Abstract
Objective
To describe first-year trajectories of medical cannabis use and identify characteristics associated with patterns of use in a cohort of adults using opioids for chronic pain.
Design
Latent class trajectory analysis of a prospective cohort study using data on the 14-day frequency of medical cannabis use.
Setting
A large academic medical center and four medical cannabis dispensaries in the New York City metropolitan area.
Subjects
Adults with chronic pain using opioids and newly certified for medical cannabis in New York between 2018 and 2020.
Methods
Using latent class trajectory analysis, we identified clusters of participants based on the 14-day frequency of medical cannabis use. We used logistic regression to determine factors associated with cluster membership, including sociodemographic characteristics, pain, substance use, and mental health symptoms.
Results
Among 99 participants, the mean age was 53 years; 62% were women, and 52% were White. We identified three clusters of medical cannabis use: infrequent use (n = 30, mean use = 1.5 days/14-day period), occasional use (n = 28, mean = 5.7 days/14-day period), and frequent use (n = 41, mean = 12.1 days/14-day period). Within clusters, use patterns did not vary significantly over 52 weeks. Differences were observed in two sociodemographic variables: Frequent (vs infrequent) use was associated with non-Hispanic White race/ethnicity (adjusted odds ratio 4.54, 95% confidence interval 1.49–14.29), while occasional (vs infrequent) use was associated with employment (adjusted odds ratio 13.84, 95% confidence interval 1.21–158.74).
Conclusions
Three clusters of medical cannabis use patterns emerged and were stable over time. Results suggest that structural factors related to race/ethnicity and employment may be major drivers of medical cannabis use, even among adults certified for its use.
Keywords: Medical Cannabis, Cannabis, Chronic Pain, Complementary Medicine, Pain Management
Introduction
Over the past 25 years, medical cannabis has become increasingly accessible in the United States for a variety of medical conditions. Currently, medical cannabis is legal in 37 states and four territories [1], with chronic or severe pain among the most commonly approved indications [2]. Although the Schedule I status of cannabis has limited research into its therapeutic potential, there is growing evidence suggesting that it is effective for managing pain [3–6].
Although increasing numbers of individuals in the United States consume medical cannabis for chronic pain, few studies have described patterns of use. Studies examining trajectories of nonmedical cannabis have described wide variations in use and have largely reported data collected annually over many years, providing less detail about use within shorter time frames [7–11]. The few published reports of patterns of medical cannabis use have been cross-sectional and have typically reported frequent use among most patients [12–16]. A more nuanced understanding of patterns of medical cannabis use can help providers anticipate patients’ clinical needs and can identify potential disparities in access; yet, to our knowledge, no studies have described trajectories of medical cannabis use.
The Medical Marijuana and Opioids (MEMO) Study is a longitudinal cohort study of adults taking opioids for chronic pain who are newly certified for medical cannabis in New York State (NYS) [17]. In this study, participants report the amount and frequency of medical cannabis consumption every 14 days, allowing for a granular examination of use. In the present analysis, we sought to understand how medical cannabis use varies over time, using latent class analysis to cluster participants by medical cannabis trajectories and identify characteristics associated with cluster assignment.
Methods
Overview
We conducted an exploratory latent class analysis of data from the first 99 participants in the MEMO Study. The MEMO Study follows participants through quarterly research visits and brief Internet-based questionnaires every 14 days to examine whether medical cannabis use is associated with a reduction in opioid use among adults with chronic pain. Written informed consent is obtained from all participants. Approved by the Montefiore Medical Center / Albert Einstein College of Medicine institutional review board and conducted according to the principles of the Helsinki Declaration, the MEMO Study is described elsewhere [17] and reported in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement [18].
Setting
The NYS Medical Marijuana Program began operating in 2016; as of March 1, 2021, more than 3,100 registered practitioners had certified more than 140,000 individuals for medical cannabis. Although recently passed legislation in NYS will change the medical cannabis program, during the study time frame, providers certified patients with specific qualifying conditions and complications, the most common of which was severe, chronic pain [19]. Providers could certify patients for up to 12 months and could recommend the ratio of Δ9-tetrahydrocannabinol (THC) to cannabidiol (CBD) (e.g., “high THC : low CBD,” “equal THC : CBD,” or “low THC : high CBD”), as well as the administration method (e.g., inhalation, oral, oromucosal). Medical cannabis products could be purchased from one of only 39 state-licensed dispensaries, which reported dispensed products, including THC and CBD content and route of administration, to the New York Prescription Monitoring Program [20]. All dispensary products are tested for THC and CBD content by a NYS Department of Health laboratory.
MEMO participants are recruited from Montefiore Health System (Montefiore) and four medical cannabis dispensaries in the New York City metropolitan area that are operated by Vireo Health and Columbia Care. Together, these dispensaries provide medical cannabis products to more than 30,000 active patients. Montefiore is the largest health care system in Bronx, NY, with primary, specialty, surgical, and acute care at four hospitals and more than 20 ambulatory clinics. The Montefiore Medical Cannabis Program is based in six primary care clinics; since its inception in 2016, 10 physicians have certified more than 2,000 patients.
Participants
MEMO participants are recruited via direct referrals from providers at Montefiore or dispensaries, letters mailed to Montefiore patients, and flyers posted in dispensary buildings and on websites. Study inclusion criteria for patients are 1) age ≥18 years, 2) fluency in English or Spanish, 3) a new certification for medical cannabis in NYS within the prior 90 days, 4) no medical cannabis use within 6 months before certification, 5) a NYS Medical Marijuana Program qualifying condition of “chronic or severe pain” or “pain that degrades health and functional capability as an alternative to opioid use” or a qualifying complication of “severe or chronic pain resulting in substantial limitation of function,” and 6) use of prescribed opioids or illicit opioids within the prior 30 days. Exclusion criteria are 1) inability to provide informed consent, 2) inability to complete study visits over 18 months, 3) NYS Medical Marijuana Program qualifying conditions likely to cause unique pain syndromes (cancer, epilepsy, multiple sclerosis, spinal cord injury, amyotrophic lateral sclerosis, Parkinson’s disease, inflammatory bowel disease, Huntington’s disease), 4) terminal illness, or 5) current or prior psychotic disorder.
Data Collection
During quarterly research visits, data are collected from MEMO participants via questionnaires. Participants also answer brief (2- to 5-minute) Internet-based questionnaires about pain and use of cannabis, opioids, and other substances every 14 days. For the present analysis, we included data from participants’ first 52 weeks of follow-up.
From September 2018 to March 2020, quarterly visits were conducted in person and included questionnaires administered via Audio Computer-Assisted Self-Interview (ACASI) technology. ACASI displays questions on a computer while playing an audio recording of the questions, and participants enter responses directly onto the computer. Because of the COVID-19 pandemic, no in-person visits have occurred since March 2020. During the pandemic, questionnaires were administered by study staff over the phone every 3 months.
In addition, MEMO participants receive automated, personalized links to the Internet-based questionnaire every 14 days via text message or e-mail. Participants are asked whether they used medical cannabis (i.e., purchased at a dispensary) in the preceding 14 days, which dispensaries they purchased cannabis from, the type of product purchased (selected from dispensary-specific drop-down menus of available products), number of days used, and number of times used per day. The Internet-based questionnaire also includes questions on pain, nonmedical cannabis use (i.e., obtained from any source other than a dispensary), and opioid use during the preceding 14 days. Participants receive $40 for quarterly visits and $5 for Web-based questionnaires; if all Web-based questionnaires are completed between each quarterly visit, a $10 bonus is provided.
Key Variables
The main exposure variable for this analysis was days of medical cannabis use per 14-day period, calculated from the Internet-based questionnaires. Because THC is intoxicating and may differentially impact pain and mental health, we also calculated the number of days of use of high-THC–containing medical cannabis products in each 14-day period. We classified any medical cannabis product with a THC:CBD ratio >2:1 as high THC, using a list of products available at all NYS dispensaries.
Sociodemographic variables measured at the baseline visit included age, gender, race/ethnicity (non-Hispanic White, non-Hispanic Black, non-Hispanic of other race, Hispanic of any race), employment, and medical insurance status. Additional variables included pain severity and interference, measured by the Pain, Enjoyment of Life and General Activity Scale (PEG-3) [21] (range 0–10, with ≥4 indicating moderate to severe pain); pain catastrophizing, measured by the Pain Catastrophizing Scale (range 0–52, high > 30) [22]; depressive symptoms, measured by the Patient Health Questionnaire (PHQ-9) (range 0–27, high >10) [23]; anxiety symptoms, measured by the General Anxiety Disorder-7 (GAD-7; range 0–21, high >10) [24]; post-traumatic stress disorder (PTSD) symptoms, measured by the Post-Traumatic Stress Disorder Questionnaire—Civilian Version (PCL-C) (range 6–30, high >14) [25]; attention deficit hyperactivity disorder symptoms, measured by the World Health Organization Adult Attention-Deficit Hyperactivity Disorder Self-Report Screening Scale for DSM-5 (ASRS-5) (range 0–25, high >14) [26]; insomnia symptoms, measured by the Insomnia Severity Index (ISI) (range 0–28, high >15) [27]; cannabis dependence or abuse (per DSM-4), measured by the Mini-International Neuropsychiatric Interview (MINI) [28]; current tobacco cigarette use [29]; problematic alcohol use, measured by the Alcohol Use Disorders Identification Test (AUDIT) (range 0–21, problematic use >7) [30]; and other nonmedical drug use, measured by the Addiction Severity Index (ASI) [31].
Latent Class Trajectory Modeling
We used longitudinal latent class analysis to group participants into clusters based on the trajectory of days of medical cannabis use over 52 weeks. Using Mplus software (Muthén & Muthén, Los Angeles, CA, USA) [32], we developed latent class analysis models fitted to days of cannabis use for each 14-day period, treating this variable as a censored normal variable in the models. We set each of the trajectory polynomials to be quadratic, because preliminary analyses showed a better fit to the data as compared with a linear model. All available data were used, even if participants had not yet completed 52 weeks of follow-up. We included a time-variant indicator variable for the COVID-19 pandemic (March 15, 2020, or later) to control for its potential influence. We applied the full-information maximum-likelihood approach for the missing data in the analysis. To assure finding the maximum of the likelihood function, we used 200 random sets of starting values.
Starting with a one–latent class model that assumed all participants had the same trajectory of medical cannabis use over time, we fit successive models with increasing numbers of clusters. The appropriate number of clusters was determined on the basis of both statistical criteria and the clinical experience of the investigators, five of whom are internal medicine physicians with long-standing experience certifying patients for medical cannabis. Statistical tests included the value of the Bayesian information criterion (BIC ), where lower values indicate better fit; the significance of the Lo-Mendell-Rubin adjusted-likelihood ratio test (LMR-LRT), with significant values indicating a better fit of the X model compared with X – 1; model entropy, where values closer to 1.0 indicate a higher accuracy of classifying individuals into latent classes; and average classification probabilities for cluster membership. After determining the appropriate number of clusters, we created an indicator variable for each cluster, which had a value of 1 if the participant had the largest Bayesian posterior probability for that cluster and 0 otherwise. We then repeated the analysis, using the same methods to group participants into clusters based on trajectories of days of high-THC medical cannabis use.
For overall medical cannabis use, compared with the two-cluster solution, the three-cluster solution had a lower BIC (7,386 vs 7,693), had similar entropy (0.87 vs 0.89), was not significantly different when the LMR-LRT statistic was used (P = 0.14), and had a lower, though overlapping, range of average classification probabilities for cluster membership (91–95% vs 94–99%). In discussing the trajectories, we felt that a two-cluster solution did not adequately describe the variability in frequency of use observed in our clinical practice. Compared with the three-cluster solution, four- and five-cluster solution models had similar statistical profiles (BIC 7,386 vs 7,276 and 7,112, respectively; entropy 0.87 vs 0.87 and 0.85, respectively; nonsignificant LMR-LRT statistics) but included very small (n < 10) clusters that were believed to not be clinically meaningful. Because some investigators have cautioned against overextraction of latent classes due to the presence of non-normal data [33], we reached a consensus that the three-cluster solution was preferred. We repeated analyses examining days of high-THC medical cannabis use per 14-day period. Compared with the two-cluster solution, the three-cluster solution had a lower BIC (4,893 vs 5,142), had lower entropy (0.90 vs 0.96), was not significantly different when the LMR-LRT statistic was used (P = 0.54), and had a lower range of average classification probabilities for cluster membership (93–97% vs 98–99%). Again, compared with the three-cluster solution, four- and five-cluster solution models had similar statistical profiles (BIC 4,719 and 4,660, respectively; entropy 0.88 and 0.86, respectively) but included very small clusters. Our consensus was that a three-cluster solution was optimal.
Finally, for both any medical cannabis use and high-THC medical cannabis use, we examined whether clusters differed on sociodemographic and clinical covariates by using one-way analysis of variance tests to compare continuous variables and chi-squared or Fisher exact tests to compare categorical variables. We then conducted multivariate logistic regression to examine factors related to cluster memberships, separately comparing frequent with infrequent use clusters and occasional with infrequent use clusters. We included the following factors, which were associated with either any medical cannabis or high-THC medical cannabis trajectories in bivariate analyses at the P < 0.10 level: race/ethnicity, employment status, type of insurance, days of opiate use, current tobacco use, and PTSD symptoms. For these analyses, race/ethnicity was examined as a two-level variable (non-Hispanic White vs other race/ethnicity). Because of the small number of employed individuals in the infrequent use cluster, we conducted a sensitivity analysis for overall medical cannabis use with this variable excluded from the regression model. Statistical significance for all tests was two sided at P < 0.05. Analyses reported in this article were not prespecified.
Results
Of 537 individuals screened, 117 enrolled (Supplementary Data). Of these, 99 were included in the present analysis. Mean age was 53 years (standard deviation = 13.5 years), 62 (63%) were female, 25 (25%) were Hispanic, and 23 (23%) were non-Hispanic Black (Table 1). Most participants were not employed (78%) and had health insurance (99%). At baseline, 40% reported current nonmedical cannabis use, 8% screened positive for cannabis dependence or abuse, 6% screened positive for problematic alcohol use, and 32% were current tobacco smokers. Mean score on the Pain, Enjoyment of Life and General Activity Scale was 7.2 out of 10. Many had moderate or severe depression symptoms (42%), high PTSD symptoms (40%), and high attention deficit-hyperactivity disorder scores (45%).
Table 1.
Participants’ characteristics by trajectories of days of overall medical cannabis use and days of high-THC medical cannabis use
| Total Sample | Clusters of Overall Medical Cannabis Use |
Clusters of High-THC Medical Cannabis Use |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 99 | Infrequent Use (n=30) | Occasional Use (n=28) | Frequent Use (n=41) | P | Infrequent Use (n=50) | Occasional Use (n=24) | Frequent Use (n=25) | P | |
| Sociodemographic characteristics | |||||||||
| Age, years, mean±SD | 53.1±13.5 | 53.4±14.6 | 51.4±13.5 | 54±12.8 | 0.72 | 55.3±13.3 | 50.5±15.8 | 51.2±10.8 | 0.16 |
| Female gender, n (%) | 62 (62.6) | 17 (56.7) | 20 (71.4) | 25 (61) | 0.49 | 34 (68) | 15 (62.5) | 13 (52) | 0.40 |
| Race/ethnicity, n (%) | 0.004 | 0.03 | |||||||
| Non-Hispanic White | 45 (45.5) | 7 (23.3) | 12 (42.9) | 26 (63.4) | – | 16 (32) | 12 (50) | 17 (68) | – |
| Non-Hispanic Black | 23 (23.2) | 9 (30) | 11 (39.3) | 3 (7.3) | – | 14 (28) | 8 (33.3) | 1 (4) | – |
| Hispanic (any race) | 25 (25.3) | 12 (40) | 4 (14.3) | 9 (22) | – | 16 (32) | 3 (12.5) | 6 (24) | – |
| Non-Hispanic other race | 6 (6.0) | 2 (6.7) | 1 (3.5) | 3 (7.3) | – | 4 (8) | 1 (4.2) | 1 (4) | – |
| Currently employed, n (%) | 22 (22.2) | 3 (10) | 11 (39.3) | 8 (19.5) | 0.02 | 12 (24) | 5 (20.8) | 5 (20) | 0.91 |
| Health insurance, n (%)^ | 0.098 | 0.73 | |||||||
| Public | 72 (72.7) | 26 (86.7) | 19 (67.9) | 27 (65.9) | – | 38 (76) | 16 (66.7) | 18 (72) | – |
| Private | 26 (26.3) | 3 (10) | 9 (32.1) | 14 (34.2) | – | 11 (22) | 8 (33.3) | 7 (28) | – |
| Substance use, n (%) | |||||||||
| Current nonmedical cannabis use | 40 (40.4) | 14 (46.7) | 12 (42.9) | 14 (34.2) | 0.54 | 18 (36) | 11 (45.8) | 11 (44) | 0.66 |
| Cannabis dependence or abusea | 8 (8.1) | 2 (6.7) | 1 (3.6) | 5 (12.2) | 0.41 | 2 (4) | 3 (12.5) | 3 (12) | 0.32 |
| >7 days of opioid use in the past 14 days | 40 (40.4) | 14 (46.7) | 6 (21.4) | 20 (48.8) | 0.053 | 21 (42) | 10 (41.7) | 9 (36) | 0.87 |
| Problematic alcohol useb | 6 (6.1) | 1 (3.3) | 3 (10.7) | 2 (4.9) | 0.46 | 2 (4) | 2 (8.3) | 2 (8) | 0.69 |
| Current tobacco use | 31 (31.3) | 12 (40) | 4 (14.3) | 16 (36.6) | 0.07 | 15 (30) | 5 (20.8) | 11 (44) | 0.21 |
| Pain and mental health | |||||||||
| PEG score,c mean±SD | 7.3±2.0 | 7.6±2.2 | 7.6±1.5 | 6.9±2.1 | 0.20 | 7.3±2.2 | 7.4±1.7 | 7.3±1.9 | 0.92 |
| Moderate or severe anxiety symptoms,d n (%) | 32 (32.3) | 7 (23.3) | 11 (39.3) | 14 (34.2) | 0.41 | 12 (24) | 8 (33.3) | 12 (48) | 0.11 |
| Moderate or severe depression symptoms,e n (%) | 42 (42.4) | 15 (50) | 12 (42.9) | 15 (36.6) | 0.53 | 20 (40) | 9 (37.5) | 13 (52) | 0.52 |
| High PTSD symptoms,f n (%) | 40 (40.4) | 11 (36.7) | 12 (42.9) | 17 (41.5) | 0.88 | 16 (32) | 9 (37.5) | 15 (60) | 0.06 |
| High ADHD symptoms,g n (%) | 45 (45.5) | 13 (43.3) | 13 (46.4) | 19 (46.3) | 0.96 | 22 (44) | 9 (37.5) | 14 (56) | 0.41 |
| Moderate or severe insomnia symptoms,h n (%) | 35 (35.4) | 14 (46.7) | 10 (35.7) | 11 (26.8) | 0.22 | 20 (40) | 5 (20.8) | 10 (40) | 0.23 |
| Clinically relevant pain catastrophizing,i n (%) | 41 (41.4) | 12 (40) | 13 (46.4) | 16 (39) | 0.81 | 21 (42) | 9 (37.5) | 11 (44) | 0.89 |
SD = standard deviation; PEG = Pain, Enjoyment of life, and General activity scale; ADHD = attention deficit-hyperactivity disorder.
Proportions exclude one person who did not have health insurance.
Mini-International Neuropsychiatric Interview [28].
Score >4 for men and >3 on the Alcohol Use Disorders Identification Test [30].
Ref. [21].
Score >10 on the General Anxiety Disorder-7 [24].
Score >10 on the Patient Health Questionnaire-9 [23].
Score >14 on the PTSD CheckList—Civilian Version [25].
Score >14 on the World Health Organization ADHD self-report scale [26].
Score >15 on the Insomnia Severity Index [27].
Score >30 on the Pain Catastrophizing Scale [22].
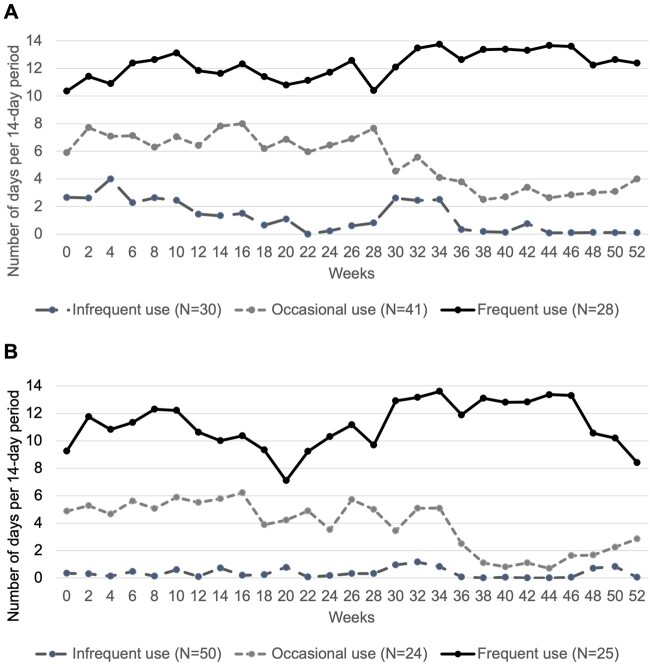
Figure 1A presents three distinct trajectories of days of medical cannabis use per 14-day period over participants’ first 52 weeks of use: 1) infrequent use (n = 30), with a mean of 1.5 days of any medical cannabis use per 14-day period; 2) occasional use (n = 28), with a mean of 5.7 days of use per 14-day period; and 3) frequent use (n = 41), with a mean of 12.1 days per 14-day period. Vaped medical cannabis use differed by cluster (infrequent use: 27%; occasional use: 71%; frequent use: 81%), as did the proportion of participants who had discontinued use of medical cannabis by the end of the study period (infrequent use: 75%; occasional use: 20%; frequent use: 0%; P < 0.001). We observed fewer days of medical cannabis use after, compared with before, week 30 in both the infrequent use and occasional use clusters, although differences in the slope of each trajectory over time were not significant. When adjusted for baseline characteristics, those in the frequent (vs infrequent) medical cannabis use cluster were significantly less likely to report a race/ethnicity other than non-Hispanic White (adjusted odds ratio [aOR] 0.22, 95% confidence interval [CI] 0.07–0.67) (Table 2). Compared with infrequent use, those in the occasional use cluster were more likely to be employed (aOR 13.84, 95% CI 1.21–158.74), less likely to use tobacco (aOR 0.15, 95% CI 0.03–0.82), and less likely to use opioid analgesics most days (aOR 0.18, 95% CI 0.04–0.76). Similar results were observed in the sensitivity analysis in which employment status was excluded from the model (Supplementary Data). We observed no differences between clusters with respect to nonmedical cannabis use, cannabis dependence or abuse, or mental health symptoms.
Figure 1.
Trajectories of days of (A) overall medical cannabis use and (B) high-THC medical cannabis use per 14-day period.
Table 2.
Participant characteristics associated with membership in clusters of overall medical cannabis and high-THC medical cannabis trajectories
| Overall Medical Cannabis |
High-THC Medical Cannabis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Occasional vs Infrequent Use |
Frequent vs Infrequent Use |
Occasional vs Infrequent Use |
Frequent vs Infrequent Use |
|||||
| Participant Characteristics | aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P | aOR (95% CI) | P |
| Non-Hispanic Black, non-Hispanic other race, or Hispanic (vs non-Hispanic White race) | 0.89 (0.22–3.59) | 0.867 | 0.22 (0.07–0.67) | 0.008 | 0.52 (0.18–1.50) | 0.223 | 0.11 (0.03–0.43) | 0.002 |
| Employed (vs not) | 13.84 (1.21–158.74) | 0.034 | 0.60 (0.09–4.21) | 0.607 | 0.36 (0.07–1.92) | 0.232 | 0.17 (0.03–1.04) | 0.055 |
| Private (vs public) insurance | 0.59 (0.05–7.00) | 0.675 | 4.15 (0.70–24.75) | 0.118 | 2.72 (0.55–13.37) | 0.218 | 5.17 (0.75–35.39) | 0.095 |
| >7 days of opioid use in the past 14 days (vs ≤7 days) | 0.18 (0.04–0.76) | 0.020 | 1.18 (0.40–3.45) | 0.764 | 0.96 (0.34–2.70) | 0.941 | 0.64 (0.19–2.15) | 0.467 |
| Current tobacco use (vs not) | 0.15 (0.03–0.82) | 0.028 | 1.08 (0.35–3.34) | 0.899 | 0.74 (0.22–2.47) | 0.625 | 3.13 (0.90–10.90) | 0.073 |
| High PTSD symptomsa (vs not) | 2.05 (0.53–7.97) | 0.299 | 1.75 (0.57–5.36) | 0.326 | 1.34 (0.45–4.01) | 0.607 | 7.93 (1.94–32.44) | 0.004 |
Defined as a score >14 on the PTSD CheckList—Civilian Version [25].
Bold font indicates statistical significance.
Figure 1B presents three distinct trajectories of days of high-THC medical cannabis use over 52 weeks: 1) infrequent use (n = 50), with a mean of 0.4 days of high-THC medical cannabis use per 14-day period; 2) occasional use (n = 24), with a mean of 4.3 days of high-THC medical cannabis use per 14-day period; and 3) frequent use (n = 25), with a mean of 11.2 days of high-THC medical cannabis use per 14-day period. The proportion of participants who had discontinued use of high-THC medical cannabis by the end of the study period differed by cluster (infrequent use: 88%; occasional use: 31%; frequent use: 0%; P < 0.001). We observed fewer days of high-THC medical cannabis use after week 30 in the occasional use cluster, although again differences in the slope of each trajectory over time were not significant. We observed a higher frequency of vaped product consumption in the frequent use cluster (92%) than in the occasional (58%) or infrequent use (48%) clusters. In multivariable analyses, those in the frequent (vs infrequent) high-THC medical cannabis use cluster were less likely to report a race/ethnicity other than non-Hispanic White (aOR 0.11, 95% CI 0.03–0.43) and more likely to report high PTSD symptoms (aOR 7.93, 95% CI 1.94–32.44). We did not observe differences in clusters with respect to nonmedical cannabis use, cannabis use dependence or abuse, or mental health symptoms other than PTSD.
Discussion
Among a cohort of adults in NYS newly certified for medical cannabis for chronic pain, we identified three distinct trajectories over 52 weeks of any medical cannabis use, as well as high-THC medical cannabis use. Notably, for both overall and high-THC product use, differences were evident at baseline and remained stable throughout the first year. To our knowledge, this is the first published study describing trajectories of medical cannabis use over time. We observed few differences between clusters with respect to clinical characteristics, including nonmedical cannabis use, pain burden, and mental health symptoms. Rather, major differences between clusters were participant race/ethnicity and employment status. These findings suggest that structural socioeconomic factors limiting access to medical cannabis may be a major driver of utilization, even among adults certified for medical cannabis use.
Understanding patterns of medical cannabis use is important for clinicians certifying patients and for policy makers seeking to understand how end users engage with the medical cannabis system. Our results suggest that medical cannabis patients are likely to fall into categories of frequent, occasional, or infrequent use, and that similar patterns exist among adults using high-THC formulations. Moreover, the observed stability of medical cannabis consumption suggests that adults with chronic pain may quickly establish therapeutic levels of use and not require substantial escalation over time to maintain this effect, although some patients may discontinue use over time. Previous cross-sectional studies of medical cannabis patients have described substantial heterogeneity in the frequency, dose, and route of cannabis administration, although the large majority (75–88%) of participants in those studies reported daily medical cannabis use [12, 14, 16, 34]. The lower frequency of use observed in our study may more accurately reflect use patterns, given the frequent and repeated measurements of cannabis use in the MEMO study. Alternatively, this finding may reflect more limited access to medical cannabis, potentially because of high cost [35], individual sociodemographic characteristics, or the NYS market, which is one of the most tightly regulated markets in the United States with respect to the density of both dispensaries and certifying providers [36].
We observed substantial differences between clusters with respect to race/ethnicity, with non-Hispanic White participants more likely to report frequent use of any and high-THC medical cannabis products than were non-Hispanic Black and Hispanic participants, even after adjustment for other characteristics. This finding suggests that some of the observed difference in frequency of medical cannabis use may be related to structural factors impacting access to medical cannabis. Prior studies suggest that Black and Hispanic individuals are underrepresented among medical cannabis patients [37, 38]. Our study adds to these findings, showing that even among persons certified for medical cannabis, race differentially impacts use. This may reflect race-related structural factors, including poorer access to dispensaries for Black and Hispanic populations [39], racial disparities in how providers treat pain [40], and potential reluctance to use medical cannabis given years of disproportionately aggressive enforcement of cannabis laws among Black and Hispanic populations [41, 42]. Although questions remain about the relative benefits and risks of cannabis, given the long history of racial disparities that pervade both the health care and criminal justice systems in the United States, additional studies are urgently needed to examine the impact of structural racism on access to medical cannabis.
We found no significant differences across clusters of overall medical cannabis use with respect to baseline pain, mood disorder symptoms, or signs of problematic alcohol or cannabis use. These results stand in contrast to multiple studies of nonmedical cannabis use reporting significant positive associations between frequency of cannabis consumption and use of alcohol [7, 11] and other substances [11, 43] or mental health disorders [11, 44]. Similarly, significant associations between mood disorders, pain, and cannabis use have been described in the few studies of medical cannabis that have examined these relationships [16, 45]. The lack of differences in most clinical characteristics between clusters in our study may reflect relatively narrow inclusion criteria or may be a function of publication bias toward studies reporting positive associations. We did observe a lower frequency of tobacco and opioid analgesic use and a higher frequency of employment in the cluster with occasional medical cannabis use than in the infrequent and frequent use clusters. These nonlinear, U-shaped results may reflect higher levels of social functioning or competing work-related priorities in the cluster with occasional medical cannabis use or could potentially indicate lower functioning due to severe pain or intoxication in the cluster with frequent cannabis use. Additional studies investigating associations between medical cannabis and social functioning would be valuable.
This study has several notable strengths and limitations. The frequent measurements of medical cannabis use every 14 days allowed us to describe trajectories much more granularly than any other published medical cannabis study to date. Even so, these are self-reported data and therefore are subject to recall and social desirability bias. The relatively small sample size may have limited the ability to detect all differences across clusters, particularly for substance use measures, as well as statistically significant differences in use over time within each cluster. Finally, this study included participants in the New York City area with chronic pain who used opioids and were seeking medical cannabis certification, a population that has not been well characterized in the literature. Therefore, our results may not be fully generalizable to the individuals in the target population or the broader population of medical cannabis patients in the United States.
Conclusions
In conclusion, in one of the first studies to describe trajectories of medical cannabis use, distinct stable patterns of use emerged over the first year among adults in the New York City area initiating medical cannabis for chronic pain. Although cluster membership did not differ by clinical characteristics, significant differences emerged with respect to participant race and ethnicity. These findings can inform individual- and policy-level recommendations and suggest that efforts are necessary to reduce racial disparities in the access and use of medical cannabis.
Supplementary Data
Supplementary Data may be found online at http://painmedicine.oxfordjournals.org.
Supplementary Material
Funding sources: This work was supported by the National Institute on Drug Abuse (R01DA044171, K24DA036955, K24DA046309); by the National Institute of Mental Health (K23 MH114752); and by the Einstein-Rockefeller-CUNY Center for AIDS Research (P30-AI124414), which is supported by the following Co-funding and Participating Institutes and Centers of the National Institutes of Health: National Institute of Allergy and Infectious Diseases; National Cancer Institute; Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Heart, Lung, and Blood Institute; National Institute on Drug Abuse; National Institute of Mental Health; National Institute on Aging; Fogarty International Center; and the Office of AIDS Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health .
Conflicts of interest: The authors have no conflicts to disclose.
Trial registration: ClinicalTrials.gov (NCT03268551).
References
- 1. Karmen Hanson AG. State medical marijuana laws. Available at: https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx (accessed 27 July 2021).
- 2. Park J-Y, Wu L-T.. Prevalence, reasons, perceived effects, and correlates of medical marijuana use: A review. Drug Alcohol Depend 2017;177:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015;313(24):2456–73. [DOI] [PubMed] [Google Scholar]
- 4.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Press; 2017:486. [PubMed] [Google Scholar]
- 5. Joy JE, Watson SJ, Benson JA; Division of Neuroscience and Behavioral Health; Institute of Medicine. Marijuana and Medicine: Assessing the Science Base. Washington, DC: National Academy Press; 1999. [Google Scholar]
- 6. Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: A clinical review. JAMA 2015;313(24):2474–83. [DOI] [PubMed] [Google Scholar]
- 7. Terry-McElrath YM, O’Malley PM, Johnston LD, et al. Longitudinal patterns of marijuana use across ages 18--50 in a US national sample: A descriptive examination of predictors and health correlates of repeated measures latent class membership. Drug Alcohol Depend 2017;171:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang C, Brook JS, Leukefeld CG, et al. Trajectories of marijuana use from adolescence to adulthood as predictors of unemployment status in the early forties. Am J Addict 2016;25(3):203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Passarotti AM, Crane NA, Hedeker D, et al. Longitudinal trajectories of marijuana use from adolescence to young adulthood. Addict Behav 2015;45:301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly BC, Vuolo M.. Cognitive aptitude, peers, and trajectories of marijuana use from adolescence through young adulthood. PLoS One 2019;14(10):e0223152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boden JM, Dhakal B, Foulds JA, et al. Life-course trajectories of cannabis use: A latent class analysis of a New Zealand birth cohort. Addiction 2020;115(2):279–90. [DOI] [PubMed] [Google Scholar]
- 12. Lucas P, Baron EP, Jikomes N.. Medical cannabis patterns of use and substitution for opioids & other pharmaceutical drugs, alcohol, tobacco, and illicit substances; results from a cross-sectional survey of authorized patients. Harm Reduct J 2019;16(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lucas P, Walsh Z.. Medical cannabis access, use, and substitution for prescription opioids and other substances: A survey of authorized medical cannabis patients. Int J Drug Policy 2017;42:30–5. [DOI] [PubMed] [Google Scholar]
- 14. Sexton M, Cuttler C, Finnell JS, et al. A cross-sectional survey of medical cannabis users: Patterns of use and perceived efficacy. Cannabis Cannabinoid Res 2016;1(1):131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaufmann CN, Kim A, Miyoshi M, et al. Patterns of medical cannabis use among older adults from a cannabis dispensary in New York state. Cannabis Cannabinoid Res 2020. doi: 10.1089/can.2020.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haug NA, Padula CB, Sottile JE, et al. Cannabis use patterns and motives: A comparison of younger, middle-aged, and older medical cannabis dispensary patients. Addict Behav 2017;72:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cunningham CO, Starrels JL, Zhang C, et al. Medical Marijuana and Opioids (MEMO) Study: Protocol of a longitudinal cohort study to examine if medical cannabis reduces opioid use among adults with chronic pain. BMJ Open 2020;10(12):e043400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med 2007;147(8):573–7. [DOI] [PubMed] [Google Scholar]
- 19.New York State Medical Marijuana Program. Available at: https://www.health.ny.gov/regulations/medical_marijuana/ (accessed 27 July 2021).
- 20.I-STOP/Prescription Monitoring Program (PMP) Internet system for tracking over-prescribing /Prescription Monitoring Program. Available at: https://www.health.ny.gov/professionals/narcotic/prescription_monitoring/ (accessed 27 July 2021).
- 21. Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med 2009;24(6):733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sullivan MJL, Bishop SR, Pivik J.. The pain catastrophizing scale: Development and validation. Psychol Assess 1995;7(4):524–32. [Google Scholar]
- 23. Kroenke K, Spitzer RL, Williams JB.. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med 2006;166(10):1092–7. [DOI] [PubMed] [Google Scholar]
- 25. Lang AJ, Stein MB.. An abbreviated PTSD checklist for use as a screening instrument in primary care. Behav Res Ther 2005;43(5):585–94. [DOI] [PubMed] [Google Scholar]
- 26. Ustun B, Adler LA, Rudin C, et al. The World Health Organization adult attention-deficit/hyperactivity disorder self-report screening scale for DSM-5. JAMA Psychiatry 2017;74(5):520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morin CM, Belleville G, Bélanger L, et al. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34(5):601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(20):22–33. [PubMed] [Google Scholar]
- 29.US Department of Commerce, Census Bureau (2020). National Cancer Institute and Food and Drug Administration co-sponsored Tobacco Use Supplement to the Current Population Survey. 2018–2019. Available at: https://cancercontrol.cancer.gov/brp/tcrb/tus-cps/questionnaires-data (accessed 2 September 2021).
- 30. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 31. McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat 1992;9(3):199–213. [DOI] [PubMed] [Google Scholar]
- 32.Muthén & Muthén. MPlus. 3463 Stoner Avenue, Los Angeles, CA 90066; Available at: http://www.statmodel.com/ (accessed 27 July 2021).
- 33. Bauer DJ, Curran PJ.. Distributional assumptions of growth mixture models: Implications for overextraction of latent trajectory classes. Psychol Methods 2003;8(3):338–63. [DOI] [PubMed] [Google Scholar]
- 34. Bonn-Miller MO, Boden MT, Bucossi MM, et al. Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse 2013;40(1):23–30. [DOI] [PubMed] [Google Scholar]
- 35. Guercio D. Why is New York’s medical marijuana program insanely expensive? 2020. Available at: https://www.leafly.com/news/industry/why-is-new-yorks-medical-marijuana-program-insanely-expensive (accessed 27 July 2021).
- 36. Richard EL, Althouse AD, Arnsten JH, et al. How medical are states’ medical cannabis policies?: Proposing a standardized scale. Int J Drug Policy 2021;94:103202. [DOI] [PubMed] [Google Scholar]
- 37. Salazar CA, Tomko RL, Akbar SA, et al. Medical cannabis use among adults in the Southeastern United States. Cannabis 2019;2(1):53–65. [PMC free article] [PubMed] [Google Scholar]
- 38. Mahabir VK, Merchant JJ, Smith C, et al. Medical cannabis use in the United States: A retrospective database study. J Cannabis Res 2020;2(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cooke A, Freisthler B, Mulholland E.. Examination of market segmentation among medical marijuana dispensaries. Subst Use Misuse 2018;53(9):1463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pletcher MJ, Kertesz SG, Kohn MA, et al. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA 2008;299(1):70–8. [DOI] [PubMed] [Google Scholar]
- 41. Golub A, Johnson BD, Dunlap E.. The race/ethnicity disparity in misdemeanor marijuana arrests in New York City. Criminol Public Policy 2007;6(1):131–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Solomon R. Racism and its effect on cannabis research. Cannabis Cannabinoid Res 2020;5(1):2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Okafor CN, Cook RL, Chen X, et al. Trajectories of marijuana use among HIV-seropositive and HIV-seronegative MSM in the Multicenter AIDS Cohort Study (MACS), 1984–2013. AIDS Behav 2017;21(4):1091–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Swift W, Coffey C, Carlin JB, et al. Adolescent cannabis users at 24 years: Trajectories to regular weekly use and dependence in young adulthood. Addiction 2008;103(8):1361–70. [DOI] [PubMed] [Google Scholar]
- 45. Nugent SM, Yarborough BJ, Smith NX, et al. Patterns and correlates of medical cannabis use for pain among patients prescribed long-term opioid therapy. Gen Hosp Psychiatry 2017;50:104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.