Abstract
We previously reported a 39-year-old man who presented with pulmonary and cerebral Cryptococcus gattii (genotype VGIIa) infection and was successfully treated with liposomal amphotericin B and flucytosine induction therapy. Following induction therapy, oral fluconazole treatment was initiated as consolidation therapy. However, the patient complained of progressively worsening headache, presenting an elevated cerebrospinal fluid (CSF) cell count. The minimum inhibitory concentrations of the CSF isolate were 8 and 0.12 μg/mL for fluconazole and voriconazole, respectively. The oral administration of voriconazole for more than 18 months alleviated his symptoms. Voriconazole might be useful for controlling refractory cases of C. gattii infection.
Keywords: Cryptococcus gattii, voriconazole, fluconazole
Introduction
Globally, Cryptococcus infections are regarded as an invasive mycosis mainly caused by Cryptococcus neoformans and C. gattii, and in Japan, nearly all reported cryptococcosis cases are caused by C. neoformans infections (1,2). Although we reported the sixth Japanese case of C. gattii infection in 2016, cryptococcosis caused by C. gattii remains extremely rare in Japan (1,3,4).
The clinical features of cryptococcosis caused by C. neoformans and C. gattii are similar; however, some strains of C. gattii (VG IIa, VG IIb, and VG IIc) show resistance to antifungal drugs (2,5). Particularly in C. gattii-infected patients with central nervous systems (CNS) lesions and/or large pulmonary lesions, the recommended duration of induction therapy is approximately 6 weeks, with a total duration of therapy of 18 to 24 months (2,6). Furthermore, aggressive management, such as drainage of cerebrospinal fluid (CSF) and/or surgical resection of cryptococcomas may be considered (2,6).
We herein report a refractory case of C. gattii infection despite consolidation therapy with oral fluconazole in which voriconazole effectively controlled the infection. To our knowledge, this is the first reported case of refractory C. gattii infection successfully treated with voriconazole monotherapy in Japan.
Case Report
We previously reported a 39-year-old man with pulmonary and cerebral C. gattii (genotype VGIIa) infection, successfully treated with liposomal amphotericin B and flucytosine induction therapy (1); the size of the cryptococcomas was reportedly reduced after induction therapy. The patient had previously been healthy, presenting with chief complaints of headache and a low-grade fever. He was a current smoker with no history of recent overseas travel.
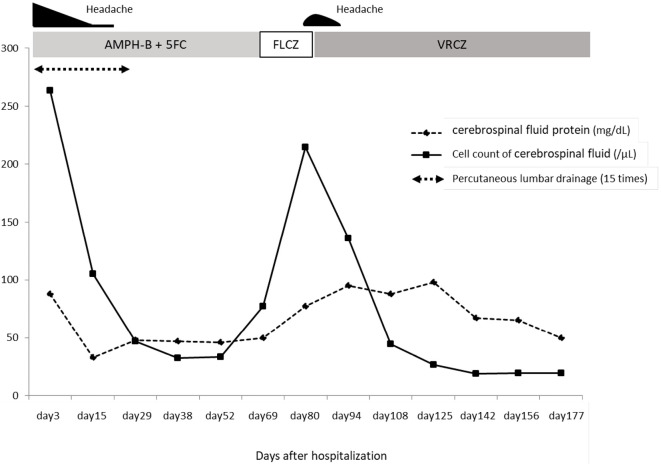
After induction therapy, oral fluconazole treatment was initiated as consolidation therapy on 63 days after hospirtalization. However, during consolidation therapy, he complained of progressively worsening headache, with an increased CSF cell count (215/mm2 cells, 82.7% of which were lymphocytes) (Fig. 1). Although the cryptococcal strain was not isolated from the CSF after the initiation of induction therapy, there was no further shrinkage of the cerebral or pulmonary cryptococcomas (Fig. 2a-d). We considered performing surgical resection of cryptococcomas, but since the cerebral lesions were small and multiple in number, resection could not be performed. We checked the results of antifungal drug susceptibility of the isolate [drug susceptibility tests (Clinical Laboratory Standards Institute (CLSI) M27-A3 method) were performed at the Medical Mycology Research Center, Chiba University] and noted minimum inhibitory concentrations (MICs) of 8 and 0.12 μg/mL for fluconazole and voriconazole, respectively (Table).
Figure 1.
Clinical course after hospitalization. The cerebrospinal fluid protein level and cell count are increased after initiating oral fluconazole treatment, gradually decreasing after starting voriconazole treatment. AMPH-B: amphotericin B, 5FC: flucytosine, FLCZ: fluconazole, VRCZ: voriconazole
Figure 2.
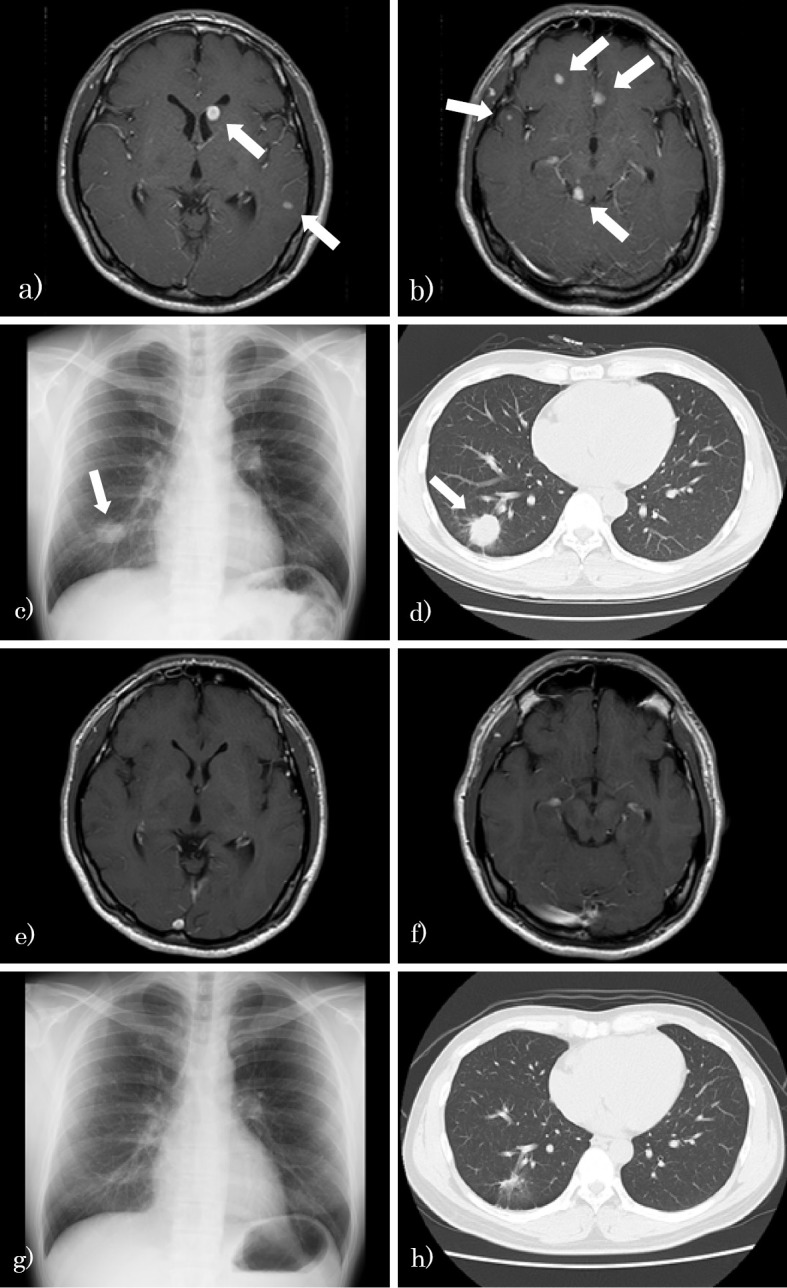
Pulmonary and cerebral cryptococcomas after induction therapy (73 days after hospitalization; a, b, c, d) and 2 years after maintenance therapy (e, f, g, h). a, b: Gadolinium-enhanced brain MRI shows multiple brain tumors. c: Chest X-ray shows a tumor in the right lower field. d: Chest CT shows a tumor in the right S9/10 (arrows indicate cryptococcomas). e, f: Gadolinium-enhanced brain MRI shows the disappearance of multiple brain tumors. g: Chest X-ray shows a shrunken tumor in the right lower field. h: Chest CT shows a scarred lesion in the right S9/10. MRI: magnetic resonance imaging, CT: computed tomography
Table.
MIC of Anti-fungal Drugs for the Isolate from Cerebrospinal Fluid.
| Anti-fungal drugs | MIC (μg/mL) |
|---|---|
| MCFG | >16 |
| CPFG | 8 |
| AMPH-B | 1 |
| 5FC | 2 |
| FLCZ | 8 |
| ITCZ | 0.25 |
| VRCZ | 0.12 |
| MCZ | 0.25 |
MIC: minimun inhibitory concentration, MCFG: micafungin, CPFG: caspofungin, AMPH-B: amphotericin B, 5FC: flucytosine, FLCZ: fluconazole, ITCZ: itraconazole, VRCZ: voriconazole, MCZ: miconazole
We suspected the case of being resistant to fluconazole and administered oral voriconazole (approximately 300-400 mg/day) as a salvage treatment 84 days after hospitalization. We then examined the plasma and CSF voriconazole concentrations. In the CSF, the voriconazole concentration was almost half of that detected in the serum; the CSF concentrations ranged from 0.64 to 1.89 μg/mL, and the CSF-to-plasma concentration ratio ranged from 0.49 to 0.53. Treatment with voriconazole ameliorated the patient's symptoms, and we continued administering voriconazole for over 18 months as consolidation and maintenance therapy.
Two years after the final voriconazole administration, the serological cryptococcal antigen test was negative, with no recurrence of pulmonary or cerebral cryptococcomas (Fig. 2e-h). The patient demonstrated no residual disability except for a marginally elevated creatinine level (creatinine, 1.12 mg/dL; normal range, 0.60-1.10) owing to long-term amphotericin B treatment.
Discussion
Traditionally, C. gattii has been recognized as a pathogen endemic in tropical and subtropical climates. However, since the 1990s, outbreaks of C. gattii have been reported in the Pacific Northwest in North America (5,6). C. gattii was first isolated from Australian eucalypts and now has been isolated from more than 50 tree species, including angiosperms and gynosperms (3,6). Furthermore, C. gattii is an emerging pathogen in a broad range of animals, including dogs, cats, horses, sheep, cows, koalas, and birds (6). Reportedly, the most common sites of C. gattii infections include the CNS and lungs, demonstrating an incubation period of 2 to 11 months (6).
In Japan, C. gattii infection is recognized as an imported infectious disease, and only sporadic cases have been documented since 2001 (1,7). The present case presented no recent history overseas travel and no known exposure to any imported timber or animals. In addition to our case, only two other cases are considered to have been infected with C. gattii within Japan (1,8). C. gattii infection is typically likely to be associated with mass lesions, neurological complications, and antifungal resistance (4-6). Therefore, clinical practice guidelines recommend surgical resection of cryptococcomas if their size cannot be reduced after four weeks of therapy (2). In the present case, the cryptococcoma size failed to be reduced after fluconazole therapy initiation; however, owing to the small size and multiple number of cerebral lesions, surgical resection could not be performed. Furthermore, as C. gattii was not isolated from the CSF after the initiation of induction therapy, we should have considered the possibility of immune reconstitution inflammatory syndrome (IRIS). However, based on the brain magnetic resonance imaging findings, we clinically suspected a refractory case of cerebral cryptococcosis. We checked the antifungal drug susceptibility of the CSF isolate and then proceeded to administer voriconazole. To the best of our knowledge, this is the first Japanese case of C. gattii infection achieving complete remission with voriconazole consolidation and maintenance therapy without surgical resection.
Regarding the antifungal susceptibility of C. gattii, resistance to amphotericin B and flucytosine has rarely been reported in previous investigations; however, elevated MICs of C. gattii (genotype VG IIa and VG IIc) against fluconazole have been reported in the Pacific Northeast (6). In addition, a previous report found that all clinical isolates that had not been exposed to azole drugs and environmental strains manifested heteroresistance to fluconazole (9). Some studies have shown that voriconazole is more effective in vitro against C. gattii than fluconazole, and fluconazole MICs against the C. gattii genotype VG II, detected in clinical and environmental isolates in the Pacific Northeast, were higher than those of voriconazole and itraconazole (6). Although itraconazole fails to penetrate the CSF adequately, voriconazole penetrates the CSF and brain tissue, making it a promising option in patients presenting with CNS fungal infections (10,11). In addition, as the concentration of voriconazole in the CSF is almost half of the serum concentration (9), monitoring of voriconazole in the CSF is not necessary. If antifungal susceptibilities of an isolate are examined and the MIC of voriconazole is low, voriconazole may be a useful treatment option in C. gattii-infected patients who are refractory to other available antifungal therapies. Generally, in Cryptococcus infection, an MIC of fluconazole exceeding 16 μg/mL suggests resistance to antifungal drugs. However, as data regarding the relationship between the MICs of antifungal drugs and the clinical outcomes are lacking, further investigations are required to determine the efficacy of voriconazole against C. gattii infection.
In conclusion, we encountered a refractory case of C. gattii infection that was successfully treated with voriconazole monotherapy. Although voriconazole is not recommended for use in treating C. gattii infection according to the clinical guidelines, if the voriconazole MIC against the isolate is low, voriconazole may be beneficial for controlling C. gattii infection. Additional studies are warranted to validate our findings.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank Katsuhiko Kamei at Chiba University (Medical Mycology Research Center) for performing the mycological analysis during patient treatment.
References
- 1. Nakao M, Muramatsu H, Takahashi T, et al. Cryptococcus gattii genotype VGIIa infection in an immunocompetent Japanese patient: a case report and mini-review. Intern Med 55: 3021-3024, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50: 291-322, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kawamura I, Kamei K, Yarita K, et al. Cryptococcus gattii genotype VGIIb infection in Japan. Med Mycol J 55: E51-E54, 2014. [DOI] [PubMed] [Google Scholar]
- 4. Kitaura T, Takahashi M, Umeyama T, et al. Cryptococcus gattii genotype VGIIa infection imported from Vancouver Island to Japan. J Infect Chemother 24: 573-575, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Harris JR, Lockhart SR, Debess E, et al. Cryptococcus gattii in the United States: clinical aspects of infection with an emerging pathogen. Clin Infect Dis 53: 1188-1195, 2011. [DOI] [PubMed] [Google Scholar]
- 6. Chen SC, Meyer W, Sorrell TC. Cryptococcus gattii infections. Clin Microbiol Rev 27: 980-1024, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsunemi T, Kamata T, Fumimura Y, et al. Immunohistochemical diagnosis of Cryptococcus neoformans var. gattii infection in chronic meningoencephalitis: the first case in Japan. Intern Med 40: 1241-1244, 2001. [DOI] [PubMed] [Google Scholar]
- 8. Okamoto K, Hatakeyama S, Itoyama S, et al. Cryptococcus gattii genotype VGIIa infection in man, Japan, 2007. Emerg Infect Dis 16: 1155-1157, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varma A, Kwon-Chung KJ. Heteroresistance of Cryptococcus gattii to fluconazole. Antimicrob Agents Chemother 54: 2303-2311, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wood N. Pharmacokinetics of voriconazole. Jpn J Chemother 53: 16-23, 2005. [Google Scholar]
- 11. Lutsar I, Roffey S, Troke P. Voriconazole concentrations in the cerebrospinal fluid and brain tissue of guinea pigs and immunocompromised patients. Clin Infect Dis 37: 728-732, 2003. [DOI] [PubMed] [Google Scholar]