Abstract
An elderly patient with multiple myeloma (MM) was being treated with several regimens and developed a severe drug eruption, necessitating the use of atovaquone instead of trimethoprim-sulfamethoxazole for pneumocystis pneumonia (PCP) prophylaxis. For progressive MM, treatment with isatuximab, an anti-CD38 monoclonal antibody, was started. During the treatment, he developed Listeria monocytogenes bacteremia and recovered quickly with ampicillin administration. CD38 is closely related to the innate immune response against L. monocytogenes, and isatuximab may increase the risk of infection. Therefore, trimethoprim-sulfamethoxazole may be useful in the prevention of not only PCP but also L. monocytogenes infection.
Keywords: Listeria monocytogenes, CD38, isatuximab, multiple myeloma
Introduction
Listeria monocytogenes is a Gram-positive bacterium that enters the human body orally through food, such as meat and dairy products. Although L. monocytogenes is rarely pathogenic to humans, it can cause listeriosis, leading to meningitis and bacteremia in pregnant women, infants, elderly individuals, and cancer patients, with a high mortality rate (1,2).
Invasive listeriosis sometimes develops in patients with hematologic malignancies, especially in those who have undergone stem cell transplantation because of severe insufficiency of cell-mediated immunity (3). In recent years, new agents with different mechanisms of action have been developed for the treatment of various hematologic malignancies. These agents can cause complex changes in immunity.
Isatuximab, a monoclonal antibody that binds a specific epitope on the human CD38 receptor, is highly effective against multiple myeloma (MM). CD38 is closely related to the regulation of innate immunity against L. monocytogenes infection (4). Therefore, the use of isatuximab may be associated with an increase in the rate of Listeria infections.
We herein report a patient with MM who developed L. monocytogenes bacteremia during isatuximab therapy.
Case Report
The patient, a 68-year-old Japanese man, presented with initial complaints of back pain. He was diagnosed with IgG lambda-type, symptomatic MM and thereafter received treatment at our institution for three years. At the time of the diagnosis, the patient's serum IgG level was 6,458 mg/dL, while the levels of serum-free lambda and kappa light chains were 970 and 13.7 mg/L, respectively. Bone marrow aspiration showed hyperplastic bone marrow with 48% abnormal plasma cells. A chromosome test revealed deletion of the Y chromosome but no other high-risk chromosomal abnormalities. Based on the revised international staging system for MM, the case was categorized as stage III.
The patient was treated with bortezomib, lenalidomide, and dexamethasone (VRD) as first-line therapy. Trimethoprim-sulfamethoxazole (TMP-SMX) was also started at the beginning of the treatment to prevent pneumocystis pneumonia (PCP). After two cycles of VRD, the patient achieved a partial response and was subsequently treated with lenalidomide and dexamethasone (RD) therapy. During RD therapy, the patient developed a severe skin rash suspected of being drug eruption; treatment for MM was therefore discontinued, and corticosteroids were started. Since the drug-induced lymphocyte stimulation test (DLST) was positive for several drugs, including TMP-SMX, the suspected drugs were discontinued, and PCP prophylaxis was substituted with aerosolized pentamidine.
The patient's MM worsened with mass formation in the sternum and clavicle. Therefore, the patient was subjected to several treatment modalities to address the relapse. Carfilzomib, lenalidomide, and dexamethasone (KRD) therapy was first administered, followed by daratumumab, bortezomib, and dexamethasone (DVD) and then pomalidomide and dexamethasone (PD) therapy. Following that, ixazomib, lenalidomide, and dexamethasone (IRD) therapy was concurrently administered with radiation, which was followed by PD therapy. The patient was subsequently treated with elotuzumab, pomalidomide, and dexamethasone (EPD) therapy. However, despite receiving the above-mentioned treatments, his MM progressively worsened. Therefore, isatuximab, pomalidomide, and dexamethasone (Isa-PD) therapy was started. At this time, atovaquone was being used for PCP prophylaxis.
The Isa-PD schedule was planned with isatuximab 10 mg/kg on days 1, 8, 15, and 22; pomalidomide 2 mg (maximum tolerated dose) on days 1 to 21; and dexamethasone 40 mg on days 1, 8, 15, and 22. Atovaquone was used for PCP prophylaxis, and a small dose of a corticosteroid was continued to treat the drug eruption. On day 5 after the onset of Isa-PD therapy, the patient developed a high fever and hypotension and was administered cefepime under suspicion of febrile neutropenia (FN).
The laboratory test data collected at that time are summarized in Table. Blood culture samples were positive for a Gram-positive bacterium, which was confirmed to be L. monocytogenes. The isolate was found to be sensitive to ampicillin (minimum inhibitory concentration of 0.5 μg/mL). The patient had no gastrointestinal symptoms prior to the development of L. monocytogenes bacteremia and no symptoms or signs suggestive of meningitis or encephalitis. Treatment was then switched from cefepime to ampicillin for 14 days, and his symptoms resolved rapidly with ampicillin treatment. Furthermore, MM showed a good response despite the interruption of Isa-PD treatment after day 21. Whether or not TMP-SMX treatment was the main cause of the drug eruption was unclear, since the DLST was positive for several drugs, and the skin rash was clearly improved at the time. Therefore, TMP-SMX was resumed in small doses on a trial basis during the second cycle of Isa-PD therapy, and neither the skin rash nor L. monocytogenes bacteremia relapsed.
Table.
Laboratory Findings at the Onset of Bacteremia.
| Complete blood cell count | Blood chemistry | |||||||
| White blood cell | 10,200 | /µL | Total protein | 6.7 | g/dL | Blood urea nitrogen | 49.8 | mg/dL |
| Neutrophil | 94.9 | % | Albumin | 1.9 | g/dL | Creatinine | 1.4 | mg/dL |
| Lymphocyte | 1.2 | % | Total bilirubin | 0.6 | mg/dL | Creatine kinase | 14 | U/L |
| Monocyte | 3.3 | % | Aspartate transaminase | 32 | U/L | Amylase | 125 | U/L |
| Eosinophil | 0.2 | % | Alanine aminotransferase | 35 | U/L | Uric acid | 6.4 | mg/dL |
| Basophil | 0.4 | % | Lactate dehydrogenase | 128 | U/L | C-reactive protein | 7.35 | mg/dL |
| Hemoglobin | 9.3 | g/dL | Alkaline phosphatase | 222 | U/L | |||
| Platelet | 20.8×104 | /µL | γ-Glutamyltransferase | 91 | U/L | |||
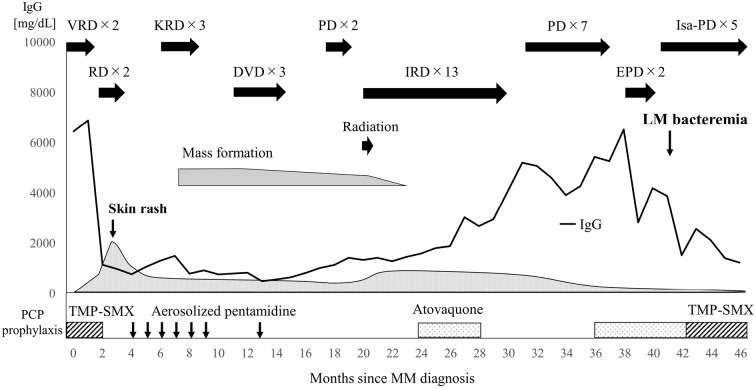
Figure shows the complete treatment regimen of the patient after MM was diagnosed.
Figure.
Clinical course of the patient. DVD: daratumumab, lenalidomide, and dexamethasone, EPD: elotuzumab, pomalidomide, and dexamethasone, IRD: ixazomib, lenalidomide, and dexamethasone, Isa-PD: isatuximab, pomalidomide, and dexamethasone, KRD: carfilzomib, lenalidomide, and dexamethasone, LM: Listeria monocytogenes, MM: multiple myeloma, PCP: pneumocystis pneumonia, PD: pomalidomide and dexamethasone, RD: lenalidomide and dexamethasone, TMP-SMX: trimethoprim-sulfamethoxazole, VRD: bortezomib, lenalidomide, and dexamethasone
Discussion
L. monocytogenes is an intracellular parasitic bacterium that can survive and proliferate within macrophages. Cell-mediated immunity by immune cells, such as activated macrophages and dendritic cells, plays an important role in the innate immune response against L. monocytogenes (5). CD38 is expressed in activated macrophages and is essential for their regulation. It has been shown that CD38-knockout mice have increased susceptibility to L. monocytogenes infection due to the inhibition of macrophage migration to the infection site (4). This may also be observed in humans, since CD38 is also induced in human macrophages upon infection (6). Therefore, monoclonal antibodies, such as daratumumab, which targets CD38, also highly expressed in myeloma cells, may increase the risk of L. monocytogenes infection. In a nested case-control study conducted on patients amid an outbreak at a commercial eatery, patients who received daratumumab were at a 340-fold higher risk of developing L. monocytogenes infection than other cancer patients. In addition, patients treated with daratumumab had a 75-fold higher risk of listeriosis than all other MM patients, although the study included high-risk patients who underwent stem cell transplantation and received experimental therapies for relapsed refractory disease (7). In fact, there have been two case reports of L. monocytogenes infections after the administration of daratumumab (8,9). Isatuximab is a human anti-CD38 monoclonal antibody similar to daratumumab; however, the antibody targets different amino acid sequences and possesses strong proapoptotic activity independent of cross-linking agents (10). As demonstrated in this case, isatuximab may be a risk factor for the development of L. monocytogenes infection, similar to daratumumab.
The other risk factors that may lead to L. monocytogenes infection include pregnancy, organ transplant, tumor necrosis factor antagonists, cancer chemotherapy, old age, diabetes, and high-dose steroids (1,2). Therefore, the L. monocytogenes infection in our patient may not have been due to isatuximab alone but rather to multiple factors, such as MM therapy, old age, and long-term use of corticosteroids. However, throughout the course of treatment in this case, which included daratumumab therapy as well, L. monocytogenes infection did not develop. The infection first developed after the administration of isatuximab, suggesting that isatuximab was an important factor in the establishment of the infection.
Routine stool culture is not recommended for L. monocytogenes detection, as asymptomatic fecal carriage complicates the prediction of the risk of infection (2). This difficulty in reliably predicting conditions that cause L. monocytogenes infection can result in severe infections in patients with hematologic malignancies when the bacterium becomes pathogenic (3). Therefore, prevention and early treatment of L. monocytogenes infection are important. The primary way of preventing L. monocytogenes infection is to avoid consuming contaminated foods, such as dairy products and meat. Second, TMP-SMX, which has been reported as an independent protective factor against L. monocytogenes infection, should be used prophylactically as part of the treatment regimen (11). L. monocytogenes meningoencephalitis and cerebral abscesses have been reported after switching from TMP-SMX to atovaquone for preventing PCP (12). In the present case, L. monocytogenes bacteremia developed after TMP-SMX treatment was switched to atovaquone and did not recur after the administration of TMP-SMX was re-started, suggesting the effectiveness of TMP-SMX in preventing the infection. Ampicillin is the first treatment of choice for L. monocytogenes infection; other therapeutic options include TMP-SMX and meropenem, whereas cephem antibiotics are usually ineffective (2,3). Fourth-generation cephem drugs, which are commonly used in the management of FN, may be inadequate for the initial treatment of L. monocytogenes infection. Therefore, meropenem, which is effective against both listeriosis and FN, should be considered, especially after the use of anti-CD38 antibodies.
In conclusion, we described a patient with MM who developed L. monocytogenes bacteremia during isatuximab therapy. Isatuximab can cause L. monocytogenes infection and may necessitate prophylaxis with TMP-SMX and dietary guidance.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank the nursing staff who cared for the patient at Kitakyushu Municipal Medical Center and the technical staff at the hospital's bacteriology laboratory.
References
- 1. Vázquez-Boland JA, Kuhn M, Berche P, et al. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev 14: 584-640, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shoham S, Bartlett JG. Listeria monocytogenes. Johns Hopkins Medicine POC-IT guides [Internet]. [cited 2021 Feb 27]. Available from: https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540318/all/Listeia_monocytogenes
- 3. Rivero GA, Torres HA, Rolston KVI, Kontoyiannis DP. Listeria monocytogenes infection in patients with cancer. Diagn Microbiol Infect Dis 47: 393-398, 2003. [DOI] [PubMed] [Google Scholar]
- 4. Lischke T, Heesch K, Schumacher V, et al. CD38 controls the innate immune response against Listeria monocytogenes. Infect Immun 81: 4091-4099, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maudet C, Levallois S, Disson O, Lecuit M. Innate immune responses to Listeria in vivo. Curr Opin Microbiol 59: 95-101, 2021. [DOI] [PubMed] [Google Scholar]
- 6. Amici SA, Young NA, Narvaez-Miranda J, et al. CD38 is robustly induced in human macrophages and monocytes in inflammatory conditions. Front Immunol 9: 1593, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khan S, Vaisman A, Hota SS, et al. Listeria susceptibility in patients with multiple myeloma receiving daratumumab-based therapy. JAMA Oncol 6: 293-294, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hung DLL, Lau SKP, Woo PCY. Severe sepsis caused by Listeria monocytogenes in a patient given monoclonal antibodies against CD38 and proteosome inhibitor. Infect Microbes Dis 1: 30-31, 2019. [Google Scholar]
- 9. Horikita F, Hashiguchi J, Nagashima T. Post colonoscopic Listeria monocytogenes meningitis in a patient with multiple myeloma during daratumumab-based therapy. Rinsho Ketsueki (Jpn J Clin Hematol) 61: 1611-1615, 2020. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 10. Deckert J, Wetzel MC, Bartle LM, et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res 20: 4574-4583, 2014. [DOI] [PubMed] [Google Scholar]
- 11. Fernàndez-Sabé N, Cervera C, López-Medrano F, et al. Risk factors, clinical features, and outcomes of listeriosis in solid-organ transplant recipients: a matched case-control study. Clin Infect Dis 49: 1153-1159, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Adjei PC. Listeria monocytogenes meningoencephalitis and cerebral abscess in a heart transplant recipient. Case Rep Infect Dis 2020: 8498216, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]