Abstract
Members of the HECT family of E3 ubiquitin ligases have emerged as prominent regulators of PTEN function, subcellular localization and levels. In turn this unfolding regulatory network is a allowing for the identification of genes directly involved in both tumorigenesis at large and cancer susceptibility syndromes. While the complexity of this regulatory network is still being unraveled, these new findings are paving the way for novel therapeutic modalities for cancer prevention and therapy as well as for other diseases. Here we will review the signal transduction and therapeutic implications of the cross-talk between HECT family members and PTEN.
Keywords: HECT family of E3 ubiquitin ligase, NEDD4, PTEN, WWP1, I3C
Introduction
The phosphatidylinositol 3-kinase (PI3K) signaling pathway regulates fundamental biological processes, including stemness, cell growth, survival, differentiation, glucose metabolism, and motility through direct lipid phosphorylation and production of phosphatidylinositol 3,4,5-trisphosphate (PIP3). Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) dephosphorylates PIP3, with specific affinity for the phosphate group at the D3 position of inositol ring, to phosphatidylinositol 4,5-bisphosphate (PIP2) [1,2]. As such, PTEN serves as a critical negative upstream regulator of the PI3K signal transduction cascade through PIP3 dephosphorylation at plasma membrane, hence terminating the propagation of the signal to the downstream AKT pathway [3, 4]. PTEN can also act as a dual-specificity protein phosphatase, dephosphorylating tyrosine-, serine- and threonine-phosphorylated polypeptides in vitro [5, 6]. Indeed, PTEN dephosphorylates several protein substrates, including focal adhesion kinase (FAK) in inhibiting cell adhesion to extracellular matrix, cAMP-responsive element-binding protein (CREB) in suppressing transcriptional activation, tyrosine kinases SRC and PTK6 in inhibiting kinase activities, and insulin receptor substrate-1 (IRS1) in controlling insulin-induced glucose metabolism, to exert its tumor-suppressive function [7–11]. Moreover, phosphatase-independent, mostly scaffolding, activities of PTEN for tumor suppression have also been identified [12–14].
PTEN is by now recognized as one of the most frequently mutated, down-regulated, silenced, and functionally inactivated tumor suppressor genes [15, 16]. Partial loss of PTEN is pervasive and observed in cancers of various histology. PTEN is also found mutated in heterozygosity in the germ line of individuals affected by a number of syndromes commonly referred as PTEN hamartomas tumour syndromes (PHTS), which invariably share two features: cancer susceptibility and developmental defects [17, 18]. Additionally, PTEN loss causes resistance to a number of cancer therapies including resistance to selective inhibitors of PI3Ks [19–21]. On this basis, there is currently tremendous interest in studying the mechanisms that control PTEN levels, subcellular localization and functional activation with the goal of finding ways to reactivate its function for cancer prevention and therapy.
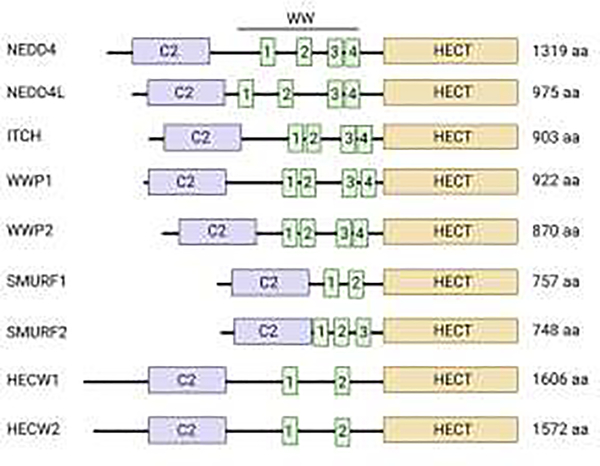
The enzymatic activity of the HECT-E3 ligases has been recently implicated in the control of PTEN function, localization and expression levels. Specifically, members of a subgroup of HECT-E3 ligases known as C2-WW-HECT (NEDD4-like) comprising at least nine member in humans (NEDD4, NEDD4L, ITCH, SMURF1, SMURF2, WWP1, WWP2, HECW1, and HECW2), have been found to converge on PTEN regulation through distinct and cooperative mechanisms, which will be the focus of this review. This subgroup is characterized by a common modular architecture composed of a N-terminal C2 domain related to protein kinase C, two to four domains with central tryptophan - tryptophan (WW), and a C-terminal HECT enzymatic domain. WW domains mediate protein - protein interactions through the recognition of Pro-rich motifs (PPxY, LPxY or related sequences) and phosphorylated Ser/Thr-Pro. These domains provide a scaffold for recruiting protein substrates and regulators (refs. [22–24] and Figure 1).
Figure 1. Schematic diagram of domain structure of human NEDD4-subfamily HECT E3 ubiquitin ligases.
C2, calcium-binding domain; WW, tryptophan - tryptophan (WW) domain; HECT, homologous to the E6-AP carboxyl terminus (HECT) domain. NEDD4, neuronal precursor cell-expressed developmentally downregulated 4; NEDD4L, NEDD4 like E3 ubiquitin protein ligase; ITCH, itchy E3 ubiquitin protein ligase; WWP1, WW domain containing E3 ubiquitin protein ligase 1; WWP2, WV, domain containing E3 ubiquitin protein ligase 2; SMURF1, SMAD specific E3 ubiquitin protein ligase 1; SMURF2, SMAD specific E3 ubiquitin protein ligase 2; HUWE1, HECT, UBA and WWE domain containing E3 ubiquitin protein ligase 1; HUWE2, HECT, UBA and WWE domain containing E3 ubiquitin protein ligase 2.
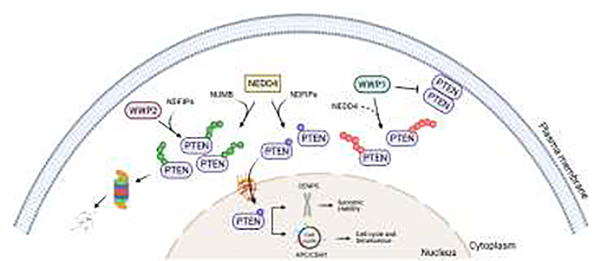
PTEN has been found to be regulated essentially at three levels (Figure 2): i) its full enzymatic activation towards lipids dephosphorylation through dimerization at plasma membrane; ii) its proteasome-depended degradation aberrantly observed in human cancers, and iii) its ability to move from the plasma membrane, to the cytosol, to the nucleus to exert additional lipid-independent functions [3, 4]. Each of these steps are regulated by members of NEDD4-like family of HECT E3 ubiquitin ligases singly or cooperatively.
Figure 2. Proposed model for the HECTs fate decision of PTEN in a cell.
While NEDD4 and WWP2 promote PTEN polyubiquitination, with the assistance of adaptor proteins NDFIPs and NUMB, and subsequent proteasomal degradation in the cytoplasm, NEDD4-mediated PTEN monoubiquination leads to its translocation into the nucleus where it controls the cell cycle and genomic stability. Furthermore, WWP1 mediates PTEN K27-linked polyubiquitination, ablating its dimerization and plasma membrane localization (Created with BioRender.com). C2, calcium-binding domain; PTEN, phosphatase and tensin homolog deleted on chromosome 10; NEDD4, neuronal precursor cell-expressed developmentally downregulated 4; WWP1, WW domain containing E3 ubiquitin protein ligase 1; WWP2, WW domain containing E3 ubiquitin protein ligase 2; NDFIPs, NEDD4 family interacting proteins; NUMB, NUMB endocytic adaptor protein; APC/CDH1, anaphase-promoting complex/CDC20 homolog 1; CENPC, centromere protein C; U, ubiquitin.
NEDD4 and PTEN
NEDD4 (neuronal precursor cell-expressed developmentally downregulated 4; also known as NEDD4–1) was the first identified E3 ubiquitin ligase of PTEN, resulting in PTEN polyubiquitination and subsequent proteasomal degradation [25]. Correlative expression analyses have shown an inverse correlation between (increased) NEDD4 and (decreased) PTEN in many different types of human cancer. Monoubiquitination of PTEN at Lys13 and Lys289 by NEDD4 was also shown to lead to PTEN nuclear import, which is a prerequisite for nuclear PTEN to fully exert its tumor suppression activities [13, 26–28]. Likewise NEDD4-mediated monoubiquitination at Lys13 PTEN is required for exosomal transport of PTEN to exert phosphatase activity beyond the cell [29]. Thus NEDD4 can act as a proto-oncogene to degrade PTEN, or in a tumor-suppressive role to direct PTEN to the nucleus and exosomes to stimulate (non-)cell-autonomous PTEN activity. Although how NEDD4 controls both mono- and polyubiquitination of PTEN remains mechanistically unclear, several possibilities can be envisioned: first, NEDD4 modification and/or association with different adaptor modules [e.g. p34 (ref. [30]), NDFIPs, and NUMB (see below)] can switch it from mono- to polyubiquitination mode; second, NEDD4 may utilize a specific ubiquitin code and poly-ubiquitin chains of specific linkage for PTEN mono- and polyubiquitination; third, the cytoplasmic/nuclear localization of NEDD4 [31] may dictate PTEN stability in the cytoplasmic compartment by a polyubiquitination mode and maintain nuclear residence of PTEN by a monoubiquitination mode. While loss of Pten induces excessive neurite growth in mice [32], Nedd4 KO mutants show impairment of neuronal and glial development [33–35], indicating that PTEN and NEDD4 have antagonistic functions in the control of neurite growth. However, Nedd4 KO does not have a large impact on stability and nuclear import of PTEN [33, 36, 37], implying that the role of NEDD4 in regulation of PTEN is likely to be complex and context specific. In addition, unraveling the physiological role of NEDD4 in tumorigenesis using transgenic mouse models is also needed. NEDD4 binds to PTEN via an interaction between their respective C2 domains [25, 38], suggesting that the NEDD4/PTEN interaction can be modulated by (de)stabilization of their C2 domains. Indeed, CK1α (casein kinase 1 alpha 1) antagonizes the NEDD4/PTEN interaction by unfavoring PTEN phosphorylation on Thr366/Ser385 [39], which is considered as PTEN destabilization sites [40, 41]. Conversely, Rak phosphorylates PTEN on Tyr336, thereby reducing the binding of PTEN to NEDD4 and protecting it from proteasomal degradation [42], while PRL2 (phosphatase of regenerating liver 2) dephosphorylates PTEN at Tyr336 to trigger the opposite outcome [43]. On the other hand, the N-terminal C2 domain of NEDD4 renders its C-terminal catalytic HECT domain inactive, a well-established mechanism of autoinhibition among NEDD4 family members [44, 45]. Thus proteins or compounds that restrict this autoinhibitory conformation of NEDD4-family HECT enzymes can serve as their activators. More recently, we have found that WWP1 (WW domain-containing ubiquitin E3 ligase 1) interacts with and promotes K27-linked PTEN polyubiquitination, subsequently reducing the PTEN dimerization and membrane localization [46] (see next section); in this setting, combined expression of NEDD4 and WWP1 synergistically increases PTEN polyubiquitination, although NEDD4 singularly triggers PTEN monoubiquitination but not K27-linked polyubiquitination. NEDD4 and WWP1 are found to physically interact [46] and it is therefore possible that NEDD4 interaction with the C2 domain of WWP1 releases WWP1 from its autoinhibitory conformation, in turn targeting PTEN for K27-linked polyubiquitination. An alternative possibility is that NEDD4 and WWP1 both interact with PTEN resulting in sequential modifications of PTEN; the initiating NEDD4, that may necessarily have no strong lysine preference, modifies PTEN with monoubiquitin or short chains, and the NEDD4-mediated PTEN monoubiquitination could be then elongated by WWP1 which exhibits a strong preference for ubiquitin’s K27 linkage. Future work should investigate the presumed complex interplay between NEDD4 and WWP1 in the coordinate regulation of PTEN.
WWP1 and PTEN
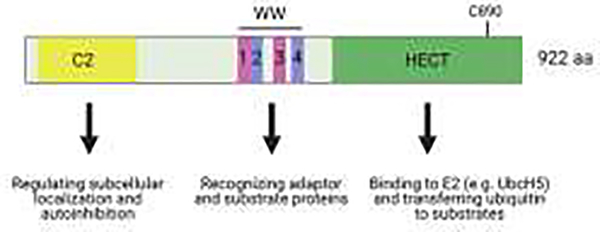
WWP1, as NEDD4, is a prototypical member of the HECT family of E3 ligases. It structurally displays a C2 N-terminal domain, followed by four WW domains centrally located, and an C-terminal HECT catalytic domain (Figure 3A). As for other members of this family of enzymes, the C2 domain is thought to mediate WWP1 localization at plasma membrane while the WW domains allow WWP1 to interact with regulatory proteins as well as with ubiquitination substrates [47]. The WWP1 gene is located on chromosome 8q21.3, clove to the c-myc proto-oncogene, in a region very frequently amplified in human cancers of various histology [48]. Indeed, WWP1 is often found mutated, genetically amplified, and overexpressed in prevalent human cancers including prostate, breast, colon, pancreatic, liver cancers as well as hemopoietic malignancies including leukemias and lymphomas [49–51]. While WWP1 overexpression was soon reported to favor cell growth and tumorigenesis, hence attributing to WWP1 proto-oncogenic activities [52, 53], the consequence and critical relevance of WWP1 mutations were only very recently unraveled as we will discuss below.
Figure 3. WWP1 acting as a ‘brake’ on PTEN activation.
(A) Schematic diagram of the WWP1 protein domains. (B) Proposed model for the role of the MYC-WWP1 axis in triggering PTEN K27-linked polyubiquitination to suppress its dimerization, plasma membrane recruitment, and antagonism of PI3K/AKT signaling (Created with BioRender.com). C2, calcium-binding domain; WW, tryptophan - tryptophan (WW) domain; HECT, homologous to the E6-AP carboxyl terminus (HECT) domain; C890, active-site cysteine 890 of WWP1; E2, ubiquitin-conjugating enzyme; UbcH5, ubiquitin-conjugating enzyme H5; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PI3K, phosphatidylinositol 3-kinase; AKT, AKT serine/threonine kinase; PTEN, phosphatase and tensin homolog deleted on chromosome 10; WWP1, WW domain containing E3 ubiquitin protein ligase 1; MYC, MYC proto-oncogene; U, ubiquitin.
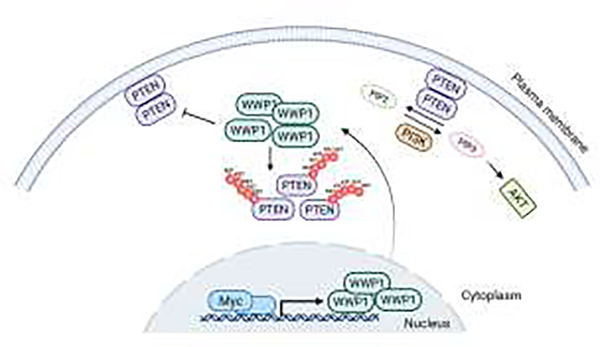
WWP1 can ubiquitinate several tumor suppressive substrates targeting them for degradation. These include, for example, TP63, the TP53 homologues, and TGFBR1 and Smad2 [54–56]. While WWP1 targets for degradation its substrates by adding classic K11 and K48 ubiquitin chains, this enzyme is also able to modify PTEN with K27 ubiquitin chains, which by contrasts does not result in the degradation of PTEN but rather prevents PTEN from dimerizing/oligomerizing triggering its dissociation from the plasma membrane [46] (Figure 3B). This in turn results in the transient and reversible inactivation of the phosphatase activity of PTEN towards lipid at plasma membrane, and the consequent activation of the PI3-kinase signal transduction pathway [46]. It is worth noticing that these effects are transient and reversible because PTEN is not degraded as a result of WWP1-mediated modification. Indeed, PTEN goes back to plasma membrane when WWP1 is inhibits d or silenced [46].
As aforementioned, NEDD4 primes PTEN for subsequent modification by WWP1 and in so doing NEDD4 and WWP1 synergize in favoring PTEN ubiquitination [46]. The inactivation of Wwp1 in the mouse is compatible with adult’s life [46, 57]. Wwp1 KO mutants display striking similarities with transgenic mutants that harbor extra copies of the PTEN gene [58], such as smaller body size, a suppressed PI3K pathway, tumor resistance and metabolic fitness with metabolic features reminiscent of a systemic anti-Warburg effect [46]. Intriguingly, c-Myc can transcriptionally upregulate WWP1, which in turn links c-Myc to the activation of PI3K signaling, with devastating consequences in the cancers where c-Myc and WWP1 are found to be genetically co-amplified [46, 48].
This hardwired c-MYC-WWP1-PI3K pathway for cell growth and proliferation is conserved from C. elegans to mammals further underscoring its evolutionary importance. Of additional importance, WWP1 gain of function mutations have been reported in the germ line of PHTS individuals and families, which do not harbor PTEN mutations and yet display comparable syndromic characteristics, as well as in the germ line of patients with sporadic form of cancer [59, 60]. These findings are three-fold relevant: i) they genetically link WWP1 and PTEN through the development of syndromes with similar phenotypes underpinning the genetic and biochemical link between these two enzymes; ii) they identify WWP1 as an important cancer susceptibility gene when mutated; iii) they identify the mutations of WWP1 observed in human cancer as “gain of function” mutations hence explaining their frequent incidence in tumors of various histology. Perhaps, however, the most relevant aspect of these recent findings resides in their therapeutic implications. Indeed a natural compound found in Brassicaceae, Indole-3-carbinol (I3C for brief), previously reported to be able to inhibit NEDD4 [61] is now identified as a more potent inhibitor of WWP1 [46], paving the way to clinical trials for cancer prevention and therapy in PHTS and in human cancer at large.
WWP2 and PTEN
As WWP1, WW domain-containing protein 2 (WWP2) is prototypical member of the NEDD-like protein family. WWP2 shares with its paralogues similar structural features with a C2 domain located N terminally, 4 tandem WW domains of 35 to 40 amino acids characterized by the classic 4 conserved aromatic residues, and a catalytic HECT domain located C-terminally [22–24]. WWP2 is alternatively spliced and three variants encoding distinct isoforms have been reported for this gene: Full-length WWP2 (870 amino acids), together with N-terminal (WWP2-N, 336 amino acids); C-terminal (WWP2-C, 440 amino acids) isoforms. WWP2 triggers the ubiquitination and subsequent degradation of its targets. As WWP1, WWP2 can negatively regulate TGF-β signaling and PTEN. The full-length WWP2 interacts with and targets SMAD2, SMAD3 and SMAD7 while WWP2-C interacts only with SMAD7 in the TGF-β pathway favoring epithelial-mesenchymal transition and tumorigenesis [62, 63]. Of interest, WWP2-N, which lacks the HECT ubiquitin ligase domain, can still interact with full-length WWP2 thereby inducing auto-activation. Additionally, WWP2, most likely the full-length isoform, triggers the degradation of PTEN and the subsequent activation of PI3K/AKT signaling [64, 65]. In line with these findings, Wwp2 KO mice display features similar to Wwp1 KO mutants such as reduced body size, suppressed AKT phosphorylation and cellular proliferation accompanied by PTEN elevation [66]. As WWP1, WWP2 is frequently overexpressed and mutated in human cancers of various histology even though the functional consequences of WWP2 mutations are still uncharacterized [24, 67]. WWP2 ubiquitination activity is enhanced by NDFIP1 and NDFIP2 as we will discuss below.
All together, these findings along with the ability of WWP2 to trigger the degradation of TRIF and RBP, in turn suppressing anti-tumor responses [68, 69], identify this gene as a bona ide proto-oncogene and a critical regulator of PTEN.
NDFIPs and NUMB
NEDD4-family HECT E3s exploit several unique mechanisms to regulate their activity and function. These include the aforementioned autoinhibition by intramolecular interaction and the binding of adaptor proteins altering E3’s catalytic activity and E3-substrate interaction. NEDD4 family interacting proteins (NDFIPs; NDFIP1 and NDFIP2) are the earliest known mammalian adaptors of HECT E3s [70, 71]. The NDFIP proteins contain three N-terminal Pro-rich (PY) motifs, three transmembrane domains, and a short C-terminal region [72]. NDFIPs can enhance the HECT catalytic activities of multiple NEDD4 family members by binding to the WW domains of NEED4 via their PY motifs to relieve the autoinhibitory conformation between the C2 and HECT domains of NEDD4 [73]. Besides their roles as co-activators of NEDD4, NDFIPs can also stimulate recruitment of NEDD4 to ubiquitinate its target proteins - such as PTEN - that lack the classical PY motifs. Intriguingly, by mediating the interaction between PTEN and different NEDD4-family members, NDFIPs can determine the fates of PTEN. For example, NDFIP1 promotes NEDD4 binding to and monoubiquitination of PTEN, which facilitates the nuclear import and the exosomal export of PTEN, with consequences for (non-)cell-autonomous PTEN activity [29, 37, 74]. Of particular importance, Ndfip1 KO mice display features similar to nuclear PTEN-deficient (PTENK13R, D384V) mice such as increased susceptibility to excitotoxic brain damage and normal PI3K/AKT signaling [37, 75]. On the other hand, recruitment of WWP2 and Itch by NDFIP1 and NDFIP2 leads to polyubiquitination of PTEN and its subsequent proteasomal degradation, with critical outcomes for signaling through AKT activation [76, 77].
NUMB endocytic adaptor protein (NUMB) functions as another adaptor for NEDD4 to polyubiquitinate PTEN and target it for degradation [78], suggesting that the ability of NEDD4 to switch from mono- to polyubiquitination of PTEN resulting in its different fates can be modulated by availability and accessibility of distinct adaptor proteins. In future work, it will be interesting to understand the mechanisms that determine activation of a specific adaptor for PTEN ubiquitination in cancer and other diseases and to examine how distinct adaptors impact the specificity of NEDD4-family ligases in determining the type of ubiquitin linkages and thus the cell fate decisions of PTEN.
The role of HECTs homo- and heterodimerization
Besides the aforementioned intramolecular and adaptors associations, homodimerization and oligomerization in HECT E3s can also play a regulatory role. For example, homodimerization of HECT domains of HUWE1 leads to autoinhibition, but engagement of N-terminal activation segment to the dimerization region can relieve autoinhibition [79]. In addition, autoubiquitination-dependent trimerization of NEDD4 renders the ligase inactive [80]. SMURF1 can form a head-to-toe homodimer via interaction between C2 and HECT domains to inhibit HECT activity [81]. WWPs undergo autoinhibition, accompanied by decreased PTEN polyubiquitination, when they form homodimers via interaction between the HECT domain and WW2-WW3 linker region [46, 60, 76]. Furthermore, WWP1 K740N and N745S (within the HECT domain), two germline variants that had been implicated in cancer susceptibility as well as high risk for fatal COVID-19, were found to lead to aberrant WWP1 enzymatic activation with subsequent PTEN inactivation, thereby triggering hyperactive growth-promoting PI3K signaling in cancer models [60, 82]. Critically, these mutations offer insights on the mechanisms that lead to gain of function of WWPs by suppressing their ability to homodimerize and oligomerize and thereby relieving the WW linker autoinhibition towards PTEN.
The mono- and heterodimeric state of WWPs can also play a distinct role, and thus define the role of a specific WWP in context. Indeed, WWP2 was found to exist in two functionally distinct forms, wherein WWP2 in monomeric form ubiquitinates and degrades full-length p73, a member of the p53 protein family, but in a heterodimeric complex with WWP1 regulates N-terminally truncated ΔNp73 isoform, thus maintaining a fine balance between these two isoforms for cell survival [83]. More recently, we have found that while NEDD4 alone monoubiquitinates PTEN it also c operates with WWP1 in promoting K27-linked polyubiquitination of PTEN, through heterodimeric interactions between as-yet-uncharacterized regions [46], highlighting the potential importance of HECTs heterodimerization in the regulation of PTEN. However, as aforementioned, it is also plausible that these HECTs act sequentially towards PTEN polyubiquitination in a two-step manner: i) NEDD4 adds monoubiquitin or a short ubiquitin chain linked via ubiquitin K27 or other Ks which might be likely trimmed by deubiquitinating enzymes (DUBs) although not determined; ii) Based on this monoubiquitin moiety on PTEN, WWP1 next produces K27-linked polyubiquitin chains. This perspective has a way of reinforcing the idea that PTEN polyubiquitination requires cooperation between distinct, sequentially acting E3s, and raise the enthralling possibility that other members of NEDD4-family HECTs also require the assistance of a second E3 when targeting substrates. In a further working model, heterodimerization of HECT family members could dictate their chain specificity and hence the subsequent fate of the substrate. For example, HECT1 with HECT2 would yield a ubiquitin chain towards degradation (e.g. K48 chain) while HECT1 and HECT3 would yield a ubiquitin chain towards subcellular compartmentalization (e.g. K27 chain).
Transcriptional and post-translational regulations of HECTs
Several important cancer-associated signaling pathways and cancer genes ave been found to regulate HECT E3s at a transcriptional level: While Ras, NF-κB and STAT3 signaling pathways stimulate the transcription of NEDD4 [84–86], MYC functions as a transcription factor for WWP1 towards PTEN K27-linked polyubiquitination and subsequent inactivation [46]. On the other hand, PI3K/PTE N-mTORC1 signaling can regulate NEDD4 expression at the translational level [33].
Additionally, many different post-translational modifications of HECT E3 ligases can regulate their catalytic activity by triggering conformational changes in HECTs or by affecting the interactions with adaptor proteins. For example, growth factor signaling [e.g. FGFR1 (fibroblast growth factor receptor 1)] and glucose signaling activate NEDD4 by inducing SRC-family tyrosine kinases, SRC and VES respectively, dependent phosphorylation of specific tyrosine residues in the C2 (Tyr43) and HECT (Tyr585) domains, relieving an inhibitory intra- or intermolecular interaction [87, 88]. HECT E3 ligases can be also ubiquitinated by other E3 ligases: for instance, the SCF (Skp1-Cullin1-F-box protein) β-TRCP E3 ligase promotes ubiquitination and degradation of NEDD4 [89]. The interferon inducible ISG15, a ubiquitin-like protein, can also regulate the activity of NEDD4 [90]: indeed, the binding of ISG15 to NEDD4 has been shown to block the interaction with its E2 thereby inhibiting the catalytic activity [91,92]. A key question for the future is how post-translational modifications of HECTs contribute to the regulation of PTEN in tumorigenesis, in turn identifying novel strategies to remedy PTEN dysfunction for cancer prevention and therapy.
Implication for therapy
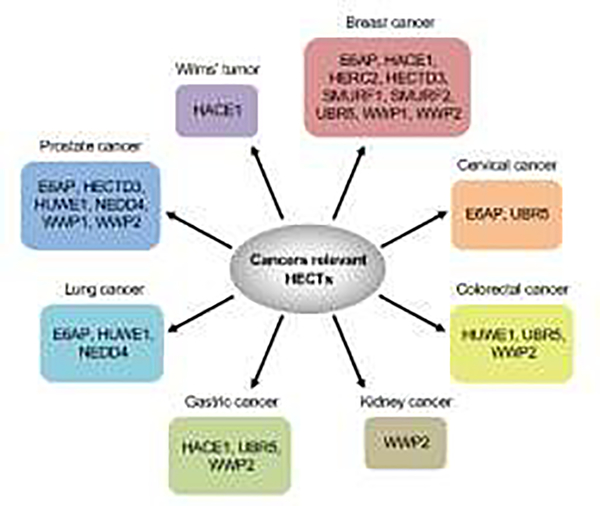
To date several members of the HECT family have been implicated in cancer pathogenesis in tumors of various histology as well as in cancer susceptibility syndromes (refs. [23, 24] and Figure 4), while further studies will expand and refine their implication in tumorigenesis. HECT family members have been also implicated the pathogenesis of other diseases including viral infections, such as COVID-19, or neurological disorders [82, 93, 94]. On this basis, it would be tempting to speculate that a portfolio of potent and very selective HECT inhibitors would represent a formidable array of new drugs for multiple indications. However, while this would be desirable for certain indications or tumor types, there is merit in exploring the development of inhibitors that can simultaneously hit multiple members of this family with differential potency. This would be desirable in the case of PTEN for a number of reasons. It is already known that multiple members of the family, (e.g. NEDD4, WWP1 and WWP2) can regulate PTEN levels and subcellular localization, in certain instances cooperatively as we discussed in this review. Thus, the selective inactivation of one member, say WWP2, could trigger tumor escape mechanisms through the upregulation of another member of the same family, say WWP1. Additionally, in certain cases it would be desirable to employ inhibitors with variable potency towards HECT family members. In this respect the example of the natural compound I3C is paradigmatic. I3C is known to inhibit both NEDD4 and WWP1 albeit with differential potency, being more potent towards WWP1 than NEDD4. This is a rather desirable outcome in the case of PTEN because a more potent inhibition of NEDD4 in its ability to monoubiquitinate PTEN could result in PTEN exclusion from the nucleus, hence impairing its nuclear tumor suppressive functions, and enhanced toxicity due to the fact that Nedd4 complete inactivation results in cellular and embryonic lethality [95–97].
Figure 4. Relevance of HECTs to different human cancer types.
E6AP, E6-associated protein; HACE1, HECT domain and ankyrin repeat containing E3 ubiquitin protein ligase 1; HERC2, HECT and RLD domain containing E3 ubiquitin protein ligase 2; HECTD3, HECT domain E3 ubiquitin protein ligase 3; SMURF1, SMAD specific E3 ubiquitin protein ligase 1; SMURF2, SMAD specific E3 ubiquitin protein ligase 2; UBR5, ubiquitin protein ligase E3 component n-recognin 5; WWP1, WW domain containing E3 ubiquitin protein ligase 1; WWP2, WW domain containing E3 ubiquitin protein ligase 2; HUWE1, HECT, UBA and WWE domain containing E3 ubiquitin protein ligase 1; NEDD4, neuronal precursor cell-expressed developmentally downregulated 4.
While the intricacy of the cross-talks between HECT E3 ligases further unravels, the opportunity to pharmacologically modulate and inactivate HECT family members, selectively and combinatorically, remains a critical area of investigation for the treatment of cancer and other diseases.
Future directions
PTEN is a bona fide lipid phosphatase that antagonizes the PI3K/AKT pathway and is recognized as a major dose-dependent tumor suppressor. The cellular mechanisms that control PTEN levels therefore offer potential routes to cancer prevention and therapy. Ubiquitin-proteasome system (UPS) is essential for the downregulation of PTEN, and HECT E3 ubiquitin ligases, such as NEDD4 and WWP2, mediate PTEN polyubiquitination and degradation. However, it still remains challenge for future research to further validate their genetic and functional roles for the regulation of PTEN in cancer development and progression and to identify the physiological signals and the availability of their interaction partners that trigger PTEN destruction in tumorigenic processes. In an emerging field of study, PTEN was found to homodimerize at the plasma membrane towards its full activation and be secreted in exosomes to exert a cell non-autonomous effect on neighboring cells, which led to the discovery of new modes of PTEN function and regulation. We now appreciate that the dimeric configuration and localization of PTEN in subcellular compartments (nucleus, plasma membrane, and exosome) rely on coordination between diverse types of ubiquitin chain linkage, which are facilitated by different HECT E3s with matching specificities. It still has remained elusive at a structural level how HECT E3s achieve linkage specificity for PTEN mono- and polyubiquitination. Although for RING (really interesting new gene)-type E3s the structural determinants of linkage-specific ubiquitin chain formation have been delineated for several E2 ubiquitin-conjugating enzymes [98], for HECT E3s the E2s do not appear to play a determining role. For HECT E3s, the chain linkage specification lies largely with the catalytic HECT domain, but the situation is likely complicated due to the high conservation of the HECT domain with HECT E3s. Additional players, reviewed herein, include different HECT adaptor proteins that contribute to higher binding affinity and linkage preference for PTFN and HECTs heterodimers that act cooperatively and sequentially on PTEN polyubiquitination. On the other hand, many deubiquitinating enzymes (DUBs) display selectivity for particular ubiquitin linkage types or positions within ubiquitin chains. It is plausible that following deubiquitination of PTEN mono- and short-chain polyubiquitin that is conferred by one HECT and has been linked to its nuclear and exogenous tumor-suppressive activities, certain DUBs can allow the other HECT to generate on PTEN K27-linked long-chains to suppress its dimerization and plasma membrane recruitment, and vice versa. Thus, further investigation is needed to identify new DUBs that orchestrate short-to-long chain and ubiquitin linkage switching on PTEN. In addition, researchers have begun to identify PTEN DUBs: HAUSP (Herpesvirus-associated ubiquitin-specific protease, also known as USP7), which specifically removes the monoubiquitination of PTEN for its nuclear export [27], and USP11 (ubiquitin specific peptidase 11), USP13 (ubiquitin specific peptidase 13) and OTUD3 (OTU deubiquitinase 3), which reverse the polyubiquitination of PTEN leading to its stabilization [99–101]. However, the DUBs specifically involved in the disassembly of K27-linked polyubiquitin chains from PTEN and subsequent PTEN dimerization and plasma membrane accumulation have not been identified to date.
These novel findings in turn offer exciting therapeutic entry points for increasing PTEN dosage by small molecule WWP2 inhibitor (e.g. ref. [102]; NSC288387), or enhancing PTEN activity through potentiation of its ability to dimerize at plasma membrane taking advantage of the NEDD4/WWP1 natural inhibitor indole-3-carbinol or other synthetic HECT inhibitors. With t e linkage or the aforementioned DUBs to PTEN reactivation, their pharmacological manipulation is undoubtedly promising venture on the horizon.
An additional intriguing puzzle has arisen following the recent studies of the existence of distinct PTEN translational variants within a cell. Like canonical PTEN, PTEN-long isoform associates with membranes and is secreted from the cell and can function as a secretory PI3K antagonist and exogenous tumor suppressor. Precisely therefore, a goal of future studies is to take advantage of administration of PTEN-long protein for therapeutic benefit. In addition, other activities for PTEN isoforms have been conceived including localization of PTEN to mitochondria to regulate energy metabolism and a role for ribosome biogenesis in the nucleolus. The canonical PTEN and various PTEN isoforms may be modulated by the same or similar mechanisms on the basis of their sequence homology, but nevertheless it will be interesting to understand how HECT E3s regulate expression, localization and activity of canonical PTEN versus distinct PTEN isoforms.
Ultimately, a plethora of roles of PTEN in crucial anticancer mechanisms hold it as an important therapeutic target. Indeed PTEN loss causes resistance to a number of cancer therapies including resistance to PI3K inhibitors, and hence, it is conceivable that identifying therapeutic PTEN reactivation regimens, including PTEN protein delivery using cationic lipidoids, increasing or restoring PTEN dosage, and enhancing PTEN lipid and protein phosphatase activities by small-molecule activators, yet unidentified, could overcome resistance to both targeted and conventional cancer therapies, while also offering major opportunities for cancer prevention and treatment.
Acknowledgements
We are thankful to Mi Kyung Park for technical support for figure illustration. This work was supported in part by National Institutes of Health grants (CA196740, CA258100) and Department of Defense grant (W81XWH-20-1-0379) to M.S.S and a grant from the PTEN Research Foundation to P.P.P.
Funding Source
This work was supported in part by National Institutes of Health grants (CA196740, CA258100) and Department of Defense grant (W81XWH-20-1-0379) to M.S.S and a grant from the PTEN UK Foundation to P.P.P.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest. P.P.P. is a cofounder of Rekindle Pharmaceuticals. The company is developing novel therapies for cancer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Maehama T, Dixon JE, The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate, J Biol Chem 273(22) (1998) 13375–13378. [DOI] [PubMed] [Google Scholar]
- [2].Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW, Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN, Cell 95(1) (1998) 29–39. [DOI] [PubMed] [Google Scholar]
- [3].Lee YR, Chen M, Pandolfi PP, The functions and regulation of the PTEN tumour suppressor: new modes and prospects, Nat Rev Mol Cell Biol 19(9) (2018) 547–562. [DOI] [PubMed] [Google Scholar]
- [4].Song MS, Salmena L, Pandolfi PP, The functions and regulation of the PTEN tumour suppressor, Nat Rev Mol Cell Biol 13(5) (2012) 283–296. [DOI] [PubMed] [Google Scholar]
- [5].Li DM, Sun H, TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta, Cancer Res 57(11) (1997) 2124–2129. [PubMed] [Google Scholar]
- [6].Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK, P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase, Proc Natl Acad Sci U S A 94(17) (1997) 9052–9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gu T, Zhang Z, Wang J, Guo J, Shen WH, Yin Y, CREB is a novel nuclear target of PTEN phosphatase, Cancer Res 71(8) (2011) 2821–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shi Y, Wang J, Chandarlapaty S, Cross J, Thompson C, Rosen N, Jiang X, PTEN is a protein tyrosine phosphatase for IRS1, Nat Struct Mol Biol 21(6) (2014) 522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tamura M, Gu J, Danen EH, Takino T, Miyamoto S, Yamada KM, PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway, J Biol Chem 274(29) (1999) 20693–20703. [DOI] [PubMed] [Google Scholar]
- [10].Wozniak DJ, Kajdacsy-Balla A, Macias V, Ball-Kell S, Zenner ML, Bie W, Tyner AL, PTEN is a protein phosphatase that targets active PTK6 and inhibits PTK6 oncogenic signaling in prostate cancer, Nat Commun 8(1) (2017) 1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z, Sahin AA, Esteva FJ, Hortobagyi GN, Yu D, Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways, Nat Med 17(4) (2011) 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kuchay S, Giorgi C, Simoneschi D, Pagan J, Missiroli S, Saraf A, Florens L, Washburn MP, Collazo-Lorduy A, Castillo-Martin M, Cordon-Cardo C, Bebti SM, Pinton P, Pagano M, PTEN counteracts FBXL2 to promote IP3R3- and Ca(2+)-mediated apoptosis limiting tumour growth, Nature 546(7659) (2017) 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, Pandolfi PP, Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner, Cell 144(2) (2011) 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhao D, Lu X, Wang G, Lan Z, Liao W, Li J, Liang X, Chen JR, Shah S, Shang X, Tang M, Deng P, Dey P, Chakravarti D, Chen P, Spring DJ, Navone NM, Troncoso P, Zhang J, Wang YA, DePinho RA, Synthetic essentiality of chromatin remodelling factor CHD1 in PTEN-deficient cancer, Nature 542(7642) (2017) 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hollander MC, Blumenthal GM, Dennis PA, PTEN loss in the continuum of common cancers, rare syndromes and mouse models, Nat Rev Cancer 11 (4) (2011) 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Salmena L, Carracedo A, Pandolfi PP, Tenets of PTEN tumor suppression, Cell 133(3) (2008) 403–414. [DOI] [PubMed] [Google Scholar]
- [17].Eng C, PTEN: one gene, many syndromes, Hum Mutat 22(3) (2003) 183–198. [DOI] [PubMed] [Google Scholar]
- [18].Tan MH, Mester J, Peterson C, Yang Y, Chen JL, Rybicki LA, Milas K, Pederson H, Remzi B, Orloff MS, Eng C, A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands, Am J Hum Genet 88(1) (2011) 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Papa A, Pandolfi PP, The PTEN(−)PI3K Axis in Cancer, Biomolecules 9(4) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dillon LM, Miller TW, Therapeutic targeting of cancers with loss of PTEN function, Curr Drug Targets 15(1) (2014) 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Juric D, Castel P, Griffith M, Griffith OL, Won HH, Ellis H, Ebbesen SH, Ainscough BJ, Ramu A, Iyer G, Shah RH, Huynh T, Mino-Kenudson M, Sgroi D, Isakoff S, Thabet A, Elamine L, Solit DB, Lowe SW, Quadt C, Peters M, Derti A, Schegel R, Huang A, Mardis ER, Berger MF, Baselga J, Scaltriti M, Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor, Nature 518(7538) (2015) 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Weber J, Polo S, Maspero E, HECT E3 Ligases: A Tale With Multiple Facets, Front Physiol 10 (2019) 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bernassola F, Karin M, Ciechanover A, Melino G, The HECT family of E3 ubiquitin ligases: multiple players in cancer development, Cancer Cell 14(1) (2008) 10–21. [DOI] [PubMed] [Google Scholar]
- [24].Bernassola F, Chillemi G, Melino G, HECT-Type E3 Ubiquitin Ligases in Cancer, Trends Biochem Sci 44(12) (2019) 1057–1075. [DOI] [PubMed] [Google Scholar]
- [25].Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, Jiang X, NEDD4–1 is a proto-oncogenic ubiquitin ligase for PTEN, Cell 128(1) (2007) 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y, Essential role for nuclear PTEN in maintaining chromosomal integrity, Cell 128(1) (2007) 157–170. [DOI] [PubMed] [Google Scholar]
- [27].Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, Pandolfi PP, The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PM L network, Nature 455(7214) (2008) 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, Tempst P, Chi SG, Kim HJ, Misteli T, Jiang X, Pandolfi PP, Ubiquitination regulates PTEN nuclear import and tumor suppression, Cell 128(1) (2007) 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J, Tan SS, The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells, Sci Signal 5(243) (2012) ra70. [DOI] [PubMed] [Google Scholar]
- [30].Hong SW, Moon JH, Kim JS, Shin JS, Jung KA, Lee WK, Jeong SY, Hwang JJ, Lee SJ, Suh YA, Kim I, Nam KY, Han S, Kim JE, Kim KP, Hong YS, Lee JL, Lee WJ, Choi EK, Lee JS, Jin DH, Kim TW, p34 is a novel regulator of the oncogenic behavior of NEDD4–1 and PTEN, Cell Death Differ 21(1) (2014) 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hamilton MH, Tcherepanova I, Huibregtse JM, McDonnell DP, Nuclear import/export of hRPF1/Nedd4 regulates the ubiquitin-dependent degradation of its nuclear substrates, J Biol Chem 276(28) (2001) 26324–26331. [DOI] [PubMed] [Google Scholar]
- [32].Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ, Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease, Nat Genet 29(4) (2001) 404–411. [DOI] [PubMed] [Google Scholar]
- [33].Hsia HE, Kumar R, Luca R, Takeda M, Courchet J, Nakashima J, Wu S, Goebbels S, An W, Eickholt BJ, Polleux F, Rotin D, Wu H, Rossner MJ, Bagni C, Rhee JS, Brose N, Kawabe H, Ubiquitin F3 ligase Nedd4–1 acts as a downstream target of PI3K/PTEN-mTORC1 signaling to promote neurite growth, Proc Natl Acad Sci U S A 111(36) (2014) 13205–13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ding X, Jo J, Wang CY, Cristobal CD, Zuo Z, Ye Q, Wirianto M, Lindeke-Myers A, Choi JM, Mohila CA, Kawabe H, Jung SY, Bellen HJ, Yoo SH, Lee HK, The Daam2-VHL-Nedd4 axis governs developmental and regenerative oligodendrocyte differentiation, Genes Dev 34(17–18) (2020) 1177–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu Y, Oppenheim R.w., Sugiura Y, Lin W, Abnormal development of the neuromuscular junction in Nedd4-deficient mice, Dev Biol 330(1) (2009) 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fouladkou F, Landry T, Kawabe H, Neeb A, Lu C, Brose N, Stambolic V, Rotin D, The ubiquitin ligase Nedd4–1 is dispensable for the regulation of PTEN stability and localization, Proc Natl Acad Sci U S A 105(25) (2008) 8585–8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Howitt J, Lackovic J, Low LH, Naguib A, Macintyre A, Goh CP, Callaway JK, Hammond V, Thomas T, Dixon M, Putz U, Silke J, Bartlett P, Yang B, Kumar S,Trotman LC, Tan SS, Ndfip1 regulates nuclear Pten import in vivo to promote neuronal survival following cerebral ischemia, J Cell Biol 196(1) (2012) 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang X, Shi Y, Wang J, Huang G, Jiang X, Crucial role of the C-terminus of PTEN in antagonizing NEDD4–1-mediated PTEN ubiquitination and degradation, Biochem J 414(2) (2008) 221–229. [DOI] [PubMed] [Google Scholar]
- [39].Cai J, Li R, Xu X, Zhang L, Lian R, Fang L, Huang Y, Feng X, Liu X, Li X, Zhu X, Zhang H, Wu J, Zeng M, Song E, He Y, Yin Y, Li J, Li M, CK1alph suppresses lung tumour growth by stabilizing PTEN and inducing autophagy, Nat Cell Biol 20(4) (2018) 465–478. [DOI] [PubMed] [Google Scholar]
- [40].Al-Khouri AM, Ma Y, Togo SH, Williams S, Mustelin T, Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta, J Biol Chem 280(42) (2005) 35195–35202. [DOI] [PubMed] [Google Scholar]
- [41].Maccario H, Perera NM, Davidson L, Downes CP, Leslie NR, PTEN is destabilized by phosphorylation on Thr366, Biochem J 405(3) (2007) 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yim EK, Peng G, Dai H, Hu R, Li K, Lu Y, Mills GB, Meric-Bernstam F, Hennessy BT, Craven RJ, Lin SY, Rak functions as a tumor suppressor by regulating PTEN protein stability and functor, Cancer Cell 15(4) (2009) 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Li Q, Bai Y, Lyle LT, Yu G, Amarasinghe O, Nguele Meke F, Carlock C, Zhang ZY, Mechanism of PRL2 phosphatase-mediated PTEN degradation and tumorigenesis, Proc Natl Acad Sci U S A 117(34) (2020) 20538–20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mari S, Ruetalo N, Maspero E, Stoffregen MC, Pasqualato S, Polo S, Wiesner S, Structural and functional framework for the autoinhibition of Nedd4-family ubiquitin ligases, Structure 22(11) (2014) 1639–1649. [DOI] [PubMed] [Google Scholar]
- [45].Wiesner S, Ogunjimi AA, Wang HR, Rotin D, Sicheri F, Wrana JL, Forman-Kay JD, Autoinhibition of the HECT-type ubiquitin ligase Smurf2 through its C2 domain, Cell 130(4) (2007) 651–662. [DOI] [PubMed] [Google Scholar]
- [46].Lee YR, Chen M, Lee JD, Zhang J, Lin SY, Fu TM, Chen H, Ishikawa T, Chiang SY, Katon J, Zhang Y, Shulga YV, Bester AC, Fung J, Monteleone E, Wan L, Shen C, Hsu CH, Papa A, Clohessy JG, Teruya-Feldstein J, Jain S, Wu H, Matesic L, Chen RH, Wei W, Pandolfi PP, Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway, Science 364(6441) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhi X, Chen C, WWP1: a versatile ubiquitin E3 ligase in signaling and diseases, Cell Mol Life Sci 69(9) (2012) 1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chen C, Zhou Z, Ross JS, Zhou W, Dong JT, The amplified WWP1 gene is a potential molecular target in breast cancer, Int J Cancer 121(1) (2007) 80–87. [DOI] [PubMed] [Google Scholar]
- [49].Chen C, Sun X, Guo P, Dong XY, Sethi P, Zhou W, Zhou Z, Petros J, Frierson HF Jr., Vessella RL, Atfi A, Dong JT, Ubiquitin E3 ligase W WP1 as an oncogenic factor in human prostate cancer, Oncogene 26(16) (2007) 2386–2394. [DOI] [PubMed] [Google Scholar]
- [50].Zhang XF, Chao J, Pan QZ, Pan K, Weng DS, Wang QJ, Zhao JJ, He J, Liu Q, Jiang SS, Chen CL, Zhang HX, Xia JC, Overexpression of WWP1 promotes tumorigenesis and predicts unfavorable prognosis in patients with hepatocellular carcinoma, Oncotarget 6(38) (2015) 40920–40933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chen C, Matesic LE, The Nedd4-like family of E3 ubiquitin ligases and cancer, Cancer Metastasis Rev 26(3–4) (2007) 587–604. [DOI] [PubMed] [Google Scholar]
- [52].Cheng Q, Cao X, Yuan F, Li G, Tong T, Knockdown of WWP1 inhibits growth and induces apoptosis in hepatoma carcinoma cells through the activation of caspase3 and p53, Biochem Biophys Res Commun 448(3) (2014) 248–254. [DOI] [PubMed] [Google Scholar]
- [53].Zhang L, Wu Z, Ma Z, Liu H, Wu Y, Zhang Q, WWP1 as a potential tumor oncogene regulates PTEN-Akt signaling pathway in human gastric carcinoma, Tumour Biol 36(2) (2015) 787–798. [DOI] [PubMed] [Google Scholar]
- [54].Li Y, Zhou Z, Chen C, WW domain-containing E3 ubiquitin protein ligase 1 targets p63 transaction factor for ubiquitin-mediated proteasomal degradation and regulates apoptosis, Cell Death Differ 15(12) (2008) 1941–1951. [DOI] [PubMed] [Google Scholar]
- [55].Komuro A, Imamura T, Saitoh M, Yoshida Y, Yamori T, Miyazono K, Miyazawa K, Negative regulation of transforming growth factor-beta (TGF-beta) signaling by WW domain-containing protein 1 (WWP1), Oncogene 23(41) (2004) 6914–6923. [DOI] [PubMed] [Google Scholar]
- [56].Seo SR, Lallemand F, Ferrand N, Pessah M, L’Hoste S, Camonis J, Atfi A, The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation, Embo J 23(19) (2004) 3780–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hoshino S, Kobayashi M, Tagawa R, Konno R, Abe T, Furuya K, Miura K, Wakasawa H, Okita N, Sudo Y, Mizunoe Y, Nakagawa Y, Nakamura T, Kawabe H, Higami Y, WWP1 knockout in mice exacerbates obesity-related phenotypes in white adipose tissue but improves whole-body glucose metabolism, FEBS Open Bio 10(3) (2020) 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Garcia-Cao I, Song MS, Hobbs RM, Laurent G, Giorgi C, de Boer VC, Anastasiou D, Ito K, Sasaki AT, Rameh L, Carracedo A, Vander Heiden MG, Cantley LC, Pinton P, Haigis MC, Pandolfi PP, Systemic elevation of PTEN induces a tumor-suppressive metabolic state, Cell 149(1) (2012) 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jiang H, Dempsey DR, Cole PA, Ubiquitin Ligase Activities of WWP1 Germline Variants K740N and N745S, Biochemistry 60(5) (2021) 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lee YR, Yehia L, Kishikawa T, Ni Y, Leach B, Zhang J, Panch N, Liu J, Wei W, Eng C, Pandolfi PP, WWP1 Gain-of-Function Inactivation of PTEN in Cancer Predisposition, N Engl J Med 382(22) (2020) 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Quirit JG, Lavrenov SN, Poindexter K, Xu J, Kyauk C, Durkin KA, Aronchik I, Tomasiak T, Solomatin YA, Preobrazhenskaya MN, Firestone GL, Indole-3-carbinol (I3C) analogues are potent small molecule inhibitors of NEDD4–1 ubiquitin ligase activity that disrupt proliferation of human melanoma cells, Biochem Pharmacol 127 (2017) 13–27. [DOI] [PubMed] [Google Scholar]
- [62].Soond SM, Chantry A, Selective targeting of activating and inhibitory Smads by distinct W WP2 ujiquitin ligase isoforms differentially modulates TGFbeta signalling and EMT, Oncogene 30(21) (2011) 2451–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Soond SM, Smith PG, Wahl L, Swingler TE, Clark IM, Hemmings AM, Chantry A, Novel WWP2 ubiquitin ligase isoforms as potential prognostic markers and molecular targets in cancer, Biochimica et biophysica acta 1832(12) (2013) 2127–2135. [DOI] [PubMed] [Google Scholar]
- [64].Fang S, Zhang D, Weng W, Lv X, Zheng L, Chen M, Fan X, Mao J, Mao C, Ye Y, Xu M, Ji J, CPSF7 regulates liver cancer growth and metastasis by facilitating WWP2-FL and targeting the WWP2/PTEN/AKT signaling pathway, Biochim Biophys Acta Mol Cell Res 1867(2) (2020) 118624. [DOI] [PubMed] [Google Scholar]
- [65].Maddika S, Kavela S, Rani N, Palicharla VR, Pokorny JL, Sarkaria JN, Chen J, WWP2 is an E3 ubiquitin ligase for PTEN, Nat Cell Biol 13(6) (2011) 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Li H, Zhang P, Zhang Q, Li C, Zou W, Chang Z, Cui CP, Zhang L, WWP2 is a physiological ubiquitin ligase for phosphatase and tensin homolog (PTEN) in mice, J Biol Chem 293(23) (2018) 8886–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fukumoto C, Nakashima D, Kasamatsu A, Unozawa M, Shida-Sakazume T, Higo M, Ogawara K, Yokoe H, Shiiba M, Tanzawa H, Uzawa K, VWP2 is overexpressed in human oral cancer, determining tumor size and poor prognosis in patients: downregulation of WWP2 inhibits the AKT signaling and tumor growth in mice, Oncoscience 1(12) (2014) 807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yang Y, Liao B, Wang S, Yan B, Jin Y, Shu HB, Wang YY, E3 ligase WWP2 negatively regulates TLR3-mediated innate immune response by targeting TRIF for ubiquitination and degradation, Proc Natl Acad Sci U S A 110(13) (2013) 5115–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Marcucci R, Brindle J, Paro S, Casadio A, Hempel S, Morrice N, Bisso A, Keegan LP, Del Sal G, O’Connell MA, Pin1 and WWP2 regulate GluR2 Q/R site RNA editing by ADAR2 with opposing effects, Embo J 30(20) (2011) 4211–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Jolliffe CN, Harvey KF, Haines BP, Parasivam G, Kumar S, Identification of multiple proteins expressed in murine embryos as binding partners for the WW domains of the ubiquitin-protein ligase Nedd4, Biochem J 351 Pt 3 (2000) 557–565. [PMC free article] [PubMed] [Google Scholar]
- [71].Shearwin-Whyatt LM, Brown DL, Wylie FG, Stow JL, Kumar S, N4WBP5A (Ndfip2), a Nedd4-interacting protein, localizes to multivesicular bodies and the Golgi, and has a potential role in protein trafficking, J Cell Sci 117(Pt 16) (2004) 3679–3689. [DOI] [PubMed] [Google Scholar]
- [72].Shah SS, Kumar S, Adaptors as the regulators of HECT ubiquitin ligases, Cell Death Differ 28(2) (2021) 455–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mund T, Pelham HR, Control of the activity of WW-HECT domain E3 ubiquitin ligases by NDFIP proteins, EMBO Rep 10(5) (2009) 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Howitt J, Low LH, Putz U, Doan A, Lackovic J, Goh CP, Gunnersen J, Silke J, Tan SS, Ndfip1 represses cell proliferation by controlling Pten localization and signaling specificity, J Mol Cell Biol 7(2) (2015) 119–131. [DOI] [PubMed] [Google Scholar]
- [75].Igarashi A, Itoh K, Yamada T, Adachi Y, Kato T, Murata D, Sesaki H, lijima M, Nuclear PTEN deficiency causes microcephaly with decreased neuronal soma size and increased seizure susceptibility, J Biol Chem 293(24) (2018) 9292–9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jiang H, Thomas SN, Chen Z, Chiang CY, Cole PA, Comparative analysis of the catalytic regulation of NEDD4–1 and WWP2 ubiquitin ligases, J Biol Chem 294(46) (2019) 17421–17436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mund T, Pelham HR, Regulation of PTEN/Akt and MAP kinase signaling pathways by the ubiquitin ligase activators Ndfip1 and Ndfip2, Proc Natl Acad Sci U S A 107(25) (2010) 11429–11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Shao C, Li Z, Ahmad N, Liu X, Regulation of PTEN degradation and NEDD4–1 E3 ligase activity by Numb, Cell Cycle 16(10) (2017) 957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sander B, Xu W, Eilers M, Popov N, Lorenz S, A conformational switch regulates the ubiquitin ligase HUWE1, Elife 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Attali I, Tobelaim WS, Persaud A, Motamedchaboki K, Simpson-Lavy KJ, Mashahreh B, Levin-Kravets O, Keren-Kaplan T, Pilzer I, Kupiec M, Wiener R, Wolf DA, Rotin D, Prag G, Ubiquitylation-dependent oligomerization regulates activity of Nedd4 ligases, Embo J 36(4) (2017) 425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wan L, Zou W, Gao D, Inuzuka H, Fukushima H, Berg AH, Drapp R, Shaik S, Hu D, Lester C, Eguren M, Malumbres M, Glimcher LH, Wei W, Cdh1 regulates osteoblast function through an APC/C-independent modulation of Smurf1, Mol Cell 44(5) (2011) 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Novelli G, Liu J, Biancolella M, Alonzi T, Novelli A, Patten JJ, Cocciadiferro D, Agolini E, Colona VL, Rizzacasa B, Giannini R, Bigio B, Goletti D, Capobianchi MR, Grelli S, Mann J, McKee TD, Cheng K, Amanat F, Krammer F, Guarracino A, Pepe G, Tomino C, Tandjaoui-Lambiotte Y, Uzunhan Y, Tubiana S, Ghosn J, Effort CHG, French CCSG, Co VCC, Notarangelo LD, Su HC, Abel L, Cobat A, Elhanan G, Grzymski JJ, Latini A, Sidhu SS, Jain S, Davey RA, Casanova JL, Wei W, Pandolfi PP, Inhibition of HECT E3 ligases as potential therapy for COVID-19, Cell Death Dis 12(4) (2021) 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Chaudhary N, Maddika S, WWP2-WWP1 ubiquitin ligase complex coordinated by PPM1G maintains the balance between cellular p73 and DeltaNp73 levels, Mol Cell Biol 34(19) (2014) 3754–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zeng T, Wang Q, Fu J, Lin Q, Bi J, Ding W, Qiao Y, Zhang S, Zha o W, Lin H, Wang M, Lu B, Deng X, Zhou D, Yin Z, Wang HR, Impeded Ne dd4–1-mediated Ras degradation underlies Ras-driven tumorigenesis, Cell Rep 7(3) (2014) 871–882. [DOI] [PubMed] [Google Scholar]
- [85].Wang Y, Yuan B, Qiao L, Yang H, Li X, STAT3 operates as a novel transcription factor that regulates NEDD4 in Keloid, Biochem Biophys Res Commun 518(4) (2019) 638–643. [DOI] [PubMed] [Google Scholar]
- [86].Fujita M, Yamamoto Y, Jiang JJ, Atsumi T, Tanaka Y, Ohki T, Murao N, Funayama E, Hayashi T, Osawa M, Maeda T, Kamimura D, Murakami M, NEDD4 Is Involved in Inflammation Development during Keloid Formation, J Invest Dermatol 139(2) (2019) 333–341. [DOI] [PubMed] [Google Scholar]
- [87].Persaud A, Alberts P, Mari S, Tong J, Murchie R, Maspero E, Safi F, Moran MF, Polo S, Rotin D, Tyrosine phosphorylation of NEDD4 activates its ubiquitin ligase activity, Sci Signal 7(346) (2014) ra95. [DOI] [PubMed] [Google Scholar]
- [88].Zhang X, Li B, Rezaeian AH, Xu X, Chou PC, Jin G, Han F, Pan BS, Wang CY, Long J, Zhang A, Huang CY, Tsai FJ, Tsai CH, Logothetis C, Lin HK, H3 ubiquitination by NEDD4 regulates H3 acetylation and tumorigenesis, Nat Commun 8 (2017) 14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Liu J, Wan L, Liu P, Inuzuka H, Liu J, Wang Z, Wei W, SCF(beta-TRCP)-mediated degradation of NEDD4 inhibits tumorigenesis through modulating the PTEN/Akt signaling pathway, Oncotarget 5(4) (2014) 1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sluimer J, Distel B, Regulating the human HECT E3 ligases, Cell Mol Life Sci 75(17) (2018) 3121–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Malakhova OA, Zhang DE, ISG15 inhibits Nedd4 ubiquitin E3 activity and enhances the innate antiviral response, J Biol Chem 283(14) (2008) 8783–8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Okumura A, Pitha PM, Harty RN, ISG15 inhibits Ebola VP40 VLP budding in an L-domain-dependent manner by blocking Nedd4 ligase activity, Proc Natl Acad Sci U S A 105(10) (2008) 3974–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Rougeulle C, Glatt H, Lalande M, The Angelman syndrome candidate gene, UBE3A/E6-AP, is imprinted in brain, Nat Genet 17(1) (1997) 14–15. [DOI] [PubMed] [Google Scholar]
- [94].Vu TH, Hoffman AR, Imprinting of the Angelman syndrome gene, UBE3A, is restricted to brain, Nat Genet 17(1) (1997) 12–13. [DOI] [PubMed] [Google Scholar]
- [95].Boase NA, Rychkov GY, Townley SL, Dinudom A, Candi E, Voss AK, Tsoutsman T, Semsarian C, Melino G, Koentgen F, Cook DI, Kumar S, Respiratory distress and perinatal lethality in Nedd4–2-deficient mice, Nat Commun 2 (2011) 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Fouladkou F, Lu C, Jiang C, Zhou L, She Y, Walls JR, Kawabe H, Brose N, Henkelman RM, Huang A, Bruneau BG, Rotin D, The ubiquitin ligase Nedd4–1 is required for heart development and is a suppressor of thrombospondin-1, J Biol Chem 285(9) (2010) 6770–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kawabe H, Neeb A, Dimova K, Young SM Jr., Takeda M, Katsurabayashi S, Mitkovski M, Malakhova OA, Zhang DE, Umikawa M, Kariya K, Goebbels S, Nave KA, Rosenmund C, Jahn O, Rhee J, Brose N, Regulation of Rap2A by the ubiquitin ligase Nedd4–1 controls neurite development, Neuron 65(3) (2010) 358–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Stewart MD, Ritterhoff T, Klevit RE, Brzovic PS, E2 enzymes: more than just middle men, Cell research 26(4) (2016) 423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Park MK, Yao Y, Xia W, Setijono SR, Kim JH, Vila IK, Chiu HH, Wu Y, Billalabeitia EG, Lee MG, Kalb RG, Hung MC, Pandolfi PP, Song SJ, Song MS, PTEN self-regulates through USP11 via the PI3K-FOXO pathway to stabilize tumor suppression, Nat Commun 10(1) (2019) 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yuan L, Lv Y, Li H, Gao H, Song S, Zhang Y, Xing G, Kong X, Wang L, Li Y, Zhou T, Gao D, Xiao ZX, Yin Y, Wei W, He F, Zhang L, Deubiquitylase OTUD3 regulates PTEN stability and suppresses tumorigenesis, Nat Cell Biol 17(9) (2015) 1169–1181. [DOI] [PubMed] [Google Scholar]
- [101].Zhang J, Zhang P, Wei Y, Piao HL, Wang W, Maddika S, Wang M, Chen D, Sun Y, Hung MC, Chen J, Ma L, Deubiquitylation and stabilization of PTEN by USP13, Nat Cell Biol 15(12) (2013) 1486–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Watt JE, Hughes GR, Walpole S, Monaco S, Stephenson GR, Bulman Page PC, Hemmings AM, Angulo J, Chantry A, Discovery of Small Molecule WW /P2 Ubiquitin Ligase Inhibitors, Chemistry 24(67) (2018) 17677–17680. [DOI] [PubMed] [Google Scholar]