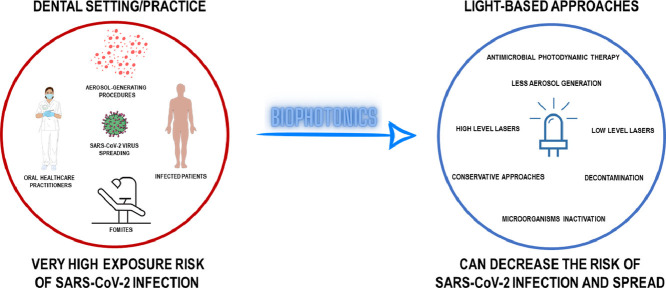
Abstract
Biophotonics is defined as the combination of biology and photonics (the physical science of the light). It is a general term for all techniques that deal with the interaction between biological tissues/cells and photons (light). Biophotonics offers a great variety of techniques that can facilitate the early detection of diseases and promote innovative theragnostic approaches. As the COVID-19 infection can be transmitted due to the face-to-face communication, droplets and aerosol inhalation and the exposure to saliva, blood, and other body fluids, as well as the handling of sharp instruments, dental practices are at increased risk of infection. In this paper, a literature review was performed to explore the application of Biophotonics approaches in Dentistry focusing on the COVID-19 pandemic and how they can contribute to avoid or minimize the risks of infection in a dental setting. For this, search-related papers were retrieved from PubMED, Scielo, Google Schoolar, and American Dental Association and Centers for Disease Control and Prevention databases. The body of evidence currently available showed that Biophotonics approaches can reduce microorganism load, decontaminate surfaces, air, tissues, and minimize the generation of aerosol and virus spreading by minimally invasive, time-saving, and alternative techniques in general. However, each clinical situation must be individually evaluated regarding the benefits and drawbacks of these approaches, but always pursuing less-invasive and less aerosol-generating procedures, especially during the COVID-19 pandemic.
Keywords: COVID-19, SARS-CoV-2, Biophotonics, Dentistry, Aerosol, Cross-infection, COVID-19 dentistry biosafety, Antimicrobial blue light, UVC decontamination, Photobiomodulation, Laser therapy, Antimicrobial photodynamic therapy, Biofilm, Oral decontamination, Dental caries, Endodontics, Endodontic treatment, Periodontics, Periodontal disease, Oral soft tissues
Graphical abstract
1. Introduction
In the beginning of December 2019, an outbreak of pneumonia with an unknown cause emerged in Wuhan, China. Clinically, according to Huang et al., in 2020, the symptoms were similar to a viral pneumonia, including fever, dizziness and cough [1]. Sequencing analysis of samples obtained from the respiratory tract, revealed an infection caused by a novel coronavirus that was phylogenetically related to the severe acute respiratory syndrome virus (SARS-CoV). This virus was later named SARS-CoV-2, which was responsible for this novel coronavirus disease, later named COVID-19. In March 2020, as the COVID-19 cases began to grow exponentially, spreading globally, it led the World Health Organization (WHO) to declare COVID-19 as a pandemic disease and a public health emergency [2].
According to the WHO, as of January 2021 more than 95.3 million cases have now been confirmed worldwide, with over 2 million deaths occurring as a result. Human-to-human transmission of SARS- CoV-2 is through exposure to respiratory fluids carrying infectious virus. Exposure can occurs by inhalation of very fine respiratory droplets and aerosol particles, deposition of respiratory droplets and particles on exposed mucous membranes in the mouth, nose, or eye by direct splashes and sprays, and touching mucous membranes with hands that have been soiled either directly by virus-containing respiratory fluids or indirectly by touching surfaces with virus on them [3].
The high level of viral load in the upper respiratory tract, even among pre-symptomatic or asymptomatic patients are associated to SARS-CoV-2 shedding [4], which distinguishes it from SARS-CoV-1, where replication occurs mainly in the lower respiratory tract [5], as well as with transmission risk for other respiratory viruses [6,7].
Health care practitioners are under higher risk for this infection due to their close contact with infected patients [8]. Around the world, thousands of health professionals have had to leave their positions due to COVID-19 infection. Also, the psychological pressure and fear that can cause anxiety, depressive and distress symptoms [9] can contribute to health professionals leave their positions during the pandemic outbreak Many of them have unfortunately died as a result of the daily direct contact with the coronavirus [10]. Oral healthcare practitioners work close to patients and also, they are exposed to aerosol and droplets splashing out of patients’ oral cavity [8,11]. Therefore, dentists are under a high risk to get infection from patients and potentially spreading it to their peers, families, and other patients. Under these circumstances, it may be natural for dentists to develop a fear of being infected by their patients [12].
SARS-CoV-2 has been identified in the saliva of infected patients [13], suggesting that the aerosols generated during dental procedures from an infected person may pose a risk to dental staff. These droplets can remain even after the patient has left the clinic, leading to infection of dental professionals via aerosols and contaminated surfaces [3]. Therefore, precautionary decontamination strategies should be considered for all patients, including asymptomatic individuals, to prevent cross contamination [14].
Special care is required in dental facilities. Especially in the beginning of the coronavirus outbreak, dentists were recommended to only attend dental emergencies under strict measures wearing specific professional protection equipment to minimize the risk and maintain social distancing [11,15]. It is feasible that this extreme care is responsible for the low percentage of dentists infected during the pandemic outbreak.
According to the guidelines highlighted by the United States Center for Disease control (CDC) published in December 2020, aerosol generating procedures should be postponed or treated using alternative approaches to minimize aerosolization [16]. The dentists should prioritize the most critical dental services and provide any care in a way to minimize harm to patients from delaying care and harm to personnel and patients from potential exposure to SARS-CoV-2 infection [16]. In addition to these recommendations, a triage strategy has been adopted for oral care patient to determine whether immediate treatment is warranted or could be delayed, especially during the pandemic outbreak. In this way, we believe that Biophotonics could significantly contribute to Dentistry during this pandemic outbreak to avoid or minimize the aerosol or droplets production during oral treatments.
Biophotonics is defined as the science of generating and harnessing light (photons) to image, detect and manipulate biological materials. It is applied successfully in Medicine and Dentistry to aid in diagnosis and treatment of different diseases. Biophotonics mainly involves the interaction between light with biological tissues, and it is used to study biological tissues and biological processes at scales that range from micro to nano-levels. Biophotonics integrates lasers, photonics, nanotechnology and biotechnology. This integrated approach can provides a new dimension for diagnostics and therapeutics [17], [18], [19].
In Dentistry, Biophotonics is crucial for the early detection of diseases, to carry out more effective and minimally-invasive targeted-therapies, and to restore diseased tissues to full function and aesthetics [20]. Since Biophotonics is based on conservative and minimally invasive techniques, it could be a good option in Dentistry, especially during these pandemic outbreak [21].
In this way, the goal of this literature review was two-fold. First, we stated the risk and peculiar characteristics of the dental setting and practice especially during the COVID-19 pandemic and we described the care that dental staff should take. Second, we aimed to describe the mechanisms and discuss the potential role of several light-based approaches to avoid or minimize infection by SARS-CoV-2 during the COVID-19 pandemic.
2. Search strategy of the literature
A search of the literature was carried out in PubMED, Scielo, Google Schoolar, American Dental Association and Centers for Disease Control and Prevention (CDC – USA) to determine the current information regarding COVID-19 pandemic outbreak and applications of Biophotonics in dentistry specially under this situation. Two hundred three papers were retrieved by inserting by inserting the keywords “COVID-19″, “SARS-CoV-2″, “Biophotonics”, “Dentistry”, “Aerosol”, “Cross-infection”, “Transmission”, “COVID-19 safety protocols”, “COVID-19 dentistry biosafety”, “Dental practice management”, “Antimicrobial blue light,” “UVC decontamination”, “Photobiomodulation”, “Laser”, “Low level laser”, “Laser therapy”, “High Level Laser”, “High intensity laser”, “Photodynamic therapy”, “Antimicrobial photodynamic therapy”, “Photochemotherapy”, “Photodynamic inactivation”, “Photosensitizer”, “Biofilm”, “Curcumin”, “Oral decontamination”, “Oral infections”, “Dental caries”, “Endodontics”, “Endodontic treatment”, “Systematic review”, “Meta-analysis”, “Periodontics”, “Periodontal disease”, “Periodontitis”, “Oral mucosa” and “Oral soft tissues”. It is anticipated that this overview will create a specific picture in the dental care practitioner's mind regarding the current status and use of Biophotonics in Dentistry during the pandemic outbreak.
3. Dental settings and practical considerations
Due to the particular ergonomic scenario of the dental setting, the dental practice should be carefully revised during COVID-19 outbreak in order to offer safe care to both oral healthcare professionals and patients. In this section, important considerations regarding dental setting and dental practice and how it is related to the SARS-CoV-2 virus are given.
3.1. Cross-infection and aerosol transmission
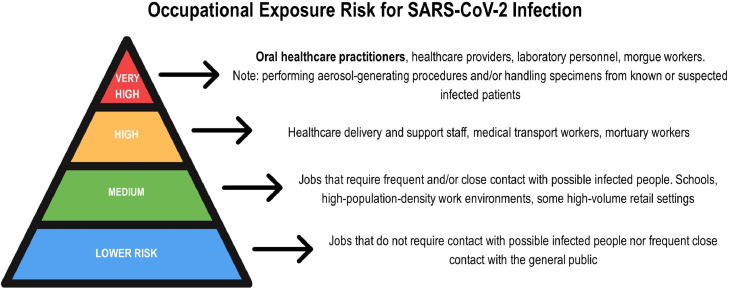
Healthcare providers are in the front-line of the COVID-19 outbreak under a notable risk of exposure to the virus. However, the peculiar characteristics of the dental setting put oral healthcare practitioners (dental clinicians, assistants and hygienists) and their patients under a high risk of cross-infection, especially by the transmission of respiratory infectious diseases, such as more recently by SARS-CoV-2 [11,22]. Moreover, the Occupational Safety and Health Administration has highlighted the concern of safe dental practice, when ranked oral healthcare practitioners under “very high exposure risk” for SARS-CoV-2 infection (Fig. 1 ) [23]. Thus, COVID-19 outbreak has claimed that preventing disease by transmission-based measures is crucial.
Fig. 1.
Pyramid of occupational exposure risk for SARS-CoV-2 infection according to the Occupational Safety and Health Administration. Note that oral healthcare practitioners are ranked under “very high” exposure risk due to the close contact with patients and the use of aerosol-generating procedures during the dental practice.
Many factors are involved in cross-transmission. Microbial species, virulence, risk of transmission, region of exposure, and frequency of exposure are the major factors involved in contracting an infectious disease [24]. Furthermore, these factors in association can increase the infection risk for both patients and oral healthcare practitioners, since they can be host/reservoir of pathogenic and non-pathogenic microorganisms [24].
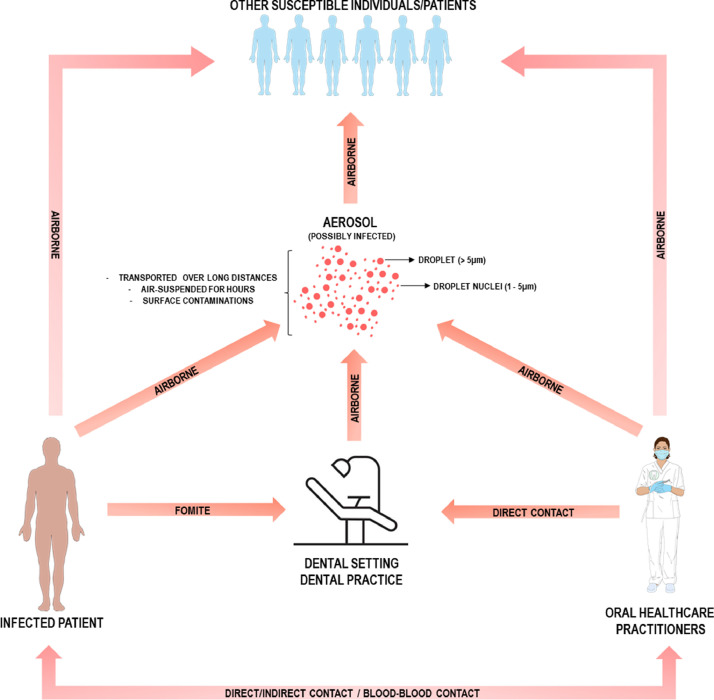
Saliva, respiratory secretion and respiratory droplets are expelled during coughs, sneezes and talking [25], which represent important routes for spreading SARS-CoV-2 worldwide. Aerosols are air-suspended small particles which can exhibit various characteristics depending on humidity, airflow and temperature [12]. Regarding aerosol size, they can range from droplets (> 5 μm) to droplet nuclei (1–5 μm). These very small particles are claimed to stay suspended in the air for hours, be carried over long distances and contaminate surfaces by up to 1 m distance [12,25,26]. In addition, SARS-CoV-2 virus can be viable even after 24–72 h, depending on the material surface [27].
In a dental setting, microorganism transmission can occur by the following routes: direct contact, blood-blood contact or dental unit waters and aerosols [24]. For SARS-CoV-2, the most common transmission route is by inhalation of droplets or aerosols from infected individuals or through direct inoculation by fomites (Fig. 2 ) [25,28].
Fig. 2.
Different contamination routes that are present during the dental practice. Note that airborne transmission and aerosol play a crucial role in virus transmission.
Generic dental practice that pre-disposes oral healthcare practitioners to infection are: saliva, blood, aerosol/droplets and infected instruments [3,11]. Additionally, many aerosol-generating procedures are performed by using high and low-speed handpieces, ultrasonic scalers, and air-water syringes [12]. Consequently, these procedures can aerosolize saliva and/or blood and spread infectious agents around the dental offices. It is important to address that ACE2-positive epithelial cells from salivary gland ducts are potential targets for SARS-CoV-2 [29], which suggests that salivary glands could behave as infection reservoirs [30] and saliva as a spread-source of the virus [31]. However, further studies are required to substantiate these hypotheses.
Aerosol plays a crucial role in airborne infections and it should be avoided especially right now. Furthermore, auxiliary biosafety approaches such as: individual dental biosafety barrier and rubber dam should be used in all procedures in addition to performing minimally invasive procedures capable to decrease aerosol formation, the pathogenicity and spreading are valuable during the SARS-CoV-2 outbreak [32,33]. In addition, extra high-volume suction for aerosol and spatter should be used during the procedures along with regular suction [3,34].
3.2. Biosafety in dentistry
The biosafety protocols were revised to reduce the risk of COVID-19 transmission to patients and dental staff [16]. The implementation of protective measures before, during, and immediately after dental care was necessary [33]. Current protocols recommend initial screening via telephone to identify patients with suspected or symptoms consistent with COVID-19 infection and, in positive cases, avoid non-emergent dental care [35]. Everyone entering the dental healthcare facility should be screened for fever and COVID-19 symptoms or exposure to people with SARS-CoV-2 infection [16,35]. Physical distancing should be adopted in the waiting rooms and in the dental office, where only the operator and assistant are within six feet of the treatment aerosol generating area [16]. Dentists should reduce aerosol production as much as possible [36] through the use of hand instrumentation, rubber dam, high-volume suction, and they should take extra-oral radiographs, since the intraoral ones may induce coughing [37]. It is advisable to perform dental practice with prevention-centered and/or minimally invasive approaches [36].
During aerosol generating procedures, dental staff should use an N95 respirator or a respirator that offers an equivalent or high level of protection [16], full-face shield, goggles, gown or protective clothing and gloves [16,37]. The proper cleaning and disinfection of environments (including waiting room), and the change of protective barriers after each patient should be ensured [16,33].
The removal of personal protective equipment (PPE) should follow the correct order, as follow: remove gloves, gown or protective clothing, the patient should leave the room or care area, wash your hands, remove eye protection and surgical mask or respirator, and perform hand hygiene [16]. However, even following all biosafety measures, SARS-CoV-2 can remain in the air, putting the dental care team at risk, after removing the PPE, and the next patients [38].
In case of a dental emergency of patient under or suspected of having COVID-19, in addition to the aforementioned care, dental treatment should be provided in an individual room with a closed door. Once aerosol generating cannot be totally avoid, the procedure should ideally take place in an airborne infection isolation room, and involves a limited number of people. In addition, it is important to consider scheduling the patient at the end of the day, and do not schedule any other patients at that time [16].
4. Biophotonics approaches
In this section, the Biophotonics approaches that may help dental healthcare practitioners to avoid or minimize the risk of SARS-CoV-2 infection are addressed. Table 1 displays an overview of all the Biophotonics approaches that will be presented in this paper.
Table 1.
Potential biophotonics approaches to be used for the dental staff during COVID-19 pandemic.
| Biophotonics approaches | Indications | Advantages | Limitations |
|---|---|---|---|
| UVC light (100–260 nm) |
Surface and air decontamination | Non-contact decontamination method; High rates of viruses’ inactivation, including SARS-CoV-2 (> 99%), in a short time |
Prolonged and continuous exposure might cause unwanted effects, such as skin damage and eye diseases |
| Antimicrobial blue light (405 nm) |
surface and air decontamination | Non-toxic to mammalian tissues | Higher radiant exposure is needed; Limited evidence regarding their efficacy against viruses |
| Low-level lasers (1 – 100 mW/cm²) | Pain control; Mitigation of aphthous ulceration and oral mucositis; Muscle relaxation; Management of dentinal hypersensitivity |
Abbreviation of chair time; Acceleration of healing and repair processes; Non-thermal and non-mutagenic effects; Management of COVID-19-related oral lesions with successfully healing after few days; Control and mitigation of TMD symptoms due to psychological pressure of quarantine |
Limited evidence of the different aspects of COVID-19 and their oral manifestations; Lack of randomized clinical trials to safely state the efficacy in COVID-19 patients |
| High-level laser (> 500 mW) (CO2, Nd:YAG, Er:YAG Er,Cr:YSGG, and diode lasers) |
Soft tissue oral surgeries (gingivectomy, ulectomy, frenectomies, fiberotomy); Cavity preparation; Caries removal; Remineralization of enamel and dentin; Root canal cleaning and disinfection |
Microbial reduction; Diminished aerosol generation; Decontamination of the irradiated area; Bloodless and atraumatic interventions; Better hemostasis; Decreased postoperative pain, trauma, edema, scarring, and infection rate; Minimally invasive; Time-saving; Shorten the number of dental visits |
Ablation plume generated may harbor bacteria, fungi, and viruses (including SARS-CoV-2); Lack of information regarding biosafety care during laser operation; High-volume suction close to the irradiation site is recommended |
| Antimicrobial photodynamic therapy | Oral decontamination; Dental caries; Endodontic infections; Periodontal lesions; Oral candidiasis; Denture stomatitis; Herpes virus |
Efficacy against enveloped viruses like SARS-CoV-2; Decrease of infection recurrence; Does not promote microbial resistance; Non-invasive and non-toxic treatment; Minimize and inactivate microorganisms’ load; Low-cost treatment; Minimally invasive; Non-surgical treatment; Wound healing; Reduced bleeding |
Lack of standardization in aPDT protocol applications |
Abbreviations: TMD (Temporomandibular disorder); CO2 (Carbon dioxide); Nd:YAG (Neodymium-doped yttrium aluminium garnet); Er:YAG (Erbium-doped yttrium aluminium garnet); Er;Cr;YSGG (Erbium, chromium-doped yttrium, scandium, gallium and garnet).
4.1. Decontamination of the surfaces and air
Given the nature of dental procedures that frequently require oral irrigation and the use of high or low speed handpiece and ultrasonic scalers, the propensity for spreading oral microbiota to surfaces and via aerosols is high [39]. Furthermore, as SARS-CoV-2 has been shown to be carried in high titers in saliva of both symptomatic and asymptomatic individuals [13,31], the risk of cross contamination within the dental clinics is a genuine concern. There are several Biophotonics approaches that have been demonstrated to effectively decontaminate surfaces and aerosols within clinical settings. Ultraviolet C (UVC; 100–260 nm wavelength), which possesses potent microbicidal properties [40], [41], [42], has been a particularly effective approach for the decontamination of patient rooms [43]. In a study by Boyce et al. [44], it was found that following the application of UVC using a mobile UVC device, the risk of positive microbial culture within patient rooms dropped by 88% illustrating the effectiveness of UVC for decontamination purposes. These findings were supported by another study by Mahida et al. [45] who found that 99.9% of bacteria could be eliminated within patient rooms as short as 5 min following the application of UVC. The authors from both studies asserted that ‘shadowing’ could be a mitigating factor that could reduce the effectiveness of UVC, however, they conceded that the use of reflective UV paint could improve the overall exposure area. Importantly, the use of UVC has also been validated for use against SARS-CoV-2. For example, in a study by Kitagawa et al. [46] found that the low UVC dose of 3 mJ/cm2 reflecting a 30 s exposure time was sufficient to reduce the viability of SARS-CoV-2 by 99.7%. Put together, these findings strongly suggest that UVC could be an effective Biophotonics approach to decontaminate surfaces within the high-risk environment of the dental clinic.
Some studies have also suggested that UVC may be a possible approach for decontaminating SARS-CoV-2 within aerosols. For example, in a study by Buonanno et al. [47] determined that UVC could inactivate 99.9% of seasonal coronaviruses within the air. While they had not directly evaluated the effectiveness against SARS-CoV-2, the authors were confident that due to genomic similarities between the seasonal coronaviruses and SARS-CoV-2 that this method would be equally effective.
As an alternative Biophotonics approach to UVC, antimicrobial blue light (aBL; 405 nm wavelength) has been attracting attention because of its intrinsic antimicrobial properties [48]. The dominant mechanism of aBL mediated antimicrobial effects is through photo-excitation of endogenous porphyrins that induce the production of reactive oxygen species (ROS) [49]. In dentistry, aBL has been shown to be a potentially efficacious approach for the treatment of gingivitis and periodontal diseases, as evidenced by in vitro evaluations [50] as well as denoted in clinical studies [51]. In fact, aBL has also been used as a method for hospital disinfection which may suggest that it could be a viable approach for the disinfection of dental clinics. In a study by Bache et al. [52] they found that a 7-day exposure under very low irradiances of aBL (0.0096 mW/cm2 – 0.2310 mW/cm2) over all surfaces present within the hospital room, resulted in an average viability reduction of 60%, with certain surfaces being completely decontaminated. These findings suggest that aBL may be a potential approach for decontaminating dental clinics. Unlike UVC, significantly higher radiant exposures are required to inactivate microbes, however, aBL has the benefit that it is not toxic to mammalian tissue even at high radiant exposures [53]. Therefore, it is feasible that aBL could be present even during procedures within the dental clinics to limit contamination of surfaces and the air. The literature regarding the efficacy of aBL against viruses are limited [49], and to date there is no evidence to show that aBL would be effective at inactivating SARS-CoV-2. However, in a recent study, it was determined that SARS-CoV-2 requires porphyrins which it derives from hemoglobin, resulting in perturbation of heme metabolism [54]. This hypothesis was brought into question by the in-silico analyses and thus it still requires experimental evidence to corroborate. Nevertheless, if SARS-CoV-2 indeed results in an accumulation of porphyrins within the host, it is feasible that aBL could inactivate SARS-CoV-2. However, as far as decontamination from surfaces are concerned, as there would be no interaction with host cells, it is difficult to predict the potential efficacy of aBL against SARS-CoV-2 on surfaces or aerosols.
Despite the proven efficiency of UV radiation for pathogen control, the safety of UV still deserves attention, since its use may cause unwanted adverse effects including skin damage and eye diseases [55]. Thus, safety guidelines of UV-based approaches are needed to ensure its general use to control or prevent SARS-CoV-2 and other infections [55].
4.2. Low-Level lasers
Low-level lasers have been widely used in dentistry to provide modulation of the inflammatory process, analgesic effects and to accelerate the healing process over soft and hard oral tissues. It is also used in the treatment of several pathologies without causing a thermal response and without mutagenic effects [18,[56], [57], [58], [59]]. The mechanisms of the low-level lasers are based on the photobiomodulation of intracellular compounds [60].
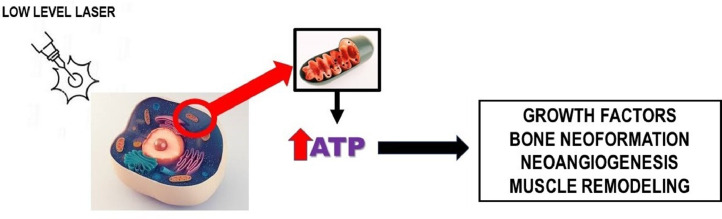
Photobiomodulation is described as a simple, efficient and low-cost treatment for acute and chronic pain [57]. The light under specific wavelengths penetrates tissues and is absorbed by mitochondria photoreceptors that activate chemical reactions resulting in increased ATP synthesis, promoting tissue regeneration, for example, in the nerves of the skin, muscle, bone and peripheral (Fig. 3 ).
Fig. 3.
Mechanism of action of low-level lasers.
Photobiomodulation provided by the use of low-level lasers can be applied in a variety of clinical oral conditions including pain control in orthodontics, the mitigation of aphthous ulceration, the management of dentinal hypersensitivity as well as the prevention and mitigation of cancer radio- and chemo-therapy-related oral mucositis among others [61], [62], [63], [64], [65], [66], [67]. In addition, low-level lasers can act on muscle relaxation and adaptation of the temporomandibular joint, to improve the quality of life [68], [69], [70], [71], during the orthodontic/orthopedic treatment and to immediate relief in a stressful situation of muscle contraction or even headaches due to malocclusion, lymphatic drainage to accelerate repair processes and also in the reduction of pain episodes in cases of trigeminal neuralgia [69] and composite resin restorations [72].
Photobiomodulation typically uses low-intensity light exposure (energy densities in the range of 1–100 mW/cm2) for a few minutes [58]. Thus, given the many possibilities including during the pandemic outbreak.
It has been reported that COVID-19 positive patients may present oral lesions such as aphthous-like ulcer, erythema, and lichen planus [73]. The same study reported that more than 78% of the COVID-19 infected patients exhibited some oral lesion [73]. In this context, combined phototherapy approaches have been used to manage these COVID-19-related oral lesions [74,75]. The use of photobiomodulation has been claimed even in severe cases of acute respiratory distress syndrome in COVID-19 infected patients [76]. Clinical outcomes related to the application of photobiomodulation have shown successfully healing of oral lesions after few days [74,75]. These benefits appear to be related on the photobiomodulation's capacity to reduce or inhibit important substances involved in pain and inflammatory processes [74], leading to an enhanced healing and improved quality of life for patients. A recent systematic review reported that photobiomodulation can significantly decrease the production of pro-inflammatory cytokines and some interleukins, reducing inflammation processes, regenerating damaged tissues [77], and balancing the immune system [76].
Moreover, during the pandemic outbreak, people with a positive diagnosis or with suspected COVID-19 case have experienced great psychological pressure [78]. In addition, people who are quarantined, fulfilling social isolation, restricted to leave, concerned about infection, afraid of death, lack information, and who have lost daily social relationships, can further experience high levels of anxiety and depression [79]. Once psychological factors are associated with the development of disorders, including temporomandibular disorders (TMD) [80], low-level lasers could contribute to control and minimize the symptoms of TMD, especially during the pandemic outbreak.
Although the use of photobiomodulation to treat COVID-19 positive patients is promising, further information regarding the different aspects of COVID-19 [75] and randomized clinical trials [77] are required in order to safely state their use and effectiveness.
4.3. High-level lasers
In Dentistry, lasers that work beyond the 500 mW range are called high-level lasers. These lasers have been applied to high-intensity laser therapies given their soft tissue and/or hard tissue cutting hability. For such uses, CO2, Nd:YAG, Erbium (Er:YAG, and Er,Cr:YSGG) and diode lasers are among the most used in Dentistry [81].
New insights or scientific evidence could allow dentists to improve the biosafety measures and prevent cross contamination when dealing with new infectious diseases such as COVID-19. High-level lasers used in Dentistry meet guidelines to minimize risk of cross infection by SARS-CoV-2 mainly through microbial reduction and diminished aerosol production [18]. Moreover, high-level lasers are widely used in dentistry as a minimally invasive approach that allows dental professionals to perform different procedures more rapidly as well as few follow-ups and patient visits, which is very important specially during the pandemic outbreak [18].
4.3.1. Soft tissues applications
High-level lasers are used to quickly and effectively addressing soft tissue complications through bloodless and atraumatic surgical interventions. The main indication for high level lasers in soft tissues is based on surgeries such as gingivectomy, ulectomy, frenectomies, and fiberotomy. The benefits of using high-level lasers for soft tissue oral surgery include better hemostasis, decreased postoperative pain and infection rate, minimal tissue contraction, low or no need for sutures, short surgical stages, decreased trauma, edema and scarring, besides the reduced need for local anesthetics. As high-powered lasers act by increasing the temperature, their use also has the advantage of decontaminating the irradiated area. Thus, there is a great probability of the tissue repair process occurs with no presence of infection in the surgical wound [82].
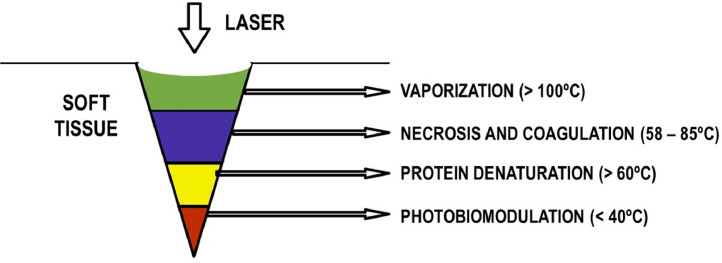
Among the high-level lasers used to the soft tissue oral surgery, the diode and CO2 lasers play a special role given their cutting ability, providing safer procedures. Diode laser wavelength ranges between 810 and 1064 nm and it is absorbed by pigmented tissues that contain hemoglobin, melanin, and collagen. The laser/tissue interaction may lead to coagulation, denaturation of proteins, vaporization, and carbonization of the tissues depending on the amount of energy emitted (Fig. 4 ). This process can seal the blood vessels promoting hemostasis, inhibits the pain receptors on the incision area, decreases the risk of infection, and may improve the healing process. Diode laser has been used for soft tissue surgery in the oral mouth like periodontal surgery due to effective tissue ablation, hemostatic and bactericidal effects, and a more adequate and clear visualization of the surgical area during its use [83].
Fig. 4.
Mechanism of action of high-level lasers. They act by ablation, in which laser energy is absorbed by water and hydroxyapatite and result in particle removal by micro-explosions.
Likewise, CO2 laser has been increasingly used in oral surgeries as an efficient alternative to conventional surgeries, as it shows good absorption by the tissue water, resulting in the vaporization of the intra and extracellular liquid with disintegration of the cells. The advantages of the CO2 laser are related to the precise elimination of soft tissues with hemostasis and simultaneous microorganism reduction, and postoperative process with a mild inflammatory response by reducing infection [83,84].
Moreover, soft-tissues surgical procedures such as frenectomy and mucocele removal with diode or Nd:YAG lasers could be performed within a few minutes, reducing the need for anesthesia or wound care and therefore reducing the fear of the patient [85]. Besides, diode laser has positively influenced the post-surgical wound healing by the acceleration of the epithelial regeneration, providing lower postoperative pain and discomfort than conventional techniques [86], [87], [88].
Er:YAG, Er,Cr:YSGG, CO2, diode, and Nd:YAG lasers have been also employed for gingival tissue ablation. Among that, Er:YAG laser has shown to be the most efficient and refined for gingival ablation with minimal or no thermal damage on surrounding tissues [86]. Er,Cr:YSGG laser has been used in nonsurgical periodontal treatment showing additional improvements in terms of dental pocket reduction and gingival bleeding compared to traditional nonsurgical therapy [89].
4.3.2. Hard tissues applications
The lasers used to hard tissues act by ablation, a mechanism in which laser energy is absorbed by water and hydroxyl group in hydroxyapatite, causing rapid heating and swelling that result in high internal pressures leading to the removal of the substrate by micro-explosions. Cavity preparations with high-level laser allows maximum conservation of dental structure, low need for anesthesia with additional characteristics making the enamel, dentin and cement more resistant to the acid attack of bacteria [82].
For the dental hard tissues, Er:YAG laser is mainly used for cavity preparations, caries removal, polymer restorations removal, remineralization of enamel and dentin, and to decrease the amount of microorganisms, apart from be able to work with reduced water spray, reducing the contamination of the environment through the aerosol produced during the procedures, which could prevent or minimize the risk of transmission for COVID-19 as much as possible [18]. For endodontic application, Er:YAG laser-activated irrigation has been shown as an effective method of root canal cleaning and disinfection [90].
According to Brugnera Jr. et al., in 2020, Er:YAG laser can be an important tool to create a less contaminated clinical environment once significantly decreases the production of aerosol during the dental procedures. It is important for both, the health of the dental staff and also to minimize the contamination of the environment and equipments. Especially right now, during the COVID-19 pandemic outbreak, this could be an important tool to prevent or minimize the risk of transmission.
A previous published literature review [83] comparing surgical methods of treatment for soft tissue showed that the use of high-level laser was considered as minimally invasive and showed superior advantages to those of the conventional scalpel, such as reduction of bleeding, inflammation, post-operative pain and lower probability of scars. These reports meet guidelines to minimize risk of cross infection by SARS-CoV-2, insofar as high-level lasers allow dental professionals to perform quickly procedures which requires shorter operative time, as well as fewer follow-ups and patient visits. Furthermore, high level lasers are able to operate with reduced water spray decreasing the aerosol produced that could help to avoid or minimize cross infection in a dental setting [18].
Although less aerosol-generation is observed, high-level laser generates a spray, ablation plume, or vaporization due to their increased temperature during the use. Few studies have investigated the composition of laser ablation plume, but it is reported that the plume may contain bacteria, viruses, and fungi [91], which suggests that the ablation plume can harbor SARS-CoV-2 when using the laser in infected patients [92]. In addition, a recent published systematic review highlighted the lack of information regarding biosafety care when dental clinicians use laser equipment [92]. The use of high-volume suction close to the irradiation site is strongly recommended during the procedure [92].
4.4. Antimicrobial photodynamic therapy
The term “photodynamic action” describes a reaction that depends on the presence of molecular oxygen and the use of a chemical compound called as photosensitizer and a light source under specific wavelength [93], [94], [95]. Hermann von Tappeiner firstly noted that isolated light and dye, in the absence of oxygen, did not cause cell death. This author continued to develop the concept of photodynamic action and presented the first results in humans using eosin as a photosensitizer to treat different skin diseases [94].
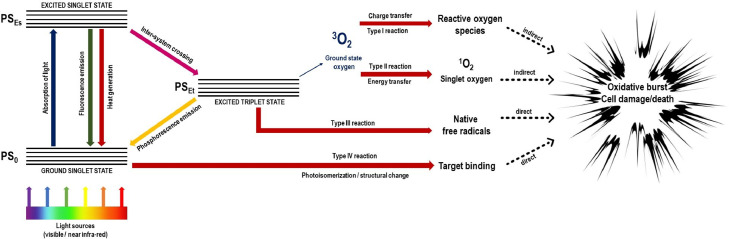
Antimicrobial photodynamic therapy (aPDT) basically consists on the interaction among three components [93,[96], [97], [98], [99], [100], [101]], resulting in photo-physical-chemical reactions responsible to produce reactive oxygen species (ROS) with high oxidation ability of the cellular components, causing cell damage [93] (Fig. 5 ).
Fig. 5.
Adapted Jablonski's diagram showing the photo-physical and the photo-chemical mechanisms involved in aPDT reactions.
The mechanism of action of the photosensitizer on the cellular targets determines the type of reaction that occurs during antimicrobial photodynamic therapy (aPDT). The photosensitizer can act directly or indirectly. Direct reactions depend on the presence of oxygen (reactions type I and II), while indirect reactions do not depend on the presence of oxygen (reactions type III and IV) [102]. During aPDT reaction, the photosensitizer absorbs photons emitted by the light source. In this way, the photosensitizer moves from the fundamental state to excited singlet state. Once the photosensitizer undergoes this initial transformation, it can return to the ground state by releasing excitation energy (fluorescence or heat) through an internal conversion or move to a less unstable state of excitation, called triplet state [103], [104], [105], [106], [107]. Thus, upon reaching this state, the photosensitizer can return to the fundamental state through the emission of phosphorescence or by two reactions that generate reactive oxygen species (ROS) [103], [104], [105], [106], [107], [108].
In the type I reaction, the photosensitizer under excited state reacts with the target tissues. The reaction occurs by the transfer of electrons, forming superoxide radicals (O2 −) that can undergo chemical redox reactions and become hydrogen peroxide (H2O2) which is a highly-reactive precursor of free hydroxyl radicals. The formation of these hydroxyl radicals is caused by an oxidative process (Fenton-like reactions) that are delivered by the decomposition of hydrogen peroxide (H2O2) [103], [104], [105], [106], [107]. From this stage, the reaction can interact with any biomolecule in the environment, causing cell death [109].
In the type II reaction, the energy of the photosensitizer under the excited triplet state is transferred to the fundamental triplet state of oxygen, generating singlet oxygen, with high toxicity [109,110]. The singlet oxygen generated can interact with various molecules inside the cell, causing cell death [110,111]. The type II reaction has a toxic effect of singlet oxygen, causing a rapid and indiscriminate reaction with all types of biomolecules [112]. Because it has no defense mechanism against these molecules, the cell dies [112]. Type I and II reactions can occur at the same time, regardless of whether they have different paths. The ratio between the two reactions is directly influenced by the intracellular substrates, the characteristics of the photosensitizers used and the concentration of oxygen present in the cellular environment [113].
Type III and IV reactions are not dependent on oxygen to promote microbial cell death during secondary reactions caused by the direct activation of the photosensitizer. In the type III reaction, an interaction of the photosensitizer under triplet state with free radicals occurs which promotes cytotoxicity inside the microbial cell. Finally, in the type IV reaction, an intramolecular remodeling occurs, so-called photoisomerization after excitation of the photosensitizer under specific wavelength, helping the connection to the target cell [102].
The application of aPDT in Dentistry has been described as a widely used method for the treatment of bacterial and fungal infections, oral cancer, diagnosis of oral malignant lesions, disinfection of the oral cavity and for the treatment of dental caries and other oral infections, such as periodontal and endodontic infections [114], [115], [116]. In addition, aPDT can be considered a simple technique, with attractive characteristics for use, since it has some important advantages, such as: broad spectrum of action (photosensitizer acts on several microorganisms), low or no occurrence of side effect and has no resistance to treatment regardless of the number of applications [114].
The use of aPDT as an alternative method for disinfecting the oral cavity, treating pneumonia and/or respiratory tract infections and also against enveloped viruses like SARS-CoV-2 has proven effective [117,118]. In addition, aPDT may be an effective method against SARS-CoV-2, due to the formation of reactive oxygen species (ROS) that are able to damage viruses [117], [118], [119] and prevents microorganisms to penetrate the mucous membrane, controlling possible secondary infections [120]. Considering the mutation ability of SARS-CoV-2, the use of aPDT for oral decontamination becomes even more important, since it is a method that does not promote microbial resistance mechanisms [120], [121], [122]. Therefore, an alternative to perform this procedure with the purpose of disinfection of the oral cavity [123].
According to Nunes et al. [123], aPDT can be useful to reduce the spread of COVID-19 in dental offices. According to recent published studies, aPDT showed in vitro antiviral activity against SARS-CoV-2 [117,118]. Moreover, a systematic review of 27 studies showed that aPDT can inactivate enveloped and non-enveloped DNA and RNA viruses, which suggest their promising potential against SARS-CoV-2 [124].
Thus, in order to contribute to the safety of all dentistry professionals, the association of other protocols with aPDT can be a useful method to prevent infections by COVID-19 in a dental care facility.
4.4.1. Oral decontamination
The oral cavity is an appropriate environment for the development of various microorganisms, since it has different surfaces, secretions and nutrients. As a result, the environment becomes favorable for the colonization of several microorganisms [125,126]. Furthermore, hard tissues, such as dental enamel, may facilitate bacteria attachment and the consequently development of biofilms. However, some dental materials used in dental practice to restore the integrity of the teeth and reestablish the patient's function, such as resin-based composite, amalgams, ceramics, orthodontic wires, among others, can also facilitate the formation of the biofilms [127,128].
Due to the various factors that contribute to the formation of biofilm and the colonization of microorganisms in the oral cavity, the control of the biofilms in the oral cavity is considered a great challenge. As an alternative, chlorhexidine-based mouthwashes represent the most used chemical strategy for biofilm control [114,[129], [130], [131]]. Chlorhexidine has a large absorption spectrum, which acts on bacteria (Gram-positive and Gram-negative), yeasts, and virus. In addition, this antimicrobial agent reduces 90% of bacteria for more than 7 h [132]. However, several adverse effects are related to the continued use of chlorhexidine in the mouth, such as altered taste, discolored teeth, burning sensation [114,129], increased calculus formation and soft tissues discoloration [133], [134], [135].
The application of aPDT has been used against pathogenic microorganisms as an antibacterial, antifungal and antiviral non-invasive treatment [136]. For this reason, aPDT has been increasingly investigated to verify the effectiveness in oral cavity disinfection. In a systematic review published by Kallesariam et al. [137], it was observed that aPDT can be effective for decontamination of the oral cavity, with important clinical applications, since this procedure is performed, a reduction in the microorganisms’ load from oral biofilms (fungus, bacteria, yeasts and virus) occurs. The reduction and inactivation of microorganisms consequently mitigate their possible spread during aerosol-generating procedures, which may include the SARS-CoV-2 virus. According to Syyatchenko et al., in 2021, aPDT shows high antiviral activity against SARS-CoV-2 by the use of methylene blue combined in vitro to Radachlorin [118]. Thus, in pandemic times, aPDT could be a good option to decontaminate the oral cavity previously to different clinical procedures in order to minimize and inactivate microorganisms load, as well as, reduce their pathogenicity. However, we emphasize that aPDT does not completely eliminate the risk of cross-transmission, making the biosafety measures indispensable.
4.4.2. Dental caries
Dental caries can be defined as a multifactorial, dynamic and biofilm-mediated disease [138], [139], [140]. The disease development and progression results from the biofilm imbalance and acid production from dietary carbohydrate fermentation which causes demineralization of the tooth surface [141,142]. In the long term, dental caries can cause not only biological and dental consequences, but also negative impacts on the individual's quality of life and general health. Although a significant reduction in the prevalence of dental caries in many countries around the world [141], except in Scandinavian countries [143] has been observed, caries disease still remains a major public health problem, affecting more than 2.4 billion people of all age groups around the world [141,144].
During the dental caries progression, it is possible to observe the development of visible changes on the tooth structure, or incipient caries lesions, which initially do not cause any discomfort/pain, but whether untreated, can progress to cavitation [142,145,146]. New strategies, the improvement of conventional techniques and the development of new equipment for the treatment of carious lesions are important not only to reduce the prevalence of caries, but also to promote oral health [147]. Therefore, minimally invasive dentistry aims the prevention, remineralization and, once necessary, minimal intervention during restorative procedures, preserving as much as possible the sound dental structure [142,148].
Traditionally, complete caries removal was performed (infected and affected) during cavity preparation for future restoration. However, contemporary dentistry aims to prepare the cavity in a minimally invasive manner, removing only the infected dentin [149,150]. This type of treatment has been increasingly used in order to avoid pulp exposure [151,152]. The complete removal of decayed tissue can promote pulp exposure due to the depth of the cavity and can also promote a reduction in the thickness of the remaining dentin (thickness between the bottom of the cavity and the dental pulp) [149,153]. Therefore, more conservative approaches are being adopted [149].
During the pandemic outbreak, the protocols in a dental setting were reviewed and changed [154]. Usually, to remove the dental caries rotary instruments, such as high and low-speed handpiece and dental ultrasound are used. Both of them are not recommended at this moment, as they can induce coughing and generate aerosols. If necessary, it is up to the dentist to prefer atraumatic restorative techniques that uses only a sharp spoon excavator to remove the dental caries [154,155].
Adjunctive antimicrobial techniques should be used as a complimentary technique after dental caries removal for providing the disinfection of the remaining bacterially contaminated dentine before placing a restoration [156]. In this way, aPDT could be a valuable clinical possibility [156]. aPDT can acts as a promising adjuvant method to promote dentin decontamination prior to the future restoration [156], reducing the bacteria load at the cavity preparation. Some studies in the literature using dentin substrate and different in vitro models of carious lesion induction and in vivo models, showed promising results provided by aPDT [157], [158], [159].
4.4.3. Endodontic infections
Endodontic infections are one of the main causes of emergency dental visits [160]. During the pandemic of SARS-CoV-2, endodontic emergencies have increased more than normally [161] which highlight that dental healthcare practitioners can manage these emergencies more often.
The endodontic treatment mainly aims to control the infection of the root canal system [162], [163], [164]. Conventionally, mechanical debridement and chemical irrigation are employed to obtain suitable disinfection [164], [165], [166]. However, the anatomical complexity of the root canal system hinders a complete debridement and removal of the microorganisms’ load [167], which can lead the endodontic treatment to fail due to persistent contamination.
In the last few years, many laboratorial and clinical findings have reported aPDT as a adjunctive strategy to reduce bacterial load, post-operative pain, and to enhance the chances of tissue healing [162,163,168,169]. Moreover, the easy application and unlikely generation of microbial resistance also encourage the use of aPDT combined with endodontic procedures [170].
However, there is no standard protocol for aPDT application since the aPDT parameters, including type of photosensitizer, irradiation time and power density used can vary among different studies [171]. Regarding the light source, diode lasers were the most used for aPDT in endodontics due to the hinder access for light irradiation in the root canal systems [171]. Although the promising results for aPDT, a need for high-quality and well-conducted studies which aims to state an aPDT protocol was reported [171].
Taking the SARS-CoV-2 outbreak and the scientific reports into account, aPDT can be a useful strategy in endodontic treatment since it can promote enhanced microorganism elimination which may decrease infection recurrence, the number of emergency dental visits, and the risk of SARS-CoV-2 transmission. Moreover, rubber dam and high-power saliva ejectors are strongly recommended as protective barriers to avoid contaminated spread during endodontic interventions [34,161]. According to Marshall K., in 2017 [172], with the use of rubber dam, 98.5% of the microbial content of such aerosols is shown to be eliminated. In addition, the use of rubber dam can avoid the continued touch recontamination of the gloved hands of dentist and nurse with the patient's oral mucosa.
4.4.4. Periodontics
The COVID-19 pandemic represents a challenging environment for periodontology [173]. Furthermore, the global burden of periodontal diseases remains high [174]. Periodontitis is one of the most common inflammatory diseases [175] associated with the presence of bacteria and their subproducts, mediated by the host immune response, resulting in attachment and bone loss [176]. Non-surgical mechanical therapy for removal of bacterial biofilms and mineralized deposits on the root surface remains the treatment of choice for periodontitis [177].
The high infectivity of SARS CoV-2 makes ultrasonic devices units not recommended due to the high generation of aerosols. Therefore, scalers and curettes should be the instruments of choice for non-surgical mechanical therapy [3]. Likewise, the application of aPDT stands out during this pandemic time, as a low-cost local treatment, minimally invasive, non-toxic to tissues, without causing bacterial resistance [104] and killing a broad spectrum of pathogens: bacteria, fungi, parasites and viruses [136]. aPDT has become a promising adjunctive therapy to non-surgical mechanical therapy in the treatment of periodontitis, showing similar clinical results compared to antibiotic therapy [178]. Another feature that aPDT can provide is the bleeding reduction during the probing of the treated sites [179], [180], [181] which is important due to the transmission routes of SARS CoV-2 [3].
In addition, the combination of aPDT to non-surgical mechanical therapy during supportive periodontal therapy may lead to improvements in clinical parameters such as probing depth and clinical attachment level [182]. A single session of mechanical therapy followed by three aPDT sessions may enhance clinical outcomes [183], thus avoiding repeated instrumentation that can cause damage to the root surface, hypersensitivity, gingival recession and bleeding [184]. Also, the application of this adjunctive approach during surgical therapy has shown superior clinical parameters than surgical therapy alone [185].
Different systematic reviews and meta-analysis have assessed the role of aPDT as an adjunct to surgical and non-surgical mechanical therapy in the treatment of periodontal diseases [178,182,[185], [186], [187], [188], [189]]. A total of 26 randomized clinical trials using as test group (aPDT + surgical/non-surgical mechanical therapy) and usually as control group (surgical/non-surgical mechanical therapy alone or in combination with antibiotic therapy) were reviewed. Diverse clinical parameters such as probing depth, bleeding on probing, clinical attachment level, gingival recession, plaque index and gingival index, as well as immunological and bacterial profiles were evaluated, showing better or comparable results to those of the control groups. However, these positive outcomes described for the adjunctive aPDT should be interpreted carefully due the studies heterogeneity involving multiple variables such as clinical, photosensitizer and laser parameters [190]. Therefore, the scientific community needs to establish and require a minimum of clinical parameters for aPDT research to be able to replicate or compare therapies with greater precision [177].
The performance of procedures safely for both patients and staff in the face of this pandemic is mandatory. In this way, aPDT following accurate protocols can serve as a safe, minimally invasive and efficient complement in the treatment of periodontal diseases.
4.4.5. Soft tissues
Microorganisms are involved in several diseases that can affect oral soft tissues [190]. The fungi Candida albicans can act as opportunistic pathogens associated with oral mucosa diseases such as oral candidiasis and denture stomatitis [191]. Similarly, the Herpes Simplex virus can cause infectious diseases in the orofacial region [192]. The implementation of aPDT mediated by blue methylene as an adjunct treatment in oral candidiasis has been supported to a certain extent in terms of reduction of microbial load [193]. With regard to denture stomatitis, aPDT seems to offer results comparable to those of conventional antifungal therapies [194]. Likewise, the application of this therapy is promising for the herpes simplex virus, within the available scientific evidence, the aPDT can be a possible effective treatment for recurrent herpes labialis [195].
In addition, aPDT has been described as an effective alternative treatment option for potentially malignant oral disorders, such as oral leukoplakia, oral lichen planus, erythroleukoplakia, and oral verrucous hyperplasia [196]. Furthermore, cancer patients who received chemotherapy can be affected by oral mucositis that are often infected by microorganisms such as Candida Albicans and Herpes Simplex virus [197]. aPDT may represent an adequate treatment for oral mucositis lesions, which shows positive results in wound healing in addition to its antimicrobial properties [198]. Therefore, aPDT can play a key role for elderly patients and patients who have underlying health conditions, which are a more susceptible population for the COVID-19 pandemic [199]. aPDT has demonstrated promising outcomes when used as isolated therapy or in combination with other conventional treatments such as surgery, chemotherapy and radiotherapy in the treatment of malignant and premalignant oral, head and neck lesions [200]. According to the available research, aPDT is a potential adjuvant to conventional therapies for the treatment of several diseases [190]. However, further research is needed using well-designed randomized controlled clinical trials to establish accurate clinical parameters for the treatment and prevention of different pathologies.
SARS-CoV-2 outbreak can be seen as an opportunity to improve resources and provide safer procedures for both health workers and patients [201] in which aPDT stands out as a non-invasive safe therapy, which shows several remarkable effects, such as wound healing, reducing bleeding, effectively against pathogenic microorganisms and that does not cause bacterial resistance, compared with the use of antibiotic [190].
5. Safety use of biophotonics approaches in the SARS-CoV-2 pandemic
According to Arnabat-Dominguez et al. [202], in addition to all common and routine biosafety recommendations for dentistry, and especially right now during the pandemic outbreak, specific care should be followed to the use of different approaches based on the use of Biophotonics.
For the safety use of high-level lasers, the lowest laser parameters [202] that could provide adequate clinical effects in combination with a reduction of air flow under a correct water spray during laser ablation may reduce the aerosol production and viral spread. Under any clinical situation, special glasses/google for each laser wavelength, the use a rubber dam to avoid viral contamination from the mouth and saliva, and a high-volume saliva ejector/volume suction or a vacuum system close to the treated site should be employed. For the clinical applications of low-level lasers i.e., photobiomodulation, the handpieces of the devices should be protected through the application of transparent removable plastic films like PVC to avoid any contact with the skin, mucosa and oral fluids. According to Rodrigues et al. [203] PVC plastic film is the most suitable for this purpose and it could cause minor changes in the power output. In addition, it is an easily accessible material that is able to prevent cross-contamination.
Special care should also be provided to the disinfection of the light-based devices before and after their clinical use.
6. Conclusions
SARS-CoV-2 outbreak has forcibly changed the dental practice and biosafety measures due to the high exposure risk of infection by oral healthcare practitioners. Aerosol-generating procedures are commonly employed in dental practice and may be an important route for virus transmission and spread. In this context, light-based approaches have claimed to reduce the microorganism load, to decontaminate surfaces, air, tissues, and the generation of aerosol and virus spreading. At the same time, the Biophotonics approaches majorly represent minimally invasive, time-saving, and alternative techniques that can help oral healthcare practitioners to combat or avoid infection by SARS-CoV-2 and to provide safe care for patients.
Declaration of Competing Interest
The authors deny any conflict of interest in this paper.
Acknowledgments
The authors would like to thank CAPES (Coordination for the Improvement of Higher Education Personnel, Finance Code 001, Grant Number: 001/1776257) and FAPESP (São Paulo Research Foundation, Process Numbers: 2013/07276-1 and 2019/10851-4) for the financial support.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus Disease (COVID-19) Situation Reports, (n.d.). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed February 2, 2021).
- 3.Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020;12 doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 5.Cheng P.K.C., Wong D.A., Tong L.K.L., Ip S.M., Lo A.C.T., Lau C.S., Yeung E.Y.H., Lim W.W.L. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet. 2004;363:1699–1700. doi: 10.1016/S0140-6736(04)16255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreira L.P., Watanabe A.S.A., Camargo C.N., Melchior T.B., Granato C., Bellei N. Respiratory syncytial virus evaluation among asymptomatic and symptomatic subjects in a university hospital in Sao Paulo, Brazil, in the period of 2009-2013. Influenza Other Respir. Viruses. 2018;12:326–330. doi: 10.1111/irv.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsang T.K., Cowling B.J., Fang V.J., Chan K.H., Ip D.K.M., Leung G.M., Peiris J.S.M., Cauchemez S. Influenza a virus shedding and infectivity in households. J. Infect. Dis. 2015;212:1420–1428. doi: 10.1093/infdis/jiv225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ather A., Patel B., Ruparel N.B., Diogenes A., Hargreaves K.M. Coronavirus disease 19 (COVID-19): implications for clinical dental care. J. Endod. 2020;46:584–595. doi: 10.1016/j.joen.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfefferbaum B., North C.S. Mental health and the Covid-19 pandemic. N. Engl. J. Med. 2020;383:510–512. doi: 10.1056/nejmp2008017. [DOI] [PubMed] [Google Scholar]
- 10.The Lancet COVID-19: protecting health-care workers. Lancet. 2020;395:922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng L., Hua F., Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J. Dent. Res. 2020;99:481–487. doi: 10.1177/0022034520914246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zemouri C., De Soet H., Crielaard W., Laheij A. A scoping review on bio-aerosols in healthcare & the dental environment. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.To K.K.W., Tsang O.T.Y., Yip C.C.Y., Chan K.H., Wu T.C., Chan J.M.C., Leung W.S., Chik T.S.H., Choi C.Y.C., Kandamby D.H., Lung D.C., Tam A.R., Poon R.W.S., Fung A.Y.F., Hung I.F.N., Cheng V.C.C., Chan J.F.W., Yuen K.Y. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020;71:841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., Zimmer T., Thiel V., Janke C., Guggemos W., Seilmaier M., Drosten C., Vollmar P., Zwirglmaier K., Zange S., Wölfel R., Hoelscher M. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382:970–971. doi: 10.1056/nejmc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bescos R., Casas-Agustench P., Belfield L., Brookes Z., Gabaldón T. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J. Dent. Res. 2020;99:1113. doi: 10.1177/0022034520932149. [DOI] [PubMed] [Google Scholar]
- 16.C. for D.C. and Prevention, Guidance for dental settings, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html.
- 17.Kishen A., Asundi A. Imperial College Press; 2006. Fundamentals and Applications of Biophotonics in Dentistry. Series on. [DOI] [Google Scholar]
- 18.Brugnera Junior A., Zanin F., Nammour S., Groisman S. Biophotonics in health care and its relevance in fighting the coronavirus disease. Photobiomodul. Photomed. Laser Surg. 2020;38:521–523. doi: 10.1089/photob.2020.4883. [DOI] [PubMed] [Google Scholar]
- 19.Rahman S.U., Mosca R.C., Govindool Reddy S., Nunez S.C., Andreana S., Mang T.S., Arany P.R. Learning from clinical phenotypes: low-dose biophotonics therapies in oral diseases. Oral Dis. 2018;24:261–276. doi: 10.1111/odi.12796. [DOI] [PubMed] [Google Scholar]
- 20.Singh S., Gupta I., Amarnath J., Gupta R., Pandey A., Gupta S. Biophotonics: a magical ray of hope in periodontics. Rama Univ. J. Dent. Sci. 2016;3:11–18. [Google Scholar]
- 21.Saito Nogueira M. Biophotonics for pandemic control: large-area infection monitoring and microbial inactivation of COVID-19. Photodiagn. Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajaj N., Granwehr B.P., Hanna E.Y., Chambers M.S. Salivary detection of SARS-CoV-2 (COVID-19) and implications for oral health-care providers. Head Neck. 2020;42:1543–1547. doi: 10.1002/hed.26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Occupational Safety and Health Administration, Guidance on Preparing Workplaces for COVID-19, 2020.
- 24.Volgenant C.M.C., de Soet J.J. Cross-transmission in the dental office: does this make you ill? Curr. Oral Health. Rep. 2018;5:221–228. doi: 10.1007/s40496-018-0201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization Transmission of SARS-CoV-2: implications for infection prevention precautions. Sci. Brief. 2020:1–10. 09 July 2020. [Google Scholar]
- 26.Barker J., Jones M.V. The potential spread of infection caused by aerosol contamination of surfaces after flushing a domestic toilet. J. Appl. Microbiol. 2005;99:339–347. doi: 10.1111/j.1365-2672.2005.02610.x. [DOI] [PubMed] [Google Scholar]
- 27.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., De Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J., Liao X., Qian S., Yuan J., Wang F., Liu Y., Wang Z., Wang F.S., Liu L., Zhang Z. Community transmission of severe acute respiratory syndrome Coronavirus 2, Shenzhen, China, 2020. Emerg. Infect. Dis. 2020;26:1320–1323. doi: 10.3201/eid2606.200239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L., Wei Q., Alvarez X., Wang H., Du Y., Zhu H., Jiang H., Zhou J., Lam P., Zhang L., Lackner A., Qin C., Chen Z. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J. Virol. 2011;85:4025–4030. doi: 10.1128/jvi.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J., Li Y., Gan F., Du Y., Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J. Dent. Res. 2020;99:989. doi: 10.1177/0022034520918518. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Ren B., Peng X., Hu T., Li J., Gong T., Tang B., Xu X., Zhou X. Saliva is a non-negligible factor in the spread of COVID-19. Mol. Oral Microbiol. 2020;35:141–145. doi: 10.1111/omi.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montalli V.A.M., Garcez A.S., Montalli G.A.M., França F.M.G., Suzuki S.S., Mian L.M.T., Motta R.H.L., Napimoga M.H., Junqueira J.L.C. Individual biosafety barrier in dentistry: an alternative in times of covid-19. Preliminary study. RGO Rev. Gaúcha Odontol. 2020;68 doi: 10.1590/1981-863720200001820200088. [DOI] [Google Scholar]
- 33.Cabrera-Tasayco F.D.P., Rivera-Carhuavilca J.M., Atoche-Socola K.J., Peña-Soto C., Arriola-Guillén L.E. Disaster Medicine and Public Health Preparedness; 2020. Biosafety Measures at the Dental Office After the Appearance of COVID-19: A Systematic Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samaranayake L.P., Fakhruddin K.S., Buranawat B., Panduwawala C. The efficacy of bio-aerosol reducing procedures used in dentistry: a systematic review. Acta Odontol. Scand. 2021;79:69–80. doi: 10.1080/00016357.2020.1839673. [DOI] [PubMed] [Google Scholar]
- 35.Madurantakam P. How can dentistry get back to work safely? Evid. Based Dent. 2020;21:48. doi: 10.1038/s41432-020-0090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Halabi M., Salami A., Alnuaimi E., Kowash M., Hussein I. Assessment of paediatric dental guidelines and caries management alternatives in the post COVID-19 period. A critical review and clinical recommendations. Eur. Arch. Paediatr. Dent. 2020;21:543–556. doi: 10.1007/s40368-020-00547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Dental Association . ADA; 2020. Summary of ADA Guidance During the COVID-19 Crisis; pp. 19–20. https://www.ada.org/en/press-room/news-releases/2020-archives/april/summary-of-ada-guidance-during-the-covid-19-crisis?utm_source=mouthhealthy&utm_medium=covid-19-mh&utm_content=cv-gov—interim-statement&utm_campaign=covid-19. [Google Scholar]
- 38.Bizzoca M.E., Campisi G., Muzio L.Lo. Covid-19 pandemic: what changes for dentists and oral medicine experts? A narrative review and novel approaches to infection containment. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17113793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rautemaa R., Nordberg A., Wuolijoki-Saaristo K., Meurman J.H. Bacterial aerosols in dental practice - a potential hospital infection problem? J. Hosp. Infect. 2006;64:76–81. doi: 10.1016/j.jhin.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh C., Sun P.P., Araud E., Nguyen T.H. Mechanism and efficacy of virus inactivation by a microplasma UV lamp generating monochromatic UV irradiation at 222nm. Water Res. 2020;186 doi: 10.1016/j.watres.2020.116386. [DOI] [PubMed] [Google Scholar]
- 41.Kim D.K., Kang D.H. UVC LED irradiation effectively inactivates aerosolized viruses, bacteria, and fungi in a chamber-type air disinfection system. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00944-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welch D., Buonanno M., Grilj V., Shuryak I., Crickmore C., Bigelow A.W., Randers-Pehrson G., Johnson G.W., Brenner D.J. Far-UVC light: a new tool to control the spread of airborne-mediated microbial diseases. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-21058-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nogueira M.S. Ultraviolet-based biophotonic technologies for control and prevention of COVID-19, SARS and related disorders. Photodiagn. Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boyce J.M., Havill N.L., Moore B.A. Terminal decontamination of patient rooms using an automated mobile UV light unit. Infect. Control Hosp. Epidemiol. 2011;32:737–742. doi: 10.1086/661222. [DOI] [PubMed] [Google Scholar]
- 45.Mahida N., Vaughan N., Boswell T. First UK evaluation of an automated ultraviolet-C room decontamination device (Tru-D™) J. Hosp. Infect. 2013;84:332–335. doi: 10.1016/j.jhin.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Kitagawa H., Nomura T., Nazmul T., Omori K., Shigemoto N., Sakaguchi T., Ohge H. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am. J. Infect. Control. 2020 doi: 10.1016/j.ajic.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buonanno M., Welch D., Shuryak I., Brenner D.J. Far-UVC light (222nm) efficiently and safely inactivates airborne human coronaviruses. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-67211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leanse L.G., Goh X.S., Cheng J.X., Hooper D.C., Dai T. Dual-wavelength photo-killing of methicillin-resistant Staphylococcus aureus. JCI Insight. 2020;5 doi: 10.1172/JCI.INSIGHT.134343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y., Wang Y., Wang Y., Murray C.K., Hamblin M.R., Hooper D.C., Dai T. Antimicrobial blue light inactivation of pathogenic microbes: state of the art. Drug Resist. Updat. 2017;33–35:1–22. doi: 10.1016/j.drup.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee H., Kim Y.G., Um H.S., Chang B.S., Lee S.Y., Lee J.K. Efficacy of an LED toothbrush on a Porphyromonas gingivalis biofilm on a sandblasted and acid-etched titanium surface: an in vitro study. J. Periodontal Implant Sci. 2018;48:164–173. doi: 10.5051/jpis.2018.48.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Genina E.A., Titorenko V.A., Belikov A.V., Bashkatov A.N., Tuchin V.V. Adjunctive dental therapy via tooth plaque reduction and gingivitis treatment by blue light-emitting diodes tooth brushing. J. Biomed. Opt. 2015;20 doi: 10.1117/1.jbo.20.12.128004. [DOI] [PubMed] [Google Scholar]
- 52.Bache S.E., Maclean M., Gettinby G., Anderson J.G., MacGregor S.J., Taggart I. Universal decontamination of hospital surfaces in an occupied inpatient room with a continuous 405nm light source. J. Hosp. Infect. 2018;98:67–73. doi: 10.1016/j.jhin.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y., Wu X., Chen J., Amin R., Lu M., Bhayana B., Zhao J., Murray C.K., Hamblin M.R., Hooper D.C., Dai T. Antimicrobial blue light inactivation of gram-negative pathogens in biofilms: in vitro and in vivo studies. J. Infect. Dis. 2016;213:1380–1387. doi: 10.1093/infdis/jiw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.W. Liu, H. Li, COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism, (2020). doi:10.26434/chemrxiv.11938173.v4.
- 55.Wiwanitkit V. Ultraviolet-based biophotonic technologies and COVID-19. Photodiagn. Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayyildiz S., Emir F., Sahin C. Evaluation of low-level laser therapy in TMD patients. Case Rep. Dent. 2015;2015 doi: 10.1155/2015/424213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Sousa M.V.P., Kawakubo M., Ferraresi C., Kaippert B., Yoshimura E.M., Hamblin M.R. Pain management using photobiomodulation: mechanisms, location, and repeatability quantified by pain threshold and neural biomarkers in mice. J. Biophotonics. 2018;11 doi: 10.1002/jbio.201700370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hambling M.R., de Sousa M.V.P., Agrawal T. 1st ed. Pan Standford Publishing; 2016. Handbook of Low-Level Laser Therapy. [Google Scholar]
- 59.Gonnelli F.A.S., Palma L.F., Giordani A.J., Deboni A.L.S., Dias R.S., Segreto R.A., Segreto H.R.C. Low-level laser therapy for the prevention of low salivary flow rate after radiotherapy and chemotherapy in patients with head and neck cancer. Radiol. Bras. 2016;49:86–91. doi: 10.1590/0100-3984.2014.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Freitas L.F., Hamblin M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron. 2016;22:348–364. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cronshaw M., Parker S., Anagnostaki E., Lynch E. Systematic review of orthodontic treatment management with photobiomodulation therapy, photobiomodulation, photomedicine. Laser Surg. 2019;37:862–868. doi: 10.1089/photob.2019.4702. [DOI] [PubMed] [Google Scholar]
- 62.Sonesson M., De Geer E., Subraian J., Petrén S. Efficacy of low-level laser therapy in accelerating tooth movement, preventing relapse and managing acute pain during orthodontic treatment in humans: a systematic review. BMC Oral Health. 2016;17 doi: 10.1186/s12903-016-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Machado A.C., Viana Í.E.L., Farias-Neto A.M., Braga M.M., de Paula Eduardo C., de Freitas P.M., Aranha A.C.C. Is photobiomodulation (PBM) effective for the treatment of dentin hypersensitivity? A systematic review. Lasers Med. Sci. 2018;33:745–753. doi: 10.1007/s10103-017-2403-7. [DOI] [PubMed] [Google Scholar]
- 64.Sgolastra F., Petrucci A., Severino M., Gatto R., Monaco A. Lasers for the treatment of dentin hypersensitivity: a meta-analysis. J. Dent. Res. 2013;92:492–499. doi: 10.1177/0022034513487212. [DOI] [PubMed] [Google Scholar]
- 65.Lalla R.V., Bowen J., Barasch A., Elting L., Epstein J., Keefe D.M., McGuire D.B., Migliorati C., Nicolatou-Galitis O., Peterson D.E., Raber-Durlacher J.E., Sonis S.T., Elad S., Al-Dasooqi N., Brennan M., Gibson R., Fulton J., Hewson I., Jensen S.B., Logan R., Öhrn K.E.O., Sarri T., Saunders D., von Bültzingslöwen I., Yarom N., Allemano J., Al-Azri A.R., Antunes H.S., Ariyawardana A., Bateman E., Blijlevens N., Boers-Doets C.B., Bossi P., Brown C.G., Chang Y.C., Cheng K.K., Cooksley C., Correa E.P., Dennis K., Di Palma M., Drucker S., Eilers J., Escalante C., Estilo C.L., Everaus H., Fijlstra M., Fliedner M., Freidank A., Gerber E., Gibson F., Gomez J.G., Halm J., Hita G., Hutchins R.D., Hodgson B., Hovan A., Jarvis V., King E.E., Kouloulias V.E., Latortue M.C., Lees J., Lopes N.N.F., Loprinzi C., Michelet M., Mori T., Nair R.G., Niscola P., Oberle-Edwards L.K., Osaguona A., Parelkar P., Park J., Parker I., Pettersson B.G., Potting C., Rao N.G., Riesenbeck D., Rouleau T., Schubert M.M., Silverman S., Soga Y., Spijkervet F.K.L., Stokman M., Stringer A.M., Tissing W.J.E., van der Velden W.J.F.M., van de Wetering M.D., Vithala M., Weikel D.S., Yazbeck R., Yeoh E., Zadik Y. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120:1453–1461. doi: 10.1002/cncr.28592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cronshaw M., Parker S., Anagnostaki E., Mylona V., Lynch E., Grootveld M. Photobiomodulation and oral mucositis: a systematic review. Dent. J. 2020;8:87. doi: 10.3390/DJ8030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zadik Y., Arany P.R., Fregnani E.R., Bossi P., Antunes H.S., Bensadoun R.J., Gueiros L.A., Majorana A., Nair R.G., Ranna V., Tissing W.J.E., Vaddi A., Lubart R., Migliorati C.A., Lalla R.V., Cheng K.K.F., Elad S. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer. 2019;27:3969–3983. doi: 10.1007/s00520-019-04890-2. [DOI] [PubMed] [Google Scholar]
- 68.Zwiri A., Alrawashdeh M.A., Khan M., Ahmad W.M.A.W., Kassim N.K., Ahmed Asif J., Suan Phaik K., Husein A., Ab-Ghani Z. Effectiveness of the laser application in temporomandibular joint disorder: a systematic review of 1172 patients. Pain Res. Manag. 2020;2020:1–10. doi: 10.1155/2020/5971032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de F.Z. Lizarelli R., Maciel V. Novas Técnicas Ópticas Para as Áreas Da Saúde. 1st ed. Editoria Livraria da Física; São Paulo: 2008. Fototerapia de baixa intensidade em odontologia e fisioterapia; pp. 185–209. [Google Scholar]
- 70.Shukla D., Muthusekhar M. Efficacy of low-level laser therapy in temporomandibular disorders: a systematic review. Natl. J. Maxillofac. Surg. 2016;7:62. doi: 10.4103/0975-5950.196127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tamae P.E., Vital A., Santos D., Luise M., Simão D.S., Carolina A., Canelada N., Regina K., Vital T., Santos D., Eduardo A., Junior D.A., Bagnato V.S. Can the associated use of negative pressure and laser therapy be a new and efficient treatment for Parkinson 's pain ? A comparative study Alzheimer's disease & Parkinsonism. J. Alzheimer's Dis. Park. 2020;10 [Google Scholar]
- 72.de S. Rastelli A.N., Jacomassi D.P., Bagnato V.S. Novas Técnicas Ópticas Para as Áreas Da Saúde. 1st ed. Editoria Livraria da Física; São Paulo: 2008. Fotoclareamento e fotoprocessamento de resinas compostas; pp. 53–80. [Google Scholar]
- 73.Fidan V., Koyuncu H., Akin O. Oral lesions in Covid 19 positive patients. Am. J. Otolaryngol. Head Neck Med. Surg. 2021;42 doi: 10.1016/j.amjoto.2021.102905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramires M.C.C.H., Mattia M.B., Tateno R.Y., Palma L.F., Campos L. A combination of phototherapy modalities for extensive lip lesions in a patient with SARS-CoV-2 infection. Photodiagn. Photodyn. Ther. 2021;33 doi: 10.1016/j.pdpdt.2021.102196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teixeira I.S., Leal F.S., Tateno R.Y., Palma L.F., Campos L. Photobiomodulation therapy and antimicrobial photodynamic therapy for orofacial lesions in patients with COVID-19: a case series. Photodiagn. Photodyn. Ther. 2021:34. doi: 10.1016/j.pdpdt.2021.102281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soheilifar S., Fathi H., Naghdi N. Photobiomodulation therapy as a high potential treatment modality for COVID-19. Lasers Med. Sci. 2020 doi: 10.1007/s10103-020-03206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nejatifard M., Asefi S., Jamali R., Hamblin M.R., Fekrazad R. Probable positive effects of the photobiomodulation as an adjunctive treatment in COVID-19: a systematic review. Cytokine. 2021:137. doi: 10.1016/j.cyto.2020.155312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiang Y.T., Yang Y., Li W., Zhang L., Zhang Q., Cheung T., Ng C.H. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7:228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brooks S.K., Webster R.K., Smith L.E., Woodland L., Wessely S., Greenberg N., Rubin G.J. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Theroux J., Stomski N., Cope V., Mortimer-Jones S., Maurice L. A cross-sectional study of the association between anxiety and temporomandibular disorder in Australian chiropractic students. J. Chiropr. Educ. 2019;33:111–117. doi: 10.7899/JCE-18-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sant'Anna E.F., de S. Araújo M.T., Nojima L.I., da Cunha A.C., da Silveira B.L., Marquezan M. High-intensity laser application in orthodontics. Dental Press J. Orthod. 2017;22:99–109. doi: 10.1590/2177-6709.22.6.099-109.sar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pendyala C., Tiwari R.V., Dixit H., Augustine V., Baruah Q., Baruah K., Pendyala C., et al. LASERS and its applications in dentistry A contemporary apprise on LASERS and its applications in dentistry. Int. J. Oral Heal. Med. Res. 2017;4:47–51. www.ijohmr.com [Google Scholar]
- 83.Ortega-Concepción D., Cano-Durán J.A., Peña-Cardelles J.F., Paredes-Rodríguez V.M., González-Serrano J., López-Quiles J. The application of diode laser in the treatment of oral soft tissues lesions. A literature review. J. Clin. Exp. Dent. 2017;9:e925–e928. doi: 10.4317/jced.53795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Komori S., Matsumoto K., Matsuo K., Suzuki H., Komori T. Clinical study of laser treatment for frenectomy of pediatric patients. Int. J. Clin. Pediatr. Dent. 2017;10:272–277. doi: 10.5005/jp-journals-10005-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calisir M., Ege B. Evaluation of patient perceptions after frenectomy operations: a comparison of neodymium-doped yttrium aluminum garnet laser and conventional techniques in the same patients. Niger. J. Clin. Pract. 2018;21:1059–1064. doi: 10.4103/njcp.njcp_2_18. [DOI] [PubMed] [Google Scholar]
- 86.Kawamura R., Mizutani K., Lin T., Lin T., Lin T., Kakizaki S., Mimata A., Watanabe K., Saito N., Meinzer W., Iwata T., Izumi Y., Izumi Y., Aoki A. Ex vivo evaluation of gingival ablation with various laser systems and electroscalpel, photobiomodulation. Photomed. Laser Surg. 2020;38:364–373. doi: 10.1089/photob.2019.4713. [DOI] [PubMed] [Google Scholar]
- 87.Bagher S.M., Sulimany A.M., Kaplan M., Loo C.Y. Treating mucocele in pediatric patients using a diode laser: three case reports. Dent. J. 2018;6 doi: 10.3390/dj6020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gobbo M., Bussani R., Perinetti G., Rupel K., Bevilaqua L., Ottaviani G., Biasotto M. Blue diode laser versus traditional infrared diode laser and quantic molecular resonance scalpel: clinical and histological findings after excisional biopsy of benign oral lesions. J. Biomed. Opt. 2017;22 doi: 10.1117/1.jbo.22.12.121602. [DOI] [PubMed] [Google Scholar]
- 89.Ustun K., Hatipoglu M., Daltaban O., Felek R., Firat M. Clinical and biochemical effects of erbium, chromium: yttrium, scandium, gallium, garnet laser treatment as a complement to periodontal treatment. Niger. J. Clin. Pract. 2018;21:1150–1157. doi: 10.4103/njcp.njcp_51_18. [DOI] [PubMed] [Google Scholar]
- 90.Do Q.L., Gaudin A. The efficiency of the ER: YAG laser and photon-induced photoacoustic streaming (PIPS) as an activation method in endodontic irrigation: a literature review. J. Lasers Med. Sci. 2020;11:316–331. doi: 10.34172/jlms.2020.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pierce J.S., Lacey S.E., Lippert J.F., Lopez R., Franke J.E., Colvard M.D. An assessment of the occupational hazards related to medical lasers. J. Occup. Environ. Med. 2011;53:1302–1309. doi: 10.1097/JOM.0b013e318236399e. [DOI] [PubMed] [Google Scholar]
- 92.Lago A.D.N., Cordon R., Gonçalves L.M., Menezes C.F.S., Furtado G.S., Rodrigues F.C.N., Marques D.M.C. How to use laser safely in times of COVID-19: systematic review. Spec. Care Dent. 2021 doi: 10.1111/scd.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rkein A.M., Ozog D.M. Photodynamic therapy. Dermatol. Clin. 2014;32:415–425. doi: 10.1016/j.det.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 94.von Tappeiner H. Die photodynamische Erscheinung (Sensibilisierung durch fluoreszierende Stoffe) Ergebn Physiol. 1909;8:698–741. [Google Scholar]
- 95.Ackroyd R., Kelty C., Brown N., Reed M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001;74:656. doi: 10.1562/0031-8655(2001)074<0656:thopap>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 96.Kwiatkowski S., Knap B., Przystupski D., Saczko J., Kędzierska E., Knap-Czop K., Kotlińska J., Michel O., Kotowski K., Kulbacka J. Photodynamic therapy – mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Q., Li L. Photodynamic combinational therapy in cancer treatment. J. B.U.ON. 2018;23:561–567. [PubMed] [Google Scholar]
- 98.Gomez G.F., Huang R., MacPherson M., Ferreira Zandona A.G., Gregory R.L. Photo inactivation of streptococcus mutans biofilm by violet-blue light. Curr. Microbiol. 2016;73:426–433. doi: 10.1007/s00284-016-1075-z. [DOI] [PubMed] [Google Scholar]
- 99.Haag P.A., Steiger-Ronay V., Schmidlin P.R. The in Vitro antimicrobial efficacy of PDT against periodontopathogenic bacteria. Int. J. Mol. Sci. 2015;16:27327–27338. doi: 10.3390/ijms161126027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fumes A.C., da Silva Telles P.D., Corona S.A.M., Borsatto M.C. Effect of aPDT on streptococcus mutans and Candida albicans present in the dental biofilm: systematic review. Photodiagn. Photodyn. Ther. 2018;21:363–366. doi: 10.1016/j.pdpdt.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 101.Khorsandi K., Hosseinzadeh R., Shahidi F.K. Photodynamic treatment with anionic nanoclays containing curcumin on human triple-negative breast cancer cells: cellular and biochemical studies. J. Cell. Biochem. 2019;120:4998–5009. doi: 10.1002/jcb.27775. [DOI] [PubMed] [Google Scholar]
- 102.Scherer K.M., Bisby R.H., Botchway S.W., Parker A.W. New approaches to photodynamic therapy from Types I, II and III to Type IV using one or more photons. Anticancer Agents Med. Chem. 2016;17:171–189. doi: 10.2174/1871520616666160513131723. [DOI] [PubMed] [Google Scholar]
- 103.Cieplik F., Deng D., Crielaard W., Buchalla W., Hellwig E., Al-Ahmad A., Maisch T. Antimicrobial photodynamic therapy–what we know and what we don't. Crit. Rev. Microbiol. 2018;44:571–589. doi: 10.1080/1040841X.2018.1467876. [DOI] [PubMed] [Google Scholar]
- 104.Wainwright M., Maisch T., Nonell S., Plaetzer K., Almeida A., Tegos G.P., Hamblin M.R. Photoantimicrobials—are we afraid of the light? Lancet Infect. Dis. 2017;17:e49–e55. doi: 10.1016/S1473-3099(16)30268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maisch T., Baier J., Franz B., Maier M., Landthaler M., Szeimies R.M., Bäumler W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7223–7228. doi: 10.1073/pnas.0611328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alves E., Faustino M.A.F., Neves M.G.P.M.S., Cunha A., Tome J., Almeida A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014;6:141–164. doi: 10.4155/fmc.13.211. [DOI] [PubMed] [Google Scholar]
- 107.Da M., Baptista S., Cadet J., Di Mascio P., Ghogare A.A., Greer A., Hamblin M.R., Lorente C., Cristina Nunez S., Ribeiro M.S., Thomas A.H., Vignoni M., Yoshimura T.M., Author P.P. Type I and II photosensitized oxidation reactions: guidelines and mechanistic pathways † HHS public access author manuscript. Photochem. Photobiol. 2017;93:912–919. doi: 10.1111/php.12716.Type. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mello R., Martínez-Ferrer J., Alcalde-Aragonés A., Varea T., Acerete R., González-Núñez M.E., Asensio G. Reactions at interfaces: oxygenation of n-butyl ligands anchored on silica surfaces with methyl(trifluoromethyl)dioxirane. J. Org. Chem. 2011;76:10129–10139. doi: 10.1021/jo2019703. [DOI] [PubMed] [Google Scholar]
- 109.Ghorbani J., Rahban D., Aghamiri S., Teymouri A., Bahador A. Photosensitizers in antibacterial photodynamic therapy: an overview. Laser Ther. 2018;27:293–302. doi: 10.5978/islsm.27_18-RA-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu Z., Tang Z., Phillips J.A., Yang R., Wang H., Tan W. Regulation of singlet oxygen generation using single-walled carbon nanotubes. J. Am. Chem. Soc. 2008;130:10856–10857. doi: 10.1021/ja802913f. [DOI] [PubMed] [Google Scholar]
- 111.Zhu T.C., Finlay J.C. The role of photodynamic therapy (PDT) physics. Med. Phys. 2008;35:3127–3136. doi: 10.1118/1.2937440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Demidova T.N., Hamblin M.R. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob. Agents Chemother. 2005;49:2329–2335. doi: 10.1128/AAC.49.6.2329-2335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang L., Xuan Y., Koide Y., Zhiyentayev T., Tanaka M., Hamblin M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: an in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012;44:490–499. doi: 10.1002/lsm.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Azizi A., Shohrati P., Goudarzi M., Lawaf S., Rahimi A. Comparison of the effect of photodynamic therapy with curcumin and methylene Blue on streptococcus mutans bacterial colonies. Photodiagn. Photodyn. Ther. 2019;27:203–209. doi: 10.1016/j.pdpdt.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 115.da Mota A.C.C., Leal C.R.L., Olivan S., Gon??alves M.L.L., de Oliveira V.A., Pinto M.M., Bussadori S.K. Case report of photodynamic therapy in the treatment of dental caries on primary teeth. J. Lasers Med. Sci. 2016;7:131–133. doi: 10.15171/jlms.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gursoy H., Ozcakir-Tomruk C., Tanalp J., Yilmaz S. Photodynamic therapy in dentistry: a literature review. Clin. Oral Investig. 2013;17:1113–1125. doi: 10.1007/s00784-012-0845-7. [DOI] [PubMed] [Google Scholar]
- 117.Gendrot M., Andreani J., Duflot I., Boxberger M., Bideau M.Le, Mosnier J., Jardot P., Fonta I., Rolland C., Bogreau H., Hutter S., Scola B.La, Pradines B. Methylene blue inhibits replication of SARS-CoV-2 in vitro. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Svyatchenko V.A., Nikonov S.D., Mayorov A.P., Gelfond M.L., Loktev V.B. Antiviral photodynamic therapy: inactivation and inhibition of SARS-CoV-2 in vitro using methylene blue and Radachlorin. Photodiagn. Photodyn. Ther. 2021;33 doi: 10.1016/j.pdpdt.2020.102112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moghissi K., Dixon K., Gibbins S. Does PDT have potential in the treatment of COVID 19 patients? Photodiag. Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dias L.D., Blanco K.C., Bagnato V.S. COVID-19: beyond the virus. The use of photodynamic therapy for the treatment of infections in the respiratory tract. Photodiagn. Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kipshidze N., Yeo N., Kipshidze N. Photodynamic and sonodynamic therapy of acute hypoxemic respiratory failure in patients with COVID-19. Photodiagn. Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Almeida A., Faustino M.A.F., Neves M.G.P.M.S. Antimicrobial photodynamic therapy in the control of COVID-19. Antibiotics. 2020;9:1–10. doi: 10.3390/antibiotics9060320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nunes L.P., Chalub L.O., Strazzi-Sahyon H.B., dos Santos P.H., Cintra L.T.Â., Sivieri-Araujo G. Photodynamic therapy as a potential oral disinfection protocol during COVID-19 outbreak. Photodiagn. Photodyn. Ther. 2021;33 doi: 10.1016/j.pdpdt.2021.102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Conrado P.C.V, Sakita K.M., Arita G.S., Galinari C.B., Gonçalves R.S., Lopes L.D.G., Lonardoni M.V.C., Teixeira J.J.V, Bonfim-Mendonça P.S., Kioshima E.S. A systematic review of photodynamic therapy as an antiviral treatment: potential guidance for dealing with SARS-CoV-2. Photodiagn. Photodyn. Ther. 2021;34 doi: 10.1016/J.PDPDT.2021.102221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perea E.J. Oral flora in the age of molecular biology. Chem. Nat. Compd. 2004;10:1–5. [PubMed] [Google Scholar]
- 126.Thomas J.G., Nakaishi L.A. Managing the complexity of a dynamic biofilm. J. Am. Dent. Assoc. 2006;137:S10–S15. doi: 10.14219/jada.archive.2006.0409. [DOI] [PubMed] [Google Scholar]
- 127.Mártha K., Lorinczi L., BicǍ C., Gyergyay R., Petcu B., LazǍr L. Assessment of periodontopathogens in subgingival biofilm of banded and bonded molars in early phase of fixed orthodontic treatment. Acta Microbiol. Immunol. Hung. 2016;63:103–113. doi: 10.1556/030.63.2016.1.8. [DOI] [PubMed] [Google Scholar]
- 128.An J.S., Kim K., Cho S., Lim B.S., Ahn S.J. Compositional differences in multi-species biofilms formed on various orthodontic adhesives. Eur. J. Orthod. 2017;39:528–533. doi: 10.1093/ejo/cjw089. [DOI] [PubMed] [Google Scholar]
- 129.Marwah N., Gupta S., Padiyar N., Padiyar B. Comparative evaluation of effects of triphala, garlic extracts, and chlorhexidine mouthwashes on salivary streptococcus mutans counts and oral hygiene status. Int. J. Clin. Pediatr. Dent. 2018;11:299–306. doi: 10.5005/jp-journals-10005-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dos Santos G.O., Milanesi F.C., Greggianin B.F., Fernandes M.I., Oppermann R.V., Weidlich P. Chlorhexidine with or without alcohol against biofilm formation: efficacy, adverse events and taste preference. Braz. Oral Res. 2017;31:e32. doi: 10.1590/1807-3107BOR-2017.vol31.0032. [DOI] [PubMed] [Google Scholar]
- 131.Martínez-Hernández M., Reda B., Hannig M. Chlorhexidine rinsing inhibits biofilm formation and causes biofilm disruption on dental enamel in situ. Clin. Oral Investig. 2020 doi: 10.1007/s00784-020-03250-3. [DOI] [PubMed] [Google Scholar]
- 132.Donato H.A.R., Pratavieira S., Grecco C., Brugnera-Júnior A., Bagnato V.S., Kurachi C. Clinical comparison of two photosensitizers for oral cavity decontamination. Photomed. Laser Surg. 2017;35:105–110. doi: 10.1089/pho.2016.4114. [DOI] [PubMed] [Google Scholar]
- 133.T. Walsh, O. Jm, D. Moore, T. Walsh, O. Jm, D. Moore, Chlorhexidine treatment for the prevention of dental caries in children and adolescents (Review) SUMMARY OF FINDINGS FOR THE MAIN COMPARISON, (2015). doi:10.1002/14651858.CD008457.pub2.www.cochranelibrary.com. [DOI] [PMC free article] [PubMed]
- 134.Chye R.M.L., Perrotti V., Piattelli A., Iaculli F., Quaranta A. Effectiveness of different commercial chlorhexidine-based mouthwashes after periodontal and implant surgery: a systematic review. Implant Dent. 2019;28:74–85. doi: 10.1097/ID.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 135.James P., Worthington H.V., Parnell C., Harding M., Lamont T., Cheung A., Whelton H., Riley P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017:2017. doi: 10.1002/14651858.CD008676.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hamblin M.R. Antimicrobial photodynamic inactivation: a bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016;33 doi: 10.1016/j.mib.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kellesarian S.V., Qayyum F., de Freitas P.C., Akram Z., Javed F. Is antimicrobial photodynamic therapy a useful therapeutic protocol for oral decontamination? A systematic review and meta-analysis. Photodiagn. Photodyn. Ther. 2017;20:55–61. doi: 10.1016/j.pdpdt.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 138.Pitts N.B., Zero D.T., Marsh P.D., Ekstrand K., Weintraub J.A., Ramos-Gomez F., Tagami J., Twetman S., Tsakos G., Ismail A. Dental caries. Nat. Rev. Dis. Prim. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 139.Santin G.C., Oliveira D.S.B., Galo R., Borsatto M.C., Corona S.A.M. Antimicrobial photodynamic therapy and dental plaque: a systematic review of the literature. Sci. World J. 2014;2014 doi: 10.1155/2014/824538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Silva A.R.S., Alves F.A., Antunes A., Goes M.F., Lopes M.A. Patterns of demineralization and dentin reactions in radiation-related caries. Caries Res. 2009;43:43–49. doi: 10.1159/000192799. [DOI] [PubMed] [Google Scholar]
- 141.Fontana M., Wolff M., Featherstone J.D. Introduction to ICNARA 3. Adv. Dent. Res. 2018;29:3. doi: 10.1177/0022034517746372. [DOI] [PubMed] [Google Scholar]
- 142.Fontana M., Gonzalez-Cabezas C. Evidence-based dentistry caries risk assessment and disease management. Dent. Clin. N. Am. 2019;63:119–128. doi: 10.1016/j.cden.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 143.Manji F., Dahlen G., Fejerskov O. Caries and periodontitis: contesting the conventional wisdom on their aetiology. Caries Res. 2018;52:548–564. doi: 10.1159/000488948. [DOI] [PubMed] [Google Scholar]
- 144.Kassebaum N.J., Bernabé E., Dahiya M., Bhandari B., Murray C.J.L., Marcenes W. Global burden of untreated caries: a systematic review and metaregression. J. Dent. Res. 2015;94:650–658. doi: 10.1177/0022034515573272. [DOI] [PubMed] [Google Scholar]
- 145.Fontana M., Young D.A., Wolff M.S., Pitts N.B., Longbottom C. Defining dental caries for 2010 and beyond. Dent. Clin. N. Am. 2010;54:423–440. doi: 10.1016/j.cden.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 146.Steiner-Oliveira C., Longo P.L., Aranha A.C.C., Ramalho K.M., Mayer M.P.A., de C., Eduardo Paula. Randomized in vivo evaluation of photodynamic antimicrobial chemotherapy on deciduous carious dentin. J. Biomed. Opt. 2015;20 doi: 10.1117/1.jbo.20.10.108003. [DOI] [PubMed] [Google Scholar]
- 147.Banerjee A., Frencken J.E., Schwendicke F., Innes N.P.T. Contemporary operative caries management: consensus recommendations on minimally invasive caries removal. Br. Dent. J. 2017;223:215–222. doi: 10.1038/sj.bdj.2017.672. [DOI] [PubMed] [Google Scholar]
- 148.Alves L.V.G.L., Curylofo-Zotti F.A., Borsatto M.C., de S. Salvador S.L., Valério R.A., Souza-Gabriel A.E., Corona S.A.M. Influence of antimicrobial photodynamic therapy in carious lesion. Randomized split-mouth clinical trial in primary molars. Photodiagn. Photodyn. Ther. 2019;26:124–130. doi: 10.1016/j.pdpdt.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 149.Ricketts D., Lamont T., Innes N.P., Kidd E., Clarkson J.E. Operative caries management in adults and children. Cochrane Database Syst. Rev. 2013;2013 doi: 10.1002/14651858.CD003808.pub3. [DOI] [PubMed] [Google Scholar]
- 150.Schwendicke F., Frencken J.E., Bjørndal L., Maltz M., Manton D.J., Ricketts D., Van Landuyt K., Banerjee A., Campus G., Doméjean S., Fontana M., Leal S., Lo E., Machiulskiene V., Schulte A., Splieth C., Zandona A.F., Innes N.P.T. Managing carious lesions: consensus recommendations on carious tissue removal. Adv. Dent. Res. 2016;28:58–67. doi: 10.1177/0022034516639271. [DOI] [PubMed] [Google Scholar]
- 151.Orhan A.I., Oz F.T., Ozcelik B., Orhan K. A clinical and microbiological comparative study of deep carious lesion treatment in deciduous and young permanent molars. Clin. Oral Investig. 2008;12:369–378. doi: 10.1007/s00784-008-0208-6. [DOI] [PubMed] [Google Scholar]
- 152.Whitworth J.M., Myers P.M., Smith J., Walls A.W.G., McCabe J.F. Endodontic complications after plastic restorations in general practice. Int. Endod. J. 2005;38:409–416. doi: 10.1111/j.1365-2591.2005.00962.x. [DOI] [PubMed] [Google Scholar]
- 153.Murray P.E., Smith A.J., Windsor L.J., Mjör I.A. Remaining dentine thickness and human pulp responses. Int. Endod. J. 2003;36:33–43. doi: 10.1046/j.0143-2885.2003.00609.x. [DOI] [PubMed] [Google Scholar]
- 154.Bouguezzi A., Cherni I., Sioud S., Hentati H., Selmi J. COVID-19: special precautions in dentistry. Open Access J. Biomed. Sci. 2020;2:283–284. doi: 10.38125/OAJBS.000164. [DOI] [Google Scholar]
- 155.Lakhani B.P., Sharma A., Sanwalka V., Lakhani P. Aerosol reduction in dentistry: minimizing risk of Covid 19. Eur. J. Med. Health Sci. 2020;2 doi: 10.24018/ejmed.2020.2.3.294. [DOI] [Google Scholar]
- 156.Cieplik F., Buchalla W., Hellwig E., Al-Ahmad A., Hiller K.A., Maisch T., Karygianni L. Antimicrobial photodynamic therapy as an adjunct for treatment of deep carious lesions—A systematic review. Photodiagn. Photodyn. Ther. 2017;18:54–62. doi: 10.1016/j.pdpdt.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 157.Melo M.A.S., Rolim J.P.M.L., Passos V.F., Lima R.A., Zanin I.C.J., Codes B.M., Rocha S.S., Rodrigues L.K.A. Photodynamic antimicrobial chemotherapy and ultraconservative caries removal linked for management of deep caries lesions. Photodiagn. Photodyn. Ther. 2015;12:581–586. doi: 10.1016/j.pdpdt.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 158.de A.B. Guglielmi C., Simionato M.R.L., Ramalho K.M., Imparato J.C.P., Pinheiro S.L., Luz M.A.A.C. Clinical use of photodynamic antimicrobial chemotherapy for the treatment of deep carious lesions. J. Biomed. Opt. 2011;16 doi: 10.1117/1.3611009. [DOI] [PubMed] [Google Scholar]
- 159.Neves P.A.M., Lima L.A., Rodrigues F.C.N., Leitão T.J., Ribeiro C.C.C. Clinical effect of photodynamic therapy on primary carious dentin after partial caries removal. Braz. Oral Res. 2016;30 doi: 10.1590/1807-3107BOR-2016.vol30.0047. [DOI] [PubMed] [Google Scholar]
- 160.Huang S.M., Huang J.Y., Yu H.C., Su N.Y., Chang Y.C. Trends, demographics, and conditions of emergency dental visits in Taiwan 1997–2013: a nationwide population-based retrospective study. J. Formos. Med. Assoc. 2019;118:582–587. doi: 10.1016/j.jfma.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 161.Yu J., Zhang T., Zhao D., Haapasalo M., Shen Y. Characteristics of endodontic emergencies during coronavirus disease 2019 outbreak in Wuhan. J. Endod. 2020;46:730–735. doi: 10.1016/j.joen.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Coelho M.S., Vilas-Boas L., Tawil P.Z. The effects of photodynamic therapy on postoperative pain in teeth with necrotic pulps. Photodiagn. Photodyn. Ther. 2019;27:396–401. doi: 10.1016/j.pdpdt.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 163.Vieira G.C.S., Antunes H.S., Pérez A.R., Gonçalves L.S., Antunes F.E., Siqueira J.F., Rôças I.N. Molecular analysis of the antibacterial effects of photodynamic therapy in endodontic surgery: a case series. J. Endod. 2018;44:1593–1597. doi: 10.1016/j.joen.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 164.Chugal N., Mallya S.M., Kahler B., Lin L.M. Endodontic treatment outcomes. Dent. Clin. N. Am. 2017;61:59–80. doi: 10.1016/j.cden.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 165.Prazmo E.J., Kwaśny M., Łapiński M., Mielczarek A. Photodynamic therapy as a promising method used in the treatment of oral diseases. Adv. Clin. Exp. Med. 2016;25:799–807. doi: 10.17219/acem/32488. [DOI] [PubMed] [Google Scholar]
- 166.Dioguardi M., Di Gioia G., Illuzzi G., Laneve E., Cocco A., Troiano G. Endodontic irrigants: different methods to improve efficacy and related problems. Eur. J. Dent. 2018;12:459–466. doi: 10.4103/ejd.ejd_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Paqué F., Boessler C., Zehnder M. Accumulated hard tissue debris levels in mesial roots of mandibular molars after sequential irrigation steps. Int. Endod. J. 2011;44:148–153. doi: 10.1111/j.1365-2591.2010.01823.x. [DOI] [PubMed] [Google Scholar]
- 168.da Silva C.C., Chaves Júnior S.P., Pereira G.L.D., d. C. Fontes K.B.F., Antunes L.A.A., Póvoa H.C.C., Antunes L.S., Iorio N.L.P.P. Antimicrobial photodynamic therapy associated with conventional endodontic treatment: a clinical and molecular microbiological study. Photochem. Photobiol. 2018;94:351–356. doi: 10.1111/php.12869. [DOI] [PubMed] [Google Scholar]
- 169.de Miranda R.G., Colombo A.P.V. Clinical and microbiological effectiveness of photodynamic therapy on primary endodontic infections: a 6-month randomized clinical trial. Clin. Oral Investig. 2018;22:1751–1761. doi: 10.1007/s00784-017-2270-4. [DOI] [PubMed] [Google Scholar]
- 170.Okamoto C.B., Motta L.J., Prates R.A., da Mota A.C.C., Gonçalves M.L.L., Horliana A.C.R.T., Mesquita Ferrari R.A., Fernandes K.P.S., Bussadori S.K. Antimicrobial photodynamic therapy as a co-adjuvant in endodontic treatment of deciduous teeth: case series. Photochem. Photobiol. 2018;94:760–764. doi: 10.1111/php.12902. [DOI] [PubMed] [Google Scholar]
- 171.Pourhajibagher M., bahador A. Adjunctive antimicrobial photodynamic therapy to conventional chemo-mechanical debridement of infected root canal systems: a systematic review and meta-analysis. Photodiagn. Photodyn. Ther. 2019;26:19–26. doi: 10.1016/j.pdpdt.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 172.Marshall K. `Dam it-it's easy!’-or is it? Br. Dent. J. 2017;222:839–840. doi: 10.1038/sj.bdj.2017.489. [DOI] [PubMed] [Google Scholar]
- 173.Nibali L., Ide M., Ng D., Buontempo Z., Clayton Y., Asimakopoulou K. The perceived impact of Covid-19 on periodontal practice in the United Kingdom: a questionnaire study. J. Dent. 2020;102 doi: 10.1016/j.jdent.2020.103481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tonetti M.S., Jepsen S., Jin L., Otomo-Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J. Clin. Periodontol. 2017;44:456–462. doi: 10.1111/jcpe.12732. [DOI] [PubMed] [Google Scholar]
- 175.Jepsen S., Blanco J., Buchalla W., Carvalho J.C., Dietrich T., Dörfer C., Eaton K.A., Figuero E., Frencken J.E., Graziani F., Higham S.M., Kocher T., Maltz M., Ortiz-Vigon A., Schmoeckel J., Sculean A., Tenuta L.M.A., van der Veen M.H., Machiulskiene V. Prevention and control of dental caries and periodontal diseases at individual and population level: consensus report of group 3 of joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J. Clin. Periodontol. 2017;44:S85–S93. doi: 10.1111/jcpe.12687. [DOI] [PubMed] [Google Scholar]
- 176.Tonetti M.S., Greenwell H., Kornman K.S. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J. Periodontol. 2018;89:S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 177.Lang N.P., Salvi G.E., Sculean A. Nonsurgical therapy for teeth and implants—when and why? Periodontol. 2000;79(2019):15–21. doi: 10.1111/prd.12240. [DOI] [PubMed] [Google Scholar]
- 178.Souza E.Q.M., da Rocha T.E., Toro L.F., Guiati I.Z., Ervolino E., Garcia V.G., Wainwright M., Theodoro L.H. Antimicrobial photodynamic therapy compared to systemic antibiotic therapy in non-surgical treatment of periodontitis: systematic review and meta-analysis. Photodiagn. Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101808. [DOI] [PubMed] [Google Scholar]
- 179.Christodoulides N., Nikolidakis D., Chondros P., Becker J., Schwarz F., Rössler R., Sculean A. Photodynamic therapy as an adjunct to non-surgical periodontal treatment: a randomized, controlled clinical trial. J. Periodontol. 2008;79:1638–1644. doi: 10.1902/jop.2008.070652. [DOI] [PubMed] [Google Scholar]
- 180.Chondros P., Nikolidakis D., Christodoulides N., Rössler R., Gutknecht N., Sculean A. Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: a randomized controlled clinical trial. Lasers Med. Sci. 2009;24:681–688. doi: 10.1007/s10103-008-0565-z. [DOI] [PubMed] [Google Scholar]
- 181.Ge L., Shu R., Li Y., Li C., Luo L., Song Z., Xie Y., Liu D. Adjunctive effect of photodynamic therapy to scaling and root planing in the treatment of chronic periodontitis. Photomed. Laser Surg. 2011;29:33–37. doi: 10.1089/pho.2009.2727. [DOI] [PubMed] [Google Scholar]
- 182.Xue D., Zhao Y. Clinical effectiveness of adjunctive antimicrobial photodynamic therapy for residual pockets during supportive periodontal therapy: a systematic review and meta-analysis. Photodiagn. Photodyn. Ther. 2017;17:127–133. doi: 10.1016/j.pdpdt.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 183.Grzech-Leśniak K., Gaspirc B., Sculean A. Clinical and microbiological effects of multiple applications of antibacterial photodynamic therapy in periodontal maintenance patients. A randomized controlled clinical study. Photodiagn. Photodyn. Ther. 2019;27:44–50. doi: 10.1016/j.pdpdt.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 184.Garcia V., Theodoro L. Lasers in Dentistry: Guide for Clinical Practice. Wiley-Blackwell Publishing Ltd; Hoboken, NJ: 2015. Antimicrobial photodynamic therapy in the treatment of periodontal diseases; pp. 423–435. [Google Scholar]
- 185.Akram Z., Shafqat S.S., Niaz M.O., Raza A., Naseem M. Clinical efficacy of photodynamic therapy and laser irradiation as an adjunct to open flap debridement in the treatment of chronic periodontitis: a systematic review and meta-analysis. Photodermatol. Photoimmunol. Photomed. 2020;36:3–13. doi: 10.1111/phpp.12499. [DOI] [PubMed] [Google Scholar]
- 186.Akram Z., Abduljabbar T., Sauro S., Daood U. Effect of photodynamic therapy and laser alone as adjunct to scaling and root planing on gingival crevicular fluid inflammatory proteins in periodontal disease: a systematic review. Photodiagn. Photodyn. Ther. 2016;16:142–153. doi: 10.1016/j.pdpdt.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 187.Akram Z., Hyder T., Al-Hamoudi N., Binshabaib M.S., Alharthi S.S., Hanif A. Efficacy of photodynamic therapy versus antibiotics as an adjunct to scaling and root planing in the treatment of periodontitis: a systematic review and meta-analysis. Photodiagn. Photodyn. Ther. 2017;19:86–92. doi: 10.1016/j.pdpdt.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 188.Akram Z. How effective is adjunctive antimicrobial photodynamic therapy in treating deep periodontal pockets in periodontal disease? A systematic review. J. Investig. Clin. Dent. 2018;9:e12345. doi: 10.1111/jicd.12345. [DOI] [PubMed] [Google Scholar]
- 189.Vohra F., Akram Z., Safii S.H., Vaithilingam R.D., Ghanem A., Sergis K., Javed F. Role of antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: a systematic review. Photodiagn. Photodyn. Ther. 2016;13:139–147. doi: 10.1016/j.pdpdt.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 190.Carrera E.T., Dias H.B., Corbi S.C.T., Marcantonio R.A.C., Bernardi A.C.A., Bagnato V.S., Hamblin M.R., Rastelli A.N.S. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: a critical review. Laser Phys. 2016;26:1–23. doi: 10.1088/1054-660X/26/12/123001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Singh A., Verma R., Murari A., Agrawal A. Oral candidiasis: an overview. J. Oral Maxillofac. Pathol. 2014;18:81–85. doi: 10.4103/0973-029X.141325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lewis M.A.O. Herpes simplex virus: an occupational hazard in dentistry. Int. Dent. J. 2004;54:103–111. doi: 10.1111/j.1875-595X.2004.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 193.Boltes Cecatto R., Siqueira de Magalhães L., Fernanda Setúbal Destro Rodrigues M., Pavani C., Lino-dos-Santos-Franco A., Teixeira Gomes M., Fátima Teixeira Silva D. Methylene blue mediated antimicrobial photodynamic therapy in clinical human studies: the state of the art. Photodiagn. Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101828. [DOI] [PubMed] [Google Scholar]
- 194.Davoudi A., Ebadian B., Nosouhian S. Role of laser or photodynamic therapy in treatment of denture stomatitis: a systematic review. J. Prosthet. Dent. 2018;120:498–505. doi: 10.1016/j.prosdent.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 195.Lotufo M.A., Tempestini Horliana A.C.R., Santana T., de Queiroz A.C., Gomes A.O., Motta L.J., Ferrari R.A.M., dos Santos Fernandes K.P., Bussadori S.K. Efficacy of photodynamic therapy on the treatment of herpes labialis: a systematic review. Photodiagn. Photodyn. Ther. 2020;29 doi: 10.1016/j.pdpdt.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 196.Jin X., Xu H., Deng J., Dan H., Ji P., Chen Q., Zeng X. Photodynamic therapy for oral potentially malignant disorders. Photodiagn. Photodyn. Ther. 2019;28:146–152. doi: 10.1016/j.pdpdt.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 197.O.M. Maria, N. Eliopoulos, T. Muanza, Radiation-induced oral mucositis, Front. Oncol. 7 (2017). doi:10.3389/fonc.2017.00089. [DOI] [PMC free article] [PubMed]
- 198.Lavaee F., Amanati A., Ramzi M., Naseri S., Shakiba Sefat H. Evaluation of the effect of photodynamic therapy on chemotherapy induced oral mucositis. Photodiagn. Photodyn. Ther. 2020;30 doi: 10.1016/j.pdpdt.2020.101653. [DOI] [PubMed] [Google Scholar]
- 199.Ioannidis J.P.A., Axfors C., Contopoulos-Ioannidis D.G. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Di Stasio D., Romano A., Gentile C., Maio C., Lucchese A., Serpico R., Paparella R., Minervini G., Candotto V., Laino L. Systemic and topical photodynamic therapy (PDT) on oral mucosa lesions: an overview. J. Biol. Regul. Homeost. Agents. 2018;32:123–126. [PubMed] [Google Scholar]
- 201.Ren Y., Feng C., Rasubala L., Malmstrom H., Eliav E. Risk for dental healthcare professionals during the COVID-19 global pandemic: an evidence-based assessment. J. Dent. 2020;101 doi: 10.1016/j.jdent.2020.103434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Arnabat-Dominguez J., Vecchio A.D., Todea C., Grzech-Leśniak K., Vescovi P., Romeo U., Nammour S. Laser dentistry in daily practice during the COVID-19 pandemic: benefits, risks and recommendations for safe treatments. Adv. Clin. Exp. Med. 2021;30:119–125. doi: 10.17219/ACEM/130598. [DOI] [PubMed] [Google Scholar]
- 203.Rodrigues F.C.N., de Araújo J.G.L., Dos Santos Araújo E.M., Lago A.N.D., Mantilla T.F., de Freitas P.M. Influence of biosafety materials of the laser output power. Lasers Med. Sci. 2021;36:311–315. doi: 10.1007/S10103-020-03030-1. [DOI] [PubMed] [Google Scholar]