Abstract
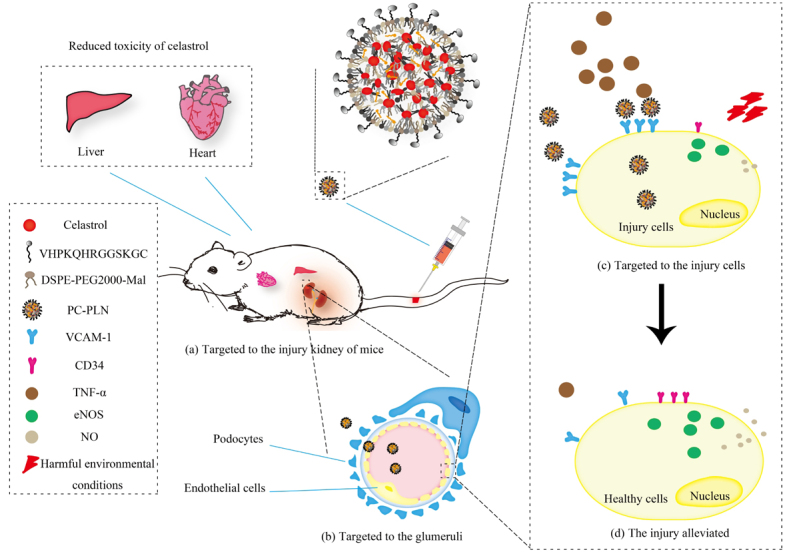
The etiology of chronic kidney disease (CKD) is complex and diverse, which could be briefly categorized to glomerular- or tubular-originated. However, the final outcomes of CKD are mainly glomerular sclerosis, endothelial dysfunction and injury, and chronic inflammation. Thus, targeted delivery of drugs to the glomeruli in order to ameliorate glomerular endothelial damage may help alleviate CKD and help enrich our knowledge. The herb tripterygium wilfordii shows therapeutic effect on kidney disease, and celastrol (CLT) is one of its active ingredients but with strong toxicity. Therefore, based on the unique structure and pathological characteristics of the glomerulus, we designed a targeted delivery system named peptides coupled CLT-phospholipid lipid nanoparticles (PC-PLNs) to efficiently deliver CLT to damaged endothelial cells and podocytes in the glomerulus for CKD treatment and research. PC-PLNs could effectively inhibit inflammation, reduce endothelial damage, alleviate CKD severity, and reduce the toxicity of CLT. We also studied the mechanism of CLT in the treatment of nephropathy and found that CLT can increase the level of NO by increasing eNOS while inhibiting the expression of VCAM-1, thus provides an anti-inflammatory effect. Therefore, our study not only offered an efficient CKD drug formulation for further development, but also provided new medical knowledge about CKD.
Electronic Supplementary Material
Supplementary material (attached with all the supporting tables and figures mentioned in this work) is available in the online version of this article at 10.1007/s12274-021-3894-x.
Keywords: celastrol, chronic kidney disease (CKD), glomerulus, endothelial cells, VCAM-1
Electronic Supplementary Material
Targeted delivery of celastrol to glomerular endothelium and podocytes for chronic kidney disease treatment
Acknowledgements
This work was supported by the Regional Innovation and Development Joint Fund (No. U20A20411) and the National Science Fund for Excellent Young Scholars (No. 82022070).
Contributor Information
Zhirong Zhang, Email: zrzzl@vip.sina.com.
Ling Zhang, Email: zhangling83@scu.edu.cn.
References
- [1].Lal M A, Young K W, Andag U. Targeting the podocyte to treat glomerular kidney disease. Drug Discov. Today. 2015;20:1228–1234. doi: 10.1016/j.drudis.2015.06.003. [DOI] [PubMed] [Google Scholar]
- [2].Liu C P, Hu Y, Lin J C, Fu H L, Lim L Y, Yuan Z X. Targeting strategies for drug delivery to the kidney: From renal glomeruli to tubules. Med. Res. Rev. 2019;39:561–578. doi: 10.1002/med.21532. [DOI] [PubMed] [Google Scholar]
- [3].Cascão R, Vidal B, Lopes I P, Paisana E, Rino J, Moita L F, Fonseca J E. Decrease of CD68 synovial macrophages in celastrol treated arthritic rats. PLoS One. 2015;10:e0142448. doi: 10.1371/journal.pone.0142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu J L, Lee J, Hernandez M A S, Mazitschek R, Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Venkatesha S H, Dudics S, Astry B, Moudgil K D. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog. Dis. 2016;74:ftw059. doi: 10.1093/femspd/ftw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xie G S, Zhu L, Song Y Y, Huang W, Hu D, Cai Z W. An integrated quantitative proteomics strategy reveals the dual mechanisms of celastrol against acute inflammation. Chin. Chem. Lett. 2021;32:2164–2168. doi: 10.1016/j.cclet.2020.11.064. [DOI] [Google Scholar]
- [7].Cascão R, Fonseca J E, Moita L F. Celastrol: A spectrum of treatment opportunities in chronic diseases. Front. Med. 2017;4:69. doi: 10.3389/fmed.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gao Y F, Zhou S, Pang L Z, Yang J C, Li H J, Huo X W, Qian S Y. Celastrol suppresses nitric oxide synthases and the angiogenesis pathway in colorectal cancer. Free Radic. Res. 2019;53:324–334. doi: 10.1080/10715762.2019.1575512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sha M, Ye J, Zhang L, Luan Z, Chen Y, Huang J. Celastrol induces apoptosis of gastric cancer cells by miR-21 inhibiting PI3K/Akt-NF-κB signaling pathway. Pharmacology. 2014;93:39–46. doi: 10.1159/000357683. [DOI] [PubMed] [Google Scholar]
- [10].Venkatesha S H, Moudgil K D. Celastrol and its role in controlling chronic diseases. Adv. Exp. Med. Biol. 2016;928:267–289. doi: 10.1007/978-3-319-41334-1_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guo L, Luo S, Du Z W, Zhou M L, Li P W, Fu Y, Sun X, Huang Y, Zhang Z R. Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nat. Commun. 2017;8:878. doi: 10.1038/s41467-017-00834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim J E, Lee M H, Nam D H, Song H K, Kang Y S, Lee J E, Kim H W, Cha J J, Hyun Y Y, Han S Y, et al. Celastrol, an NF-κB inhibitor, improves insulin resistance and attenuates renal injury in db/db mice. PLoS One. 2013;8:e62068. doi: 10.1371/journal.pone.0062068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li R, Li Y P, Zhang J H, Liu Q H, Wu T, Zhou J, Huang H, Tang Q, Huang C Y, Huang Y, et al. Targeted delivery of celastrol to renal interstitial myofibroblasts using fibronectin-binding liposomes attenuates renal fibrosis and reduces systemic toxicity. J. Control. Release. 2020;320:32–44. doi: 10.1016/j.jconrel.2020.01.017. [DOI] [PubMed] [Google Scholar]
- [14].Xu S H, Feng Y Q, He W S, Xu W, Xu W, Yang H J, Li X Y. Celastrol in metabolic diseases: Progress and application prospects. Pharmacol. Res. 2021;167:105572. doi: 10.1016/j.phrs.2021.105572. [DOI] [PubMed] [Google Scholar]
- [15].Zhao Y, Tan Y N, Meng T T, Liu X, Zhu Y, Hong Y, Yang X Q, Yuan H, Huang X, Hu F Q. Simultaneous targeting therapy for lung metastasis and breast tumor by blocking the NF-κB signaling pathway using celastrol-loaded micelles. Drug Deliv. 2018;25:341–352. doi: 10.1080/10717544.2018.1425778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhou L L, Lin Z X, Fung K P, Cheng C H K, Che C T, Zhao M, Wu S H, Zuo Z. Celastrol-induced apoptosis in human HaCaT keratinocytes involves the inhibition of NF-κB activity. Eur. J. Pharmacol. 2011;670:399–408. doi: 10.1016/j.ejphar.2011.09.014. [DOI] [PubMed] [Google Scholar]
- [17].Li L, Liao J L, Yuan Q, Hong X, Li J, Peng Y L, He M Z, Zhu H L, Zhu M S, Hou F F, et al. Fibrillin-1-enriched microenvironment drives endothelial injury and vascular rarefaction in chronic kidney disease. Sci. Adv. 2021;7:eabc7170. doi: 10.1126/sciadv.abc7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sharp C N, Doll M A, Megyesi J, Oropilla G B, Beverly L J, Siskind L J. Subclinical kidney injury induced by repeated cisplatin administration results in progressive chronic kidney disease. Am. J. Physiol. Renal Physiol. 2018;315:F161–F172. doi: 10.1152/ajprenal.00636.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jourde-Chiche N, Fakhouri F, Dou L, Bellien J, Burtey S, Frimat M, Jarrot P A, Kaplanski G, Le Quintrec M, Pernin V, et al. Endothelium structure and function in kidney health and disease. Nat. Rev. Nephrol. 2019;15:87–108. doi: 10.1038/s41581-018-0098-z. [DOI] [PubMed] [Google Scholar]
- [20].Kitching A R, Hutton H L. The players: Cells involved in glomerular disease. Clin. J. Am. Soc. Nephrol. 2016;11:1664–1674. doi: 10.2215/CJN.13791215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ma Y H, Cai F H, Li Y Y, Chen J H, Han F, Lin W Q. A review of the application of nanoparticles in the diagnosis and treatment of chronic kidney disease. Bioact. Mater. 2020;5:732–743. doi: 10.1016/j.bioactmat.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang Y F, Wu Q S, Wang J D, Li L, Sun X, Zhang Z R, Zhang L. Co-delivery of p38α MAPK and p65 siRNA by novel liposomal glomerulus-targeting nano carriers for effective immunoglobulin a nephropathy treatment. J. Control. Release. 2020;320:457–468. doi: 10.1016/j.jconrel.2020.01.024. [DOI] [PubMed] [Google Scholar]
- [23].Xu C, Chang A, Hack B K, Eadon M T, Alper S L, Cunningham P N. TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis. Kidney Int. 2014;85:72–81. doi: 10.1038/ki.2013.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang G W, Li Q Y, Chen D F, Wu B H, Wu Y L, Tong W J, Huang P T. Kidney-targeted rhein-loaded liponanoparticles for diabetic nephropathy therapy via size control and enhancement of renal cellular uptake. Theranostics. 2019;9:6191–6208. doi: 10.7150/thno.37538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Assady S, Wanner N, Skorecki K L, Huber T B. New insights into podocyte biology in glomerular health and disease. J. Am. Soc. Nephrol. 2017;28:1707–1715. doi: 10.1681/ASN.2017010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lu C C, Wang G H, Lu J, Chen P P, Zhang Y, Hu Z B, Ma K L. Role of podocyte injury in glomerulosclerosis. Adv. Exp. Med. Biol. 2019;1165:195–232. doi: 10.1007/978-981-13-8871-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Visweswaran G R R, Gholizadeh S, Ruiters M H J, Molema G, Kok R J, Kamps J A A M. Targeting rapamycin to podocytes using a vascular cell adhesion molecule-1 (VCAM-1)-harnessed SAINT-based lipid carrier system. PLoS One. 2015;10:e0138870. doi: 10.1371/journal.pone.0138870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sol M, Kamps J A A M, van den Born J, van den Heuvel M C, van der Vlag J, Krenning G, Hillebrands J L. Glomerular endothelial cells as instigators of glomerular sclerotic diseases. Front. Pharmacol. 2020;11:573557. doi: 10.3389/fphar.2020.573557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu X Y, Guo R Q, Chen P L, Wang Q, Cunningham P N. TNF induces caspase-dependent inflammation in renal endothelial cells through a Rho- and myosin light chain kinase-dependent mechanism. Am. J. Physiol. Renal Physiol. 2009;297:F316–F326. doi: 10.1152/ajprenal.00089.2009. [DOI] [PubMed] [Google Scholar]
- [30].Ailuno G, Baldassari S, Zuccari G, Schlich M, Caviglioli G. Peptide-based nanosystems for vascular cell adhesion molecule-1 targeting: A real opportunity for therapeutic and diagnostic agents in inflammation associated disorders. J. Drug Deliv. Sci. Technol. 2020;55:101461. doi: 10.1016/j.jddst.2019.101461. [DOI] [Google Scholar]
- [31].Zhong F, Mallipattu S K, Estrada C, Menon M, Salem F, Jain M K, Chen H Y, Wang Y J, Lee K, He J C. Reduced krüppel-like factor 2 aggravates glomerular endothelial cell injury and kidney disease in mice with unilateral nephrectomy. Am. J. Pathol. 2016;186:2021–2031. doi: 10.1016/j.ajpath.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu D, Huang Y, Chen B J, Zeng J, Guo N, Zhang S F, Liu L X, Xu H, Mo X M, Li W M. Activation of mammalian target of rapamycin pathway confers adverse outcome in nonsmall cell lung carcinoma. Cancer. 2011;117:3763–3773. doi: 10.1002/cncr.25959. [DOI] [PubMed] [Google Scholar]
- [33].He C B, Hu Y P, Yin L C, Tang C, Yin C H. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- [34].Levchenko T S, Rammohan R, Lukyanov A N, Whiteman K R, Torchilin V P. Liposome clearance in mice: The effect of a separate and combined presence of surface charge and polymer coating. Int. J. Pharm. 2002;240:95–102. doi: 10.1016/S0378-5173(02)00129-1. [DOI] [PubMed] [Google Scholar]
- [35].Chen D F, Han S P, Zhu Y Q, Hu F, Wei Y H, Wang G W. Kidney-targeted drug delivery via rhein-loaded polyethyleneglycol-co-polycaprolactone-co-polyethyleneimine nanoparticles for diabetic nephropathy therapy. Int. J. Nanomed. 2018;13:3507–3527. doi: 10.2147/IJN.S166445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Haque S, Pouton C W, McIntosh M P, Ascher D B, Keizer D W, Whittaker M R, Kaminskas L M. The impact of size and charge on the pulmonary pharmacokinetics and immunological response of the lungs to PLGA nanoparticles after intratracheal administration to rats. Nanomed.: Nanotechnol., Biol. Med. 2020;30:102291. doi: 10.1016/j.nano.2020.102291. [DOI] [PubMed] [Google Scholar]
- [37].Kong D H, Kim Y K, Kim M R, Jang J H, Lee S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int. J. Mol. Sci. 2018;19:1057. doi: 10.3390/ijms19041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li R R, Kowalski P S, Morselt H W M, Schepel I, Jongman R M, Aslan A, Ruiters M H J, Zijlstra J G, Molema G, van Meurs M, et al. Endothelium-targeted delivery of dexamethasone by anti-VCAM-1 SAINT-O-Somes in mouse endotoxemia. PLoS One. 2018;13:e0196976. doi: 10.1371/journal.pone.0196976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Calin M, Stan D, Schlesinger M, Simion V, Deleanu M, Constantinescu C A, Gan A M, Pirvulescu M M, Butoi E, Manduteanu I, et al. VCAM-1 directed target-sensitive liposomes carrying CCR2 antagonists bind to activated endothelium and reduce adhesion and transmigration of monocytes. Eur. J. Pharm. Biopharm. 2015;89:18–29. doi: 10.1016/j.ejpb.2014.11.016. [DOI] [PubMed] [Google Scholar]
- [40].Kelly K A, Nahrendorf M, Yu A M, Reynolds F, Weissleder R. In vivo phage display selection yields atherosclerotic plaque targeted peptides for imaging. Mol. Imaging Biol. 2006;8:201. doi: 10.1007/s11307-006-0043-6. [DOI] [PubMed] [Google Scholar]
- [41].Tousoulis D, Kampoli A M, Papageorgiou C T N, Stefanadis C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012;10:4–18. doi: 10.2174/157016112798829760. [DOI] [PubMed] [Google Scholar]
- [42].Verma S K, Molitoris B A. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin. Nephrol. 2015;35:96–107. doi: 10.1016/j.semnephrol.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Whiting C, Castillo A, Haque M Z, Majid D S A. Protective role of the endothelial isoform of nitric oxide synthase in ANG II-induced inflammatory responses in the kidney. Am. J. Physiol. Renal Physiol. 2013;305:F1031–F1041. doi: 10.1152/ajprenal.00024.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Adler S, Huang H, Loke K E, Xu X B, Tada H, Laumas A, Hintze T H. Endothelial nitric oxide synthase plays an essential role in regulation of renal oxygen consumption by NO. Am. J. Physiol. Renal Physiol. 2001;280:F838–F843. doi: 10.1152/ajprenal.2001.280.5.F838. [DOI] [PubMed] [Google Scholar]
- [45].Baylis C. Nitric oxide deficiency in chronic kidney disease. Am. J. Physiol. Renal Physiol. 2008;294:F1–F9. doi: 10.1152/ajprenal.00424.2007. [DOI] [PubMed] [Google Scholar]
- [46].Coleman J W. Nitric oxide: A regulator of mast cell activation and mast cell-mediated inflammation. Clin. Exp. Immunol. 2002;129:4–10. doi: 10.1046/j.1365-2249.2002.01918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dolinina J, Sverrisson K, Rippe A, Öberg C M, Rippe B. Nitric oxide synthase inhibition causes acute increases in glomerular permeability in vivo, dependent upon reactive oxygen species. Am. J. Physiol. Renal Physiol. 2016;311:F984–F990. doi: 10.1152/ajprenal.00152.2016. [DOI] [PubMed] [Google Scholar]
- [48].Fiore M C, Jimenez P M, Cremonezzi D, Juncos L I, García N H. Statins reverse renal inflammation and endothelial dysfunction induced by chronic high salt intake. Am. J. Physiol. Renal Physiol. 2011;301:F263–F270. doi: 10.1152/ajprenal.00109.2010. [DOI] [PubMed] [Google Scholar]
- [49].Liu B, Xu L L, Yu X M, Li W, Sun X Z, Xiao S, Guo M J, Wang H F. Protective effect of KLF15 on vascular endothelial dysfunction induced by TNF-α. Mol. Med. Rep. 2018;18:1987–1994. doi: 10.3892/mmr.2018.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Roumeliotis S, Mallamaci F, Zoccali C. Endothelial dysfunction in chronic kidney disease, from biology to clinical outcomes: A 2020 update. J. Clin. Med. 2020;9:2359. doi: 10.3390/jcm9082359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Guo Y M, Li P F, Gao L, Zhang J M, Yang Z R, Bledsoe G, Chang E, Chao L, Chao J L. Kallistatin reduces vascular senescence and aging by regulating microRNA-34a-SIRT1 pathway. Aging Cell. 2017;16:837–846. doi: 10.1111/acel.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kim J, Lee K S, Kim J H, Lee D K, Park M, Choi S, Park W, Kim S, Choi Y K, Hwang J Y, et al. Aspirin prevents TNF-α-induced endothelial cell dysfunction by regulating the NF-κB-dependent miR-155/eNOS pathway: Role of a miR-155/eNOS axis in preeclampsia. Free Radic. Biol. Med. 2017;104:185–198. doi: 10.1016/j.freeradbiomed.2017.01.010. [DOI] [PubMed] [Google Scholar]
- [53].Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, et al. TNF-α downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J. Clin. Invest. 2006;116:2791–2798. doi: 10.1172/JCI28570.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Choi S, Kim J, Kim J H, Lee D K, Park W, Park M, Kim S, Hwang J Y, Won M H, Choi Y K, et al. Carbon monoxide prevents TNF-α-induced eNOS downregulation by inhibiting NF-κB-responsive miR-155-5p biogenesis. Exp. Mol. Med. 2017;49:e403. doi: 10.1038/emm.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang J D, Patel M B, Griffiths R, Mao A, Song Y S, Karlovich N S, Sparks M A, Jin H X, Wu M, Lin E E, et al. Tumor necrosis factor-α produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension. 2014;64:1275–1281. doi: 10.1161/HYPERTENSIONAHA.114.03863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Liu X, Gao R W, Li M, Si C F, He Y P, Wang M, Yang Y, Zheng Q Y, Wang C Y. The ROS derived mitochondrial respirstion not from NADPH oxidase plays key role in celastrol against angiotensin II-mediated HepG2 cell proliferation. Apoptosis. 2016;21:1315–1326. doi: 10.1007/s10495-016-1294-6. [DOI] [PubMed] [Google Scholar]
- [57].Matavelli L C, Huang J Q, Siragy H M. Angiotensin AT2 receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57:308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pandey A, Goru S K, Kadakol A, Malek V, Gaikwad A B. Differential regulation of angiotensin converting enzyme 2 and nuclear factor-κB by angiotensin II receptor subtypes in type 2 diabetic kidney. Biochimie. 2015;118:71–81. doi: 10.1016/j.biochi.2015.08.005. [DOI] [PubMed] [Google Scholar]
- [59].Kruse N T. Nutraceuticals as a potential adjunct therapy toward improving vascular health in CKD. Am. J. Physiol. Regul., Integr. Comp. Physiol. 2019;317:R719–R732. doi: 10.1152/ajpregu.00152.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pushpakumar S, Kundu S, Sen U. Hydrogen sulfide protects hyperhomocysteinemia-induced renal damage by modulation of caveolin and eNOS interaction. Sci. Rep. 2019;9:2223. doi: 10.1038/s41598-018-38467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Targeted delivery of celastrol to glomerular endothelium and podocytes for chronic kidney disease treatment


