Abstract
Objective
Epstein-Barr virus (EBV) and Human Herpes virus 6 (HHV-6) are believed to involve in multiple sclerosis (MS) pathogenesis. Natural killer (NK) and CD8+ T cells have essential roles in handling viral infections and their phenotypic and functional properties may be influenced following exposure to viral infections. Here, we investigated the association of NK and CD8+ T cells subpopulations frequency with EBV and HHV-6 viral load in MS patients.
Materials and Methods
In this case-control study, EBV and HHV-6 viral load were evaluated in plasma of newly diagnosed relapsing-remitting MS (RRMS) patients at relapse phase (n=23), who were not on disease-modifying therapy (DMT), and sex- and age-matched healthy controls (n=19) using real-time polymerase chain reaction (PCR). The frequency of NK and CD8+ T cells subsets were assessed by CD27, CD28, CD45RO, CD56, and CD57 markers using flow cytometry.
Results
Despite the increased level of EBV viral load in RRMS patients compared to the control group, there was no statistically significant difference in EBV and HHV-6 copy numbers between the studied groups. In addition, a significant decrease was observed in the percentages of CD56brightCD57- and CD56dimCD57+CD8lowCD45RO- NK cells in RRMS patients in comparison to healthy controls. Analysis of CD8+ T cell subsets showed a substantially high proportion of CD27+CD28+CD45RO+CD57-CD8hiT cells in patients at relapse phase compared to controls. The frequency of NK and T cells subtypes was not associated with EBV and HHV6 plasma viral loads.
Conclusion
These findings further highlight the variation of NK and CD8+ T cells subsets frequency in clinically active RRMS patients. Since the composition of cells was not associated with EBV and HHV-6 viral load, perhaps other viral infections may be involved in altered NK and CD8+ T cells subpopulation. Larger cohort studies are needed to confirm these results.
Keywords: CD8+ T Cell, Epstein-Barr Virus, Human Herpes Virus 6, Multiple Sclerosis, Natural Killer Cell
Introduction
Multiple sclerosis (MS) is the most common demyelinating autoimmune disease of the central nervous system which shows a considerable rising trend around the world. With a heterogeneous clinical manifestation, more than 85% of patients show relapsing-remitting MS (RRMS) at the disease onset. It seems that environmental factors trigger disease activity in genetically predisposed individuals (1).
Even though MS has a vague etiology, a possible role has been speculated for Human Herpes viruses (HHV) such as HHV-6 and Epstein-Barr virus (EBV). However, no specific virus has been definitively proven as a causative factor yet (2, 3). Cross-reactivity between these viruses and central nervous system (CNS) antigens, bystander activation, and epitope spreading are mechanisms that have been proposed to explain the role of herpesviruses in the disease progression (4). Besides their potential role in MS pathogenesis, it is well established that natural killer (NK) and CD8+ T cells are vital mediators of controlling virus infection (5-8).
NK cells cover the front line of defense against viral infections and are recognized as CD3- cells with downregulated CD8, high or low expression levels of CD56 (CD56bright or CD56dim), and the presence or absence of CD57. CD56bright NK cells constitute approximately 10% of peripheral NK cells lacking expression of CD57 and have immunomodulatory functions. Though CD56dim subsets (CD57+ or CD57-) are the most circulating NK cells (nearly 90%) and are commonly accepted for cytolytic activity (9, 10). On the other hand, cytotoxic CD8+ T cells are crucial cells of adaptive immunity known via high expression of CD8 surface marker (CD8hi cells) (11). They could be categorized based on the expression of CD27, CD28, CD57 and CD45RO, as naïve (CD27+ CD28+ CD45RO- CD57-), early-differentiated/ central memory (CM) (CD27+ CD28+ CD45RO+ CD57-), intermediate-(CD27+ CD28- CD45RO-/+ CD57+) and latedifferentiated (CD27- CD28- CD45RO- CD57+) cells (12, 13).
Recent studies have shown that viral infections might be responsible for the diverse composition of NK and CD8+ T cells populations. Expansion of early differentiated NK cells (with CD56dim CD57- phenotype) during EBV infection has been identified (14). Furthermore, EBV- and HCV- specific CD8+ T cells frequently have early-differentiated/memory phenotype, whereas intermediate and late-differentiated CD8+ T cells are the most frequent subsets in individuals infected with human immunodeficiency virus (HIV) and Cytomegalovirus (CMV) (13). Of note, the vast majority of evidence has described the potential role of diverse subpopulations of NK and CD8+ T cells in MS pathogenesis (7, 15, 16). For instance, a recent study has shown elevated numbers of circulating CD3− CD56dim perforin+ NK cells in MS patients (17). Also, the higher frequency of CD56bright NK cells has been demonstrated following MS drugs regimens (18). Furthermore, a lower number of effector memory (CD45RA– CD62L–) CD8+ T cells has been illustrated in MS patients than healthy controls (19), that can be raised with the Fingolimod therapy (20).
In the present study, we hypothesized that elevated/ lower levels of differentiated CD8low NK and CD8high T cells subsets in RRMS patients might be associated with an increase or a decrease of HHV-6/EBV viral load which could reflect a specific virus infection in MS.
Materials and Methods
Participants
In the present case-control study, twenty-three RRMS patients (18 females, 5 males; mean age: 32.3 ± 9.6 Y, EDSS: 2 ± 0.5) were examined. Patients were referred to the Iranian Center of Neurological Research at Sina General Hospital, Tehran University of Medical Sciences, Tehran, Iran. The disease was diagnosed according to revised McDonald’s criteria (21) by an experienced neurologist. To quantify the disability of patients, the Kurtzke Expanded Disability Status Scale (EDSS) (22) was measured. All patients were at the relapse phase, and not on disease-modifying therapy (DMT); i.e. most of them were newly diagnosed. They also met the inclusion criteria of not being treated with any kind of immunomodulatory/immunosuppressive drugs such as interferon (IFN)-β and corticosteroid for at least 3 months before initiation of study. Nineteen sex- and age-matched healthy controls (14 females, 5 males; mean age: 29.3 ± 7.2 Y) who had no history of MS or other autoimmune and inflammatory diseases, even in their families, were also enrolled in this study. All patients and controls were of Iranian origin. Informed consent was obtained from all participants prior to the study.
The study was approved according to the ethical guidelines of the Tehran University of Medical Sciences (90-10-18-15044), and conducted in accordance with the Declaration of Helsinki (23).
Real-time polymerase chain reaction: detection and quantification of EBV and HHV-6 specific DNA
EBV and HHV-6 DNAs were isolated efficiently from 200 μL of plasma using the High Pure Viral Nucleic Acid Kit (Roche, Germany) as per the manufacturer’s instruction. For detection and quantification of virus-specific DNA, Real Star® EBV and HHV-6 PCR Kits 1.0 (Altona Diagnostics, Germany) were used. Both HHV-6A and HHV-6B subtypes were identified by HHV-6 PCR Kit (24).
Flow cytometry: NK and CD8+ T cell subsets
To identify NK and CD8+ T cells subsets, Blood samples (about 10 ml) were collected in EDTA-containing tubes. The peripheral blood mononuclear cells (PBMCs) were first isolated using Ficoll-Hypaque (Innotrain, Germany) density gradient centrifugation (1000×g at room temperature) and then washed two times with phosphate-buffered saline (PBS, Euroimmun, Germany). Centrifugation of samples was at 300 ×g and 4°C. Subsequently, isolated PBMCs (106 cells/100 µl) were stained with anti-CD8-APC, CD45RO-PE, CD27-Percp-eFlour780, CD28-Pe-Cy7, CD57-FITC, and CD56-PerCp-eFlour710 monoclonal antibodies (mAbs) (all from eBioscience, USA) and incubated at 4°C for 30 minutes. The samples were fixed with formaldehyde and analyzed within 24 hours by flow cytometer (BD FACSAria, USA). At least 50,000 events were counted for each sample.
Statistical analysis
Statistical analysis was performed using SPSS v.20 software. Data are expressed as mean ± standard error of the mean (SEM). The difference between groups was considered statistically significant when P<0.05. All numeric variables were tested for normality of distribution by the Kolmogorov-Smirnov test. The Chi-square test was used to identify if there was a significant relationship between the prevalence of viruses among RRMS patients and healthy controls. Additionally, an independent t test was utilized to compare the median of EBV/HHV-6 viral load and the mean difference of NK and CD8+ T cells between groups. The link between HHV-6 and EBV viral load with NK and CD8+ T cells were assessed with the Pearson’s correlation.
Results
Selection of RRMS patients and healthy controls
Twenty-three clinically active RRMS patients and 19 healthy individuals were investigated in the current study. There was no significant difference in the mean age of patients and healthy controls (P=0.25). Furthermore, the gender distribution was also not significantly different between the studied groups (P=0.13).
Higher EBV viral load in RRMS patients
In order to determine the EBV and HHV-6 DNA copy numbers, we measured the viruses in plasma obtained from RRMS patients and healthy controls. The analysis showed that 34.8% of patients and 10.5% of controls were EBV positive (P=0.06). Moreover, 21.7% of patients and 42.1% of controls were positive for HHV-6 (P=0.15). Higher EBV viral load was found in MS patients compared to controls (median: 250.95 copies/ml, range: 0-3430 copies/ml vs median: 4.15 copies/ml, range 0-73 copies/ml). However, there was no significant difference between the groups (P=0.12). The median number of HHV-6 viral copies was 18.91 copies/ml (range 0-304 copies/ml) in RRMS patients and 17.57 copies/ml (range 0-79 copies/ml) in healthy subjects (P=0.93), the difference was not statistically significant (P=0.93, Table 1).
Table 1.
EBV and HHV-6 DNA plasma viral load in RRMS patients and healthy controls
|
| ||||
|---|---|---|---|---|
| Viruses | Viral load | RRMS | HCs | P value |
|
| ||||
| EBV | Prevalence (%) | 34.8 | 10.5 | 0.06 |
| Median viral load (copies/ml) | 250.95 | 4.15 | 0.12 | |
| HHV-6 | Prevalence (%) | 21.7 | 42.1 | 0.15 |
| Median viral load (copies/ml) | 18.91 | 17.57 | 0.93 | |
|
| ||||
Higher EBV viral load was found in RRMS patients compared to controls. P values for the prevalence and median of EBV/HHV-6 were calculated via Chi-square and Independent t test, respectively. Statistical significance was defined at P <0.05. EBV; Epstein-Barr v irus, HHV-6; H uman Herpes virus-6, RRMS; Relapsing-remitting multiple sclerosis, and HCs; Healthy controls.
The frequency of NK and CD8+ T cells and their correlation with EBV/HHV-6 viral load
To categorize NK and CD8+ T cell subsets proportion, data analysis was performed using the FlowJo software package v.7.6.1 (Tree Star). Lymphocytes were first gated on a forward versus side scatter dot plot and the cells
were investigated based on the expression of CD3 and CD8. The majority of CD8high cells (approximately more than 90%) were CD3 positive while CD8low cells were Cell J, Vol 23, No 6, November 2021 either CD3+ or CD3- (Fig .1). Based on our results and also previous studies (11), CD8high CD3+ populations are conventional T cells while NK cells are located in CD8low CD3- cells.
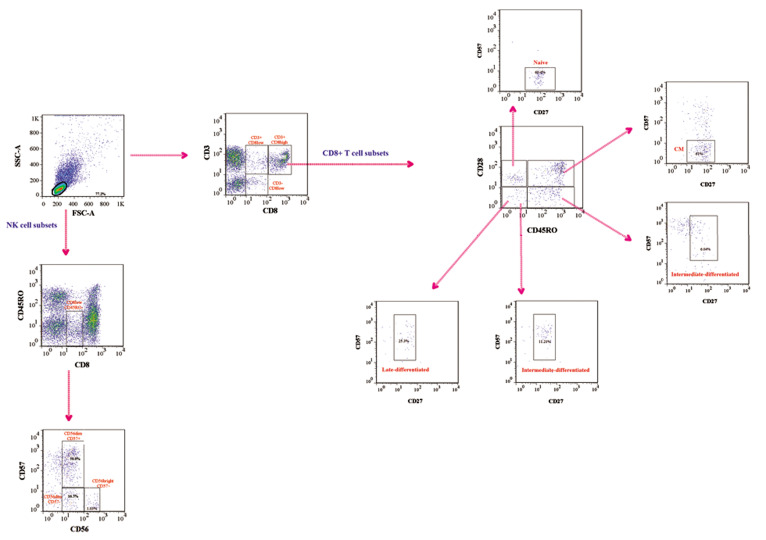
Fig.1.
Representative gating strategy for different NK and CD8+ T cell subsets. Peripheral blood mononuclear cells (PBMCs) were first gated for lymphocytes (SSC-A vs. FSC-A). Gated cells were displayed on a plot of CD3 vs. CD8 expression. CD8+ cells consisted of two subpopulations, including CD8low and CD8high cells were considered as NK cells and various subsets were determined via CD56 and CD57. Since all CD8high expressed CD3, they proposed as CD8+ T cells. Afterward, different CD8+ subpopulations were identified using CD27, CD28, CD45, and CD57 markers. NK; Natural killer cell, SSC-A; Side scatter area, and FSC-A; Forward scatter area.
Interestingly, the CD3- CD8low cells were CD45RO- . These cells which seem to be NK cells were analyzed according to the expression of CD56 and CD57. The NK cells categorized into CD56bright CD57- , CD56dim CD57- and CD56dim CD57+ subsets. Furthermore, different subsets of CD8high T cells, naïve (CD27+ CD28+ CD45RO- CD57-), early-differentiated (CD27+ CD28+ CD45RO+ CD57-), intermediate-differentiated (CD27+ CD28- CD45RO- / + CD57+) and late-differentiated (CD27- CD28- CD45RO- CD57+) cells were also determined. A representative example of the gating strategy is illustrated in Figure 1.
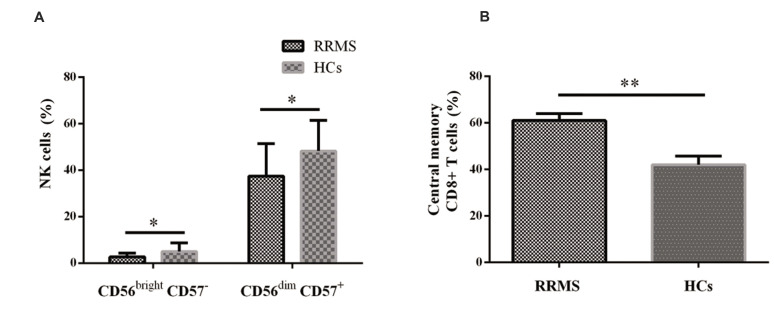
Analysis of cells within CD8low CD45RO- population revealed that the frequency of CD56brightCD57- and CD56dim CD57+ NK cell subsets decreased in RRMS patients at relapse phase in comparison to healthy controls (P=0.02 and P=0.01, respectively). Additionally, the percentage of CD8high CD27+ CD28+ CD45RO+ CD57- (CM) cells significantly decreased in RRMS patients (P=0.001, Fig .2). No differences were observed in the frequency of other cell subsets between the studied groups. The detail of different NK and CD8+ T cells frequency was listed in Table 2.
Fig.2.
The frequency of NK and CD8+ T cell subsets in RRMS patients. The Frequency of A. NK cells and B. CD8+ T cell subsets in RRMS patients and healthy controls. An Independent t test, among. P<0.05 was considered statistically significant. NK; Natural killer cell, RRMS; Relapsing-remitting multiple sclerosis, HCs; Healthy controls, *; P<0.05, and **; P<0.01.
We next analyzed the association of EBV and HHV-6 viral load with the proportion of different NK and CD8+ T cells subpopulations. We found no correlation between the frequency of these cells and EBV/HHV-6 viral load in neither RRMS patients nor healthy controls.
Table 2.
NK and CD8+ T cells subsets frequency in RRMS patients and healthy controls
|
| |||
|---|---|---|---|
| Cell subsets | RRMS | HCs | P value |
|
| |||
| NK cell subsets (within CD8low CD45RO- cells) | |||
| CD56bright CD57- | 2.76 ± 1.70 | 5.09 ± 3.7 | 0.02* |
| CD56dim CD57- | 20.46 ± 10 | 17.11 ± 6.62 | 0.37 |
| CD56dim CD57+ | 37.46 ± 14.02 | 48.33 ± 13.17 | 0.01* |
| T cell subsets (within CD8high cells) | |||
| CD27+ CD28+ CD45RO- CD57- (Naïve) | 67.96 ± 5.23 | 58.76 ± 25.42 | 0.25 |
| CD27+ CD28+ CD45RO+ CD57- (Early-differentiated/central memory)a | 61.00 ± 3.05 | 42.00 ± 3.72 | 0.001** |
| CD27+ CD28- CD45RO- CD57+ (Intermediate-differentiated) | 10.80 ± 1.12 | 14.32 ± 1.59 | 0.09 |
| CD27+ CD28- CD45RO+ CD57+ (Intermediate-differentiated)b | 6.67 ± 1.21 | 8.08 ± 1.03 | 0.38 |
| CD27- CD28- CD45RO- CD57+ (Late differentiated, effector)c | 23.57 ± 4.41 | 27.77 ± 5.48 | 0.56 |
|
| |||
Data are expressed as mean of percentage ± SEM, P values were calculated using Independent t test. a ; The most frequently reported phenotype in EBV, HCV, and Influenza infection, b ; The most frequently reported phenotype in HIV infection, c ; The most frequently reported phenotype in CMV infection, RRMS; Relapsing-remitting multiple sclerosis, HCs; Healthy controls, EBV; Epstein-Barr virus, HCV; Hepatitis C virus, CMV; Cytomegalovirus, NK; Natural killer cell, * ; P<0.05, and **; P<0.01.
Discussion
In the current study, our purpose was to assess whether a virus-mediated inflammatory response is associated with the modified frequency of NK and CD8+ T cell subsets in RRMS patients. Consequently, we have examined: (1) EBV and HHV-6 prevalence and viral load, and (2) the proportion of particular subsets of NK and CD8+ T cells in newly diagnosed RRMS patients at relapse phase and healthy individuals. Notably, our data show for the first time that decreased regulatory NK cells (CD56bright CD57-) and increased CM CD8+ T cells (CD27+ CD28+ CD45RO+ CD57-) in clinically active RRMS patients were not associated with EBV and HHV-6 plasma viral loads.
Several types of research have been carried out to determine the involvement of viruses in MS pathogenesis. Of those, EBV and HHV-6 are believed to be the most noticeable neurotropic viruses that may be associated with the disease onset and progress (2). Our results showed an increasing trend of plasma EBV DNA positivity in RRMS patients at the acute phase compared to healthy individuals. However, there was no significant difference neither in EBV nor in HHV-6 copy number between studied groups. Previous studies have identified no alterations in the prevalence of EBV DNA in serum, plasma, and CSF of MS patients vs. healthy individuals, even with sensitive standardized methods (25, 26). Despite the higher HHV-6 DNA load in the brain and CSF samples of MS patients (27, 28), few studies have reached a statistically significant increase in the periphery (25, 29). Consistent with our finding, Álvarez-Lafuente et al. (25) also did not find a significant difference in HHV-6 median viral load of RRMS patients in comparison to healthy donors. It was speculated that HHV-6 activation is perhaps tissue-restricted (30).
NK cells are vital cells of innate immunity in combating viral infections. In human peripheral blood, two major subsets of NK cells exist; regulatory (CD56bright CD57-) and cytotoxic (CD56dim CD57-/+) subpopulations (9, 10). Particularly, the acquisition of CD57 expression represents the terminally-differentiated NK cells and CD56dim CD57+ produces more IFN-gamma than CD56dim CD57- (31). Here, we described significantly decreased proportions of both CD56bright CD57- and CD56dim CD57+ CD8low NK cells (within CD45RO- population) in RRMS patients. The frequency of CD56dim CD57- was also increased in patients, although it was not significant. Similarly, a reduced number of CD3- CD8low CD56+ and CD56bright NK cells have been discovered in untreated MS patients (32, 33). A number of MS approved drugs, including daclizumab, dimethyl-fumarate (DMF), and IFN-β, exert their anti-inflammatory activity through the expansion of CD56bright immature NK cells which in turn inhibits CD4+ and CD8+ T cell survival (18, 34). Recently, Marastoni et al. (35), showed an increased percentage of CD3− CD56+ NK cells during DMF treatment. These observations support the evidence that both CD56bright CD57- and CD56dim CD57+ NK cells subsets have an immunoregulatory role in MS.
Controlling viral infection is mediated not only through NK cells but also via CD8+ T cells. The most common markers used for discrimination of CD8+ T cells subsets are CD27, CD28, CD45RO, and CD57. According to the expression of these markers, diverse subsets of CD8+ T cells including naïve, early differentiated (CM), intermediate differentiated and effector memory (EM) have been reported (13). Another finding in our study was a higher frequency of CD27+ CD28+ CD45RO+ CD57- CD8+ T cells (early-differentiated/CM cells) in RRMS patients. Our results are in keeping with those obtained by Liu et al. (36), demonstrating an increased number of CD8+ CCR7+ CD45RA- CM T cells in MS patients during the early stages of the disease. Fingolimod as an immunomodulatory drug for the treatment of MS exerts its effects by inhibiting recirculation of CM autoreactive T cells, resulting in decreased numbers of CM (CCR7+ CD45RO+) and increased number of effector cells (CCR7- CD45RA-) within CD8+ T cells (20).
Herpesviruses, which may impact on MS development, have been ascertained for remodeling of NK and CD8+ T cells phenotype, suggesting the importance of their phenotypes in predicting specific pathogens infections in MS. While EBV infection could stimulate the expansion of early differentiated NK cells (CD56dim CD57-) (14), terminally differentiated compartments (CD56dim CD57+) have been defined following human cytomegalovirus (HCMV) and varicella-zoster virus (VZV) infections (37, 38). Moreover, remarkable changes in the expression pattern of NK cells-associated miRNAs and transcription factors have also been perceived following HHV-6A/B infections (39). Recently, an adaptive NK cell phenotype in MS was also correlated to the HCMV infection (8). Besides, within the acute phase of infection, CD8+ T cells express CD27 and CD28, while in chronic viral infection the cells are CD27+ and CD28- (12). Interestingly, the existence of CD27+ CD28+ CD45RO+ CD57- T cells has been shown during EBV, HCV, and Influenza infections (40). However, the role of such cells formed following viral infections in MS pathogenesis is unknown. Despite former studies, we did not explore a significant correlation between the EBV or HHV-6 viral load and frequency of NK and CD8+ T cell subpopulations in RRMS patients. Given the evidence that other mechanisms may contribute to the configuration of different immune cells in MS, additional studies are required to reveal whether the development of specific subsets of NK and CD8+ T cells occurs following a previous viral infection or it is related to the disease process.
Conclusion
Altogether, the study showed a lesser frequency of CD56bright CD57- and CD56dim CD57+ CD8low NK cells and more CD27+ CD28+ CD45RO+ CD57- CD8+ T-cells in RRMS patients during relapse phase. However, these were not associated with either EBV or HHV6 plasma viral load. While studies such as the present study may help to reveal other microbial triggers in MS pathogenesis, further investigations are required to reveal the role of specific subsets of NK and CD8+ T cells in MS.
Acknowledgements
This research was financially sponsored by the Tehran University of Medical Sciences, School of Medicine (grant number: 90-03-30-15044). We acknowledge the great contribution of technicians of the Tehran University of Medical Sciences, Sina General Hospital, and patients. We declare that the authors have no conflict of interest.
Authors’ Contributions
M.I., Z.S.; Conceived and designed the experiments. M.I.; Provided reagents and materials. Z.S., M.B., M.N., B.N., P.K., M.I.; Contributed to all experimental work, data and statistical analysis, and interpretation of data. M.A.S.; Diagnosed multiple sclerosis patient. All authors participated in the finalization of the manuscript and approved the final draft.
References
- 1.Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, et al. Author Correction: Multiple sclerosis. Nat Rev Dis Primers. 2018;4:49–49. doi: 10.1038/s41572-018-0050-3. [DOI] [PubMed] [Google Scholar]
- 2.Fierz W. Multiple sclerosis: an example of pathogenic viral interaction? Virol J. 2017;14:42–42. doi: 10.1186/s12985-017-0719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKay KA, Kwan V, Duggan T, Tremlett H. Risk factors associated with the onset of relapsing-remitting and primary progressive multiple sclerosis: a systematic review. Biomed Res Int. 2015;2015:817238–817238. doi: 10.1155/2015/817238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mentis AA, Dardiotis E, Grigoriadis N, Petinaki E, Hadjigeorgiou GM. Viruses and multiple sclerosis: from mechanisms and pathways to translational research opportunities. Mol Neurobiol. 2017;54(5):3911–3923. doi: 10.1007/s12035-017-0530-6. [DOI] [PubMed] [Google Scholar]
- 5.Münz C, Chijioke O. Natural killer cells in herpesvirus infections. F1000Res. 2017;6:F1000 Faculty Rev–1231. doi: 10.12688/f1000research.11197.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Lier RAW, Ten Berge IJM, Gamadia LE. Human CD8+ Tcell differentiation in response to viruses. Nat Rev Immunol. 2003;3(12):931–939. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 7.Salehi Z, Doosti R, Beheshti M, Janzamin E, Sahraian MA, Izad M. Differential frequency of CD8+ T cell subsets in multiple sclerosis patients with various clinical patterns. PLoS One. 2016;11(7):e0159565–e0159565. doi: 10.1371/journal.pone.0159565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreira A, Alari-Pahissa E, Munteis E, Vera A, Zabalza A, Llop M, et al. Adaptive features of natural killer cells in multiple sclerosis. Front Immunol. 2019;10:2403–2403. doi: 10.3389/fimmu.2019.02403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcenaro E, Notarangelo LD, Orange JS, Vivier E. NK cell subsets in health and disease: new developments. Front Immunol. 2017;8:1363–1363. doi: 10.3389/fimmu.2017.01363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang EC, Lehner PJ, Graham S, Borysiewicz LK. CD8high (CD57+) T cells in normal, healthy individuals specifically suppress the generation of cytotoxic T lymphocytes to Epstein-Barr virus-transformed B cell lines. Eur J Immunol. 1994;24(11):2903–2909. doi: 10.1002/eji.1830241148. [DOI] [PubMed] [Google Scholar]
- 12.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28- and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134(1):17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appay V, van Lier RAW, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73(11):975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 14.Azzi T, Lünemann A, Murer A, Ueda S, Béziat V, Malmberg KJ, et al. Role for early-differentiated natural killer cells in infectious mononucleosis. Blood. 2014;124(16):2533–2543. doi: 10.1182/blood-2014-01-553024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mimpen M, Smolders J, Hupperts R, Damoiseaux J. Natural killer cells in multiple sclerosis: a review. Immunol Lett. 2020;222:1–11. doi: 10.1016/j.imlet.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Kaskow BJ, Baecher-Allan C. Effector T cells in multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(4):a029025–a029025. doi: 10.1101/cshperspect.a029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plantone D, Marti A, Frisullo G, Iorio R, Damato V, Nociti V, et al. Circulating CD56dim NK cells expressing perforin are increased in progressive multiple sclerosis. J Neuroimmunol. 2013;265(1-2):124–127. doi: 10.1016/j.jneuroim.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Rommer PS, Milo R, Han M, Satyanarayan S, Sellner J, Hauer L, et al. Immunological aspects of modern MS therapeutics. Front Immunol. 2019;10:1564–1564. doi: 10.3389/fimmu.2019.01564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pender MP, Csurhes PA, Pfluger CM, Burrows SR. Deficiency of CD8+ effector memory T cells is an early and persistent feature of multiple sclerosis. Mult Scler. 2014;20(14):1825–1832. doi: 10.1177/1352458514536252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song ZY, Yamasaki R, Kawano Y, Sato S, Masaki K, Yoshimura S, et al. Peripheral blood T cell dynamics predict relapse in multiple sclerosis patients on fingolimod. PLoS One. 2015;10(4):e0124923–e0124923. doi: 10.1371/journal.pone.0124923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 23.Williams JR. The declaration of helsinki and public health. Bull World Health Organ. 2008;86(8):650–652. doi: 10.2471/BLT.08.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sam SS, Rogers R, Gillani FS, Tsongalis GJ, Kraft CS, Caliendo AM. Evaluation of a next-generation sequencing metagenomics assay to detect and quantify DNA viruses in plasma from transplant recipients. J Mol Diagn. 2021;23(6):719–731. doi: 10.1016/j.jmoldx.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Álvarez-Lafuente R, Heras VD, Bartolomé M, García-Montojo M, Arroyo R. Human herpesvirus 6 and multiple sclerosis: a one-year follow-up study. Brain Pathol. 2006;16(1):20–27. doi: 10.1111/j.1750-3639.2006.tb00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cocuzza CE, Piazza F, Musumeci R, Oggioni D, Andreoni S, Gardinetti M, et al. Quantitative detection of Epstein-Barr virus DNA in cerebrospinal fluid and blood samples of patients with relapsing-remitting multiple sclerosis. PLoS One. 2014;9(4):e94497–e94497. doi: 10.1371/journal.pone.0094497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cermelli C, Berti R, Soldan SS, Mayne M, D’Ambrosia JM, Ludwin SK, et al. High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J Infect Dis. 2003;187(9):1377–1387. doi: 10.1086/368166. [DOI] [PubMed] [Google Scholar]
- 28.Soldan SS, Leist TP, Juhng KN, McFarland HF, Jacobson S. Increased lymphoproliferative response to human herpesvirus type 6A variant in multiple sclerosis patients. Ann Neurol. 2000;47(3):306–313. [PubMed] [Google Scholar]
- 29.Ahmadi Jalali Moghadam M, Honarmand H. Human herpesvirus 6 viremia in patients with classic multiple scelerosis in guilan, Iran. J Med Microbiol Infec Dis. 2014;2(4):133–137. [Google Scholar]
- 30.Agut H, Bonnafous P, Gautheret-Dejean A. Laboratory and clinical aspects of human herpesvirus 6 infections. Clin Microbiol Rev. 2015;28(2):313–335. doi: 10.1128/CMR.00122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116(19):3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Jager PL, Rossin E, Pyne S, Tamayo P, Ottoboni L, Viglietta V, et al. Cytometric profiling in multiple sclerosis uncovers patient population structure and a reduction of CD8low cells. Brain. 2008;131(7):1701–1711. doi: 10.1093/brain/awn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodríguez-Martín E, Picón C, Costa-Frossard L, Alenda R, Sainz De La Maza S, Roldán E, et al. Natural killer cell subsets in cerebrospinal fluid of patients with multiple sclerosis. Clin Exp Immunol. 2015;180(2):243–249. doi: 10.1111/cei.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina S, Villarrubia N, Sainz de la Maza S, Lifante J, CostaFrossard L, Roldán E, et al. Optimal response to dimethyl fumarate associates in MS with a shift from an inflammatory to a tolerogenic blood cell profile. Mult Scler. 2018;24(10):1317–1327. doi: 10.1177/1352458517717088. [DOI] [PubMed] [Google Scholar]
- 35.Marastoni D, Buriani A, Pisani AI, Crescenzo F, Zuco C, Fortinguerra S, et al. Increased NK cell count in multiple sclerosis patients treated with dimethyl fumarate: a 2-year longitudinal study. Front Immunol. 2019;10:1666–1666. doi: 10.3389/fimmu.2019.01666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu GZ, Fang LB, Hjelmström P, Gao XG. Increased CD8+ central memory T cells in patients with multiple sclerosis. Mult Scler. 2007;13(2):149–155. doi: 10.1177/1352458506069246. [DOI] [PubMed] [Google Scholar]
- 37.Wu Z, Sinzger C, Frascaroli G, Reichel J, Bayer C, Wang L, et al. Human cytomegalovirus-induced NKG2Chi CD57hi natural killer cells are effectors dependent on humoral antiviral immunity. J Virol. 2013;87(13):7717–7725. doi: 10.1128/JVI.01096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell TM, McSharry BP, Steain M, Ashhurst TM, Slobedman B, Abendroth A. Varicella zoster virus productively infects human natural killer cells and manipulates phenotype. PLoS Pathog. 2018;14(4):e1006999–e1006999. doi: 10.1371/journal.ppat.1006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizzo R, Soffritti I, D’Accolti M, Bortolotti D, Di Luca D, Caselli E. HHV-6A/6B infection of NK cells modulates the expression of miRNAs and transcription factors potentially associated to impaired NK activity. Front Microbiol. 2017;8:2143–2143. doi: 10.3389/fmicb.2017.02143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GMA, Papagno L, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8(4):379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]