive controls (e.g. native tenocytes). However, it is plausible that the expression levels of mature tenocyte markers, including collagen type 1, could continue to increase with more time in culture. Furthermore, it is also unclear if a specific threshold exists regarding the ratio of tenocytes and ADMSCs required to promote tenogenic differentiation and ultimately tissue repair / regeneration in vivo.56 Additionally, direct contact co-culture of tenocytes and ADMSCs could have resulted in enhanced differentiation as there would be no ‘dilution’ effect on the soluble signals due to mixing conditioned media with fresh media as was performed in the current study. While direct contact co-culture methods have been shown to improve MSC differentiation compared to indirect methods,57 it can challenging to elucidate cell-specific responses without first sorting the cells – a process that may influence cell behavior and make interpretation of results difficult. Regardless, others have shown similar results in co-culture studies of healthy (uninjured) tenocytes and MSCs together using direct and indirect cultures.58–60
Of note, while tenocytes from degenerative tissue have been previously shown to have an altered phenotype which could impact their ability to promote differentiation,61 this was not observed here possibly due to the mutually beneficial paracrine crosstalk that has been observed previously between ADMSCs and tenocytes.62 From a clinical application perspective, this data demonstrates the potential that MSC fate can be controlled without in vitro manipulation and pre-differentiation prior to use. ADMSCs were used in this study due to their greater abundance,23,63 and the ease by which an enriched population of ADMSCs can be obtained from lipoaspirate via a 30-minute centrifugation period prior to shoulder surgery.64 However, it should be noted that centrifuged stromal vascular fraction is not a pure isolation of ADMSCs, and that their numbers may be limited in a point of care application. Moreover, ADMSC isolation from fat requires enzymatic techniques. Further, other MSC or orthobiologic sources, including bone marrow aspirate concentrate (BMAC),65 PRP,66 or bursal tissue cells67 may be alternative options for cellular augmentation of the repair at the point of care.
Finally, the propensity ADMSCs seeded on LHBT scaffolds to attach and align along the collagen fibers was noted. This suggests that the LHBT scaffolds provide topographical cues that guide the organized arrangement of cells which will ultimately direct the deposition of ECM for tendon repair. This was evidenced by ADMSCs exhibiting a transition in cell morphology from a rhombohedral-shape two hours after seeding, to an elongated / spindle-shape (similar to that observed in native tenocytes) after 7 days of culture. If the ADMSCs were not able to attach and spread out, they would have remained round. Others have demonstrated that maintenance of an elongated cell morphology is essential for achieving and supporting an appropriate tenocyte-like gene transcript expression profile.68 Taken together, LHBT scaffolds appear to promote a cellular morphology that is conducive to supporting a tenocyte-like phenotype which may ultimately contribute to improved tendon regeneration.
Limitations
As with any laboratory study, several limitations were noted. First, meshing was performed on cadaveric tendons to determine changes in dimensional characteristics, however prior pilot studies using fresh autograft yielded the similar results and thus the results herein can be extrapolated to live tendons. Another limitation was that in vitro expansion of ADMSCs prior to the study was performed which would not be feasible in a point of care approach. However, the tenocyte to ADMSC ratio (1:1) and the respective quantities used to generate conditioned media and undergo differentiation, respectively, were based on conservative estimates of the number of tenocytes and ADMSCs that can be harvested clinically using the proposed technique without having to undergo in vitro expansion.64,69 A third limitation was that the harvested tendons used in the studies were degenerate, however this could represent a relevant clinical scenario. Finally, this model of cell culture likely underestimates the complexity of an in vivo microenvironment (e.g. in the presence of inflammatory cytokines, growth factors and mechanical cues as would be expected at the healing repair site) and thus re-evaluation of these cell studies in a culture system that mimics the in vivo repair microenvironment is warranted in addition to in vivo studies.
CONCLUSIONS
The ability to produce a viable autologous scaffold from the proximal biceps tendon having dimensions, porosity, mechanical characteristics, native ECM components, and viable tenocytes that produce bioactive signals conducive to supporting the biologic augmentation of rotator cuff repair surgery has been demonstrated.
Supplementary Material
Clinical Relevance:
This biologically active construct may help to improve the quality of healing and regeneration at the repair site of rotator cuff tears, especially those at high risk for re-tear.
Acknowledgements:
Research support has been provided in part by the National Institute of General Medical Sciences of the National Institutes of Health [award number: P20GM103444]. The authors would also like to acknowledge the generous support from the John Witherspoon Gilpin M.D., ’82 Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Social Medial Handles:
@MercuriLab
@CU_BioE
@hawkfoundation
@SptbgRegional
@mayoclinicsport
REFERENCES
- 1.Research Id. Sports Medicine Market Analysis, Size, Trends - U.S; 2019. [Google Scholar]
- 2.Domb BG, Glousman RE, Brooks A, Hansen M, Lee TQEN. High-tension double-row footprint repair compared with reduced-tension single-row repair for massive rotator cuff tears. J Bone Joint Surg Am. 2008;90(Supplemental 4):35–39. [DOI] [PubMed] [Google Scholar]
- 3.Smith CD, Alexander S, Hill AM, Huijsmans PE, Bull AM, Amis AA, De Beer JFWAL. A biomechanical comparison of single and double-row fixation in arthroscopic rotator cuff repair. J Bone Jt Surg Am Vol. 2006;88(11):2425–2431. [DOI] [PubMed] [Google Scholar]
- 4.Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon-bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surgery, Sport Traumatol Arthrosc. 2009;17(12):1466–1472. doi: 10.1007/s00167-009-0771-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ménard J, Léger-St-Jean B, Balg F, Petit Y , Beauchamp MRD. Suture bridge transosseous equivalent repair is stronger than transosseous tied braided-tape. J Orthop Sci. 2017;22(6):1120–1125. [DOI] [PubMed] [Google Scholar]
- 6.Galatz LM, Ball CM, Teefey SA, Middleton WDYK. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86(2):219–24. [DOI] [PubMed] [Google Scholar]
- 7.Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear Rates after Arthroscopic Single-Row, Double-Row, and Suture Bridge Rotator Cuff Repair at a Minimum of 1 Year of Imaging Follow-up: A Systematic Review. Arthrosc - J Arthrosc Relat Surg. 2015;31(11):2274–2281. doi: 10.1016/j.arthro.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 8.Duquin TR, Buyea C, Bisson LJ. Which Method of Rotator Cuff Repair Leads to the Highest Rate of Structural Healing? Am J Sports Med. 2010;38(4):835–841. doi: 10.1177/0363546509359679 [DOI] [PubMed] [Google Scholar]
- 9.Le BTN, Wu XL, Lam PH, Murrell GAC. Factors predicting rotator cuff retears: An analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med. 2014;42(5):1134–1142. doi: 10.1177/0363546514525336 [DOI] [PubMed] [Google Scholar]
- 10.Mirzayan R, Weber AE, Petrigliano FACJ. Rationale for Biologic Augmentation of Rotator Cuff Repairs. J Am Acad Orthop Surg. 2019;27(13):468–478. [DOI] [PubMed] [Google Scholar]
- 11.Patel S, Gualtieri AP, Lu HHLW. Advances in biologic augmentation for rotator cuff repair. Ann N Y Acad Sci. 2016;1383(1):97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charles MD, Christian DRCB. The Role of Biologic Therapy in Rotator Cuff Tears and Repairs. Curr Rev Musculoskelet Med. 2018;11(1):150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: A systematic review. Br Med Bull. 2010;94(1):165–188. doi: 10.1093/bmb/ldp051 [DOI] [PubMed] [Google Scholar]
- 14.Ricchetti ET, Aurora A, Iannotti JP, Derwin K a. Scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2012;21(2):251–265. doi: 10.1016/j.jse.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Jo CH, Shin JS, Lee YG, et al. Platelet-Rich Plasma for Arthroscopic Repair of Large to Massive Rotator Cuff Tears. Am J Sports Med. 2013;41(10):2240–2248. doi: 10.1177/0363546513497925 [DOI] [PubMed] [Google Scholar]
- 16.Castricini R, Longo UG, De Benedetto M, et al. Platelet-Rich Plasma Augmentation for Arthroscopic Rotator Cuff Repair. Am J Sports Med. 2011;39(2):258–265. doi: 10.1177/0363546510390780 [DOI] [PubMed] [Google Scholar]
- 17.Dohan Ehrenfest DM, Bielecki T, Mishra A, et al. In search of a consensus terminology in the field of platelet concentrates for surgical use: platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin gel polymerization and leukocytes. Curr Pharm Biotechnol. 2012;13(7):1131–1137. http://www.ncbi.nlm.nih.gov/pubmed/21740379. Accessed November 21, 2017. [DOI] [PubMed] [Google Scholar]
- 18.Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular Matrix Bioscaffolds for Orthopaedic Applications<sbt aid="1062029">A Comparative Histologic Study</sbt> J Bone Jt Surg. 2006;88(12):2673. doi: 10.2106/JBJS.E.01008 [DOI] [PubMed] [Google Scholar]
- 19.Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(6):1238–1244. doi: 10.2106/JBJS.E.00524 [DOI] [PubMed] [Google Scholar]
- 20.Alberti KA, Sun J-Y, Illeperuma WR, Suo Z, Xu Q. Laminar tendon composites with enhanced mechanical properties. J Mater Sci. 2015;50(6):2616–2625. doi: 10.1007/s10853-015-8842-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawelec KM, Wardale RJ, Best SM, Cameron RE. The effects of scaffold architecture and fibrin gel addition on tendon cell phenotype. J Mater Sci Mater Med. 2015;26(1):5349. doi: 10.1007/s10856-014-5349-3 [DOI] [PubMed] [Google Scholar]
- 22.Zitnay JL, Reese SP, Tran G, Farhang N, Bowles RD, Weiss JA. Fabrication of dense anisotropic collagen scaffolds using biaxial compression. Acta Biomater. November 2017. doi: 10.1016/j.actbio.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caplan AI. Mesenchymal stem cells: Cell based reconstructive therapy in orthopedics. Tissue Eng. 2005;11(7):1198–1211. doi: 10.1089/ten.2005.11.1198 [DOI] [PubMed] [Google Scholar]
- 24.Gulotta LV, Chaudhury S, Wiznia D. Stem cells for augmenting tendon repair. Stem Cells Int. 2012;2012(i):291431. doi: 10.1155/2012/291431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loeffler BJ, Scannell BP, Peindl RD, et al. Cell-based tissue engineering augments tendon-to-bone healing in a rat supraspinatus model. J Orthop Res. 2013;31(3):407–412. doi: 10.1002/jor.22234 [DOI] [PubMed] [Google Scholar]
- 26.Kurtz CA, Loebig TG, Anderson DD, DeMeo PJ, Campbell PG. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am J Sports Med. 1999;27(3):363–369. doi: 10.1177/03635465990270031701 [DOI] [PubMed] [Google Scholar]
- 27.Gonçalves AI, Rodrigues MT, Lee S-J, et al. Understanding the role of growth factors in modulating stem cell tenogenesis. Neves NM, ed. PLoS One. 2013;8(12):e83734. doi: 10.1371/journal.pone.0083734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulotta LV, Rodeo SA. Growth Factors for Rotator Cuff Repair. Clin Sports Med. 2009;28(1):13–23. doi: 10.1016/j.csm.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 29.Kovacevic D, Gulotta LV., Ying L, Ehteshami JR, Deng X-H, Rodeo SA. rhPDGF-BB Promotes Early Healing in a Rat Rotator Cuff Repair Model. Clin Orthop Relat Res. 2015;473(5):1644–1654. doi: 10.1007/s11999-014-4020-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.James R, Kesturu G, Balian G, Chhabra a B. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33(1):102–112. doi: 10.1016/j.jhsa.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Suen CW, Zhang J fang, Li G. Current concepts on tenogenic differentiation and clinical applications. J Orthop Transl. 2017;9:28–42. doi: 10.1016/j.jot.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanco D, Caprara C, Ciardelli G, et al. Tenogenic differentiation protocol in xenogenic-free media enhances tendon-related marker expression in ASCs. PLoS One. 2019;14(2):1–21. doi: 10.1371/journal.pone.0212192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ditsios K, Agathangelidis F, Boutsiadis A, Karataglis D, Papadopoulos P. Long head of the biceps pathology combined with rotator cuff tears. Adv Orthop. 2012;2012:405472. doi: 10.1155/2012/405472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gill TJ, McIrvin E, Mair SD, Hawkins RJ. Results of biceps tenotomy for treatment of pathology of the long head of the biceps brachii. J Shoulder Elb Surg. 2001;10(3):247–249. doi: 10.1067/mse.2001.114259 [DOI] [PubMed] [Google Scholar]
- 35.Sano H, Mineta M, Kita A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310–316. doi: 10.1007/s00776-010-1453-5 [DOI] [PubMed] [Google Scholar]
- 36.Rhee YG, Cho NS, Lim CT, Yi JW, Vishvanathan T. Bridging the gap in immobile massive rotator cuff tears: augmentation using the tenotomized biceps. Am J Sports Med. 2008;36(8):1511–1518. doi: 10.1177/0363546508316020 [DOI] [PubMed] [Google Scholar]
- 37.Obma PR. Free biceps tendon autograft to augment arthroscopic rotator cuff repair. Arthrosc Tech. 2013;2(4):e441–5. doi: 10.1016/j.eats.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sano H, Mineta M, Kita A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310–316. [DOI] [PubMed] [Google Scholar]
- 39.Jeon YS, Lee J, Kim RG, Ko YW, Shin SJ. Does Additional Biceps Augmentation Improve Rotator Cuff Healing and Clinical Outcomes in Anterior L-Shaped Rotator Cuff Tears? Clinical Comparisons With Arthroscopic Partial Repair. Am J Sports Med. 2017;45(13):2982–2988. doi: 10.1177/0363546517720198 [DOI] [PubMed] [Google Scholar]
- 40.Ahrens PM, Boileau P. The long head of biceps and associated tendinopathy. J Bone Jt Surg - Ser B. 2007;89(8):1001–1009. doi: 10.1302/0301-620X.89B8.19278 [DOI] [PubMed] [Google Scholar]
- 41.Obma PR. Free Biceps Tendon Autograft to Augment Arthroscopic Rotator Cuff Repair. Arthrosc Tech. 2013;2(4):e441–e445. doi: 10.1016/j.eats.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sano H, Mineta M, Kita A, Itoi E. Tendon patch grafting using the long head of the biceps for irreparable massive rotator cuff tears. J Orthop Sci. 2010;15(3):310–316. doi: 10.1007/s00776-010-1453-5 [DOI] [PubMed] [Google Scholar]
- 43.Ruotolo C, Fow JENW. The supraspinatus footprint: an anatomic study of the supraspinatus insertion. Arthroscopy. 2004;20(3):246–9. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Li B, Wang JHC. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials. 2011;32(29):6972–6981. doi: 10.1016/j.biomaterials.2011.05.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang G, Rothrauff BB, Lin H, Gottardi R, Alexander PG, Tuan RS. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials. 2013;34(37):9295–9306. doi: 10.1016/j.biomaterials.2013.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bokor DJ, Sonnabend D, Deady L, et al. Preliminary investigation of a biological augmentation of rotator cuff repairs using a collagen implant: A 2-year MRI follow-up. Muscles Ligaments Tendons J. 2015;5(3):144–150. doi: 10.n138/mltj/2015.5.3.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Kampen C, Arnoczky S, Parks P, Hackett E, Ruehlman D, Turner AST. Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: a histological evaluation in sheep. Muscles Ligaments Tendons J. 2013;3(3):229–235. [PMC free article] [PubMed] [Google Scholar]
- 48.Virk MS, Cole BJ. Proximal Biceps Tendon and Rotator Cuff Tears. Clin Sports Med. 2016;35(1):153–161. doi: 10.1016/j.csm.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 49.Takahashi N, Sugaya H, Matsuki K, et al. Hypertrophy of the extra-articular tendon of the long head of biceps correlates with the location and size of a rotator cuff tear. Bone Joint J. 2017;99-B(6):806–811. doi: 10.1302/0301-620X.99B6.BJJ-2016-0885.R1 [DOI] [PubMed] [Google Scholar]
- 50.Desai SS, Mata HK. Long Head of Biceps Tendon Pathology and Results of Tenotomy in Full-Thickness Reparable Rotator Cuff Tear. Arthrosc J Arthrosc Relat Surg. 2017;33(11):1971–1976. doi: 10.1016/j.arthro.2017.06.018 [DOI] [PubMed] [Google Scholar]
- 51.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136(8): 1351–1361. doi: 10.1242/dev.027342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Killian ML, Thomopoulos S. Scleraxis is required for the development of a functional tendon enthesis. FASEB J. 2016;30(1):301–311. doi: 10.1096/fj.14-258236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.James R, Kesturu G, Balian G, Chhabra a B. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33(1):102–112. doi: 10.1016/j.jhsa.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 54.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6(2):181–190. [PubMed] [Google Scholar]
- 55.Järvinen TA, Józsa L, Kannus P, Järvinen TL, Hurme T, Kvist M, Pelto-Huikko M, Kalimo HJM. Mechanical loading regulates the expression of tenascin-C in the myotendinous junction and tendon but does not induce de novo synthesis in the skeletal muscle. J Cell Sci. 2003;116(5):857–866. [DOI] [PubMed] [Google Scholar]
- 56.Kraus A, Woon C, Raghavan S, Megerle K, Pham H, Chang J. Co-culture of human adipose-derived stem cells with tenocytes increases proliferation and induces differentiation into a tenogenic lineage. Plast Reconstr Surg. 2013;132(5):754e–766e. doi: 10.1097/PRS.0b013e3182a48b46 [DOI] [PubMed] [Google Scholar]
- 57.Chiu LLY, Bianco J, Giardini-Rosa RCK and WS. Direct and indirect co-culture of bone marrow stem cells and adipose-derived stem cells with chondrocytes in 3D scaffold-free culture. J Regen Med Tissue Eng. 2016;5(1):1–9. [Google Scholar]
- 58.Luo Q, Song G, Song Y, Xu B, Qin J, Shi Y. Indirect co-culture with tenocytes promotes proliferation and mRNA expression of tendon/ligament related genes in rat bone marrow mesenchymal stem cells. Cytotechnology. 2009;61(1-2):1–10. doi: 10.1007/s10616-009-9233-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barboni B, Curini V, Russo V, et al. Indirect co-culture with tendons or tenocytes can program amniotic epithelial cells towards stepwise tenogenic differentiation. PLoS One. 2012;7(2):e30974. doi: 10.1371/journal.pone.0030974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraus A, Woon C, Raghavan S, Megerle K, Pham H, Chang J. Co-culture of human adipose-derived stem cells with tenocytes increases proliferation and induces differentiation into a tenogenic lineage. Plast Reconstr Surg. 2013;132(5):754e–766e. doi: 10.1097/PRS.0b013e3182a48b46 [DOI] [PubMed] [Google Scholar]
- 61.Taylor SE, Vaughan-Thomas A, Clements DN, et al. Gene expression markers of tendon fibroblasts in normal and diseased tissue compared to monolayer and three dimensional culture systems. BMC Musculoskelet Disord. 2009;10(27):1–10. doi: 10.1186/1471-2474-10-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ekwueme EC, Shah JV, Mohiuddin M, et al. Cross-Talk Between Human Tenocytes and Bone Marrow Stromal Cells Potentiates Extracellular Matrix Remodeling In Vitro. J Cell Biochem. 2016;117(3):684–693. doi: 10.1002/jcb.25353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. http://www.ncbi.nlm.nih.gov/pubmed/10102814. Accessed November 21, 2017. [DOI] [PubMed] [Google Scholar]
- 64.Condé-Green A, de Amorim NFG, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg. 2010;63(8):1375–1381. doi: 10.1016/j.bjps.2009.07.018 [DOI] [PubMed] [Google Scholar]
- 65.Nazal MR, McCarthy MBR, Mazzocca AD, Martin SD. Connective Tissue Progenitor Analysis of Bone Marrow Aspirate Concentrate Harvested From the Body of the Ilium During Arthroscopic Acetabular Labral Repair. Arthrosc - J Arthrosc Relat Surg. 2020:1–10. doi: 10.1016/j.arthro.2019.11.125 [DOI] [PubMed] [Google Scholar]
- 66.Barber FA. PRP as an adjucnt to rotator cuff tendon repair. Sports Med Arthrosc. 2018;26(2):42–47. [DOI] [PubMed] [Google Scholar]
- 67.Morikawa D, Muench LN, Baldino JB, et al. Comparison of Preparation Techniques for Isolating Subacromial Bursa-Derived Cells as a Potential Augment for Rotator Cuff Repair. Arthrosc - J Arthrosc Relat Surg. 2020;36(1):80–85. doi: 10.1016/j.arthro.2019.07.024 [DOI] [PubMed] [Google Scholar]
- 68.Zhu J, Li J, Wang B, et al. The regulation of phenotype of cultured tenocytes by microgrooved surface structure. Biomaterials. 2010;31(27):6952–6958. doi: 10.1016/j.biomaterials.2010.05.058 [DOI] [PubMed] [Google Scholar]
- 69.Wagenhäuser MU, Pietschmann MF, Sievers B, et al. Collagen type I and decorin expression in tenocytes depend on the cell isolation method. BMC Musculoskelet Disord. 2012;13(140):1–8. doi: 10.1186/1471-2474-13-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


