Abstract
Objective
Sulforaphane (SFN) is a natural free radical scavenger that can reduce oxidative stress (OS) through mediating nuclear factor (erythroid-derived 2)-like 2 (NF-E2-related factor 2 or NRF2)/antioxidant response element (ARE) signaling pathway and the downstream antioxidant enzymes. Here, we intended to study the role of SFN in OS- induced human granulosa cells (GCs) by investigating the intracellular levels of reactive oxygen species (ROS), cell death, and NRF2-ARE pathway.
Materials and Methods
This experimental study was conducted on GCs of 12 healthy women who had normal menstrual cycles with no history of polycystic ovary syndrome (PCOS), endometriosis, menstrual disorders, hyperprolactinemia, or hormonal therapy. After isolation of GCs, the MTT assay was performed to explore GCs viability after treatment with SFN in the presence or absence of H2O2. Flow cytometry was utilized to determine the intracellular ROS production and the apoptosis rate. Evaluation of the mRNA and protein expression levels of NRF2 and phase II enzymes including superoxide dismutase (SOD) and catalase (CAT) was performed by quantitative real-time polymerase chain reaction (PCR) and western blotting. Finally, the data were analyzed by SPSS software using One-way ANOVA and the suitable post-hoc test. Significance level was considered as P<0.05.
Results
Pretreatment of GCs with SFN attenuated intracellular ROS production and apoptosis rate in the H2O2-exposed cells. Moreover, SFN treatment increased the mRNA expression level of NRF2, SOD, and CAT. Higher expression of NRF2 and SOD was also observed at the protein level.
Conclusion
Our study demonstrated that SFN protects human GCs against H2O2induced-OS by reducing the intracellular ROS production and the following apoptosis through a mechanism by which NRF2 increases the antioxidant enzymes such as SOD and CAT. This result may have a potential application in assisted reproduction cycles by improving the quality of GCs and the embedded oocyte, especially in PCOS patients.
Keywords: Granulosa Cells, NF-E2-Related Factor 2, Oxidative Stress, Sulforaphane
Introduction
Oxidative stress (OS) occurs when there is a disruption in redox homeostasis, a state which refers to the natural capacity of a cell to handle the challenges that produce electrophile molecules such as reactive oxygen species (ROS) (1). Indeed, excessive ROS production takes place during OS and results in damage to cellular components including DNA, proteins, and lipids. Hydrogen peroxide (H2 O2), superoxide (•O2), and hydroxyl radical (•OH), the most important types of ROS, are produced as byproducts during cellular metabolism activities (2). These factors participate in the modulation of molecular and biological mechanisms in reproduction (3). However, excessive production of ROS leads to the disruption of endogenous redox homeostasis and consequently activation of cell death mechanisms such as apoptosis (4). Therefore, ROS are considered as a double-edged sword (5). In this manner, an adapted antioxidant system conducts the elimination of excessive ROS and maintains the balance between ROS production and cellular antioxidant capacity, which finally protects the cell from the damages of OS (1).
It has been revealed that ROS plays a regulatory role in the female reproductive system, especially during folliculogenesis, steroidogenesis, oocyte maturation, and luteolysis (6). However, when the level of ROS exceeds the normal value, as describes above as an OS condition, several reproductive disorders may occur including polycystic ovary syndrome (PCOS) (7). Hence, there is a link between OS and pathophysiology of disorders of the female reproductive system (6). Granulosa cells (GCs), somatic steroidogenic cells surrounding the oocyte, play a vital role in oocyte maturation (8). These cells produce nutrients and growth factors needed for the oocyte maturation and have a complicated antioxidant system preserving oocytes from the damages of OS (9). Nevertheless, a high level of ROS production caused by disruption of the intrinsic antioxidant system in GCs, leads to apoptosis in GCs and contributes to poor oocyte maturation especially in the cases of PCOS (10).
Kelch‑like ECH‑associated protein 1 (KEAP1)‑nuclear factor erythroid 2‑related factor 2 (NRF2)‑antioxidant response element (ARE) signaling pathway is one of the most important mechanisms in OS regulation (11). NRF2, is an essential transcription factor for the expression of different antioxidant genes and thus the primary cellular mean against OS (12). Under normal conditions, KEAP1 molecule deactivates NRF2 by binding to it and sequestering it from nuclei, where its target genes reside (11). During OS, oxidation of cysteine residue inactivates KEAP1, leading to activation and translocation of NRF2 into the nucleus where it binds to ARE promoter region (13). Consequently, NRF2 enhances cellular antioxidant capacity by upregulating the gene expression of downstream antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT), the first-line defense enzymes in the elimination process of ROS (14). It has been reported that NRF2-ARE pathway plays an essential role against OS in murine, bovine, and human GCs (15-18). Therefore, induction of this signaling pathway using exogenous activators may potentially be a beneficial route for the management of ROS generation in GCs.
Sulforaphane (SFN) is an organic isothiocyanate with antioxidant activity found in the Brassicaceae family, e.g. broccoli (19). SFN has a lipophilic nature and translocates into cells by passive diffusion due to its low molecular weight, and has various impacts such as antioxidant, anti-apoptotic, and anti-inflammatory effects (20). Several in vivo and in vitro studies have indicated that SFN can induce NRF2 pathway and promotes the downstream antioxidant genes (16, 21). It has also been reported that SFN improves cell viability and decreases the cytotoxicity in bovine OS-induces GCs (16). Nevertheless, there is no evidence of its protective effects on human OS-induced GCs. This study aimed to explore the effects of SFN in activation of NRF2-ARE pathway and its downstream antioxidant enzymes, SOD and CAT, in OS-induced cultured human GCs.
Materials and Methods
Study population
This experimental study was conducted on GCs of healthy women aged from 20-38 years old referred to the Infertility Department of Shariati Hospital, Tehran, Iran. All the participants had a normal ovulatory function and menstrual cycle (25-35 days), without a history of PCOS, endometriosis, hirsutism, menstrual disorders, hyperprolactinemia, or hormonal therapy. The GCs were isolated from follicular fluid during ovum pickup. The Research Ethics Committee of Tehran University of Medical Sciences approved the study (IR.TUMS. MEDICINE.REC.1397.230) and informed consent was obtained from all participants. A total of 12 individuals participated in the study.
Ovarian hyperstimulation protocol
For ovarian hyperstimulation, gonadotropin-releasing hormone (GnRH) antagonist protocol was performed in all participants. Briefly, recombinant follicle stimulating hormone (rFSH,150-225 IU, Gonal-F®, Merck Serono SA, Switzerland) was administered on day 3 from the beginning of the menstrual cycle and sustained until at least 2 follicles achieve the size of 14 to 15 mm. To assess the follicular growth, transvaginal ultrasonography was performed. Next, GnRH antagonist (0.25 mg, Cetrotide®, Merck Serono SA, Switzerland) was administered and continued until the dominant follicles achieve the size of 18 mm. Human chorionic gonadotropin (hCG, 10,000 IU, Choriomon®, IBSA, Lugano, Switzerland) was utilized for the final maturation of the oocytes. After 36 hours of hCG administration, transvaginal ultrasound-guided follicular aspiration was performed for oocyte retrieval. The follicles with a size above 18 mm were used for the isolation of GCs.
Granulosa cells isolation and culture
Human GCs were isolated and purified as previously described (22), which provides the highest percentage yield of live purified GCs using density gradient centrifugation. In brief, the isolated follicular fluids were pooled from differentpatients to reduce the variability of individual samples. Then, 3000 rpm centrifugation was performed for 10 minutes to remove the supernatant and the pellet was resuspended in 2.5 ml of Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12, Gibco, USA). GCs were isolated using Ficoll-Paque Plus solution (GE Healthcare, United Kingdom), followed by another centrifugation at 3000 rpm for 10 min. Next, GCs were washed and cultured in a complete medium containing DMEM/F-12 supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco, USA), 100 U/ml of penicillin (Gibco, USA), 100 mg/ml of (Gibco, USA), 2 mmol/l of glutmax (Sigma-Aldrich, USA), and 2 mg/ ml of amphotericin B (PAN Biotech, Germany) at 37°C and 5% CO2 . The medium was changed after 48 hours.
Experimental groups
After 48 hours of culture, the cells were divided into 4 experimental groups:
Group 1: GCs were cultured for 24 hours in the presence of dimethyl sulfoxide (DMSO) as the vehicle of SFN
Group 2: GCs were cultured for 22 hours and then exposed to 200 µM of H2 O2 for another 2 hours to induce OS
Group 3: GCs were cultured for 24 hours in the presence of SFN
Group 4: GCs pretreated with SFN for 22 hours and then exposed to 200 µM of H2 O2 for another 2 hours
The dose and duration of H2 O2 exposure were selected according to our recently published model for OS induction in human GCs (23).
Cell viability assay
3 - (4, 5- d i m e t h y l t h i a z o l- 2- y l) - 2 , 5-diphenyltetrazolium bromide (MTT) (Alfa Aesar by Thermo Fisher Scientific, England) assay was conducted to evaluate the cytotoxicity of SFN (S4441, Sigma-Aldrich, USA) and to determine its optimal dose for treating cells. First, SFN (stock concentration: 5 mg/mL) was diluted in DMSO (Sigma-Aldrich, USA) and different concentrations of it were prepared by diluting in the culture medium including 5, 10, 15, 20, 25, and 30 µM. Then, GCs were seeded on a 96-well plate at a density of 1×104 cells per well and exposed to the mentioned doses of SFN for 24 hours. Furthermore, we also pretreated GCs with the mentioned concentrations of SFN for 22 hours and then exposed them to 200 µM of H2 O2 for 2 hours to determine an optimal dose of SFN for protecting cells against H2 O2 induced OS. Next, 0.5 mg/ml of MTT solution was added to the wells and the cells were incubated at 37°C for 4 hours in dark. Then the produced colorful crystals were dissolved in DMSO and, the optical density (OD) was measured at 570 nm wavelength using a microplate reader (BioTek, USA).
Intracellular reactive oxygen species levels measurement
The intracellular ROS levels of experimental groups were measured by flow cytometric analysis using 2ˊ-7ˊ-Dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma-Aldrich, USA) fluorescent probe according to the manufacturer’s protocol. In detail, GCs were seeded at a density of 2×105 cells per well in a 6 well plate and treated as described above. Next, the cells were incubated with DCFH-DA at a concentration of 1 µM for 30 minutes at 37°C and then washed and resuspended in phosphate-buffered saline (PBS). The fluorescence intensity was measured in the FL-1 channel at a wavelength between 500 and 530 nm by BD FACScan flow cytometry (Becton Dickinson, USA). About 10,000 cells were analyzed for each group. Data were analyzed using Flowjo software (Flowjo 7.6.1).
Apoptosis assay
The Annexin V-fluorescein isothiocyanate (FITC)/ propidium iodide (PI) apoptosis detection kit (Thermo Fisher Scientific, USA) was used as per the manufacturers’ protocol to define the total apoptotic cells and distinguish apoptosis from necrosis. In brief, GCs were seeded at a density of 2×105 cells per well in a 6-well plate in the defined groups. Then, cells were suspended in 1X annexin-binding buffer and, incubated with Annexin V-FITC and PI at room temperature for 15 minutes in dark. The fluorescence emission was evaluated using BD FACScan flow cytometry (Becton Dickinson, USA) and the results were analyzed using Flowjo software (Flowjo 7.6.1). Briefly, the following criteria were used for interpretation of the data obtained from flow cytometry:
Q1 area: Annexin V-negative, PI-positive GCs are necrotic GCs,
Q2 area: Annexin V-positive, PI-positive GCs are late apoptotic GCs,
Q3 area: Annexin V-positive, PI-negative GCs are early apoptotic GCs,
Q4 area: Annexin V-negative, PI-negative GCs are viable GCs.
Quantitative real-time polymerase chain reaction
The total RNA was extracted by TRIzol reagent (Life Technologies, USA) based on the manufacturer’s instructions. Then, cDNA was synthesized using 1 μg of total RNA by a First-Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA). Quantitative real-time polymerase chain reaction (PCR) was conducted to investigate the levels of target mRNAs using a RealQ Plus Master Mix Green (Ampliqon, Denmark) by Applied Biosystems StepOne real-time PCR (Applied Biosystems, USA). The 2-ΔΔCt method was applied for data analysis. GAPDH was considered as a housekeeping gene. The specific primers for target genes are provided in Table 1.
Table 1.
Specific primers used for quantitative real-time polymerase chain reaction
|
| |
|---|---|
| Gene | Primer sequence (5ˊ-3ˊ) |
|
| |
| SOD | F: CGAGCAGAAGGAAAGTAATGGA |
| R: TGGATAGAGGATGAAAGTGAGGA | |
| CAT | F: TGGTGAATGGAAATGGGGAGG |
| R: CAAGTGGGATGAGAGGGTAGT | |
| NRF2 | F: TTCCTTCAGCAGCATCCTCTC |
| R: AATCTGTGTTGACTGTGGCATC | |
| GAPDH | F: AGTCCACTGGCGTCTTCAC |
| R: ATCTTGAGGCTGTTGTCATACTTC | |
|
| |
Western blotting
Total cellular proteins were extracted using a ReadyPrep™ Protein Extraction Kit (Bio-Rad, USA) using the manufacturer’s protocol. Bradford reagent (Bio-Rad, USA) was applied to estimate the protein concentration. Next, lysates (20 μg of protein) were loaded and resolved on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membranes (Bio-Rad, USA). After blocking in a solution of 3% skim milk, the membranes were incubated with each primary antibody at 4°C overnight. The used antibodies were as follow: i. Antibody against SOD (ab16831, Abcam, UK), ii. Antibody against CAT (ab16731, Abcam, UK), iii. Antibody against NRF2 (ab137550, Abcam, UK), and iv. Antibody against β-actin (ab8226, Abcam, UK). After washing the blots, incubation with corresponding horseradish peroxidase-conjugated secondary antibodies (Abcam, UK) was performed at room temperature for 1 hour. Signals were detected using an enhanced chemiluminescent (ECL) detection system (Amersham Pharmacia Biotech, UK). β-actin was used to normalize the relative band densities of SOD, CAT, and NRF2. Data were analyzed using ImageJ software (V1.48, NIH, USA).
Statistical analysis
All data were presented as mean values ± standard deviation (SD). The normality of the data was checked by the Kolmogorov-Smirnov test. The comparisons of the groups’ means were conducted by One-way ANOVA and the suitable post-hoc test. The SPSS v.19 software (Chicago, IL, USA) was used for the statistical analysis of the data. P<0.05 was considered statistically significant.
Results
Granulosa cells viability after treatment with SFN and H2 O2
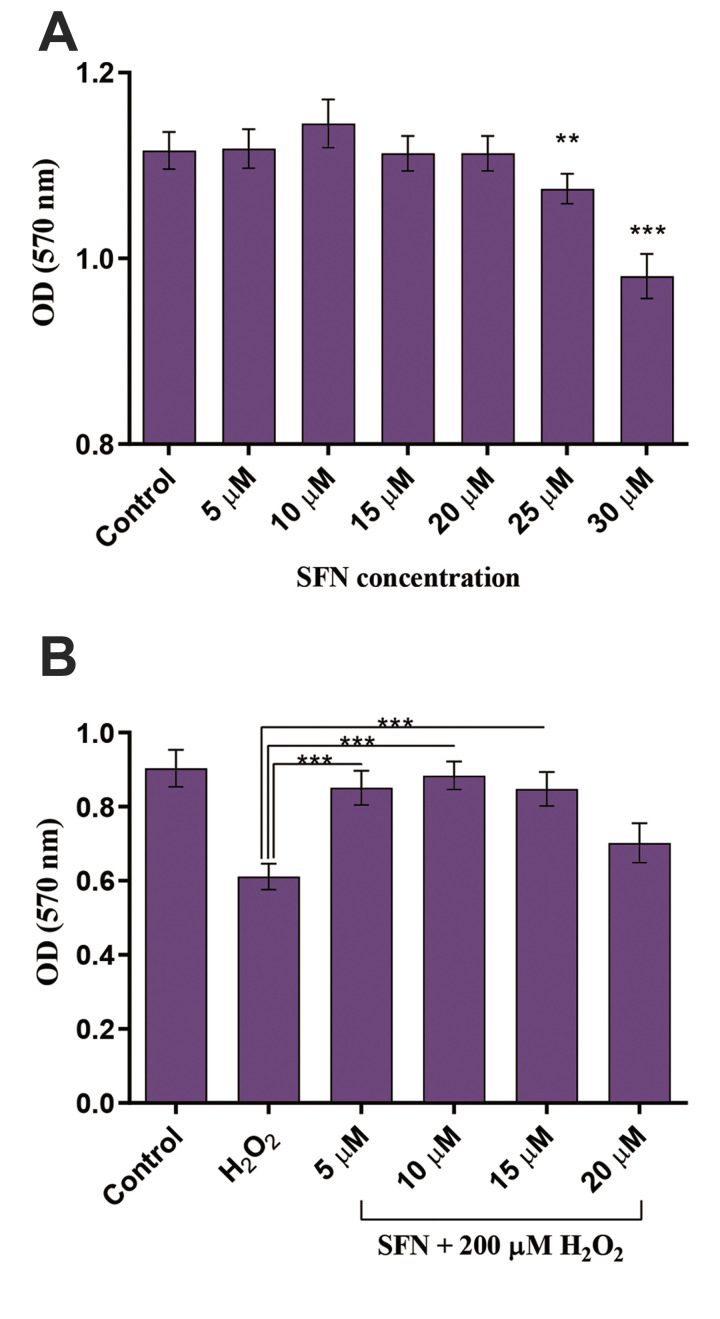
To discover an optimal concentration of SFN for protecting cells against OS-induced cytotoxicity, we pretreated GCs with different concentrations of SFN, and cell viability was measured by MTT assay. The results revealed a significant reduction in the viability of GCs at concentrations of ≥ 25 µM of SFN in comparison to the control group after 24 hours (P≤0.01, Fig .1A). Hence, GC cells were treated with 5, 10, 15, and 20 µM of SFN for 22 hours and then exposed to 200 µM of H2 O2 for 2 hours.
Fig.1.
SFN cytotoxicity evaluation and its protective effect against H2 O2 -induced OS in GCs. A. A significant reduction in the viability of GCs was detected in SFN-treated cells at concentrations of ≥ 25 µM in comparison to the control group. B. SFN pretreatment at concentrations of 5, 10, and 15 µM protected GCs from H2 O2 cytotoxicity. Results are presented as the mean ± SD of 3 independent experiments. **; P<0.01, ***; P<0.001, SFN; Sulforaphane, H2 O2 ; Hydrogen peroxide, OS; Oxidative stress, GCs; Granulosa cells, and OD; Optical density.
Results showed that SFN pretreatment can protect GCs from H2 O2 cytotoxicity (P<0.001, Fig .1B). We chose 10 µM as the optimal protective concentration of SFN against H2 O2 -induced OS in GCs to be used in the next experiments.
SFN inhibits intracellular reactive oxygen species production in granulosa cells exposed to H2 O2
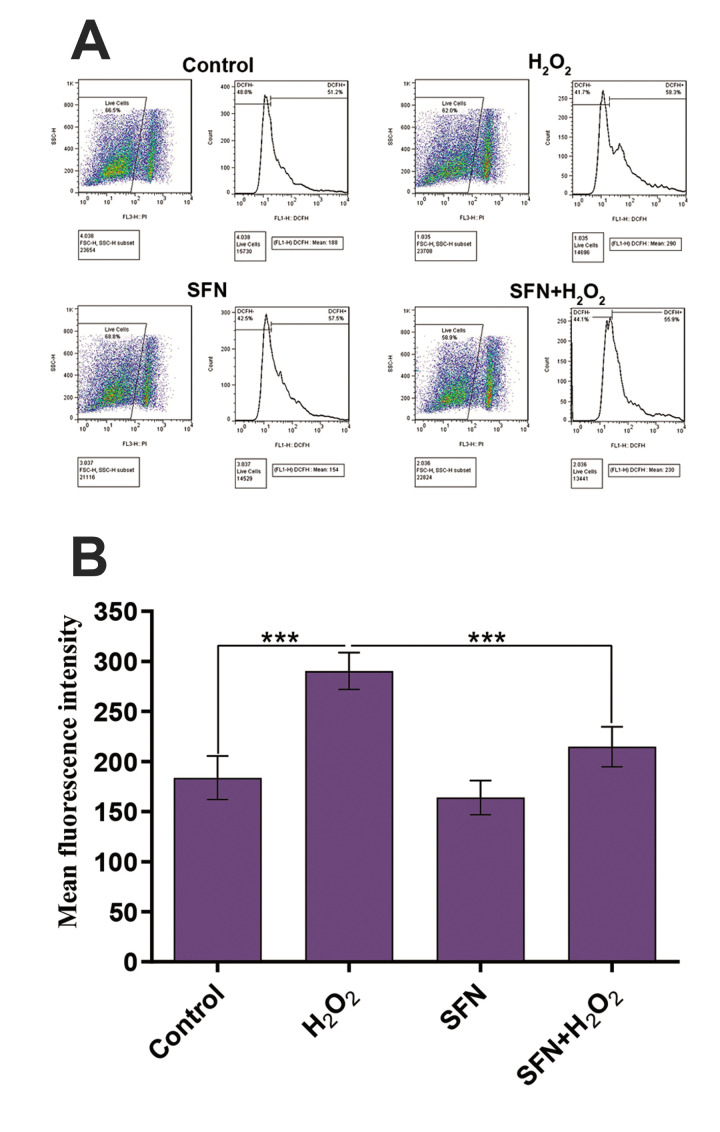
To determine the intracellular ROS levels in the GCs exposed to H2 O2 , a DCFH-DA fluorescent probe was used. The results confirmed that H2 O2 treatment induced a significant elevation in the level of intracellular ROS when compared to the control group (mean fluorescence intensity: 290.33 vs. 183.67). However, this value significantly decreased in the group pretreated with SFN and then exposed to the H2 O2 (mean fluorescence intensity: 214.67, Fig .2).
Fig.2.
Protective effects of SFN on H2 O2 -induced intracellular ROS production in GCs. A. Flow cytometry using a DCFH-DA fluorescent probe showed alteration in the level of intracellular ROS in the experimental groups. B. H2 O2 treatment induced a significant elevation in the level of intracellular ROS compared to the control group, whereas pretreatment with SFN before H2 O2 exposure significantly decreased the level of intracellular ROS. Results are presented as the mean ± SD of 3 independent experiments. ***; P<0.001, SFN; Sulforaphane, H2 O2 ; Hydrogen peroxide, and GCs; Granulosa cells.
SFN prevents granulosa cells from H2 O2 -induced apoptosis
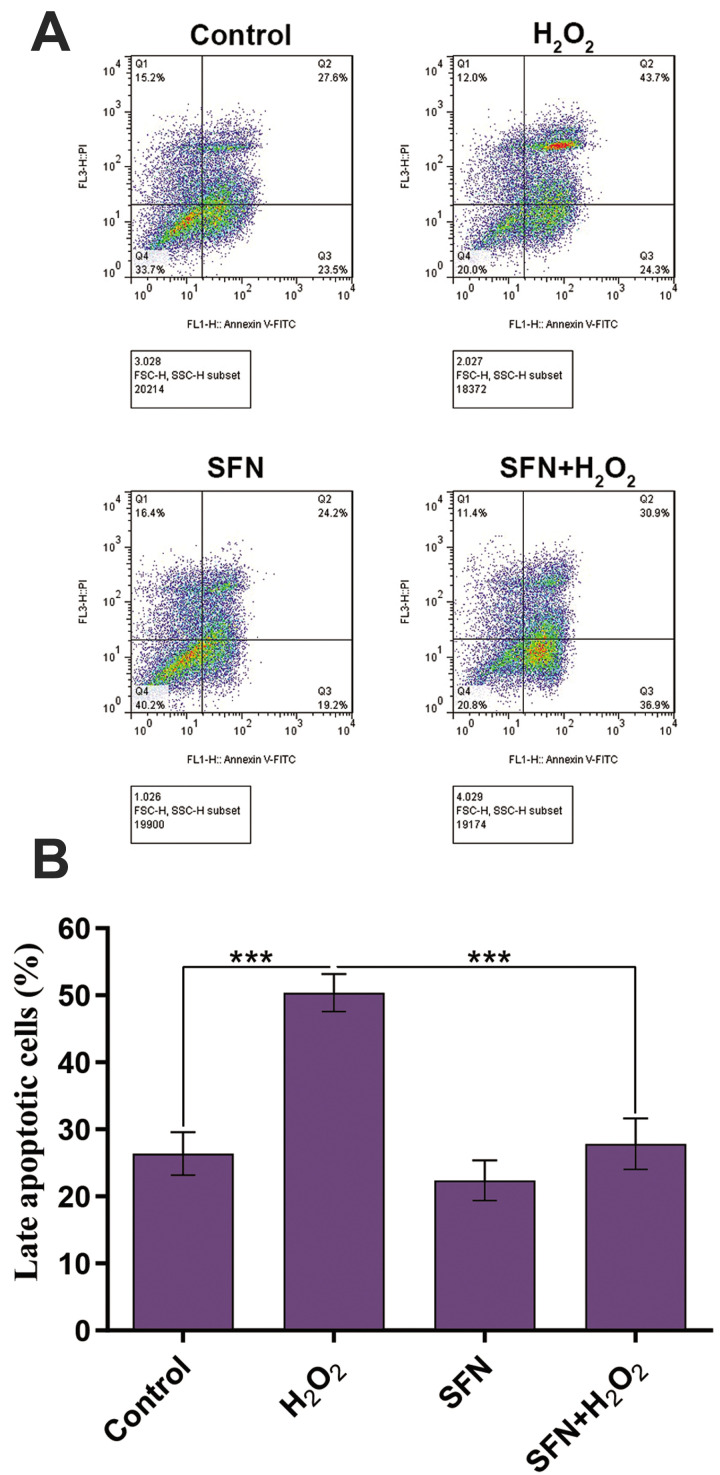
The annexin V/PI staining method was utilized to investigate cell death pathways (necrosis or apoptosis) of GCs in the experimental groups by flow cytometry. Our focus was on the detection of late apoptotic cells (annexin V+ /PI+) that typically locate in the Q2 area as described in the method section. Results demonstrated that the percentage of the cells in the late apoptotic state was significantly higher in the group exposed to H2 O2 (50.37%) in comparison to the control group (26.40%). The percentage of annexin V+ /PI+ cells significantly decreased in the group pretreated with SFN and then exposed to H2 O2 (27.83%, Fig .3).
Fig.3.
Protective effects of SFN on H2 O2 -induced GCs death. A. Annexin V/PI assay showed a difference in cell death pathways (necrosis and apoptosis) of GCs in the experimental groups. B. The percentage of cells in the late apoptotic state was significantly higher in the group exposed to H2 O2 in comparison to the control group, whereas the percentage of late apoptotic cells significantly decreased in the group pretreated with SFN and then exposed to the H2 O2 . The results are presented as the mean ± SD of 3 independent experiments. ***; P<0.001, SFN; Sulforaphane, H2 O2 ; Hydrogen peroxide, PI; Propidium iodide, and GCs; Granulosa cells.
SFN affects the gene and protein expression level of NRF2, SOD, and CAT in granulosa cells
The results so far showed that H2 O2 treatment elevated ROS production and apoptosis in GCs; however, SFN pretreatment protected GCs against these detrimental effects of H2 O2 . Therefore, it was assumed that OS regulation pathways were activated. To investigate the alterations in gene and protein expression level of key regulator genes in the pathway, including NRF2, SOD, and CAT, quantitative real-time PCR (Fig .4) and western blot analysis (Fig .5) were performed, respectively.
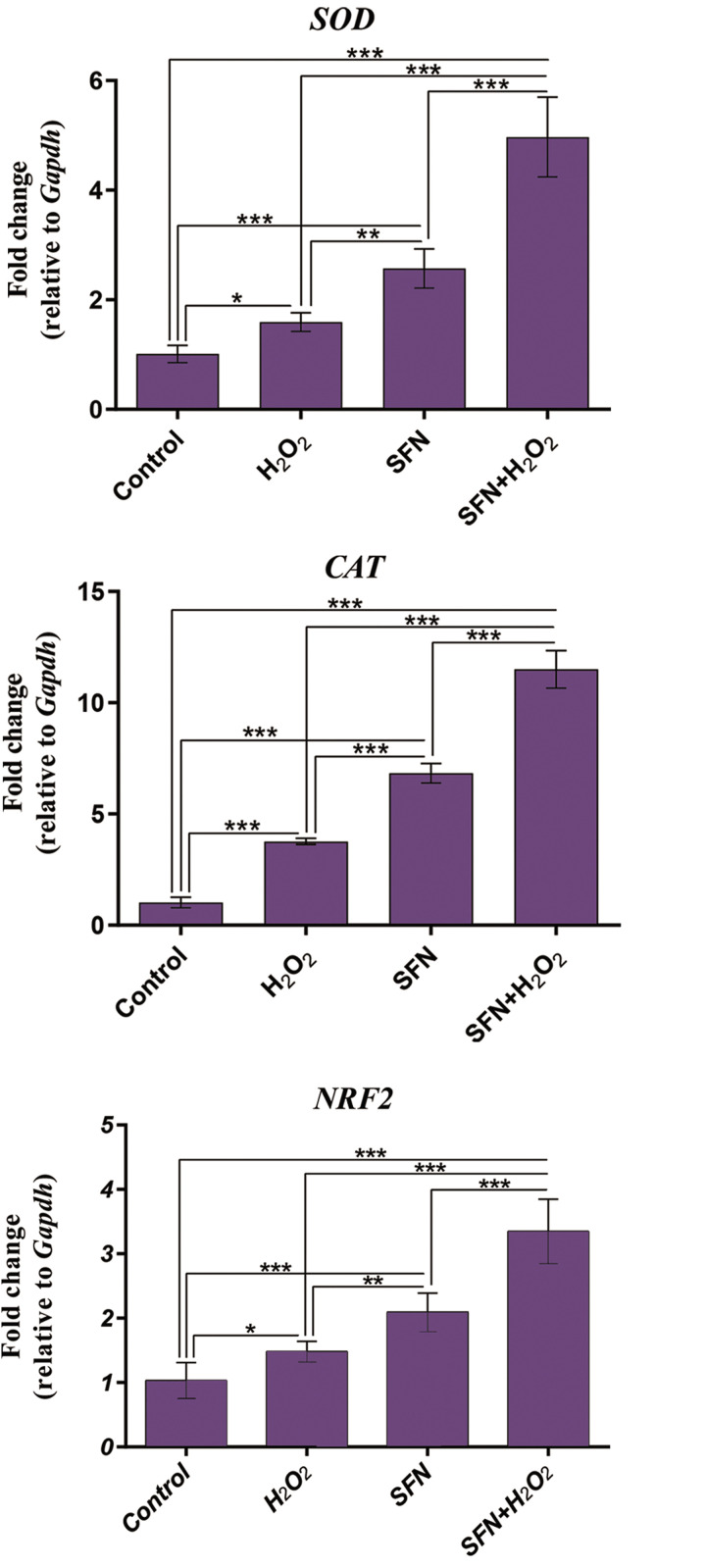
Fig.4.
Evaluation of the mRNA expression level of NRF2, SOD, and CAT in the experimental groups by quantitative real-time PCR. GAPDH was utilized as the internal standard for the normalization of the data. H2 O2 treatment caused a significant increase in the expression of NRF2, SOD, and CAT at mRNA level compared to the control group. Likewise, SFN pretreatment increased the mRNA expression of NRF2, SOD, and CAT compared to both control and H2 O2 -treated groups. Results are presented as mean ± SD. *; P<0.05, **; P<0.01, ***; P<0.001, NRF2; Nuclear factor erythroid 2-related factor 2, SOD; Superoxide dismutase, CAT; Catalase, SFN: Sulforaphane, H2 O2 ; Hydrogen peroxide, and PCR; Polymerase chain reaction.
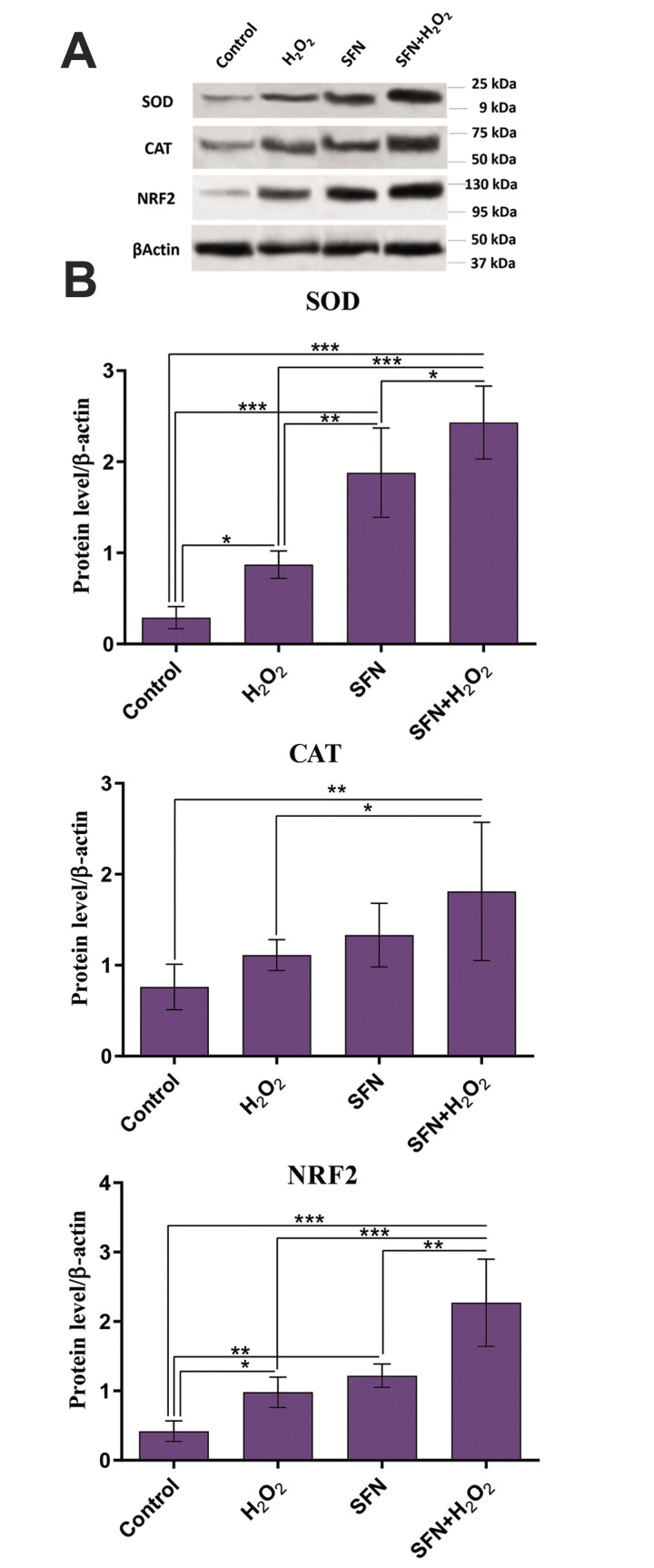
Fig.5.
Evaluation of the protein expression level of NRF2, SOD, and CAT in the experimental. A. NRF2, SOD, and CAT protein levels were measured by western blot. B. The bands’ densities of NRF2, SOD, and CAT proteins were normalized in comparison to β-actin and analyzed by the semiquantitative method. H2 O2 treatment caused a significant increase in the expression of NRF2 and SOD proteins but had no significant effect on CAT protein expression. A significantly higher expression of NRF2, SOD, and CAT proteins was observed in SFN+H2 O2 treated group compared to both control and H2 O2 -exposed groups. Values are presented as the mean ± SD. *; P<0.05, **; P<0.01, ***; P<0.001. NRF2; Nuclear factor-E2-related factor 2, SOD; Superoxide dismutase, CAT; Catalase, SFN; Sulforaphane, and H2 O2 ; Hydrogen peroxide.
Results showed that H2 O2 treatment caused an increase in the expression of NRF2 and SOD at both mRNA and protein levels (P<0.05). However, while the level of CAT gene expression was significantly higher in the H2 O2 -exposed group (P<0.001); it was not reflected at the protein level.
SFN treatment in both SFN alone and SFN+H2 O2 groups significantly increased the mRNA expression of NRF2, SOD, and CAT compared to the control group (P<0.001). Moreover, SFN significantly augmented the mRNA expression of NRF2, SOD, and CAT in the SFN+H2 O2 treated group compared to the H2 O2 -exposed group (P<0.001). At the protein levels, we also observed significantly higher expression of NRF2 and SOD in both SFN and SFN+H2 O2 treated groups compared to the control group.
When the protein levels of these genes were assessed, NRF2 and SOD showed a significant increase in the SFN+H2 O2 treated group compared to the group exposed to H2 O2 (P<0.001). CAT did not show a significant difference at the protein level in the group treated with SFN compared to the control group. However, its protein level was significantly elevated in the SFN+H2 O2 treated group compared to the control (P<0.01) and H2 O2 -exposed groups (P<0.05).
Discussion
The present study investigated the effect of SFN on NRF2-ARE pathway and the downstream antioxidant enzymes, SOD and CAT, in human GCs under H2 O2 - induced OS condition. Following the determination of nontoxic doses of SFN, we first studied the protective effects of these concentrations on GCs viability under H2 O2 -induced OS to choose the optimal dose. The results revealed that SFN has a protective effect at concentrations of 5, 10, and 15 μM, and 10 μM was chosen as the optimal dose to be used for the rest of the study. Then, we investigated the effect of SFN on the intracellular ROS production, apoptosis, mRNA, and protein expression levels of NRF2, SOD, and CAT in GCs treated by H2 O2 . The main finding of the present study was that the protective effect of SFN against H2 O2 - induced intracellular ROS production and cell death may be conducted through mediating the NRF2-ARE pathway and its downstream antioxidant enzymes including SOD and CAT at both mRNA and protein levels.
As mentioned above, GCs are important cells during follicular development and oocyte maturation. In the process of follicular rupture and ovulation, a great level of ROS is produced by neutrophils and macrophages at the site of follicular rupture, where GCs come to direct contact with (7). GCs are sensitive to the damage caused by this OS, so lack of a protective system results in a wide spectrum of disorders in GCs which have inevitable effects on the fertility of the oocyte (10). Therefore, there is a need for the presence of an antioxidant system in GCs against oxidative damage, apoptosis, and follicular atresia during the ovulatory process. GCs have a complex antioxidant system that defends oocytes from the damage of homeostasis imbalances (9).
GCs are equipped with both enzymatic and non-enzymatic antioxidant systems that are pivotal for their survival under OS conditions (24). Among several endogenous antioxidants involved in this manner, the NRF2-ARE pathway has drawn attention in GCs recently (16, 18). During the follicular growth and ovulation, GCs establish an inherent defense system including the NRF2-ARE pathway against various stressors (25). The first-line defense of the antioxidant system is identified as SOD and CAT enzymes regulated by a promoter sequence recognized as ARE in the NRF2-ARE pathway. These antioxidant enzymes are induced when NRF2, as the main transcription factor, is stimulated and translocated into the nucleus for attaching to the ARE region and thereby inducing their expression in OS condition (26). Compounds like SFN, as a natural product targeting this enzymatic system, have achieved growing attention in this context and seem to have potential effects against OS in various cell types (16, 21). Indeed, numerous studies have shown that SFN is able to increase the expression of phase II antioxidant system enzymes and protects against oxidative damage in different types of cells (27, 28). For this purpose, we intended to explore the protective effects of SFN in human GCs under the condition of OS. It should be noted that one of the most common models for OS induction in vitro is H2 O2 exposure (29). Our recent study developed a similar model for OS induction in human GCs using H2 O2 at the concentration of 200 µM by 2 hours incubation.
In line with our findings regarding the noncytotoxic effect of SFN at the concentration of 10 μM, other studies have also reported that 10 μM of SFN is not toxic and can be considered as the optimal dose to investigate its protective effects (30). Indeed, we showed that 5-20 µM of SFN had no adverse effect on GCs viability, whereas higher concentrations (≥25 µM) displayed cytotoxic effects identified by the lower number of viable cells. This finding was almost supported by a study that introduced higher concentrations of SFN (>15 μM) as a cytotoxic dose leading to cell loss in bovine GCs (31).
Herein, we showed that 10 μM of SFN protects GCs against H2 O2 -induced OS and the following apoptosis as supported by the previous studies (16). For instance, Carrasco-Pozo et al. (32) reported that 10 μM of SFN can protect pancreatic beta cell line MIN6 against OS induced by high levels of cholesterol. According to the present study, the observed effect of SFN on attenuating H2 O2 -induced ROS production and apoptosis seems to be mediated by the NRF2-ARE pathway as the results showed a remarkable higher expression of NRF2 at both gene and protein levels in GCs treated with a medium concentration of SFN. This effect may be modulated by both direct and indirect effects of SFN on the expression of Nrf2 as described in previous studies (33). Indeed, the direct effect of SFN on the NRF2 promoter hypomethylation was reported before (34). Moreover, in the indirect effect of SFN, the overexpression of NRF2 may result from the process of its positive autoregulation (16). In addition, it is reported that this powerful antioxidant modifies cysteine residues of Keap1 chemically and enhances the dissociation of the NRF2-KEAP1 complex (35).
Remarkably, our selected downstream antioxidant enzymes, SOD and CAT, were also upregulated at both gene and protein levels after treatment with SFN. In line with our data, a study reported that SFN treatment at a similar concentration (10 μM) induces the NRF2-ARE pathway and the expression of SOD and CAT almost 2 to 5 folds in bovine GCs compared to the control group. They also indicated that a higher concentration of SFN (20 μM) is cytotoxic and induces the accumulation of ROS and cell death in GCs. Hence, they propose a dose-dependent antioxidative effect of SFN in these cells (31). Another study also supported these findings regarding the inducible effect of SFN on the expression of Nrf2 and its downstream target antioxidant genes (SOD and CAT) in bovine GCs (16).
Our findings are also consistent with the data described in other model systems. For instance, it was reported that SFN was able to induce the expression of NRF2 and reduce the production of intracellular ROS in rat lung epithelial cells (36). SFN treatment also induced the NRF2-ARE pathway and SOD and CAT, and reduced cell death in rats with stress urinary incontinence (37). Moreover, SFN induced the NRF2-ARE pathway and CAT expression in a dose-dependent manner in human and rat lens epithelial cells (LECs) and aging human lenses which were halted after ARE area mutation, confirming the SFN-induced NRF2-ARE pathway (38).
We also observed that the protective effect of SFN against intracellular ROS production and cell death was remarkable when cells were treated with H2 O2 and not SFN alone. One interesting finding of the study is that H2 O2 induced the expression of NRF2 and antioxidant system enzymes which was remarkably amplified when cooperated with SFN treatment. This points toward this hypothesis that a low or moderate level of ROS acts as an activator of stress-responsive mechanisms, but an extra level of ROS, with damaging effects, needs the presence of powerful antioxidants to scavenge them through inducing the gene expression of antioxidant enzymes such as SOD and CAT as downstream factors of NRF2-ARE pathway.
Hence, these explanations along with our data support the protective role of SFN in preserving GCs against H2 O2 -induced OS. Then, we may reach this point that administration of foods rich in SFN, such as broccoli sprout with approximately 7.5 g of SFN, may have beneficial effects in improving disorders linked to the process of ovulation. Moreover, the current study may bring to mind a potential role for SFN in providing a noble strategy for treating PCOS, infertility, and other ovarian diseases linked to oxidative damage and improving the quality of ovarian follicles. It should be noted that the use of SFN as a therapeutic agent was demonstrated in cancer therapy before (39). Therefore, different in vivo and in vitro studies have provided convincing evidence for using SFN to induce the antioxidant enzymes in several types of cells in the OS condition. One important notion is the appropriate dose of SFN for clinical usage as its different concentrations display different consequences (40). Here, we found 10 μM of SFN as an optimal dose in our in vitro study in human GCs. Conversely, a high concentration of SFN is cytotoxic to GCs as indicated by a previous study showing higher levels of intracellular ROS and lower viability of bovine GCs (31). Hence, the exact dose for use in clinical practice must be well-recognized in pre-clinical or in vivo studies.
The present study is one of the first studies, to the best of our knowledge, to draw attention to the possible role of SFN in protecting human GCs against H2 O2 -induced OS; however, the limitations deserve to be declared. An important point which warrants consideration is the need for using a specific inhibitor of Nrf2, such as Trigonelline (23), to specify the study pathway as a target of SFN and to improve the validation of the results. Therefore, we propose the use of specific inhibitors in future investigations associated with this subject.
Conclusion
The present study indicated that SFN induces the expression of NRF2 and its downstream antioxidant enzymes, SOD and CAT, at both gene and protein expression levels in human GCs under OS conditions. Moreover, SFN reduces the levels of intracellular ROS and the apoptosis rate of the GCs. It is tempting to speculate that the stimulation of the NRF2-ARE pathway by SFN attenuates the damage by OS in human GCs via the activation of SOD and CAT. Hence, this study may have applicable information for improving the outcomes of assisted reproduction cycles, especially in PCOS patients.
Acknowledgements
This study was supported by the Tehran University of Medical Sciences (Grant No. 96-03-30-36210). All authors have read the journal’s authorship agreement and the manuscript has been reviewed and approved by all the authors. The authors declare that there is no conflict of interest in this study.
Authors’ Contributions
S.E., A.A., F.A.; Conceptualized the study and provided resources. S.E., Z.N., M.T., M.Kh., M.E., Z.R., F.A.; Designed and performed the experiments, analyzed and interpreted the data. S.E., Z.N., M.T., M.Kh., M.E., Z.R.; Prepared the original draft. A.A., F.A.; Reviewed, prepared the final format, contributed in funding acquisition, and supervised the whole project. All authors read and approved the final manuscript.
References
- 1.Ursini F, Maiorino M, Forman HJ. Redox homeostasis: the golden mean of healthy living. Redox Biol. 2016;8:205–215. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujii J, Iuchi Y, Okada F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod Biol Endocrinol. 2005;3:43–43. doi: 10.1186/1477-7827-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863(12):2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Martin KR, Barrett JC. Reactive oxygen species as doubleedged swords in cellular processes: low-dose cell signaling versus high-dose toxicity. Hum Exp Toxicol. 2002;21(2):71–75. doi: 10.1191/0960327102ht213oa. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28–28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49–49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canipari R. Oocyte--granulosa cell interactions. Hum Reprod Update. 2000;6(3):279–289. doi: 10.1093/humupd/6.3.279. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Xie Y, Yang D, Ren D. Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget. 2017;8(15):25310–25322. doi: 10.18632/oncotarget.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai Q, Xiang W, Li Q, Zhang H, Li Y, Zhu G, et al. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front Med. 2018;12(5):518–524. doi: 10.1007/s11684-017-0575-y. [DOI] [PubMed] [Google Scholar]
- 11.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10(11):549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018;29(17):1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao F, Li K, Zhao L, Liu J, Suo Q, Zhao J, et al. Effect of Nrf2 on rat ovarian tissues against atrazine-induced anti-oxidative response. Int J Clin Exp Pathol. 2014;7(6):2780–2789. [PMC free article] [PubMed] [Google Scholar]
- 16.Sohel MMH, Amin A, Prastowo S, Linares-Otoya L, Hoelker M, Schellander K, et al. Sulforaphane protects granulosa cells against oxidative stress via activation of NRF2-ARE pathway. Cell Tissue Res. 2018;374(3):629–641. doi: 10.1007/s00441-018-2877-z. [DOI] [PubMed] [Google Scholar]
- 17.Khadrawy O, Gebremedhn S, Salilew-Wondim D, Taqi MO, Neuhoff C, Tholen E, et al. Endogenous and exogenous modulation of Nrf2 mediated oxidative stress response in bovine granulosa cells: Potential implication for ovarian function. Int J Mol Sci. 2019;20(7):1635–1635. doi: 10.3390/ijms20071635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akino N, Wada-Hiraike O, Terao H, Honjoh H, Isono W, Fu H, et al. Activation of Nrf2 might reduce oxidative stress in human granulosa cells. Mol Cell Endocrinol. 2018;470:96–104. doi: 10.1016/j.mce.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Guerrero-Beltrán CE, Calderón-Oliver M, Pedraza-Chaverri J, Chirino YI. Protective effect of sulforaphane against oxidative stress: recent advances. Exp Toxicol Pathol. 2012;64(5):503–508. doi: 10.1016/j.etp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Dinkova-Kostova AT, Fahey JW, Wade KL, Jenkins SN, Shapiro TA, Fuchs EJ, et al. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol Biomarkers Prev. 2007;16(4):847–851. doi: 10.1158/1055-9965.EPI-06-0934. [DOI] [PubMed] [Google Scholar]
- 21.Wise RA, Holbrook JT, Criner G, Sethi S, Rayapudi S, Sudini KR, et al. Lack of effect of oral sulforaphane administration on Nrf2 expression in COPD: a randomized, double-blind, placebo controlled trial. PLoS One. 2016;11(11):e0163716–e0163716. doi: 10.1371/journal.pone.0163716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chilvers RA, Bodenburg YH, Denner LA, Urban RJ. Development of a novel protocol for isolation and purification of human granulosa cells. J Assist Reprod Genet. 2012;29(6):547–556. doi: 10.1007/s10815-012-9739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rashidi Z, Aleyasin A, Eslami M, Nekoonam S, Zendedel A, Bahramrezaie M, et al. Quercetin protects human granulosa cells against oxidative stress via thioredoxin system. Reprod Biol. 2019;19(3):245–254. doi: 10.1016/j.repbio.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, He G, Chen M, Zuo T, Xu W, Liu X. The role of antioxidant enzymes in the ovaries. Oxid Med Cell Longev. 2017;2017:4371714–4371714. doi: 10.1155/2017/4371714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poljsak B, Šuput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev. 2013;2013:956792–956792. doi: 10.1155/2013/956792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Awad MA, Aldosari SR, Abid MR. Genetic alterations in oxidant and anti-oxidant enzymes in the vascular system. Front Cardiovasc Med. 2018;5:107–107. doi: 10.3389/fcvm.2018.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Kim DS, Yadav RK, Kim HR, Chae HJ. Sulforaphane prevents doxorubicin-induced oxidative stress and cell death in rat H9c2 cells. Int J Mol Med. 2015;36(1):53–64. doi: 10.3892/ijmm.2015.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He C, Li B, Song W, Ding Z, Wang S, Shan Y. Sulforaphane attenuates homocysteine-induced endoplasmic reticulum stress through Nrf-2-driven enzymes in immortalized human hepatocytes. J Agric Food Chem. 2014;62(30):7477–7485. doi: 10.1021/jf501944u. [DOI] [PubMed] [Google Scholar]
- 29.Upadhyay S, Vaish S, Dhiman M. Hydrogen peroxide-induced oxidative stress and its impact on innate immune responses in lung carcinoma A549 cells. Mol Cell Biochem. 2019;450(1-2):135–147. doi: 10.1007/s11010-018-3380-2. [DOI] [PubMed] [Google Scholar]
- 30.Kim BG, Fujita T, Stankovic KM, Welling DB, Moon IS, Choi JY, et al. Sulforaphane, a natural component of broccoli, inhibits vestibular schwannoma growth in vitro and in vivo. Sci Rep. 2016;6:36215–36215. doi: 10.1038/srep36215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sohel MMH, Konca Y, Akyuz B, Arslan K, Sariozkan S, Cinar MU. Concentration dependent antioxidative and apoptotic effects of sulforaphane on bovine granulosa cells in vitro. Theriogenology. 2017;97:17–26. doi: 10.1016/j.theriogenology.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Carrasco-Pozo C, Tan KN, Gotteland M, Borges K. Sulforaphane protects against high cholesterol-induced mitochondrial bioenergetics impairments, inflammation, and oxidative stress and preserves pancreatic β-cells function. Oxid Med Cell Longev. 2017;2017:3839756–3839756. doi: 10.1155/2017/3839756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwak MK, Itoh K, Yamamoto M, Kensler TW. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol. 2002;22(9):2883–2892. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su ZY, Zhang C, Lee JH, Shu L, Wu TY, Khor TO, et al. Requirement and epigenetics reprogramming of Nrf2 in suppression of tumor promoter TPA-induced mouse skin cell transformation by sulforaphane. Cancer Prev Res (Phila) 2014;7(3):319–329. doi: 10.1158/1940-6207.CAPR-13-0313-T. [DOI] [PubMed] [Google Scholar]
- 35.Hu C, Eggler AL, Mesecar AD, van Breemen RB. Modification of keap1 cysteine residues by sulforaphane. Chem Res Toxicol. 2011;24(4):515–521. doi: 10.1021/tx100389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiao Z, Chang J, Li J, Nie D, Cui H, Guo D. Sulforaphane increases Nrf2 expression and protects alveolar epithelial cells against injury caused by cigarette smoke extract. Mol Med Rep. 2017;16(2):1241–1247. doi: 10.3892/mmr.2017.6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan X, Liu C, Chen YB, Gu M, Cai ZK, Chen Q, et al. Sulforaphane treatment of stress urinary incontinence via the Nrf2- ARE pathway in a rat model. Cell Physiol Biochem. 2017;44(5):1912–1922. doi: 10.1159/000485880. [DOI] [PubMed] [Google Scholar]
- 38.Kubo E, Chhunchha B, Singh P, Sasaki H, Singh DP. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017;7(1):14130–14130. doi: 10.1038/s41598-017-14520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenzi M, Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Cancer Treat Res. 2014;159:207–223. doi: 10.1007/978-3-642-38007-5_12. [DOI] [PubMed] [Google Scholar]
- 40.Boddupalli S, Mein JR, Lakkanna S, James DR. Induction of phase 2 antioxidant enzymes by broccoli sulforaphane: perspectives in maintaining the antioxidant activity of vitamins a, C, and e. Front Genet. 2012;3:7–7. doi: 10.3389/fgene.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]