Abstract
Objective:
Radiation exposure of the lens during a neck CT may increase a patient’s risk of developing cataracts. Radiologists in our institution worked with technicians to modify neck CT scan procedure to include a reduction in scan range and tube potential (kVp), and neck positioning using a head tilt. We objectively quantified the organ dose changes after this procedure modification by using computer simulation.
Methods:
We retrospectively analyzed CT images of 40 patients (20 males and 20 females) scanned before and after the procedure change. Radiation dose to the lens delivered before and after the procedure change was calculated using an in-house CT dose calculator combined with computational human phantoms deformed to match head tilt angles. We also calculated the doses to other radiosensitive organs including brain, pituitary gland, eye globes, and salivary glands before and after the procedure change.
Results:
Our dose calculations demonstrated that modifying neck position and shortening scan range reduced the dose to the lens by 89% on average (p<0.0001). Brain, pituitary gland, globes, and salivary gland doses also decreased by 59%, 52%, 66%, and 29% on average, respectively. We found overranging significantly affects the lens dose.
Conclusion:
Combining head tilt and scan range reduction is an easy and effective method that significantly reduces radiation dose to the lens and other radiosensitive head and neck organs.
Keywords: neck CT, lens, radiation dose, dose reduction
Introduction
The lens of the eye is one of the radiosensitive tissues in the human body (1). Radiation induced cataracts are of concern to patients undergoing head or neck computed tomography (CT) exams as well as to radiologists, technicians and other clinical staff involved in fluoroscopy procedures. In 1984, International Commission on Radiological Protection (ICRP) recommended 5 Sv for a single acute exposure and 8 Sv for fractionated or protracted exposure as the threshold equivalent dose for cataract (2,3). More recently, based on recent epidemiological studies reporting an increasing cataract latency period as dose decreased, ICRP lowered the threshold for radiation-induced cataract to 0.5 Sv with 90–95% confidence intervals (4), which is lower by a factor of 10 than the previous value.
Several techniques to reduce radiation dose to the lens of the eye in CT imaging procedures have been introduced. Bismuth-coated latex shielding of the eye reduces lens dose by 25–45% and orbit dose by 25–50% (5–12). Lead shielding produces similar results with a 44% reduction in entrance doses of the lens in CT exams of the brain (13). Gantry titling is another common technique, resulting in a 75% reduction in lens dose and slightly more reduction in orbit dose during brain CT imaging (10,12,14). A more recently developed technique is organ-based tube current modulation (TCM), which has been shown to reduce lens and orbit dose by about 30% (10,11). Additionally, the use of iterative reconstruction with tube current modulation reduces lens dose up to 34%, when compared to standard filtered back-projection (15–18). A global reduction in tube current also results in an eye dose reduction of about 30% (11).
In 2013, radiologists at our institution worked with technicians to modify our neck CT imaging procedure, aiming to reduce lens dose as well as the dose to other radiosensitive head and neck organs. The new procedure includes a reduction in scan range (upper limit at the sellar floor and lower limit at the sternal notch), a reduction in tube potential, and a modification in neck positioning using a head tilt (orbitomeatal line perpendicular to table). In contrast to other dose reduction methods described above, the new procedure at our institution does not require sophisticated technical solutions.
After about four years of conducting the new scan procedure, we wanted to verify the effectiveness of our procedure change by comparing the radiation dose to the lens and other critical organs before and after the procedure change. We hypothesized that the procedure change would result in a reduction of the lens dose. In the current study, we objectively quantified the significance of the organ dose change by collecting sample CT images of patients scanned before and after the procedure change and calculating dose to major radiosensitive organs.
Materials and Methods
Patient CT
In this Institutional Review Board exempt analysis, we retrospectively analyzed anonymized CT images of 20 adult male and 20 adult female patients, randomly selected from two time periods: 3/2010 – 10/2013 and 1/2014 – 3/2016 before and after the procedure change, respectively. Each patient had two sets of CT images: before and after the procedure change. The new procedure was introduced during the end of the calendar year of 2013. The 40 patients scanned before and after the procedure change were on average 52 years old (20 – 76 years) and 55 years old (24 – 79 years), respectively. All scans were obtained on any of the following scanners: Siemens Definition, Flash or Force. Scan protocols for the before and after scans were similar except that tube potential (kVp) was reduced from 120 to 100 kVp after the procedure change.
Scan Parameters
In spiral CT scanning, additional gantry rotations are added at the scan start and end for data interpolation to reconstruct the first and last slice of the scan. This results in an elongation of the scan length called overranging (19). Overranging may cause organs that are not seen in the CT scan, but are very close to the scan start and end, to be directly exposed to radiation (20). For this reason, we calculated overranging values for each scan and incorporated this into our dose calculations. We derived overranging from DLP and CTDIvol obtained from DICOM headers and the imaged scan length measured from CT images as described in the following equation (19),
| (1) |
| (2) |
where LOV is overranging length (cm), LTOT is total exposed scan length (cm), and LIM is the imaged length (cm). We added the half of overranging length, LOV derived from Equation 1 and 2 to the scan start location and the other half to the scan end location as illustrated in the right column of Figure 2.
Figure 2.
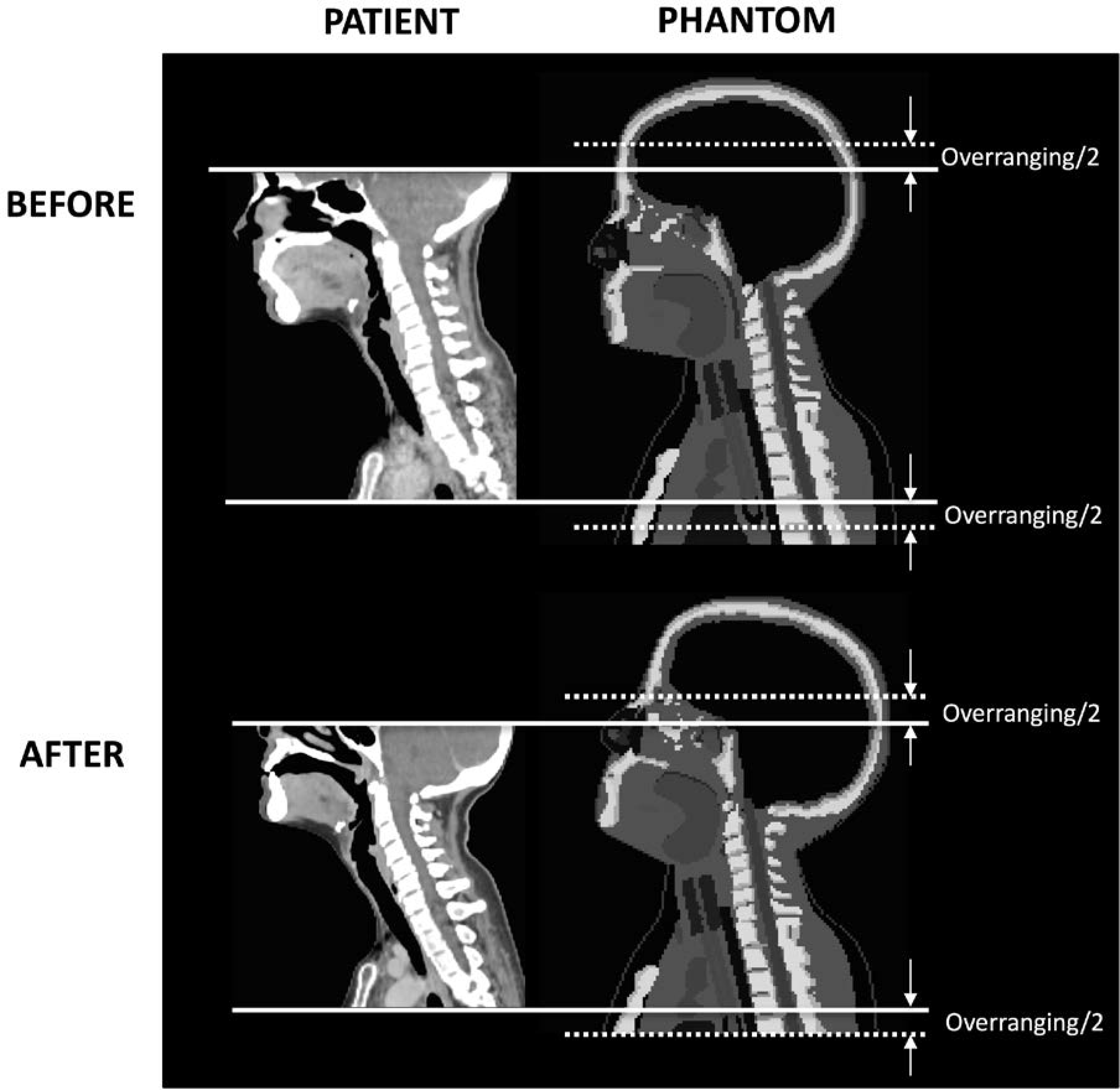
Mapping of patient-specific scan locations (left column) onto phantoms (right column) to define scan start and scan end (solid lines) with overranging (dotted lines) added for dose calculations.
We measured the head tilt angle between the Frankfurt plane (plane passing through inferior margins of the orbits and the superior margin of the external acoustic meatus) and the horizontal reference plane on the topograms of the patients obtained before and after the procedure change (Figure 1). We obtained the average head tilt angles of −5 degrees and +17 degrees for the before and after scans, respectively.
Figure 1.
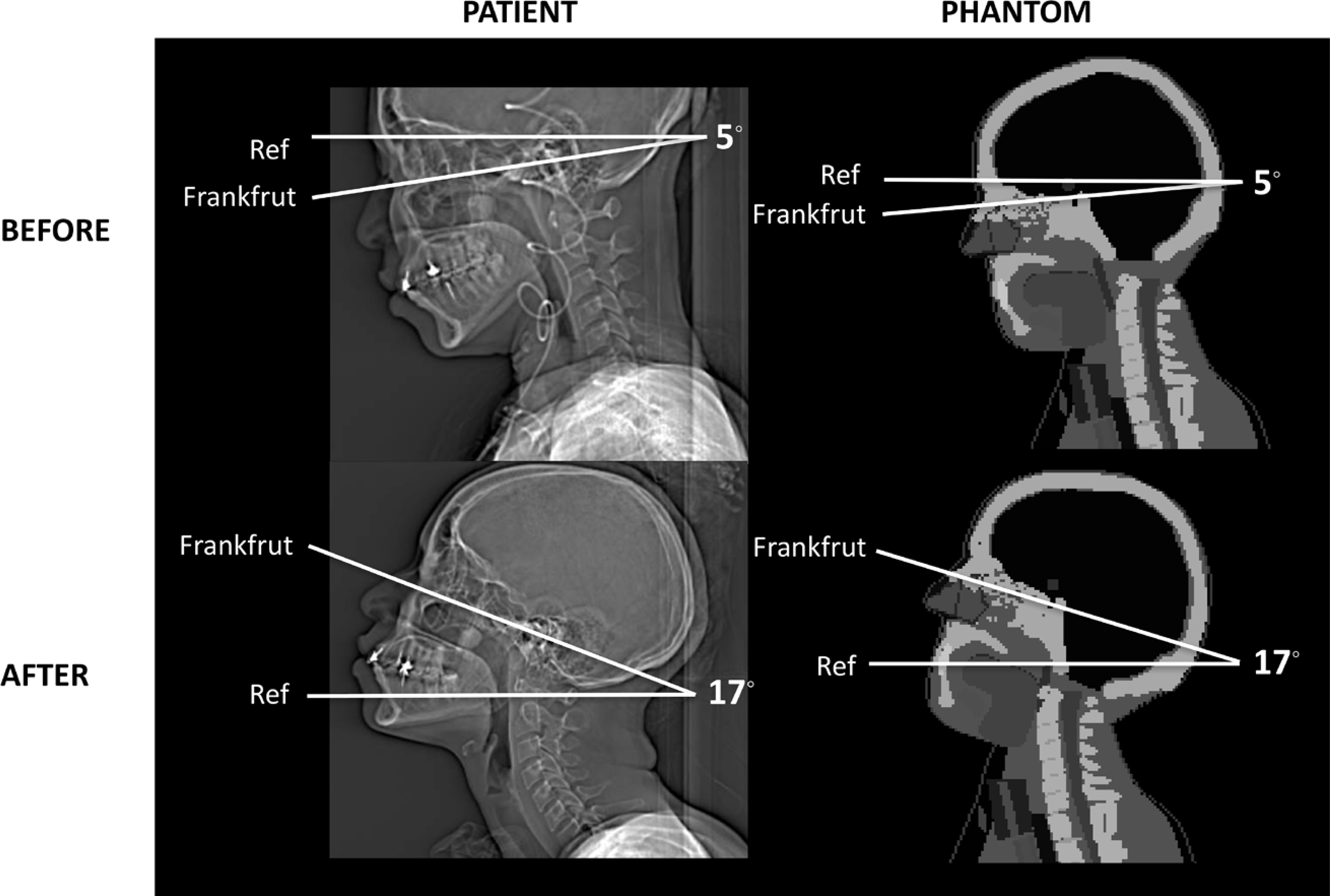
Measurement of the head tilt angle of the patients before (top row) and after (bottom row) the procedure change from topograms and the modification of the head tilt angle of the computational phantoms to match the measurements of the patient head tilt (right column).
Calculation of Organ Dose
We adopted organ dose calculation algorithm we previously published, where organ dose is derived from multiplying dose coefficients (organ dose normalized to reference CTDIvol) by scan-specific CTDIvol (21). The dose coefficients were calculated using an in-house CT dose calculator, National Cancer Institute dosimetry system for CT (NCICT)1 (21), for both before and after scans. NCICT is based on a comprehensive library of organ dose coefficients (mGy/mGy), organ dose (mGy) normalized to the CTDIvol (mGy) of the reference CT scanner used for the scanner simulation, calculated from a series of reference computational human phantoms coupled with the Monte Carlo simulation of a reference CT scanner (21,22). NCICT requires the age and gender of patients, CTDIvol, and scan location mapped on the phantoms for the calculation of dose to 30 organs and tissues.
For the scans before the procedure change, we confirmed that the head tilt angle in the reference adult phantoms built in NCICT is close to −5 degrees, which was the average angle measured from the 40 patients scanned before the procedure change as shown in the top row in Figure 1. To determine scan location on the phantom, we manually measured scan start and end from the patient CT images and mapped the scan locations on the reference adult phantoms, as shown in the top row in Figure 2. Dose coefficients to the lens, brain, pituitary gland, globes, and salivary glands (including parotid, submandibular, and sublingual glands) were estimated using the patient-specific scan locations for the 40 scans conducted before the procedure change. The doses to the parotid, submandibular, and sublingual glands were volume-weighted to obtain mean salivary gland dose.
For the scans after the procedure change, we modified the head tilt angle in the existing adult male and female phantoms for the scans after the procedure change by matching the tilt angle to 17 degrees (23) as shown in the bottom row in Figure 1. To simplify the process, we rotated only the whole head and its substructures without bending the cervical spine of the original computational human phantom in surface format using a 3D modeling software, Rhinoceros™ (McNeel North America, Seattle, WA). A new set of organ dose coefficients for the lens, brain, pituitary gland, globes, and salivary glands were calculated using the modified adult male and female phantoms combined with NCICT. Patient-specific scan locations were mapped onto the modified phantoms with head tilt, as shown in the bottom row in Figure 2, to derive the dose coefficients for the 40 scans conducted after the procedure change.
To evaluate the impact of the reduction of tube potential (eventually reducing CTDIvol) and overranging on organ dose, we calculated the lens dose by gradually adding those two factors. First, we calculated the lens dose without CTDIvol and overranging considered. This is the same quantity with the lens dose coefficients which is the lens dose normalized to the CTDIvol of the reference CT scanner simulated in NCICT. Second, we added overranging to the scan range but still did not account for CTDIvol in the calculations. Third, we took both CTDIvol and overranging into account.
Results
CTDIvol and Overranging
We found CTDIvol decreased by 28% after the procedure change compared to before: from 17.3 mGy (9.6 – 28.6 mGy) to 12.5 mGy (7.7 – 21.9 mGy), which is mainly due to the reduction in tube potential from 120 to 100 kVp (24). The overranging length was 2.5 cm (1.5 – 3.9 cm) and 3.0 cm (2.0 – 3.3 cm) on average in the scans before and after the procedure change, respectively. In the after scans with head tilt, the lens is not included in the planned scan length. However, when half of the overranging length is added to the scan start, the lens is fully or partially included in the scan range for some patients (Figure 2).
In the following sections, we evaluated the impact of CTDIvol change and overranging on the dose reduction. We compared doses before and after in three steps: (1) without both CTDIvol reduction and overranging considered; (2) without CTDIvol reduction but with overranging considered; (3) both CTDIvol reduction and overranging considered.
Dose Reduction without CTDIvol and Overranging Considered
Figure 3A shows the change in the lens dose without CTDIvol and overranging considered. If we did not reduce tube potential in the new procedure and there was no overranging involved, the median lens dose would reduce from 0.82 (0.74 – 0.87) mGy/mGy to 0.06 (0.04 – 0.22) mGy/mGy by 93% on average. In the scans before the new procedure, the lens was fully or partially included in the scan range. In contrast, the lens was completely outside the scan coverage after the procedure change. The use of a head tilt resulted in a lower scan start location: the average scan start changed from 9 cm to 11 cm (the distance from the top of the head) after the procedure change. The images before the procedure change had an average scan length of 25 cm, while after procedure images decreased to 21 cm.
Figure 3.
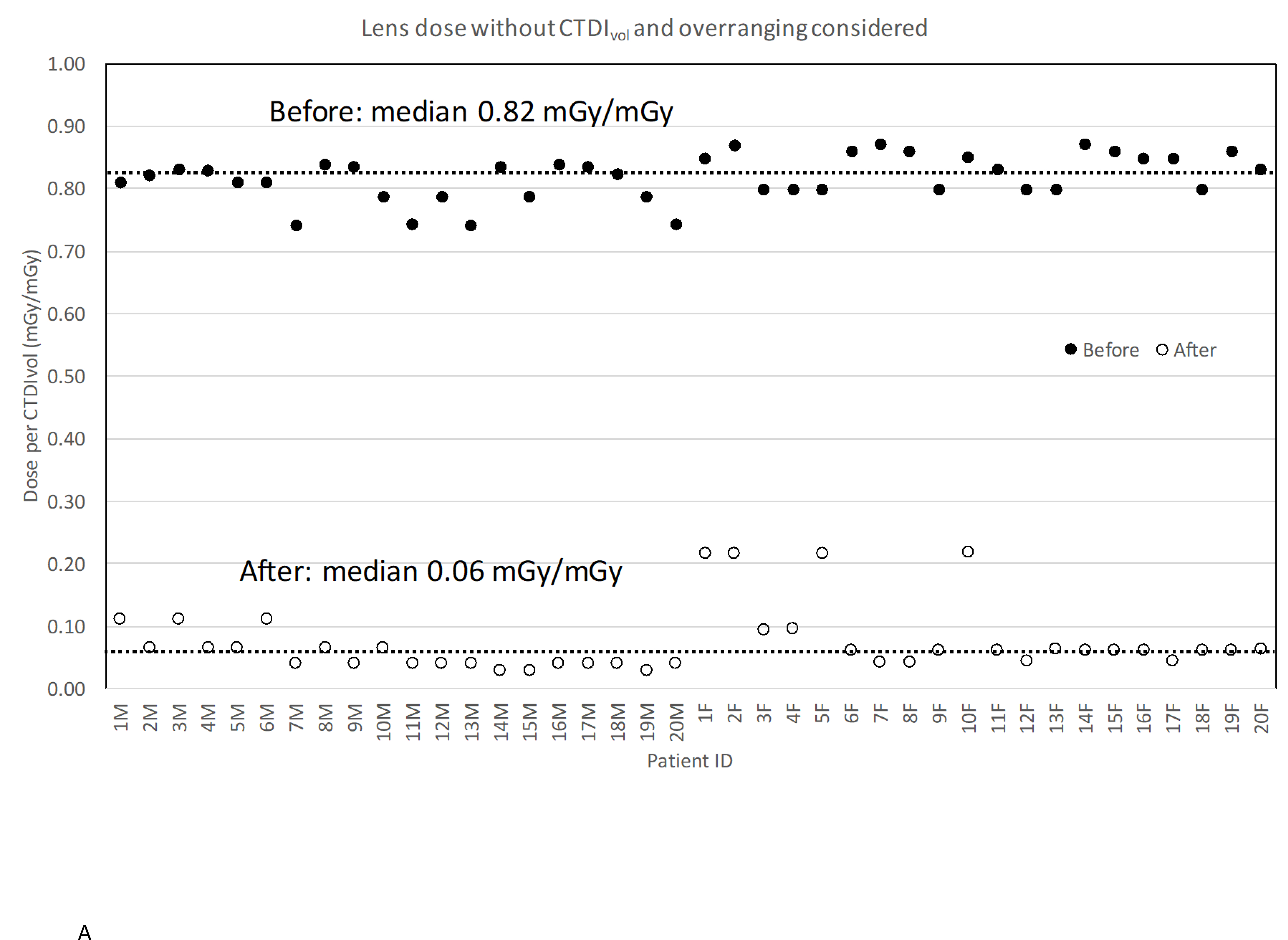
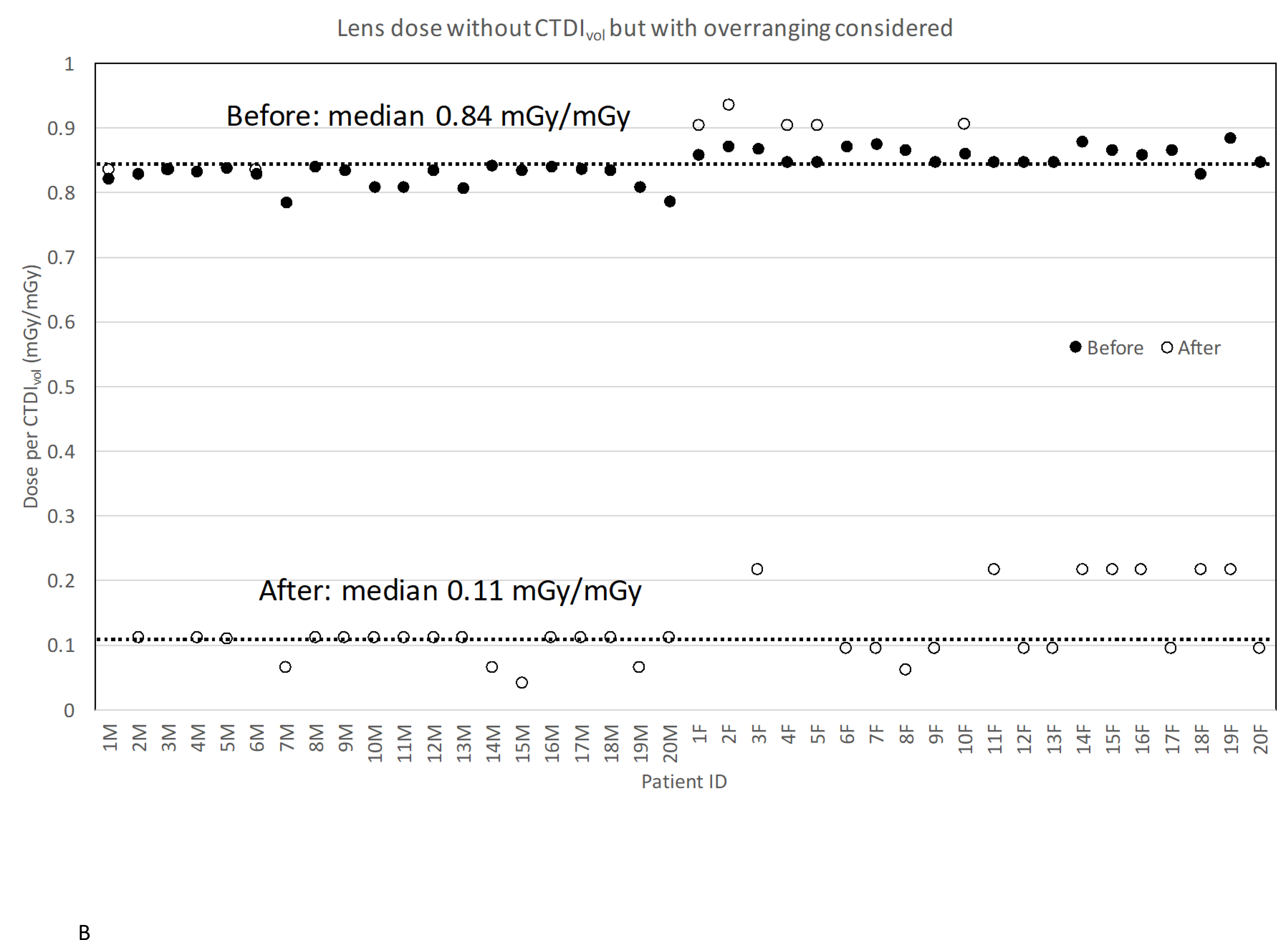
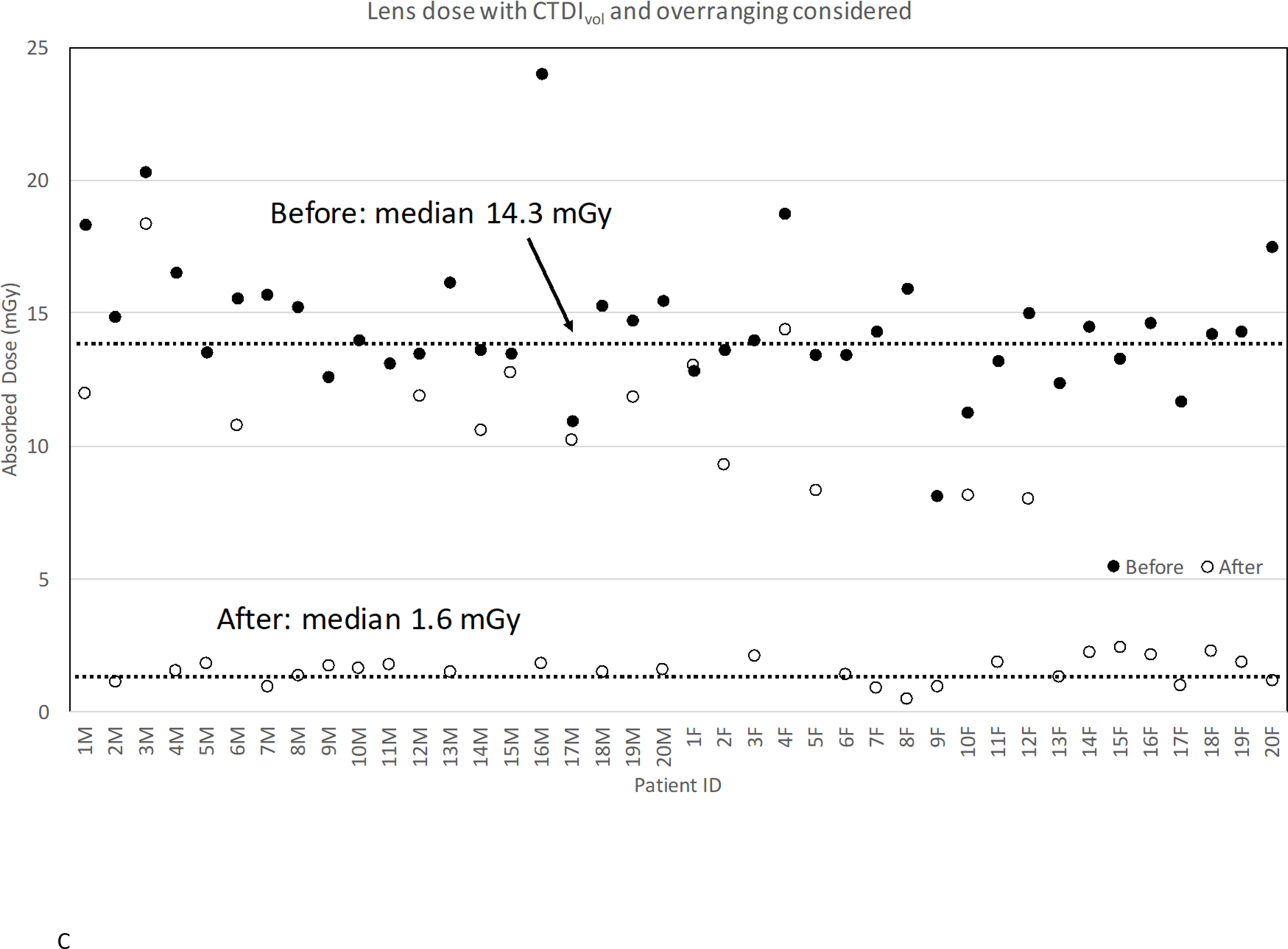
Lens dose reduction for scans before and after the procedure change: (a) without CTDIvol and overranging considered; (b) without CTDIvol but with overranging considered; (c) with CTDIvol and overranging considered.
Dose Reduction without CTDIvol but with Overranging Considered
We added the impact of overranging to the lens dose change but still without CTDIvol considered (Figure 3B). With overranging added, the median lens dose after the procedure change increased from 0.06 mGy/mGy to 0.11 mGy/mGy because the lens now became closer or included to the scan coverage. Even so, there is still about 87% lens dose reduction in the scans after the procedure change compared to the ones before the change. In some female patients, the lens dose after the procedure change was even higher than that before the change. For those patients, the overranging after the procedure change was slightly greater than the value before, which further increased the lens dose.
Dose Reduction with CTDIvol and Overranging Considered
Finally, we took both CTDIvol and overranging into account. Figure 3C shows the change of organ doses with both CTDIvol and overranging considered before and after the procedure change. The median lens dose reduced by 89% (p<0.0001) after the procedure change from 14.3 mGy to 1.6 mGy. The dose reduction was improved from 87% without CTDIvol incorporated because of the reduction in CTDIvol now incorporated. Table 1 shows the dose reduction in other organs. Doses to brain, pituitary gland, globes, and salivary glands show a reduction by 59%, 52%, 66%, and 29%. The doses to the parotid, submandibular, and sublingual glands were volume-weighted to obtain mean salivary gland dose. The head tilt might not significantly reduce radiation dose to the submandibular and sublingual glands, which are always included in the scan coverage both before and after the procedure change.
Table 1.
Comparison of the absorbed dose to major radio-sensitive organs in the head for 40 adult patients before and after the procedure change. CTDIvol and Overranging for each patient was incorporated in the dose calculations.
| Patient ID | Brain |
Pituitary gland |
Globes |
Salivary glands |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mGy) |
% Diff | Dose (mGy) |
% Diff | Dose (mGy) |
% Diff | Dose (mGy) |
% Diff | |||||
| Before | After | Before | After | Before | After | Before | After | |||||
|
| ||||||||||||
| 1M | 9.0 | 4.3 | 52 | 10.8 | 5.2 | 52 | 17.1 | 9.5 | 44 | 20.7 | 12.2 | 41 |
| 2M | 8.5 | 2.5 | 70 | 9.2 | 2.9 | 69 | 13.9 | 3.3 | 76 | 16.5 | 8.6 | 48 |
| 3M | 14.2 | 6.6 | 54 | 13.4 | 8.0 | 40 | 19.1 | 14.6 | 24 | 22.6 | 18.8 | 17 |
| 4M | 10.6 | 3.5 | 67 | 10.6 | 4.0 | 62 | 15.5 | 4.5 | 71 | 18.1 | 11.7 | 35 |
| 5M | 9.5 | 4.1 | 57 | 8.9 | 4.6 | 48 | 12.7 | 5.3 | 59 | 15.1 | 13.7 | 9 |
| 6M | 8.9 | 3.9 | 56 | 9.6 | 4.7 | 51 | 14.5 | 8.5 | 41 | 17.3 | 11.0 | 36 |
| 7M | 5.4 | 3.0 | 45 | 8.0 | 1.8 | 77 | 14.5 | 1.2 | 92 | 18.3 | 12.3 | 33 |
| 8M | 11.3 | 3.0 | 73 | 10.3 | 3.5 | 66 | 14.3 | 3.9 | 73 | 16.9 | 10.3 | 39 |
| 9M | 8.1 | 3.9 | 52 | 8.1 | 4.4 | 45 | 11.8 | 5.0 | 58 | 14.0 | 13.1 | 6 |
| 10M | 5.8 | 3.7 | 36 | 7.7 | 4.2 | 45 | 13.0 | 4.8 | 63 | 16.0 | 12.5 | 22 |
| 11M | 5.4 | 4.0 | 26 | 7.2 | 4.6 | 36 | 12.2 | 5.2 | 57 | 15.0 | 13.7 | 9 |
| 12M | 8.7 | 4.3 | 50 | 8.6 | 5.2 | 40 | 12.6 | 9.4 | 25 | 15.1 | 12.2 | 19 |
| 13M | 6.7 | 3.4 | 49 | 8.9 | 3.9 | 56 | 15.0 | 4.4 | 71 | 18.3 | 11.5 | 37 |
| 14M | 10.6 | 3.8 | 64 | 9.3 | 4.6 | 51 | 12.8 | 8.4 | 34 | 15.1 | 10.9 | 28 |
| 15M | 8.7 | 4.6 | 47 | 8.6 | 5.6 | 36 | 12.6 | 10.1 | 20 | 15.1 | 13.1 | 13 |
| 16M | 17.9 | 4.1 | 77 | 16.2 | 4.7 | 71 | 22.6 | 5.3 | 76 | 26.6 | 14.0 | 48 |
| 17M | 7.7 | 3.7 | 52 | 7.2 | 4.5 | 38 | 10.3 | 8.1 | 21 | 12.1 | 10.5 | 14 |
| 18M | 9.8 | 3.4 | 65 | 9.8 | 3.9 | 60 | 14.3 | 4.4 | 69 | 16.9 | 11.5 | 32 |
| 19M | 6.1 | 4.3 | 30 | 8.1 | 5.2 | 37 | 13.7 | 9.4 | 31 | 16.8 | 12.2 | 28 |
| 20M | 5.3 | 3.6 | 33 | 7.8 | 4.1 | 48 | 14.3 | 4.6 | 68 | 18.1 | 12.1 | 33 |
|
| ||||||||||||
| 1F | 7.5 | 5.5 | 26 | 9.3 | 7.4 | 20 | 12.8 | 11.5 | 10 | 13.9 | 12.5 | 10 |
| 2F | 10.2 | 4.4 | 57 | 10.5 | 5.5 | 47 | 13.7 | 8.5 | 38 | 14.7 | 8.7 | 41 |
| 3F | 9.4 | 3.1 | 67 | 10.6 | 4.5 | 57 | 14.0 | 4.4 | 69 | 15.2 | 8.4 | 45 |
| 4F | 9.2 | 6.1 | 34 | 13.1 | 8.2 | 37 | 18.5 | 12.7 | 32 | 20.5 | 13.9 | 32 |
| 5F | 6.6 | 3.5 | 47 | 9.4 | 4.8 | 49 | 13.3 | 7.3 | 45 | 14.8 | 8.0 | 46 |
| 6F | 10.1 | 3.8 | 62 | 10.3 | 4.0 | 62 | 13.5 | 1.9 | 86 | 14.5 | 12.5 | 14 |
| 7F | 11.6 | 2.5 | 78 | 11.2 | 2.6 | 77 | 14.4 | 1.3 | 91 | 15.4 | 8.3 | 46 |
| 8F | 10.7 | 1.6 | 85 | 12.0 | 1.2 | 90 | 15.9 | 0.6 | 96 | 17.2 | 6.6 | 62 |
| 9F | 4.0 | 2.5 | 36 | 5.7 | 2.6 | 53 | 8.0 | 1.3 | 84 | 8.9 | 8.3 | 6 |
| 10F | 6.6 | 3.5 | 47 | 8.2 | 4.7 | 43 | 11.2 | 7.2 | 36 | 12.3 | 7.9 | 36 |
| 11F | 6.5 | 2.8 | 57 | 9.2 | 4.0 | 57 | 13.1 | 3.9 | 70 | 14.5 | 7.5 | 48 |
| 12F | 7.4 | 3.4 | 54 | 10.5 | 4.6 | 56 | 14.9 | 7.0 | 53 | 16.5 | 7.7 | 53 |
| 13F | 6.1 | 3.6 | 40 | 8.6 | 3.8 | 57 | 12.2 | 1.8 | 85 | 13.6 | 11.9 | 13 |
| 14F | 12.4 | 3.3 | 73 | 11.5 | 4.8 | 58 | 14.6 | 4.7 | 68 | 15.5 | 9.0 | 42 |
| 15F | 8.9 | 3.6 | 60 | 10.0 | 5.2 | 48 | 13.3 | 5.0 | 62 | 14.4 | 9.7 | 33 |
| 16F | 8.6 | 3.2 | 63 | 10.7 | 4.6 | 57 | 14.6 | 4.5 | 69 | 16.0 | 8.6 | 46 |
| 17F | 7.9 | 2.7 | 66 | 8.8 | 2.8 | 69 | 11.7 | 1.3 | 88 | 12.7 | 8.8 | 31 |
| 18F | 5.8 | 3.4 | 41 | 9.4 | 4.9 | 48 | 14.0 | 4.8 | 66 | 15.9 | 9.1 | 42 |
| 19F | 12.8 | 2.8 | 78 | 11.5 | 4.0 | 65 | 14.4 | 3.9 | 73 | 15.4 | 7.5 | 52 |
| 20F | 8.6 | 3.2 | 63 | 12.3 | 3.3 | 73 | 17.4 | 1.6 | 91 | 19.3 | 10.5 | 46 |
|
| ||||||||||||
| Median | 8.7 | 3.5 | 59 | 9.4 | 4.5 | 52 | 13.9 | 4.8 | 66 | 15.5 | 10.9 | 29 |
Discussion
We objectively verified that a simple modification of scan technique combining head tilt, reduced tube potential, and decreased scan range resulted in a significant lens dose reduction. The organ doses including the lens dose were very sensitive to the scan start location. We found that it is difficult to completely avoid the exposure of the lens without accurate quantification of overranging prior to scans. Some patients did not benefit from the head tilt method although all patients benefitted from the reduction in tube potential.
The median lens dose of 14.3 mGy per scan before the procedure change is still much lower than the threshold for cataract, 500 mGy, which cannot be reached until about 35 repeated neck CT scans. The new scan procedure made the neck scanning much safer by reducing the lens dose per scan from 14.3 to 1.6 mGy. Positioning the neck in extension will limit a patient’s risk for cataracts, while still allowing for the inclusion of critical neck structures in the CT image.
The authors are aware of the following limitations. First, we used surrogate patient anatomy, computational human phantoms, in lieu of the patient-specific CT images. Dosimetric uncertainty may come from anatomical differences between phantoms and patients and mapping scan range on phantoms. However, the same surrogate method was used consistently for all CT scans so that the dose uncertainty would be uniform across all scans both before and after the procedure change. Second, the dose reduction could vary when different scanner models (different built-in dose reduction techniques) and scan protocols are used for the scans before and after the procedure change. Finally, our dose reduction method is not applicable to patients who cannot tilt their neck due to neck pain or cervical spine surgery.
Conclusion
We confirmed significant radiation absorbed dose reduction to the lens and other radiosensitive organs in head and neck regions before and after simple modification of patient positioning and scan range. We found that the lens dose was reduced by 89% (p<0.0001) after the procedure change and observed significant dose reduction for other radiosensitive organs in the head up to 66% (e.g., globes). The head tilt procedure is an easy and effective method to significantly reduce radiation exposure to the lens and other radiosensitive organs, without the need for sophisticated technical solutions. The proposed dose reduction method can be tested for other studies, where much higher dose to the lens and other radiosensitive organs is involved (e.g., neuro-perfusion studies) in the future.
Acknowledgement
This research was funded by the intramural research program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The authors thank Dr. Jianhua Yao at the NIH Clinical Center for extracting anonymized patient CT images for analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
NCICT is available from http://ncidose.nci.nih.gov
References
- 1.ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103, Ann ICRP. 1st ed. Oxford; Pergamon Press: International Commission on Radiological Protection; 2007;37(2–4):1–332. [DOI] [PubMed] [Google Scholar]
- 2.ICRP. Nonstochastic Effects of Ionizing Radiation. ICRP Publication 41, Ann ICRP. 1984;14(3). [PubMed] [Google Scholar]
- 3.ICRP I. ICRP Publication 60: 1990 Recommendations of the International Commission on Radiological Protection. ICRP publication 60, Ann ICRP. 1991. [PubMed] [Google Scholar]
- 4.ICRP. ICRP Statement on Tissue Reactions / Early and Late Effects of Radiation in Normal Tissues and Organs – Threshold Doses for Tissue Reactions in a Radiation Protection Context. ICRP Publication 118, Ann ICRP. 2012;41. [DOI] [PubMed] [Google Scholar]
- 5.Mukundan S Jr., Yoshizumi T, Kloeblen E. MOSFET Dosimetry for Radiation Dose Assessment of Bismuth Shielding of the Eye in Children. American Journal of Roentgenology. 2007. June;188(6):1648–50. [DOI] [PubMed] [Google Scholar]
- 6.Hopper KD, Neuman JD, King SH, Kunselman AR. Radioprotection to the eye during CT scanning. American Journal of Ophthalmology. 2001. November;132(5):808. [PMC free article] [PubMed] [Google Scholar]
- 7.Hopper KD. Orbital, thyroid, and breast superficial radiation shielding for patients undergoing diagnostic CT. Seminars in Ultrasound, CT and MRI. 2002. October;23(5):423–7. [DOI] [PubMed] [Google Scholar]
- 8.Meloni T, Mistretta L, Tofani S. Dose reduction in multislice CT by means of bismuth shields: results of in vivo measurements and computed evaluation. Radiol med. 2009. December 14;115(1):152–69. [DOI] [PubMed] [Google Scholar]
- 9.Mendes M, Costa F, Figueira C, Teles P. Assessment of patient dose reduction by bismuth shielding in CT using measurements, GEANT4 and MCNPX simulations. Radiation Protection Dosimetry. 2015. July 13;165(1–4):175–81. [DOI] [PubMed] [Google Scholar]
- 10.Nikupaavo U, Kaasalainen T, Reijonen V, Ahonen S-M, Kortesniemi M. Lens Dose in Routine Head CT: Comparison of Different Optimization Methods With Anthropomorphic Phantoms. American Journal of Roentgenology. 2015. January;204(1):117–23. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Duan X, Christner JA, Leng S, Grant KL, McCollough CH. Bismuth Shielding, Organ-based Tube Current Modulation, and Global Reduction of Tube Current for Dose Reduction to the Eye at Head CT. Radiology. Radiological Society of North America, Inc; 2012. January;262(1):191–8. [DOI] [PubMed] [Google Scholar]
- 12.Heaney DE, Norvill CAJ. A Comparison of reduction in CT dose through the use of gantry angulations or bismuth shields. Australas Phys Eng Sci Med. Springer Netherlands; 2006. June;29(2):172–8. [DOI] [PubMed] [Google Scholar]
- 13.Uiso CBS, Msaki P, Kazema R. Use of lead shields for radiation protection of superficial organs in patients undergoing head CT examinations. Radiation Protection Dosimetry. 2008. March 28;130(4):490–8. [DOI] [PubMed] [Google Scholar]
- 14.Yeoman LJ, Howarth L, Britten A, Cotterill A, Adam EJ. Gantry angulation in brain CT: dosage implications, effect on posterior fossa artifacts, and current international practice. Radiology. 1992. July;184(1):113–6. [DOI] [PubMed] [Google Scholar]
- 15.Kvasnicka T, Klzo L, Grepl J. Reduction of effective dose and organ dose to the eye lens in head mdct using iterative image reconstruction and automatic tube current modulation. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013. October 3;:1–8. [DOI] [PubMed] [Google Scholar]
- 16.Kilic K, Erbas G, Guryildirim M, Arac M, Ilgit E, Coskun B. Lowering the Dose in Head CT Using Adaptive Statistical Iterative Reconstruction. American Journal of Neuroradiology. 2011. October 7;32(9):1578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korn A, Fenchel M, Bender B, Danz S, Hauser TK, Ketelsen D, et al. Iterative Reconstruction in Head CT: Image Quality of Routine and Low-Dose Protocols in Comparison with Standard Filtered Back-Projection. American Journal of Neuroradiology. 2012. February 13;33(2):218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korn A, Bender B, Fenchel M, Spira D, Schabel C, Thomas C, et al. Sinogram affirmed iterative reconstruction in head CT: Improvement of objective and subjective image quality with concomitant radiation dose reduction. European Journal of Radiology. Elsevier Ireland Ltd; 2013. September 1;82(9):1431–5. [DOI] [PubMed] [Google Scholar]
- 19.W H. Overranging at Multi- section CT : An Underes- timated Source of Excess Radiation Exposure 1. Radiographics. 2010;30:1057–67. [DOI] [PubMed] [Google Scholar]
- 20.Schilham A, van der Molen AJ, Prokop M, de Jong HW. Overranging at Multisection CT: An Underestimated Source of Excess Radiation Exposure. Radiographics. Radiological Society of North America; 2010. July;30(4):1057–67. [DOI] [PubMed] [Google Scholar]
- 21.Lee C, Kim KP, Bolch WE, Moroz BE, Folio Les. NCICT: a computational solution to estimate organ doses for pediatric and adult patients undergoing CT scans. Journal of Radiological Protection. IOP Publishing; 2015. November 25;35(4):891–909. [DOI] [PubMed] [Google Scholar]
- 22.Lee C, Kim KP, Long D, Fisher R, Tien C, Simon SL, et al. Organ doses for reference adult male and female undergoing computed tomography estimated by Monte Carlo simulations. Medical Physics. 2011;38(3):1196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee C, Lodwick D, Hurtado J, Pafundi D, Williams JL, Bolch WE. The UF family of reference hybrid phantoms for computational radiation dosimetry. Physics in Medicine and Biology. IOP Publishing; 2010;55(2):339–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNitt-Gray MF. AAPM/RSNA physics tutorial for residents: topics in CT . Radiographics. Radiological Society of North America; 2002;22(6):1541. [DOI] [PubMed] [Google Scholar]


