Abstract
Soil-borne diseases are the major problems in mono cropping. A mixture (designated LTM-m) composed of agricultural wastes and a beneficial microorganism Streptomyces saraceticus SS31 was used as soil amendments to evaluate its efficacy for managing Rhizoctonia solani and root knot nematode (Meloidogyne incognita). In vitro antagonistic assays revealed that SS31 spore suspensions and culture broths effectively suppressed the growth of R. solani, reduced nematode egg hatching, and increased juvenile mortality. Assays using two Petri dishes revealed that LTM-m produced volatile compounds to inhibit the growth of R. solani and cause mortality to the root knot nematode eggs and juveniles. Pot and greenhouse tests showed that application of 0.08% LTM-m could achieve a great reduction of both diseases and significantly increase plant fresh weight. Greenhouse trials revealed that application of LTM-m could change soil properties, including soil pH value, electric conductivity, and soil organic matter. Our results indicate that application of LTM-m bio-organic amendments could effectively manage soil-borne pathogens.
Keywords: bio-organic amendment, Meloidogyne incognita, organic farming, Rhizoctonia solani, soil property
Organic vegetables have become increasingly popular worldwide because of the issue and concern in food safety and environmental problems resulting from excessive uses of toxic chemicals. To control plant diseases in organic farming, development of biological control measures using beneficial microorganisms is a promising alternative. Most of the managing strategies in organic agriculture rely on physical and biological controls, which have achieved some success on controlling foliar diseases. However, biological control has limited success on controlling soilborne plant parasitic pathogens, which are often the main obstacles for the production in organic farms (Huang et al., 2013). To combat soil-borne pathogens in organic farming, application of organic soil amendments can be beneficial and cost-effective.
Organic soil amendments using agricultural products or wastes have been known to improve soil chemical and physical properties, enrich beneficial organisms in soil environments, increase enzymatic activity, and elevate the rate of decomposition (Goyal et al., 1999). Recycling agricultural wastes can reduce production cost and benefit environmental conservation (Al-Barakah et al., 2013). Many agricultural wastes such as chitinous materials (shrimp and crab shell powder), oil cakes, and plant residues have been used to control plant diseases (Bonanomi et al., 2007; Sikora and Roberts, 2018). Chitinous materials have been shown to enrich the population of chitinolytic microorganisms. Chitinase produced by chitinolytic microorganisms has been known to be vital for controlling soil-borne pathogens because it can break down fungal cell wall and nematode egg shells (Oka, 2010). Thus, agricultural wastes are often used as soil amendments to elevate the population of chitinolytic microorganisms.
Many Streptomyces spp. can produce antibiotics and chitinases (Tang-um and Niamsup, 2012). Others have hyperparasitic abilities on fungal pathogens (Prabavathy et al., 2006; Sutherland and Papavizas, 1991; Zuberer et al., 1988). Many Streptomyces spp. collected in our lab have been shown to inhibit different pathogens. Streptomyces saraciticas SS31 originally isolated from a citrus grove has been proven to inhibit a number of plant pathogens, including Pytihum aphanidermatum, Rhizoctonia solani AG4 HGII, Sclerotium rolfsii, Fusarium solani Vn4-2, Alternaria brassicicola, Xanthomonas axonopodis pv. vesicatoria, X. vesicatoria, X. axonopodis subsp. citri, X. campestris pv. campestris, Acidovorax avenae subsp. citrulli, and Pectobacterium carotovorum subsp. carotovorum (Chen, 2008; Huang, 2016). The SS31 strain is capable of promoting plant growth and producing indoleacetic acid, siderophore, and hydrolytic enzymes such as amylase, protease, cellulase, and chitinase (Tu, 2014).
In 2019, an organic farm in Kaohsiung, Taiwan, reported severe yield losses due to several soil-borne diseases caused by Rhizoctonia damping off, club root disease of Brassicaceae, bacterial soft rot and root knot nematode (Meloidogyne incognita). Of them, Rhizoctonia damping off and M. incognita are the most commonly identified problems in the region. In this study, S. saraciticas SS31 was mixed with several agricultural products or wastes, including soybean meal, crab and shrimp shells, neem seed oilcake, sugar, and dolomite to form a bio-organic soil amendment designated LTM-m. Pot and greenhouse trials were carried out to evaluate the efficacy of LTM-m for controlling R. solani and M. incognita. LTM-m produced toxic metabolites and volatile compounds, both of which could suppress the growth of R. solani and reduce damping-off. Application of LTM-m also effectively suppressed egg hatching and the formation of root knot. Nematode eggs after being treated with LTM-m became deformed or lysed. Overall, our results have shown that a combination of SS31 and agricultural products is effective in controlling soil-borne plant pathogens. This present study provides an example of how to utilize eco-friendly natural products and microorganisms as a soil amendment for disease management in organic agriculture.
Materials and Methods
Identification and maintenance of microorganisms
Two soil-borne pathogens: R. solani and root knot nematode (RKN) M. incognita were collected from diseased plants in Yonglin organic farm (Kaohsiung, Taiwan). R. solani was isolated from okra (Abelmoschus esculentus) and M. incognita was from spoon cabbage (Brassica chinensis L. cv. Ching-Geeng). R. solani was cultured on potato dextrose agar (PDA) at 28°C for 4 days and stored in 10% soil mixed with 1% agar. M. incognita was maintained on water spinach for 30 days. Nematode eggs were harvested and washed by NaOCl according to Hussey (1973). Fungal DNA was extracted using Tissue & Cell Genomic DNA Purification Kit (GeneMark, Taipei, Taiwan). R. solani was identified to the species by sequence similarity of a DNA fragment amplified by PCR using two primers V9G (5′-TTACGTCCCTGCCCTTTGTA-3′) (van den Ende and de Hoog, 1999) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (Al-Fadhal et al., 2018; Damm et al., 2019). Nematode DNA was extracted using Direct PCR lysis reagent (Viagen Biotech, Los Angeles, CA, USA). M. incognita was identified to species based on restriction fragment length polymorphism patterns. DNA fragments were amplified by PCR using two primers TRNAH (5′-TGAATTTTTTATTGTGATTAA-3′) and MRH106 (5′-AATTTCTAAAGACTTTTCTTAGT-3′) (Stanton et al., 1997), digested by the restriction enzyme HinfI (New England BioLabs, Ipswich, MA, USA), and analyzed by gel electrophoresis (Pagan et al., 2015).
Streptomyces saraceticus 31 (SS31) originally isolated from a citrus grove was revitalized and cultured on potato sucrose agar (PSA) plates. Fully grown SS31 was inoculated into sterilized 2% soybean powder sugar broth, and cultured for 5 days on a shaker set at 130 rpm. Culture filtrates containing secondary metabolites were obtained by centrifugation at 10,000 rpm for 1 minute and passing through a 0.22 μm Millipore filter. SS31 spores were collected by washing off with 0.5% Tween 20 from a PSA plate, and the concentration of spores was adjusted to 108 cfu (OD = 0.3). LTM-m was prepared by mixing 40% crab shell powder, 30% soybean powder, 15% neem seed meal, 10% sugar, 5% dolomite, and S. saraceticus SS31.
In vitro antagonistic tests against R. solani
In-vitro antagonistic activity against R. solani was assayed on a 9-cm dia. PDA plate. A 8-mm paper disc was placed in the center and R. solani agar discs (5 mm) were placed 4 cm away at each side of the paper disc. For each test, 50 μl of SS31 spore suspensions, SS31 culture broths (2% soybean powder sugar broth), SS31 filtrates, or water (mock control) was placed on the paper disc, and the plates were incubated at 30°C until R. solani hyphae reached to the edge of the control plate. Percentage of growth inhibition was calculated using the following equation: (A−B)/A × 100%, where A is the distance between test pathogen and SS31 and B is the radius of the pathogen colony. Each treatment had three replicates and all tests were repeated three times.
In vitro antagonistic tests against M. incognita
Egg masses of M. incognita were hatched in water. Second-staged juvenile (J2) was collected, and its concentration was adjusted to 200 J2/ml. Eggs embedded in a gelatinous matrix were released by adding 10% commercial bleach, and relocated to fresh water, and the concentration was adjusted to 200 eggs/ml. Approximately 100 J2 or eggs (in 500 μl water) were added to a 1.5 ml micro-centrifuge tube and mixed with equal volume of spore suspensions, culture broths or filtrates of SS31 (108 cfu). To ensure complete homogeneity, tubes were inverted several times. Percentage of J2 mortality and egg hatching rate were recorded after 24 h and 5 days of incubation, respectively. Each treatment had three replicates and all tests were repeated three times.
Assays for antagonistic volatiles against R. solani
Volatile antimicrobial activity was assayed in a petri dish filling with LTM-m. LTM-m (2, 5, or 10 g) was placed in a petri dish (90 mm diameter, 20 mm height), and 10 g sterilized sand was used as mock controls. A smaller PDA plate (50 mm diameter) inoculated with a 5-mm R. solani hyphae disc was placed on top of LTM-m. The whole set of plates was sealed with parafilm to allow volatile compounds to interact with R. solani. R. solani culture placing on top of sand was used as a mock control. Plates were kept at room temperature (~25°C) for 7 days and radial growth of R. solani were measured.
Assays for antagonistic volatiles against M. incognita
Either egg masses or approximately 100 J2 of M. incognita were placed in the center of a 0.08% water agar plate (50 mm), which was placed on top of LTM-m or sterilized sand as stated above. After 7 days, percentage of egg hatch and J2 mortality was determined and morphology of eggs and juveniles after treatment were also recorded with an aid of a light microscope.
Growth chamber trials for controlling R. solani
R. solani inoculum was modified from Yang (1994). R. solani was first grown on sterilized potato cubes for 7 days, and 100 g of potato were mixed with 900 g of peat moss (BVB substrates, C100B) to make R. solani inoculum. R. solani inoculum were then mixed with King root substrates (Dayi, Pingtung County, Taiwan) in a 1:10 ratio to made R. solani-infested soils for seedling tray tests. In a clean plastic box, 600 g of R. solani-infested soils and LTM-m (0.08, 0.16, and 0.24%) or ddH2O (mock control) were mixed. The mixture was incubated for 14 days in the closed boxes and used as planting materials. A 64 compartment (8 × 8) seedling tray was filled with planting materials, and two seeds of spoon cabbages (Qing-gang variety) were sown in each hole. The trays were kept in a growth chamber (set at 28°C, 12 h/12 h, light/dark cycle). Emergent percentage of seeds and disease severity were examined 7 and 14 days after sowing. Disease severity was classified using the following scale: 0, symptomless; 1, lesions at the base of the stem less than 0.5 cm; 2, lesion areas between 0.5 and 1 cm; 3, lesions greater than 1 cm and showing dampingoff, or post-emergence damping-off; and 4, showing pre-emergence damping-off.
Growth chamber trials for controlling M. incognita
Sand and peat moss after being sterilized were mixed at the ratio of 2:3 (w/w). After adding M. incognita eggs (two per g of soil), soil (2 kg) were mixed with LTM-m (0.08, 0.16 or 0.24%, w/w). Sterilized soil alone was used as a negative control, and soil containing M. incognita eggs only was used a positive control. All treatments were individually sealed in a plastic box, kept at 28°C for 14 days, and used to grow water spinach (Ipomoea aquatica) in a 3-inch pot (three seeds per pot). Plants were kept in a growth chamber set 28°C and 12 h light/12 h darkness cycle for 30 days. Number, fresh weight, and height of plants in each pot were recorded. Percentage of root gall formation (Zeck, 1971) and the number of egg mass were also recorded. Each treatment had seven pots, and the experiment was repeated three times.
Greenhouse trials
Trials were conducted in two greenhouses (B02 and K07) located in Yonglin organic farm where plants were infested with RKN. Field infestation rate was evaluated by randomly sampling 100 plants for galling index in a greenhouse. In a 320 m2 greenhouse, 100 kg of LTM-m containing 100 l of 100×SS31 culture broths was mixed with soil. Soils were covered with black plastic sheets for 14 days. Water spinach plants (n = 100) after growing in soils for 30 days were randomly selected to examine for gall formation and shoot weight. Galling index (0–10) was classified using the following scale: 0, no galls, 1, 1–10% root system galled; 2, 11–20% galls; 3, 21–30% galls; 4, 31–40% galls; 5, 41–50% galls; 6, 51–60% galls; 7, 61–70% galls; 8, 71–80% galls; 9, 81–90% galls; and 10, 91–100% galls (Zeck, 1971). The experiment was conducted for three successive seasons, from Nov 22, 2019 till May 8, 2020. The dates of harvest were January 17, March 21, and May 8.
Measurement of soil pH and electrical conductive (EC)
Soil samples from greenhouse trials were collected from the rhizosphere of the plants used for disease assessment and analyzed for physical and chemical properties after each of growing seasons. Soil samples were air-dried for one week and passed through a 2-mm diameter test sieve. Soil was mixed with deionized water at a 1:5 (w/v) ratio in a beaker with agitation for 30 min, stood for 10 min, and measured using a FiveEasy pH meter F20 (Mettler Toledo, Columbus, OH, USA) and a EC meter (inoLab Cond 7110, Taiwan).
Soil texture was determined using the Robinson’s pipette method (Buchan et al., 1993) with modifications, which clay sedimentation was processed for 7 h, and 25 ml of soil solution was sampled from 7–10 cm fraction. Sand and clay contents were calculated by the following equations (Gee and Bauber, 1986).
Soil organic matter (SOM) was determined by mixing 0.5 g of air-dried soil with 10 ml 1 N K2Cr2O7 and 20 ml of sulfuric acid (H2SO4) overnight, and then adding 10 ml phosphoric acid, 50 ml of deionized water and 30 drops of diphenylamine. The resultant solution was tittered to the endpoint with 0.5 N of Fe(NH4)2(SO4)2 according to the method described by Nelson and Sommer (1996) and calculated using the following formula.
Inorganic N and P were quantified followed the methods described by Maynard (1997) and Murphy and Riley (1962), respectively. The exchangeable K, Ca, and Mg ions were quantified following the method of Haby et al. (1990). Exchangeable K was detected by a Flame photometer (Sherwood 420 Flame photometer, Sherwood Scientific Ltd, Cambridge, UK). Exchangeable Ca and Mg were detected by an atomic absorption spectrophotometer (Hitachi, Chiyoda City, Tokyo, Japan).
Statistical analysis
The data of the root galling rate, root galling index, cabbage emergent rate, disease severity, disease incidence and control rate were arcsine transformed, and analyzed using one-way ANOVA followed by least significant difference test in PASW Statistics version 18.0 (SPSS Inc., Chicago, IL, USA). Data of the inhibition rate of SS31 to R. solani, the mortality rate of M. incognita J2 and the egg hatching rate of M. incognita were analyzed using one-way ANOVA followed by Tukey’s b test.
Results
Using SS31 against R. solani and M. incognita
Antagonistic activities assayed on paper discs revealed that spore suspensions and culture broths of SS31 showed growth inhibitory activities against R. solani at rates ranging from 33.2% to 42.4% (Table 1). SS31 spore suspensions had greater inhibitory effects than culture broths in three different experiments. Growth inhibitory rates of SS31 culture broths decreased slightly after dilution. However, SS31 culture filtrates had no inhibitory effects on R. solani.
Table 1.
Growth inhibitory rates of Rhizoctonia solani treated with spore suspensions or culture broths of SS31
| Treatment | Inhibition rate (%) | |||
|---|---|---|---|---|
|
| ||||
| 1st exp. | 2nd exp. | 3rd exp. | Average | |
| SS31 spore suspension | 36.4 ± 0.8 aa | 43.7 ± 1.9 a | 47.3 ± 1.4 a | 42.4 ± 1.7 ab |
| SS31 culture broth 30× | 34.7 ± 1.1 ab | 36.6 ± 0.7 b | 41.3 ± 0.7 a | 37.5 ± 1.0 ab |
| SS31 culture broth 50× | 31.9 ± 0.3 b | 32.9 ± 0.3 c | 38.3 ± 3.7 a | 34.4 ± 1.5 b |
| SS31 culture broth 100× | 27.8 ± 0.4 c | 31.3 ± 0.6 c | 40.4 ± 1.5 a | 33.2 ± 1.9 b |
Means (n = 3) in the same column followed by the same letter are not significantly different (P = 0.05) according to Tukey’s b test. Values are presented as mean ± SE.
Means (n = 9) in the same column followed by the same letter are not significantly different (P = 0.05) according to Tukey’s b test. Values are presented as mean ± SE.
Mortality assays conducted in a centrifuge tube revealed that M. incognita juveniles (J2) remained viable in sterilized distilled water alone (mock control). M. incognita juveniles after being treated with spore suspensions, culture broths, or filtrates of SS31 resulted in a high mortality rate compared to mock controls in all tests (Table 2). The mortality rate of J2 after treating with SS31 culture broths ranged from 89.3% to 94.3%. SS31 culture broths resulted in greater mortality than spore suspensions. After 5-day incubation in water, ~28% of eggs hatched (Table 3). Egg masses incubated in spore suspensions, culture broths, or filtrates resulted in low hatching rates ranging from 0.3 to 4.3%. Egg masses incubated in 30×SS31 culture broths resulted in the lowest hatching rate (average ~0.32%).
Table 2.
The mortality of root knot nematode J2 treated with spore suspensions, culture broths or filtrates of SS31
| Treatment | J2 mortality (%)a,b | ||||
|---|---|---|---|---|---|
|
| |||||
| 1st exp. | 2nd exp. | 3rd exp. | 4th exp. | Average | |
| SS31 spore suspension | 68.9 ± 1.2 bc | 65.8 b ± 3.0 | 74.3 ± 2.3 ab | 92.1 ± 3.0 ab | 75.3 ± 3.2 bcd |
| SS31 culture broth | |||||
| 30× | 100 ± 0 a | 100 ± 0 a | 81.1 ± 2.7 ab | 96.3 ± 1.9 a | 94.3 ± 2.5 a |
| 50× | 98.3 ± 0.3 a | 98.4 ± 1.6 a | 86.0 ± 1.8 a | 95.2 ± 0.6 a | 94.5 ± 1.6 a |
| 100× | 94.0 ± 3.0 a | 97.8 ± 1.3 a | 75.2 ± 0.3 ab | 90.3 ± 2.6 ab | 89.3 ± 2.7 ab |
| SS31 filtrate | |||||
| 30× | 90.7 ± 0.8 a | 67.1 ± 0.6 b | 70.1 ± 2.0 b | 83.8 ± 1.3 b | 77.9 ± 3.0 bc |
| 50× | 92.6 ± 2.9 a | 66.2 ± 2.2 b | 83.4 ± 4.3 a | 84.6 ± 3.5 b | 81.7 ± 3.2 abc |
| 100× | 26.7 ± 13.1 c | 69.8 ± 13.1 b | 80.5 ± 2.9 ab | 93.9 ± 1.0 ab | 67.7 ± 8.6 c |
J2 mortality was determined 24 h after being treated with SS31 culture broth. Each replicate had approximately 100 J2.
Mortality = (number of dead juvenile/total juveniles) × 100%. The natural mortality of J2 in the water control after 24 h in four experiments was zero.
Means (n = 3) in the same column followed by the same letter are not significantly different (P = 0.05) according to Tukey’s b test. Values are presented as mean ± SE.
Means (n = 12) in the same column followed by the same letter are not significantly different (P = 0.05) according to Tukey’s b test. Values are presented as mean ± SE.
Table 3.
The egg hatching rate of root knot nematodes after being treated with culture broths or filtrates of SS31
| Treatment | Egg hatching rate (%)a,b | |||
|---|---|---|---|---|
|
| ||||
| 1st exp. | 2nd exp. | 3rd exp. | Average | |
| SS31 spore suspension | 00.7 ± 0.7 bc | 0.9 ± 0.5 b | 1.8 ± 0.4 b | 1.1 ± 0.3 bd |
| SS31 culture broth | ||||
| 30× | 0 ± 0 b | 0 ± 0 b | 1.0 ± 0.3 b | 0.3 ± 0.2 b |
| 50× | 0 ± 0 b | 0 ± 0 b | 4.4 ± 1.0 b | 1.5 ± 0.8 b |
| 100× | 1.1 ± 0.3 b | 0.8 ± 0.8 b | 6.1 ± 1.3 b | 2.7 ± 1.0 b |
| SS31 filtrate | ||||
| 30× | 0.8 ± 0.8 b | 0.4 ± 0.4 b | 6.8 ± 3.8 b | 2.7 ± 1.5 b |
| 50× | 0 ± 0 b | 1.8 ± 0.7 b | 11.1 ± 2.7 b0 | 4.3 ± 0.2 b |
| 100× | 0.8 ± 0.8 b | 4.8 ± 0.5 b | 9.6 ± 3.0 b | 3.6 ± 1.8 b |
| Water control | 38.4 ± 4.8 a0 | 22.5 ± 6.7 a 0 | 23.9 ± 1.3 a0 | 28.3 ± 3.5 a0 |
Egg hatching rate was determined 5 days after treatment.
Egg hatching rate = (the number of juvenile/(eggs + juveniles)) × 100%.
Means (n = 3) in the same column followed by the same letter are not significantly different (P = 0.05) according to least significant difference test (LSD). Values are presented as mean ± SE.
Means (n = 9) in the same column followed by the same letter are not significantly different (P = 0.05) according to LSD. Values are presented as mean ± SE.
Volatile compounds of LTM-m
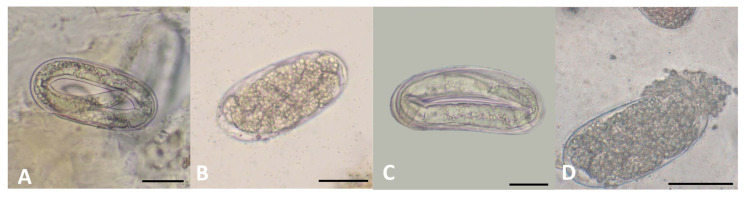
Assays using a petri dish plate inside a bigger plate revealed that growth of R. solani upon exposure to LTM-m was inhibited to varying degrees when compared to mock controls (Table 4). R. solani never physically contacted with LTM-m, indicating the involvement of toxic volatile compounds that had an inhibitory effect on the growth of R. solani. Similar tests were performed using eggs and juveniles of M. incognita as targets, revealing that volatile compounds produced from LTM-m significantly inhibited egg hatch and resulted in up to 93.2% mortality rate of juveniles (Table 5). Untreated nematode eggs showed well-differentiated juveniles inside (Fig. 1A). Many eggs after being exposed to LTM-m failed to develop into juveniles and displayed darker color. Some eggs had dense materials inside (Fig. 1B), and others had deformed juveniles (Fig. 1C). Some egg shells were apparently lysed (Fig. 1D).
Table 4.
The fumigant antagonistic activity of LTM-m against Rhizoctonia solani
| Treatment | Inhibition rate (%) | |||
|---|---|---|---|---|
|
| ||||
| 1st exp. | 2nd exp. | 3rd exp. | Average | |
| LTM-m 2 g | 52 ± 3.5 aa | 20.7 ± 1.8 a | 29 ± 2.6 a | 33.9 ± 4.9 ab |
| LTM-m 5 g | 67 ± 3.5 a | 29.7 ± 3.8 a | 14.7 ± 14.7 a | 37.1 ± 9.0 a |
| LTM-m 10 g | 50 ± 14.0 a | 6.0 ± 4.2 b | 32 ± 16.0 a | 29.3 ± 8.9 a |
| CKc | 0 b | 0 b | 0 a | 0 b |
Means (n = 3) in the same column followed by the same letter are not significantly different (P = 0.05) according to least significant difference test (LSD). Values are presented as mean ± SE.
Means (n = 9) in the same column followed by the same letter are not significantly different (P = 0.05) according to LSD. Values are presented as mean ± SE.
CK: 10 g sterilized sandy soil with 70% (v/v) distilled water.
Table 5.
The egg hatching rate and the J2 mortality of root knot nematode 7 days after LTM-m fumigation treatment
| Treatment | Egg hatching rate (%) | |||
|---|---|---|---|---|
|
| ||||
| 1st exp. | 2nd exp. | 3rd exp. | Average | |
| Egg hatching rate (%) | ||||
| LTM-m 2 g | 8.6 ± 4.4 ba | 11.4 ± 3.8 b | 5.2 ± 1.2 b | 8.4 ± 1.9 bb |
| LTM-m 5 g | 5.2 ± 1.4 b | 10.3 ± 1.4 b | 1.8 ± 0.2 b | 5.8 ± 1.4 b |
| LTM-m 10 g | 2.3 ± 0.3 b | 7.2 ± 1.3 b | 0.9 ± 0.4 b | 3.4 ± 1.0 b |
| CKe | 47.6 ± 12.7 a | 78.2 ± 17.0 a | 74.7 ± 6.8 a | 66.8 ± 8.0 a |
| J2 mortality (%) | ||||
| LTM-m 2 g | 92.8 ± 2.3 ac | 70.7 ± 13.8 a | 64.7 ± 7.0 a | 76.1 ± 6.2 ad |
| LTM-m 5 g | 94.5 ± 1.7 a | 91.6 ± 3.2 a | 83.1 ± 6.7 a | 89.7 ± 2.8 a |
| LTM-m 10 g | 96.2 ± 0 a | 90.9 ± 3.9 a | 92.5 ± 3.2 a | 93.2 ± 1.7 a |
| CKe | 3.8 ± 0.3 b | 3.1 ± 0.4 b | 3.4 ± 1.7 b | 3.4 ± 0.5 b |
Means (n = 3) in the same column followed by the same letter are not significantly different (P = 0.05) according to least significant difference test (LSD). Values are presented as mean ± SE.
Means (n = 9) in the same column followed by the same letter are not significantly different (P = 0.05) according to LSD. Values are presented as mean ± SE.
CK: 10 g sterilized sandy soil with 70% (v/v) distilled water.
Fig. 1.
Morphology of Meloidogyne incognita eggs after LTM-m fumigation treatments for 7 days. (A) Normal unhatched egg without any treatment. (B) Unhatched egg morphology in LTM-m treatments. (C) Deformed juvenile unhatched after 5 g and 10 g LTM-m treatment. (D) Lysed egg morphology after 10 g LTM-m treatment. Scale bars = 10 μm (A–C), 20 μm (D).
Disease severity of seedling trays and pot assays
Disease control tests assayed in seedling trays revealed that LTM-m significantly increased the emergent rate of spoon cabbage seeds and decreased R. solani-induced seedling damping-off (Table 6). Pot assays revealed that nearly 38% of water spinach roots grown in RKN-infested soil bore galls 30 days after planting (Table 7). The number of egg masses was estimated to be around 21.3 per pot. Plants grown in soil mixing with LTM-m decreased the rate of gall formation as low as 5.9%. Increasing the amount of LTM-m decreased the rate of gall formation and the number of egg masses. The number of egg masses was significantly reduced in soil mixing with LTM-m. No galls or egg masses were found in plants grown in control soil. Plants grown in soil containing LTM-m were significantly higher than those grown in soil without LTM-m. However, plant weights showed no significant differences among all treatments.
Table 6.
The emergent rate (%) and disease severity (%) of spoon cabbage grown in the Rhizoctonia solani-infested planting material with pre-plant amending of different LTM-m concentrations for 14 days
| Treatment | 1st exp. | 2nd exp. | Average | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Emergent rate | Disease severity | Emergent rate | Disease severity | Emergent rate | Disease severity | |
| CKa | 43.7 ± 5.3 bb | 66.4 ± 5.3 a | 58.6 ± 7.3 b | 47.9 ± 9.8 a | 51.2 ± 5.0 ac | 57.1 ± 6.2 a |
| 0.08% | 78.1 ± 3.4 a | 38.5 ± 2.4 b | 84.4 ± 3.8 a | 24.4 ± 3.9 a | 58.3 ± 7.7 a | 31.5 ± 3.4 b |
| 0.16% | 67.2 ± 3.7 a | 47.1 ± 2.2 b | 61.7 ± 6.4 b | 42.8 ± 5.4 a | 57.1 ± 4.3 a | 44.9 ± 2.8 a |
| 0.24% | 62.5 ± 6.9 a | 50.4 ± 5.4 b | 46.9 ± 10.8 b | 54.9 ± 11.2 a | 56.5 ± 4.6 a | 52.6 ± 5.8 a |
CK: R. solani-infested soil with 40–50% (v/v) distilled water.
Data was presented as means ± SE (n = 4), means in the same column followed by the same letter are not significantly different (P = 0.05) according to least significant difference test (LSD).
Data was presented as means± SE (n = 8), means in the same column followed by the same letter are not significantly different (P = 0.05) according to LSD.
Table 7.
The root galling rate (%), egg mass numbers and the plant height and weight of water spinach planted in RKN-infested soil treated with different concentrations of LTM-m for 14 days before planting
| Treatment | Disease control | Plant growth | ||
|---|---|---|---|---|
|
|
|
|||
| Galling rate | Egg mass number | Plant height per pot | Plant weight per pot (g) | |
| Blank (water only) | - | - | 37.3 ± 2.7 aa | 4.9 ± 0.6 a |
| CK (nematode only) | 38.3 ± 6.1 ab | 21.3 ± 4.3 a | 27.8 ± 1.8 b | 5.2 ± 0.6 a |
| 0.08% | 8.8 ± 1.8 b | 5.1 ± 1.4 b | 32.6 ± 2.0 a | 6.2 ± 0.8 a |
| 0.16% | 8.4 ± 1.6 b | 4.9 ± 0.8 b | 31.3 ± 2.2 a | 5.0 ± 0.7 a |
| 0.24% | 5.9 ± 1.3 b | 3.6 ± 0.8 b | 33.1 ± 1.9 a | 6.1 ± 0.8 a |
RKN, root knot nematode.
Data was presented as means ± SE (n = 21), means in the same column followed by the same letter are not significantly different (P = 0.05) according to least significant difference test.
Disease severity and plant yield after LTM-m application in greenhouses
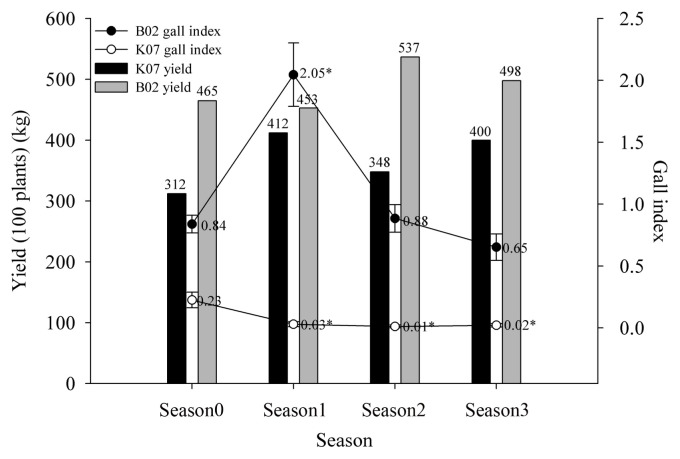
Field sampling of water spinach over four successive seasons in two greenhouses (B02 and K07) revealed that application of LTM-m significantly reduced the formation of root knots, particularly in greenhouse B02 (Fig. 2). In greenhouse B02, galling index increased from 0.84 to 2.05 after the first growing season. LTM-m was added into soil at the beginning of the second growing season, and galling index decreased considerably (decreasing from 2.05 to 0.88) as assayed at the end of the second growing season. Application of LTM-m at the beginning of the third season resulted in further reduction in galling index. Although galling index was much lower in greenhouse K07, it appeared that application of LTM-m also significantly reduced galling index as assayed from samples collected from successive growing seasons. Application of LTM-m increased biomass yields of water spinach shoots in three growing seasons compared to those of the first season.
Fig. 2.
The root knot galling index and the yield (100 plants) of water spinach with three successive LTM-m pre-plant treatments. The galling index was classified into 11 grades, where 0 = no galls, and every 10% was classified as one grade till 10 = 91–100% roots galled.
Soil physical and chemical properties after successive LTM-m applications
The type of soil texture in both greenhouses is silt loam. Soil pH values increased, and EC values decreased after LTM-m treatment (Table 8). SOM increased after LTM-m treatment in greenhouse B02, whereas no significant changes of organic matter were observed in greenhouse K07. The content of soil inorganic nitrogen fluctuated between growing seasons, showing no significant difference between growing seasons with and without LTM-m treatment. The content of soil inorganic phosphorus increased in greenhouse B02 after LTM-m treatment. In greenhouse K07, the content of inorganic phosphorus had no significant difference in soil with or without LTM-m treatment after three growing seasons. The value of exchangeable cations K, Mg and Ca decreased in both greenhouses (Table 8).
Table 8.
The soil properties in two greenhouse trails with three successive LTM-m pre-plant treatments
| GHa | Date (2020) | pH (1:5) | EC (1:5) (dS/m) | OM (%) | Content (mg/kg soil) | Soil texture | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Inorganic-N | Bray-1 P | Ex-K | Ex-Ca | Ex-Mg | Sand (%) | Silt (%) | Clay (%) | Texture | |||||
| B02 | 01/17 | 7.1 ab | 0.2 a | 3.4 b | 11.3 a | 208.0 c | 187.3 a | 1,850.4 a | 470.2 a | 39 | 60.1 | 0.9 | Silt loam |
| 03/21 | 7.2 a | 0.1 b | 3.6 b | 1.1 b | 218.2 b | 183.4 ab | 1,289.8 b | 271.9 b | |||||
| 05/08 | 7.3 a | 0.04 c | 4.2 a | 5.6 ab | 282.0 a | 176.0 b | 895.6 b | 221.8 b | |||||
| K07 | 01/17 | 7.0 b | 0.3 b | 2.3 a | 9.0 b | 64.0 a | 169.4 a | 1905.0 a | 402.5 a | 36 | 63 | 1 | Silt loam |
| 03/21 | 7.0 b | 0.5 a | 2.4 a | 16.9 a | 62.0 a | 120.7 b | 983.8 b | 182.3 b | |||||
| 05/08 | 7.4 a | 0.2 c | 2.4 a | 5.6 b | 60.5 a | 116.1 b | 895.6 b | 157.5 b | |||||
GH, greenhouse code; pH (1:5), the soil pH value was tested by adding 1 g soil in 5 ml water; EC (1:5), the soil electrical conductivity was tested with 1 g soil in 5 ml water; OM, organic matter; Bray-1 P, inorganic phosphorus; EX-K, Ca, Mg, exchangeable cation of K, Ca, Mg.
Means (n = 3) in the same column followed by the same letter are not significantly different (P = 0.05) according to Tukey’s b test.
Discussion
In this present study, we have demonstrated that the SS31 isolate of S. saraceticus applied as soil amendments could reduce soil-borne pathogens and increase plant yields. This study points to the notion that SS31 with great potential on biological control could be suitable for organic farming system. As assayed on plates, the results clearly showed that SS31 spore suspensions resulted in the best inhibitory efficacy against R. solani. Surprisingly, SS31 broth filtrates, from which S. saraceticus biomases were removed, had no inhibitory activity, indicating the vital requirements of SS31 spores for a successful disease management. The results also suggested that secondary metabolites produced by SS31 for 5 days in the culture broth might not be sufficient to suppress the growth of R. solani. It is also possible that antifungal substances are not secreted into medium. Dual culture assays revealed that R. solani hyphae cocultured with SS31 spore suspensions or culture broth (containing SS31 spores) showed necrosis, indicating a fungicidal activity of SS31 spore suspensions that could be diffused into the PSA plates. SS31 bioactivities against RKN revealed further that culture broths or filtrates of SS31 effectively killed RKN J2 and suppressed egg hatching. A large amount of unhatched eggs was observed to contain condensed protoplasm. Similar results have been reported in Heterodera glycines after being treated with geldanamycin (at 10 μg/ml) produced by Streptomyces hygroscopicus (Skantar et al., 2005). This supports further the notion that SS31 after culturing in soybean powder and sugar-containing broth might be able to produce toxic compounds to inhibit M. incognita.
In addition to culture broths and spore suspensions, the results of two-plate assays also indicated that LTM-m emitted volatile compounds that are toxic to R. solani and M. incognita. The fumigants released by LTM-m effectively inhibited hyphal growth of R. solani and RKN egg hatching, and even caused the death of RKN juveniles. Statistics showed that LTM-m applied at 5 g per plate had the best inhibitory effect on the growth of R. solani. However, some discrepancies, which could likely due to an incomplete mixing of LTM-m ingredients, were observed among different treatments and experiments. Nevertheless, all LTM-m treatments had inhibitory effects compared to the control treatment. For assays using RKN as a target, 10 g of LTM-m resulted in the highest J2 mortality and inhibition of egg hatching. Many eggs treated by LTM-m volatile compounds failed to hatch, became lysed or had deformed juveniles, suggesting that the toxic substances could dissolve in soft water agar, in which RKN juveniles and eggs were embedded. The results strongly suggested that amending LTM-m before planting in the fields for appropriate length could suppress the population of R. solani and RKN.
LTM-m contains organic ingredients, which are easily accessible and eco-friendly. A combination of those natural materials with S. saraceticus SS31 apparently can mitigate soil-borne diseases. Crab and shrimp shell powder, which contains abundant of chitin, has been proven to enhance the population of Streptomyces spp. in soil (Mitchell and Alexander, 1962; Sneh et al., 1971; Williams and Robinson, 1981). Soybean meal can release NH3 during decomposition, and NH3 can also inhibit soil-borne pathogens and nematodes (van Bniggen and Termorskuizen, 2003). Dolomite is well-known for soil acidity improvement, and its high magnesium content and high soil pH value have been known for the better soil suppressiveness (Wang and Hsieh, 1986; Young et al., 1991). High soil pH could hydrolyze more urea and keep more nitrogen in soil, both of which can lead to a better suppression to diseases and pathogens (Zasada and Tenuta, 2008). In this study, a combination of organic soil amendment and Streptomyces saraceticus SS31 has been demonstrated to suppress two economically important pathogens. Seedling tray tests showed that 0.08% LTM-m pre-planting treatment could achieve the best results on the promotion of seed germination and the suppression of disease severity caused by R. solani. Pot experiments showed that all LTM-m, particularly at 0.24%, pre-planting treatments successfully decreased the galling rate and egg mass number of M. incognita on the roots of water spinach. In M. incognita-infested soil, application of LTM-m at 0.08% and 0.24% slightly but not significantly increased plant weight compared to the control treatment. It appears that application of 0.08 % LTM-m is sufficient to decrease the damages caused by RKN and increase shoot weight.
Soil suppressiveness to plant pathogens are closely influenced by soil microorganisms, soil fertility, and soil properties. Soil amendments have been shown to improve soil aeration, soil structure, drainage, moisture holding capacity, nutrient availability, and microbial ecology (Bailey and Lazarovits, 2003). Due to those improvements, soil amendments have been demonstrated to alleviate a number of plant diseases (Abawi and Widmer, 2000; Akhtar and Malik, 2000; Bonanomi et al., 2007; Conn and Lazarovits, 2000; Cook, 1986; Gamliel et al., 2000; Lazarovits et al., 2001). In greenhouse studies, our results have shown that three successive pre-planting applications of LTM-m significantly reduced root galling index and increased plant fresh weight, indicating the practical application of LTM-m under the field conditions.
Application of LTM-m increased the pH value in both greenhouses, likely due to the presence of dolomite in LTM-m. Soil pH could greatly influence the availability of nutrients (McCauley et al., 2009) because some nutrients exist in different forms under different pH values, and not all the forms could be taken up by plants. Studies have shown that a prolonged application of organic fertilizer will increase soil pH values to neutral or slightly alkaline (Fließbach et al., 2007). Under the alkaline conditions, NH4 and OH− could generate ammonia, which may cause low soil fertility and prevent plant growth (Zhu et al., 2011). Thus, increasing soil pH after LTM-m application may benefit the growth of water spinach.
Although soil EC values slightly decreased after LTM-m treatment in two greenhouses, the changes were insignificant. It has been long known that the EC value of soil saturated solution, if higher than 4 dS/m, may cause salt accumulation, which could inhibit plant growth (Shahid and Rahman, 2011). SOM, which is relevant to soil fertility and yields is also an important factor for soil quality (Reeves, 1997). After applying LTM-m, SOM in both greenhouses increased, which might have an effect on the increase of plant fresh weight.
The amount of inorganic nitrogen decreased after LTM-m treatment. The amount of inorganic nitrogen can be affected by many factors, including mineralization, nitrogen fixation, nitrification, leaching and plant assimilation, etc. (Nguyen et al., 2017). Organic amendments provide not only nutrients to plants, but also enrich the population of microorganisms in the soil, and increasing soil microorganisms might lower soil inorganic nitrogen due to better mineralization. Because the texture of soil in our test sites is silt loam, it might be prone to a leaching effect (de Vos et al., 2000) and lower inorganic nitrogen in the soil. Soil pH value and properties could affect the available phosphorus concentration (Larsen, 1967), which might explain why soil inorganic phosphorus increased in greenhouse B02 and no significant changes in greenhouse K07. It is noticeable that the amount of soil Ca, Mg or K, which is antagonistic to each other decreased after LTM-m application in both greenhouses. The Ca/Mg ratio in greenhouse B02 is between 3 and 4, which is an ideal ratio for plants as proposed by (Kopittke and Menzies, 2007). In contrast, the Ca/Mg ratio in greenhouse K07 is around 5.75, which might decrease the availability of Mg to plants. High Ca concentration might lead to low availability of phosphorus as observed in the greenhouse K07.
In this study, LTM-m containing S. saraceticus SS31 was formulated as an organic soil amendment and tested for its efficacy for controlling Rhizoctonia blight and RKN in both laboratory and greenhouse conditions. Application of LTM-m could also reduce the outbreak of downy mildew in two greenhouse trails on Chinese kale and the hybrid of bok choy and spoon cabbage (data not shown). This warrants further investigation in the future. Preliminary tests in the lab also revealed that volatile compounds emitted from LTM-m had growth inhibitory against Sclerotinia sclerotiorum and Botrytis sp. (data not shown), suggesting a broad spectrum of volatile compounds against plant pathogens. Overall, our studies indicate that improving LTM-m efficacy by integrating it with other cultural practices shall offer more control strategies to organic farming system.
Acknowledgments
We would like to thank Dr. Kuang-Ren Chung for his editorial suggestions, and Dr. Hung-Yu Lai kindly let us using the soil property analysis apparatuses. The research is partially supported by Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, Executive Yuan, Project 109A164.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- Abawi GS, Widmer TL. Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Appl Soil Ecol. 2000;15:37–47. [Google Scholar]
- Akhtar M, Malik A. Roles of organic soil amendments and soil organisms in the biological control of plantparasitic nematodes: a review. Bioresour Technol. 2000;74:35–47. [Google Scholar]
- Al-Barakah FN, Radwan SMA, Abdel-Aziz RA. Using biotechnology in recycling agricultural waste for sustainable agriculture and environmental protection. Int J Curr Microbiol Appl Sci. 2013;2:446–459. [Google Scholar]
- Al-Fadhal FA, Al-Abedy AN, Al-Janabi MM. Molecular identification of novel isolates of Rhizoctonia solani Kühn and Fusarium spp. (Matsushima) isolated from petunia plants (Petunia hybrida L.) Plant Arch. 2018;18:703–711. [Google Scholar]
- Bailey KL, Lazarovits G. Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res. 2003;72:169–180. [Google Scholar]
- Bonanomi G, Antignani V, Pane C, Scala F. Suppression of soilborne fungal diseases with organic amendments. J Plant Pathol. 2007;89:311–324. [Google Scholar]
- Buchan GD, Grewal KS, Claydon JJ, Mcpherson RJ. A comparison of sedigraph and pipette methods for soil particle-size analysis. Australas J Soil Res. 1993;31:407–417. [Google Scholar]
- Chen Y-C. MS thesis. National Chung Hshing University; Taichung, Taiwan: 2008. Application of Streptomyces spp. for controlling disease caused by plant pathogenic fungi and plant parasitic nematodes. (in Chinese) [Google Scholar]
- Conn KL, Lazarovits G. Soil factors influencing the efficacy of liquid swine manure added to soil to kill Verticillium dahliae . Can J Plant Pathol. 2000;22:400–406. [Google Scholar]
- Cook RJ. Plant health and the sustainability of agriculture, with special reference to disease control by beneficial microorganisms. Biol Agric Hortic. 1986;3:211–232. [Google Scholar]
- Damm U, Sato T, Alizadeh A, Groenewald JZ, Crous PW. The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Stud Mycol. 2019;92:1–46. doi: 10.1016/j.simyco.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos JA, Hesterberg D, Raats PAC. Nitrate leaching in a tile-drained silt loam soil. Soil Sci Soc Am J. 2000;64:517–527. [Google Scholar]
- Fließbach A, Oberholzer H-R, Gunst L, Mäder P. Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric Ecosyst Environ. 2007;118:273–284. [Google Scholar]
- Gamliel A, Austerweil M, Kritzman G. Non-chemical approach to soilborne pest management: organic amendments. Crop Prot. 2000;19:847–853. [Google Scholar]
- Gee GW, Bauder JW. Particle size analysis. In: Klute A, editor. Methods of soil analysis. Part 1. 2nd ed. Vol. 9. The American Society of Agronomy, Soil Science Society of America; Madison, WI, USA: 1986. pp. 383–411. [Google Scholar]
- Goyal S, Chander K, Mundra MC, Kapoor KK. Influence of inorganic fertilizers and organic amendments on soil organic matter and soil microbial properties under tropical conditions. Biol Fertil Soils. 1999;29:196–200. [Google Scholar]
- Haby VA, Russelle MP, Skogley EO. Testing soils for potassium, calcium, and magnesium. In: Westerman RL, editor. Soil testing and plant analysis. 3rd ed. The Soil Science Society of America, Inc; Madison, WI, USA: 1990. pp. 181–227. [Google Scholar]
- Huang CY. MS thesis. National Chung Hshing University; Taichung, Taiwan: 2016. Studies on the potency of applying two species of Streptomyces with chemicals to control Meloidogyne incognita and Xanthomonas vesicatoria . (in Chinese) [Google Scholar]
- Huang L-F, Song L-X, Xia X-J, Mao W-H, Shi K, Zhou Y-H, Yu J-Q. Plant-soil feedbacks and soil sickness: from mechanisms to application in agriculture. J Chem Ecol. 2013;39:232–242. doi: 10.1007/s10886-013-0244-9. [DOI] [PubMed] [Google Scholar]
- Hussey RS. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis Rep. 1973;57:1025–1028. [Google Scholar]
- Kopittke PM, Menzies NW. A review of the use of the basic cation saturation ratio and the “ideal” soil. Soil Sci Soc Am J. 2007;71:259–265. [Google Scholar]
- Larsen S. Soil phosphorus. Adv Agron. 1967;19:151–210. [Google Scholar]
- Lazarovits G, Tenuta M, Conn KL. Organic amendments as a disease control strategy for soilborne diseases of high-value agricultural crops. Australas Plant Pathol. 2001;30:111–117. [Google Scholar]
- Maynard DG. Soil nutrient dynamics in a boreal mixed-wood cutover following the application of hexazinone. Ecol Appl. 1997;7:416–430. [Google Scholar]
- McCauley A, Jones C, Jacobsen J. Soil pH and organic matter. Nutr Manag Modul. 2009;8:1–12. [Google Scholar]
- Mitchell R, Alexander M. Microbiological processes associated with the use of chitin for biological control. Soil Sci Soc Am J. 1962;26:556–558. [Google Scholar]
- Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36. [Google Scholar]
- Nelson DW, Sommers LE. Total carbon, organic carbon, and organic matter. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnston CT, Sumner ME, editors. Methods of soil analysis: part 3 chemical methods, 5.3. The Soil Science Society of America, Inc; Madison, WI, USA: 1996. pp. 961–1010. [Google Scholar]
- Nguyen TTN, Xu C-Y, Tahmasbian I, Che R, Xu Z, Zhou X, Wallace HM, Bai SH. Effects of biochar on soil available inorganic nitrogen: a review and meta-analysis. Geoderma. 2017;288:79–96. [Google Scholar]
- Oka Y. Mechanisms of nematode suppression by organic soil amendments: a review. Appl Soil Ecol. 2010;44:101–115. [Google Scholar]
- Pagan C, Coyne D, Carneiro R, Kariuki G, Luambano N, Affokpon A, Williamson VM. Mitochondrial haplotype-based identification of ethanol-preserved root-knot nematodes from Africa. Phytopathology. 2015;105:350–357. doi: 10.1094/PHYTO-08-14-0225-R. [DOI] [PubMed] [Google Scholar]
- Prabavathy VR, Mathivanan N, Murugesan K. Control of blast and sheath blight diseases of rice using antifungal metabolites produced by Streptomyces sp. PM5. Biol Control. 2006;39:313–319. [Google Scholar]
- Reeves DW. The role of soil organic matter in maintaining soil quality in continuous cropping systems. Soil Tillage Res. 1997;43:131–167. [Google Scholar]
- Shahid SA, Rahman K. Soil salinity development, classification, assessment, and management in irrigated agriculture. In: Pessarakli M, editor. Handbook of plant and crop stress. 3rd ed. CRC Press; Boca Raton, FL, USA: 2011. pp. 23–39. [Google Scholar]
- Sikora RA, Roberts PA. Management practices: an overview of integrated nematode management technologies. In: Sikora RA, Coyne D, Hallmann J, Timper P, editors. Plant parasitic nematodes in subtropical and tropical agriculture. 3rd ed. CAB International; Wallingford, Oxfordshire, UK: 2018. pp. 795–838. [Google Scholar]
- Skantar AM, Agama K, Meyer SLF, Carta LK, Vinyard BT. Effects of geldanamycin on hatching and juvenile motility in Caenorhabditis elegans and Heterodera glycines . J Chem Ecol. 2005;31:2481–2491. doi: 10.1007/s10886-005-7114-z. [DOI] [PubMed] [Google Scholar]
- Sneh B, Katan J, Henis Y. Mode of inhibition of Rhizoctonia solani in chitin-amended soil. Phytopathology. 1971;61:1113–1117. [Google Scholar]
- Stanton J, Hugall A, Moritz C. Nucleotide polymorphisms and an improved PCR-based mtDNA diagnostic for parthenogenetic root-knot nematodes (Meloidogyne spp.) Fundam Appl Nematol. 1997;20:261–268. [Google Scholar]
- Sutherland ED, Papavizas GC. Evaluation of oo-spore hyperparasites for the control of phytophthora crown rot of pepper. J Phytopathol. 1991;131:33–39. [Google Scholar]
- Tang-um J, Niamsup H. Chitinase production and antifungal potential of endophytic Streptomyces strain P4. Maejo Int J Sci Technol. 2012;6:95–104. [Google Scholar]
- Tu K-I. MS thesis. National Chung Hshing University; Taichung, Taiwan: 2014. Using Streptomyces spp. for promoting plant growth and investigating their root growth regulators. (in Chinese) [Google Scholar]
- van Bniggen AHC, Termorskuizen AJ. Integrated approaches to root disease management in organic farming systems. Integrated approaches to root disease management in organic farming systems. Australas Plant Pathol. 2003;32:141–156. [Google Scholar]
- van den Ende AHGG, de Hoog GS. Variability and molecular diagnostics of the neurotropic species Cladophialophora bantiana . Stud Mycol. 1999;43:151–162. [Google Scholar]
- Wang JF, Hsieh WH. Studies on the suppressive factors and characteristics of suppressive soils of clubroot in crucifers. Plant Prot Bull. 1986;28:363–370. [Google Scholar]
- Williams ST, Robinson CS. The role of streptomycetes in decomposition of chitin in acidic soils. J Gen Microbiol. 1981;127:55–63. [Google Scholar]
- Yang S-H. MS thesis. National Chung Hshing University; Taichung, Taiwan: 1994. Control of Rhizoctonia damping-off of garden peas. (in Chinese) [Google Scholar]
- Young CC, Cheng KT, Waller GR. Phenolic compounds in conducive and suppressive soils on clubroot disease of crucifers. Soil Biol Biochem. 1991;23:1183–1189. [Google Scholar]
- Zasada IA, Tenuta M. Alteration of the soil environment to maximize Meloidogyne incognita suppression by an alkaline-stabilized biosolid amendment. Appl Soil Ecol. 2008;40:309–317. [Google Scholar]
- Zeck WM. A. rating scheme for field evaluation of rootknot nematode infestations. Pflanzenschutz Nachrichten Bayer. 1971;24:141–144. [Google Scholar]
- Zhu T, Zhang J, Cai Z. The contribution of nitrogen transformation processes to total N2O emissions from soils used for intensive vegetable cultivation. Plant Soil. 2011;343:313–327. [Google Scholar]
- Zuberer DA, Kenerley CM, Jeger MJ. Populations of bacteria and actinomycetes associated with sclerotia of Phymatotrichum omnivorum buried in Houston black clay. Plant Soil. 1988;112:69–76. [Google Scholar]