Abstract
Objective
The clinical studies of acute myeloid leukaemia (AML) revealed that antigen escaping variants cause cancer recurrence even after treatment with chimeric antigen receptor (CAR)-T cells that target a single tumour antigen. Due to the heterogeneous expression of antigens on leukaemia blasts, we hypothesized that a novel bispecific CAR, directed to the folate receptor beta (FRβ)-binding single-chain variable fragment (scFv) and an IL3α-binding receptor (CD123) that has more expression in AML blasts, can decrease CAR-T cell exhaustion and increase the efficacy of CAR-T cells to prevent antigen escaping and consequent recurrence of AML.
Materials and Methods
In this experimental study, the survival, proliferation, and cytolysis of CAR-T cells remains suboptimal even with a costimulatory endodomain. Hence, we designed and constructed a tandem CAR that joins an FRβ and CD123 in the second generation retroviral vector to generate a bispecific tandem CAR (TanCAR-T cell).
Results
TanCAR FRβ-CD123 T cells showed distinct binding to FRβ or CD123 expressing cells. They could lyse the leukaemia cell lines (66.1 ± 11%) comparable to the single CAR-T cells against these determinants. TanCAR FRβ- CD123 T cells simultaneously engaged FRβ and CD123, which promoted T cell activation in targeting and lysis of the examined leukaemia cell lines. TanCAR-T cell significantly induced interferon gamma (IFNγ) and interleukin 2 (IL-2) production more than single CAR-T cells, which produced a synergistic enhancement of TanCAR FRβ-CD123 T cell function when dual antigens faced simultaneously.
Conclusion
Dual-specific TanCAR FRβ-CD123 T cells showed therapeutic potential to improve AML control by co- engaging FRβ and CD123 molecules in a robust, divalent immune system. This strategy may be a useful therapeutic approach in patients with relapsed B-cell malignancies.
Keywords: Acute Myeloid Leukaemia, Chimeric Antigen Receptor, CD123, Folate Receptor β
Introduction
Current research intends to redirect the immune system for specific recognition of transformed cells to cure cancers. The genetic engineering of protein structures such as immune cell antigen receptors has led to the production of chimeric antigen receptors (CARs) (1) and bispecific CARs that could redirect T cells to interact with specific targets on transformed cells (2, 3).
Acute myeloid leukaemia (AML) is a poor prognosis disease with a five-year survival rate below 50%. Although the treatments cause remission in the majority of patients, most relapse and require subsequent chemotherapy or hematopoietic stem cell transplant (HSCT) (4). In this setting, anti-CD19 CAR-T cells and anti-CD19/CD3 bispecific antibodies have achieved successful outcomes in some clinical studies (5). Blinatumomab is a dual-specific structure that is a bispecific T lymphocyte engager. Blinatumomab contains two different single-chain variable fragments (scFv) derived from monoclonal antibodies (mAb) against CD19 and CD3ε (6).
The dual-specific mAb concept is extensively used for B cell acute lymphoblastic leukaemia (ALL) (6, 7). The fusion of scFv from a mAb with the intracellular signalling domain of a T cell receptor (TCR) generates a CAR-T cell for starting an immunotherapy process. Over the last decade, dual-specific mAbs and CAR-T cells have proven their potential to regress cancer in patients with poor prognosis (8, 9). The Philadelphia Children’s Hospital of the University of Pennsylvania report of their CTL019 CAR-T cell indicated that out of 59 patients, 93% were in complete remission (CR) at one month. Patients had a median follow-up of 12 months with a reported relapse-free survival of 55% and overall survival of 79%. Despite the excellent responses of patients to CD19-directed T lymphocytes, a significant number of patients relapse. The data shows that of the 93% that have CR one month after therapy, only 55% have disease-free survival (DFS) after one year (10).
Further investigations show that CD19-loss is a mechanism of transformed cell escape from the CD19- directed immunotherapies (11). An alternative strategy to avoid antigen loss is co-targeting of multiple markers of the transformed cells. Possible targets expressed in B-cells and B-AML blasts include interleukin 3 (IL-3) receptor α (CD123) (12, 13) and folate receptor beta (FRβ) (14). An important target that is expressed on CD19-negative blasts relapsing after CAR-T cell therapy is CD123 (15). Studies show that the anti-CD123 CAR-T cells can eradicate CD19-negative blasts in xenograft animal models (16). The results of previous studies showed that the only CD123 targeting agent had limited efficacy against leukaemia cells (17). On the other hand, FRβ is expressed in about 70% of AML cases (18),thus making it an interesting target molecule for targeted immunotherapy (19). FRβ has limited expression in normal tissues and increased expression in B-AML blasts (20). Here, we sought to develop the first bispecific TanCAR that targets both CD123 and FRβ in order to overcome the problems reported for other CARs. We generated and characterized fully human CD123-FRβ bispecific CAR constructs that effectively expressed and targeted AML blast cell lines in vitro.
Materials and Methods
This experimental study included in silico simulations and in vitro studies to evaluate the function of a new bi-CAR-T cell against AML blasts. The methodology of this study was based on previous tandem CAR designs (17, 21) and IL-3 receptor blockade by an anticancer antibody PDB:4JZJ (13, 22).
In silico design of the structural functionality of a TanCAR FRβ-CD123
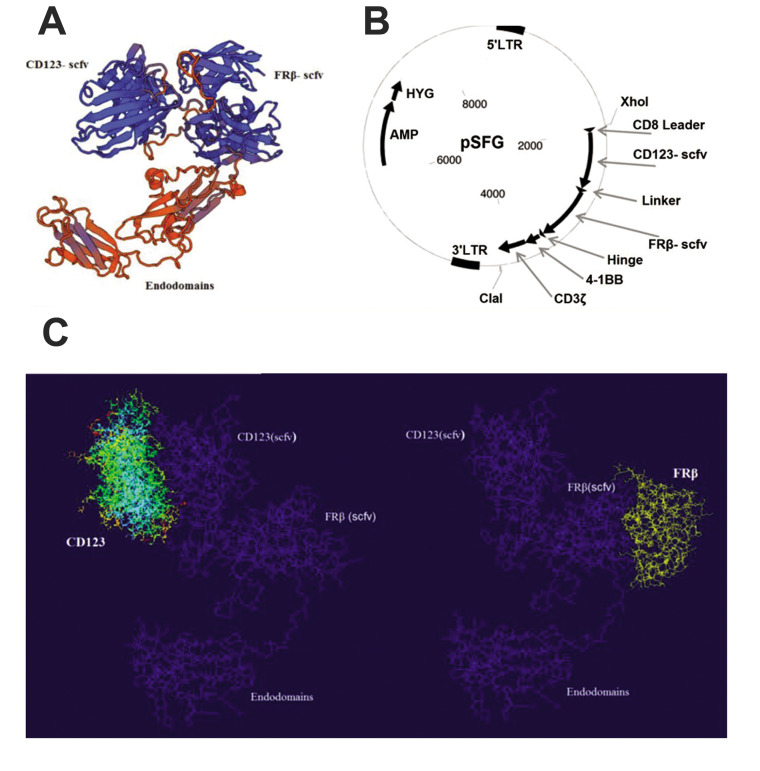
In order to redirect the specificity of T lymphocytes toward both CD123 and FRβ simultaneously using a CAR molecule, we constructed a tandem CAR. This is a bivalent CAR molecule that can engage two determinants with a single exo-domain (Fig .1A). The pattern of the TanCAR exo-domain was composed of the CD123- and FRβ-scFv fragments in tandem and separated by a linker. It was assembled on Clone Manager (3), modified to remove unwanted restriction enzyme sites, and optimized for maximum protein production using GeneOptimizer software, as previously described (23). The CAR exo-domain consisted of a CD8 leader sequence, followed by a CD123 scFv, and was joined to the FRβ-specific scFv by a five amino acid sequence [glycine (4)-serine (1)] linker. The cellular binding domain has an IgG1 hinge moiety. A CD8 transmembrane region connected the exo-domain to a second generation endodomain that incorporated the 4-1BB and CD3ζ signalling domain (Fig .1B).
Fig.1.
The CD123-FRβ bi-TanCAR structure, its encoding transgene, and in silico design. A. A computational rendition of the TanCAR structure. The CD123-scFv (left) and folate receptor beta (FRβ) (right) are shown in blue. The glycine/serine linker is highlighted in yellow. B. The pSFG vector construct that encodes the bispecific TanCAR. C. Schematic figure depicts the TanCAR docking to its respective targets. The structure prediction shows that the two designed chimeric single-chain variable fragment (scFv) chimeras are well-separated and capable to engage their own specific antigens.
Chimeric antigen receptor construction
The cassette encoded a single-chain antibody, the 4-1BB endodomain. The ζ chain of the TCR complex was cloned into the pSFG retroviral backbone (Fig .1B). The cassette was chemically synthesized (Biomatik, Canada). The synthesized cassette in pUC19 was amplified in an Escherichia coli TOP10 strain. The amplified plasmid was extracted and the insert was isolated by the double digestion of the Xho I and Cla I restriction enzymes. The entire sequence of the insert was amplified via polymerase chain reaction (PCR), which was conducted using F: GATGGATCAACGCTGGCAAT and R: GCCCCAGTAGTCAAACGAAC primers. The PCR product was digested and ligated into a second generation pSFG retroviral vector (Addgene, US) that contained 4-1BB and CD3ζ signalling chains (Fig .1B).
Production of the retroviral vector and T cell transduction
The vectors were produced in HEK293T cells as previously described by the Lanitis group (24). Briefly, a high-titre retroviral vector was produced in HEK293T cells that were seeded at 107 cells per T175 cell culture flask, 24 hours before transfection. All plasmid DNA were purified using the QIAGEN Maxi Prep Kit (Qiagen, Germany). The pELNS transfer plasmid by Express Inn (Open Biosytems, CA, US) was used for the transfection system. Cells were transfected with pVSV-G, pRSV.REV, pMDLg/p.RRE, and transfer plasmid (Open Biosytems, CA, US). The viral supernatant was harvested at 48 hours after the transfection. The viral particles were concentrated by centrifugation for three hours at 6000 g (Beckman Coulter, CA, US). Peripheral blood mononuclear cells (PBMCs) were obtained from three healthy donors. The T lymphocytes were enriched by CD3+ Ab (Miltenyi Biotec, Bergisch Gladbach, Germany). The lymphocytes were taken from a healthy donor that signed a written informed consent according to the Ethical Committee of the Life Sciences, Faculty of North Tehran Branch of Islamic Azad University, Tehran, Iran (IR.IAU.TNB.REC.1397.65). The CD8+ lymphocytes were isolated from a population of mononuclear cells via a MACS Isolation Kit (130-096-495, Miltenyi Biotech, Germany). The T lymphocytes were activated with CD3/4-1BB Dynabeads (Thermo Fisher, US) and transduced at 48 hours after activation in plates coated with recombinant fibronectin fragments (RetroNectin, Takara, Japan). The T cells were cultured in complete medium that contained RPMI 1640 (Gibco, UK), GlutaMAX (Gibco, UK), MEM NEAA (Gibco, UK), 10% foetal bovine serum (FBS, Gibco, UK), 100 U/ mL of penicillin-100 μg/mL of streptomycin (Pen-Strep, Gibco, UK), with 50 IU/mL of recombinant human IL-2 (Biolegend, UK).
Cell lines
Human AML cell lines THP1 (12) and MV4-11 were purchased from the Persian Type Culture Collection (PTCC), Pasture Institute, Tehran, Iran. All cells were grown at 37°C in complete media, as previously described. The CD123 and FRβ cells were previously transduced with a retroviral vector.
T cell activation and cytokine release assays
CAR-T cells (2×105) were co-cultured with 2×105 target cells (1:1 ratio) in 500 μL of complete media in triplicate. After 24 hours, the supernatants were tested for the presence of IL-2 and interferon gamma (IFN-γ) by enzyme-linked immunosorbent assay (ELISA) (BioLegend, UK).
T cell proliferation
In order to evaluate the growth of the T cells, we cultured the control and CAR-T cells and stimulated them once a week with B-AML cell lines (ratio 2:1) without the addition of any exogenous cytokines. The cell cultures were performed for five weeks. Finally, the cells were counted weekly using Trypan blue (Merck, Germany) staining.
Cytotoxicity
The 51Cr assay was used to detect cytolytic activity of the T lymphocytes as previously described. The percentage of specific lysis in the wells (in triplicate) was calculated based on the following formula: (test release-spontaneous release)/(maximal release-spontaneous release)×100 (25).
Flow cytometry
All of the cells were washed and suspended in FACS buffer that contained PBS with 0.1% sodium azide and 0.4% human serum albumin. The cells were stained with fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated mAbs. We used CD19, CD123, and FRβ (Becton Dickinson, Mountain View, CA, US) to stain the AML blasts. CD3, CD4, CD8, CD56, CD45RA, CD45RO, CD62L, CD27, CCR7, and PD-1 (Becton Dickinson, Mountain View, CA, US) were used to stain the T lymphocytes. PD-1 was the exhaustion marker of the T cells. In order to detect CAR expression, the cells were incubated at 4o C for 20 minutes with biotin-labelled polyclonal goat anti-mouse F(ab)2 antibodies (Santa Cruz Biotechnology Dallas, Texas, USA) and then washed twice with FACS buffer. Apoptosis was measured using Annexin V and 7AAD staining (Becton Dickinson, US). Cells were analysed by FACSCalibur (Becton Dickinson, US) equipped with a triple fluorescence signal filter.
Statistical analysis
The data are reported as mean ± standard error. Statistical analysis was performed using the unpaired 2-tailed Student’s t test. GraphPad Prism 6.0 software (GraphPad Software Inc., USA) was used for statistical calculations. A 'single-step Tukey’s range test' was used to test for statistical significance. P≤0.05 was considered significant.
Results
In silico design of the TanCAR
Docking of the tandem CAR to the cognate receptors was modelled for the CD123 and the FRβ-scFv (Fig .1A). The TanCAR recombinant vector was designed and constructed (Fig .1B). Models for CD123 (Protein Data Bank ID: 4JZJ) and FRβ-scFv were optimized for each molecule with PatchDock, then gathered in the context of a bispecific TanCAR (Fig .1C). The arrangement structure of the TanCAR domains allowed for simultaneous binding of both receptors.
Generation of chimeric antigen receptor-expressing T cells with detectable lytic activity
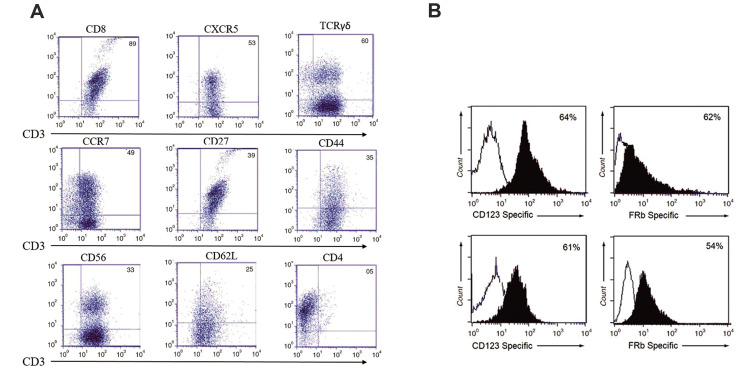
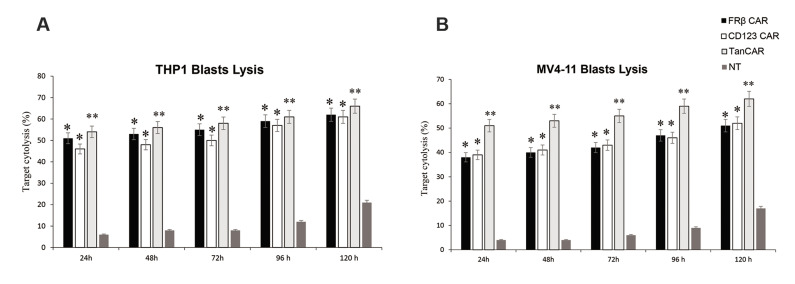
We evaluated the persistence expression and cytolytic activity of our bispecific CAR FRβ-CD123 in vitro. CARs expression persisted for more than 10 weeks in vitro before turning down toward baseline non-expressing T cells. The markers associated with memory (e.g., CD27, CD28, CD62L, and CCR7) were expressed by CAR-T cells (Fig .2A). Flow cytometry results showed that a transduction efficiency of up to 54% with a multiplicity of infection of 1.0. The ability to react with both CD123 and the FRβ could provide an advantage to CAR-T cells over their monospecific counterparts by increasing the overall avidity to their targets. We detected the surface expression of the TanCAR using a Fab-specific antibody and specific FRβ protein on THP1 leukaemia cells and on the tandem CAR-T cells (Fig .2B). We assessed the cytolytic ability of our designed TanCAR FRβ-CD123, CD123-CAR, and FRβ CAR-expressing cells. All CAR-T cells had specific cytotoxic activity against THP1 and MV4-11 cells (66 ± 11% and 62 ± 14% specific lysis for bispecific Tan CAR; 61 ± 12% and 52 ± 15% specific lysis for FRβ CAR-T cells; and 62 ± 14% and 51 ± 11% specific lysis for CD123 CAR-T cells at a ratio of 10:1 for the THP1 and MV4-11 cells, respectively, **P≤0.005, P≤0.05). Control T cells showed no significant cytotoxic activity against any of these target cell lines (Fig .3). Hyperstimulation of T cells, which we induced in TanCAR-T cells upon co-simultaneous excitation with both CD123 and FRβ, could result in a drained phenotype. In order to compare the activity period of bispecific TanCAR and monospecific CAR-T cells, we calculated the density of AML cell lines at 10 days after transduction (Fig .3A, B). At first, all CAR-T cell products significantly lysed the tumour cell lines. However, at and beyond 72 hours, bispecific TanCAR-T cells were significantly better able to cytolyse tumour cell lines compared with the other cells (*P≤0.05 at 72 hours and **P≤0.005 at five days). In all of the experiments, the transduction rate of the TanCAR was normalized to the CD123-CAR and FRβ CAR-T cell populations. Significantly higher cytotoxicity ratios were seen with TanCAR-T cells compared with monospecific CAR-T cells. There was very weak cytolytic activity by NT primary T cell blasts (Fig .3) and no significant cytotoxicity of the TanCAR-T cells against CD123-FRβ-non-expressing targets (data not shown).
Fig.2.
Surface expressions of the T cell determinants and TanCAR molecule. A. Expression of cell surface markers associated with T cells as gated on CD3+CAR+ cells by flow cytometry. B. Detection of the surface expression of the TanCAR using a CD123 Fab-specific antibody and specific FRβ protein on THP1 leukaemia cells (top) and using anti-Fab and FRβ protein on tandem CAR-T cells (bottom). FRβ; Folate receptor beta and CAR; Chimeric antigen receptor
Fig.3.
Activity of bispecific TanCAR-T cells against THP1 and MV4-11 leukaemia cell lines. 51Cr-release assays of bispecific TanCAR-CD123-FRβ T cells against leukaemia lines A. THP-1 and B. MV4-11 compared with the monospecific CAR-T cells and non-transduced T cells that were all generated from a healthy donor. The two-tailed t test was performed between TanCAR CD123-FRβ and the T cell product that exhibited the highest cytotoxic degree. CAR; Chimeric antigen receptor, *; P≤0.05, and h; Hours.
Cytokine release by TanCAR-T cells
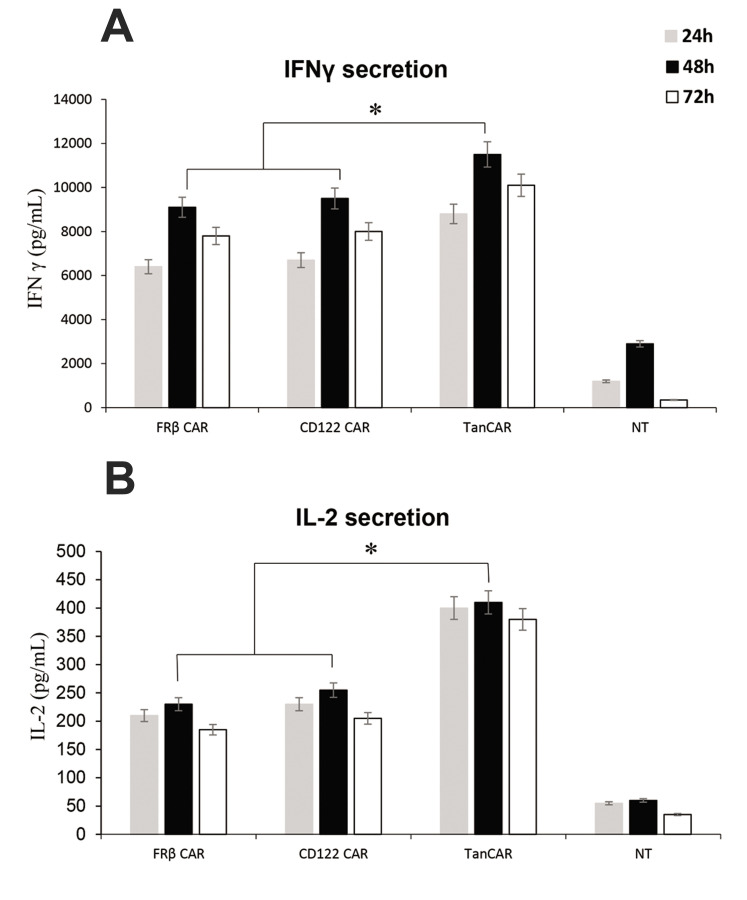
We co-cultured the B-AML blast lines with bispecific TanCAR, CAR-CD123, and CAR-FRβ T cells at a ratio of 10:1 (CAR:blast). The supernatants of the co-cultures were collected after 24 to 72 hours and tested by ELISA for the presence of IFN-γ and IL-2. There were significantly more cytokines in supernatants of the TanCAR-T cells and the amounts were compared in all groups. Neither non-transduced T lymphocytes, nor control blasts had detectable levels of IL-2 and IFN-γ cytokines (Fig .4).
Fig.4.
Cytokine expression of bi-TanCAR-T cells against the THP1 leukaemia cell line. Cytokine production of T cells detected in the supernatant of 24- to 72-hour co-culture with the THP1 cell line. Analysis of IFN-γ and IL-2 from supernatants of the co-cultures of bispecific TanCAR CD123-FRβ T cells, CD123CAR-T cells, FRβ chimeric antigen receptor (CAR)-T cells, and non-transduced cells detected by enzyme-linked immunosorbent assay (ELISA). The data are pooled from three independent experiments, each done in triplicate. A single-step Tukey’s range test was used. IFN-γ; Interferon gamma, IL-2; Interleukin 2, FRβ; Folate receptor beta, ELISA; Enzyme-linked immunosorbent assay, *; P≤0.05, and h; Hours.
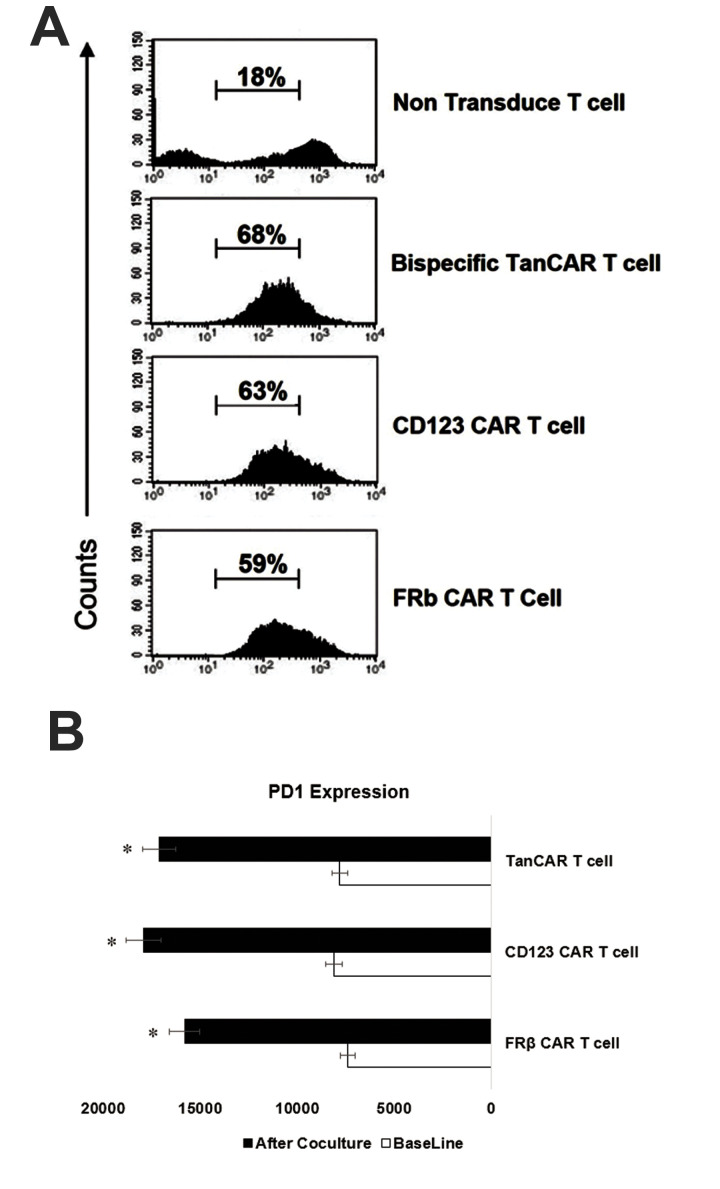
In vitro proliferation and exhaustion of chimeric antigen receptor-T cells
We observed continuous proliferation of all CAR-T cell groups (Fig .5A). In order to compare the period of TanCAR FRβ-CD123 antitumour activity, we assessed the expression of the exhaustion marker PD-1 after co-culture with leukemic blasts for one week at a ratio of 10:1 (T cells to blasts). The THP1 cells were checked at 72 hours after continuous stimulation of T lymphocytes. The T cells were subsequently gated for CAR expression and compared with unstimulated CAR-T cells. The CD8+ part of all the CAR products showed significant upregulation of surface PD-1 following co-culture with THP1. All CAR-T cells exhibited a close increase in PD-1 (Fig .5B).
Fig.5.
Assessment of TanCAR-T cell proliferation and expressions of their exhaustion markers PD-1. A. Flow cytometry output during incubation of TanCAR CD123-FRβ, monospecific chimeric antigen receptor (CAR-T) cells, and non-transduced cells with the THP1 leukaemia cell line after 10 days of co-culture. B. Median fluorescence intensity results for expression of PD-1 on the CD8+ CAR-T cells before and after repeated stimulation with the leukaemia cell line for one week. Representative data are from three independent experiments, each done in triplicate. A single-step Tukey’s range test was used. *; P≤0.05.
Discussion
CARs have human leukocyte antigen (HLA)-unrestricted activity when compared to the nature of TCRs (26). This is important in tumours, which adopt immune evasions by affecting major histocompatibility complex (MHC) processing and presentation (27). Most antigens targeted by CARs are not actually tumour-specific, but simply tumour-associated. This means the antigens are shared with normal tissues. This problem could be lessened by the use of dual-specific CARs. A single CAR that targets two antigens affects a broader variety of haematologic malignancies, and targets them more efficiently. In this experiment, we used predictive molecular modelling to interrogate the TanCAR structure and provide evidence for synthesis of a functional structure. We described, for the first time, the design and production of an anti-CD123 and FRβ linkage into a single TanCAR-T cell specific for two AML blasts-associated antigens. There are many studies that used each of the CD123 and FRβ determinants (12-15, 17, 28) as the CAR-T cell specific targets. The CAR construct is able to be activated via the binding of either CD123 or FRβ tumour molecules. Four structural templates with more than 60% identity were recognized, from which a model for residues 41-279 of TanCAR was constructed. We confirmed that TanCAR-transduced cells effectively recognize antigen epitopes in a distinctive manner and have a sustainable activity after simultaneously targeting them. This bivalent targeting of the TanCAR also counteracts antigen escape of the target cell and could eliminate them more efficiently in comparison with monospecific CAR-T cells. As seen with chemotherapy and HSCT, B-AML treatment requires conjunctive use of multiple agents to avoid relapse (29). So, the same concept would be true for CAR-T cell therapies. The use of a single target on transformed cells can lead to the specific escape mechanisms and it is undoubtedly the use of dual-directed approaches that will reduce these events.
The expression of the exo-domain of CARs in its entirety was confirmed by flow cytometry, which was specific for both the CD123 and FRβ domains. The specific function of the TanCARs toward the targets indicated that the correct folding of this complex exo-domain was achieved in our designing process. Because cytokine secretion is important for in vivo efficacy of CAR-T cells, we conducted the cytokine assay for both the IL-2 and IFN-γ molecules. T lymphocyte activation, which was measured by IL-2 and IFN-γ cytokine secretion, was proportional to the density of the target cells cultured in the plate. The bispecific interaction to both targets caused a significantly higher cytokine secretion by TanCAR-T cells than monospecific CAR-T cells. Furthermore, while exposure to a single antigen leads to a decline in cytokine release over a certain antigen density in monospecific CAR-T cells, the TanCAR-T cells continued to secrete elevated levels of cytokines. This extra IL-2 and IFN-γ cytokine production increases the functional capacity of the TanCAR-T cells due to its simultaneous engagement. However, Schneider et al. (30) reported that the CD20- specific CAR was superior to tandem CAR constructs in the production of T cell cytokines such as IFNγ, IL-2, and TNF-alpha, but their co-culture of TanCAR with the target resulted in fewer remaining cells.
The docking process of TanCAR-T cells to both targets, which was supported by the in silico design model helped us in the production of TanCAR-T cells that had enhanced cytotoxicity against target cells. The potential to recruit target tumor-associated antigens (TAAs) to the T cell-AML blast interface was revealed by the mass clustering at the dual TAA IS, with robust T cell activation.
The innate robust activity of the TanCAR-T cells is attributed to its bi-directional functionality. Regular CAR-T cells recognize only one antigen. For instance, when B-AML blasts are treated with CD19 CAR-T cells, CD19 under-expressing or non-expressing cells survive and they will be selected to generate a new population that is unresponsive to CAR-T cell therapy or other therapies (31). The TanCAR molecule allows T lymphocytes to capture both single- and double-positive determinant populations. The general applicability of the TanCARs to other antigens or extracellular binding domains remains to be determined and should be studied more precisely. We do not know if conformational changes induced after binding of the primary antigen in the TanCAR-T cells change the specificity of the secondary epitope for its antigen.
Gene transfer by the viral vectors typically requires cell division for stable transfer. The CARs introduced into T cells should be expanded effectively. Thus, stable expression of CAR can be achieved without prior T cell propagation. Despite the long-time antigenic stimulation in our assay, TanCAR-T cells preserved their cytolytic strength. Lymphocyte exhaustion is a condition of their dysfunction, which would be determined by their sustained expression of the inhibitory surface receptors (ISRs) (32). The ISRs that include TIM3, LAG3, and PD-1 contribute to T lymphocyte exhaustion (33, 34). Impairment of immunity due to T cell exhaustion is the main cause of death in patients with sepsis after the acute phase (34). Expressions of these ISRs were not significantly different between the bispecific TanCAR-T cell and other cells, and suggested the same levels of susceptibility to the ISRs. The robust activation without exhaustion of this bispecific TanCAR-T cell enables this structure to have the potential to become ubiquitous.
However, our work lacks an animal preclinical study. Two long-term follow-up preclinical animal studies have shown that some tumours re-emerged when the doses of CAR-T cells were small (35). These re-emerged tumours were double-negative, which indicated that TanCAR-T cells could overcome just a single antigen escape (8, 36). The late aspect of double-negative tumour cells raise the question about the threshold number of TAAs to be targeted. The previous work by Hegde et al. (35) partly answered this question in a mathematical model built in data sets from a primary glioblastoma multiform model (GBM) that predicted complete tumour elimination. They are currently substantiating this discovery in a larger cohort of primary GBM. This profile includes TAAs as well as components from the tumour microenvironment and vasculature (1). However, the current in vitro investigation provides us a proof of principle of the improved functionality and effectiveness of dual-targeting T cells that exert their effector function by means of a bispecific CAR structure.
Until now, the safety of CAR-T cells remains an important concern. CARs off-targets, cross-reactivity, and unintentional T cell stimulation remain to be solved. Bispecific TanCAR-T cells exhibit reactivity against two antigens with conditional extra activation. It is predictable that targeting two antigens could increase the risk to the normal cells. Therefore, to reduce the risk, "gated" activation of CAR-T cells has been described. In this way, an individual TAA-specific CAR structure mediates signalling adequate to induce cytolysis and a second CAR with specificity to another TAA mediates a costimulatory signal that promotes the full activation of the T lymphocytes (37). In cases where a TAA is same as the normal tissue, a CAR molecule that provides a costimulatory receptor domain that identifies the second antigen could selectively lyse tumour cells that express dual antigens but do not affect malignant cells that express either antigen alone (38-40). The robust T cell functionality through tandem repeat has many advantages in tumour suppression and better activation dynamics (3). However, the risk of insertional mutagenesis is less with a single transgene, which can destabilize the T cell genome. There are reports about the development of CD19 escape variants after CD19 CAR-T cell therapy (8, 9); therefore, we are in need of the production of bispecific TanCAR. Finally, our particular choice of antigens, CD123 and FRβ, allows for a T cell product that is effective in AML therapy.
Conclusion
CAR-T cells are an emerging, efficient tool for cancer therapy. Numerous molecules, however, present as the antigenic aspect of heterogeneous malignancies. Hence, redirecting tumour-specific profiles rather than a single marker could strength the efficacy of anticancer therapies. We have confirmed that a dual-specific tandem CAR FRβ-CD123 molecule can target cytotoxic T lymphocytes toward two different antigenic epitopes and result in significant control of B-AML blasts.
Acknowledgements
We want to gratefully thank Dr. Alireza Shoae-Hassani for his helpful comments. We are thankful for the time and patience of the anonymous referees. This study is sponsored by the Council for Development of Stem Cell Sciences and Technologies, Vice -Presidency for Science and Technology, Presidency of the Islamic Republic of Iran. The authors declare that there is no conflict of interest.
Authors’ Contributions
A.Gh., A.A.H., P.P.; Participated in study design, data collection and evaluation, drafting, and statistical analysis. M.E., A.M.; Performed docking and analytical process of the study. A.Gh., A.A.H.; Contributed extensively to data interpretation and the conclusion. A.Gh., P.P.; Conducted the molecular experiments. All authors performed edited and approved the final version of this manuscript for submission.
References
- 1.Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest. 2019;129(8):3464–3464. doi: 10.1172/JCI131246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruella M, Maus MV. Catch me if you can: leukemia escape after CD19-directed T cell immunotherapies. Comput Struct Biotechnol J. 2016;14:357–362. doi: 10.1016/j.csbj.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, et al. TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Mol Ther Nucleic Acids. 2013;2(7):e105–e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roboz GJ. Current treatment of acute myeloid leukemia. Curr Opin Oncol. 2012;24(6):711–719. doi: 10.1097/CCO.0b013e328358f62d. [DOI] [PubMed] [Google Scholar]
- 5.Ruella M, Gill S. How to train your T cell: genetically engineered chimeric antigen receptor T cells versus bispecific T-cell engagers to target CD19 in B acute lymphoblastic leukemia. Expert Opin Biol Ther. 2015;15(6):761–766. doi: 10.1517/14712598.2015.1009888. [DOI] [PubMed] [Google Scholar]
- 6.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 7.Topp MS, Gökbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 8.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. The Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain T, Bar M, Kansagra AJ, Chong EA, Hashmi SK, Neelapu SS, et al. Use of chimeric antigen receptor T Cell therapy in clinical practice for relapsed/refractory aggressive B cell non-hodgkin lymphoma: an expert panel opinion from the American society for transplantation and cellular therapy. Biol Blood Marrow Transplant. 2019;25(12):2305–2321. doi: 10.1016/j.bbmt.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li F, Sutherland MK, Yu C, Walter RB, Westendorf L, Valliere-Douglass J, et al. Characterization of SGN-CD123A, a potent CD123- directed antibody-drug conjugate for acute myeloid leukemia. Mol Cancer Ther. 2018;17(2):554–564. doi: 10.1158/1535-7163.MCT-17-0742. [DOI] [PubMed] [Google Scholar]
- 13.Arcangeli S, Rotiroti MC, Bardelli M, Simonelli L, Magnani CF, Biondi A, et al. Balance of Anti-CD123 chimeric antigen receptor binding affinity and density for the targeting of acute myeloid leukemia. Mol Ther. 2017;25(8):1933–1945. doi: 10.1016/j.ymthe.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynn RC, Poussin M, Kalota A, Feng Y, Low PS, Dimitrov DS, et al. Targeting of folate receptor β on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells. Blood. 2015;125(22):3466–3476. doi: 10.1182/blood-2014-11-612721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arai N, Homma M, Abe M, Baba Y, Murai S, Watanuki M, et al. Impact of CD123 expression, analyzed by immunohistochemistry, on clinical outcomes in patients with acute myeloid leukemia. Int J Hematol. 2019;109(5):539–544. doi: 10.1007/s12185-019-02616-y. [DOI] [PubMed] [Google Scholar]
- 16.Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126(10):3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mardiros A, Dos Santos C, McDonald T, Brown CE, Wang X, Budde LE, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122(18):3138–3148. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynn RC, Feng Y, Schutsky K, Poussin M, Kalota A, Dimitrov DS, et al. High-affinity FRβ-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia. 2016;30(6):1355–1364. doi: 10.1038/leu.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross JF, Wang H, Behm FG, Mathew P, Wu M, Booth R, et al. Folate receptor type beta is a neutrophilic lineage marker and is differentially expressed in myeloid leukemia. Cancer. 1999;85(2):348–357. doi: 10.1002/(sici)1097-0142(19990115)85:2<348::aid-cncr12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Lu YJ, Chu H, Wheeler LW, Nelson M, Westrick E, Matthaei JF, et al. Preclinical evaluation of bispecific adaptor molecule controlled folate receptor CAR-T cell therapy with special focus on pediatric malignancies. Front Oncol. 2019;9:151–151. doi: 10.3389/fonc.2019.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang X, Tang Q, Mao Y, Huang X, Jia L, Zhu J, et al. CD137 costimulation improves the antitumor effect of LMP1-specific chimeric antigen receptor T cells in vitro and in vivo. Onco Targets Ther. 2019;12:9341–9350. doi: 10.2147/OTT.S221040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broughton SE, Hercus TR, Hardy MP, McClure BJ, Nero TL, Dottore M, et al. Dual mechanism of interleukin-3 receptor blockade by an anti-cancer antibody. Cell Rep. 2014;8(2):410–419. doi: 10.1016/j.celrep.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 23.Raab D, Graf M, Notka F, Schodl T, Wagner R. The GeneOptimizer Algorithm: using a sliding window approach to cope with the vast sequence space in multiparameter DNA sequence optimization. Syst Synth Biol. 2010;4(3):215–225. doi: 10.1007/s11693-010-9062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanitis E, Poussin M, Hagemann IS, Coukos G, Sandaltzopoulos R, Scholler N, et al. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Mol Ther. 2012;20(3):633–643. doi: 10.1038/mt.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottschalk S, Edwards OL, Sili U, Huls MH, Goltsova T, Davis AR, et al. Generating CTLs against the subdominant Epstein-Barr virus LMP1 antigen for the adoptive immunotherapy of EBV-associated malignancies. Blood. 2003;101(5):1905–1912. doi: 10.1182/blood-2002-05-1514. [DOI] [PubMed] [Google Scholar]
- 26.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 27.Singh R, Paterson Y. Immunoediting sculpts tumor epitopes during immunotherapy. Cancer Res. 2007;67(5):1887–1892. doi: 10.1158/0008-5472.CAN-06-3960. [DOI] [PubMed] [Google Scholar]
- 28.Lynn RC, Feng Y, Schutsky K, Poussin M, Kalota A, Dimitrov DS, et al. High-affinity FRβ-specific CAR T cells eradicate AML and normal myeloid lineage without HSC toxicity. Leukemia. 2016;30(6):1355–1364. doi: 10.1038/leu.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeVita VT Jr, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68(21):8643–8653. doi: 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- 30.Schneider D, Xiong Y, Wu D, Nlle V, Schmitz S, Haso W, et al. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer. 2017;5:42–42. doi: 10.1186/s40425-017-0246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Sun Q, Liang X, Chen Z, Zhang X, Zhou X, et al. Mechanisms of relapse after CD19 CAR T-cell therapy for acute lymphoblastic leukemia and its prevention and treatment strategies. Front Immunol. 2019;10:2664–2664. doi: 10.3389/fimmu.2019.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 33.Dong Y, Li X, Zhang L, Zhu Q, Chen C, Bao J, et al. CD4(+) T cell exhaustion revealed by high PD-1 and LAG-3 expression and the loss of helper T cell function in chronic hepatitis B. BMC Immunol. 2019;20(1):27–27. doi: 10.1186/s12865-019-0309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niu B, Zhou F, Su Y, Wang L, Xu Y, Yi Z, et al. Different expression characteristics of LAG3 and PD-1 in sepsis and their synergistic effect on T cell exhaustion: a new strategy for immune checkpoint blockade. Front Immunol. 2019;10:1888–1888. doi: 10.3389/fimmu.2019.01888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Invest. 2016;126(8):3036–3052. doi: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hegde M, Corder A, Chow KK, Mukherjee M, Ashoori A, Kew Y, et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther. 2013;21(11):2087–2101. doi: 10.1038/mt.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20(1):70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 38.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6(261):261ra151–261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duong CP, Westwood JA, Berry LJ, Darcy PK, Kershaw MH. Enhancing the specificity of T-cell cultures for adoptive immunotherapy of cancer. Immunotherapy. 2011;3(1):33–48. doi: 10.2217/imt.10.81. [DOI] [PubMed] [Google Scholar]
- 40.Wilkie S, van Schalkwyk MC, Hobbs S, Davies DM, van der Stegen SJ, Pereira AC, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2012;32(5):1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]