Abstract
Objective
The interaction of tumor cells with surrounding stem cells such as adipose-derived mesenchymal stem cells (ASCs) would be a crucial mechanism of tumor progression. It has been shown that irradiation can affect tumor microenvironment through different mechanisms. Given that, we aimed to examine the bystander radiation-induced effects of ASCs on different cancer cell lines.
Materials and Methods
In this experimental study, ASCs were extracted from five healthy donors, cultured and then irradiated with a 5Gy of gamma radiation. Following 72 hours of incubation, irradiated ASCs-conditioned media (IACM) and non-irradiated ASCs-conditioned media (NIACM) were collected. Following incubation of different cell lines, Jurkat, LNCaP, U87-MG, MDA-MB-231 and MCF-7, in different media, DMEM, NIACM, and IACM, ALDEFLUOR assay and wound healing assays, were conducted. Using quantitative real-time polymerase chain reaction (qRT-PCR), the expression of ATP-binding cassette transporter genes, ABCA1 and ABCG2, was measured in these cell lines.
Results
NIACM significantly increased ALDH activity in MDA-MB-231 cell (P=0.02), while IACM was associated with significant decrease in the LNCaP and MCF-7 cell lines, respectively P=0.02, P=0.03, compared to DMEM as the control. The area of the scratch site was significantly reduced in MDA-MB-231 cells cultured with NIACM compared to DMEM (P=0.04). Furthermore, ABCA1 mRNA expression was considerably decreased in IACM- but not in DMEMtreated LNCaP line (P=0.01).
Conclusion
It seems, after exposing to radiation, ASCs modify to prevent tumor development and metastasis through their radiation-induced bystander effects. Therefore, a better understanding of ASCs function in the tumor microenvironment may provide new insights into therapeutic strategies to surmount radio-resistance in cancer treatment.
Keywords: Cancer, Radiation, Stem Cells, Tumor Microenvironment
Introduction
Radiotherapy is one of the main cancer treatment modalities. It has been shown that irradiation can affect and sensitize tumor microenvironment through different mechanisms such as immunomodulation and angiogenesis (1). Certain scientists believe that besides tumor cells, manipulating tumor microenvironment components can be beneficial to promising cancer treatment (2). The interaction of tumor cells and surrounding stem cells such as adipose-derived mesenchymal stem cells (ASCs) has been recently reported as a crucial mechanism of tumor progression and development (3). On the other hand, irradiated tumor cells release certain cytokines and growth factors which lead to the recruitment of stem cells, and promotion of cell proliferation and metastatic behavior of nearby tumor cells (4). This phenomenon is equivalent to bystander effects in radiobiology which reveals certain effects in the non-irradiated cells located in the neighboring irradiated cells. It has been reported that tumor cells induce mesenchymal stem cells (MSCs) to express factors such as aldehyde dehydrogenase (ALDH) (5). Also, it has been shown that radiation-conditioned media increase ALDH activity (6). Hence, evaluation of this factor can help us understand the aggressive behavior of different cancer cell lines due to culture with irradiated ASCs-conditioned media (IACM). On the other hand, regulation and expression of different genes in human stem cells can be essential. ATP-binding cassette (ABC) transporters genes which encode membrane-bound pumps have pivotal roles in the biological communication systems. Among the seven subfamilies of ABC proteins, ABCG2 has a multidrug-resistant phenotype which considered as a cancer stem cell indicator (7). A recent study has shown that the highest expression of ABCG2 in various malignancies increases resistance to drugs or radiotherapy treatment (8). ABCA1 is another member of this family that regulates cholesterol efflux protein across the plasma membrane and probably stimulates carcinogenesis (9). Therefore, increasing the expression of ABC transporters can affect radiotherapy outcomes (10).
Today, the capacity of MSCs to modulate the tumor microenvironment is of great importance in the field of cancer research (11-13). On the other hand, little is known about the effects of irradiated ASCs located in the tumor microenvironment on tumor progression. Due to the lack of attention to ASCs in radiation therapy regimens, the objective was to examine the probability of bystander radiation-induced effects of irradiated ASCs-conditioned media on different cancer cell lines.
Materials and Methods
Ethical approval
In this experimental study, ASC samples were obtained from five healthy participants recruited for mammoplasty surgery. Participants with a history of chemotherapy, radiotherapy and medical conditions such as autoimmunity were not included in this study. Prior to the study, participation consent and approval were obtained by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.REC.1396.S290). All participants were assured that their information would be kept confidential.
Adipose-derived mesenchymal stem cell isolation and culture
ASCs were obtained using a previously reported method (14). Briefly, adipose tissues were washed twice with phosphate buffered saline (PBS), fragmented with surgical scalpels and incubated with 0.2% collagenase type I (Cat. No. 17100-017, Gibco, USA) at 37°C for 40 minutes. After that, the cell suspension was centrifuged at 400G for 5 minutes and was cultured in the Dulbecco’s Modified Eagle’s Medium (DMEM, Cat. No. LM-D1099, Biosera, UK) supplemented with 1% penicillin/streptomycin (Cat. No. XC-A4122, Biosera, UK) and 10% fetal bovine serum (FBS, Cat. No. 10270-106, Gibco, USA). Following the growth and increase in the number of cells to a confluency of 90%, they were transferred to other flasks using trypsin-EDTA (Cat. No. LM-T1706, Biosera, UK). Flow cytometry was used to characterize the isolated cells. In brief, the extracted cells were detached from the surface of the flasks with trypsin-EDTA. After centrifuging the cells with PBS, they were stained with fluorescin isothiocyanate (FITC)-coupled or phycoerythrin (PE)-conjugated CD14, CD34, CD45, CD44, CD105, CD166. Isotype-matched mouse monoclonal antibodies (BD-Pharmingen, USA) were used to exclude non-specific staining of the cells. The characterized cells were thoroughly washed with PBS and analyzed on a FACSCalibur machine (BD Biosciences, USA).
Irradiation geometry
At 90% confluent, ASCs were exposed to 5 Gy of gamma radiation at a dose rate of 0.28 Gy/minutes based on a previous study (15). We used a non-clinical Theratron cobalt-60 therapy unit (MDS Nordion, Canada) of our Radiotherapy and Oncology Department. To further ensure the correctness of the delivered doses, a Farmertype ionization chamber (PTW-Freiburg, Germany) was utilized in the same cells irradiated geometry. The dimensions of the radiation field and the distance of Cobalt-60 source to the cells were considered 35×35 cm2 and 80 cm, respectively. Simulating the same conditions for ASCs, the control group of the ASCs was placed outside the radiation treatment room when the cells in the treatment groups were irradiated. Following radiation, the ASCs’ flasks were placed in an icebox and quickly transferred to the incubator (37˚C and 5% CO2 with 95% humidity). Seventy two hours after incubation, ASCs’ cell culture supernatant was collected and used for the rest of the study.
Cancer cell lines
Herein, we used U87MG as a malignant cell line of glioblastoma (C531, Pasteur Institute, Iran). Also, two breast cancer cell lines, including MCF-7 (C135, Pasteur Institute, Iran) as a non-invasive cell line and MDA-MB-231 (C578, Pasteur Institute, Iran) as an invasive triple negative cell line were employed. Other cell lines such as LNCaP, a human prostatic carcinoma cell line (C439, Pasteur Institute, Iran), and Jurkat, an immortalized line of human T lymphocyte (C121, Pasteur Institute, Iran), were also used. All these cell lines were obtained from the National Cell Bank of Iran. They were cultured in RPMI-1640 (Cat. No. LM-R1637, Biosera, UK) containing 10% FBS, and 1% penicillin/streptomycin and gradually adapted to DMEM media. Different cell lines were separately cultured in DMEM, NIACM and IACM at 37˚C, 5% CO2 with 95% humidity. After 72 hours, the tests were performed.
ALDEFLUOR assay and flow cytometry
The cancer cell lines including MDA-MB-231, U87- MG, MCF-7, LNCaP, and Jurkat were cultured with the collected conditioned media of irradiated and non-irradiated ASCs for 72 hours. ALDEFLUOR kit (Cat. No. 01700, Stem Cell Technologies, Vancouver, BC, Canada) was utilized to detect the different levels of ALDH enzymatic activity in cancer cells. For ALDEFLUOR assay, 3×105 cells/ml were harvested from cell cultures and resuspended in 1ml ALDEFLUOR assay buffer. Then, 5 μl ALDH inhibitor diethylaminobenzaldehyde (DEAB) was added to each negative control tube and recapped immediately. Following this step, 5 μl of activated substrate, ALDH, was added to the test tubes, while ALDH is BODIPY™-aminoacetaldehyde (BAAA) substrate. It is recommended to make aliquots of activated substrate and use them in your experiments. After that, 0.5 ml of suspension cells in the test tubes were moved to the same negative control. Different cell lines were incubated for 40- 50 minutes at 37˚C so the cells have enough time to convert the substrate to the fluorescent product. Subsequently, the cells were centrifuged, washed and suspended in 0.3 ml of ALDEFLUOR™ assay buffer. Flow cytometry (FACSCALIBUR- BD Company-USA) was employed to perceive the brightly fluorescent ALDH+ cells in the FL1- channel. Finally, acquired data were analyzed by FLOWJO software (Tree Star Inc, OR, USA). According to the kit manufacturer’s instructions, the SKBR-3 cell line (C207, Pasteur Institute, Iran) was used as a positive control, and at least 2×105 events were collected per samples.
Scratch assay (wound healing assay)
To investigate the wound healing effect of ASCs, the scratch assay was done. Following a 72-hour culture, the cell conditioned media were collected from irradiated ASCs and non-irradiated ASCs and frozen at -70˚C until required. Then, 2×105 of each cancer cell line (MCF-7, MDA-MB-231, LNCaP, U87-MG), adapted to the DMEM, were seeded in quadruplicates in a 24-well plate overnight and permitted to adhere and form a confluent monolayer. Next, with the use of a 10 μl pipette tip, we created a scratch as a straight line through the center of each well containing different cancer cells and the wells washed with PBS to remove cell debris. After that, 400 µl of IACM or NIACM were added to each cell line. At time zero and 8 hours after incubation with the conditioned media of ASCs, wound healing was monitored by measuring the gap area for each frame in the wound healing experiment. The staining was done with crystal violet, after which, the cell imaging was performed (eight images for each sample). To obtain reliable results, the tests were repeated twice for each sample. Finally, images were processed with ImageJ software (National Institute of Health, Bethesda, MD, USA).
RNA isolation and cDNA synthesis
Also, 72 hours following gamma radiation, supernatant of ASCs culture was collected and the adherent ASCs were washed twice with PBS. After co-culture of ASCs with LNCaP, MDA-MB-231, and MCF-7 cell lines, total RNA was extracted using 1ml of cold RNX-Plus (Cat. No. EX6101, Sinaclon, Iran) from cell lines. To check the accuracy, optical densities of isolated RNA were read at 260 nm and 280 nm wavelengths using a NanoDrop spectrophotometer (Wilmington, DE, USA). In the next step, cDNA was produced using cDNA synthesis kit (Cat. No. K1632, Thermo Fisher Scientific, USA) following on the manufacturer’s instruction.
Quantitative real-time polymerase chain reaction
Using quantitative real-time polymerase chain reaction (qRT-PCR), ABC transporter genes (A1 and G2) expression of cell lines was assessed after cultured with DMEM, NIACM, and IACM. All qPCR reactions were measured at least two times using 2xSYBR Green Master Mix (Cat. No. 4309155, Thermo Fisher Scientific, USA). The amplification was done for 40 cycles (95˚C 20 seconds, 60˚C 20 seconds, 72˚C 40 seconds). The specificity of qRT-PCR amplification was verified by melting curve analysis. Here, β-actin gene was used as the housekeeping gene. Finally, ΔΔCt method was used to determine the relative expression level of mRNA in the samples. The primers were designed by AllelID software (Oligo Perfect Designer, Invitrogen, USA.
Statistical analysis
SPSS software version 21 and Graph Pad Prism 5 were utilized for data analysis and graph presentation, respectively. We used Kruskal-Wallis H test for the analysis of non-parametric data. Also, P<0.05 was considered significant.
Results
Subjects and ASCs characterization
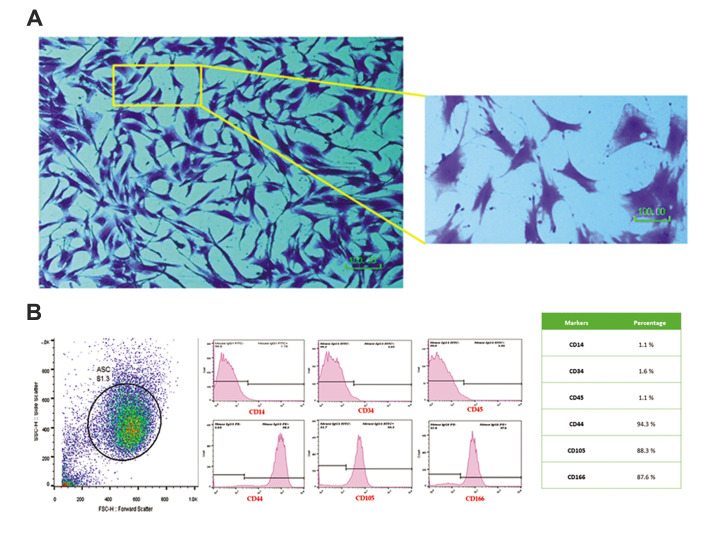
Based on medical records, the mean ± SD age of the participants was 38 ± 4 years. Harvested in passage 3, ASCs was demonstrated a spindle-shape appearance in 0.1% crystal violet staining (Fig .1A). Moreover, ASCs were harvested and characterized for the expressions of MSC markers. Flow cytometry characterization revealed that ASCs were extremely positive for the expressions of CD166, CD44, and CD105, yet negative for CD34, CD14, and CD45 (Fig .1B).
Fig.1.
Adipose-derived mesenchymal stem cells (ASCs) characterization. A. Morphology and B. Phenotype. ASCs were seen with spindle shape appearance and expression of MSC specific CD markers (scale bar: 100 μm).
ALDEFLUOR assay and flow cytometry analysis
After test set up by using SK-BR-3 breast cancer cell line, ALDH+ cells population were detected (Fig .2A). We obsereved that ALDH activity in the MDA-MB-231 breast cancer cell line increased with both of NIACM and IACM which was statistically significant in the case of using NIACM (P=0.02). We observed no significant difference in the percentage of ALDH+ between MDA-MB-231 cells cultured with DMEM and IACM (P=0.53). The mean ± SEM percentages of ALDH+ cells in the DMEM, NIACM, and IACM were 4.81 ± 0.23%, 14.63 ± 0.48%, and 6.01 ± 0.4%, respectively (Fig .2B, C).
Fig.2.
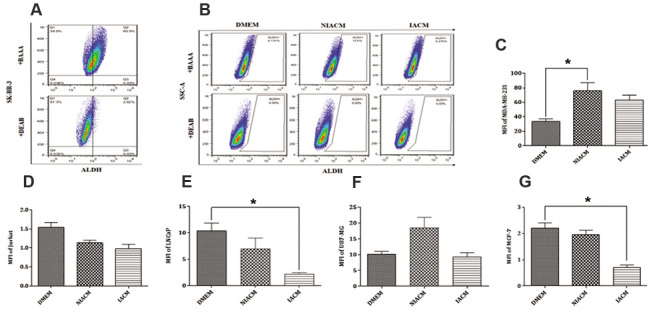
Evaluation of ALDH activity in different cancer cell lines. A. Detection of ALDH+ cells population in SKBR3 breast cancer cell line as a positive control. B. The schematic representation of ALDH positive cells by flow cytometry. Statistical analysis of ALDH activity post culture of C. MDA-MB-231, D. Jurkat, E. LNCaP, F. U87-MG, and G. MCF-7 with DMEM, NIACM of ASCs and IACM of ASCs. ALDH-DEAB was used as a negative control. Kruskal-Wallis test was used for the analysis of non-parametric data. ASCs; Adipose-derive mesenchymal stem cells, IACM; Irradiated ASCs-conditioned media, NIACM; Non-irradiated ASCs-conditioned media, ALDH; Aldehyde dehydrogenase, DMEM; Dulbecco’s Modified Eagle’s Medium, and *; P<0.05.
Also, the effects of IACM and NIACM on the Jurkat were examined. The results showed that NIACM and IACM, in comparison to the DMEM, reduces the ALDH enzyme in the Jurkat, although the change was not statistically significant (P=0.69, P=0.05). These changes were more noticeable in IACM. The mean ± SEM percentages of ALDH+ cells in DMEM, NIACM, and IACM were 6.04 ± 0.45%, 5.11 ± 0.43%, and 3.87 ± 0.42%, respectively (Fig .2D). The results of LNCaP revealed that NIACM decreased ALDH enzyme activity when compared to DMEM, however, these changes were not statistically significant (P=0.53).The culture of LNCaP with IACM significantly reduced ALDH activity in comparison with DMEM (P=0.02). The mean ± SEM percentages of ALDH+ cells in DMEM, NIACM, and IACM were 3.56 ± 0.14%, 2.23 ± 0.6% and 0.81 ± 0.07%, respectively (Fig .2E).
Evaluation of ALDH activity in U87-MG showed that when U87-MG cells were cultured with NIACM and IACM, the activity of ALDH enzyme increased, but not significantly (P=0.05, P=0.9). The ALDH activity was 1.47-fold higher in the NIACM cultured U87-MG cells than DMEM. The mean ± SEM percentages of ALDH+ cells in DMEM, NIACM, and IACM were 2.39 ± 0.14%, 3.70 ± 0.23% and 2.7 ± 0.16%, respectively (Fig .2F).
The assessment of the expression of ALDH in the MCF-7 breast cancer cell line showed that ALDH activity was barely reduced while, MCF-7 cell line was cultured with NIACM (P=0.40). In contrast, IACM significantly reduced ALDH activity in the MCF-7 cell line, also in comparison with DMEM cells cultured we observed down to 2-fold (P=0.03). The mean ± SEM percentages of ALDH+ cells in DMEM, NIACM, and IACM were 2.75 ± 0.16%, 1.95 ± 0.17%, and 1.35 ± 0.22%, respectively (Fig .2G).
Scratch wound healing assay
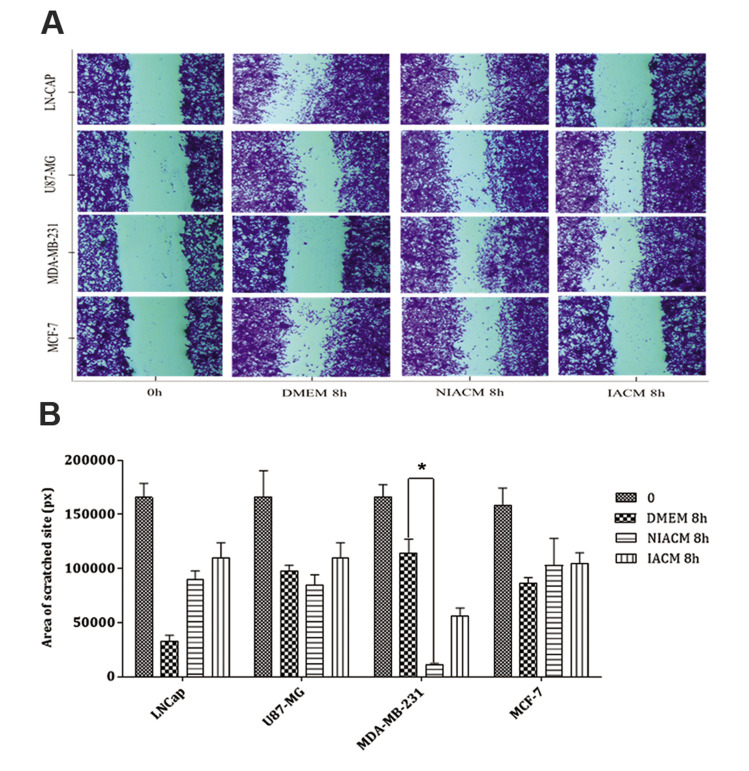
For wound healing assay, all images were taken at the beginning (0 hour) and 8 hours after culturing the different tumor cell lines with different culture media: standard (DMEM 10%), IACM, and NIACM (Fig .3A). Images analysis showed less migration in LNCaP, U87- MG, and MCF-7 cell lines cultured with IACM (P=0.9), in comparison with DMEM. A reduction was further observed in the area of the scratched site in the U87- MG and MDA-MB-231cancer cell lines cultured with NIACM, which was statistically significant for MDA-MB-231 (P=0.041, Fig .3B).
Fig.3.
Wound healing assay at the beginning (0 hour) and 8 hours of different tumor cell lines in different culture media. A. A scratch was made in a confluent monolayer of LN-CAP, U87MG, MDA-MB-231, and MCF-7 cancer cell lines, and they were then cultured in the presence of DMEM, NIACM of ASCs and IACM of ASCs. Images were acquired at 0 and 8 hours post-incubation (scale bar: 100 μm). B. Analysis of wound healing response of cancer cell lines following exposure to DMEM, NIACM, and IACM of ASCs. Data are presented as mean ± SEM. P<0.05 was considered significant. Kruskal-Wallis test was used for the analysis of non-parametric data. ASCs; Adipose-derive mesenchymal stem cells, IACM; Irradiated ASCs-conditioned media, NIACM; Non-irradiated ASCs-conditioned media, h; Hours, DMEM; Dulbecco’s Modified Eagle’s Medium, and *; P<0.05.
ABC transporters (A1 and G2) expression in the LNCaP, MDA-MB-231, and MCF-7
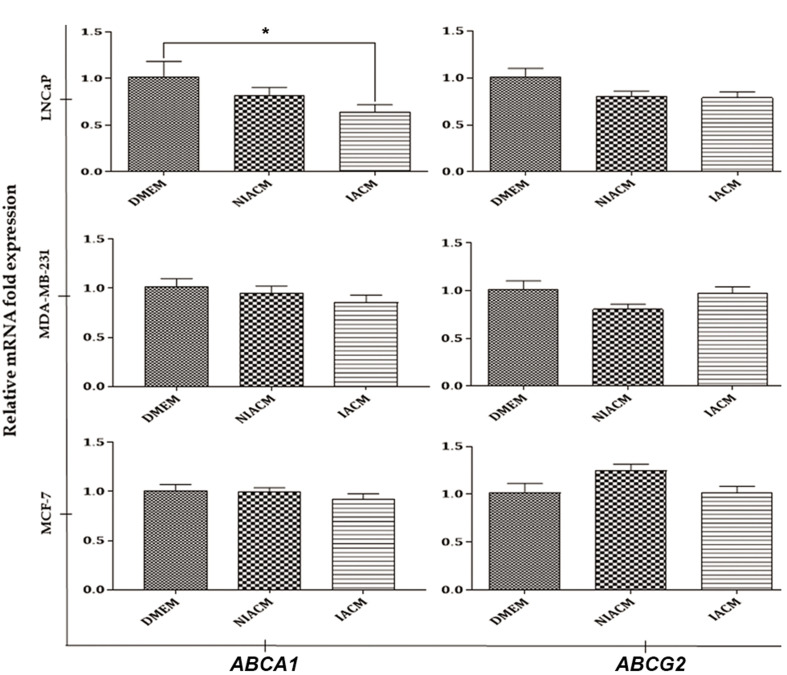
Based on the results of the ALDH activity, ABCA1 and ABCG2 expression was investigated in LNCaP, MDA-MB-231 and MCF-7 cancer cell lines. This may shed light on the molecular mechanisms responsible for the effects of IACM and NIACM. As shown in Figure 4, IACM reduced the mRNA expression of ABCA1 and ABCG2 in the LNCaP, in comparison with DMEM, which was statistically significant for ABCA1 (P=0.01). No difference was found in the mRNA expression of ABCA1 and ABCG2 in the both of MDA-MB 231 and MCF-7 cell lines among the different studied groups.
Fig.4.
The mRNA expression of ABCA1 and ABCG2 in LNCaP, MDA-MB-231, and MCF-7 cultured in the presence of DMEM, NIACM, and IACM of ASCs. Each experiment was conducted twice independently, with each sample measured in duplicate. Error bars indicate the standard error of the mean and statistical significance was set at P<0.05 (*). Kruskal-Wallis test was used to analyze non-parametric data. ASCs; Adipose-derive mesenchymal stem cells, IACM; Irradiated ASCs-conditioned media, DMEM; Dulbecco’s Modified Eagle’s Medium, and NIACM; non-irradiated ASCs -conditioned media.
Discussion
Over recent years, scientists have turned their attention to the tumor microenvironment as an effective target for overcoming tumor therapeutic resistance (16, 17). Also, the bystander irradiation effects assessment showed that besides direct effects, irradiated cells release various mediators to their neighbors, causing a variety of potential effects on bystander cells (18). The present research examined the effects of ASCs irradiated and non-irradiated conditioned media on the ALDH activity of different cancer cell lines. We exhibited that NIACM caused a significant upregulation in ALDH activity, as well as the migration of MDA-MB-231 cells. However, co- culturing with IACM led to the downregulation of ALDH activity in all our studied cell lines, particularly LNCap, MDA-MB-231, and MCF-7. Also, IACM reduces LNCap and MDA-MB-231 migration compared to NIACM and DMEM. Moreover, our findings showed that the expression of ABC transporter A1 in the LNCap was significantly reduced due to culturing with IACM, in comparison with the control group.
Li et al. (5) reported that the co-culture of carcinoma cells with bone marrow-derived MSCs increases ALDH positive cells. They also showed that prostaglandin E2 (PGE2) secretion by MSCs can lead to cancer stem-cell generation and tumor progression. Moreover, MSC-conditioned medium prevents A549 cell migration but stimulates MDA-MB-231 cell migration through modulating insulin receptor and human epidermal growth factor receptor 3 phosphorylation (19). Based on Sherman et al. study, the culture of fibroblasts with autologous bone marrow-derived MSCs and MSCs-conditioned media increase the migration of these cells, that lead to reducing the scratch area percentage (20). Based on our results, it can be speculated that the presence of ASCs in the tumor microenvironment conduce tumor cell progression, especially triple-negative breast cancer cells. This finding is in line with the results of previous study conducted by Razmakah et al. (21). Observing non-significant results in this study regarding other studied cancer cell lines, the role of the NIACM and IACM would be less effective on the metastasis of other types of tumor cells compared to breast cancer. This mechanism might be conducted through communicating with tumor cells and increasing mediators such as ALDHs in these cells. It leads to increase tumor growth and cancer stem cell production and survival. However, it seems that this scenario is different in various types of cancers; in this regard, Maj et al. (22) reported that ASC-conditioned media reduced viability of human renal carcinoma cell line (786-0) and bladder carcinoma cell line (T24). They also cleared that amniotic fluid-derived stem cells (AFSCs) media reduced the metabolic activity of 786-0 cells significantly. Also, further studies showed that MSC-conditioned media may decrease the resistance of tumor cells to drug and radiation treatment (23, 24). These findings are inconsistent with our results; an increase in the MDA-MB-231 cell line proliferation in NIACM co-cultured condition. This may reflect the differential effects of MSCs in relation to the tumor cell type.
It has been shown that irradiation augments the ALDH expression in different cell lines (25, 26). Scientists have demonstrated that ALDHhiCD44+ breast cancer cells contribute to radiation and chemotherapy resistance of breast cancer. Also, ALDH expression, a cancer stem cell biomarker, is capable to increase DNA repair potential, that results in the tumors radioresistance and chemoresistance (27). Our results showed that IACM reduces the ALDH activity in all cancer cell lines, particularly LNCaP, MCF-7 and MDA-MB-231 cells, and suppresses proliferation in LNCaP and MDA-MB-231 and U87-MG compared to NIACM co-cultured condition. In this regard, He et al. (28) reported that MSC-conditioned media reduced the ALDH positive cancer stem cells activity and tumor cell migration and metastasis. Based on this study, MSC-conditioned media can increase radio sensitivity of MDA-MB-231 cell line through down-regulating Stat3 signaling pathway. According to this report and our findings, it seems that radiation to stromal cells would be an effective step to alleviate the radiation resistance specially in the MDA-MB-231 cancer cells. Moreover, although ASCs may promote tumor growth and development, they would be modified under the influence of distinct radiation doses. Also, radiation shows bystander effects on tumor cells, and acts in favor of anti-tumor immune responses, particularly in prostate and breast cancers. It is supposed that the nature of the tumor and hormone receptors may impact the results of the study. Furthermore, it seems that ASCs does not play a role in the expression of ALDH in U87-MG and Jurkat cancer cells.
It has been reported that MSC culture supernatant had no significant effect on the Jurkat cell line proliferation (29). Also, previous studies showed that irradiation is capable of decreasing the MSCs proliferation and migration (30, 31). Based on our results NIACM decreases the proliferation of MDA-MB-231 cells. In line with our finding, de Araujo Farias et al. (32) also reported that MSCs conditioned media which exposed to 5 Gy of irradiation reduced the proliferation of tumor cells. According to this report, irradiated MSCs secrete certain anti-tumor cytokines with negative effects on the melanoma tumor cells (32). On the other hand, Haubner et al. (33) showed that co-culturing fibroblasts with ASCs improves wound healing due to cell proliferation stimulation and also, matrix metalloproteinases modulation following radiotherapy. In yet another study, Gómez-Millán et al. (34) evaluated the effects of umbilical cord MSC conditioned media on melanoma cancer cell lines. They reported that the presence of some cytokines in the irradiated-conditioned media regulates inflammation and protects bystander cells following irradiation. In the present research, in addition to the origin of our conditioned media, that were extracted from ASCs, we used different cancer cell lines. Given these results, it is likely that the source of stem cells influences the stimulating or inhibiting role of stem cells.
Normal stem cells express a high level of ABC transporters to retain a relatively stable intracellular environment, and also, export cytotoxic agents to the outside of the cell (35). It has been reported that ABC transports activity also serves as a cellular radioresistance (36). Yeh et al. (37) exhibited that human adipose-derived stem cells enhances the expression of ABCG2 in the triple negative breast cancer through CXCL1 secretion , which may lead to the tumor chemoresistance. In our study, we showed the statistically significant down regulation of ABCA1 in LNCaP cell line treated with IACM. Therefore, irradiation may modify ASCs through which chemoresistance in distinct cancer cells might be induced by altering the expression of related genes such as ABC transporters. This may not be the case in all types of cancers although, the effect of ABC transporters expression on the protein level has to be determined in future studies. Also, our results showed non-significant change in the expression of ABCA1 and ABCG2 in different types of triple positive (MCF-7) and negative (MDA-MB-231) breast cancer cell lines. Nevertheless, after co-culturing with NIACM and IACM, we recorded a rarely decrease in the expression of ABCA1 and ABCG2 in both of MDA-MB-231 and MCF-7 cancer cell lines. Previous study reported that co-culturing of breast cancer cells with MSCs leads to an enhanced expression of vimentin, Snail, and Slug and a reduced expression of E-cadherin and β-catenin (38). Also, it has been shown that silencing β-catenin leads to down-regulation of ABCG2 transporter expression and activity (39, 40).
Conclusion
Our finding highlighted that following exposure to radiation, ASCs may convert to positive operators. This character is associated with preventing tumor growth and the emergence of metastasis signs such as cancer stem cell production in certain types of tumors. Thus, more accurate insight into the importance of ASCs in the tumor microenvironment may disclose novel therapeutic strategies to overcome radio-resistance in cancer.
Acknowledgements
The authors would like to thank the patients and all the participants for their kind contribution to this project. This work was financially supported by Shiraz University of Medical Sciences, Shiraz, Iran (Grant no. 12468) and Shiraz Institute for Cancer Research (Grant no. ICR-100- 504). There is no conflict of interest in this study.
Authors’ Contributions
S.T.; All in vitro experiments performance, data analysis and manuscript writing. M.A.M.-S.; In vivo experiment performance and in vivo data analysis. A.Gh., M.R., S.M.J.M.; Study concept and design, sing the data analysis supervision, financial support, and manuscript final approvement. All authors read and approved the final manuscript.
References
- 1.Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Najafi M, Goradel N H, Farhood B, Salehi E, Solhjoo S, Toolee H, et al. Tumor microenvironment: Interactions and therapy. J Cell Physiol. 2019;234(5):5700–5721. doi: 10.1002/jcp.27425. [DOI] [PubMed] [Google Scholar]
- 3.Arena S, Salati M, Sorgentoni G, Barbisan F, Orciani M. Characterization of tumor-derived mesenchymal stem cells potentially differentiating into cancer-associated fibroblasts in lung cancer. Clin Transl Oncol. 2018;20(12):1582–1591. doi: 10.1007/s12094-018-1894-4. [DOI] [PubMed] [Google Scholar]
- 4.Ilmer M, Vykoukal J, Recio Boiles A, Coleman M, Alt E. Two sides of the same coin: stem cells in cancer and regenerative medicine. FASEB J. 2014;28(7):2748–2761. doi: 10.1096/fj.13-244640. [DOI] [PubMed] [Google Scholar]
- 5.Li HJ, Reinhardt F, Herschman HR, Weinberg RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem-cell niche via Prostaglandin E2 signaling. Cancer Discov. 2012;2(9):840–855. doi: 10.1158/2159-8290.CD-12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong J, Lang BJ, Weng D, Eguchi T, Murshid A, Borges TJ, et al. Genotoxic stress induces Sca‐1‐expressing metastatic mammary cancer cells. Mol Oncol. 2018;12(8):1249–1263. doi: 10.1002/1878-0261.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7(9):1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 8.Westover D, Li F. New trends for overcoming ABCG2/BCRP-mediated resistance to cancer therapies. J Exp Clin Cancer Res. 2015;34:159–159. doi: 10.1186/s13046-015-0275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attie A D. ABCA1: at the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem Sci. 2007;32(4):172–179. doi: 10.1016/j.tibs.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Al-Dimassi S, Abou-Antoun T, El-Sibai M. Cancer cell resistance mechanisms: a mini review. Clin Transl Oncol. 2014;16(6):511–516. doi: 10.1007/s12094-014-1162-1. [DOI] [PubMed] [Google Scholar]
- 11.Pennings S, Liu KJ, Qian H. The stem cell niche: Interactions between stem cells and their environment. Stem Cells Int. 2018:4879379–4879379. doi: 10.1155/2018/4879379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shojaei S, Hashemi SM, Ghanbarian H, Salehi M, Mohammadi‐Yeganeh S. Effect of mesenchymal stem cells‐derived exosomes on tumor microenvironment: tumor progression versus tumor suppression. J Cell Physiol. 2019;234(4):3394–3409. doi: 10.1002/jcp.27326. [DOI] [PubMed] [Google Scholar]
- 13.Zhang LN, Kong CF, Zhao D, Cong XL, Wang SS, Ma L, et al. Fusion with mesenchymal stem cells differentially affects tumorigenic and metastatic abilities of lung cancer cells. J Cell Physiol. 2019;234(4):3570–3582. doi: 10.1002/jcp.27011. [DOI] [PubMed] [Google Scholar]
- 14.Razmkhah M, Jaberipour M, Hosseini A, Safaei A, Khalatbari B, Ghaderi A. Expression profile of IL-8 and growth factors in breast cancer cells and adipose-derived stem cells (ASCs) isolated from breast carcinoma. Cell Immunol. 2010;265(1):80–85. doi: 10.1016/j.cellimm.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 15.D’Andrea FP, Safwat A, Burns JS, Kassem M, Horsman MR, Overgaard J. Tumour microenvironment and radiation response in sarcomas originating from tumourigenic human mesenchymal stem cells. Int J Radiat Biol. 2012;88(6):457–465. doi: 10.3109/09553002.2012.683509. [DOI] [PubMed] [Google Scholar]
- 16.Cheki M, Yahyapour R, Farhood B, Rezaeyan A, Shabeeb D, Amini P, et al. COX-2 in radiotherapy: a potential target for radioprotection and radiosensitization. Curr Mol Pharmacol. 2018;11(3):173–183. doi: 10.2174/1874467211666180219102520. [DOI] [PubMed] [Google Scholar]
- 17.Farhood B, Goradel NH, Mortezaee K, Khanlarkhani N, Salehi E, Nashtaei MS, et al. Intercellular communications-redox interactions in radiation toxicity; potential targets for radiation mitigation. J Cell Commun Signal. 2019;13(1):3–16. doi: 10.1007/s12079-018-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szatmári T, Kis D, Bogdándi EN, Benedek A, Bright S, Bowler D, et al. Extracellular vesicles mediate radiation-induced systemic bystander signals in the bone marrow and spleen. Front Immunol. 2017;8:347–347. doi: 10.3389/fimmu.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761–773. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherman AB, Gilger BC, Berglund AK, Schnabel LV. Effect of bone marrow-derived mesenchymal stem cells and stem cell supernatant on equine corneal wound healing in vitro. Stem Cell Res Ther. 2017;8(1):120–120. doi: 10.1186/s13287-017-0577-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razmkhah M, Mansourabadi Z, Mohtasebi MS, Talei AR, Ghaderi A. Cancer and normal adipose‐derived mesenchymal stem cells (ASCs): do they have differential effects on tumor and immune cells? Cell Biol Int. 2018;42(3):334–343. doi: 10.1002/cbin.10905. [DOI] [PubMed] [Google Scholar]
- 22.Maj M, Bajek A, Nalejska E, Porowinska D, Kloskowski T, Gackowska L, et al. Influence of mesenchymal stem cells conditioned media on proliferation of urinary tract cancer cell lines and their sensitivity to ciprofloxacin. J Cell Biochem. 2017;118(6):1361–1368. doi: 10.1002/jcb.25794. [DOI] [PubMed] [Google Scholar]
- 23.Cortes-Dericks L, Froment L, Kocher G, Schmid RA. Human lungderived mesenchymal stem cell-conditioned medium exerts in vitro antitumor effects in malignant pleural mesothelioma cell lines. Stem Cell Res Ther. 2016;7(5):1–8. doi: 10.1186/s13287-016-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raj AT, Kheur S, Bhonde R, Gupta AA, Patil S. Assessing the effect of human mesenchymal stem cell-derived conditioned media on human cancer cell lines: a systematic review. Tissue Cell. 2021;71:101505–101505. doi: 10.1016/j.tice.2021.101505. [DOI] [PubMed] [Google Scholar]
- 25.Ames E, Canter RJ, Grossenbacher SK, Mac S, Smith RC, Monjazeb AM, et al. Enhanced targeting of stem-like solid tumor cells with radiation and natural killer cells. Oncoimmunology. 2015;4(9):e1036212–e1036212. doi: 10.1080/2162402X.2015.1036212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vares G, Cui X, Wang B, Nakajima T, Nenoi M. Generation of breast cancer stem cells by steroid hormones in irradiated human mammary cell lines. PLoS One. 2013;8(10):e77124–e77124. doi: 10.1371/journal.pone.0077124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark DW, Palle K. Aldehyde dehydrogenases in cancer stem cells: potential as therapeutic targets. Ann Transl Med. 2016;4(24):518–518. doi: 10.21037/atm.2016.11.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He N, Kong Y, Lei X, Liu Y, Wang J, Xu C, et al. MSCs inhibit tumor progression and enhance radiosensitivity of breast cancer cells by down-regulating Stat3 signaling pathway. Cell Death Dis. 2018;9(10):1026–1026. doi: 10.1038/s41419-018-0949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mousavi Niri N, Jaberipour M, Razmkhah M, Ghaderi A, Habibagahi M. Mesenchymal stem cells do not suppress lymphoblastic leukemic cell line proliferation. Iran J Immunol. 2009;6(4):186–194. [PubMed] [Google Scholar]
- 30.Fekete N, Erle A, Amann EM, Fürst D, Rojewski MT, Langonné A, et al. Effect of high-dose irradiation on human bone-marrowderived mesenchymal stromal cells. Tissue Eng Part C Methods. 2014;21(2):112–122. doi: 10.1089/ten.tec.2013.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SH, Jin SY, Song JS, Seo KK, Cho KH. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol. 2012;24(2):136–143. doi: 10.5021/ad.2012.24.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Araujo Farias V, O’Valle F, Lerma BA, Ruiz de Almodovar C, Lopez-Penalver JJ, Nieto A, et al. Human mesenchymal stem cells enhance the systemic effects of radiotherapy. Oncotarget. 2015;6(31):31164–31180. doi: 10.18632/oncotarget.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haubner F, Muschter D, Pohl F, Schreml S, Prantl L, Gassner HG. A co-culture model of fibroblasts and adipose tissue-derived stem cells reveals new insights into impaired wound healing after radiotherapy. Int J Mol Sci. 2015;16(11):25947–25958. doi: 10.3390/ijms161125935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gómez-Millán J, Katz ISS, de Araujo Farias V, Linares-Fernández JL, López-Peñalver J, Ortiz-Ferrón G, et al. The importance of bystander effects in radiation therapy in melanoma skin-cancer cells and umbilical-cord stromal stem cells. Radiother Oncol. 2012;102(3):450–458. doi: 10.1016/j.radonc.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Di C, Zhao Y. Multiple drug resistance due to resistance to stem cells and stem cell treatment progress in cancer. Exp Ther Med. 2015;9(2):289–293. doi: 10.3892/etm.2014.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ingram WJ, Crowther LM, Little EB, Freeman R, Harliwong I, Veleva D, et al. ABC transporter activity linked to radiation resistance and molecular subtype in pediatric medulloblastoma. Exp Hematol Oncol. 2013;2(1):26–26. doi: 10.1186/2162-3619-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeh WL, Tsai CF, Chen DR. Peri-foci adipose-derived stem cells promote chemoresistance in breast cancer. Stem Cell Res Ther. 2017;8(1):177–177. doi: 10.1186/s13287-017-0630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klopp AH, Lacerda L, Gupta A, Debeb BG, Solley T, Li L, et al. Mesenchymal stem cells promote mammosphere formation and decrease E-cadherin in normal and malignant breast cells. PLoS One. 2010;5(8):e12180–e12180. doi: 10.1371/journal.pone.0012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chikazawa N, Tanaka H, Tasaka T, Nakamura M, Tanaka M, Onishi H, et al. Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer Res. 2010;30(6):2041–2048. [PubMed] [Google Scholar]
- 40.Vesel M, Rapp J, Feller D, Kiss E, Jaromi L, Meggyes M, et al. ABCB1 and ABCG2 drug transporters are differentially expressed in non-small cell lung cancers (NSCLC) and expression is modified by cisplatin treatment via altered Wnt signaling. Respir Res. 2017;18(1):52–52. doi: 10.1186/s12931-017-0537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]