Abstract
Hypersensitivity pneumonitis (HP) sometimes develops in people working in specific environments. We herein report a case of occupation-related HP in a citrus farmer in Japan. A 66-year-old man developed a fever, dyspnea, and general malaise in March after working near a trash dump filled with moldy tangerines. He presented with leukocytosis, bilateral lung opacities on chest radiographs, and intra-alveolar and interstitial lymphocytic inflammation with fibrotic change on a lung biopsy. His symptoms disappeared after admission and recurred on a revisit to the workplace. Fungal culture and a mycobiome analysis using next-generation sequencing suggested an association with exposure to Penicillium digitatum.
Keywords: fungi, hypersensitivity pneumonitis, next generation sequencing, occupational hypersensitivity pneumonitis, Penicillium digtatum
Introduction
Hypersensitivity pneumonitis (HP) is an allergic, diffuse pneumonia caused by repeated inhalation of antigenic substances (1). Causal antigens of HP can be classified into six categories: i) fungi/molds, ii) bacteria, iii) animal proteins, iv) plant proteins, v) low-molecular-weight chemicals, and vi) metals (1-3). Bird-related hypersensitivity pneumonitis accounts for 66-68% of all HP in Japan and in other countries, followed by farmer's lungs and HP caused by housing-related fungi, such as summer-type hypersensitivity pneumonitis (4-8). Clinical characteristics of HP differ depending on the type of antigen and the magnitude and duration of environmental exposure.
In some cases, HP develops in association with specific occupations, termed occupation-related HP (OHP). Various types of agricultural workers may develop OHP, such as farmer's lung (9), greenhouse worker's lung (10), and mushroom worker's lung (11). Molds and fungi are the most common cause of OHP: indeed, 98% of newly-developed OHP in Finland were attributed to molds (3).
We herein report a case with OHP in a citrus farmer possibly associated with exposure to Penicillium digitatum.
Case Report
A 66-year-old man who was a current smoker of 30 pack-years was admitted to a hospital due to a fever, dyspnea, and general malaise. He had been working in a citrus farm for decades, where he harvested two varieties of tangerines (one at the end of autumn, and one at the end of winter) and dumped damaged fruits before shipping in a storehouse. In March, he felt dyspnea and malaise 10 days before the admission. Three days later, he developed a fever of 38℃.
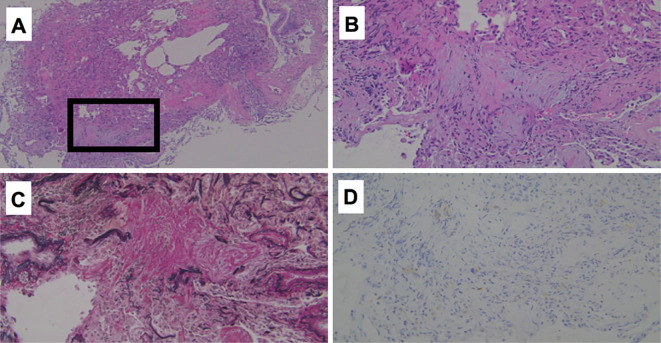
Chest X-radiography showed consolidation in the bilateral lungs, and chest computed tomography demonstrated diffuse, small-nodular shadows, consolidation along with bronchovascular bundles and traction bronchiectasis (Fig. 1). Peripheral blood tests demonstrated leukocytosis (12,200/μL) and elevated serum levels of C-reactive protein (CRP, 14.8 mg/dL). His forced vital capacity had decreased from 4.01 L in the previous summer to 2.55 L (74.8% of the predicted value), and the carbon monoxide transfer coefficient had decreased from 101% to 87% of the predicted value. A bronchoscopic examination was performed 5 days after admission, showing increased total cell counts (4.47×105/mL) and lymphocytosis (21%) with an increased CD4/CD8 ratio (4.55) in the bronchoalveolar lavage fluid (BALF) from the middle lobe of the right lung. Pathologically, intra-alveolar and interstitial lymphocytic inflammation was observed in the right lung specimen obtained by a transbronchial biopsy, with collagen deposition on Elastica van Gieson staining (Fig. 2). There was no granuloma or aggregation of CD68-positive macrophages.
Figure 1.
Radiographic findings of the lungs one week prior to the admission. Chest X radiography (A) showed consolidation in the bilateral lungs, and high-resolution computed tomography demonstrated centrilobular nodules (B) and consolidation along with bronchovascular bundles and traction bronchiectasis (C).
Figure 2.
Pathological findings of a transbronchial lung biopsy sample. Hematoxylin and Eosin staining (A: ×40, B: ×200), Elastica van Gieson staining (C: ×200), and immunohistochemistry of CD68 (D: ×200).
His symptoms as well as laboratory abnormalities had mostly disappeared spontaneously within 10 days of admission, but radiographic improvement was only partial at this point. When he re-visited the storehouse and his home for 1 hour as a test of environmental exposure, his serum CRP levels gradually increased from 2.0 to 6.0 mg/dL within 5 days. No changes in the symptoms, body temperatures, or radiographic findings were observed. The serum CRP levels decreased and remained negative even after being discharged home while avoiding any visit to the storehouse.
We inspected his house, finding no fungal contamination, duvets, or humidifiers inside the house, but moldy tangerines were found stacked in a trash dump next to the storehouse. Culture of the moldy tangerines demonstrated multiple species of fungi, including Candida californica, Dipodascus geotrichum, Debaryomyces hansenii, Hanseniaspora uvarum, Kregervanrija fluxuum, and Penicillium digitatum. A mycobiome analysis of the tangerine peels using next-generation sequencing of internal transcribed spacer 1 region of fungal ribosomal RNA gene amplicon found that P. digitatum accounted for 98.4% of the fungus-derived gene sequences. However, precipitating antibodies against various fungi, including three Penicilium spp. (P. digitatum, P. luteum, P. glabrum), six Aspergillus spp., Trichosporon cutaneum, and Aureobasidium pullulans, were all negative in the serum of the patient.
The storehouse was extensively cleaned, and the patient worked there while wearing a mask. During the next winter and spring, there was no symptomatic recurrence, and the radiographic abnormalities of the lungs except for traction bronchiectasis gradually disappeared.
Discussion
There have been a few cases of OHP in citrus farmers reported to date, exclusively from Japan (12-14). Tangerines are harvested in late fall and stocked from December to February in storehouses, which was when and where the previous patients developed OHP (Table), whereas the current patient grew a different variety of citrus harvested in late winter and developed symptoms in March. Peripheral blood leukocytosis was common, but the serum levels of CRP, Krebs von den Lungen-6, and lactate dehydrogenase were variable among cases. The modest lymphocytosis in the BALF and a high CD4/CD8 ratio in the present case may have been related to the fibrotic changes observed radiographically and pathologically due to a longer and more insidious exposure to the antigen. CD4-positive lymphocytes are increased in the fibrotic lungs (15,16) and localized in the fibrotic alveoli and lymphoid follicles, whereas CD8-positive T lymphocytes distribute diffusely in relatively normal alveoli (17).
Table.
Characteristics and Laboratory Findings of Hypersensitivity Pneumonitis in Citrus Farmers.
| Age/gender | 50 F | 67 F | 53 M | 62 F | 66 M |
|---|---|---|---|---|---|
| Reference | 12 | 12 | 12 | 13 | |
| Symptoms | fever, cough, dyspnea | dyspnea, appetite loss | fever, cough, dyspnea | fever, cough | fever, dyspnea, fatigue |
| Admission | January | February | January | February | March |
| Medical histories | - | tuberculosis | - | dyslipidemia ectopic pregnancy |
- |
| Tobacco | never | never | never | never | 30 pack-years |
| Laboratory data | |||||
| WBC (/μL) Neutrophils (%) |
15,120 (95.3) |
4,520 (60.0) |
8,060 (82.1) |
7,800 (78.2) |
12,200 (84.9) |
| LDH (IU/mL) | 251 | 412 | 195 | 278 | 185 |
| CRP (mg/dL) | 0.1 | 0.2 | 6.8 | 9.08 | 14.83 |
| KL-6 (IU/mL) | 1,100 | 10,400 | 400 | 336 | 154 |
| Bronchoalveolar lavage fluid |
|||||
| TCC (/mL) | 3.05×105 | 1.9×105 | 1.92×105 | 6.3×105 | 4.47×105 |
| Lymphocytes (%) | 37 | 83.5 | 40.7 | 59 | 21 |
| CD4/CD8 | 0.2 | 0.63 | 1.54 | 1.64 | 4.55 |
| Serum precipitation antibody |
Penicillium luctem
Aspergillus fumigatus Candida albicans Alternaria kikuchiana |
negative | Aspergillus fumigatus |
Candida albicans
Penisillium lutem Cladosporium |
negative |
| Environmental culture |
Penicillium digitatum
Aspergillus niger Cladosporium cladosporioides |
Penicillim sp. |
Penicillium sp. Mucor |
Aspergillus niger Paecilomyces sp. |
Penicillium digitatum |
CRP: C-reactive protein, KL-6: Krebs von den Lungen-6, LDH: lactate dehydrogenase, TCC: total cell count, WBC: white blood cell
We performed fungal culture and a mycobiome analysis of the molded tangerine in the storehouse, finding that P. digitatum was the dominant fungus. P. digitatum and other Penicillium spp. were also the major air-borne fungi in the tangerine storehouses in other cases of citrus farmer's OHP (12,13). However, we and other researchers failed to detect precipitating antibody to P. digitatum in the serum of the patients with citrus farmer's OHP, suggesting that 1) other pathogens had caused the disease; 2) this type of OHP is dominantly caused by different pathogenic mechanisms, such as Type IV hypersensitivity reactions (18); or 3) the tests using crude fungal extracts lacked sufficient sensitivity for the diagnosis of chronic HP. In fact, the sensitivity of serum IgG and IgA for recurrent and insidious types of chronic bird-related HP was only 48-61%, in contrast to 85-91% in cases of acute or recurrent-type disease (19). Further analyses are necessary to identify the causal antigen for tangerine worker's OHP.
Tangerine farms are mostly maintained as a family business in Japan, making it difficult for patients with OHP to avoid antigens by job change. It is, therefore, necessary to invent procedures that can reduce the amount of airborne antigen content and exposure in the workplace environment.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Kirill Kryukov, PhD for his contribution to mycobiome analysis.
References
- 1. Fink JN, Ortega HG, Reynolds HY, et al. Needs and opportunities for research in hypersensitivity pneumonitis. Am J Respir Crit Care Med 171: 792-798, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Baur X. Hypersensitivity pneumonitis (extrinsic allergic alveolitis) induced by isocyanates. J Allergy Clin Immunol 95: 1004-1010, 1995. [DOI] [PubMed] [Google Scholar]
- 3. Quirce S, Vandenplas O, Campo P, et al. Occupational hypersensitivity pneumonitis: an EAACI position paper. Allergy 71: 765-779, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Selman M, Lacasse Y, Pardo A, Cormier Y. Hypersensitivity pneumonitis caused by fungi. Proc Am Thorac Soc 7: 229-236, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Xaubet A, Ancochea J, Morell F, et al. Report on the incidence of interstitial lung diseases in Spain. Sarcoidosis Vasc Diffuse Lung Dis 21: 64-70, 2004. [PubMed] [Google Scholar]
- 6. Roelandt M, Demedts M, Callebaut W, et al. Epidemiology of interstitial lung disease (ILD) in flanders: registration by pneumologists in 1992-1994. Working group on ILD, VRGT. Vereniging voor Respiratoire Gezondheidszorg en Tuberculosebestrijding. Acta Clin Belg 50: 260-268, 1995. [DOI] [PubMed] [Google Scholar]
- 7. Hanak V, Golbin JM, Ryu JH. Causes and presenting features in 85 consecutive patients with hypersensitivity pneumonitis. Mayo Clin Proc 82: 812-816, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Okamoto T, Miyazaki Y, Ogura T, et al. Nationwide epidemiological survey of chronic hypersensitivity pneumonitis in Japan. Respir Investig 51: 191-199, 2013. [DOI] [PubMed] [Google Scholar]
- 9. Malmberg P, Rask-Andersen A, Rosenhall L. Exposure to microorganisms associated with allergic alveolitis and febrile reactions to mold dust in farmers. Chest 103: 1202-1209, 1993. [DOI] [PubMed] [Google Scholar]
- 10. Yoshida K, Ueda A, Yamasaki H, Sato K, Uchida K, Ando M. Hypersensitivity pneumonitis resulting from Aspergillus fumigatus in a greenhouse. Arch Environ Health 48: 260-262, 1993. [DOI] [PubMed] [Google Scholar]
- 11. Akizuki N, Inase N, Ishiwata N, et al. Hypersensitivity pneumonitis among workers cultivating Tricholoma conglobatum (shimeji). Respiration 66: 273-278, 1999. [DOI] [PubMed] [Google Scholar]
- 12. Yasui H, Matsui T, Yokomura K, Nakano Y, Suda T, Chida K. Three cases of hypersensitivity pneumonitis in citrus farmers. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 48: 172-177, 2010. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 13. Kato F, Ogasawara T, Kasai H, Nishimura R, Kasamatsu N, Hashizume I. A case of hypersensitivity pneumonitis in a citrus fruit farmer. J Jpn Soc Respir Endoscopy 34: 21-25, 2012. (in Japanese, Abstract in English). [Google Scholar]
- 14. Yoshida K, Ando M, Araki S. Acute pulmonary edema in a storehouse of moldy oranges: a severe case of the organic dust toxic syndrome. Arch Environ Health 44: 382-384, 1989. [DOI] [PubMed] [Google Scholar]
- 15. Chizzolini C, Brembilla NC, Montanari E, Truchetet ME. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun Rev 10: 276-281, 2011. [DOI] [PubMed] [Google Scholar]
- 16. Falta MT, Bowerman NA, Dai S, Kappler JW, Fontenot AP. Linking genetic susceptibility and T cell activation in beryllium-induced disease. Proc Am Thorac Soc 7: 126-129, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamadori I, Fujita J, Kajitani H, et al. Lymphocyte subsets in lung tissues of non-specific interstitial pneumonia and pulmonary fibrosis associated with collagen vascular disorders: correlation with CD4/CD8 ratio in bronchoalveolar lavage. Lung 178: 361-370, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Bellanger AP, Lignon T, Godet Y, et al. Fungal peptides from pneumonitis hypersensitivity etiologic agents are able to induce specific cellular immune response. J Immunol Methods 440: 67-73, 2017. [DOI] [PubMed] [Google Scholar]
- 19. Shirai T, Tanino Y, Nikaido T, et al. Screening and diagnosis of acute and chronic bird-related hypersensitivity pneumonitis by serum IgG and IgA antibodies to bird antigens with ImmunoCAPⓇ. Allergol Int 70: 208-214, 2021. [DOI] [PubMed] [Google Scholar]