Abstract
The CFTR modulator combination elexacaftor/tezacaftor/ivacaftor (ETI) is a genetic mutation-targeted treatment in cystic fibrosis that results in profound improvements in clinical outcomes. Each of the compounds are substrates of CYP3A4/5, the cytochrome P450 enzyme family for which tacrolimus is also a substrate. The use of these compounds in an individual with a solid organ transplant has not been previously studied and there is potential for a drug interaction. In this report, we describe a pediatric liver transplant recipient with clinical decline related to cystic fibrosis who improved substantially with ETI, without significant impact on the systemic exposure of either ETI or tacrolimus.
Keywords: Ivacaftor, Tezacaftor, Elexacaftor, Liver transplantation, CFTR, tacrolimus, Drug interaction, Cytochrome P450
Introduction
Genetic mutations in the Cystic Fibrosis (CF) Transmembrane Conductance Regulator (CFTR) result in a multi-organ disease with significant morbidity and mortality, which has been significantly alleviated in recent years with the advent of CFTR modulator therapy targeting certain mutation defects. Most recently, a triple combination therapy (elexacaftor/tezacaftor/ivacaftor, ETI) was approved for approximately 90% of people with cystic fibrosis on the basis of their specific genetic mutations. For patients who have received a solid organ transplant, the use of modulators is controversial because of the potential for drug interactions and uncertain benefit.(1) Patients who receive transplants are typically treated with a combination of a glucocorticoid, a calcineurin inhibitor (CNI) and a nucleotide blocking agent that are adjusted over time as well as antimicrobials to prevent opportunistic infections. The combination of drug regimens for immunosuppression, prevention of opportunistic infections and CFTR modulators poses a high risk for drug interactions. There are limited studies available regarding interactions between these agents and immunosuppressive therapy, specifically the calcineurin inhibitor tacrolimus, and ETI. All three components of ETI are metabolized through cytochrome P450 isozymes (CYP3A),(2) and each have the potential for either inhibition or induction of metabolic enzymes and transporters. Tacrolimus is a CYP3A4 and P-glycoprotein (PgP) substrate and has a narrow therapeutic window for maintaining immunosuppression and avoiding adverse events. In this report, we describe the clinical outcomes and medication concentrations from a patient receiving tacrolimus and ETI.
Case
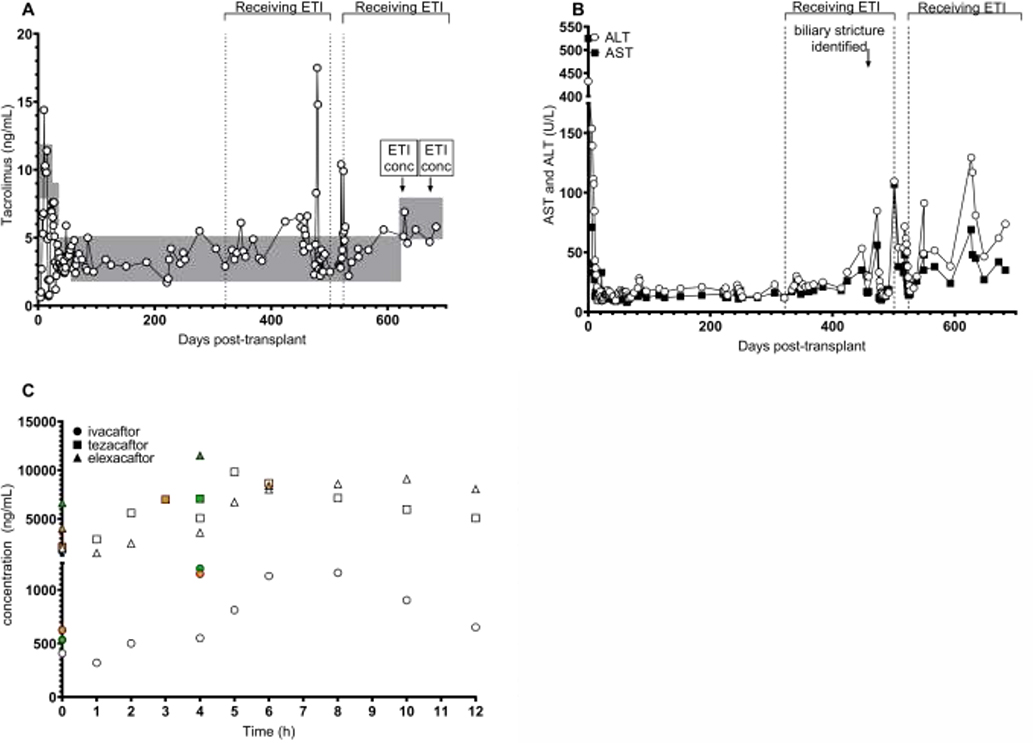
A 14 year-old white female with CF (F508/R560T) developed CF pulmonary disease, CF-related liver disease (CFLD), pancreatic insufficiency, CF-related diabetes (CFRD), and sinusitis. Over time, her CFLD progressed to biliary cirrhosis with subsequent development of portal hypertension including varices, hypersplenism, thrombocytopenia and hepatopulmonary syndrome. She had persistent elevation of her liver enzymes including aspartate aminotransaminase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT) as well as coagulopathy unresponsive to vitamin K supplementation. Due to the progressive decline in hepatic function, she was listed for liver transplant and subsequently underwent an orthotopic liver transplantation. Immunosuppression induction consisted of basiliximab and methylprednisolone, followed by maintenance immunosuppression with glucocorticoids (taper), tacrolimus and mycophenolic acid. CFRD control was a challenging immediate post-transplant due to the diabetogenicity of tacrolimus and corticosteroids; patient’s insulin was adjusted and a pump initiated with improvement. Tacrolimus concentrations (Figure 1A) were monitored frequently to establish appropriate dosing of 3 mg twice daily, and remained within goal range (shown by gray areas in Figure 1A) along with other markers of acute liver injury including ALT and AST through 321 days post-transplant (Figure 1B). At this time, ETI (200mg elexacaftor/100mg tezacaftor/150mg ivacaftor in the morning and 150mg ivacaftor in the evening) was initiated due to decline in lung function and increased frequency of pulmonary exacerbations. The patient reported less cough and sputum production after starting ETI, and clinical improvement in weight percentile (from 25 to 33), BMI percentile (from 36 to 45), and percent predicted forced expiratory volume in one second (ppFEV1(3), from a baseline of 88% to 100%). There was no significant change in CFRD control. Due to the chance of a significant interaction between ETI and tacrolimus, the patient was closely monitored with labs twice weekly the first month, weekly the second month, and then as per established liver transplant protocol. ALT and AST remained within normal ranges for three months, whereas a slight increase in tacrolimus concentrations were noted (Figure 1A–B). Five months after starting ETI, the patient developed a fever, cough and progressive fatigue and was admitted for an exacerbation of CF lung disease. At this time AST, ALT, GGT and bilirubin significantly increased, and the patient was found to have a biliary stricture, not at the transplant anastomosis, which was dilated. Tacrolimus dose was adjusted to remain within targeted range. During the admission, because of the elevated transaminases, ETI was held until these labs returned to baseline. Tacrolimus dosing was returned to 3 mg twice daily. The patient reported renewed symptoms of CF and experienced another exacerbation shortly after stopping ETI. After treatment, her lung function improved and with normalized transaminases, she restarted modulator therapy with improvement in symptoms, weight percentile (42), BMI percentile (58), and ppFEV1 (93%). At this point, concentrations of all three compounds were quantified on two separate occasions under an IRB-approved research protocol (UAB IRB #300001194) and found in both cases to be close to those reported in adolescent pharmacokinetic studies of ETI(2), (Figure 1C). Tacrolimus concentrations have remained stable within adjusted goals (slightly increased along with increased dose of mycophenolic acid) as per clinical course to manage possible rejection.
Figure 1. Drug monitoring after liver transplant.
A. Tacrolimus concentrations were obtained clinically after transplant for routine monitoring. Gray boxes indicate desired target blood exposure during each period after transplant. “ETI conc” indicates timing of blood collection for compound concentrations. ETI brackets and vertical dotted lines indicate time on and off modulator therapy. B. AST and ALT tests were obtained clinically for monitoring. C. Elexacaftor, tezacaftor, and ivacaftor concentrations were quantitated from plasma by mass spectrometry similar to previous reports.(6) White open data points indicate first ETI concentrations measured sequentially over 12 hours. Green data points indicate sparse sampling at 0 and 4 hours for the second ETI concentration assessment. Orange data points represent the steady-state minimum and maximum concentrations as reported by the manufacturer. (2) The time of maximum concentration for each compound varies: median (range) = ivacaftor 4h (3–6); tezacaftor 3h (2–4); elexacaftor 6h (4–12). One green point is hidden behind orange square at time=0.
Discussion
Calcineurin inhibitors, such as cyclosporine and tacrolimus, are key to many immunosuppressant regimens. Both cyclosporine and tacrolimus are metabolized by CYP3A4 and CYP3A5.6 If co-administered with any medications that induce or inhibit these enzymes, close monitoring is required. CFTR modulators pose a theoretical risk of interaction. Currently, there are four approved CFTR modulators: ivacaftor, lumacaftor/ivacaftor, tezacaftor/ivacaftor and ETI. As described by the manufacturer, ivacaftor is metabolized by CYP3A4 and is a CYP3A4 substrate as well as a weak CYP3A4 and PgP inhibitor; lumacaftor is also metabolized by CYP3A4 and is a strong CYP3A4 inducer; and tezacaftor and elexacaftor are both primarily metabolized by CYP3A isozymes. Co-administration of these modulators with CYP3A4 inducers or inhibitors should be done with caution and close monitoring for drug interactions, if at all.(1) For example, in studies of the use of ivacaftor in combination with midazolam, a CYP3A4 substrate, ivacaftor increased the concentration, decreased the clearance and extended the half-life of midazolam.(4) Two patient cases have been reported describing the use of lumacaftor/ivacaftor after liver transplantation managed with tacrolimus. Lumacaftor/ivacaftor with concurrent tacrolimus use in these cases was found to be safe with evidence of clinical benefit; in these cases, therapeutic tacrolimus blood concentrations were able to be maintained with appropriate dose alterations.(5)
The child described in our case received ivacaftor as part of the triple-combination therapy ETI in combination with tacrolimus. Currently, there is very little evidence to support this combination due to concern for significant drug interactions. In this patient receiving both medications, all four compounds remained within expected concentration ranges. However, despite successful treatment for cystic fibrosis lung disease and growth with ETI, the fluctuating transaminases suggest ETI-induced liver injury or the possibility of liver allograft rejection. Transaminases improved after increased dose of mycophenolic acid, suggesting possible rejection. However, without a liver biopsy a definitive answer cannot be provided. Therefore, close monitoring of tacrolimus and transaminases should be used with any patient undertaking concomitant use of tacrolimus with CFTR modulator therapy in liver transplant recipients.
Highlights.
Highly effective modulator treatment results in improvement in clinical outcomes.
Modulator use may result in drug interactions that need to be managed.
Tacrolimus and modulator use together is manageable.
Acknowledgements and Role of the funding source:
JSG is supported by the NIH/NHLBI (K23HL143167) and the Cystic Fibrosis Foundation (GUIMBE18A0-Q). The funding sources provided no input to the content of the manuscript.
Abbreviations:
- CF
cystic fibrosis
- CFTR
Cystic Fibrosis Transmembrane conductance regulator
- ppFEV1
percent predicted forced expiratory volume in one second
Footnotes
Declaration of competing interest
All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mitchell RM, Jones AM, Barry PJ. CFTR modulator therapy in patients with cystic fibrosis and an organ transplant. Paediatric respiratory reviews. 2018;27:6–8. [DOI] [PubMed] [Google Scholar]
- 2.Administration FaD. Drug Approval Package: Trikafta Food and Drug Administration [Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212273Orig1s000MultidisciplineR.pdf.
- 3.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG Jr. Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15(2):75–88. [DOI] [PubMed] [Google Scholar]
- 4.Robertson SM, Luo X, Dubey N, Li C, Chavan AB, Gilmartin GS, et al. Clinical drug-drug interaction assessment of ivacaftor as a potential inhibitor of cytochrome P450 and P-glycoprotein. Journal of clinical pharmacology. 2015;55(1):56–62. [DOI] [PubMed] [Google Scholar]
- 5.Chouchane I, Stremler-Lebel N, Reix P. Lumacaftor/ivacaftor initiation in two liver transplantation patients under tacrolimus and antifungal azoles. Clin Case Rep. 2019;7(4):616–8. [DOI] [PMC free article] [PubMed] [Google Scholar]