Summary
Vascular endothelial growth factor B (VEGF-B) is an interesting therapeutic candidate for coronary artery disease. However, it can also cause ventricular arrhythmias, potentially preventing its use in clinics. We cloned VEGF-B isoforms with different receptor binding profiles to clarify the roles of VEGFR-1 and Nrp-1 in angiogenesis and to see if angiogenic properties can be maintained while avoiding side effects. VEGF-B constructs were studied in vivo using adenovirus (Ad)-mediated intramyocardial gene transfers into the normoxic and ischemic porcine heart (n = 51). It was found that the unprocessed isoform VEGF-B186R127S is as efficient angiogenic growth factor as the native VEGF-B186 in normoxic and ischemic heart. In addition, AdVEGF-B186R127S increased myocardial perfusion reserve by 22% in ischemic heart without any side effects. AdVEGF-B127 (VEGFR-1 and Nrp-1 ligand) and AdVEGF-B109 (VEGFR-1 ligand) did not induce angiogenesis. Thus, VEGF-B186 is angiogenic only before its proteolytic processing to VEGF-B127. Only the VEGF-B186 C-terminal fragment was associated with arrhythmias.
Subject area: Cellular therapy, Biochemistry, Molecular biology
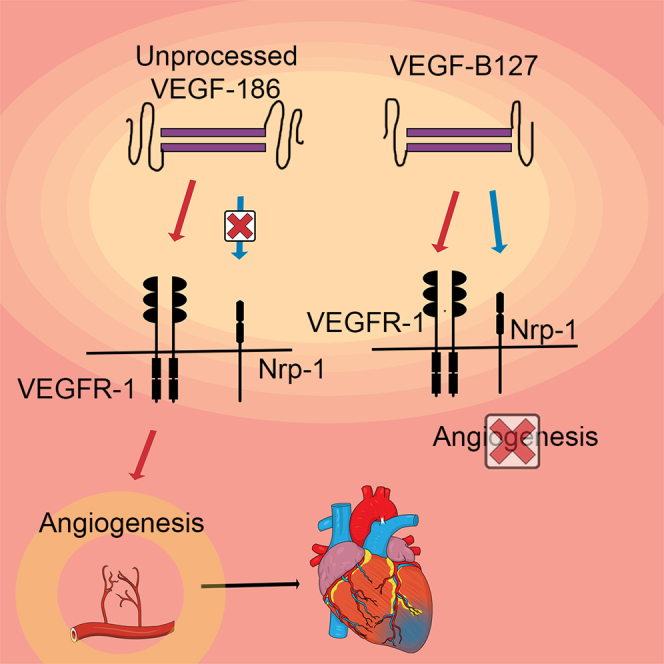
Graphical abstract
Highlights
-
•
AdVEGF-B186R127S induces angiogenesis and improves perfusion in the ischemic heart
-
•
No significant side effects were observed after AdVEGF-B186R127S therapy
-
•
VEGF-B186 is angiogenic only prior to its proteolytic processing
-
•
C-terminal fragment of VEGF-B186 is associated with ventricular arrhythmias
Cellular therapy; Biochemistry; Molecular biology;
Introduction
Despite the progress of treatments, coronary artery disease remains the leading cause of mortality and disease burden globally (Wang et al., 2016; Abbafati et al., 2020). Gene therapy-based therapeutic angiogenesis could benefit a growing number of patients suffering from severe coronary artery disease who have not gained sufficient symptom relief despite optimal pharmacological treatment and invasive procedures (Henry et al., 2014; Korpela et al., 2021). The concept of gene therapy is to induce local overexpression of a therapeutic gene in the ischemic myocardium by using local delivery of gene transfer vectors. Thus far, the most commonly used vector in myocardial gene therapy has been the replication-deficient adenovirus (Ylä-Herttuala et al., 2017), which has been used for the delivery of angiogenic growth factors, such as vascular endothelial growth factor A (VEGF-A) (Ylä-Herttuala and Baker, 2017; Cannatà et al., 2020).
Vascular endothelial growth factor B (VEGF-B) belongs to the VEGF family and is produced as two isoforms through alternative splicing. VEGF-B167 has a heparin-binding carboxyl terminus, making it bind to heparan sulfate proteoglycans in the extracellular matrix, whereas VEGF-B186 is more diffusible owing to its hydrophobic carboxyl terminus (Olofsson et al., 1996). Both isoforms are ligands for vascular endothelial growth factor receptor 1 (VEGFR-1) and Neuropilin-1 (Nrp-1), but Nrp-1 binding differs significantly because VEGF-B186 requires proteolytic processing before it can bind to Nrp-1 (Makinen et al., 1999). However, the role of these receptors in angiogenesis has remained unclear.
VEGF-B186 is expressed in skeletal muscle, brown fat, and most highly in the heart (Hagberg et al., 2010). During embryogenesis, VEGF-B has a role in the development of vasculature, but it is not vital because VEGF-B knockout mice survive but have impaired coronary vasculature (Bellomo et al., 2000). VEGF-B186 is distinct from other VEGFs owing to its favorable effects on cardiomyocyte metabolism (Hagberg et al., 2010; Kivelä et al., 2014, 2019). Although potentially useful for treating severe cardiac ischemia (Lähteenvuo et al., 2009; Nurro et al., 2016), VEGF-B186 has recently been shown to induce nerve growth in the myocardium, possibly leading to fatal ventricular arrhythmias in pigs (Lähteenvuo et al., 2020).
In this study, we cloned an unspliceable VEGF-B isoform, VEGF-B186R127S, in order to maintain the beneficial properties of VEGF-B186 while avoiding arrhythmogenic side effects. The proteolytic processing and Nrp-1 binding of VEGF-B186R127S were prevented by replacing arginine 127 with serine (Makinen et al., 1999). To investigate the properties of VEGF-B186R127S and to elucidate the role of VEGFR-1 and Nrp-1 binding in angiogenesis and arrhythmogenic events, we performed adenoviral gene transfers of different VEGF-B isoforms into the porcine myocardium.
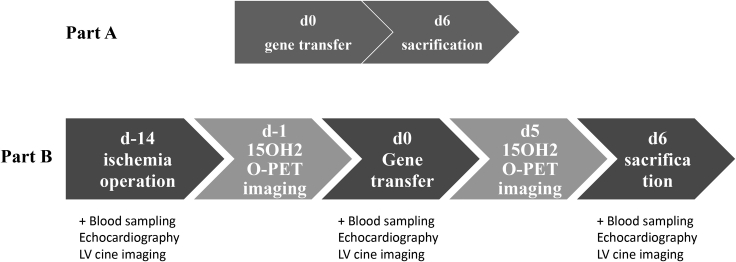
VEGF-B186R127S was compared in vitro and in vivo to the native form of VEGF-B186 and VEGF-B127, corresponding to the proteolytically processed form of VEGF-B186. We also designed and performed gene transfers with isoform VEGF-B109, a VEGFR-1 ligand lacking the Nrp-1 binding site, and with the C-terminal fragment of the VEGF-B186 (BCT 128–186). As a control, adenovirus expressing beta-galactosidase (LacZ) was used. Gene transfers were performed with a needle injection catheter into the normoxic and ischemic porcine heart, and the injections were targeted with NOGA electroanatomical mapping. The study protocol is shown in Figure 1.
Figure 1.
Study protocol and timepoints
Part A evaluated angiogenic effects of different VEGF-B isoforms in the normoxic heart. In Part B, the primary endpoints were myocardial perfusion reserve and histological quantification of the capillary area in ischemic hearts. In Part B, ischemia was induced by bottleneck stent placement to LAD two weeks before the gene transfer. 15O-H2O PET imaging was performed for nine animals one day before the gene transfer and one day before the sacrifice.
It was found that VEGF-B186R127S induces angiogenesis and increases myocardial perfusion reserve (MPR) as measured with the “golden standard” for absolute myocardial perfusion, 15O-water positron emission tomography (15O-H2O PET), without any significant side effects. We also show that VEGF-B186 is angiogenic only prior to its proteolytic processing to VEGF-B127. Arrhythmogenic side effects are most likely related to the C-terminal fragment released after the proteolytic processing of VEGF-B186.
Results
Binding profiles of the protein constructs
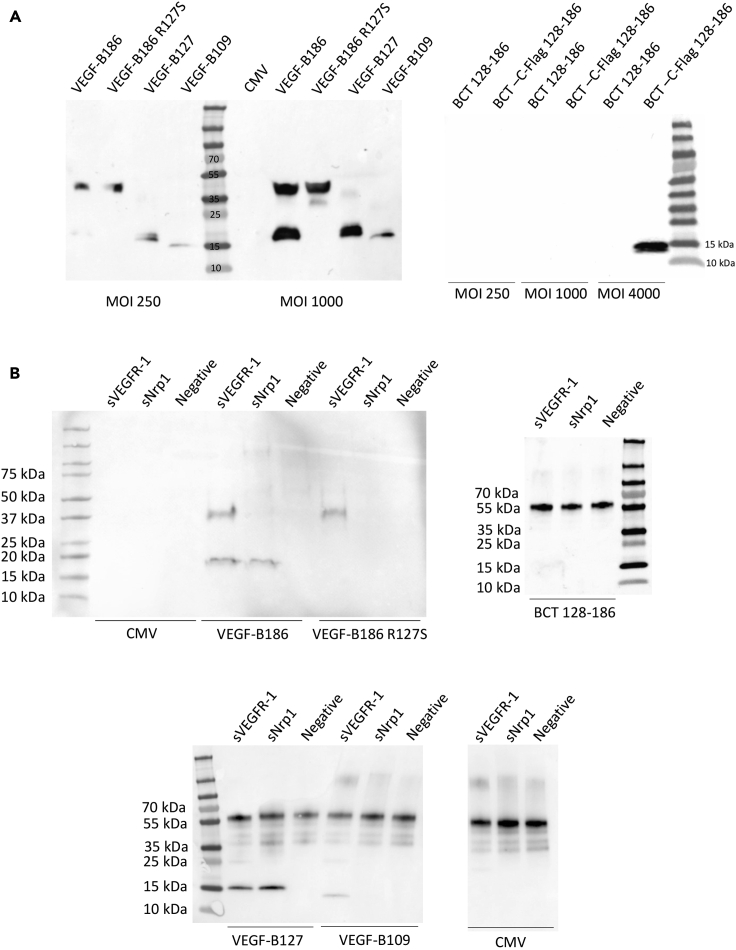
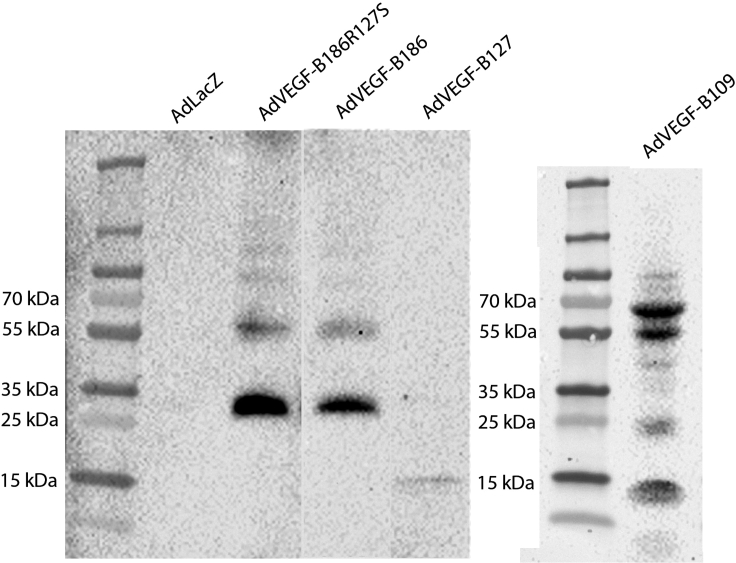
Before therapeutic use, the transduction efficacy of the adenoviral vectors was confirmed on HeLa cells (Figure 2A), and the receptor binding profiles of the different constructs were analyzed by VEGFR-1 and Nrp-1 immunoprecipitation (Figure 2B). Unprocessed isoform, VEGF-B186R127S, bound solely to VEGFR-1. Native VEGF-B186 bound VEGFR-1 and, after proteolytic processing, also to Nrp-1. VEGF-B127, corresponding to the VEGF-B186 proteolytically spliced form, bound both to Nrp-1 and VEGFR-1, whereas VEGF-B109 bound only to VEGFR-1. Study groups and each isoforms' receptor binding profiles are shown in Table 1.
Figure 2.
In vitro characterization of the receptor binding profiles of VEGF-B isoforms
(A) All the VEGF-B isoforms were detected from the HeLa cell medium after adenoviral transduction.
(B) VEGF-B immunoprecipitation with sVEGFR-1-Fc and sNrp-1-Fc from HeLa cell medium after adenoviral transduction. VEGF-B186 bound to VEGFR-1 and its proteolytically processed form (∼18 kDa) to VEGFR-1 and Nrp-1, whereas VEGF-B186R127S only bound to VEGFR-1. VEGF-B127, resembling the proteolytically processed form, was confirmed to bind both VEGFR-1 and Nrp-1, whereas VEGF-B109 bound solely to VEGFR-1. The C-terminal fragment did not bind to VEGFR-1 or Nrp-1.
Table 1.
Part A - Study groups and receptor binding profiles of the VEGF-B isoforms
| Part A. gene transfers to normoxic myocardium | Study groups | Receptor binding | |
|---|---|---|---|
| AdVEGF-B186 | N = 6 | VEGFR-1, Nrp-1a | |
| AdVEGF-B186R127S | N = 6 | VEGFR-1 | |
| AdVEGF-B127 | N = 6 | VEGFR-1, Nrp-1 | |
| AdVEGF-B109 | N = 6 | VEGFR-1 | |
| AdBCT 128–186 | N = 6 | -b | |
| AdLacZ | N = 6 | ||
after proteolytic processing.
no known receptor binding.
Neuropilin-1 binding is not required for angiogenesis induced by VEGF-B186, and the angiogenic effect of VEGF-B186 is diminished after proteolytic processing
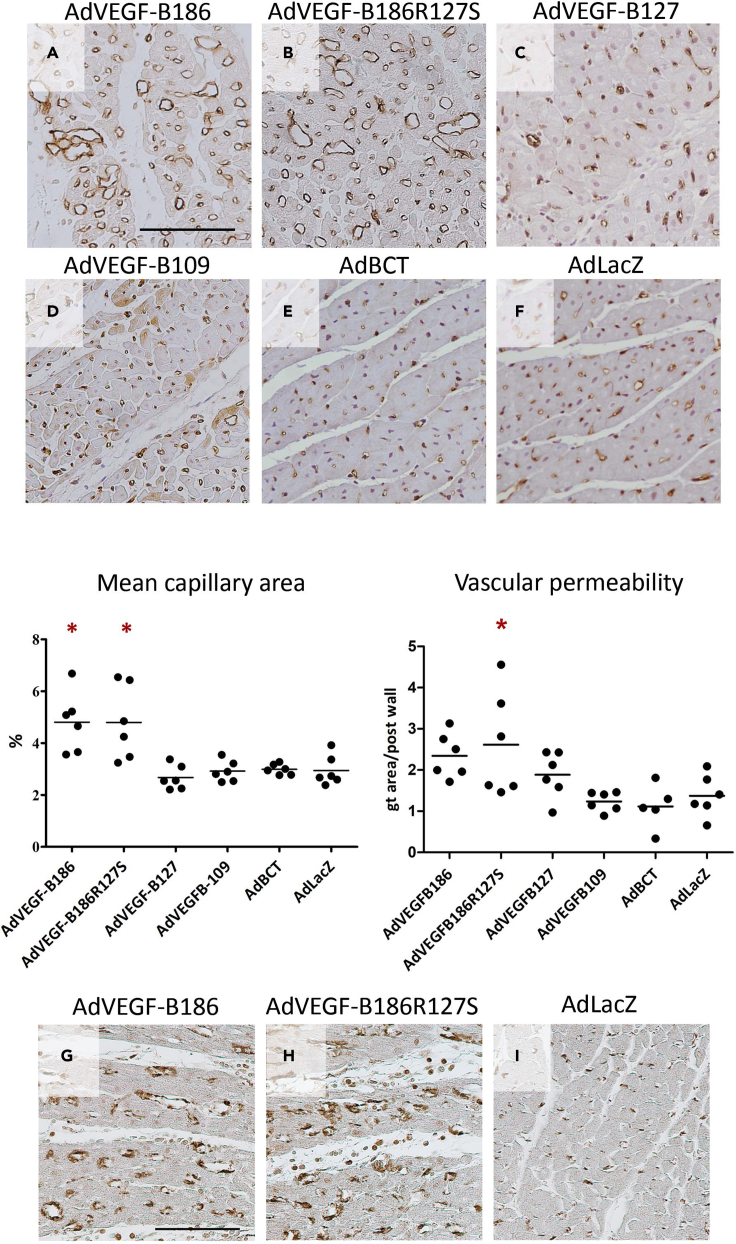
In normoxic hearts, adenoviral gene transfer of the unprocessed VEGF-B186R127S and the native VEGF-B186 induced angiogenesis, increasing the mean capillary area 1.9-fold in both groups compared to the control group (Figure 3). This implies that angiogenesis induced by VEGF-B186 is not mediated through Nrp-1 because VEGF-B186R127S only binds to VEGFR-1. Gene transfer with the isoform VEGF-B127 did not induce angiogenesis, despite its ability to bind both VEGFR-1 and Nrp-1. Thus, VEGF-B186 is angiogenic prior to the proteolytic cleaving of the VEGF-B C-terminus. However, the AdBCT 128–186 did not increase the capillary area, indicating that the VEGF-B186 C-terminal fragment does not have angiogenic potential when expressed separately. The VEGFR-1 ligand VEGF-B109 did not show angiogenic effects.
Figure 3.
AdVEGF-B186R127S induces similar angiogenesis as the native VEGF-B186
(A and B) Adenoviral gene transfer with the (A) native form of VEGF-B186 and (B) the proteolytically unprocessed form induced angiogenesis in normoxic myocardium by increasing the mean capillary area 1.9-fold compared to the control group (AdVEGF-B186 vs. AdLacZ and AdVEGF-B186R127S vs. AdLacZ, p < 0.05, 95% CI: 3.6 to 6.0 and 3.3 to 6.3).
(C) The microvascular area in VEGF-B127 transduced hearts did not differ from the control group.
(D–H) (D) VEGFR-1 ligand, VEGF-B109, did not show angiogenic potential, and expectedly, (E) no angiogenesis after gene transfer with VEGF-B186 C-terminal fragment was seen compared to the (F) control group. Myocardial sections were stained for αSMA to show pericyte coverage of the vessels in the gene transfer area of (G) VEGF-B186 and (H) VEGF-B186R127S transduced hearts. Vascular permeability measured by Miles Assay showed a statistically significant increase in permeability in AdVEGF-B186R127S transduced hearts. Results are expressed in dot plots separately for each individual animal, the line indicating the average value of the group. The asterisk indicates a statistically significant difference (p < 0.05) between the indicated group and the control group. Scale bars (A and G): 100 μm.
Vascular permeability does not increase after VEGF-B gene transfer
Pericyte coverage of the vessels in normoxic hearts treated with AdVEGF-B186 and AdVEGF-B186R127S was confirmed by αSMA staining (Figure 3), and extravasation of the plasma proteins was evaluated with Modified Miles Assay from tissue samples from normoxic hearts. A slight increase in vascular permeability was detected in the AdVEGF-B186R127S group compared to the control group (p < 0.05, 95% CI: 1.28 to 3.95 and 0.84 to 1.9, respectively) (Figure 3). However, no pericardial fluid accumulation was seen in transthoracic echocardiography.
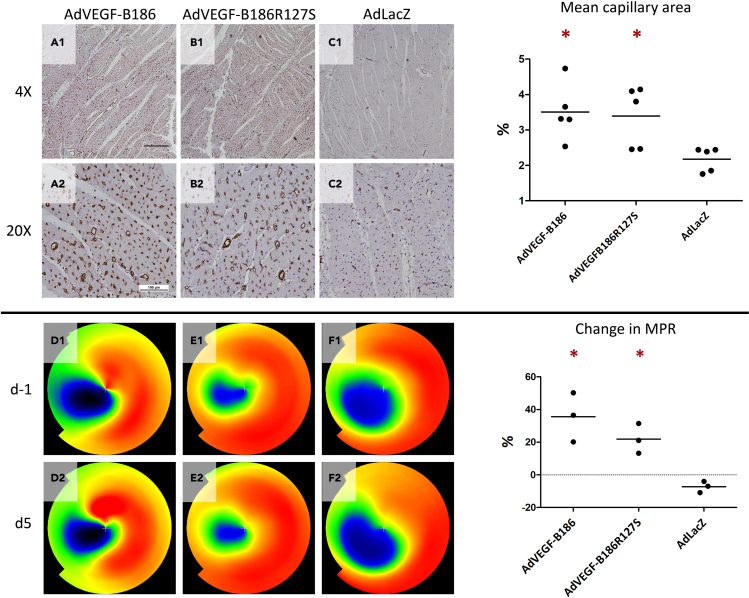
Both AdVEGF-B186 and AdVEGF-B186R127S gene transfers increase myocardial perfusion reserve in ischemic heart
In ischemic hearts, both AdVEGF-B186R127S and AdVEGF-B186 induced angiogenesis at the gene transfer area. Compared to the control group, the capillary area increased 1·6-fold both in VEGF-B186R127S and VEGF-B186 transduced hearts (Figure 4). To measure the blood perfusion of the heart, 15O-H2O PET imaging was done before gene transfer and five days after the gene transfers at Kuopio University Hospital. During the follow-up, MPR increased 36% in VEGF-B186 transduced hearts and 22% in VEGF-B186R127S transduced hearts, whereas in the control group MPR decreased 7% (Figure 4).
Figure 4.
AdVEGF-B186 and AdVEGF-B186R127S improve perfusion in ischemic heart
Mean capillary area increased (a1-a2) 1.6-fold in VEGF-B186 transduced hearts and (b1-b2) VEGF-B186R127S transduced hearts (p < 0.05, 95% CI: 2.5 to 4.5 and 2.3 to 4.5) compared to the control group (c1-c2) (95% CI-1.8 to 2.6). Improvement in MPR was seen in 15O-H2O PET in the treatment groups, because (d1-d2) AdVEGF-B186 improved MPR 36% in the gene transfer area (p < 0.05, 95% CI-1.7 to 73.1), and (e1-e2) AdVEGF-B186R127S 22%, respectively (p < 0.05, 95% CI-0.7 to 44.6) (f1-f2). In the control group, MPR decreased 7% (95% CI-15.8 to 1.2). The asterisk indicates a statistically significant difference (P<0.05) between the indicated group and the control group. Scale bar in 4x image: 500 μm. Scale bar in 20x image: 100 μm.
Overexpression of VEGF-B186 C-terminal fragment induces ventricular arrhythmias during dobutamine stress
AdBCT 128–186 animals showed a tendency to ventricular arrhythmias since ventricular bigeminy (n = 3) and non-sustained ventricular tachycardia (n = 3) were provoked during dobutamine stress. No arrhythmias were detected after the gene transfers with any other VEGF-B isoforms (data not shown).
Safety of the gene therapy
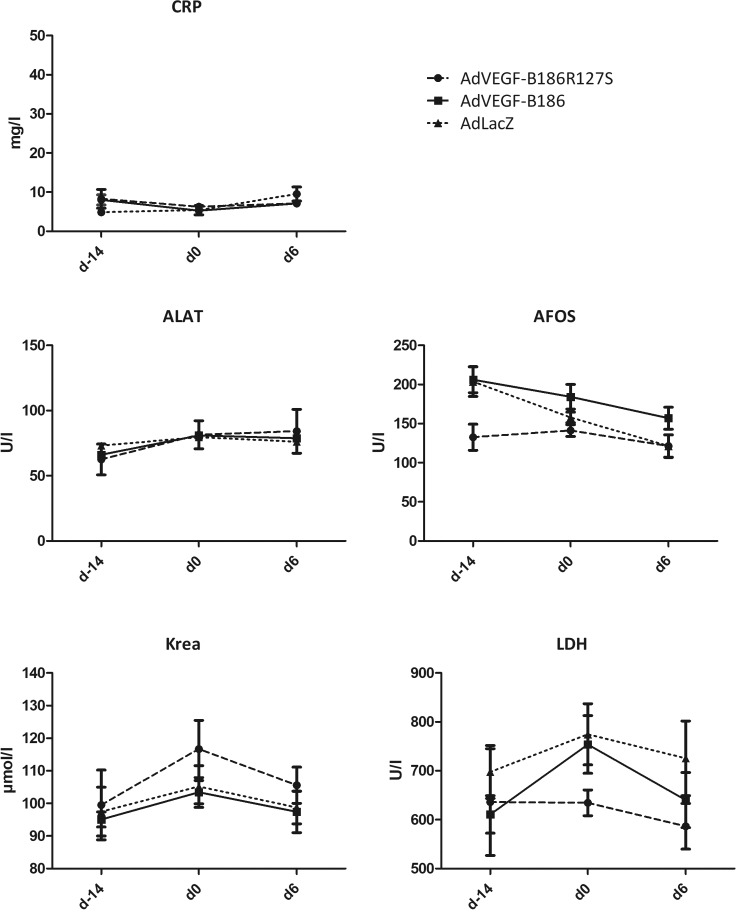
The blood samples were collected before ischemia operation, gene transfer, and six days after the gene transfer. The samples were analyzed for C-reactive protein (CRP), alanine aminotransferase (ALAT), alkaline phosphatase (AFOS), creatine, and lactate dehydrogenase (LDH). AdVEGF-B186 or AdVEGF-B186R127S gene therapy did not increase plasma CRP level or other parameters measured. Results from clinical chemistry are shown in Figure 5. No serious adverse effects, such as pericardial fluid accumulation, were detected.
Figure 5.
Clinical chemistry
There was no elevation of C-reactive protein (CRP), alanine aminotransferase (ALAT), alkaline phosphatase (AFOS), creatinine, or lactate dehydrogenase (LDH) in pig sera. Results are expressed as mean ± SEM values for each group.
Transgenes were detected from the gene transfer area
Transgene expression was detected by Western Blot from the gene transfer area of the normoxic hearts transduced with AdVEGF-B186, AdVEGF-B186R127S, AdVEGF-B127, AdVEGF-B109, and AdLacZ (Figure 6 and Figures S1–S4).
Figure 6.
Transgene expression was found in the gene transfer area
Transgene expression was detected from myocardial tissue lysates collected six days after the gene transfer by western Blotting using an anti-VEGF-B antibody (detailed in the STAR Methods section). The image has been cropped owing to AdVEGF-B186R127S and AdVEGF-B186 sample duplicates.
Discussion
Adenoviral gene therapy using VEGF-B186R127S isoform can be considered safe because no accumulation of pericardial fluid, arrhythmias, or sudden deaths were seen, and laboratory parameters from blood remained in the normal range during the follow-up. We show that VEGF-B186R127S induces angiogenesis both in normoxic and ischemic heart and improves myocardial perfusion reserve measured by 15O-H2O PET.
In this study, we analyzed for the first time the angiogenic potential of different VEGF-B186 isoforms in the porcine heart and explored the roles of VEGFR-1 and Nrp-1 in this effect. VEGF-B186 has previously been shown to bind to VEGFR-1, a tyrosine kinase receptor expressed in endothelial cells, macrophages, some hematopoietic cells, and pericytes (Barleon et al., 1996; Clauss et al., 1996). VEGFR-1 is necessary for embryonic vasculature development, having a regulatory role because VEGFR-1 deficiency manifests as fatal hypervascularization, but in adulthood, it promotes angiogenesis (Fong et al., 1999; Shibuya, 2006). After the proteolytic processing, VEGF-B186 can bind to Nrp-1 (Makinen et al., 1999), which is a co-receptor for VEGFRs binding several VEGFs, with differing specificity (Neufeld et al., 2002). In the heart, Nrp-1 is expressed along with VEGFR-1 in cardiomyocytes, coronary vessels, and myocardial capillaries, and it is essential for embryonic angiogenesis (Kawasaki et al., 1999; Lähteenvuo et al., 2009). Previously, it has been suggested that the angiogenic effect of VEGF-B186 could be explained by the occupation of VEGFR-1 as it would release VEGF-A bound to VEGFR-1 to signal via VEGFR-2 (Hiratsuka et al., 1998).
Our results also indicate that VEGF-B186 is angiogenic only prior to its proteolytic processing and the angiogenic effects are not mediated through Nrp-1. This was confirmed by the gene transfer with the unprocessed VEGF-B186R127S, which induced similar angiogenesis as the native VEGF-B186 despite its inability to bind to Nrp-1 as well as gene transfer with VEGF-B127 that did not induce angiogenesis despite its VEGFR-1 and Nrp-1 binding activity. The outcome of the VEGF-B127 gene transfer was unexpected since VEGFR-1 and Nrp-1 are the only currently known receptors for VEGF-B186.
All isoforms used in this study, except VEGF-B C–terminal fragment (BCT 128–186), bound to VEGFR-1. Considering this result, the angiogenic effect solely through VEGFR-1 occupation and the consequent release of VEGF-A from VEGFR-1 to bind to VEGFR-2 is unlikely. However, because the C-terminus is needed for the VEGF-B186 induced angiogenesis, it is possible that some additional receptors or co-receptors are yet to be identified.
Lahteenvuo et al. have previously proposed a direct Nrp-1-mediated signaling cascade for the angiogenic effects of VEGF-B186 based on the finding that angiogenesis could be inhibited by a soluble decoy Nrp-1 (Lähteenvuo et al., 2009). Considering this, Nrp-1 occupancy could enhance angiogenesis. However, based on our results, any significant direct role of Nrp-1 in VEGF-B-mediated angiogenic effects seems unlikely. During the overexpression of VEGF-B186R127S, there is also endogenous processed VEGF-B186 form (VEGF-B127) capable of binding to Nrp-1 and possibly acting as a regulator of angiogenesis. Nevertheless, we also showed that overexpression of VEGF-B127, a ligand for both VEGFR-1 and Nrp-1, did not induce angiogenesis. Thus, VEGF-B186 has a crucial role in angiogenesis before it is proteolytically processed.
Previously it has been shown that the angiogenesis induced by VEGF-B186 has distinct features when compared with other VEGFs. Unlike after VEGF-A gene therapy, vascular permeability is not increased after VEGF-B186 gene therapy, minimizing the risk for pericardial tamponade (Rissanen et al., 2003). Moreover, angiogenesis induced by VEGF-B186 mainly occurs through the enlargement of pre-existing capillaries rather than capillary sprouting (Bry et al., 2010). Besides angiogenic effects, VEGF-B186 has been shown to increase fatty acid uptake and improve cardiac contractility and cardiomyocyte metabolism under ischemia (Karpanen et al., 2008; Hagberg et al., 2010; Zentilin et al., 2010; Kivelä et al., 2014; Moessinger et al., 2020). Considering the diverse effects of VEGF-B186, indirect mechanisms could explain the angiogenic effects. It has previously been suggested that the increase in cardiomyocyte metabolism could secondarily lead to capillary enlargement and increased perfusion in VEGF-B186 transduced hearts (Huusko et al., 2010).
It has been reported that AdsVEGFR-1 with AdVEGF-B186 gene transfer increases mortality due to sudden cardiac deaths (Lähteenvuo et al., 2020). We also found that overexpression of the VEGF-B C-terminal fragment increases the tendency to ventricular tachycardia under dobutamine stress. No arrhythmias were detected after gene transfer with VEGF-B186R127S. This could indicate that VEGFR-1 signaling has a protective function in the arrhythmogenic effects of the VEGF-B186 C-terminus.
In conclusion, it was shown that the angiogenic effect of VEGF-B186 diminishes after proteolytic processing, suggesting that the C-terminal fragment of VEGF-B186 has a role in angiogenesis and after proteolytic processing also in arrhythmic events. We also show for the first time that AdVEGF-B186R127S induces angiogenesis and improves myocardial perfusion reserve in the ischemic heart without side effects, which is a clinically highly relevant finding and supports further development of this isoform toward clinical testing.
Limitations of the study
This study showed that adenoviral gene therapy with unprocessed VEGF-B186R127S is safe and efficient in ischemic heart. However, the long-term effects need to be evaluated in studies with longer follow-up time. Second, the potential alternative receptor bindings for VEGF-B C-terminus and intracellular signaling of VEGF-B186 and VEGF-B186R127S are to be determined in future studies.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-VEGF-B antibody | R&D Systems | mab3372, RRID: AB_2212999 |
| anti-VEGF-B antibody | R&D Systems | AF751, RRID:AB_355571 |
| anti-Flag | Sigma-Aldrich | F3165, RRID:AB_259529 |
| anti-mouse IgG HRP | R&D Systems | HAF018, RRID:AB_573130 |
| anti-goat IgG HRP | R&D Systems | HAF109, RRID:AB_357236 |
| Bacterial and virus strains | ||
| Serotype 5 adenoviruses | National Virus Vector Laboratory (A.I. Virtanen Institute) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| VEGF-B plasmids | GenScript | N/A |
| Software and algorithms | ||
| Fiji | https://imagej.net/software/fiji/ | N/A |
| Carimas 2 | http://www.turkupetcentre.fi/carimas | N/A |
| GraphPad 5 | https://www.graphpad.com/ | N/A |
| Other | ||
| MyoStar® intramyocardial injection catheter | Johnson & Johnson | N/A |
| NOGA® mapping system | Johnson & Johnson | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Seppo Ylä-Herttuala (seppo.ylaherttuala@uef.fi).
Materials availability
Plasmids and viral vectors generated in this study are available from the lead contact.
Experimental model and subject details
The study consisted of two parts. Part A compared different isoforms of VEGF-B in healthy porcine myocardium (n=36, six per group). Animals were randomly divided into six study groups (Table 1). Isoforms showing angiogenic effect in part A were chosen to part B, where gene transfers to ischemic myocardium were performed (n=15, five per group, see Table 2. The study protocol is shown in Figure 1. Follow-up was limited to six days, corresponding to the highest transgene expression observed in our previous studies (Huusko et al., 2010). All experiments were performed using approximately 3-month old female domestic pigs following the ARRIVE guidelines and U.K. Animals Act for animal experiments and were approved by the Animal Experiment Board in Finland.
Table 2.
Study groups in Part B
| Part B. gene transfers to ischemic myocardium | Study groups | 15O-PET imaged animals | |
|---|---|---|---|
| AdVEGF-B186 | N = 5 | 3 | |
| AdVEGF-B186R127S | N = 5 | 3 | |
| AdLacZ | N = 5 | 3 | |
In part B, ischemia was induced fourteen days before the gene transfer by placing a bottleneck stent to the left anterior descending artery in angiographic guidance. Bottleneck stent consisted of a bare metal stent (Coroflex® Blue, B.Braun Medical) covered by a polytetrafluoroethylene tube formed in a bottleneck shape to reduce coronary blood flow. The most proximal 2 stent struts were left uncovered by the tubing. The stent was inflated in the artery with stent-to-lumen-ratio of 1.3, anchoring the bottleneck in place. Size of the stent, either 3.0/3.5/4.0 x 8 mm, was chosen according to the size of the proximal LAD in the angiogram by using the automatic measurement software in the angiographic workstation (Rissanen et al., 2013).
Methods details
Expression plasmids and adenoviral vectors
VEGF-B plasmids were synthesized by GenScript (Piscataway, USA). Replication-deficient E1-E3 deleted clinical GMP-grade adenoviruses (serotype 5) were produced in HEK 293 cells in National Virus Vector Laboratory at A.I. Virtanen Institute. All viruses used in the present study were tested for sterility, mycoplasma, endotoxin and replication-competent viruses.
Pulldown assay with recombinant sVEGFR1-Fc and sNrp1-Fc
The media of transduced HeLa cells were precleared using Pierce Protein A/G Magnetic Beads (Thermo Fisher Scientific, Waltham, USA). Supernatants were collected and supplied with 5 μg sVEGFR1-Fc (R&D Systems, Minneapolis, USA) or sNrp1-Fc recombinant proteins and incubated at 4°C overnight (Nieminen et al., 2014). Controls were incubated without recombinant proteins. Protein complexes were precipitated using Pierce Protein A/G Magnetic Beads, washed, and eluted in SDS-PAGE sample buffer. Samples were analyzed using immunoblotting with anti-VEGF-B antibody (mab3372, 1:1000, R&D Systems), anti-VEGF-B antibody (AF751, 1:1000, R&D Systems) (Figure 2B 1st row) or anti-Flag antibody (1:1000, F3165, Sigma-Aldrich, Missouri, USA). Secondary antibodies used were anti-mouse IgG HRP (HAF018, R&D Systems) and anti-goat IgG HRP (HAF109, R&D Systems).
Functional measurements
Click or tap here to enter text. Transthoracic echocardiography (EPIQ 7G, Philips, Netherlands) was performed in Part A and Part B before ischemia operation, gene transfer, and sacrifice to measure any detectable pericardial fluid. Cardiac output was measured by LV cine imaging at rest and under dobutamine-induced stress at increasing infusion rates. Upon reaching the target heart rate of 160 bpm, the infusion rate was kept constant during fluoroscopic imaging. Before the operations, pigs were sedated with an intramuscular injection of 1.5 mL atropine and 6 mL of azaperone. After the sedation, animals were kept under anesthesia with propofol (15 mg/kg/h) and fentanyl (10 μg/kg/h).
Gene transfer
In part A, at the beginning of the experiment (day 0), MyoStar® intramyocardial injection catheter (Johnson & Johnson, California, USA) was introduced to the left ventricle via femoral sheath in fluoroscopic guidance (GE Innova 3100IQ 3D, GE Healthcare, Waukesha, WI). Adenoviral product was administered as ten injections of 0.2 mL each to the anterolateral wall of the left ventricle. The total dosage was 1 x 1012 vp to each heart.
In ischemic animals, gene transfers (d0) were guided by a NOGA® mapping system (Biologics Delivery Systems, Johnson & Johnson company, USA) using MyoStar® intramyocardial injection catheter (Johnson & Johnson company, USA). Gene transfers were directed into viable but hypokinetic areas of the left ventricle. For viability, a unipolar voltage over 5 mV was used as a criterion. For hypokinesia, a local linear shortening (LLS) as low as available was selected, typically at least below 12 % but preferably below 6 % (Gyöngyösi and Dib, 2011). The injection needle length was set for 3 mm. An injection duration was 30 seconds, and the injection needle was kept inside the myocardium for an additional five seconds before retraction to prevent backflow to the left ventricle.
Modified miles assay
Protein extravasation to the surrounding tissue was measured by Modified Miles Assay. Albumin-binding Evans Blue dye was given 30 mg/kg intravenously 30 minutes before sacrifice, and samples from the gene transfer area and posterior wall of the left ventricle were incubated in formamide for 24 hours at 65°C. The absorbance of the dilution was measured at 620 nm.
Western blotting
VEGF-B Western Blot was performed from tissue lysates using mab3372 antibody (1:1000, R&D Systems, USA). As a secondary antibody, anti-mouse HRP (1:1000, HAF018, R&D Systems) was used.
Sample collection
On day 6, after animals were given an intravenous KCl injection under general propofol-fentanyl-induced anesthesia, normoxic hearts were perfused with 1 % PFA, and samples were collected from the gene transfer area. Gene transfer area was defined as injection sites recognized by Evans Blue dye in normoxic hearts and by NOGA map from ischemic hearts. The samples were further fixated in 4 % PFA for 48 hours at 4°C and stored in 15 % sucrose for at least 24 hours, and then embedded in paraffin and cut to 6 μm sections.
Blood vessel measurements
Mean capillary area (%) was measured from CD31-immunostained (1:100, AF806; R&D) sections of pig myocardium at 200× magnification. All measurements were performed with Fiji software in a blinded manner from altogether 25 different randomly selected fields from 4 to 5 sections from each pig. The images were taken from samples collected from the gene transfer area.
15O-radiowater positron emission tomography
Rest and stress 15O-water PET/CT scans were performed using a Siemens Biograph mCT scanner (Siemens Healthcare, Erlangen, Germany). Computed tomography (CT) scans were performed before rest and stress imaging, and CT information was used for attenuation correction. An on-site cyclotron (PETtrace 860, GE Healthcare, UK) in-line conversion oven with Pd catalyst and radiowater generator (Hidex Oy, Finland) produced 15O-water bolus, following the GMP regulations. Rest, and stress imaging was performed using an 800 MBq 15O-water bolus. The dynamic acquisition included frames of 14x5, 3x10, 3x20, and 4x30 s (total duration 280s). After suitable decay of 12 min, stress imaging was performed with a further 800 MBq 15O-water bolus. The dynamic acquisition was performed during adenosine-induced hyperemia (200 μg/kg/min intravenous) (Tarkia et al., 2012). Images were reconstructed on a 128x128 matrix using the ordered subsets expectation maximization iterative algorithm (2 iterations, 21 subsets, zoom 2, Gaussian 6mm post-filter).
Myocardial perfusion reserve analysis
Regional myocardial perfusion (mL/g/min) was measured using Carimas 2 software (Turku PET Centre, Turku, Finland; http://www.turkupetcentre.fi/carimas). Gene transfer area was selected as a region of interest (ROI) by comparing the PET image to the NOGA map from the ischemia operation. The blood perfusion of the gene transfer area was normalized to the area of maximal perfusion of each heart both at rest and at stress. MPR was calculated as the ratio of the perfusion at stress to rest. All the analyses were performed in a blinded manner.
Clinical chemistry
Blood samples from ischemic animals were analyzed at Movet (Kuopio, Finland) for C-reactive protein, lactate dehydrogenase, alkaline phosphatase, alanine aminotransferase, creatine, and troponin from timepoints d0 and d6, respectively.
Quantification and statistical analysis
Statistical significance was evaluated by one-way analysis of variance (ANOVA) following Tukey's post hoc test when appropriate. P-value under 0.05 was considered as statistically significant (GraphPad 5 for Windows). Two-way analysis of variance was used for clinical chemistry.
Acknowledgments
The authors would like to thank the help of Marja Hedman, Tiina Laitinen, and Tomi Laitinen in 15O-H2O PET imaging at Kuopio University Hospital; Annika Viren and Severi Sormunen for assistance and PET imaging; Tiina Koponen and Sari Järveläinen for virus production at the National Virus Vector Laboratory, Biocenter Kuopio; and Heikki Karhunen, Minna Törrönen, and Riikka Venäläinen from National Laboratory Animal Center for assistance in animal work.
This study was supported by grants from Finnish Academy, ERC Advanced Grant, The Finnish Medical Foundation, Finnish Cardiac Society, and CardioReGenix EU Horizon 2020 grant.
Author contributions
H.K. designed and performed animal work, laboratory work, microscopical imaging, analyzed and made the interpretation of capillary analysis, 15H2O PET analysis, clinical chemistry interpretation, and wrote the paper. O-P.H., J.A., and K.V. performed animal experiments and laboratory work. T.N. designed the in vitro work, and T.N. and R.M. did the adenoviral transductions and immunoprecipitations on HeLa cells. P.T. designed the VEGF-B isoform protein constructs. M.H. carried out 15H2O PET imaging. P.P. was responsible for radionuclide production. J.N. performed animal experiments and revised the paper. S.Y-H. designed the study and revised the paper.
Declaration of interests
The authors declare no competing interests.
Published: December 17, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103533.
Supplemental information
Data and code availability
Data is available upon a request from the lead contact.
Analysis was performed without the need for original code.
References
- Abbafati C., Machado D.B., Cislaghi B., Salman O.M., Karanikolos M., McKee M., Abbas K.M., Brady O.J., Larson H.J., Trias-Llimós S., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barleon B., Sozzani S., Zhou D., Weich H.A., Mantovani A., Marmé D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. doi: 10.1182/blood.v87.8.3336.bloodjournal8783336. [DOI] [PubMed] [Google Scholar]
- Bellomo D., Headrick J.P., Silins G.U., Paterson C.A., Thomas P.S., Gartside M., Mould A., Cahill M.M., Tonks I.D., Grimmond S.M., et al. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ. Res. 2000;86 doi: 10.1161/01.res.86.2.e29. [DOI] [PubMed] [Google Scholar]
- Bry M., Kivelä R., Holopainen T., Anisimov A., Tammela T., Soronen J., Silvola J., Saraste A., Jeltsch M., Korpisalo P., et al. Vascular endothelial growth factor-B acts as a coronary growth factor in transgenic rats without inducing angiogenesis, vascular leak, or inflammation. Circulation. 2010;122:1725–1733. doi: 10.1161/CIRCULATIONAHA.110.957332. [DOI] [PubMed] [Google Scholar]
- Cannatà A., Ali H., Sinagra G., Giacca M. Gene therapy for the heart lessons learned and future perspectives. Circ. Res. 2020 doi: 10.1161/CIRCRESAHA.120.315855. [DOI] [PubMed] [Google Scholar]
- Clauss M., Weich H., Breier G., Knies U., Röckl W., Waltenberger J., Risaut W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J. Biol. Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- Fong G.H., Zhang L., Bryce D.M., Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development. 1999;126:3015–3025. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- Gyöngyösi M., Dib N. Diagnostic and prognostic value of 3D NOGA mapping in ischemic heart disease, Nature Reviews Cardiology. Nat. Rev. Cardiol. 2011:393–404. doi: 10.1038/nrcardio.2011.64. [DOI] [PubMed] [Google Scholar]
- Hagberg C.E., Falkevall A., Wang X., Larsson E., Huusko J., Nilsson I., van Meeteren L.A., Samen E., Lu L., Vanwildemeersch M., et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- Henry T.D., Satran D., Jolicoeur E.M. Treatment of refractory angina in patients not suitable for revascularization, Nature Reviews Cardiology. Nat. Rev. Cardiol. 2014:78–95. doi: 10.1038/nrcardio.2013.200. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S., Minowa O., Kuno J., Noda T., Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc. Natl. Acad. Sci. U S A. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huusko J., Merentie M., Dijkstra M.H., Ryhänen M.-M.M., Karvinen H., Rissanen T.T., Vanwildemeersch M., Hedman M., Lipponen J., Heinonen, et al. The effects of VEGF-R1 and VEGF-R2 ligands on angiogenic responses and left ventricular function in mice. Cardiovasc. Res. 2010;86:122–130. doi: 10.1093/cvr/cvp382. [DOI] [PubMed] [Google Scholar]
- Karpanen T., Bry M., Ollila H.M., Seppänen-Laakso T., Liimatta E., Leskinen H., Kivelä R., Helkamaa T., Merentie M., Jeltsch M., et al. Overexpression of vascular endothelial growth factor-B in mouse heart alters cardiac lipid metabolism and induces myocardial hypertrophy. Circ. Res. 2008;103:1018–1026. doi: 10.1161/CIRCRESAHA.108.178459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Kitsukawa T., Bekku Y., Matsuda Y., Sanbo M., Yagi T., Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895 LP–4902. doi: 10.1242/dev.126.21.4895. http://dev.biologists.org/content/126/21/4895.abstract [DOI] [PubMed] [Google Scholar]
- Kivelä R., Bry M., Robciuc M.R., Räsänen M., Taavitsainen M., Silvola J.M., Saraste A., Hulmi J.J., Anisimov A., Mäyränpää M.I., et al. VEGF -B-induced vascular growth leads to metabolic reprogramming and ischemia resistance in the heart. EMBO Mol. Med. 2014;6:307–321. doi: 10.1002/emmm.201303147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivelä R., Hemanthakumar K.A., Vaparanta K., Robciuc M., Izumiya Y., Kidoya H., Takakura N., Peng X., Sawyer D.B., Elenius K., et al. Endothelial cells regulate physiological cardiomyocyte growth via VEGFR2-mediated paracrine signaling. Circulation. 2019;139:2570–2584. doi: 10.1161/CIRCULATIONAHA.118.036099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpela H., Lampela J., Nurro J., Pajula J., Ylä-Herttuala S. Vascularization for Tissue Engineering and Regenerative Medicine. Springer International Publishing; 2021. Therapeutic angiogenesis: Translational and clinical experience; pp. 1–45. [DOI] [Google Scholar]
- Lähteenvuo J., Hätinen O.-P.P., Kuivanen A., Huusko J., Paananen J., Lähteenvuo M., Nurro J., Hedman M., Hartikainen J., Laham-Karam N., et al. Susceptibility to cardiac arrhythmias and sympathetic nerve growth in VEGF-B overexpressing myocardium. Mol. Ther. 2020:1731–1740. doi: 10.1016/j.ymthe.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lähteenvuo J.E., Lähteenvuo M.T., Kivelä A., Rosenlew C., Falkevall A., Klar J., Heikura T., Rissanen T.T. Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1 – and neuropilin receptor-1 – Dependent mechanisms. Circulation. 2009:845–856. doi: 10.1161/CIRCULATIONAHA.108.816454. [DOI] [PubMed] [Google Scholar]
- Makinen T., Olofsson B., Karpanen T., Hellman U., Soker S., Klagsbrun M., Eriksson U., Alitalo K. Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1. J. Biol. Chem. 1999;274:21217–21222. doi: 10.1074/JBC.274.30.21217. [DOI] [PubMed] [Google Scholar]
- Moessinger C., Nilsson I., Muhl L., Zeitelhofer M., Heller Sahlgren B., Skogsberg J., Eriksson U. VEGF-B signaling impairs endothelial glucose transcytosis by decreasing membrane cholesterol content. EMBO Rep. 2020;21 doi: 10.15252/embr.201949343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G., Kessler O., Herzog Y. Kluwer Academic/Plenum Publishers; 2002. The Interaction of Neuropilin-1 and Neurropilin-2 with Tyrosine-Kinase Receptors for VEGF, Advances in Experimental Medicine and Biology; pp. 81–90. [DOI] [PubMed] [Google Scholar]
- Nieminen T., Toivanen P.I., Rintanen N., Heikura T., Jauhiainen S., Airenne K.J., Alitalo K., Marjomäki V., Ylä-Herttuala S. The impact of the receptor binding profiles of the vascular endothelial growth factors on their angiogenic features. Biochim. Biophys. Acta. 2014;1840:454–463. doi: 10.1016/j.bbagen.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Nurro J., Halonen P.J., Kuivanen A., Tarkia M., Saraste A., Honkonen K., Lähteenvuo J., Rissanen T.T., Knuuti J., Ylä-Herttuala S. AdVEGF-B186 and AdVEGF-DΔNΔC induce angiogenesis and increase perfusion in porcine myocardium. Heart. 2016;102:1716–1720. doi: 10.1136/heartjnl-2016-309373. [DOI] [PubMed] [Google Scholar]
- Olofsson B., Pajusola K., Von Euler G., Chilov D., Alitalo K., Eriksson U. Genomic organization of the mouse and human genes for vascular endothelial growth factor B (VEGF-B) and characterization of a second splice isoform. J. Biol. Chem. 1996;271:19310–19317. doi: 10.1074/jbc.271.32.19310. [DOI] [PubMed] [Google Scholar]
- Rissanen T.T., Markkanen J.E., Gruchala M., Heikura T., Puranen A., Kettunen M.I., Kholová I., Kauppinen R.A., Achen M.G., Stacker S.A., et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ. Res. 2003;92:1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- Rissanen T.T., Nurro J., Halonen P.J., Tarkia M., Saraste A., Rannankari M., Honkonen K., Pietilä M., Leppänen O., Kuivanen A., et al. The bottleneck stent model for chronic myocardial ischemia and heart failure in pigs. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H1297–H1308. doi: 10.1152/ajpheart.00561.2013. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Springer; 2006. Vascular Endothelial Growth Factor Receptor-1 (VEGFR-1/Flt-1): A Dual Regulator for angiogenesis., Angiogenesis; pp. 225–230. [DOI] [PubMed] [Google Scholar]
- Tarkia M., Saraste A., Saanijoki T., Oikonen V., Vähäsilta T., Strandberg M., Stark C., Tolvanen T., Teräs M., Savunen T., et al. Evaluation of 68Ga-labeled tracers for PET imaging of myocardial perfusion in pigs. Nucl. Med. Biol. 2012;39:715–723. doi: 10.1016/j.nucmedbio.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Bridges C., Katz M.G., Korpisalo P. Angiogenic gene therapy in cardiovascular diseases: dream or vision? Eur. Heart J. 2017;38:1365–1371. doi: 10.1093/eurheartj/ehw547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Naghavi M., Allen C., Barber R.M., Bhutta Z.A., Carter A., Casey D.C., Charlson F.J., Chen A.Z., Coates M.M., et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Baker A.H. Cardiovascular gene therapy: past, present, and future. Mol. Ther. 2017;25:1095–1106. doi: 10.1016/j.ymthe.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentilin L., Puligadda U., Lionetti V., Zacchigna S., Collesi C., Pattarini L., Ruozi G., Camporesi S., Sinagra G., Pepe M., et al. Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. FASEB J. 2010;24:1467–1478. doi: 10.1096/fj.09-143180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon a request from the lead contact.
Analysis was performed without the need for original code.