Abstract
Objectives
Favipiravir is an antiviral that is being evaluated for the treatment of COVID-19. Use of favipiravir is associated with elevation of serum uric acid levels. Risk factors for the occurrence of hyperuricemia are unclear.
Methods
Specimens from COVID-19 patients who received 10 days of favipiravir in a previous clinical trial (jRCTs041190120) were used. Serum favipiravir concentrations were measured by LC-MS. Factors associated with the development of hyperuricemia were investigated using logistic regression analysis. Optimal cut-off values for the baseline serum uric acid levels and steady-state serum favipiravir concentrations in predicting the occurrence of hyperuricemia were determined by ROC curve analysis.
Results
Among the 66 COVID-19 patients who were treated with favipiravir for 10 days, the steady-state serum favipiravir concentrations were significantly correlated with serum uric acid levels. High baseline serum uric acid levels and steady-state serum favipiravir concentrations during therapy were factors associated with the development of hyperuricemia. The cut‑off baseline serum uric acid level and steady-state serum favipiravir concentration during favipiravir administration determined to predict hyperuricemia were 3.7 mg/dL and 46.14 μg/mL, respectively.
Conclusions
Patients with high baseline serum uric acid levels or who achieved high steady-state serum favipiravir concentrations during therapy were susceptible to hyperuricemia.
Keywords: favipiravir, hyperuricemia, uric acid, COVID-19
Introduction
Favipiravir (FVP) is an oral RNA-dependent RNA polymerase inhibitor that is being evaluated as an antiviral for the treatment of coronavirus infectious disease 2019 (COVID-19) (Alam et al., 2021). In Japan, FVP was approved for the treatment of patients with novel or re-emerging pandemic influenza virus infection in 2011. It is well recognized that the administration of FVP frequently leads to elevated uric acid (UA) levels in patients with influenza virus infections (Pharmaceuticals and Medical Devices Agency, 2014). In a phase 3 study of influenza (312 Study), a single dose of FVP 1200 mg and a single dose of 400 mg on the first day followed by 400 mg twice daily up to 4 days induced serum UA level elevation in 21 out of 378 subjects (5.6%) (Pharmaceuticals and Medical Devices Agency, 2014).
Elevation of serum UA levels has also been reported in several clinical trials of FVP against COVID-19 (Doi et al., 2020; Udwadia et al., 2021; Zhao et al., 2021). Furthermore, a COVID-19 patient with history of hyperuricemia developed acute gouty arthritis upon receiving FVP (Hase et al., 2020). In our previous randomized, multicenter, open-label clinical trial of FVP against COVID-19, FVP 1800 mg twice on the first day followed by 800 mg twice daily for up to an additional 9 days induced hyperuricemia in 69 out of 82 patients (84.1%) (Doi et al., 2020). The dose of FVP in this trial was higher than that in other studies, such as the 312 Study for influenza. Moreover, in a recent systematic review, FVP administration was significantly more likely to induce elevation of serum UA levels than placebo or other candidate antivirals for COVID-19 (5.8% vs 1.3%) (Pilkington et al., 2020). Morikawa et al. reported that high serum FVP levels (20 μg/mL or more) were associated with elevation of UA levels in COVID-19 treatment in a single-center cohort of 27 patients (Morikawa et al., 2021). These studies suggest that the risk of developing hyperuricemia may depend on the serum FVP concentrations. However, the factors related to the elevation of serum UA levels in COVID-19 patients treated with FVP remain unclear. Hence, our study investigated the factors associated with the occurrence of hyperuricemia in COVID-19 patients from a clinical trial cohort (Doi et al., 2020).
Methods
Clinical dataset
The clinical dataset from a previously conducted randomized, multicenter, open-label clinical trial was used (jRCTs041190120) (Doi et al., 2020). In brief, patients with asymptomatic or mild COVID-19 were recruited, registered, and given FVP dosed at 1800 mg twice orally at least 4 h apart on the first day, followed by 800 mg orally twice daily for up to an additional 9 days. The patients were randomized to start FVP either immediately or 5 days after randomization, with the intention to continue FVP for a total of 10 days in both groups. Of the 89 patients who participated in the clinical trial and assigned to either the immediate or delayed treatment group, 66 were included in this substudy after excluding 23 patients for the following reasons: 1) one patient withdrew from the study; 2) 20 patients did not take FVP for the full 10 days, as intended by the study protocol; 3) two patients had no baseline serum UA levels or FVP concentrations. Serum samples for the measurement of laboratory values and FVP concentrations were collected just prior to the first dose of FVP, on the 6th day of FVP administration, which represents the steady state (Morikawa et al., 2021), and on the 11th day, which was 1 day after the last FVP dose. To investigate the factors contributing to the occurrence of hyperuricemia, patients were divided into two groups: those with hyperuricemia (UA > 10 mg/dL) on the 6th-day or 11th-day samples, and those without. The definition of hyperuricemia was adopted from that for grade 4 hyperuricemia (UA > 10 mg/dL) stated in the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
LC-MS
For the measurement of serum FVP concentrations, surplus serum samples from the underlying clinical trial were used. In preparation, 200 μl of acetonitrile, 20 μl of 75% isopropanol, and an internal standard solution (10 µg/mL [13C, 15N]-FVP in acetonitrile) were added to 50 μl of serum. The mixture was then vortexed and centrifuged (15 000 × g) for 5 min. The supernatant was collected in a new 1.5-mL polypropylene tube, and an aliquot of 1 μl was injected into a triple quadrupole mass spectrometer LCMS8060NX, coupled to a Nexera X2 liquid chromatography system (Shimadzu). The analytes were separated on a Shim-pack Scepter C18-120 column (50 mm × 2.1 mm i.d., 3 μm; Shimazdu GLC), using a mobile phase of (A) 0.05% formic acid and (B) 0.05% formic acid in acetonitrile. The gradients were as follows: 5% (v/v) solvent B for 0.3 min, 5–30% solvent B linear gradient for 0.05 min, 30–90% solvent B linear gradient for 1.15 min, and 90% solvent B for 1 min. Analysis of FVP and [13C, 15N]-FVP was conducted in the multiple reaction monitoring mode, using a specific precursor ion [M-H]− and product ions pairs as follows: FVP, m/z 157.7→87.1; [13C, 15N]-FVP, m/z 159.7→85.1.
For the LC-MS method, intraday and interday reproducibility was assessed by analyzing three and seven replicates at the lower level of quantification (LLOQ: 0.05 μg/ml) in addition to two concentrations (1 μg/ml and 10 μg/ml), respectively (Supplementary Table 1). The accuracy was defined as the deviation between the true and the measured value expressed as percentage, and the precision was characterized by the relative standard deviation. The linear range of calibration curve was also evaluated by plotting the peak area ratios of FVP/[13C, 15N]-FVP versus the concentration ratios (Supplementary Figure 1).
Statistical analysis
Statistical analysis was performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) as a graphical user interface for R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, EZR is a modified version of R Commander (version 1.54) designed to add statistical functions frequently used in biostatistics (Kanda, 2013). Continuous variables (age, body mass index [BMI], laboratory values, and serum FVP concentration) were expressed as median and range, and compared between patients with or without hyperuricemia (UA > 10 mg/dL) using the Mann-Whitney U-test. The categorical variables were summarized by presenting the frequencies and proportions of patients, and compared using Fisher's exact test. The Friedman rank sum test was performed to evaluate the changes in serum UA and FVP levels between the baseline, 6th-day, and 11th-day samples. The Mann-Whitney U-test was used for comparing the serum FVP levels between patients with or without hyperuricemia (UA > 10 mg/dL) on the 6th or 11th day. Spearman's correlation test was performed for correlation analysis. Logistic regression analysis was performed for multivariable analysis of the occurrence of hyperuricemia (UA > 10 mg/dL) on either the 6th or 11th day. The cut-off values for baseline serum UA level and steady-state serum FVP concentration in predicting the occurrence of hyperuricemia were evaluated using a receiver operating characteristic (ROC) curve. The area under the curve (AUC) was calculated with corresponding sensitivity and specificity. Results were considered statistically significant at p < 0.05.
Results
Patient characteristics
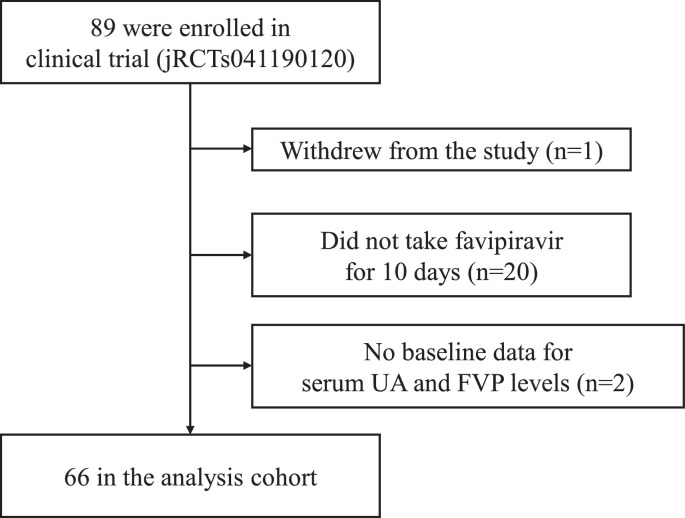
This study included 66 COVID-19 patients who received FVP for 10 days as part of a clinical trial (Figure 1 ). The baseline characteristics of the subjects are shown in Table 1 . The median age was 48.5 years (range 24.0–86.0), and the median BMI was 22.8 kg/m2 (range 16.9–37.1). Forty patients (60.6%) were male and 26 patients (39.4%) were female. The median baseline serum UA level was 4.6 mg/dL (range 1.7–7.6).
Figure 1.
Patients in this study. FVP, favipiravir; UA, uric acid
Table 1.
Baseline demographics and clinical characteristics of COVID-19 patients in the study
| Demographic and clinical characteristics | Total (n = 66) | Non-hyperuricemia(UA ≤ 10 mg/dL) (n = 26) | Hyperuricemia(UA > 10 mg/dL) (n = 40) | p-value |
|---|---|---|---|---|
| Age, median (range), yrs | 48.5 (24.0, 86.0) | 51.0 (27.0, 76.0) | 46.0 (24.0, 86.0) | 0.595 |
| Sex, no. (%) | ||||
| Male | 40 (60.6) | 17 (65.4) | 23 (57.5) | 0.610 |
| Female | 26 (39.4) | 9 (34.6) | 17 (42.5) | |
| BMI, median (range), kg/m2 | 22.8 (16.9, 37.1) | 22.0 (18.2, 34.8) | 23.5 (16.9, 37.1) | 0.978 |
| Laboratory values | ||||
| WBC, median (range), cells/μL | 4.7 (2.2, 9.2) | 4.3 (2.2, 9.2) | 4.9 (2.4, 9.1) | 0.129 |
| Platelet, median (range), × 103/μL | 207.0 (94.0, 497.0) | 179.5 (94.0, 429.0) | 220.0 (131.0, 497.0) | 0.081 |
| CRP, median (range), mg/L | 0.7 (0.0, 10.4) | 1.6 (0.0, 10.4) | 0.6 (0.0, 7.4) | 0.206 |
| ALT, median (range), U/L | 19.5 (5.0, 152.0) | 20.5 (8.0, 87.0) | 19.0 (5.0, 152.0) | 0.604 |
| AST, median (range), U/L | 23.5 (11.0, 67.0) | 28.5 (14.0, 67.0) | 22.0 (11.0, 51.0) | 0.030 |
| Urea nitrogen, median (range), mg/dL | 11.8 (6.0, 54.0) | 12.3 (6.0, 15.0) | 11.7 (6.5, 54.0) | 0.753 |
| Serum creatinine, median (range), mg/dL | 0.8 (0.4, 4.1) | 0.8 (0.5, 1.1) | 0.8 (0.4, 4.1) | 0.906 |
| Creatinine clearance, median (range), mL/min | 68.8 (16.1, 232.8) | 66.7 (23.1, 159.4) | 63.2 (16.1, 232.8) | 0.608 |
| Uric acid, median (range), mg/dL | 4.6 (1.7, 7.6) | 3.6 (1.7, 7.3) | 4.9 (2.8, 7.6) | 0.003 |
WBC, white blood cell; BMI, body mass index; CRP, C-reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase
Patients with or without subsequent hyperuricemia were divided into non-hyperuricemia (n = 26) and hyperuricemia (n = 40) groups, respectively. The two groups were comparable in their demographic and laboratory values, except for serum AST and UA levels (Table 1). The median baseline AST level in patients with hyperuricemia (22.0 U/liter; range 11.0–51.0) was significantly lower than that in patients without hyperuricemia (28.5 U/liter; range 14.0–67.0), yet there was no difference in median serum ALT levels. The median baseline serum UA level in patients with hyperuricemia (4.9 mg/dL; range 2.8–7.6) was significantly higher than that in patients without hyperuricemia (3.6 mg/dL; range 1.7–7.3). There was no difference in creatinine levels or in the creatinine clearance calculated by the Cockcroft-Gault equation as an indicator of renal function between patients with low and high baseline serum UA levels.
Serum UA and FVP levels
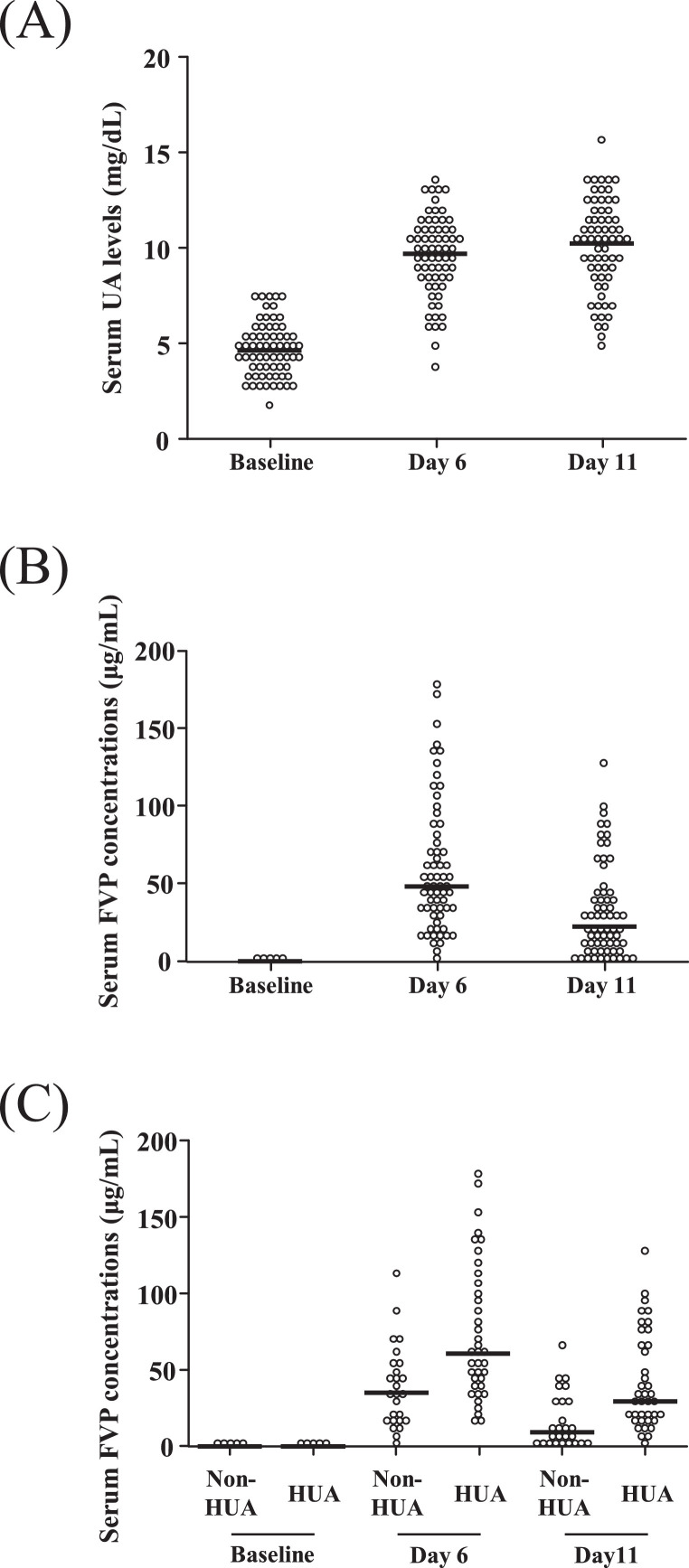
The median serum UA levels were elevated at 9.7 mg/dL (range 5.0–15.6) on the 6th day (steady state during FVP therapy) and 10.3 mg/dL (range 3.7–13.6) on the 11th day (1 day after completion of FVP therapy) (p < 0.01) (Figure 2 A).
Figure 2.
Serum UA levels and FVP concentrations in the overall cohort, and serum FVP concentrations in patients with and without hyperuricemia. Serum UA levels (A) and serum FVP concentrations (B) in patients in the overall cohort. (C) Serum FVP concentrations in patients with and without hyperuricemia (UA > 10 mg/dL). Serum samples were measured on baseline, on day 6 (steady state during therapy), and day 11 (1 day after completion of FVP therapy). For serum FVP concentrations, because of the large number of patients who had baseline levels close to 0 μg/mL — 66 in total (B), and 26 and 40 in patients without or with hyperuricemia, respectively (C) — the values are collapsed into five points. The horizontal solid lines indicate the median values. Statistical analysis was performed using the Friedman rank-sum test (p < 0.01) (A, B), and the Mann-Whitney U-test (day 6 and day 11 FVP levels, non-HUA vs HUA, p < 0.01) (C). FVP, favipiravir; HUA, hyperuricemia; UA, uric acid
Serum FVP levels in plasma were quantified by validated LC-MS methods (Supplementary Table 1 and Supplementary Figure 1). Overall, the median serum FVP concentrations were 0.00 μg/mL (range 0.00–0.02) at baseline, 48.31 μg/mL (range 3.24–178.54) on the 6th day, and 22.62 μg/mL (range 0.00–129.58) on the 11th day (p < 0.01) (Figure 2B). The median serum FVP concentrations in patients with or without hyperuricemia (UA > 10 mg/dL) at baseline were 0.00 μg/mL (range 0.00–0.01) and 0.00 μg/mL (range 0.00–0.02), respectively, which was as expected because this was prior to the first dose of FVP. After FVP was started, the median serum FVP concentrations in patients who developed hyperuricemia (60.85 μg/mL, range 17.12–178.54, on the 6th day and 29.48 μg/mL, range 0.00–129.58, on the 11th day) were significantly higher than those in patients who did not develop hyperuricemia (35.22 μg/mL, range 3.24–114.04, on the 6th day and 9.61 μg/mL, range 0.01–65.49, on the 11th day) (p < 0.01) (Figure 2C).
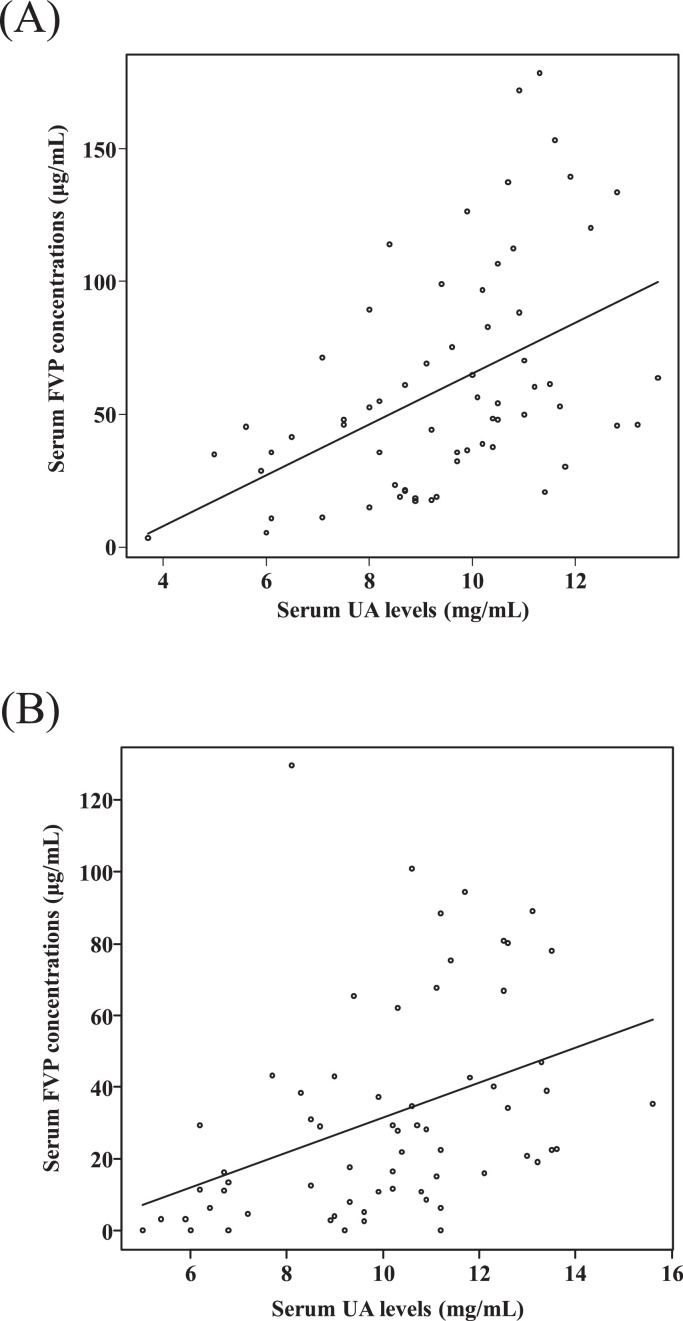
Correlation between serum FVP and UA levels
The correlations between serum FVP and UA levels on the 6th day and the 11th day were examined. The serum FVP and UA levels showed positive correlations on both days (r = 0.52 on the 6th day, p < 0.01; r = 0.49 on the 11th day, p < 0.01) (Figures 3 A, 3B). To examine the factors associated with the occurrence of hyperuricemia, multivariable analysis was performed using logistic regression analysis. Higher than median steady-state serum FVP concentrations (> 48.31 μg/mL) and higher than median baseline serum UA levels (> 4.6 mg/dL) were independent factors associated with the occurrence of hyperuricemia during FVP therapy (odds ratio for FVP concentration > median on the sixth day, 5.37 [95% CI 1.70–17.00] and odds ratio for UA level > median at baseline, 3.58 [95% CI 1.14–11.30]) (Table 2 ).
Figure 3.
Correlation between serum FVP and UA levels. (A) Correlation between serum FVP and UA levels on day 6. Spearman's r- and p-values were r = 0.52 and p < 0.01. (B) Correlation between serum FVP and UA levels on day 11. Spearman's r- and p-values were r = 0.49 and p < 0.01. FVP, favipiravir; UA, uric acid
Table 2.
Odds ratio for hyperuricemia (> 10 mg/dL) on either Day 6 or Day 11
| OR | 95% CI | p-values | |
|---|---|---|---|
| Steady-state serum FVP concentration > 48.3 µg/ml | 5.37 | 1.70–17.00 | < 0.01 |
| Baseline serum UA > 4.6 mg/dL | 3.58 | 1.14–11.30 | 0.029 |
BMI, body mass index; CI, confidence interval; FVP, favipiravir; OR, odds ratio; UA, uric acid
ROC curves for serum FVP and UA levels
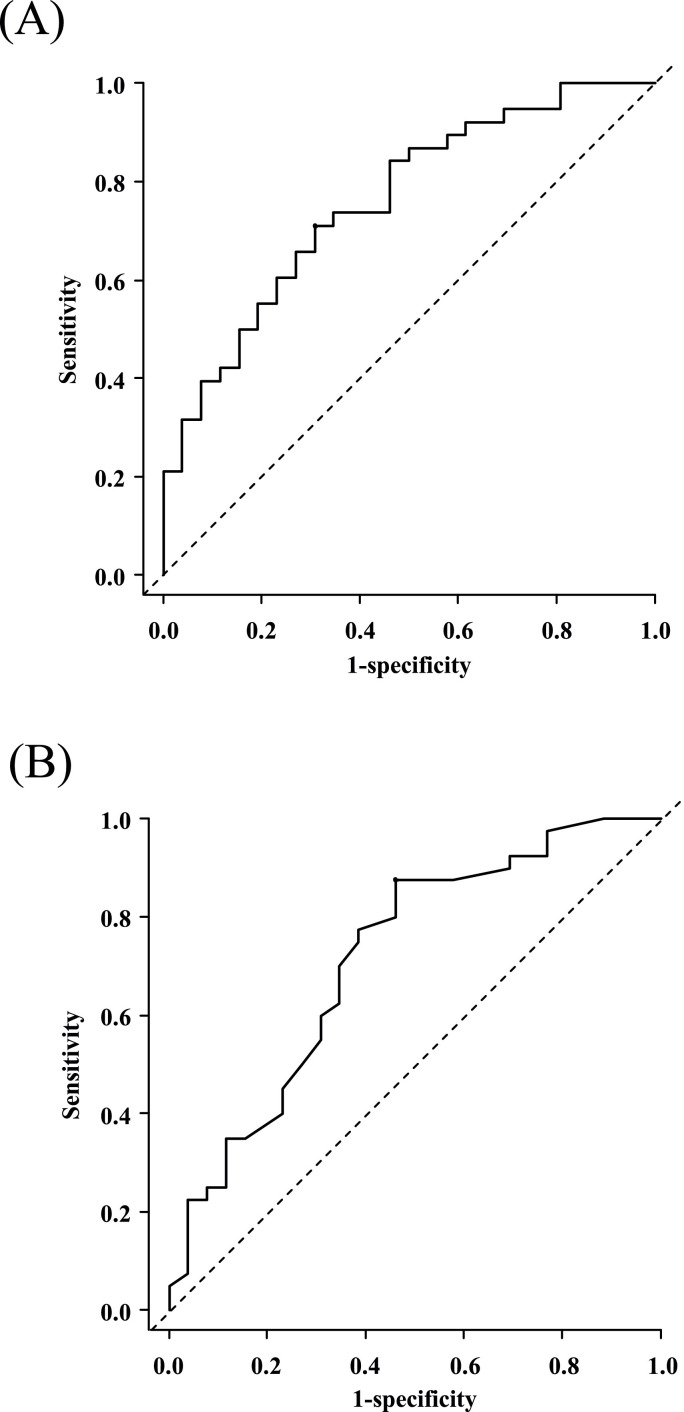
Since high serum FVP concentrations on the 6th day and high serum UA levels at baseline were independently associated with the occurrence of hyperuricemia during FVP therapy, the cut-off values for the 6th-day serum FVP concentration that approximated the steady state and the baseline serum UA level were determined by ROC curve analysis. An ROC curve identified that a 6th-day serum FVP concentration of > 46.14 μg/mL had a sensitivity of 0.71 and a specificity of 0.69 (AUC 0.76; 95% CI 0.64–0.88), while a baseline serum UA level of > 3.7 mg/dL had a sensitivity of 0.88 and a specificity of 0.54 (AUC 0.72; 95% CI 0.59–0.85) in predicting the occurrence of hyperuricemia (UA > 10 mg/dL) induced by FVP (Figures 4 A, 4B).
Figure 4.
Receiver operating characteristic (ROC) curve analysis for the occurrence of hyperuricemia. (A) ROC analysis based on steady-state serum FVP levels: cut-off, 46.14 μg/mL; sensitivity, 0.71; specificity, 0.69; AUC, 0.76 (95% CI 0.64–0.88). (B) ROC analysis based on baseline serum UA levels: cut-off, 3.70 mg/dL; sensitivity, 0.88; specificity, 0.54; AUC, 0.72 (95% CI 0.59–0.85). AUC, area under the curve; CI, confidence interval; FVP, favipiravir; UA, uric acid
Discussion
FVP is being evaluated as a potential oral treatment option for COVID-19. Transient elevation of serum UA levels occurs frequently with FVP therapy, but the risk factors for this event have not been comprehensively evaluated. Our study investigated the factors associated with the occurrence of hyperuricemia during FVP therapy by using dataset and serum samples available from a previous clinical trial of this agent against COVID-19. The serum UA and FVP levels were increased during FVP therapy, and patients who presented with hyperuricemia during or right after FVP therapy were shown to have high serum FVP concentrations compared with patients who did not develop hyperuricemia. Furthermore, high serum FVP concentrations (> 48.31 µg/ml) during FVP therapy and high baseline serum UA levels were independent factors associated with the occurrence of hyperuricemia.
In a dose–response phase II study conducted in Japanese patients with influenza (JP205 Study), dose-dependent increases of serum UA levels were reported (Pharmaceuticals and Medical Devices Agency, 2014). Our results correlating serum UA and FVP levels were consistent with this observation. FVP and its metabolite, M1, decrease UA secretion by inhibiting organic anion transporter (OAT) 1 and OAT3, which leads to decreased tubular secretion of UA, while M1 also enhances UA reabsorption of UA via urate transporter (URAT) 1 (Mishima et al., 2020; Pharmaceuticals and Medical Devices Agency, 2014). In addition, patients with renal dysfunction usually have high serum UA levels and low urinary UA clearance (Li et al., 2018). Thus, FVP-induced increases in UA levels might be influenced by renal function as well. In our study, however, baseline creatinine clearance in patients with hyperuricemia was comparable with that in patients without hyperuricemia, and creatinine clearance did not correlate with serum UA levels during FVP therapy (r = 0.10, p = 0.44; Supplementary Figure 2A). Furthermore, creatinine clearance was moderately correlated with serum FVP concentrations during FVP therapy (r = −0.44, p < 0.01; Supplementary Figure 2B). Therefore, serum FVP concentrations could be considered as a factor for hyperuricemia caused by FVP, independent of renal function. The serum FVP concentration as a cut-off value for hyperuricemia in the present study (48.3 μg/mL) was higher than in the previous study (20 μg/mL) (Morikawa et al., 2021). Since the subjects in our study were younger (median age 48.5) than in the previous study (median age 63), the difference in ages of subjects might have affected the UA levels. In addition, the UA levels after FVP administration in the previous study (6.6–9.3 mg/dL) were lower than those in the present study. The risk of acute gouty arthritis is increased by hyperuricemia (UA > 10 mg/dL) (Wilson and Saseen, 2016). Therefore, patients with a history of acute gouty arthritis may benefit from the monitoring of UA levels. Furthermore, our cut-off value might be useful in predicting the elevation of UA levels during FVP therapy.
High baseline serum UA levels were predictive of subsequent hyperuricemia (UA levels > 10 mg/dL) during FVP therapy. There are few reports on the effects of serum UA levels prior to FVP therapy on subsequent levels following therapy. One study reported that baseline serum UA levels had no effect on laboratory values during FVP administration, but it did not examine the correlation between baseline serum UA levels and those during FVP therapy (Inkaya et al., 2021). ROC analysis revealed that baseline serum UA levels > 3.7 mg/dL were predictive of hyperuricemia, which suggests that not only patients with overt hyperuricemia but also those with serum UA levels at the upper end of the normal range should be aware of the risk of hyperuricemia and its associated symptoms, such as gout attacks, that may occur with FVP therapy.
Our study had several limitations. First, blood was collected primarily for safety evaluation as part of the underlying clinical trial, and collection was not necessarily timed with FVP administration. In a Japanese phase I study for influenza (JP118 Study), in which FVP was orally administered at 1200 mg twice daily on the first day, followed by 600 mg twice daily for an additional 5 days, the serum FVP concentrations at completion of FVP administration (mean ± SD) were approximately Cmin 20 ± 20 μg/mL and Cmax 60 ± 30 μg/mL (Pharmaceuticals and Medical Devices Agency, 2014). Despite the higher FVP dose, the median FVP concentration at the same time point in our study (48.31 μg/mL, range 3.24–178.54) did not differ substantially from that assumed from the JP118 Study. It should therefore be noted that, while the timing of blood draw relative to FVP dosing was not standardized, this does not appear to fully account for this observation. Second, the specificity of baseline serum UA level in predicting hyperuricemia was limited in the ROC analysis. This was in part due to prioritization of sensitivity, since measurement of UA levels is relatively routine and inexpensive. Third, serum ferritin levels were not measured in the present study. Since ferritin levels are inversely associated with blood FPV levels (Morikawa et al., 2021), further studies to assess the impact of ferritin levels are needed.
Conclusion
Patients with COVID-19 who have high baseline serum UA levels and those who show high serum FVP concentrations during FVP treatment are susceptible to hyperuricemia induced by FVP. Not only patients with hyperuricemia, but also patients with high baseline serum UA levels within the normal range may be at elevated risk for developing hyperuricemia during FVP administration. Additionally, serum FVP concentrations might be a factor to consider for the occurrence of hyperuricemia during FVP therapy.
Acknowledgments
Funding
This research was supported by grants from the Japan Agency for Medical Research and Development (AMED) under grant numbers 19fk0108150s0001 and 20fk0108150s0001.
Conflicts of interest
YD has served on a scientific advisory board of, and received a speaking fee from, FujiFilm Toyama Chemical, the manufacturer of favipiravir in Japan. The other authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the Institutional Review Board of Fujita Health University (HM20-169).
Author contributions
TK, YD, and TM conceived and designed the study. KN and HI performed the experimental work. TK and TM contributed to the statistical analysis. SY and KT contributed to the study concept and design. TK, YD, and TM drafted the manuscript. All authors contributed to the critical input and endorsed the final version of the manuscript.
Acknowledgments
The authors would like to thank Masahiro Suzuki for helping with the processing of serum samples, and Eishi Imoto (Shimadzu Corporation) for validating the LC-MS method for quantifying FVP.
Footnotes
Authorship statement: All authors meet the ICMJE authorship criteria.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.12.324.
Appendix. Supplementary materials
References
- Alam S, Kamal TB, Sarker MMR, Zhou JR, Rahman SMA, Mohamed IN. Therapeutic effectiveness and safety of repurposing drugs for the treatment of COVID-19: position standing in 2021. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.659577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi Y, Hibino M, Hase R, Yamamoto M, Kasamatsu Y, Hirose M, et al. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020;64(12):e01897. doi: 10.1128/AAC.01897-20. –20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase R, Kurata R, Ishida K, Kurita T, Muranaka E, Mito H. Acute gouty arthritis during favipiravir treatment for coronavirus disease 2019. Intern Med. 2020;59(18):2327–2329. doi: 10.2169/internalmedicine.5377-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkaya AC, Kara E, Calik Basaran N, Sahin TK, Uyaroglu OA, Uzun O, et al. Pretreatment serum uric acid level is not a surrogate marker for the outcome of favipiravir treatment in COVID-19 patients. Turk J Med Sci. 2021 doi: 10.3906/sag-2102-84. [DOI] [PubMed] [Google Scholar]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Guo H, Zou J, Chen W, Lu Y, Zhang X, et al. Urinary excretion of uric acid is negatively associated with albuminuria in patients with chronic kidney disease: a cross-sectional study. BMC Nephrol. 2018;19(1):95. doi: 10.1186/s12882-018-0892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima E, Anzai N, Miyazaki M, Abe T. Uric acid elevation by favipiravir, an antiviral drug. Tohoku J Exp Med. 2020;251(2):87–90. doi: 10.1620/tjem.251.87. [DOI] [PubMed] [Google Scholar]
- Morikawa G, Kubota K, Kondo D, Takanashi Y, Minami S, Kinjo T, et al. Elevated blood favipiravir levels are inversely associated with ferritin levels and induce the elevation of uric acid levels in COVID-19 treatment: a retrospective single-center study. J Infect Chemother. 2021 doi: 10.1016/j.jiac.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmaceuticals and Medical Devices Agency. Report on the deliberation results [brand name] Avigan Tablet 200 mg; 2014. Available from: https://www.pmda.go.jp/files/000210319.pdf.
- Pilkington V, Pepperrell T, Hill A. A review of the safety of favipiravir — a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020;6(2):45–51. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udwadia ZF, Singh P, Barkate H, Patil S, Rangwala S, Pendse A, et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2021;103:62–71. doi: 10.1016/j.ijid.2020.11.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L, Saseen JJ. Gouty arthritis: a review of acute management and prevention. Pharmacotherapy. 2016;36(8):906–922. doi: 10.1002/phar.1788. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhang C, Zhu Q, Chen X, Chen G, Sun W, et al. Favipiravir in the treatment of patients with SARS-CoV-2 RNA recurrent positive after discharge: a multicenter, open-label, randomized trial. Int Immunopharmacol. 2021;97 doi: 10.1016/j.intimp.2021.107702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.