Abstract
DNA interstrand cross-links (ICLs) block the strand separation necessary for essential DNA functions such as transcription and replication and, hence, represent an important class of DNA lesion. Since both strands of the double helix are affected in cross-linked DNA, it is likely that conservative recombination using undamaged homologous regions as a donor may be required to repair ICLs in an error-free manner. However, in Escherichia coli and yeast, recombination-independent mechanisms of ICL repair have been identified in addition to recombinational repair pathways. To study the repair mechanisms of interstrand cross-links in mammalian cells, we developed an in vivo reactivation assay to examine the removal of interstrand cross-links in cultured cells. A site-specific psoralen cross-link was placed between the promoter and the coding region to inactivate the expression of green fluorescent protein or luciferase genes from reporter plasmids. By monitoring the reactivation of the reporter gene, we showed that a single defined psoralen cross-link was removed in repair-proficient cells in the absence of undamaged homologous sequences, suggesting the existence of an ICL repair pathway that is independent of homologous recombination. Mutant cell lines deficient in the nucleotide excision repair pathway were examined and found to be highly defective in the recombination-independent repair of ICLs, while mutants deficient in homologous recombination were found to be proficient. Mutation analysis of plasmids recovered from transfected cells showed frequent base substitutions at or near positions opposing a cross-linked thymidine residue. Based on these results, we suggest a distinct pathway for DNA interstrand cross-link repair involving nucleotide excision repair and a putative lesion bypass mechanism.
Alkylating agents were among the first compounds found to be efficacious in cancer therapy and remain important components of many modern chemotherapeutic regimens (38). Many members of this class of drugs have bifunctional groups that can react with both strands of the DNA helix and thus form interstrand and intrastrand cross-links. As profound blocks for both transcription and DNA replication, interstrand cross-links (ICLs) appear to represent the primary cytotoxic lesion induced by most bifunctional alkylating agents.
Repair of DNA ICLs has been studied extensively in Escherichia coli (11, 12). Both genetic and biochemical evidence has established a combined nucleotide excision repair (NER)-recombination mechanism for the error-free repair of ICLs, in which the gap, created by the Uvr(A)BC excinuclease, is repaired through recA-mediated recombination with a lesion-free homologous chromosome as the donor (10, 37, 41). Although the NER-recombination pathway appears to be the primary mechanism of cross-link repair in E. coli, recent evidence has suggested a recombination-independent pathway for cross-link repair in which the gap created by the uvr(A)BC excinuclease is repaired by translesion bypass in order to circumvent a deficiency in recombination or a lack of homologous donor sequences (4, 5). In the budding yeast Saccharomyces cerevisiae, members of the RAD52 epistasis group exhibit hypersensitivity to cross-linking agents and ionizing radiation, indicating that repair of ICLs requires homologous recombination. Members of the RAD3 epistasis group, which are deficient in NER, are also highly sensitive to cross-link damage (21, 29, 36). Taken together, these observations support a combined NER-recombination mechanism for ICL repair in lower eukaryotes. As is the case in E. coli, recombination-independent mechanisms may also play a role in cross-link removal in eukaryotes. The yeast pso1 mutant is characterized by hypersensitivity to psoralen-induced ICLs (19), and genetic analysis has demonstrated allelism between the pso1-1 mutant and the rev3-1 mutant (9). The REV3 gene encodes the catalytic subunit of yeast translesion DNA polymerase ζ whose function is required for induced mutagenesis (30, 33). A more recent study has indicated that rev3p is important for the processing of ICL repair intermediates in nonreplicating cells, further substantiating a lesion bypass-based recombination-independent mechanism of ICL repair in yeast (28). A plausible role for the rev3p-polymerase ζ in cross-link repair is the translesion synthesis past the lesion upon the uncoupling of a cross-link.
In mammals, mechanisms of ICL repair are largely unknown. The combined NER-recombination model does not appear to be the major mechanism of repair since the majority of NER mutants exhibit only mild sensitivity to cross-linking agents, although mutants defective in either ERCC1 or XPF do exhibit extreme sensitivity (2, 20). However, several lines of evidence have suggested the involvement of homologous recombination in ICL repair (39). In vivo, elevated levels of sister chromosome exchange induced by bifunctional alkylating agents have been well documented and connected to the repair of ICLs (7, 14, 25). Using a triplex-mediated psoralen cross-link as the model damage, intramolecular homologous recombination has been reported to occur through both nonconservative single-strand annealing and conservative reciprocal exchange pathways (15, 16). Recently, more convincing evidence of recombinational repair of ICLs has emerged through the characterization of two hamster mutants, irs1 and irs1SF, both of which exhibit extreme sensitivity to cross-linking agents (17, 24). Both the XRCC2 and the XRCC3 genes, which complement the repair deficiency of irs1 and irs1SF mutants, respectively, exhibit structural similarity to the hRad51 recombinase family (27). Studies by Johnson et al. (23) and Pierce et al. (34) have indicated that both cell lines harbor a defect in homologous recombination marked by a severe reduction in gene conversion activity. Moreover, RAD54-deficient mouse embryonic stem cells are also hypersensitive to mitomycin C as an apparent result of reduced conservative homologous recombination (14). In addition, Li et al. (26) have shown, in vitro, that the presence of an ICL in a plasmid substrate stimulates repair synthesis in mammalian cell extracts and that this stimulated synthesis is also observed in an undamaged plasmid coincubated in the same extract, suggesting that ICLs can induce recombinational repair synthesis. Taken together, these results provide substantial evidence that recombination factors participate in ICL repair and are likely to be involved in a major pathway of ICL removal.
To investigate the mechanisms by which ICLs are repaired in mammalian cells in the absence of homologous donor sequences, we employed a gene reactivation assay in which a single defined psoralen ICL was introduced into a reporter plasmid in order to block transcription of the reporter gene. Consequently, expression of the reporter gene became dependent upon removal of the ICL. We report here that repair of the ICL present in the plasmid substrate was observed in wild-type cells and that mutants defective in NER were deficient in reactivation, while mutants defective in homologous recombination were not. Rescue and sequencing of repaired plasmids indicated a high rate of mutagenesis at the site of the psoralen cross-link. These results indicate that a recombination-independent, but error-prone, pathway of ICL repair exists in mammalian cells.
MATERIALS AND METHODS
Cell lines and tissue culture conditions.
Cell lines used in this study were obtained from either the American Type Culture Collection (Rockville, Md.) or the Human Genetic Mutant Cell Repository (Camden, N.J.), unless stated otherwise. The CHO AA8 cell line and its derived mutants UV20 (ERCC1), UV24 (XPB), UV41 (XPF), and UV135 (XPG) were grown in Dulbecco modified Eagle medium medium supplemented with 10% fetal calf serum. The E1KO7-5 and E1KO-47 CHO cell lines were maintained under similar conditions. The human cell lines HT-1080 (fibrosarcoma), RKO (colon cancer epithelial), and the Chinese hamster lung fibroblast cell line V79 were cultured in minimal essential medium (MEM) supplemented with 10% serum. Xeroderma pigmentosum (XP) fibroblast cell lines XP2OS (XPA), XP4PA (XPC), XP6BE (XPD), XP30RO (XPV), and XP3BR (XPG) were maintained in MEM supplemented with 15% serum. The XP30RO cell line was a kind gift from James Cleaver (University of California at San Francisco). The irs1 (XRCC2), and irs1SF (XRCC3) mutants were generously provided by Larry Thompson (Lawrence Livermore National Laboratory, Livermore, Calif.) and Nigel Jones (The University of Liverpool, Liverpool, United Kingdom) and maintained in MEM medium supplemented with 10% serum.
Preparation of cross-linked GFP and luciferase substrates.
The cross-linked reporter substrates were prepared by ligation of a psoralen cross-linked 22-mer at a defined position in the plasmid (26). For the green fluorescent protein (GFP) substrate, two complementary oligonucleotides with the following sequences were synthesized and kinased: 5′-GCTCTCGTCTGTACACCGAAG-3′ and 5′-GCTCTTCGGTGTACAGACGAG-3′. Boldface letters indicate nucleotide residues involved in the cross-link, and a BsrGI site is underlined. Annealing of these oligonucleotides created identical 3-nucleotide 5′ cohesive ends at both ends of the duplex. Then, 100 μg of annealed duplex oligonucleotide (oligo) was mixed with 4,5,8-trimethylpsoralen (Trioxalen) at 5 μg/ml in Tris-EDTA (pH 7.5) buffer containing 25 mM NaCl. The mixture was irradiated with 365-nm UV light (10 min at 10 mW/cm2) to effect formation of the interstrand cross-link between the internal thymidines of the BsrGI site. The cross-linked oligonucleotide was then purified from non-cross-linked DNA by denaturing polyacrylamide gel electrophoresis (PAGE). To insert the cross-linked oligonucleotide, pEGFP-N1 (Clontech) was digested by HindIII, and a single dAMP residue was added to both 3′ ends by incubation with Klenow enzyme and dATP. This latter step prevents self-ligation of the plasmid by creating ends that are only ligatable to the cross-linked oligo. After ligation, covalently closed cross-linked plasmid was purified by CsCl-ethidium bromide gradient centrifugation. For construction of the control plasmid, a non-cross-linked oligo was cloned into the HindIII site of the pEGFP-N1 plasmid as described for the cross-linked oligo. To prepare the cross-linked luciferase substrate, two complementary oligonucleotide sequences (5′-TAGCTCGTCTGTACACCGAAG-3′ and 5′-TAGCTTCGGTGTACAGACGAG-3′) were synthesized and cross-linked as described above. pCMV-luc (a kind gift from M. Hedayati and L. Grossman, Department of Biochemistry, School of Public Health, Johns Hopkins University) was digested by NheI, filled-in with a single dCMP residue, and ligated to the cross-linked oligo. Purification was performed as described above. The control luciferase substrate was prepared by insertion of an uncross-linked oligo in the NheI site. The purity of the cross-linked plasmids was determined by release of the cross-link-containing fragment by restriction enzyme digestion and examination of the products by denaturing PAGE (see Fig. 1).
FIG. 1.
Recombination-independent removal of a single defined psoralen ICL in repair-proficient HT-1080 fibroblast cells. (A) Preparation of cross-linked reporter substrates as examined by denaturing PAGE. The left panel shows a purified cross-linked oligo (lane 2) and a non-cross-linked control (lane 3). Lane 1 contains a 40-mer oligo marker. The right panel shows the purity of the cross-linked plasmid substrate. Lane 1, pEGFP-N1 plasmid with an unmodified oligo inserted at the HindIII site; lane 2, pEGFP-N1 with a cross-linked oligo inserted at the HindIII site. Both were digested with BamHI and NheI to release a 54-bp fragment followed by end labeling with T4 kinase and resolution by 15% denaturing PAGE. (B) Parallel plated HT-1080 cells were transfected with equal amounts of unmodified pEGFP-N1 plasmid (HT-1080/EGFP) or cross-linked pEGFP-N1 plasmid (HT-1080/CLT). Images in the left panels show representative fluorescent views, and images in the right panels show the bright-field view. (C) Repair of a single psoralen ICL in a luciferase reporter plasmid in the repair-proficient cell lines HT-1080, RKO, AA8, and V79. The relative efficiencies of ICL repair were calculated as the percentage of luciferase activity from cross-linked reporter to that of the unmodified reporter.
Transfections and reporter reactivation assay.
Transient transfections were performed using FuGENE-6 transfection reagent (Roche Molecular Biochemicals) according to the recommendations of the manufacturer. Carrier DNA was used to equalize the total amount of plasmid DNA when necessary. With the GFP as the reporter, 50 ng of either control or cross-linked plasmid DNA was used to transfect 1.5 × 105 cells seeded in 35-mm plates. For the visualization of the cellular green fluorescence, cells were washed twice with phosphate-buffered saline, fixed in 4% paraformaldehyde at 30 h post transfection, and examined by fluorescence microscopy. For the luciferase reactivation assay, 0.1 to 5.0 ng of cross-linked or unmodified control substrate was used for transfections. Cells were harvested 30 h after transfection, and the luciferase activity was determined by using the Luciferase Assay System (Promega) and measured on a Moonlight 3010 luminometer (Pharmingen). The linear range for the luciferase assay, both in terms of the amount of transfected DNA and the amount of protein extract, was established individually for each cell line. Each datum point was the result of three or more independent transfections.
Mutation analysis.
A total of 150 ng of cross-linked pEGFP-N1 substrate was transfected into NER-proficient RKO or HT-1080 cells (5 × 105) in a 60-mm dish. At 30 h after transfection, plasmid DNAs were recovered by either Hirt extraction or a modified alkaline lysis procedure (15) and then electroporated into the E. coli repair-recombination-deficient strain AB2480 (uvrA recA mutant) (18). A 255-bp fragment containing the cross-linked region was generated by PCR amplification directly from the resulting colonies. The PCR products were digested with BsrGI; clones resistant to BsrGI digestion were amplified in kanamycin, and isolated plasmid DNAs were sequenced to identify mutations.
RESULTS
Repair of psoralen ICLs in repair-proficient cells.
To examine specifically the repair of ICLs, we have developed a procedure to prepare plasmid substrates that contain a single defined ICL (26). Using this method, we placed a single psoralen ICL in the pEGFP-N1 vector between the cytomegalovirus promoter and the GFP coding sequence (see Materials and Methods for the position of the ICL insertion). As shown (Fig. 1A), the purified cross-linked duplex oligo and the resulting plasmid are highly pure and were not found to contain detectable amounts of non-cross-linked material by this examination. As a result of the presence of the cross-link, the expression of the GFP becomes dependent upon the removal of this lesion. Upon transfection of the reporter substrate, pEGFP/CLT, into human repair-proficient HT-1080 cells, a significant number of cells were found to exhibit the GFP signal (Fig. 1B). To determine the efficiency of ICL repair, we inserted the unmodified duplex oligo at the same position in the pEGFP-N1 plasmid. The unmodified reporter plasmid and the cross-linked pEGFP/CLT plasmid were identical except that a single defined ICL was present in the latter. When the same amount of both plasmids was used in parallel to transfect HT-1080 cells, the number of enhanced GFP (EGFP)-positive cells from the cross-linked reporter was estimated to be approximately 30% of that with respect to the unmodified reporter (Fig. 1B). Reactivation of the GFP expression cassette reflected the apparent removal and/or uncoupling of the DNA cross-link and suggested that the cross-links were removed in the absence of a homologous donor sequence, since no significant homology to the pEGFP-N1 plasmid would be expected in a mammalian genome to support homologous recombination. Moreover, sequence alignment of the pEGFP-N1 plasmid indicated that there are no direct repeats flanking the cross-linked region to support an intramolecular recombination process (i.e., single-strand annealing) that could lead to the reactivation of the GFP signal.
To achieve accurate quantification of the repair of ICLs, we prepared a similarly cross-linked substrate using firefly luciferase as the reporter gene. The cross-linked oligo was placed between the promoter and the luciferase coding region to inactivate the transcription of the reporter gene. Quantification of the ICL repair efficiency was achieved by normalizing the luciferase activity from cells transfected with cross-linked plasmid against that of cells transfected with an unmodified plasmid. We examined two human (HT-1080 and RKO) and two hamster (AA8 and V79) NER-proficient cell lines. The reactivation efficiencies of ICLs in these cell lines ranged between 40 and 60% of that observed with unmodified plasmid (Fig. 1C). These results indicate that there is a mechanism for the repair of ICLs, present in mammalian cells, that operates by a process that does not require the presence of homologous donor sequences.
ERCC1 and XPA mutants are defective in the recombination-independent repair of ICLs.
We next used the cross-linked EGFP substrate to examine the repair-deficient UV20 hamster mutant (defective in ERCC1), and a human XP group A mutant, XP2OS. We found that the number of cells expressing EGFP was drastically reduced in the ERCC1 mutant, suggesting a requirement for the ERCC1-XPF heterodimeric complex. Reactivation of the EGFP reactivation was also highly deficient in the XPA mutant, indicating that the XPA gene product was also essential for the recombination-independent removal of ICLs (data not shown).
When the cross-linked luciferase reporter substrate was used to assay both the UV20 and XP2OS mutants, we confirmed that the repair of ICLs in both cell lines was highly deficient compared with repair-proficient and parental cells (Fig. 2). To further confirm the role of ERCC1 in the reactivation assay, a CHO mutant, E1KO7-5, an ERCC1-null cell line developed by a targeted knockout of ERCC1 in the repair-proficient CHO ATS-49 cell line (1), was examined and showed defective ICL repair similar to that of the UV20 mutant. To ascertain whether the defective ICL repair of the E1KO7-5 mutant was caused by its ERCC1 disruption, we examined luciferase reactivation activity in its isogenic derivative, E1KO-47 (C-ERCC1). E1KO-47 is a stable transformant of E1KO7-5 obtained upon integration of a wild-type ERCC1 cDNA expression vector, resulting in full complementation of both UV- and mitomycin C-sensitive phenotypes (R. S. Nairn, unpublished results). We found that the E1KO-47 cells exhibited ICL repair activity comparable to that of the repair-proficient AA8 cells. To verify that the ICL repair defect of the XP2OS mutant was due to a mutated XPA gene, a vector expressing wild-type XPA cDNA was introduced via cotransfection with the cross-linked substrate. As shown (Fig. 2), the repair defect of the XP2OS mutant was fully complemented by the expression of the wild-type XPA gene (C-XPA). These results indicate that the functions of the ERCC1 and XPA genes are required to repair ICLs in the absence of homologous recombination.
FIG. 2.
Involvement of XPA and ERCC1 in recombination-independent repair of ICLs. The repair of cross-linked luciferase reporter is deficient in XPA and ERCC1 mutants but can be restored to normal levels by the reintroduction of wild-type XPA and ERCC1 genes, respectively. C-XPA, XPA mutant complemented with a XPA cDNA expression vector; E1KO7-5, ERCC1-null mutant derived from CHO ATS-49; E1KO-47 (C-ERCC1), E1KO7-5 mutant transformed by stable integration of a wild-type ERCC1 cDNA expression vector.
Involvement of the NER mechanism in the recombination-independent repair of ICLs.
The incision stage of the NER pathway requires the following enzymatic activities (3, 13, 42): the DNA damage-binding activities of XPA-RPA and XPC-hRAD23B, the incision activities of the ERCC1-XPF and XPG endonucleases, and the function of the TFIIH complex, including the helicases XPB and XPD. To determine whether the entire NER incision mechanism is required in the recombination-independent repair of the psoralen ICLs, we examined characteristic human and hamster NER mutants with the cross-linked luciferase reporter. Cross-linked substrate was transfected into each mutant cell line, and reactivation of luciferase activity, as a result of ICL removal, was determined at 30 h posttransfection. As shown (Fig. 3A), reactivation of the cross-linked luciferase reporter, compared to isogenic parental cells, was greatly reduced in hamster mutants UV24, UV41, and UV135, indicating that the functions of the XPB, XPF, and XPG gene products are essential in the recombination-independent repair of ICLs. Consistent with this observation, human mutants defective in either XPD or XPG were also found deficient in the repair-mediated reactivation of luciferase. Thus, both the incision activities of NER, residing in ERCC1-XPF and XPG, and the TFIIH complex appear to participate in the recombination-independent repair of ICLs. Interestingly, the XPC mutant, XP4PA, displayed a partial defect compared to the other mammalian mutants defective in NER. This result is consistent with the observation that XPC function is not required for transcription-coupled repair (TCR) of lesions that reside in the template strand of an actively transcribed gene (32, 40). Since the ICL was placed in the actively transcribed region in the luciferase substrate, detection and processing of the ICL could be carried out by TCR in the absence of XPC. However, XPC apparently contributes to the overall reactivation through the global repair mechanism, as evidenced by the partial reactivation of the cross-linked reporter construct.
FIG. 3.
NER mutants are defective in repair reactivation of cross-linked luciferase substrate. (A) Repair efficiency of hamster NER mutants compared to parental AA8 cells. (B) Repair efficiency of XP mutants compared to repair-proficient HT-1080 cells.
Compared to normal and other XP mutants, the XP variant has been reported to have a higher frequency and an altered spectrum of mutations in response to triplex-mediated psoralen cross-links (35). We tested the XPV mutant, XP30RO, that is defective in lesion-bypass polymerase η (Pol η), to determine if Pol η is required in the reactivation of the psoralen ICL. The repair efficiency of the XPV mutant (33.27 ± 4.35), as shown (Fig. 3B), is moderately lower than the normal control (49.77 ± 2.78), suggesting that the XPV/Pol η gene is not essential for the reactivation of psoralen ICLs.
irs1 and irs1SF mutants are normal in the recombination-independent repair of ICLs.
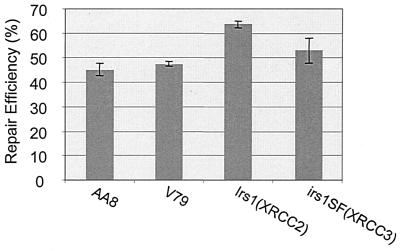
The irs1 and irs1SF mutants, which are extremely sensitive to cross-linking agents (17, 24), are defective in XRCC2 and XRCC3, respectively. These genes exhibit structural similarity to the hRad51 recombinase family (27), and recent studies have indicated that both mutant cell lines harbor a homologous recombination defect marked by greatly reduced gene conversion activity (23, 34), thus implicating conservative homologous recombination as a major mechanism of ICL repair. To determine whether recombination-independent ICL repair involves the function of the XRCC2 and XRCC3 genes, we examined the irs1 and irs1SF mutants in the luciferase reactivation assay. Both mutants exhibited wild-type recombination-independent repair activity comparable to that of the parental hamster cell lines, V79 and AA8 (Fig. 4). These results suggest that the recombination-independent ICL repair mechanism is likely a pathway distinct from the recombination-dependent mechanisms that require XRCC2 and XRCC3 gene function.
FIG. 4.
Recombination-independent repair of ICLs does not require the function of the XRCC2 and XRCC3 genes. Reactivation of the cross-linked luciferase reporter is normal in irs1 and irs1SF mutant cells compared to parental V79 and AA8 cells, respectively.
Recombination-independent repair of ICLs is error-prone.
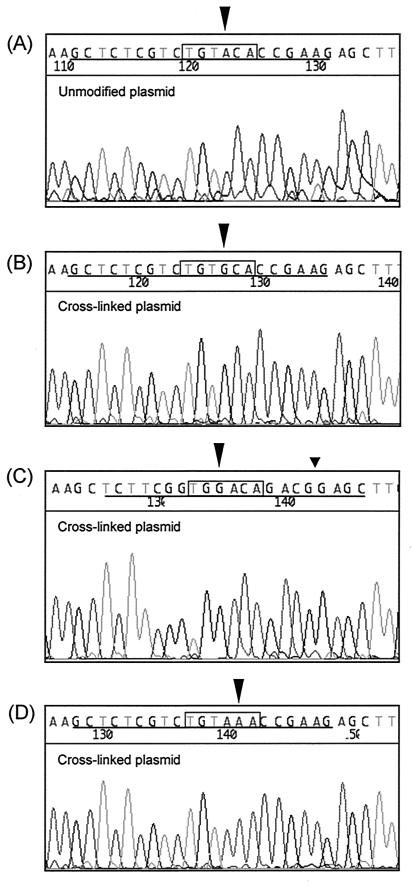
The repair of the cross-linked plasmid substrates that we have observed is most likely mediated by a recombination-independent process, since undamaged sequences that contain significant homology to the cross-linked reporter substrate would not to be available in the reactivation assay. Recombination-independent repair of ICLs is potentially mutagenic since both strands of the helix are damaged by the psoralen ICL. To determine if the recombination-independent repair of ICLs gives rise to mutations at the site of the cross-link, we transfected NER-proficient HT-1080 or RKO cells with the cross-linked pEGFP-N1 substrate. The plasmid DNA was recovered from transfected cells at 30 h posttransfection and transformed into the repair-recombination-deficient E. coli uvrA recA mutant strain AB2480 (18) to propagate lesion-free plasmid DNAs. The thymidine residues where the psoralen cross-link was attached are located within a BsrGI recognition sequence. Any mutation at or around the cross-linked thymidines should result in the inactivation of the BsrGI site. We analyzed 212 independent plasmid clones by PCR and found that approximately half, 108, contained the inserted oligo, while the other half did not. BsrGI digestion of the 108 clones indicated that 9 were refractory to cleavage and thus likely contained mutations in the BsrGI site. Sequencing of the nine clones revealed three A-to-G transversions, four A-to-C transversions, and one A-to-T transition, all of which occurred at the adenine residue opposing a cross-linked thymidine residue (Fig. 5). Interestingly, a strand bias was observed in that, of the eight mutations that occurred opposite a psoralen-adducted thymidine residue, seven were found to be opposite the thymidine residue in the nontranscribed strand, suggesting that the repair of these lesions is largely mediated by TCR as opposed to global repair. The single clone that had a mutation opposite the psoralen-adducted thymidine in the transcribed strand was found to also have a second mutation which was the deletion of a single thymidine residue eight nucleotides from the cross-linked thymidine residue (Fig. 5C). The source of this mutation is not clear, but no other mutations were observed in the sequences of the nine clones that were examined. The ninth mutation observed was a C-to-A transversion which occurred at the cytosine residue immediately 3′ to the psoralen-adducted thymidine within the BsrGI site (Fig. 5D). We did not observe deletions involving more than 1 bp as has been reported previously (8, 15), a finding which may be attributed to the lack of plasmid replication in HT-1080 cells, the lack of direct repeat sequences flanking the cross-linked site, and/or our sample size.
FIG. 5.
Mutations derived from recombination-independent repair of psoralen ICLs. The rectangle indicates the position of the BsrGI restriction site, and the cross-linked oligonucleotide sequence is underlined. (A) Sequence of the unmodified oligo inserted in the pEGFP-N1 vector. (B) A-to-G transversion found at the site opposite the psoralen-adducted thymidine residue in the nontranscribed strand (triangle). (C) T-to-G transversion found at the site opposite the psoralen-adducted thymidine in the transcribed strand (large triangle), and deletion of a T residue (small triangle). Note that the cross-linked oligo (underlined) was inserted in the opposite polarity. (D) C-to-A transversion found immediately downstream of the A residue opposite the psoralen-adducted thymidine residue (triangle).
To ensure that these observed mutations were the product of repair in the mammalian cells and not in the repair-deficient bacterial cells, cross-linked or unmodified pEGFP-N1 plasmid was directly electroporated into the AB2480 strain. The efficiency of transformation of the cross-linked plasmid was 2% of that of the unmodified plasmid and presumably arose from a low level of non-cross-linked material that contaminated the cross-linked substrate. We examined 164 of the clones resulting from the cross-linked substrate and found that approximately 80% did not contain the inserted oligo. The remaining 20% were all found to be susceptible to digestion by BsrGI, indicating that this population did not contain mutations in the restriction enzyme recognition site. Thus, among 212 clones that were passaged through mammalian cells, we observed 9 mutations, while no mutations were found among the 164 clones obtained by direct transfection in to AB2480 cells. These results indicate that the observed mutations were most likely due to repair that occurred in the mammalian cells.
The cross-linked substrate recovered from HT-1080 cells was also subjected to PCR analysis to confirm the presence of mutations prior to AB2480 transformation. A 255-bp fragment derived from plasmids containing the inserted oligo was amplified along with a 234-bp fragment of similar intensity that was likely derived from the empty vectors described above. BsrGI digestion of the PCR products showed that approximately 10% of the 255-bp fragment was resistant to the enzyme, indicating that mutations at the BsrGI site occurred as a result of passage through repair-proficient mammalian cells (data not shown).
DISCUSSION
By introducing cross-linked plasmid substrates into cultured cells, we have shown that a single defined ICL was removed through a recombination-independent pathway. Factors that are required for the incision steps of the NER pathway, but not those required for homologous recombination, appear to be essential for this mechanism. In addition, this pathway was shown to be mutagenic since frequent base substitutions were observed at or near the site of the cross-linked residues.
Mechanistic basis of recombination-independent ICL repair.
Damage to the DNA duplex can be grouped into two general categories from a molecular perspective. The first category includes damage that affects only one strand of the double helix, such as thymidine dimers, oxidative damage, adducts derived from monofunctional alkylating agents, etc. Repair mechanisms for these lesions rely on the inherent redundancy of the duplex to remove the damaged nucleotide and restore the double helix utilizing the undamaged complementary strand as a template. For instance, nucleotide excision repair, base excision repair, and mismatch repair are able to utilize this mechanism of action. The second category consists of two types of lesions that affect both strands of the double helix: DNA double-strand breaks (DSBs) and ICLs. For the repair of DSBs in mammalian cells, both nonhomologous and homologous recombination pathways have been established as major mechanisms of repair (22). However, the work described here and that reported by others (5, 28) indicates that the repair of ICLs can also be accomplished by recombination-independent mechanisms.
Perhaps the least understood stages of cross-link repair are the initial damage recognition and incision steps and the resulting uncoupling of the cross-link. These processes appear to be distinct between recombination-dependent and -independent pathways since the majority of mammalian mutants deficient in NER are only slightly sensitive to cross-linking agents, while mutants deficient in homologous recombination are extremely sensitive. Processing of monoadducts by NER involves the formation of a bubble structure at the site of the damage and the subsequent introduction of dual incisions on either side of the lesion (3, 13, 42). However, with regard to ICLs, recent results in vitro indicate that processing by mammalian NER results in dual incisions, both of which occur 5′ to the lesion (6). This may be the result of the cross-link inhibiting the formation of a proper bubble structure, and it is not clear at present whether this is a mechanism that is utilized in vivo. Indeed, Mu et al. (31) have proposed that the processing of cross-links by NER in vitro leads to a futile cycle of DNA synthesis in which the cross-links are not removed. Our results, however, clearly demonstrate that NER is involved, in vivo, in a pathway of cross-link repair, albeit an error-prone one. A potential uncoupling mechanism could result from the recently described ICL-stimulated 3′-5′ exonuclease activity of the ERCC1-XPF complex that occurs in the presence of RPA (31). This activity was shown to completely degrade one strand of a cross-linked linear duplex, leaving the complementary strand attached to a single mononucleotide. This could lead to a putative model for ICL repair in which the gap created by this activity could be filled in by a translesion DNA polymerase; the attached mononucleotide could then be removed by an additional cycle of NER. However, this model would not be compatible with incisions placed to the 5′ side of the ICL.
In mammals, a number of lesion bypass polymerases have been identified (43) and could potentially provide translesion synthesis activity for ICL repair. In yeast, a connection between psoralen ICL repair and lesion bypass synthesis has been established by the demonstration that the rev3 mutant, defective in DNA lesion bypass polymerase ζ, exhibits hypersensitivity to photoactivated psoralen (19). Since translesion synthesis is potentially error-prone, the mechanism suggested above would predict that the base opposing the cross-linked thymidine would be a mutation hotspot as a result of the bypass synthesis. Consistent with this speculation, we observed frequent base substitutions at this position in repaired plasmid substrates. However, the XPV mutant, which is defective in translesion Pol η, showed only minor decrease in the ICL reactivation. This may suggest that other lesion bypass polymerases also play important and/or redundant roles in the recombination-independent repair of ICLs. Interestingly, mutations resulting from the ICL repair were strongly biased in that the majority of misincorporations occurred opposite the psoralen-adducted thymidine in the nontranscribed strand. This observation and the finding that the XPC mutant was only partially defective in the reactivation assay suggests that TCR may be the major pathway of repair of the cross-linked reporter substrates.
Another interesting finding of the mutation analysis was that approximately half of the rescued plasmids did not contain the inserted oligo. This was surprising since our reactivation experiments indicated that approximately 50% of the plasmids had been reactivated, and less than 2% of the original substrates were without the oligo as determined by direct transfection into the AB2480 strain. If 50% of the plasmids had been fully repaired in the mammalian cells, we would expect that rescued plasmids without the oligo should have represented approximately 4% of the clones, whereas the observed fraction was actually about 50%. This observation seems to suggest that a large portion of the rescued plasmids were capable of being transcribed in the mammalian cells but were incapable of replication in the bacterial cells. Mu et al. (31) have shown that the efficiency of incision at the pyrone side of a psoralen cross-link occurs with 10-fold greater efficiency than does incision at the furan side. Thus, a plausible model to account for our findings is that if the pyrone side occurs in the transcribed strand this adduct is repaired relatively rapidly, which would allow transcription to proceed. However, the subsequent repair of the furan side should be inherently less efficient and would also only be subject to removal by the slower global repair pathway. After rescue from the mammalian cells these plasmids may thus still contain the furan adduct which would likely prevent replication in the AB2480 strain. This model also accounts for the fact that the level of reactivation was typically near 50% in wild-type cells since plasmids with the furan side in the transcribed strand would be reactivated with a greatly reduced efficiency.
Role of recombination-independent ICL repair in mammalian cells.
Repair of ICLs appears to involve two types of mechanisms: error-free repair pathways that involve conservative homologous recombination with the undamaged sister chromatid or homologous chromosome and error-prone pathways that can be either homology dependent or independent, but at the cost of increased genomic instability. Cellular resistance to ICLs is presumably established with a balance between these two types of mechanisms. In mammalian cells, the recombination-dependent mechanism appears to be the predominant pathway for ICL repair. Evidence supporting this notion comes from recent studies of the irs1 and irs1SF mutants, in which the severe cross-link sensitivity of both mutants has been linked to deficiencies in the conservative gene conversion process (23, 34). In contrast, recombination-independent ICL repair seems unlikely as a major pathway for ICL removal because the majority of mammalian NER mutants do not display a severe sensitivity to cross-link damage (2, 20). A plausible explanation is that the NER-mediated ICL uncoupling may be an inefficient process. As indicated above, introduction of the dual incisions by the NER excinucleases requires strand separation at the site of the lesion, and an ICL that covalently joins the two strands together may substantially reduce the introduction of such incisions and/or result in aberrant incisions (6). This, in turn, may lead to a low successful rate of ICL repair through the NER-lesion bypass mechanism. It is also possible that the recombination-independent pathway may function at a specific stage of the cell cycle, perhaps in G0/G1, where the homologous recombination repair of ICLs may not be efficient. In budding yeast, the rev3 mutant was found to be more sensitive to the killing by nitrogen mustard in stationary-phase cells than in exponential-phase cells (28), suggesting that recombination-independent ICL repair is employed in a cell cycle phase-specific manner. Moreover, in mutants that are defective in homologous recombination, the rev3p-mediated recombination-independent ICL repair was insufficient to support a normal level of resistance to cross-link damage (28). These results suggest that the recombination-independent ICL appears to be a minor pathway in yeast and that similar mechanisms may also play a minor role in ICL repair in mammalian cells.
ACKNOWLEDGMENTS
We thank QingYi, Wei, Lawrence Grossman, Larry Thompson, James Cleaver, and Nigel Jones for providing constructs and cell lines.
The DNA Sequencing Core facility of M. D. Anderson Cancer Center is supported by grant CA16672. This work was supported by National Cancer Institute grants CA76162 (L.L.), CA52461 (R.J.L.), CA75160 (R.J.L.), and CA36361 (R.S.N.).
REFERENCES
- 1.Adair G M, Rolig R L, Moore-Faver D, Zabelshansky M, Wilson W H, Nairn R S. Role of ERCC1 in removal of long non-homologous tail during targeted homologous recombination. EMBO J. 2000;19:5552–5561. doi: 10.1093/emboj/19.20.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson B S, Sadeghi T, Sicilano M J, Legerski R J, Murray D. Nucleotide excision repair genes as determinants of cellular sensitivity to cyclophosamide analogs. Cancer Chemother Pharmacol. 1996;38:406–416. doi: 10.1007/s002800050504. [DOI] [PubMed] [Google Scholar]
- 3.Araujo S J, Tirode F, Coin F, Pospiech H, Syvaoja J E, Stucki M, Hubscher U, Egly J M, Wood R D. Nucleotide excision repair of DNA with recombinant human proteins: definition of the minimal set of factors, active forms of TFIIH, and modulation by CAK. Genes Dev. 2000;14:349–359. [PMC free article] [PubMed] [Google Scholar]
- 4.Berardini M, Foster P L, Loechler E L. DNA polymerase II (polB) is involved in a new DNA repair pathway for DNA interstrand cross-links Escherichia coli. J Bacteriol. 1999;181:2878–2882. doi: 10.1093/gao/9781884446054.article.t031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berardini M, Mackay W, Loechler E L. Evidence for a recombination-independent pathway for the repair of DNA interstrand cross-links based on a site-specific study with nitrogen mustard. Biochemistry. 1997;36:3506–3513. doi: 10.1021/bi962778w. [DOI] [PubMed] [Google Scholar]
- 6.Bessho T, Mu D, Sancar A. Initiation of DNA interstrand cross-link repair in humans: the nucleotide excision repair system makes dual incisions 5′ to the cross-linked base and removes a 22- to 28-nucleotide-long damage-free strand. Mol Cell Biol. 1997;17:6822–6830. doi: 10.1128/mcb.17.12.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodell W J. Molecular dosimetry for sister-chromatid exchange induction and cytotoxicity by monofunctional and bifunctional alkylating agents. Mutat Res. 1990;233:203–210. doi: 10.1016/0027-5107(90)90163-x. [DOI] [PubMed] [Google Scholar]
- 8.Bredberg A, Sandor Z, Brant M. Mutational response of Fanconi anaemia cells to shuttle vector site-specific psoralen cross-links. Carcinogenesis. 1995;16:555–561. doi: 10.1093/carcin/16.3.555. [DOI] [PubMed] [Google Scholar]
- 9.Cassier-Chauvat C, Moustacchi E. Allelism between pso1–1 and rev3–1 mutants and between pso2–1 and snm1 mutants in Saccharomyces cerevisiae. Curr Genet. 1988;13:37–40. doi: 10.1007/BF00365754. [DOI] [PubMed] [Google Scholar]
- 10.Cheng S, Van Houten B, Gamper H B, Sancar A, Hearst J E. Use of psoralen-modified oligonucleotides to trap three-stranded RecA-DNA complexes and repair of these cross-linked complexes by ABC excinuclease. J Biol Chem. 1988;263:15110–15117. [PubMed] [Google Scholar]
- 11.Cole R, Levitan S D, Sinden R R. Removal of psoralen interstrand crosslinks from DNA of Escherichia coli: mechanism and genetic control. J Mol Biol. 1976;103:39–59. doi: 10.1016/0022-2836(76)90051-6. [DOI] [PubMed] [Google Scholar]
- 12.Cole R S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci USA. 1973;70:1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Laat W L, Jaspers N G, Hoeijmakers J H. Molecular mechanism of nucleotide excision repair. Genes Dev. 1999;13:768–785. doi: 10.1101/gad.13.7.768. [DOI] [PubMed] [Google Scholar]
- 14.Dronkert M L, Beverloo H B, Johnson R D, Hoeijmakers J H, Jasin M, Kanaar R. Mouse RAD54 affects DNA double-strand break repair and sister chromatid exchange. Mol Cell Biol. 2000;20:3147–3156. doi: 10.1128/mcb.20.9.3147-3156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faruqi A F, Datta H J, Carroll D, Seidman M M, Glazer P M. Triple-helix formation induces recombination in mammalian cells via a nucleotide excision repair-dependent pathway. Mol Cell Biol. 2000;20:990–1000. doi: 10.1128/mcb.20.3.990-1000.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faruqi A F, Seidman M M, Segal D J, Carroll D, Glazer P M. Recombination induced by triple-helix-targeted DNA damage in mammalian cells. Mol Cell Biol. 1996;16:6820–6828. doi: 10.1128/mcb.16.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuller L F, Painter R B. A Chinese hamster ovary cell line hypersensitive to ionizing radiation and deficient in repair replication. Mutat Res. 1988;193:109–121. doi: 10.1016/0167-8817(88)90041-7. [DOI] [PubMed] [Google Scholar]
- 18.Gurzadyan G G, Gorner H, Schulte-Frohlinde D. Ultraviolet (193, 216 and 254 nm) photoinactivation of Escherichia coli strains with different repair deficiencies. Radiat Res. 1995;141:244–251. [PubMed] [Google Scholar]
- 19.Henriques J A, Moustacchi E. Isolation and characterization of pso mutants sensitive to photo-addition of psoralen derivatives in Saccharomyces cerevisiae. Genetics. 1980;95:273–288. doi: 10.1093/genetics/95.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoy C A, Thompson L H, Mooney C L, Salazar E P. Defective DNA cross-link removal in Chinese hamster cell mutants hypersensitive to bifunctional alkylating agents. Cancer Res. 1985;45:1737–1743. [PubMed] [Google Scholar]
- 21.Jachymczyk W J, Von Borstel R C, Mowat M R A, Hastings P J. Repair of interstrand cross-links in DNA of Saccharomyces cerevisiae requires two systems of DNA repair: the RAD3 system and the RAD51 system. Mol Gen Genet. 1981;182:196–205. doi: 10.1007/BF00269658. [DOI] [PubMed] [Google Scholar]
- 22.Jeggo P A. Identification of genes involved in repair of DNA double-strand breaks in mammalian cells. Radiat Res. 1998;150:S80–S91. [PubMed] [Google Scholar]
- 23.Johnson R D, Liu N, Jasin M. Mammalian XRCC2 promotes the repair of DNA double-strand breaks by homologous recombination. Nature. 1999;401:397–399. doi: 10.1038/43932. [DOI] [PubMed] [Google Scholar]
- 24.Jones N J, Cox R, Thacker J. Isolation and cross-sensitivity of X-ray-sensitive mutants of V79–4 hamster cells. Mutat Res. 1987;183:279–286. doi: 10.1016/0167-8817(87)90011-3. [DOI] [PubMed] [Google Scholar]
- 25.Kano Y, Fujiwara Y. Roles of DNA interstrand crosslinking and its repair in the induction of sister-chromatid exchange and a higher induction in Fanconi's anemia cells. Mutat Res. 1981;81:365–375. doi: 10.1016/0027-5107(81)90123-8. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Peterson C A, Lu X, Wei P, Legerski R J. Interstrand cross-links induce DNA synthesis in damaged and undamaged plasmids in mammalian cell extracts. Mol Cell Biol. 1999;19:5619–5630. doi: 10.1128/mcb.19.8.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu N, Lamerdin J E, Tebbs R S, Schild D, Tucker J D, Shen M R, Brookman K W, Siciliano M J, Walter C A, Fan W, Narayana L S, Zhou Z Q, Adamson A W, Sorensen K J, Chen D J, Jones N J, Thompson L H. XRCC2 and XRCC3, new human Rad51-family members, promote chromosome stability and protect against DNA cross-links and other damages. Mol Cell. 1998;1:783–793. doi: 10.1016/s1097-2765(00)80078-7. [DOI] [PubMed] [Google Scholar]
- 28.McHugh P J, Sones W R, Hartley J A. Repair of intermediate structures produced at DNA interstrand cross-links in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:3425–3433. doi: 10.1128/mcb.20.10.3425-3433.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller R D, Prakash L, Prakash S. Genetical control of excision of Saccharomyces cerevisiae interstrand DNA cross-links induced by psoralen plus near-UV light. Mol Cell Biol. 1982;2:939–948. doi: 10.1128/mcb.2.8.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison A, Christensen R B, Alley J, Beck A K, Bernstine E G, Lemontt J F, Lawrence C W. REV3, a Saccharomyces cerevisiae gene whose function is required for induced mutagenesis, is predicted to encode a nonessential DNA polymerase. J Bacteriol. 1989;171:5659–5667. doi: 10.1128/jb.171.10.5659-5667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mu D, Bessho T, Nechev L V, Chen D J, Harris T M, Hearst J E, Sancar A. DNA interstrand cross-links induce futile repair synthesis in mammalian cell extracts. Mol Cell Biol. 2000;20:2446–2454. doi: 10.1128/mcb.20.7.2446-2454.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mu D, Sancar A. Model for XPC-independent transcription-coupled repair of pyrimidine dimers in humans. J Biol Chem. 1997;272:7570–7573. doi: 10.1074/jbc.272.12.7570. [DOI] [PubMed] [Google Scholar]
- 33.Nelson J R, Lawrence D W, Hinkle D C. Thymidine-thymine dimer bypass by yeast DNA polymerase zeta. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 34.Pierce A J, Johnson R D, Thompson L H, Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13:2633–2638. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raha M, Wang G, Seidman M M, Glazer P M. Mutagenesis by third-strand-directed psoralen adducts in repair-deficient human cells: high frequency and altered spectrum in a xeroderma pigmentosum variant. Proc Natl Acad Sci USA. 1996;93:2941–2946. doi: 10.1073/pnas.93.7.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon J A, Szankasi P, Nguyen D K, Ludlow C, Dunstan H M, Roberts C J, Jensen E L, Hartwell L H, Friend S H. Differential toxicities of anticancer agents among DNA repair and checkpoint mutants of Saccharomyces cerevisiae. Cancer Res. 2000;60:328–333. [PubMed] [Google Scholar]
- 37.Sladek F M, Munn M M, Rupp W D, Howard-Flanders P. In vitro repair of psoralen-DNA cross-links by RecA, UvrABC, and the 5′-exonuclease of DNA polymerase I. J Biol Chem. 1989;264:6755–6765. [PubMed] [Google Scholar]
- 38.Theicher B A. Antitumor alkylating agents. 5th ed. Vol. 1. New York, N.Y: Lippincott-Raven; 1997. [Google Scholar]
- 39.Thompson L H. Evidence that mammalian cells possess homologous recombinational repair pathways. Mutat Res. 1996;363:77–88. doi: 10.1016/0921-8777(96)00008-0. [DOI] [PubMed] [Google Scholar]
- 40.van Hoffen A, Venema J, Meschini R, van Zeeland A A, Mullenders L H. Transcription-coupled repair removes both cyclobutane pyrimidine dimers and 6-4 photoproducts with equal efficiency and in a sequential way from transcribed DNA in xeroderma pigmentosum group C fibroblasts. EMBO J. 1995;14:360–367. doi: 10.1002/j.1460-2075.1995.tb07010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Houten B, Gamper H, Holbrook S R, Hearst J E, Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci USA. 1986;83:8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood R D. Nucleotide excision repair in mammalian cells. J Biol Chem. 1997;272:23465–23468. doi: 10.1074/jbc.272.38.23465. [DOI] [PubMed] [Google Scholar]
- 43.Woodgate R. A plethora of lesion-replicating DNA polymerases. Genes Dev. 1999;13:2191–2195. doi: 10.1101/gad.13.17.2191. [DOI] [PubMed] [Google Scholar]