Abstract
Background
Intravenous cannulation is a painful procedure that can provoke anxiety and stress. Injecting local anaesthetic can provide analgesia at the time of cannulation, but it is a painful procedure. Topical anaesthetic creams take between 30 and 90 minutes to produce an effect. A quicker acting analgesic allows more timely investigation and treatment. Vapocoolants have been used in this setting, but studies have reported mixed results.
Objectives
To determine effects of vapocoolants on pain associated with intravenous cannulation in adults and children. To explore variables that might affect the performance of vapocoolants, including time required for application, distance from the skin when applied and time to cannulation. To look at adverse effects associated with the use of vapocoolants.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Latin American Caribbean Health Sciences Literature (LILACS), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Institute for Scientific Information (ISI) Web of Science and the http://clinicaltrials.gov/, http://www.controlled‐trials.com/ and http://www.trialscentral.org/ databases to 1 May 2015. We applied no language restrictions. We also scanned the reference lists of included papers.
Selection criteria
We included all blinded and unblinded randomized controlled trials (RTCs) comparing any vapocoolant with placebo or control to reduce pain during intravenous cannulation in adults and children.
Data collection and analysis
Three review authors independently assessed trial quality and extracted data, contacted study authors for additional information and assessed included studies for risk of bias. We collected and analysed data for the primary outcome of pain during cannulation, and for the secondary outcomes of pain associated with application of the vapocoolant, first attempt success rate of intravenous cannulation, adverse events and participant satisfaction. We performed subgroup analyses for the primary outcome to examine differences based on age of participant, type of vapocoolant used, application time of vapocoolant and clinical situation (emergency vs elective). We used random‐effects model meta‐analysis in RevMan 5.3 and assessed heterogeneity between trial results by examining forest plots and calculating the I2 statistic.
Main results
We found nine suitable studies of 1070 participants and included them in the qualitative analyses. We included eight studies of 848 participants in the meta‐analysis for the primary outcome (pain during intravenous cannulation). Use of vapocoolants resulted in a reduction in pain scores as measured by a linear 100 mm visual analogue scale (VAS 100) compared with controls (difference between means ‐12.5 mm, 95% confidence interval (CI) ‐18.7 to ‐6.4 mm; moderate‐quality evidence). We could not include in the meta‐analysis one study, which showed no effects of the intervention.
Use of vapocoolants resulted in increased pain scores at the time of application as measured by a VAS 100 compared with controls (difference between means 6.3 mm, 95% CI 2.2 to 10.3 mm; four studies, 461 participants; high‐quality evidence) and led to no difference in first attempt success compared with controls (risk ratio (RR) 1.00, 95% CI 0.94 to 1.06; six studies, 812 participants; moderate‐quality evidence). We documented eight minor adverse events reported in 279 vapocoolant participants (risk difference (RD) 0.03, 95% CI 0 to 0.05; five studies, 551 participants; low quality‐evidence).
The overall risk of bias of individual studies ranged from low to high, with high risk of bias for performance and detection bias in four studies. Sensitivity analysis showed that exclusion of studies at high or unclear risk of bias did not materially alter the results of this review.
Authors' conclusions
Moderate‐quality evidence indicates that use of a vapocoolant immediately before intravenous cannulation reduces pain during the procedure. Use of vapocoolant does not increase the difficulty of cannulation nor cause serious adverse effects but is associated with mild discomfort during application.
Plain language summary
Vapocoolants (cold spray) for pain treatment during intravenous cannulation
Background
Intravenous cannulation for blood tests or treatment is a common, often painful, procedure. Vapocoolant sprays or "cold sprays" are delivered onto the skin just before needle insertion to provide some pain relief. Vapocoolants offer several advantages over other pain relief techniques, particularly their rapid effects (a few seconds).
We reviewed the evidence showing how effective vapocoolants are in reducing the pain associated with inserting an intravenous cannula. The evidence is current to May 2015.
Results
We identified nine studies of 1070 participants that compared use of vapocoolants with use of placebo spray, or no spray, in children and adults undergoing intravenous cannulation in any healthcare setting. Investigators in three studies received funding from a source not reported to be involved in the study design and analysis. Vapocoolant manufacturers provided vapocoolant and placebo sprays for two studies, and were not involved in study design nor in analysis of results.
We found that vapocoolants are likely to reduce pain during intravenous cannulation and are not likely to make cannulation more difficult nor cause serious adverse events. We noted that application of vapocoolants caused some discomfort, but that using the spray resulted in reduced pain. Using a pain score range from 0 to 100 mm (0 = no pain and 100 = worst possible pain), we found that average pain scores were reduced by 12.5 mm in participants receiving vapocoolant spray.
Quality of the evidence
Overall, the quality of the evidence was moderate rather than high. However, excluding studies of poorer quality did not materially alter the results of the review.
Summary of findings
Summary of findings for the main comparison. Vapocoolant compared with placebo/no treatment for pain treatment during intravenous cannulation.
| Vapocoolant compared with placebo/no treatment for pain treatment during intravenous cannulation | ||||||
| Patient or population: pain treatment during intravenous cannulation Settings: metropolitan hospitals in UK; emergency departments in children’s hospitals in the USA; US school of dentistry; tertiary children's hospital in Canada; Army Medical Centres in the USA; metropolitan teaching hospital in Australia; emergency department of a tertiary hospital in New Zealand Intervention: vapocoolant Comparison: placebo/no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/No treatment | Vapocoolant | |||||
| Pain during cannulation | Mean pain during cannulation in the control group was 33 | Mean pain during cannulation in the intervention group was 12.5 lower (18.7 lower to 6.4 lower) | ‐ | 848 (8 RCTs) | ⊕⊕⊕⊝ Moderatea,b,c | |
| Pain at application | Mean pain at application in the control group was 0 | Mean pain at application in the intervention group was 6.3 higher (2.2 higher to 10.3 higher) | ‐ | 461 (4 RCTs) | ⊕⊕⊕⊕ Moderatec | |
| First attempt success | Study population | RR 1.00 (0.94 to 1.06) | 812 (6 RCTs) | ⊕⊕⊕⊝ Moderateb | ||
| 832 per 1000 | 832 per 1000 (782 to 882) | |||||
| Moderate | ||||||
| 853 per 1000 | 853 per 1000 (802 to 904) | |||||
| Adverse events | Study population | Not estimable | 551 (5 RCTs) | ⊕⊕⊝⊝ Lowd | ||
| Mean risk of adverse events in the control group was 0 | All minor and included 4 reports of cold sensation, 3 transient reactions of erythema at the site of spray and 1 report of burning sensation. RD = 0.03, 95% CI 0.0 to 0.05) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aFour of eight trials described no blinding of participants, but results were consistent with those of blinded studies, and so we did not downgrade for risk of bias
bHigh degree of heterogeneity was not explained by methodological and clinical differences but could be due to the small number of studies, with five studies showing good effects and 3 showing lesser or no effects of vapocoolants. Therefore, we downgraded, giving serious risk of inconsistency
cMeasurements were taken on a VAS 100 scale; although the CI does include the minimally clinically important difference, it also spans the range below the minimally important clinical difference. We downgraded on the basis of small numbers in each trial, which might overestimate treatment effects.
dTrials were not pooled, as adverse effects were reported differently across studies; so again we downgraded to serious for imprecision and for inconsistency for this outcome.
Background
Intravenous cannulation is one of the most commonly performed painful medical procedures (Kennedy 1999). Needle‐related procedures induce anxiety, fear and distress in both children and adults (Lander 2006; Uman 2006). Although psychological methods used to reduce the pain of needle procedures can be effective (Uman 2006), anaesthetic agents play an important role. These agents are used increasingly to reduce the pain of intravenous cannulation in children, but less so in adults (Lander 2006).
The ideal anaesthetic agent for intravenous cannulation would be effective, quick, pain‐free and cheap, and would cause no side effects. Injected local and topical anaesthetics are most commonly used for anaesthesia (Zempsky 2008a). However, neither is ideal, as injected local anaesthetic requires use of another needle, albeit smaller, and is effective only for insertion of larger cannulae (Zempsky 2008a). Topical anaesthetic cream, although effective for smaller cannulae, requires 30 to 90 minutes for application (Lander 2006). Investigators have explored adjuvant delivery methods such as heat, iontophoresis (Zempsky 2008a) and ultrasound (Skarbek‐Borowska 2006), but these methods are not commonly used. Newer anaesthetic delivery methods such as use of a pressured aerosolized spray may be quick and effective but remain costly (Zempsky 2008a; Zempsky 2008b).
Ethyl chloride and other vapocoolants are an attractive analgesic alternative for intravenous cannula insertion in emergency situations for which rapid analgesia is required. These sprays are delivered to the area of desired intravenous cannula application seconds before the intravenous cannula is inserted. Vapocoolants are both quick and inexpensive (Zempsky 2008a) and are thought to reduce discomfort at the intravenous cannulation site via rapid cooling of surrounding skin. Rapid cooling decreases both initiation and conduction impulses in surrounding sensory nerves, thus providing a mechanism for reducing the discomfort associated with cannulation (Burke 1999).
However, although ethyl chloride and other vapocoolants remain attractive for intravenous insertion, their clinical effectiveness remains uncertain. Individual clinical trials have reported mixed results (Moore 2009), possibly as the result of inadequate sample sizes; issues associated with analysis of pain outcomes (use of continuous data and different pain scales); and inconsistency in vapocoolant use (e.g. length of application, distance from the skin for spraying). Thus it is timely to undertake a review of the efficacy of vapocoolants for pain treatment during intravenous cannulation.
Objectives
To determine effects of vapocoolants on pain associated with intravenous cannulation in adults and children. To explore variables that might affect the performance of vapocoolants, including time required for application, distance from the skin when applied and time to cannulation. To look at adverse effects associated with the use of vapocoolants.
Methods
Criteria for considering studies for this review
Types of studies
We included in this review all randomized controlled trials (RCTs) comparing a vapocoolant with placebo or no treatment for analgesia associated with intravenous cannulation. Blinding the intervention was accepted as difficult; therefore, we included unblinded trials.
We excluded quasi‐randomized controlled trials, as these do not allow true randomization and can result in inadequate allocation concealment.
Types of participants
We included adults and children undergoing intravenous cannulation. We applied no restrictions based on sex, ethnicity, disease, diagnosis, cannulation site or study setting. We included studies that enrolled healthy volunteers.
Types of interventions
Any vapocoolant used for intravenous cannulation compared with placebo or no treatment.
Types of outcome measures
Primary outcomes
Pain during intravenous cannulation.
Secondary outcomes
Pain immediately after intravenous cannulation.
Pain at time of application of vapocoolant.
First attempt success rate of intravenous cannulation.
Adverse events (as reported by study authors, divided into minor and major).
Participant (or caregiver) satisfaction (as reported by study authors).
Search methods for identification of studies
Electronic searches
We searched the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 5; see Appendix 1), MEDLINE via Ovid SP (1966 to May 2015; see Appendix 2), EMBASE via Ovid SP (1988 to May 2015; see Appendix 3), Latin American Caribbean Health Sciences Literature (LILACS) via BIREME interface (1982 to May 2015; see Appendix 4), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO host (1982 to May 2015; see Appendix 5) and the Institute for Scientific Information (ISI) Web of Science (1900 to May 2015; see Appendix 6).
In addition, we searched the databases of ongoing trials including:
Searching other resources
We handsearched abstracts of the American Society of Anesthesiologists and reference lists of all retrieved articles (to January 2014).
We applied no language or date restrictions to our searches.
Data collection and analysis
Selection of studies
Three review authors (RG, SD, VJ) independently scanned two‐thirds of the titles and abstracts of studies identified by the search to ensure that all titles and abstracts were reviewed independently by at least two review authors. We retrieved full‐text versions of potentially relevant studies and independently assessed them for eligibility and methodological quality for possible inclusion in the review. We were not blinded with respect to the journal from which the article came, the names of study authors and institutions and the magnitude and direction of the results, because such blinding has not been shown to have a significant impact on the results of systematic reviews (Berlin 1997). We resolved discrepancies by consensus in all cases, so no adjudication was required. We recorded the selection process in sufficient detail to complete a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram (Moher 2009) and Characteristics of excluded studies tables. We imposed no language restrictions..
Data extraction and management
Three review authors (RG, SD, VJ) independently extracted data using a standardized data extraction form (Appendix 7), providing checks for discrepancies and processing as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We reported data, when possible, using intention‐to‐treat (ITT) analysis. We resolved discrepancies by consensus in all cases, so no adjudication was required.
Assessment of risk of bias in included studies
We (RG, SD, VJ) independently assessed the risk of bias of included studies. We generated a risk of bias table for each study (Higgins 2011) as part of the Characteristics of included studies table, along with a risk of bias summary figure that details all judgements made for all studies included in the review.
We graded each study for risk of bias in six domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessor, incomplete outcome data and selective reporting. For each study, we assessed these domains as having low risk of bias, high risk of bias or unclear risk of bias' if details of what happened in the study were insufficient in the report. We resolved discrepancies between review authors by consensus.
Measures of treatment effect
Primary outcome: pain during intravenous cannulation
Researchers often do not report pain in a uniform manner. Eight studies reported pain on a continuous scale (linear 100 mm visual analogue scale (VAS 100)), Ramsook 2001 used only a categorical scale and Farion 2008 and Hijazi 2009 utilized continuous and categorical scales when reporting pain. In this review, we analysed separately the meta‐analysis of continuous and categorical pain outcomes. In this clinical situation, continuous outcomes might offer advantages over dichotomized outcomes. Although dichotomizing pain outcomes can make the outcome more clinically useful and easier to understand (Higgins 2011), this dichotomization occurs at the expense of some loss of power and information (Altman 2000). In addition, this clinical situation differs from usual pain situations in that patients about to undergo insertion of an intravenous cannula usually are not in pain before the start of the procedure. Furthermore, they may experience only minimal pain during the procedure; therefore, it may be difficult to show significant pain relief with a dichotomized result.
VAS 100 is the standard method of measuring continuous pain. Although VAS 100 scores are expected to be skewed, and this may introduce bias when mean data are combined by parametric methods (Altman 2000), no suitable meta‐analysis tool that utilizes median scores is currently available. Initially, we checked the data for skew by calculating a ratio of observed mean minus lowest possible value and dividing by the standard deviation. A ratio less than two suggests an element of skew (Higgins 2011). When we noted a large degree of skew, that is, a ratio less than one, we tried to contact study authors to obtain log‐transformed data in an attempt to reduce the skew (Higgins 2011).
We combined data within the meta‐analysis and reported mean differences. When data were measured using differing scales, we combined them using standardized mean difference (SMD). A statistically significant difference observed when continuous data were compared stresses the importance of the difference between a statistical difference and a clinically important difference.
We summarized categorical data from individual studies as risk ratio (RR) with 95% confidence interval (CI) and combined data this way in the meta‐analysis.
We intended to include both self reported and observed pain scores. We included only self reported pain scores because this was the only measure available for all studies.
We found no cross‐over designed trials, as most research was carried out in an emergency department setting rather than in a setting of regular cannulization.
Secondary outcomes
We analysed pain immediately after intravenous cannulation, as described above.
We analysed pain associated with application of the vapocoolant using continuous data, as described above. We collapsed categorical data into 'little' or 'no' pain of application before performing analysis.
In this review, we summarized first attempt success rate of intravenous cannulation, adverse events and participant satisfaction as RRs (95% CIs) and combined these data in the meta‐analysis.
Unit of analysis issues
Patients can be cannulated multiple times. To avoid unit of analysis issues, we included in the meta‐analysis only trials randomized at an individual participant level along with trials that randomized participants on cannulization and provided effects adjusted for repeated interventions within individual participants.
Dealing with missing data
We contacted study authors to request missing data. We estimated missing summary data (standard deviations) from available data. We performed a sensitivity analysis of the treatment effect that included and excluded estimated data to see whether this altered the outcome of the review. No studies with more than 20% missing primary outcome data required a sensitivity analysis.
Assessment of heterogeneity
We assessed heterogeneity between trial results by examining forest plots and calculating the I2 statistic (Higgins 2003).
Assessment of reporting biases
We planned to assess reporting bias by using funnel plots, but we found insufficient numbers of studies to do this.
Data synthesis
When clinical and methodological heterogeneity was negligible, we attempted meta‐analysis in RevMan 5.3. We presented an overall mean difference (MD) for continuous data and an overall RR for dichotomous data, along with a number needed to treat for an additional beneficial outcome (NNTB), which is calculated from the risk difference (RD). In addition, we presented a narrative summary for studies that could not be included in the meta‐analysis. We weighted the overall summary statistics in accordance with recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When statistical heterogeneity was low, as indicated by an I2 statistic less than 40%, we used a fixed‐effect model meta‐analysis; otherwise we used a random‐effects model meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We analysed the following pre‐specified subgroups.
Age: children (< 18 years (< 5 years, 5 to < 18 years) or as defined by study authors) versus adults.
Observed versus reported pain scales.
Vapocoolant agent used (both types and strengths).
Application time of vapocoolant (< 5 seconds, 5 to 10 seconds, > 10 seconds).
Clinical situation (emergency vs elective care).
Adjuvant analgesics used (e.g. systemic, topical).
Cannula size (large vs small, as defined by study authors).
Source of participant population.
We used the test for subgroup differences available in RevMan 5.3 for the random‐effects model to determine whether results for subgroups were statistically significantly different.
Sensitivity analysis
We undertook sensitivity analyses to explore the robustness of results with regard to biases in included studies and studies for which data had been imputed. Furthermore, we undertook sensitivity analysis of the primary outcome to determine results if we included data derived from trials deemed at high risk of bias and thus excluded from the primary analyses. As the primary outcome of pain is a subjective one, we performed sensitivity analyses to explore the effects of including or not including studies that lacked adequate blinding of participants, investigators and outcome assessors.
'Summary of findings' tables
We used the principles of the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) system (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes (pain during intravenous cannulation, pain at time of application of vapocoolant, first attempt success rate of intravenous cannulation and adverse events) included in our review and to construct a 'Summary of findings' (SoF) table using GRADE software. The GRADE approach appraises the quality of a body of evidence according to the extent to which one can be confident that an estimate of effect or an association reflects the item being assessed. Assessment of the quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
Results of the search
We identified 2110 titles using the search strategies outlined above. After screening by title and abstract, we identified 17 articles for possible inclusion in the review. We obtained each of these articles in full text and examined them for inclusion in the review. Four did not meet inclusion criteria, and one article was an earlier abstract of a study published later in full (Costello 2006). One study had already been presented in conference abstracts and is ongoing; we placed this study in the ongoing studies section (Mace 2014). Thus we determined that nine studies were eligible for inclusion in the review. The flow diagram (Figure 1) shows results of the search and numbers excluded at each stage.
1.
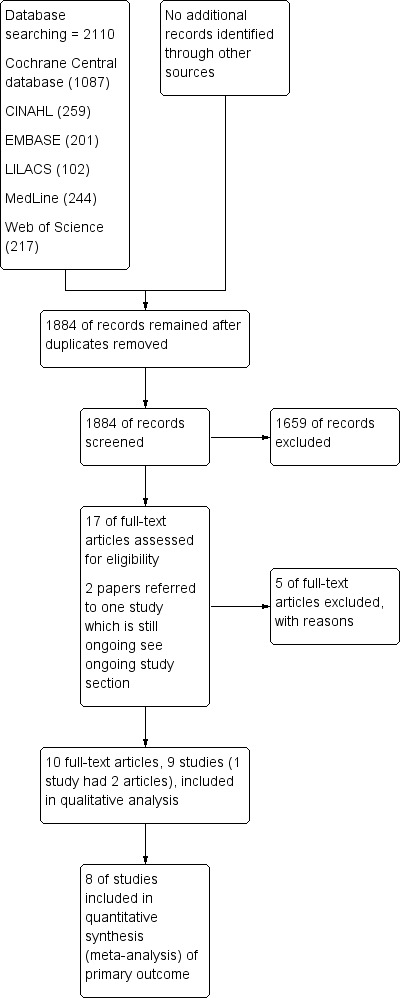
Study flow diagram.
Included studies
We included nine randomized controlled trials in our review (see Characteristics of included studies). We contacted the authors of eight of the studies by email to ask for additional data when missing from the published material. We were not able to find current contact details for the authors of one study (Selby 1995).
Investigators in all included studies reported that they obtained consent from participants (or caregivers for studies in children). Four studies occurred in the USA (Costello 2006; Crecelius 1999; Hartstein 2008; Ramsook 2001), two in the UK (Armstrong 1990; Selby 1995) and one in each of Canada (Farion 2008), Australia (Hijazi 2009) and New Zealand (Robinson 2007). Apart from Hartstein 2008 (conducted in two emergency departments), these were single‐centre studies. Six studies occurred in emergency department settings (Costello 2006; Farion 2008; Hartstein 2008; Hijazi 2009; Ramsook 2001; Robinson 2007), and the other three took place in elective settings where participants were about to undergo procedural sedation or general anaesthesia (Armstrong 1990; Crecelius 1999; Selby 1995).
Six studies recruited adult participants (Armstrong 1990; Crecelius 1999; Hartstein 2008; Hijazi 2009; Robinson 2007; Selby 1995), and three recruited children (Costello 2006; Farion 2008; Ramsook 2001).
Researchers used a range of intravenous cannula sizes within and between studies (range 24 gauge (G) to 16G). Ramsook 2001 did not report cannula size. Three studies used one intravenous cannula size for all participants (Armstrong 1990 20G; Costello 2006 22G; Selby 1995 20G), two used two intravenous cannula sizes (Crecelius 1999 22G and 20G; Farion 2008 24G and 22G), two used three intravenous cannula sizes (Hartstein 2008 22G to18G; Hijazi 2009 22G to 18G) and one used four intravenous cannula sizes (Robinson 2007 22G to 16G). Investigators in three of the five studies that used more than one intravenous cannula size did not report the numbers of participants who received each intravenous cannula size (Crecelius 1999; Hartstein 2008; Robinson 2007).
One study used vapocoolant as an adjuvant to nitrous oxide (Crecelius 1999); all other studies tested the intervention independently of other sedation or analgesics.
Excluded studies
Of 17 possible articles for inclusion, we excluded three, as they were not RCTs (Baelen 1994; Kelly 2008; Soueid 2007), and two, as investigators included no placebo/control group for comparison (Baxter 2009; Lunoe 2015) (see Characteristics of excluded studies).
Risk of bias in included studies
We presented a risk of bias graph and summary in Figure 2 and Figure 3, respectively.
2.
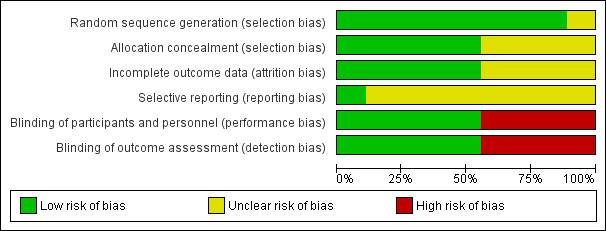
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Method of randomization
All included studies reported that participants were randomized to treatment groups. Eight studies (Armstrong 1990; Costello 2006; Crecelius 1999; Farion 2008; Hartstein 2008; Hijazi 2009, Ramsook 2001; Robinson 2007) specified the method of randomization and were classified as having low risk of bias. Selby 1995 did not specify the method of randomization and was classified as having unclear risk of bias.
Allocation concealment
Five studies (Crecelius 1999; Farion 2008; Hijazi 2009; Ramsook 2001; Robinson 2007) described adequate concealment of allocation and were classified as having low risk of bias. Researchers in the remaining four studies did not describe concealment of allocation (Armstrong 1990; Costello 2006; Selby 1995) or presented uncertain information (Hartstein 2008); we classified these studies as having unclear risk of bias.
Blinding
Blinding of participants and personnel
Four studies did not blind participants nor assessors (Armstrong 1990; Hartstein 2008; Robinson 2007; Selby 1995) and were classified as having high risk of bias; we classified the remaining five studies as having low risk of bias (Costello 2006; Crecelius 1999; Farion 2008; Hijazi 2009; Ramsook 2001).
In Costello 2006, individuals not associated with the study manufactured and labelled canisters containing study drug and placebo spray. Investigators and nursing staff performing intravenous cannulation were blinded to contents of the canisters, which were indistinguishable from one other, except for their label as "canister 1" or "canister 2". Researchers did not report participant detection of the intervention. In Crecelius 1999, the venipuncturist was not present during spray application. Approximately half of participants (51%) and venipuncturists (45%) reported that they were not able to tell whether ethyl chloride or placebo had been applied. Of the 43 (49%) participants reporting which treatment they received, six (7%) incorrectly indicated that they received placebo and three (3%) incorrectly stated that they received ethyl chloride; 34 (39%) reported their intervention correctly. Of the 48 (55%) occasions that venipuncturists reported knowing which treatment participants had received, one (1%) incorrectly reported placebo, five (6%) incorrectly reported ethyl chloride and 42 (48%) correctly reported the intervention used. Farion 2008 used similar masked canisters and indicated that the research assistant sprayed the cannulation site while all others in the room looked away. Researchers did not report participant detection of intervention. Investigators in Hijazi 2009 packed the control spray in a handheld pressurized spray can of about the same size as the intervention spray. They masked intervention and control spray cans in white paper and labelled them A and B. In all, 69% of the control group and 54% of the vapocoolant group correctly guessed which spray had been used. In Ramsook 2001, the manufacturer provided identically matched cans of isopropyl alcohol as placebo. Investigators did not report participant detection of the intervention.
Incomplete outcome data
Three studies (Crecelius 1999; Farion 2008; Ramsook 2001) provided complete data; we classified these studies as having low risk of bias. Costello 2006 excluded data for two (2%) of 129 participants because of protocol violations. Hijazi 2009 reported five (2%) protocol violations (one in the control group and four in the vapocoolant group) and 45 (22%) participants lost to follow‐up (for adverse events) but included in the analysis data for all 201 participants. We classified both studies as having low risk of bias. Armstrong 1990 did not report whether data were missing; we classified this study as having unclear risk of bias. Hartstein 2008 excluded data from analysis for two control and vapocoolant participants (4% of 92 participants) because data were missing or incomplete. However, we noted a discrepancy in participant numbers in the manuscript; the methods section reported that 47 participants were randomized to the vapocoolant group, although Figure 1 reported data from 48 vapocoolant participants; for this reason, we classified this study as having unclear risk of attrition bias. Robinson 2007 excluded four (1%) participants because they underwent more than two cannulation attempts and six (2%) because of incomplete data; we classified this study as having unclear risk of bias. In the Selby 1995 manuscript, study authors reported two different sets of figures for the first attempt success rate. These ranged from 38 to 40 out of 40 participants with first attempt success for the control group, and 36 to 37 out of 40 participants with first attempt success for the ethyl chloride group.
Selective reporting
The data collection sheet was available for Hartstein 2008; study authors reported all available outcomes, and we classified this study as having low risk of bias. Protocols were not available for the remaining eight studies (Armstrong 1990; Costello 2006; Crecelius 1999; Farion 2008; Hijazi 2009; Ramsook 2001; Robinson 2007; Selby 1995); we classified all as having unclear risk of bias, although investigators reported all expected outcomes.
Effects of interventions
See: Table 1
Primary outcome
Pain during intravenous cannulation
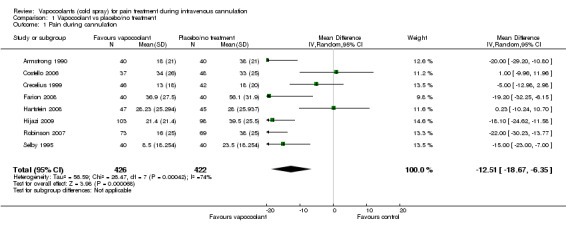
We included in the meta‐analysis eight studies (Armstrong 1990; Costello 2006; Crecelius 1999; Farion 2008; Hartstein 2008; Hijazi 2009; Robinson 2007; Selby 1995) consisting of 848 participants. We used an estimated standard deviation for data from Robinson 2007 on the mean of control groups in six other studies, as this information was not available. We estimated the standard deviation for the Selby 1995 data by using the reported 95% CI of the difference and the control group median. Use of vapocoolants resulted in a reduction in pain scores as measured by a VAS 100 compared with controls (difference between means ‐12.5 mm, 95% CI ‐18.7 to ‐6.4 mm; I2 = 74%; see Figure 4). This result showed high heterogeneity (I2 = 74%), although none of the studies favoured control over vapocoolant, and we determined this evidence to be of moderate quality. Sensitivity analysis excluding studies with unclear or high risk of bias due to lack of blinding or incomplete outcome data (Armstrong 1990; Hartstein 2008; Robinson 2007; Selby 1995; difference between means ‐10.3 mm, 95% CI ‐19.8 to ‐0.8 mm; I2 = 77%) or lack of allocation concealment (Armstrong 1990; Costello 2006; Hartstein 2008; Selby 1995; difference between means ‐15.8 mm, 95% CI ‐23.6 to ‐8.1 mm; I2 = 70%) had no material effect on the results and could not explain the heterogeneity. These results were not affected by excluding Robinson 2007 or Selby 1995 (difference between means ‐10.3 mm, 95% CI ‐18.1 to ‐2.5 mm; I2 = 76%) from the analysis, or by imputing the smallest and largest standard deviation of the other six studies. Subgroup analysis of children versus adults (see Analysis 1.2), ethyl chloride versus other vapocoolants (see Analysis 1.3), application time of spray less than five seconds versus five to 10 seconds (see Analysis 1.4) and elective versus emergency settings (Analysis 1.5) revealed no significant differences between groups and could not explain heterogeneity. Only one study used adjuvant treatment (Crecelius 1999), and one study in children (Farion 2008) reported observed and participant pain scores; again these studies did not alter results and could not explain heterogeneity. Investigators provided insufficient details for a subgroup analysis on the basis of cannula size used.
4.

Forest plot of comparison: 1 Vapocoolant vs placebo/no treatment, outcome: 1.1 Pain during cannulation.
1.2. Analysis.
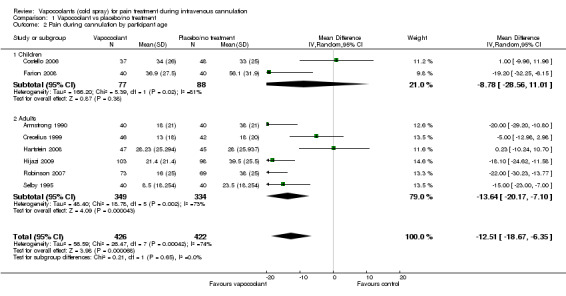
Comparison 1 Vapocoolant vs placebo/no treatment, Outcome 2 Pain during cannulation by participant age.
1.3. Analysis.
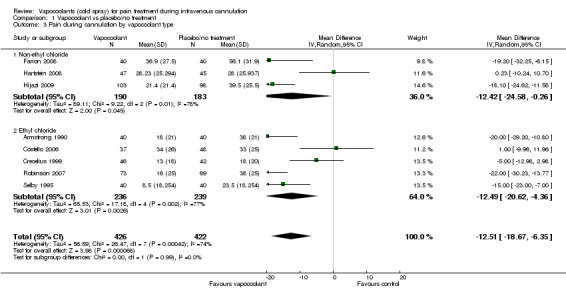
Comparison 1 Vapocoolant vs placebo/no treatment, Outcome 3 Pain during cannulation by vapocoolant type.
1.4. Analysis.
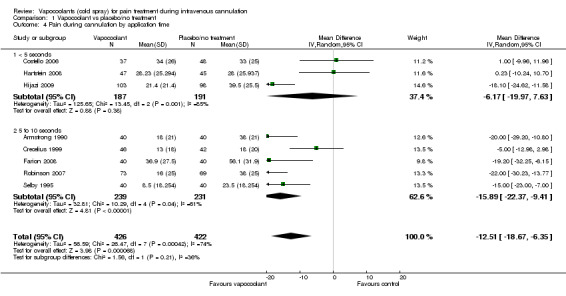
Comparison 1 Vapocoolant vs placebo/no treatment, Outcome 4 Pain during cannulation by application time.
1.5. Analysis.
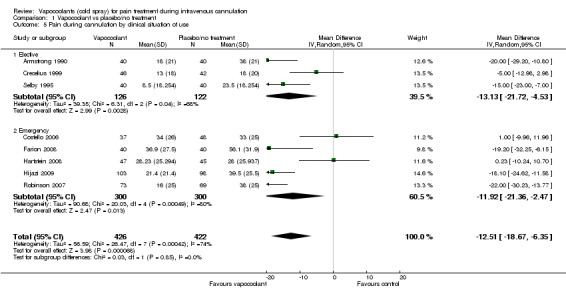
Comparison 1 Vapocoolant vs placebo/no treatment, Outcome 5 Pain during cannulation by clinical situation of use.
As this meta‐analysis included fewer than 10 included studies, we were not able to prepare a funnel plot for assessment of publication bias.
Two of the eight studies provided dichotomized data, allowing meta‐analysis of good pain relief during intravenous cannulation (Farion 2008; Hijazi 2009). Use of vapocoolants resulted in increased pain relief compared with controls (RR 1.67, 95% CI 1.3 to 2.13; I2 = 0%, NNTB = 4; see Analysis 1.6).
1.6. Analysis.
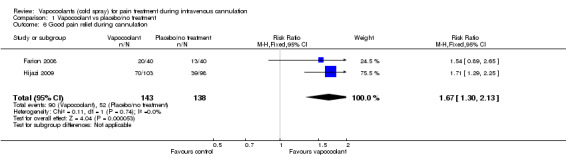
Comparison 1 Vapocoolant vs placebo/no treatment, Outcome 6 Good pain relief during cannulation.
We could not include in the meta‐analysis one study of appropriate quality (Ramsook 2001), as study authors reported only median data, using two different scales (Faces Pain Scale and Numeric Pain Scale) and three different age groups, including one age group in which only combined summary data were given for two different pain scales. Ramsook 2001, in contrast to the above findings, reported no differences in median pain scores between ethyl chloride and isopropyl alcohol for 222 children between three and 18 years of age who were undergoing intravenous cannulation in an emergency department. However, overall meta‐analysis results were not materially affected by imputing all combinations of the mean ‐ smallest and largest, VAS 100 scores and standard deviation data for placebo arms of the eight studies included for the Ramsook 2001 sample.
Secondary outcomes
Pain immediately after intravenous cannulation
Only one study (Armstrong 1990) recorded pain immediately after intravenous cannulation, at one minute. Use of vapocoolants resulted in no reduction in pain scores as measured by a VAS 100 compared with control (difference between means ‐8.0 mm, 95% CI ‐17.9 to 1.9 mm; n = 80; see Analysis 1.7).
1.7. Analysis.
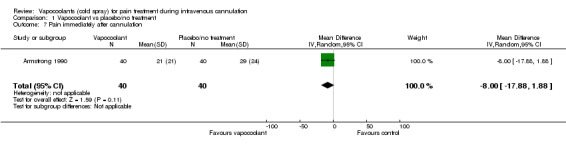
Comparison 1 Vapocoolant vs placebo/no treatment, Outcome 7 Pain immediately after cannulation.
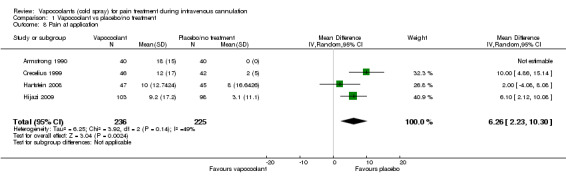
Pain at time of application of vapocoolant
Four studies (Armstrong 1990; Crecelius 1999; Hartstein 2008; Hijazi 2009) reported pain/discomfort at the time of application of vapocoolant (461 participants). High‐quality evidence shows that use of vapocoolants resulted in increased pain scores as measured by a VAS 100 compared with control (difference between means 6.3 mm, 95% CI 2.2 to 10.3 mm; I2 = 49%; see Figure 5). The largest of these studies (Hijazi 2009) confirmed that application of vapocoolant results in fewer participants experiencing little or no pain compared with controls (RR 0.67, 95% CI 0.54 to 0.84; number needed to treat for an additional harmful outcome (NNTH) = 4, 95% CI 3 to 8; see Analysis 1.9).
5.
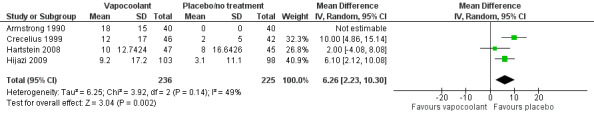
Forest plot of comparison: 1 Vapocoolant vs placebo/no treatment, outcome: 1.8 Pain at application.
1.9. Analysis.
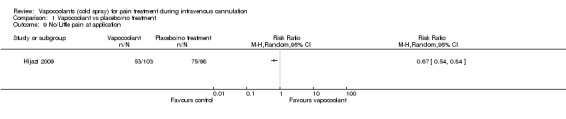
Comparison 1 Vapocoolant vs placebo/no treatment, Outcome 9 No/Little pain at application.
First attempt success rate of intravenous cannulation
Six studies of moderate quality (Costello 2006; Farion 2008; Hijazi 2009; Ramsook 2001; Robinson 2007; Selby 1995) reported data on first attempt success rate (812 participants). Use of vapocoolants resulted in no difference in first attempt success compared with controls (RR 1.00, 95% CI 0.94 to 1.06; I2 = 48%; see Figure 6).
6.
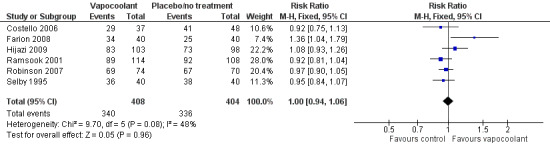
Forest plot of comparison: 1 Vapocoolant vs placebo/no treatment, outcome: 1.10 First attempt success.
Adverse events (as reported by study authors, divided into minor and major)
Five studies (Costello 2006; Crecelius 1999; Farion 2008; Hijazi 2009; Robinson 2007) reported eight adverse events in 279 vapocoolant participants and no adverse events in 272 control/placebo participants (RD 0.03, 95% CI 0 to 0.05; I2 = 10%; see Analysis 1.11). Adverse events were minor and included four reports of a cold sensation, three transient reactions of erythema at the site of spray and one report of burning sensation.
1.11. Analysis.
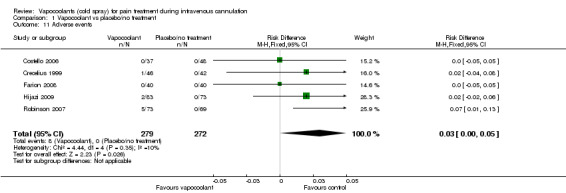
Comparison 1 Vapocoolant vs placebo/no treatment, Outcome 11 Adverse events.
Participant (or caregiver) satisfaction (as reported by study authors)
Two studies (Hartstein 2008; Hijazi 2009) reported data on participant satisfaction. Hartstein 2008 reported that 34 (72%) of 47 vapocoolant participants would choose this treatment again, although the question was not asked of control participants. Hijazi 2009 reported that 62% of adult participants in the vapocoolant group versus 39% of those in the placebo group would choose their assigned treatment group again (P value = 0.002). Farion 2008 reported caregiver, child life specialist and nurse satisfaction with pain management, but not participant satisfaction. Parent and nurse ratings of satisfaction with pain management were not significantly different between placebo and vapocoolant groups, whereas the child life specialist rating was better for the vapocoolant group (P value < 0.01). Overall, use of vapocoolants was not associated with differences in participant/caregiver satisfaction compared with that of controls (RR 1.31, 95% CI 0.82 to 2.09; I2 = 86%; see Analysis 1.12).
1.12. Analysis.
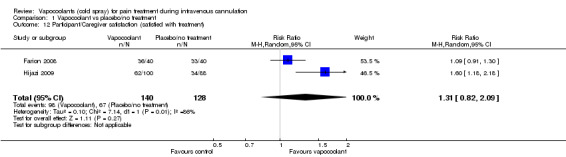
Comparison 1 Vapocoolant vs placebo/no treatment, Outcome 12 Participant/Caregiver satisfaction (satisfied with treatment).
Discussion
Summary of main results
This review found that vapocoolants provided more effective analgesia than placebo or no treatment when used just before intravenous cannulation. This effect was noted in both children and adults, and across a range of emergency and elective clinical situations. Of note, vapocoolants were not associated with any serious adverse events nor with reduction in first attempt success rates at intravenous cannulation. However, participants experienced increased discomfort at the time of vapocoolant application.
Overall completeness and applicability of evidence
A moderate number of published randomized controlled trials have addressed whether vapocoolants provide effective analgesia during intravenous cannulation. Overall, the evidence presented in this review is of low to moderate quality because of the small number of included studies, the small number of participants included in individual studies and the heterogeneity of included studies. Exploration of key methodological (ethyl chloride vs other vapocoolants, application time of spray) and clinical (children vs adults, elective vs emergency settings) differences could not explain the heterogeneity, which is likely high because of the small total number of trials, with five studies showing benefit with vapocoolants and three showing lesser or no effect. Despite these concerns, we found that use of vapocoolant suggested a clinically significant reduction in pain experienced during cannulation in analysis of both continuous and dichotomized data.
We could include in the primary meta‐analysis of this review only eight of the nine identified studies. Results of the one remaining study were not (Ramsook 2001) consistent with findings of the meta‐analysis. Ramsook 2001, a study of 222 children between three and 18 years of age undergoing intravenous cannulation in an emergency department, was the largest study identified, and its inclusion would have increased the number of participants included in the primary meta‐analysis by 21%. However, use of imputed values for Ramsook 2001 did not materially affect the results.
Four studies (Armstrong 1990; Crecelius 1999; Hartstein 2008; Hijazi 2009) comprising 461 participants reported increased pain/discomfort at the time of vapocoolant application compared with placebo or no treatment. Although this result remains statistically significant (difference between VAS 100 means 6.3 mm, 95% CI 2.2 to 10.3 mm), it is of marginal clinical significance ‐ considerably less significance than the reduction in pain during cannulation experienced by the vapocoolant group compared with placebo or no treatment (difference between VAS 100 means ‐12.5 mm, 95% CI ‐18.7 to ‐6.4 mm). However, the overall result of the meta‐analysis must be interpreted with caution, as the lower limit of the confidence level for pain during cannulation includes a margin that would be considered not clinically important. Although consensus is lacking regarding the minimum clinically significant reduction in VAS 100 score, several authors consider this to be in the region of 12 to 13 mm (Todd 1996). Our results were at the margin of this effect. However, dichotomized results, although available from only two studies (Farion 2008; Hijazi 2009), confirmed a clinically significant benefit.
Only one study examined the effects of vapocoolants with other adjuvants (Crecelius 1999) versus inhaled nitrous oxide. No reports described use of vapocoolants with routine administration of topical analgesics. In addition, it was not possible to definitively confirm the efficacy of vapocoolants across both small and large cannula sizes.
Quality of the evidence
We included in meta‐analyses of the primary outcome 848 participants in eight studies, and we included in the qualitative analyses 1070 participants in nine studies. The overall risk of bias of individual studies ranged from low to high. We classified four studies as having high risk of bias because of lack of blinding (Armstrong 1990; Hartstein 2008; Robinson 2007; Selby 1995). Although this represents the major methodological flaws of the included studies, we recognized a priori that blinding and use of a placebo were major methodological difficulties and decided to include such studies. Sensitivity analysis showed that exclusion of these studies did not alter review results. Another major methodological flaw among included studies was that protocols were not available for eight of the nine studies, leading us to classify these studies as having unclear risk of reporting bias. However, given that the primary outcome of this review (pain with intravenous cannulation) was well reported and occurred immediately after brief application of the intervention (vapocoolant), lack of available protocols was not likely to alter the results of the review. Although we planned to assess reporting bias by using funnel plots, we found that this was not possible because we identified insufficient trials for construction of meaningful plots. Although we found no negative trials, we are not likely to have missed studies because we used wide search criteria in this review.
We classified four studies (Armstrong 1990; Costello 2006; Hartstein 2008; Selby 1995) as having unclear risk of bias for concealment of allocation, and four studies (Armstrong 1990; Hartstein 2008; Robinson 2007; Selby 1995) as having unclear risk of attrition bias. Sensitivity analysis showed that exclusion of these studies did not materially alter the results.
Four of the eight studies included in the meta‐analysis had standard deviations that were larger than mean pain scores (Armstrong 1990; Crecelius 1999; Robinson 2007; Selby 1995) for the vapocoolant group, suggesting the possibility of skewed data. This variability in measurement may be a limitation of the overall conclusions of the meta‐analysis.
Overall, the quality of evidence was moderate to high but is limited by inclusion of only studies that involved relatively small sample sizes.
Potential biases in the review process
Our review process had few potential biases. Although we were not blinded to study authors, journals and institutions in our screening process, all three review authors (RG, VJ and SD) who completed the screening independently reached consensus as to which articles should be included in the review. We had difficulty in contacting some study authors to obtain supplementary information, and the absence of this information may have led to downgrading of evidence for the studies concerned. Although we reported a risk difference regarding adverse event data favouring control/placebo arms, we noted inconsistency in reporting of adverse event data. Only five of nine studies reported adverse event data (Costello 2006; Crecelius 1999; Farion 2008; Hijazi 2009; Robinson 2007), and only two (Hijazi 2009; Robinson 2007) of these studies reported details of adverse events in the methods section. We were not able to empirically assess publication bias within this review, but the electronic search was thorough and additional handsearching was undertaken, so we believe it is unlikely that we missed additional published studies. However, we acknowledge the possibility of unpublished studies on this topic that we have not been able to locate. However, if some small negative studies are not included, we think it is unlikely that these would have materially affected results of the meta‐analysis.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first high‐quality review of the role of vapocoolants in intravenous cannulation. Results are consistent with those of the previous systematic review (Moore 2009). Shah et al. undertook a meta‐analysis of interventions provided to children to reduce pain during immunization (Shah 2009). One of the interventions assessed was vapocoolants. Review authors found four studies comprising 248 participants between six weeks and six years of age that compared vapocoolants versus controls, and concluded that evidence was insufficient for or against vapocoolants for management of vaccine injection pain. However, consistent with this review, study authors reported no serious adverse events (Shah 2009). In the only study performed in adults for vaccine injection pain, fluori‐methane was associated with a reduction in vaccine injection pain (n = 172) (Mawhorter 2004). Although these findings are generally consistent with the findings of this review, they should be interpreted with caution, as needle insertion for vaccination is subcutaneous and intramuscular versus the intravenous location of peripheral catheterization.
Authors' conclusions
Implications for practice.
Vapocoolants are likely to have an analgesic effect in reducing pain during intravenous cannulation and are not likely to make cannulation more difficult nor to cause serious adverse events.
Implications for research.
Studies focusing on vapocoolants should report dichotomized outcomes, and these outcomes should be explored to determine whether they are available from studies already completed (i.e. individual participant data meta‐analysis to be undertaken). Future studies should be designed to allow for the combination of measures of participant satisfaction. Studies examining the analgesic effects of vapocoolants with different intravenous cannula sizes and different adjuvants are required to confirm the role of vapocoolants in intravenous cannulation.
Acknowledgements
We would like to thank Harald Herkner (Content and Statistical Editor) and Anthony Eidelman, Vaughan L Thomas and R Andrew Moore (Peer Reviewers) for help and editorial advice provided during preparation of this systematic review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Ethyl Chloride explode all trees #2 MeSH descriptor Cryoanesthesia explode all trees #3 (Cold near spray):ti,ab or (ethyl chloride):ti or vapocool* or (an?esthe* near skin):ti,ab or (1,1,1,2‐tetrafluoroethane):ti #4 (#1 OR #2 OR #3) #5 MeSH descriptor Infusions, Intravenous explode all trees #6 MeSH descriptor Injections, Intravenous explode all trees #7 MeSH descriptor Catheterization, Central Venous explode all trees #8 MeSH descriptor Catheterization, Peripheral explode all trees #9 (cannula* or (pain near (needl* or intravenous)) or (needle* near procedure*) or ((injection* or infusion*) near intravenous) or (catheter* near (arter* or vein*)) or pain treatment):ti,ab #10 (#5 OR #6 OR #7 OR #8 OR #9) #11 (#4 AND #10)
Appendix 2. MEDLINE (Ovid SP) search strategy
1. exp Ethyl Chloride/ or exp Cryoanesthesia/ or (Cold adj3 spray).mp. or ethyl chloride.mp. or vapocool*.af. or (an?esthe* adj3 skin).mp. or 1,1,1,2‐tetrafluoroethane.mp. 2. exp Infusions, Intravenous/ or exp Injections, Intravenous/ or catheterization, central venous/ or exp catheterization, peripheral/ or cannula*.af. or (pain adj3 (needl* or intravenous)).mp. or (needle* adj3 procedure*).mp. or ((injection* or infusion*) adj3 intravenous).mp. or (catheter* adj3 (arter* or vein*)).mp. or pain treatment.mp. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 5. 1 and 4 6. 3 or 5
Appendix 3. EMBASE (Ovid SP) search strategy
1. exp chloroethane/ or exp cryoanesthesia/ or (Cold adj3 spray).mp. or ethyl chloride.mp. or vapocool*.af. or (an?esthe* adj3 skin).mp. or 1,1,1,2‐tetrafluoroethane.mp. 2. exp intravenous drug administration/ or exp central venous catheterization/ or exp catheterization/ or cannula*.af. or (pain adj3 (needl* or intravenous)).mp. or (needle* adj3 procedure*).mp. or ((injection* or infusion*) adj3 intravenous).mp. or (catheter* adj3 (arter* or vein*)).mp. or pain treatment.mp. 3. 1 and 2 4. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. LILACS (BIREME) search strategy
"ETHYL CHLORIDE/ME" or "CRYOANESTHESIA" or (Cold and spray) or (frio and spray) or (cloreto de etilo) or (cloruro de etilo) or "vapocool$" or (anestesia and piel) or (anestesia and pele) or "1,1,1,2‐tetrafluoroethane"
Appendix 5. CINAHL (EBSCOhost) search strategy
S1 (MH "Ethyl Chloride") OR AB ( (Cold and spray) or ethyl chloride or vapocool* or (an?esthe* and skin) ) OR TI 1,1,1,2‐tetrafluoroethane S2 ( (MH "Infusions, Intravenous") OR (MH "Injections, Intravenous") OR (MH "Catheterization, Central Venous") OR (MH "Catheterization, Peripheral") ) OR AB ( cannula* or (pain and (needl* or intravenous)) or (needle* and procedure*) or ((injection* or infusion*) and intravenous) or (catheter* and (arter* or vein*)) or pain treatment ) S3 S1 and S2 S4 ( (MH "Randomized Controlled Trials") OR (MH "Random Assignment") OR (MH "Clinical Trials") OR (MH "Placebos") OR (MH "Multicenter Studies") OR (MH "Prospective Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MH "Double‐Blind Studies") ) OR ( random* or (controlled and trial*) ) S5 S3 and S4
Appendix 6. ISI Web of Science search strategy
#1 TS=((Cold SAME spray) or ethyl chloride or vapocool* or (an?esthe* SAME skin)) or TI=(1,1,1,2‐tetrafluoroethane) #2 TS=(cannula* or (pain SAME (needl* or intravenous)) or (needle* SAME procedure*) or ((injection* or infusion*) SAME intravenous) or (catheter* SAME (arter* or vein*)) or pain treatment) #3 #2 AND #1 #4 TS=(random* or (controlled SAME trial*) or placebo* or prospective or multicenter) or TS=((blind* or mask*) SAME (single or double or triple or treble)) #5 #4 AND #3
Appendix 7. Data extraction form
Data extraction form
| First author | Rv1 SD VJ | ___/___/___ | |
| Year of publication | Rv2 SD VJ | ___/___/___ | |
| Language | Arbitrator | ___/___/___ |
1. Study details
| Country of study | |
| Publication type | Journal/Abstract/Other (specify) |
2. Study eligibility/characteristics
| Inclusion criteria for systematic review | Study | |
|
Type of study |
Randomized controlled trial | Yes/No/Unclear |
| Participants | · Intravenous cannulation Age (circle those that apply): · Children · Adults Setting (circle those that apply): · Elective · Emergency · Healthy volunteers |
Yes/No/Unclear |
|
Types of intervention |
· Ethyl chloride · Other vapocoolant (state): Control group (circle one) · Placebo · No treatment |
Yes/No/Unclear Yes/No/Unclear |
|
Types of outcomes reported |
· Pain · First attempt success rate · Adverse events · Participant (caregiver) satisfaction Other: |
Yes/No/Unclear |
INCLUDE (Yes to all sections) ¨
POSSIBLE ¨
Further information required:
EXCLUDE ¨
Reason for exclusion:
| General information (Included studies table) | |
|
Trial inclusion criteria |
|
|
Trial exclusion criteria |
|
| Participants | Age: Median. Mean.. Range.. Ethnicity: Sex (percentage) Male: Female: Other: |
|
Setting (hospital or multi‐centre, country) | |
|
Trial intervention (include strength, application time, adjuvant) |
Number enrolled________ Number analysed________ |
||||
| Continuous pain scales | |||||
|
Pain (circle which measured) Pain VAS_____ (scale length) Std Dev Change in pain VAS_____ (scale length) Std Dev |
At time of vapocoolant | During cannulation | Immediately after cannulation |
||
| Dichotomized categorical pain scales | |||||
|
Pain (circle which measured) Pain categorical ____ (point scale) Number with “good pain relief" Number with “little pain” |
At time of vapocoolant | During cannulation | Immediately after cannulation |
||
| First attempt success rate | |||||
| n | N | ||||
| Adverse events | |||||
| List: | n |
N |
|||
| Patient satisfaction | |||||
| Method: | n |
N |
|||
|
Placebo/No treatment (include strength, application time, adjuvant) |
Number enrolled________ Number analysed________ |
||||
| Continuous pain scales | |||||
|
Pain (circle which measured) Pain VAS_____ (scale length) Std Dev Change in pain VAS_____ ( scale length) Std Dev |
At time of vapocoolant | During cannulation | Immediately after cannulation |
||
| Dichotomized categorical pain scales | |||||
|
Pain (circle which measured) Pain categorical ____ (point scale) Number with “good pain relief" Number with “little pain” |
At time of vapocoolant | During cannulation | Immediately after cannulation |
||
| First attempt success rate | |||||
| n | N | ||||
| Adverse events | |||||
| List: | n |
N |
|||
| Participant satisfaction | |||||
| Method: | n |
N |
|||
3. Methods: Cochrane risk of bias tool trial
| Domains | Description | Low risk of bias, high risk of bias, unclear risk of bias | |
| A |
Was the random sequence generation adequate? Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups |
||
| B |
Was the assigned treatment adequately concealed before allocation? Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment |
||
| C |
Were participants and treatment providers blinded to treatment status? Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information related to whether the intended blinding was effective |
||
| D |
Were outcome assessors blinded to treatment status? Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information related to whether the intended blinding was effective |
||
| E |
Were the outcome data complete? Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions when reported and any re‐inclusions in analyses performed by the review authors. State whether analysis was intention‐to‐treat? Pain |
||
| First attempt success rate | |
||
| Adverse events | |
||
| Participant (caregiver) satisfaction | |
||
| F |
Was evidence of selective outcome reporting noted? Were all outcomes listed in protocol or study methods reported in the results? |
||
| G |
Other sources of bias |
Data and analyses
Comparison 1. Vapocoolant vs placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain during cannulation | 8 | 848 | Mean Difference (IV, Random, 95% CI) | ‐12.51 [‐18.67, ‐6.35] |
| 2 Pain during cannulation by participant age | 8 | 848 | Mean Difference (IV, Random, 95% CI) | ‐12.51 [‐18.67, ‐6.35] |
| 2.1 Children | 2 | 165 | Mean Difference (IV, Random, 95% CI) | ‐8.78 [‐28.56, 11.01] |
| 2.2 Adults | 6 | 683 | Mean Difference (IV, Random, 95% CI) | ‐13.64 [‐20.17, ‐7.10] |
| 3 Pain during cannulation by vapocoolant type | 8 | 848 | Mean Difference (IV, Random, 95% CI) | ‐12.51 [‐18.67, ‐6.35] |
| 3.1 Non‐ethyl chloride | 3 | 373 | Mean Difference (IV, Random, 95% CI) | ‐12.42 [‐24.58, ‐0.26] |
| 3.2 Ethyl chloride | 5 | 475 | Mean Difference (IV, Random, 95% CI) | ‐12.49 [‐20.62, ‐4.36] |
| 4 Pain during cannulation by application time | 8 | 848 | Mean Difference (IV, Random, 95% CI) | ‐12.51 [‐18.67, ‐6.35] |
| 4.1 < 5 seconds | 3 | 378 | Mean Difference (IV, Random, 95% CI) | ‐6.17 [‐19.97, 7.63] |
| 4.2 5 to 10 seconds | 5 | 470 | Mean Difference (IV, Random, 95% CI) | ‐15.89 [‐22.37, ‐9.41] |
| 5 Pain during cannulation by clinical situation of use | 8 | 848 | Mean Difference (IV, Random, 95% CI) | ‐12.51 [‐18.67, ‐6.35] |
| 5.1 Elective | 3 | 248 | Mean Difference (IV, Random, 95% CI) | ‐13.13 [‐21.72, ‐4.53] |
| 5.2 Emergency | 5 | 600 | Mean Difference (IV, Random, 95% CI) | ‐11.92 [‐21.36, ‐2.47] |
| 6 Good pain relief during cannulation | 2 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.30, 2.13] |
| 7 Pain immediately after cannulation | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐8.0 [‐17.88, 1.88] |
| 8 Pain at application | 4 | 461 | Mean Difference (IV, Random, 95% CI) | 6.26 [2.23, 10.30] |
| 9 No/Little pain at application | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 First attempt success | 6 | 812 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.94, 1.06] |
| 11 Adverse events | 5 | 551 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.05] |
| 12 Participant/Caregiver satisfaction (satisfied with treatment) | 2 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.82, 2.09] |
1.1. Analysis.
Comparison 1 Vapocoolant vs placebo/no treatment, Outcome 1 Pain during cannulation.
1.8. Analysis.
Comparison 1 Vapocoolant vs placebo/no treatment, Outcome 8 Pain at application.
1.10. Analysis.

Comparison 1 Vapocoolant vs placebo/no treatment, Outcome 10 First attempt success.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Armstrong 1990.
| Methods | Randomized controlled 3‐arm parallel trial at a metropolitan hospital in Scotland | |
| Participants | 120 pre‐medicated female patients undergoing minor gynaecological day‐case surgery. "There were no statistical differences among the three groups in respect of age and weight" | |
| Interventions | Group 1 received no treatment before cannulation Group 2 received 0.2 mL lidocaine injected intradermally through a 25G needle at the puncture site Group 3 received ethyl chloride spray around the skin puncture site from a height of 8 inches for 10 seconds |
|
| Outcomes | Pain of anaesthetic application, pain of catheter insertion, skin pain 1 minute after insertion Vein visibility before and after skin anaesthesia and ease of cannulation |
|
| Funding | "Dr P Armstrong was in receipt of a grant from the Association of Anaesthetists of Great Britain and Ireland" | |
| Notes | For analysis, no treatment was used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were "allocated to one of three equal sized treatment groups using a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, but all expected outcomes reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded. Investigator present during procedure |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigator present during procedure |
Costello 2006.
| Methods | Randomized placebo‐controlled 3‐arm parallel trial in an emergency department at a children's hospital in the USA | |
| Participants | 129 children between 9 and 18 years of age needing intravenous cannulation. "Patients with impaired consciousness, cold hypersensitivity, patients unable to understand the study protocol or the visual analogue scale, those with a history of psychiatric illness or with developmental delay, coexisting painful condition, peripheral neuropathy, or cutaneous sensitivity to ethyl vinyl chloride or isopropyl chloride were excluded from study" 54% were female, 73% were white, 27% were African American |
|
| Interventions | Group I received study drug (ethyl vinyl chloride vapocoolant spray) for up to 5 seconds or until the skin blanched Group II received placebo (isopropyl alcohol spray) for up to 5 seconds or until the skin blanched Group III received no pre‐treatment "Distraction techniques and pre‐procedural educational interventions were applied without respect for group assignment" |
|
| Outcomes | Pain at first attempt at intravenous cannulation (only mean change reported), first attempt success rate | |
| Funding | None declared | |
| Notes | For analysis, the active placebo was used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned to one of three treatment groups by random number allocation" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A total of 129 subjects were approached and gave consent for entry into the study. Data from 2 subjects, each randomized to the nonintervention group, were excluded from analysis due to protocol violations" |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, but all expected outcomes reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Canisters containing study drug and placebo spray were manufactured and labelled by individuals not associated with the study. The investigators and nursing staff performing IV cannulation were blinded to the canister contents, which were indistinguishable from each other except for their label as either 'canister 1' or 'canister 2'" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Cannisters containing study drug and placebo spray were manufactured and labelled by individuals not associated with the study. The investigators and nursing staff performing IV cannulation were blinded to the canister contents, which were indistinguishable from each other except for their label as either 'canister 1' or 'canister 2'" |
Crecelius 1999.
| Methods | Randomized placebo controlled trial at a US school of dentistry | |
| Participants | 88 patients between age 18 and 80 years, scheduled to have dental surgery Patients with scarred veins were excluded "The subject population ranged in age from 18 to 72 years," mean 28 years, 60% female, 78% white |
|
| Interventions | "All subjects were given titers with incremental increases in the concentration of nitrous oxide until the patient reported feeling relaxed, light‐headed, and tingling, with a feeling or floating or heaviness. The placebo or ethyl chloride spray was then applied" "One group received room temperature distilled water spray before cannulation. The other group received a 10 second spray of ethyl chloride prior to venous cannulation" |
|
| Outcomes | Pain and anxiety before/after nitrous oxide, pain and anxiety following application of spray, pain and anxiety following venous cannulation. Adverse events | |
| Funding | "Supported in part by NIDR grant" | |
| Notes | All participants also received nitrous oxide, as described above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned" "Randomly divided" Study author contacted: "A random number table was used for the randomization rather than a random number generator" |
| Allocation concealment (selection bias) | Low risk | Not described. Study author contacted: "The research nurse kept the allocation concealed in envelops" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants enrolled included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, but all expected outcomes reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Since most subjects would not have had previous experience with ethyl chloride, they were likely unable to determine the spray treatment they received. The venipuncturist was not present during the spray application" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Most subjects (51%) and venipuncturists (46%) reported they were unable to tell if ethyl chloride or placebo was applied" "Of the 43 subjects reporting which treatment they received, 6 reported receiving placebo incorrectly and 3 reported receiving ethyl chloride incorrectly" "The venipuncturists reported knowing which treatment 48 subjects received. They reported incorrectly that 5 patients received ethyl chloride when placebo was administered and incorrectly that 1 patient received water when ethyl chloride was administered" |
Farion 2008.
| Methods | Randomized placebo‐controlled trial in the emergency department of a tertiary children's hospital in Canada | |
| Participants | 80 children, 6 to 12 years of age, requiring urgent intravenous cannulation (within 30 to 45 minutes). Mean age 9.4 years. 53% male Excluded if need for emergency vascular access, contraindications to use of vapocoolant spray (e.g. sensitivity to halogenated hydrocarbons, peripheral vascular disease), if unable to complete pain assessment or if already received a topical anaesthetic cream |
|
| Interventions | Intervention group (40) received 1,1,1,3,3‐pentafluoropropane and 1,1,1,2‐tetrafluoroethane (Pain Ease) at room temperature, sprayed from a distance of 8 to 18 cm for 4 to 10 seconds until the skin blanched Placebo group (40) "received sterile, normal saline spray at room temperature in a similar fashion" "All patients received standardized age‐appropriate preparation and distraction from 1 of 2 trained child life specialists during the cannulation attempts" |
|
| Outcomes | Self reported pain during intravenous cannulation; success rate on first attempt; parent, nurse and life specialist ratings of child's pain and satisfaction with pain management; ease of cannulation | |
| Funding | "Gebauer Company provided the Pain Ease vapocoolant spray used in the study. The company provided no other support, nor did it influence the design, conduct or reporting of the trial" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned patients to the active treatment or placebo group in blocks of 10 using a random number generator" |
| Allocation concealment (selection bias) | Low risk | "Research personnel (who were not involved in patient enrolment) masked similar canister of active treatment or placebo, labelled them with a unique identifier and placed them in sequentially numbered opaque, sealed envelopes. Once eligibility and consent were confirmed, the research assistant obtained the next envelope in sequence and recorded the envelope number and canister identifier on the enrolment log and data collection forms" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, but all expected outcomes reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Masked similar canisters" "The research assistant sprayed the cannulation site while all others in the room looked away" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Parents correctly identified which spray was given to 47 (59%) of 79 patients (P = 0.12). Nurses correctly identified the spray for 52 (65%) of 80 patients (P = 0.01) and the child life specialists were correct for 65 (81%) of 80 patients (P < 0.01)" Children not assessed for success of blinding |
Hartstein 2008.
| Methods | Randomized controlled trial in the emergency department of 2 Army Medical Centres in the USA | |
| Participants | 92 "stable emergency department (ED) patients over 18 years who required IV cannulation as part of their ED evaluation." 66 (71.8%) female Excluded subjects with "diabetes mellitus, peripheral neuropathy, poor circulation, or other skin conditions causing insensate skin. Also excluded were subjects with a history of allergy to hydrocarbon products, those premedicated with analgesic medication, unstable patients, and patients who did not demonstrate the capacity to understand study questionnaires" |
|
| Interventions | Intervention group (47) received standard skin preparation followed by 2 to 4 second spray of vapocoolant applied 3 to 5 inches (7 to 12 cm) from the skin, followed immediately by IV insertion Control group (45) received standard skin preparation |
|
| Outcomes | Pain with IV cannulation (first attempt), participant anxiety, projection of future anxiety, pain during skin preparation, participant satisfaction with method used | |
| Funding | "Skin coolant was supplied free of cost by the Gebauer Company, Cleveland, Ohio, for use in this study" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Utilizing a random number generator to assign subjects to the control or study group" |
| Allocation concealment (selection bias) | Unclear risk | "96 sequential packets containing study materials, instructions for staff placing the IV cannula and questionnaires were prepackaged" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Data from four of the approved subjects [were] not included due to incomplete documentation in one case and loss of documentation in the other three cases. An equal number of study and control subjects were included in this disqualified group" "Initial calculations were performed excluding subjects in which first IV attempt had failed due to concerns of falsely elevated VAS scored due to needle probing for the vein" "Intention to treat analysis (including the subjects with failed first attempts) did not alter the statistical significance" However, the methods section reported that 47 participants were randomized to the treatment group, yet Figure 1 reports data from 48 participants. For this review, 47 was assumed to be the correct number of participants |
| Selective reporting (reporting bias) | Low risk | Protocol provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
Hijazi 2009.
| Methods | Randomized placebo‐controlled trial | |
| Participants | 201 adult patients who required intravenous cannulation in the emergency department of a metropolitan teaching hospital in Australia. Age ≥ 18 years, mean 58.2 (standard deviation (SD) 19.5) years. 54% male. "The groups did not differ significantly (P > 0.05) in age, reason for cannulation, cannulation site, cannula size, or who cannulated the patient" "Exclusion criteria were refusal to participate, inability to provide informed consent (non‐English speaking, altered mental state, severe illness, urgent need for cannulation), moderate to severe discomfort or pain, skin disease associated with cold intolerance (such as Raynaud's phenomenon), known allergy to spray contents, peripheral neuropathy or numbness, parenteral analgesia within the previous 4 hours, and the use of other local anaesthesia" |
|
| Interventions | Intervention group (103) received vapocoolant spray (propane, butane and pentane blend) Control group (98) received water spray The principal investigator administered the allocated spray from a distance of about 12 cm for 2 seconds |
|
| Outcomes | Pain with cannulation, discomfort with spray on administration, success rate of cannulation, willingness of the participant to choose the allocated spray in the future, participant's guess at randomization status, unexpected events | |
| Funding | Funded by an advanced medical science grant from the University of Melbourne, Victoria, Australia. "The authors were entirely independent from the funders. The funders had no role in the project" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were block randomized (blocks of 6) by an independent pharmacist using a computerised random number generator" |
| Allocation concealment (selection bias) | Low risk | "Until after informed consent has been obtained, only the pharmacist knew the randomization status. At that time the principal investigator opened the sealed envelope and prepared to administer the assigned spray" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up for primary outcome of pain. However, 25 lost to follow‐up at 5 days in control group and 20 in intervention group (adverse events) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, but all expected outcomes reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The control spray "is also packed in a handheld pressurised spray can of about the same size as the intervention spray" "The intervention and control spray cans were masked in white paper and labelled A and B" "The patients, their carers in the emergency department, and independent emergency staff who collected outcome data were all blinded to randomization status" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above. "Significantly more patients in the control group correctly guessed the nature of the spray they received" (69% vs 54%) |
Ramsook 2001.
| Methods | Randomized placebo‐controlled trial in the emergency department of a children's hospital in the USA | |
| Participants | 222 patients, aged 3 to 18 years, who presented to the emergency department "requiring IV cannulation for fluids or medication administration and venipuncture for diagnostic tests" "There were no statistically significant differences for age, gender, or report of pain score for a previous intravenous cannulation and/or venipuncture between the two groups" Excluded were "pre‐verbal or developmentally delayed patients who could not complete the required self report pain scale, and patients presenting with a painful condition" 56% of intervention group and 53% of placebo group were female |
|
| Interventions | Standardized skin cleaning followed by spray from 6 inches for 5 seconds followed by immediate venipuncture. "Spraying was terminated if frosting of the skin occurred, due to cooling of skin moisture, or if the patient felt any discomfort" Intervention group (114) received ethyl chloride spray Placebo group (108) received isopropyl alcohol spray |
|
| Outcomes | Self reported pain of cannulation, difficulty of insertion, number of attempts before success, degree of impairment of the spray of vein visibility | |
| Funding | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Blinded randomization tables" |
| Allocation concealment (selection bias) | Low risk | "The pharmacist provided the appropriate spray to the nurse caring for the patient" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants enrolled included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, but all expected outcomes reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The manufacturer provided identically matched cans of isopropyl alcohol as placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Nurses may have been able to recognise ethyl chloride by the white precipitate during application. However they were not involved in pain assessment and could not have influenced these results. The nurse may have reported on the operator difficulty and impairment of vein visibility knowing which spray they had used" |
Robinson 2007.
| Methods | Randomized controlled 4‐arm parallel trial in the emergency department of a tertiary hospital in New Zealand | |
| Participants | 300 patients, older than 15 years of age, triage 3 to 5, needing cannulation. Mean age 49 years (range 16 to 92 years). 50% female. "The four groups did not differ in their demographic makeup" Inclusion criteria included a Glascow coma scale score (GCS) = 15 and conversational English Excluded if unable to give consent because of intoxication or intellectual impairment, unable to complete the visual analogue scale because of disability (e.g. blindness, inability to hold a pen), patient refusal, needing emergency cannulation, possible bowel obstruction, pneumothorax, allergy to any of the trial drugs. Excluded if more than 2 attempts at cannulation |
|
| Interventions | After standard preparation, randomized to 4 arms (number): Group 1: no anaesthesia (69) Group 2: Entonox inhaled for 1 minute before and during cannulation (77) Group 3: ethyl chloride sprayed for 5 to 10 seconds, 15 to 20 cm from intended site of cannulation (73) Group 4: 0.1 mL 1% lignocaine injected intradermally with a 26 gauge needle (71) |
|
| Outcomes | Pain with application of lignocaine or spray, pain of cannulation, side effects | |
| Funding | None declared | |
| Notes | No treatment group used in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random numbers were assigned to groups by the SAS computer program using random length blocking" |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes containing treatment instructions, data sheets and study information were opened only after the patient had given consent" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition described: 4 excluded for including more than 2 cannulation attempts; 6 excluded because of incomplete data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available, but all expected outcomes reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
Selby 1995.
| Methods | Randomized controlled 4‐arm parallel trial at a metropolitan hospital in the UK | |
| Participants | 160 unpremedicated women, American Society of Anesthesiologists (ASA) grade 1 or 2, requiring insertion of an intravenous cannula for general anaesthetic Excluded if allergies to local anaesthetics, or analgesic medication taken in last 4 hours Groups (40 in each) were similar in terms of age, weight, visibility of veins and anxiety levels |
|
| Interventions | Group 1: no local anaesthetic Group 2: 0.2 mL of EMLA cream rubbed into the skin over the vein and covered with a non‐absorbent dressing for 5 minutes before cannulation Group 3: ethyl chloride sprayed over the vein for 10 seconds from a height of 20 cm, with cannulation performed immediately Group 4: 0.2 mL of 1% lignocaine injected subcutaneously at the site of venepuncture with a 25 gauge (G) needle, and left for 30 seconds before cannulation |
|
| Outcomes | Anxiety, difficulty of cannulation, number of failed cannulations, pain during local anaesthetic application, pain of cannulation, pain 1 minute after cannulation | |
| Funding | None declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Manuscript reports 2 different set of figures for the first attempt success rate. This ranged from 38 to 40 out of 40 for the control group, and from 36 to 37 for the ethyl chloride group |
| Selective reporting (reporting bias) | Unclear risk | Protocol not described, but all expected outcomes described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baelen 1994 | Not a randomized controlled trial |
| Baxter 2009 | No placebo or control group |
| Kelly 2008 | Not a randomized controlled trial |
| Lunoe 2015 | Included no control group/no treatment group for vapocoolant |
| Soueid 2007 | Not a randomized controlled trial |
Characteristics of ongoing studies [ordered by study ID]
Mace 2014.
| Trial name or title | Trial of use of vapocoolant spray to decrease the pain of peripheral IV line placement in adults |
| Methods | Prospective randomized placebo‐controlled trial |
| Participants | Adults > 21years undergoing peripheral IV at a large urban tertiary care hospital ED 112 participants: "demographics and vital signs were not significantly different between the two groups" Mean age placebo group 48 vs 54 in vapocoolant group Male placebo 43% vs vapocoolant 40% Caucasian placebo 40% vs vapocoolant 42% African American placebo 60% vs vapocoolant 58% |
| Interventions | Intervention group vapocoolant spray (1,1,1,3,3, pentafluoropropane and 1,1,1,2 tetrafluoropropane, Gabauer Pain‐Ease); n = 78 Placebo normal saline spray n = 77 |
| Outcomes | Pain measured on a 10‐point scale at 2 points
|
| Starting date | |
| Contact information | Sahron Mace, Cleveland |
| Notes | Aims to collect data on 300 participants in total |
Differences between protocol and review
We made the following change to the protocol (Dalziel 2011); we adjusted the title of the review so that it matches that of the plain language summary by the addition of the explanatory term 'cold spray'.
Contributions of authors
Rebecca J Griffith (RG), Vanessa Jordan (VJ), David Herd (DH), Peter W Reed (PR), Stuart R Dalziel (SD).
| Drafting the protocol | SD, DH, VJ |
| Developing a search strategy | SD |
| Searching for trials (usually 2 people) | SD, RG, VJ |
| Obtaining copies of trials | SD, RG |
| Selecting which trials should be included (2 + 1 arbiter) | SD, RG, VJ, DH arbitrator |
| Extracting data from trials (2 people) | SD, RG, VJ |
| Entering data into RevMan 5.3 | SD, RG |
| Carrying out the analysis | SD, RG, VJ, DH, PR |
| Interpreting the analysis | SD, RG, VJ, DH, PR |
| Drafting the final review | SD, RG |
Sources of support
Internal sources
Starship Children's Health, New Zealand.
University of Auckland, New Zealand.
Mater Children's Hospital, Australia.
External sources
No sources of support supplied
Declarations of interest
Rebecca J Griffith: none known.
Vanessa Jordan. none known.
David Herd: none known.
Peter W Reed: none known.
Stuart R Dalziel: none known.
New
References
References to studies included in this review
Armstrong 1990 {published data only}
Costello 2006 {published data only}
- Costello M, Ramundo M, Christopher NC, Powell KR. Ethyl vinyl chloride vapocoolant spray fails to decrease pain associated with intravenous cannulation in children. Clinical Pediatrics 2006;45(7):628‐32. [PUBMED: 16928840] [DOI] [PubMed] [Google Scholar]
- Costello M, Ramundo M, Christopher NC, Powell KR. Lack of effect of etiryl vinyl chloride vapocoolant spray on decreasing pain associated with IV cannulation in children: a brief report. Pediatric Research 2002;51(4):43A. [Google Scholar]
Crecelius 1999 {published data only}
- Crecelius C, Rouhfar L, Beirne OR. Venous cannulation and topical ethyl chloride in patients receiving nitrous oxide. Anesthesia Progress 1999;46(3):100‐3. [PUBMED: 11692346] [PMC free article] [PubMed] [Google Scholar]
Farion 2008 {published data only}
- Farion KJ, Splinter KL, Newhook K, Gaboury I, Splinter WM. The effect of vapocoolant spray on pain due to intravenous cannulation in children: a randomized controlled trial. Canadian Medical Association Journal 2008; Vol. 179, issue 1:31‐6. [PUBMED: 18591524] [DOI] [PMC free article] [PubMed]
Hartstein 2008 {published data only}
Hijazi 2009 {published data only}
- Hijazi R, Taylor D, Richardson J. Effect of topical alkane vapocoolant spray on pain with intravenous cannulation in patients in emergency departments: randomised double blind placebo controlled trial. BMJ 2009; Vol. 338:457‐9. [PUBMED: 19208703] [DOI] [PMC free article] [PubMed]
Ramsook 2001 {published data only}
Robinson 2007 {published data only}
Selby 1995 {published data only}
- Selby IR, Bowles BJ. Analgesia for venous cannulation: a comparison of EMLA (5 minutes application), lignocaine, ethyl chloride, and nothing. Journal of the Royal Society of Medicine 1995; Vol. 88, issue 5:264‐7. [PUBMED: 7636819] [PMC free article] [PubMed]
References to studies excluded from this review
Baelen 1994 {published data only}
Baxter 2009 {published data only}
Kelly 2008 {published data only}
- Kelly JS. Painless vascular cannulation: ethyl chloride, vapocoolants and cryoanalgesics. Journal of the Royal College of Physicians of Edinburgh 2008; Vol. 38:232‐3.
Lunoe 2015 {published data only}
- Lunoe MM, Drendel AL, Levas MN, Weisman SJ, Dasgupta M, Hoffmann RG, et al. A randomized clinical trial of jet‐injected lidocaine to reduce venipuncture pain for young children. Annals of Emergency Medicine. 2015/05/04 2015; Vol. 66, issue 5:466‐74. [0196‐0644] [DOI] [PMC free article] [PubMed]
Soueid 2007 {published data only}
References to ongoing studies
Mace 2014 {published data only}
- Mace SE. Can we decrease the pain of peripheral intravenous line placement in adults by the use of vapocoolant spray? Preliminary results of a prospective, randomized, blinded, placebo‐controlled trial. Academic Emergency Medicine. Blackwell Publishing Ltd, 2014, issue 5 Suppl 1:S20.
- Mace SE. Prospective, randomized, blinded, placebo‐controlled trial of the use of vapocoolant spray to decrease the pain of peripheral intravenous line placement in adults in the emergency department: preliminary results. Annals of Emergency Medicine. Mosby Inc., 2014, issue 4 Suppl 1:S128.
Additional references
Altman 2000
- Altman DG. Statistics in medical journals: some recent trends. Statistics in Medicine 2000;19(23):3275‐89. [DOI: 10.1002/1097-0258(20001215)19:23%3C3275::AID-SIM626%3E3.0.CO;2-M] [DOI] [PubMed] [Google Scholar]
Berlin 1997
- Berlin JA. Does blinding of readers affect the results of meta‐analyses?. Lancet 1997;350:185‐6. [PUBMED: 9250191] [DOI] [PubMed] [Google Scholar]
Burke 1999
- Burke D, Mogyoros I, Vagg R, Kiernan MC. Temperature dependence of excitability indices of human cutaneous afferents. Muscle and Nerve 1999;22(1):51‐60. [PUBMED: 9883857 ] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kennedy 1999
- Kennedy RM, Luhmann JD. The "ouchless emergency department". Getting closer: advances in decreasing distress during painful procedures in the emergency department. Pediatric Clinics of North America 1999;46(6):1215‐47. [PUBMED: 10629683] [DOI] [PubMed] [Google Scholar]
Lander 2006
- Lander JA, Weltman BJ, So SS. EMLA and Amethocaine for reduction of children's pain associated with needle insertion. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004236.pub2] [DOI] [PubMed] [Google Scholar]
Mawhorter 2004
- Mawhorter S, Daugherty L, Ford A, Hughes R, Metzger D, Easley K. Topical vapocoolant qUickly and effectively reduces vacclne‐associated pain: results of a randomized, single‐blinded, placebo‐controlled study. Journal of Travel Medicine 2004;11:262‐72. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher 2009: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Moore 2009
- Moore A, Straube S, McQuay H. Minimising pain during intravenous cannulation. BMJ 2009;338:422‐3. [PUBMED: 19208702] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shah 2009
- Shah V, Taddio A, Rieder MJ, for the HELPinKIDS Team. Effectiveness and tolerability of pharmacologic and combined interventions for reducing injection pain during routine childhood immunizations: systematic review and meta‐analyses. Clinical Therapeutics 2009;31(Suppl B):S104‐51. [DOI] [PubMed] [Google Scholar]
Skarbek‐Borowska 2006
- Skarbek‐Borowska S, Becker BM, Lovgren K, Bates A, Minugh PA. Brief focal ultrasound with topical anesthetic decreases the pain of intravenous placement in children. Pediatric Emergency Care 2006;22(5):339‐45. [PUBMED: 16714961 ] [DOI] [PubMed] [Google Scholar]
Todd 1996
- Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Annals of Emergency Medicine 1996;27:485‐9. [DOI] [PubMed] [Google Scholar]
Uman 2006
- Uman LS, Chambers CT, McGrath PJ, Kisely S. Psychological interventions for needle‐related procedural pain and distress in children and adolescents. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD005179.pub2] [DOI] [PubMed] [Google Scholar]
Zempsky 2008a
- Zempsky WT. Pharmacological approaches for reducing venous access pain in children. Pediatrics 2008;122:S140‐53. [PUBMED: 18978008 ] [DOI] [PubMed] [Google Scholar]
Zempsky 2008b
- Zempsky WT, Bean‐Lijewski J, Kauffman RE, Koh JL, Malviya SV, Rose JB, et al. Needle‐free powder lidocaine delivery system provides rapid effective analgesia for venipuncture or cannulation pain in children: randomized, double‐blind comparison of venipuncture and venous cannulation pain after fast‐onset needle‐free powder lidocaine or placebo treatment trial. Pediatrics 2008;121(5):979‐87. [PUBMED: 18450903 ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Dalziel 2011
- Dalziel SR, Jordan V, Herd D, Reed PW. Vapocoolants for pain treatment during intravenous cannulation. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009484] [DOI] [PMC free article] [PubMed] [Google Scholar]


