Abstract
Introduction
This systematic review and meta-analysis aimed to synthesize the latest evidence on the effect of dipeptidyl peptidase-4 (DPP-IV) inhibitor in patients with COVID-19.
Methods
We performed a systematic literature search from the PubMed, Scopus, Embase, and Clinicaltrials.gov up until 15 July 2021. Studies that met the following criteria were included: prospective or retrospective observational studies or case series or randomized controlled trials (RCTs) reporting DPP-IV inhibitor use in patients with COVID-19 and mortality. The intervention group was patients receiving DPP-IV inhibitor. The control group was patients that did not receive DPP-IV inhibitor. The outcome was mortality reported as odds ratio (OR).
Results
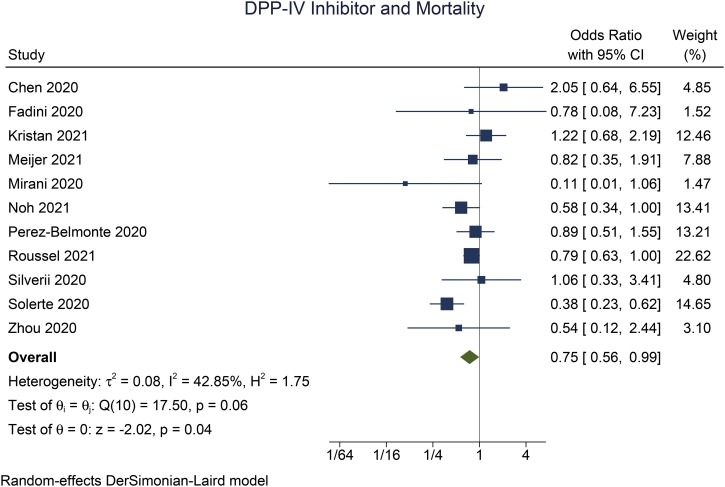
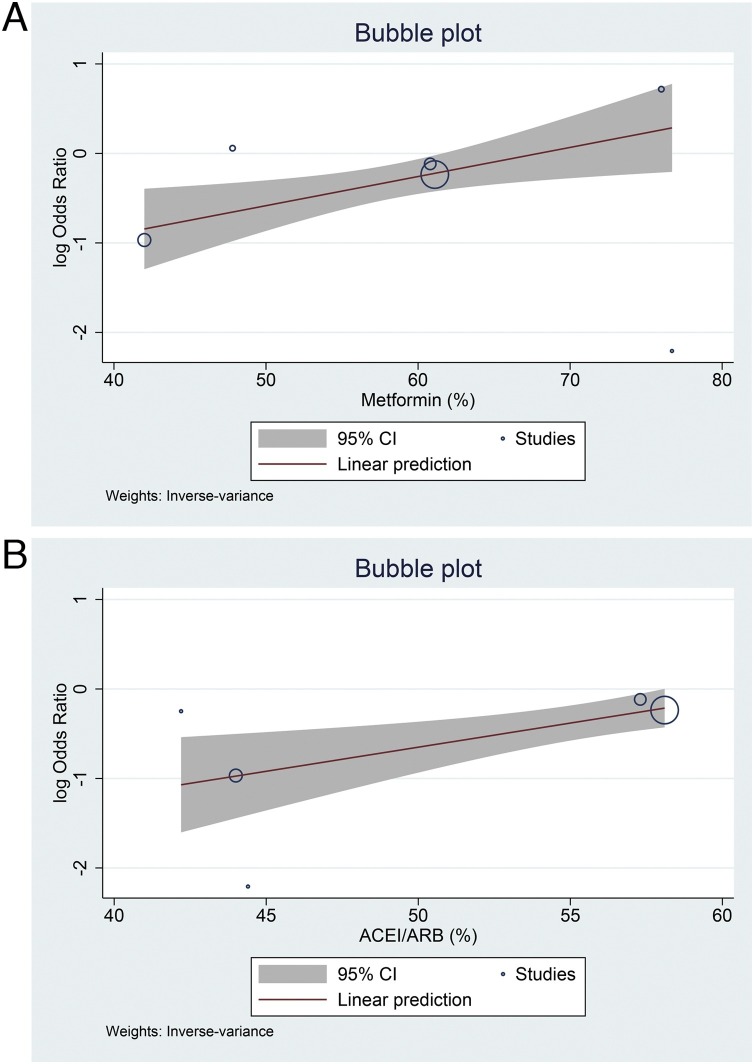
There were 11 studies consisting of 5950 patients in this meta-analysis. DPP-IV inhibitor use was associated with reduced mortality (OR 0.75 [0.56, 0.99], p = 0.043, I2: 42.9, p = 0.064) compared to those that did not receive DPP-IV inhibitor. Sensitivity analysis using the fixed-effect model (OR 0.75 [0.63, 0.88], p < 0.001, I2: 42.9, p = 0.064) also showed mortality benefit. The association between DPP-IV inhibitor and mortality was not significantly affected by age (p = 0.540), sex (p = 0.054), hypertension (p = 0.320), location (continent; p = 0.532), and retrospective/prospective nature of the study (p = 0.840). However, the association was affected by metformin (OR 1.03 [95% CI 1.01, 1.06], p = 0.010) and ACEI/ARB use (OR 1.06 [95% CI 1.02, 1.10], p = 0.004).
Conclusion
This meta-analysis showed that DPP-IV inhibitor was associated with reduced mortality in patients with COVID-19.
Keywords: Coronavirus, COVID-19, Diabetes, Dipeptidyl peptidase-4, SARS-CoV-2
1. Introduction
COVID-19 caused mortality either directly through biological mechanism or indirectly by disrupting health care system. [[1], [2], [3], [4], [5], [6]] Type 2 diabetes mellitus (T2DM) is a common comorbidity in patients with COVID-19 and also is well known as a risk factor for developing a more severe condition in COVID-19 [5,[7], [8], [9]]. Whether the glucose lowering agents that are commonly used among T2DM patients affect the outcome of the COVID-19 patients is important. The most important goal is to establish the evidence on whether these medications are harmful, neutral, or beneficial in patients with COVID-19. It is then found that antidiabetic medications may actually improve the outcome, especially by improving cardiovascular and renal function which is commonly impaired during the course of infection [[10], [11], [12]].
Previously, metformin was found to be beneficial in patients with COVID-19 [13]. One particular antidiabetic medication that has recently raised considerable interest is the relatively new dipeptidyl peptidase-4 (DPP-IV) inhibitor, which is well known for its excellent safety profile [14] and widely used as a treatment of T2DM. The use of DPP-IV inhibitor raises some concern due to its role in the regulation of T-cell activity, however, studies shown that DPP-IV inhibitor might be beneficial in patients with COVID-19. Nevertheless, although several studies showed potential benefit, the other studies showed null effect on the mortality in patients with COVID-19. This systematic review and meta-analysis aimed to synthesize the latest evidence on the effect of DPP-IV inhibitor in patients with COVID-19.
2. Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guideline.
2.1. Search strategy and study selection
We performed a systematic literature search from the PubMed, Scopus, Embase, and Clinicaltrials.gov for “(SARS-CoV-2 OR 2019-nCoV OR COVID-19) AND (dipeptidyl peptidase-4 OR DPP-4 OR DPP-IV)” from the beginning of time until 15 July 2021. Two independent authors performed title/abstract screening and eligibility assessment of the articles. Discrepancies were resolved by discussion.
2.2. Inclusion and exclusion criteria
Studies that met the following criteria were included: prospective or retrospective observational studies or case series or randomized controlled trials (RCTs) reporting DPP-IV inhibitor use in patients with COVID-19 and mortality.
Studies that met one of the following criteria were excluded: (1) review articles, (2) editorial/commentaries, (3) abstracts, (4) letters, and (5) case reports. Language restriction was not imposed.
2.3. Intervention and outcome
The intervention group was patients receiving DPP-IV inhibitor. The control group was patients that did not receive DPP-IV inhibitor. The outcome was mortality. The effect estimate was reported as odds ratio (OR).
2.4. Data extraction
Two independent authors performed data extraction of the eligible studies using standardized extraction form for the first author, study design, location of the study, inclusion criteria, sample size, age, sex, comorbidities, medication use, and the mortality in the intervention and control groups. Discrepancies during data extraction were resolved by discussion.
2.5. Risk of bias assessment
Two independent authors performed risk of bias assessment using the Newcastle-Ottawa Scale (NOS) [15]. Discrepancies during risk of bias assessment were resolved by discussion.
2.6. Statistical analysis
Random-effects meta-analysis using the Der-Simonian Laird statistical method was performed to pool the effect of DPP-IV inhibitor on mortality. Random-effects model was used regardless of heterogeneity and fixed-effects model was performed in sensitivity analysis. P-values of ≤0.05 indicate statistical significance. I2 statistics were used to inter-study heterogeneity, in which a value above 50% or p-value < 0.10 indicates substantial heterogeneity. Random-effects restricted maximum likelihood (REML) meta-regression analysis was performed to evaluate the effect of study design, location, comorbidities, and medication on the association between DPP-IV inhibitor and mortality. Statistical analysis was performed using STATA version 16.0.
3. Results
3.1. Baseline characteristics
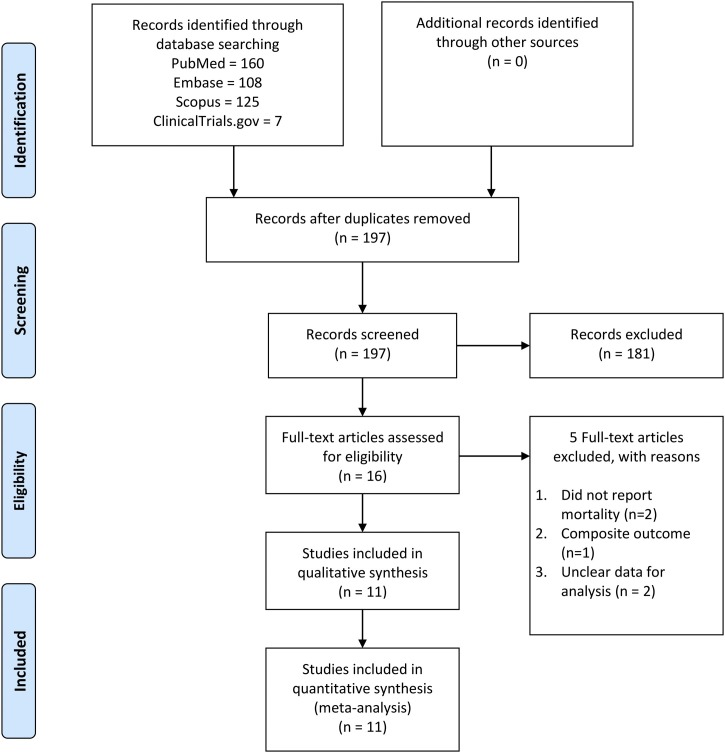
There were 11 studies consisting of 5950 patients in this meta-analysis [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]] The selection process is described in PRISMA flowchart as illustrated in Fig. 1. Characteristics of the included studies can be seen in Table 1 . There were 7 retrospective observational studies, 3 prospective observational studies, and 1 case series. 7 studies were conducted in Europe, 3 in Asia, and 1 in North America.
Fig. 1.
PRISMA flowchart.
Table 1.
Characteristics of the included studies.
| Author | Design | Location | Sample | Age (years) | Male (%) | Hypertension (%) | Statin (%) | Metformin (%) | ACEI/ARB (%) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen 2020 | RO | China | 904 | 66 vs. 65 | 46.6 | 30.20 | NA | NA | NA | 8 |
| Fadini 2020 | RO | Italy | 9 vs. 76 | 72.2 vs. 70.1 | 77.8 vs. 63.2 | 88.9 vs. 67.1 | 22.2 vs. 39.5 | NA | 22.2 vs. 25.0/44.4 vs. 15.8 | 6 |
| Kristan 2021 | RO | US | 76 vs. 832 | 62 | 51 | 78.4 | NA | NA | NA | 8 |
| Meijer 2021 | PO | Netherlands | 28 vs. 537 | 69 vs. 67 | 61 vs. 64 | 67 vs. 70 | NA | 68 vs. 77 | NA | 8 |
| Mirani 2020 | Case Series | Italy | 11 vs. 385 | 66 | 66.7 | NA | NA | NA | 44.4 | 7 |
| Noh 2021 | PO | South Korea | 453 vs 133 | NA | NA | NA | NA | NA | NA | 6 |
| Perez-Belmonte 2020 | RO | Spain | 791 vs. | 74.9 | 61.9 | 76.2 | NA | 60.8 | 27.4/29.9 | 8 |
| Roussel 2021 | PO(PSM) | France | 596 vs. 1852 | 70.3 vs. 71.1 | 67.4 vs. 62.9 | 80.1 vs. 80.2 | 52.0 vs. 47.6 | 75.2 vs.56.6 | 62.1 vs. 56.8 | 9 |
| Silverii 2020 | RO | Italy | 13 vs. 146 | 73.31 | 54.1 | NA | NA | 47.8 | NA | 6 |
| Solerte 2020 | RO | Italy | 169 vs 169 | 69 vs. 69 | 73 vs 68 | 74 vs. 67 | NA | 44 vs. 39 | 38 vs. 50 | 8 |
| Zhou 2020 | RO (PSM 1:3) | China | 111 vs. 333 | 63 vs. 64 | 51.35 vs. 47.75 | NA | NA | NA | NA | 8 |
3.2. DPP-IV inhibitor and mortality
DPP-IV inhibitor use was associated with reduced mortality (OR 0.75 [0.56, 0.99], p = 0.043, I2: 42.9, p = 0.064) (Fig. 2) compared to those that did not receive DPP-IV inhibitor. Sensitivity analysis using the fixed-effect model (OR 0.75 [0.63, 0.88], p < 0.001, I2: 42.9, p = 0.064) also showed mortality benefit.
Fig. 2.
DPP-IV inhibitor and mortality.
3.3. Meta-regression
The association between DPP-IV inhibitor and mortality was not significantly affected by age (p = 0.540), sex (p = 0.054), hypertension (p = 0.320), location (continent; p = 0.532), and retrospective/prospective nature of the study (p = 0.840). However, the association was affected by metformin (OR 1.03 [95% CI 1.01, 1.06], p = 0.010) and ACEI/ARB use (OR 1.06 [95% CI 1.02, 1.10], p = 0.004) (Fig. 3 ).
Fig. 3.
Meta-regression analysis using metformin (A) and ACEI/ARB (B) as covariates.
3.4. Publication bias
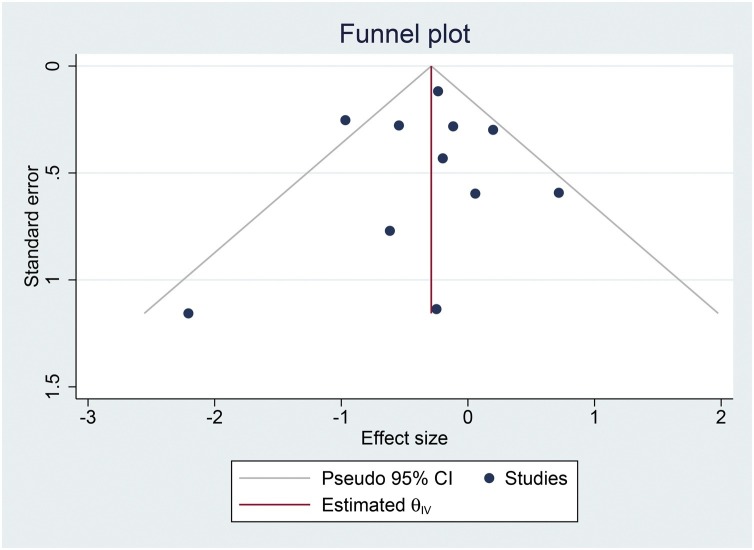
The risk of bias assessment using NOS can be seen in Table 1. Funnel-plot was symmetrical [Fig. 4 ] and there was no indication of small-study effects (p = 0.823).
Fig. 4.
Funnel-plot analysis.
4. Discussion
This meta-analysis showed that the risk of mortality was lower in COVID-19 patients receiving DPP-IV inhibitor. Meta-regression analysis indicate that the results do not vary with age, sex, hypertension, the location of the study, and retrospective/prospective nature of the studies. However, the benefit of DPP-IV inhibitor is less pronounced in patients that receive metformin and ACEI/ARB.
Although decreased mortality and respiratory complications were also found among similar studies, Patoulias et al. and Yang et al. showed no significant results possibly due to small sample size [[28], [29], [30], [31], [32], [33]]. Study that were conducted by Patoulias et al. also showed a difference between inpatient and outpatient settings in which inpatient setting is associated with significant effect of reduced mortality (RR, 0.50; 95% CI, 0.34 to 0.71; I2 = 0%; P = 0.0001) while the latter showed no significant effect (RR, 1.14; 95% CI, 0.78–1.66; I2 = 81%; P = 0.50) [28]. A baseline use of DPP-IV inhibitors is also associated with a decreased hazard of endpoints in COVID-19 infection, as demonstrated by Luk et al. [32]. However, all of these studies share a common limitation which is a relatively limited sample size and lack of data from randomized controlled trial.
The use of other glucose lowering medication is also observed in other studies. Metformin and sulfonylurea are one of the most commonly used to treat patients with T2DM. However, the use of these agents were associated with an increased hazard of composite endpoints [32]. In contrast, glucagon-like peptide-1 receptor (GLP-1R) agonists are another glucose lowering agent which also have anti-inflammatory effect and were associated with significant reduction of hospital admission, respiratory complications and incidence of mortality [33].
Metformin is often regarded as first-line antidiabetic medication and ACEI/ARB is often prescribed in patients with concurrent hypertension, cardiovascular diseases, and renal damage. Both metformin and ACEI/ARB have been shown to be associated with better prognosis in patients with COVID-19 [13,34,35], thus, the proportion of benefit from DPP-IV inhibitor may be lower when combined with other medications that may improve prognosis. Comorbidities are important to be analyzed in meta-regression because they are associated with poor outcome in patients with COVID-19 [[36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50]]. One of the study was excluded because the outcome was composite of mortality and intensive care unit admission, there was no separate data on mortality, the study showed that DPP-IV inhibitor was associated with better clinical outcome [51].
DPP-IV inhibitor is originally known as T-cell antigen CD26, is a cell receptor which is abundantly expressed in various cells such as lymphocyte, adipocyte, endothelial, lung epithelium, and also on the surface of various immune cells and regulates their function [52,53]. DPP-IV, which is closely linked to the cardiovascular biology, also plays an important role in regulating the inflammatory and immune response as it has the capability to modulate various cytokine, chemokine, peptide hormones [54,55]. Therefore, the use of DPP-IV inhibitor raises some concern over increasing susceptibility to infections, given the role of DPP-IV inhibitor in the regulation of T-cell activity [52]. While the World Health Organization’s drug adverse reaction database suggests an increased reporting of respiratory tract infection, to date there has been no evidence in large clinical trials that indicates the increased risk of infection during the use of DPP-IV inhibitor [14,56]. Moreover, due to its ability to regulate the inflammatory response through various ways, it is suggested that DPP-IV inhibitor might exert benefit for patients who are prone to cytokine storm during COVID-19 [57].
Recent studies showed high affinity between COVID-19 spike (S) protein and DPP-IV enzyme that might facilitate the viral entry into the cell, therefore contributing to the development of cytokine storm in patients with COVID-19 [58]. However, the hypothesis of DPP-IV serves as a viral entry for COVID-19 is far from the final conclusion as from a study conducted by Letko et al. suggesting beta coronavirus might enter the cells through other unknown receptor in addition to the well-known angiotensin converting enzyme-2 (ACE2) receptor [59]. The finding of lower soluble DPP-IV receptor in COVID-19 in compare to healthy subject might also indicates the role of DPP-IV in facilitating viral entry although the exact interactions between DPP-IV and coronavirus and how it influence the viral entry has not been established [[59], [60], [61]]. However, since poor glucose control, cytokine storm, and multiorgan dysfunction have been established as a risk factor for worse outcome [40,[62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74]], one might consider the benefit of excellent glucose control, cardiorenal effect and inflammatory modulation of DPP-IV inhibitor as an potential drug in treating T2DM patient with COVID-19 [5,75].
This study includes several observational studies with various populations and regions. There were 7 studies conducted in Europe, 3 in Asia, and 1 in North America. The limitation of this meta-analysis is that most of the studies were retrospective observational, RCTs are required to establish a more certain evidence. Many studies did not adequately report medications used by the patients, as shown in meta-regression analysis, medications may significantly affect the benefit of DPP-IV inhibitor in patients with COVID-19. There are other medications that were not adequately reported by most of the studies that may affect prognosis in patients with diabetes and COVID-19 [76,77].
5. Conclusion
This meta-analysis showed that DPP-IV inhibitor was associated with reduced mortality in patients with COVID-19 and results do not vary with age, sex, hypertension, the location of the study, and retrospective/prospective nature of the studies. However, further study with RCT added to the analysis is needed to establish a more certain evidence.
Conflict of interest
None.
Funding
None.
Ethical approval
Not Applicable.
Informed consent
Not Applicable.
Data availability
Data are available on reasonable request.
Contributorship statement
AFMZZ was involved in the conceptualization and design of the manuscript. AFMZZ and WMR participated in data curation and investigation. AFMZZ performed formal and statistical analysis. AFMZZ and WMR drafted the manuscript. AFMZZ and WMR review and edited the manuscript.
References
- 1.Lim M.A., Pranata R. Coronavirus disease 2019 (COVID-19) markedly increased mortality in patients with hip fracture — a systematic review and meta-analysis. J. Clin. Orthop. Trauma. 2021;12(1):187–193. doi: 10.1016/j.jcot.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.July J., Pranata R. Impact of the coronavirus disease pandemic on the number of strokes and mechanical thrombectomies: a systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2020;29(11) doi: 10.1016/j.jstrokecerebrovasdis.2020.105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pranata R., Lim M.A., Yonas E., Siswanto B.B., Meyer M. Out-of-hospital cardiac arrest prognosis during the COVID-19 pandemic. Intern. Emerg. Med. 2020;15(5):875–877. doi: 10.1007/s11739-020-02428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim M.A., Pranata R. Worrying situation regarding the use of dexamethasone for COVID-19. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620942131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pranata R., Henrina J., Raffaello W.M., Lawrensia S., Huang I. Diabetes and COVID-19: the past, the present, and the future. Metabolism. 2021;121 doi: 10.1016/j.metabol.2021.154814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pranata R., Vania R., Tondas A.E., Setianto B., Santoso A. A time-to-event analysis on air pollutants with the risk of cardiovascular disease and mortality: a systematic review and meta-analysis of 84 cohort studies. J. Evid. Based Med. 2020;13(2):102–115. doi: 10.1111/jebm.12380. [DOI] [PubMed] [Google Scholar]
- 7.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Targher G., Mantovani A., Wang X.-B., et al. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab. 2020;46(4):335. doi: 10.1016/J.DIABET.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia — a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirabelli M., Chiefari E., Puccio L., Foti D.P., Brunetti A. Potential benefits and harms of novel antidiabetic drugs during COVID-19 crisis. Int. J. Environ. Res. Public Health. 2020;17(10) doi: 10.3390/ijerph17103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pal R., Bhadada S.K. Should anti-diabetic medications be reconsidered amid COVID-19 pandemic? Diabetes Res. Clin. Pract. 2020;163 doi: 10.1016/J.DIABRES.2020.108146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomovic K., Lazarevic J., Kocic G., Deljanin-Ilic M., Anderluh M., Smelcerovic A. Mechanisms and pathways of anti-inflammatory activity of DPP-4 inhibitors in cardiovascular and renal protection. Med. Res. Rev. 2019;39(1):404–422. doi: 10.1002/MED.21513. [DOI] [PubMed] [Google Scholar]
- 13.Lukito A.A., Pranata R., Henrina J., Lim M.A., Lawrensia S., Suastika K. The Effect of Metformin Consumption on Mortality in Hospitalized COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(6):2177–2183. doi: 10.1016/j.dsx.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheen A. The safety of gliptins: updated data in 2018. Expert Opin. Drug Saf. 2018;17(4):387–405. doi: 10.1080/14740338.2018.1444027. [DOI] [PubMed] [Google Scholar]
- 15.Wells G., Shea B., O’Connell D., Peterson J. Ottawa Hosp Res Inst.; Ottawa: 2000. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 16.Roussel R., Darmon P., Pichelin M., et al. Use of dipeptidyl peptidase-4 inhibitors and prognosis of COVID-19 in hospitalized patients with type 2 diabetes: a propensity score analysis from the CORONADO study. Diabetes Obes. Metab. 2021;23(5):1162–1172. doi: 10.1111/dom.14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadini G.P., Morieri M.L., Longato E., et al. Exposure to dipeptidyl-peptidase-4 inhibitors and COVID-19 among people with type 2 diabetes: a case-control study. Diabetes Obes. Metab. 2020;22(10):1946–1950. doi: 10.1111/dom.14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristan M.M., Kim Y.K., Nelson T., et al. Predictors of severe COVID-19 disease in patients with diabetes: a multi-center review. Endocr. Pract. 2021;(January) doi: 10.1016/j.eprac.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meijer R.I., Hoekstra T., van den Oever N.C.G., et al. Treatment with a DPP-4 inhibitor at time of hospital admission for COVID-19 is not associated with improved clinical outcomes: data from the COVID-PREDICT cohort study in the Netherlands. J. Diabetes Metab. Disord. 2021 doi: 10.1007/s40200-021-00833-z. 0123456789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim M.K., Jeon J.H., Kim S.W., et al. The clinical characteristics and outcomes of patients with moderate-to-severe coronavirus disease 2019 infection and diabetes in Daegu, South Korea. Diabetes Metab. J. 2020;44:602–613. doi: 10.4093/dmj.2020.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pérez-Belmonte L.M., Torres-Peña J.D., López-Carmona M.D., et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study. BMC Med. 2020;18(1):1–10. doi: 10.1186/s12916-020-01832-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J.H., Wu B., Wang W.X., et al. No significant association between dipeptidyl peptidase-4 inhibitors and adverse outcomes of COVID-19. World J. Clin. Cases. 2020;8(22):5576–5588. doi: 10.12998/wjcc.v8.i22.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverii G.A., Monami M., Cernigliaro A., et al. Are diabetes and its medications risk factors for the development of COVID-19? Data from a population-based study in Sicily. Nutr. Metab. Cardiovasc. Dis. 2021;31(2):396–398. doi: 10.1016/j.numecd.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y., Yang D., Cheng B., et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43(7):1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 25.Noh Y., Oh I.S., Jeong H.E., Filion K.B., Yu O.H.Y., Shin J.Y. Association between dpp-4 inhibitors and covid-19–related outcomes among patients with type 2 diabetes. Diabetes Care. 2021;44(4):e64–e66. doi: 10.2337/dc20-1824. [DOI] [PubMed] [Google Scholar]
- 26.Mirani M., Favacchio G., Carrone F., et al. Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with covid-19: a case series from an academic hospital in lombardy, Italy. Diabetes Care. 2020;43(12):3042–3049. doi: 10.2337/dc20-1340. [DOI] [PubMed] [Google Scholar]
- 27.Solerte S.B., D’Addio F., Trevisan R., et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and covid-19: a multicenter case-control retrospective observational study. Diabetes Care. 2020;43(12):2999–3006. doi: 10.2337/dc20-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patoulias D., Doumas M. Dipeptidyl peptidase-4 inhibitors and COVID-19-related deaths among patients with type 2 diabetes mellitus: a meta-analysis of observational studies. Endocrinol. Metab. 2021;36(4):904. doi: 10.3803/ENM.2021.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y., Cai Z., Zhang J. DPP-4 inhibitors may improve the mortality of coronavirus disease 2019: a meta-analysis. PLoS One. 2021;16(5) doi: 10.1371/JOURNAL.PONE.0251916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonora B.M., Avogaro A., Fadini G.P. Disentangling conflicting evidence on DPP-4 inhibitors and outcomes of COVID-19: narrative review and meta-analysis. J. Endocrinol. Invest. 2021;44(7):1. doi: 10.1007/S40618-021-01515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rakhmat I.I., Kusmala Y.Y., Handayani D.R., et al. Dipeptidyl peptidase-4 (DPP-4) inhibitor and mortality in coronavirus disease 2019 (COVID-19) – a systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15(3):777–782. doi: 10.1016/j.dsx.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luk A.O.Y., Yip T.C.F., Zhang X., et al. Original research: glucose-lowering drugs and outcome from COVID-19 among patients with type 2 diabetes mellitus: a population-wide analysis in Hong Kong. BMJ Open. 2021;11(10) doi: 10.1136/BMJOPEN-2021-052310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyland J.E., Raja-Khan N.T., Bettermann K., et al. Diabetes, drug treatment and mortality in COVID-19: a multinational retrospective cohort study. Diabetes. 2021;(October) doi: 10.2337/DB21-0385. db210385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pranata R., Permana H., Huang I., et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(5):983–990. doi: 10.1016/j.dsx.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukito A.A., Widysanto A., Lemuel T.A.Y., et al. Candesartan as a tentative treatment for COVID-19: a prospective non-randomized open-label study. Int. J. Infect. Dis. 2021;108:159–166. doi: 10.1016/j.ijid.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. JRAAS. 2020;21(2) doi: 10.1177/1470320320926899. 147032032092689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akbar M.R., Wibowo A., Pranata R., Setiabudiawan B. Low serum 25-hydroxyvitamin d (Vitamin d) level is associated with susceptibility to COVID-19, severity, and mortality: a systematic review and meta-analysis. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.660420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pranata R., Huang I., Lawrensia S., et al. Proton pump inhibitor on susceptibility to COVID-19 and its severity: a systematic review and meta-analysis. Pharmacol. Rep. 2021;73(6):1642–1649. doi: 10.1007/s43440-021-00263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim M.A., Pranata R. The danger of sedentary lifestyle in diabetic and obese people during the COVID-19 pandemic. Clin. Med. Insights Endocrinol. Diabetes. 2020;13 doi: 10.1177/1179551420964487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can. J. Kidney Heal Dis. 2020;7 doi: 10.1177/2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pranata R., Supriyadi R., Huang I., et al. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin. Med. Insights Circ. Respir. Pulm. Med. 2020;14 doi: 10.1177/1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pranata R., Huang I., Raharjo S.B. Incidence and impact of cardiac arrhythmias in coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Indian Pacing Electrophysiol. J. 2020;2019(xxxx):1–6. doi: 10.1016/j.ipej.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pranata R., Huang I., Lim M.A., Wahjoepramono E.J., July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19–systematic review, meta-analysis, and meta-regression. J. Stroke Cerebrovasc. Dis. 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pranata R., Soeroto A.Y., Huang I., et al. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int. J. Tuberc. Lung Dis. 2020;24(8):838–843. doi: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- 45.Pranata R., Lim M.A., Yonas E., et al. Body mass index and outcome in patients with COVID-19: a dose–response meta-analysis. Diabetes Metab. 2021;47(2) doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atmosudigdo I.S., Lim M.A., Radi B., et al. Dyslipidemia increases the risk of severe COVID-19: a systematic review, meta-analysis, and meta-regression. Clin. Med. Insights Endocrinol. Diabetes. 2021;14 doi: 10.1177/1179551421990675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.July J., Pranata R. Prevalence of dementia and its impact on mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. Geriatr. Gerontol. Int. 2021;21(2):172–177. doi: 10.1111/ggi.14107. [DOI] [PubMed] [Google Scholar]
- 48.Pranata R., Henrina J., Lim M.A., et al. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis: clinical Frailty Scale in COVID-19. Arch. Gerontol. Geriatr. 2021;93 doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuty Kuswardhani R.A., Henrina J., Pranata R., Anthonius Lim M., Lawrensia S., Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(6):2103–2109. doi: 10.1016/j.dsx.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pranata R., Lim M.A., Huang I., et al. Visceral adiposity, subcutaneous adiposity, and severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. Clin. Nutr. ESPEN. 2021;43:163–168. doi: 10.1016/j.clnesp.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhee S.Y., Lee J., Nam H., Kyoung D.S., Shin D.W., Kim D.J. Effects of a DPP-4 inhibitor and RAS blockade on clinical outcomes of patients with diabetes and COVID-19. Diabetes Metab. J. 2021;45(2):251–259. doi: 10.4093/DMJ.2020.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deacon C.F. Physiology and pharmacology of DPP-4 in glucose homeostasis and the treatment of type 2 diabetes. Front. Endocrinol. (Lausanne) 2019;10:80. doi: 10.3389/fendo.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drucker D.J. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Ussher J.R., Drucker D.J. Cardiovascular biology of the incretin system. Endocr. Rev. 2012;33(2):187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheen A.J. Cardiovascular effects of gliptins. Nat. Rev. Cardiol. 2013;10(2):73–84. doi: 10.1038/nrcardio.2012.183. [DOI] [PubMed] [Google Scholar]
- 56.Willemen M.J., Mantel-Teeuwisse A.K., Straus S.M., Meyboom R.H., Egberts T.C., Leufkens H.G. Use of dipeptidyl peptidase-4 inhibitors and the reporting of infections: a disproportionality analysis in the World Health Organization VigiBase. Diabetes Care. 2011;34(2):369–374. doi: 10.2337/dc10-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheen A.J. DPP-4 inhibition and COVID-19: from initial concerns to recent expectations. Diabetes Metab. 2021;47(2) doi: 10.1016/j.diabet.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y., Zhang Z., Yang L., et al. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience. 2020;23(6) doi: 10.1016/j.isci.2020.101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlicht K., Rohmann N., Geisler C., et al. Circulating levels of soluble Dipeptidylpeptidase-4 are reduced in human subjects hospitalized for severe COVID-19 infections. Int. J. Obes. (Lond) 2020;44(11):1. doi: 10.1038/S41366-020-00689-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vankadari N., Wilce J.A. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 2020;9(1):601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yonas E., Alwi I., Pranata R., et al. Elevated interleukin levels are associated with higher severity and mortality in COVID 19 – a systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14(6):2219–2230. doi: 10.1016/j.dsx.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wibowo A., Pranata R., Akbar M.R., Purnomowati A., Martha J.W. Prognostic performance of troponin in COVID-19: a diagnostic meta-analysis and meta-regression. Int. J. Infect. Dis. 2021;105:312–318. doi: 10.1016/j.ijid.2021.02.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martha J.W., Pranata R., Wibowo A., Lim M.A. Tricuspid annular plane systolic excursion (TAPSE) measured by echocardiography and mortality in COVID-19: a systematic review and meta-analysis. Int. J. Infect. Dis. 2021;105(1):351–356. doi: 10.1016/j.ijid.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pranata R., Yonas E., Huang I., Lim M.A., Nasution S.A., Kuswardhani R.A.T. Fibrosis-4 index and mortality in coronavirus disease 2019: a meta-analysis. Eur. J. Gastroenterol. Hepatol. 2021 doi: 10.1097/meg.0000000000002091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sutandyo N., Kurniawati S.A., Jayusman A.M., Syafiyah A.H., Pranata R., Hanafi A.R. Repurposing FIB-4 index as a predictor of mortality in patients with hematological malignancies and COVID-19. PLoS One. 2021;16(September (9)) doi: 10.1371/journal.pone.0257775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yonas E., Alwi I., Pranata R., et al. Effect of heart failure on the outcome of COVID-19 — a meta analysis and systematic review. Am. J. Emerg. Med. 2020;46:204–211. doi: 10.1016/j.ajem.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wibowo A., Pranata R., Astuti A., et al. Left and right ventricular longitudinal strains are associated with poor outcome in COVID-19: a systematic review and meta-analysis. J. Intensive Care. 2021;9(1):9. doi: 10.1186/s40560-020-00519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pranata R., Lim M.A., Yonas E., et al. Thrombocytopenia as a prognostic marker in COVID-19 patients: diagnostic test accuracy meta-analysis. Epidemiol. Infect. 2021;149 doi: 10.1017/S0950268821000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wibowo A., Pranata R., Lim M.A., Akbar M.R., Martha J.W. Endotheliopathy marked by High Von Willebrand factor (vWF) antigen in COVID-19 is associated with poor outcome: a systematic review and meta-analysis. Int. J. Infect. Dis. 2021;(June) doi: 10.1016/j.ijid.2021.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pranata R., Huang I., Lim M.A., Yonas E., Vania R., Kuswardhani R.A.T. Delirium and mortality in coronavirus disease 2019 (COVID-19) — a systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2021;95:104388. doi: 10.1016/j.archger.2021.104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pranata R., Huang I., Lukito A.A., Raharjo S.B. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19: systematic review and meta-analysis. Postgrad. Med. J. 2020;96(1137):387–391. doi: 10.1136/postgradmedj-2020-137884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martha J.W., Wibowo A., Pranata R. Prognostic value of elevated lactate dehydrogenase in patients with COVID-19: a systematic review and meta-analysis. Postgrad. Med. J. 2021;15(January) doi: 10.1136/postgradmedj-2020-139542. postgradmedj-2020-139542. [DOI] [PubMed] [Google Scholar]
- 75.Handayani D.R., Juliastuti H., Nawangsih E.N., et al. Prognostic value of fasting hyperglycemia in patients with COVID-19 — diagnostic test accuracy meta-analysis. Obes. Med. 2021;23 doi: 10.1016/j.obmed.2021.100333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martha J.W., Pranata R., Lim M.A., Wibowo A., Akbar M.R. Active prescription of low-dose aspirin during or prior to hospitalization and mortality in COVID-19: a systematic review and meta-analysis of adjusted effect estimates. Int. J. Infect. Dis. 2021;108:6–12. doi: 10.1016/j.ijid.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zein A.F.M.Z., Sulistiyana C.S., Khasanah U., Wibowo A., Lim M.A., Pranata R. Statin and mortality in COVID-19: a systematic review and meta-analysis of pooled adjusted effect estimates from propensity-matched cohorts. Postgrad. Med. J. 2021 doi: 10.1136/postgradmedj-2021-140409. Online ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.