Abstract
Background and Aims
Currently, insufficient clinical data are available to address whether low-level viremia (LLV) observed during antiviral treatment will adversely affect the clinical outcome or whether treatment strategies should be altered if LLV occurs. This study compared the clinical outcomes of patients with a maintained virological response (MVR) and patients who experienced LLV and their treatment strategies.
Methods
A retrospective cohort of 674 patients with chronic hepatitis B virus (HBV) infection who received antiviral treatment for more than 12 months was analyzed for the development of end-stage liver disease and treatment strategies during the follow-up period. End-stage liver disease included decompensated liver cirrhosis and hepatocellular carcinoma (HCC).
Results
During a median 42-month follow-up, end-stage liver disease developed more frequently in patients who experienced LLV than in those who experienced MVR (7.73% and 15.85% vs. 0.77% and 5.52% at 5 and 10 years, respectively; p=0.000). The trend was consistent after propensity score matching. In the high-risk group of four HCC risk models, LLV patients had a higher risk of HCC development (p<0.05). By Cox proportional hazard model analysis, LLV was an independent risk factor for end-stage liver disease and HCC (hazard ratio [HR]=6.280, confidence interval [CI]=2.081–18.951, p=0.001; HR=5.108, CI=1.392–18.737, respectively; p=0.014). Patients achieved a lower rate of end-stage liver disease by adjusting treatment compared to continuing the original treatment once LLV occurred (p<0.05).
Conclusions
LLV is an independent risk factor for end-stage liver disease and HCC, and treatment adjustments can be considered.
Keywords: Low-level viremia, Chronic hepatitis B, End-stage liver disease, Hepatocellular carcinoma, Treatment strategies
Introduction
Hepatitis B virus (HBV), a major public health problem worldwide, affecting approximately 250 to 350 million people, and it is particularly prevalent in Asia and Africa.1 Individuals with chronic HBV infection can progress to cirrhosis and/or hepatocellular carcinoma (HCC), and can eventually die of progressive liver diseases.2 The REVEAL study assessed the relationship between HBV DNA levels and the risk of cirrhosis and HCC, laying the foundation for antiviral therapy.3 Approved treatments, such as interferons (IFNs) and nucleoside/nucleotide analogs (NUCs), can suppress viral replication.1 Long-term treatment with antiviral drugs can reverse cirrhosis,4,5 decrease the incidence of HCC,6 reduce hepatic decompensation,7 and modify the natural history of decompensated cirrhosis.8
Even when using potent drugs with a high genetic barrier, some patients still maintain a low level of HBV DNA (<2,000 IU/mL), especially 20 IU/mL, which is currently recommended worldwide as the lower limit of detection. Both the USA and European guidelines state that even with first-line antiviral therapies, the complete inhibition rate of HBV DNA in treatment-naïve chronic hepatitis B (CHB) patients who are hepatitis B e antigen (HBeAg)-positive is only approximately 70%.2,9 A number of real-world studies found that 20–40% of patients who take long-term antiviral therapies remained in a state of low-level viremia (LLV) (<2,000 IU/mL).10–12
At present, insufficient clinical data have demonstrated whether LLV observed during antiviral treatment will adversely affect the clinical outcome. Whether such patients should continue treatment, switch treatments, or add another drug needs confirmation by more clinical studies. Therefore, we conducted this longitudinal study in a cohort of patients with chronic HBV infection to determine whether LLV observed during antiviral therapy promoted poor clinical outcome and to compare the clinical parameters of progression vs. nonprogression to identify potential risk factors. We also explored the impact of changing the treatment or continuing the original treatment on the long-term prognosis of LLV patients.
Methods
Patients
This retrospective observational study included 1,420 patients who met all of the following inclusion criteria: (a) aged 18 years or above; (b) chronic HBV infection defined by the presence of hepatitis B surface antigen for 6 months or by clinical history; (c) no malignancy, including HCC or development of HCC within 1 year during antiviral treatment; (d) alanine aminotransferase (ALT) >2 upper limit of normal (ULN), HBV DNA > 104 IU/mL, and received NUC treatment in the Department of Infectious Disease at two hospitals in Southwest China (The Second Affiliated Hospital of Chongqing Medical University and Guizhou Provincial People’s Hospital) from August 2006 to August 2020. We excluded 746 patients who met the following exclusion criteria: (a) IFN antiviral treatment and follow-up less than 12 months (n=298); (b) decompensated cirrhosis or liver transplantation (n=45); (c) chemotherapy or immunosuppression agent (n=23); (d) coinfection with hepatitis C virus, hepatitis D virus, or human immunodeficiency virus (n=45); and (e) missing or incomplete data on liver function or hepatitis virus infection (n=335). None of the patients had a history of smoking, alcohol abuse or metabolism disorders. Finally, a total of 674 patients with HBV infection treated with NUC for >1 year were analyzed (Supplementary Fig. 1). The study conformed to the ethical guidelines of the Declaration of Helsinki and was approved by The Ethics Committee of each hospital. Informed consent was waived because of the retrospective nature of the study, and the analysis used anonymous clinical data.
Clinical evaluation
Patient demographics and baseline characteristics were regularly recorded. We collected information including treatment-naïve status, antiviral regimen, cirrhosis, HBeAg, HBV DNA, albumin (Alb), ALT, aspartate aminotransferase (AST), total bilirubin (TBil), and serum platelet count (PLT). Peripheral blood biochemistry, immunological indicators and image testing, including transient elastography (TE), abdominal sonography, computed tomography or magnetic resonance imaging, were routinely assessed every 3–6 months.
Definition of viremia
Based on the HBV DNA levels during the follow-up period, patients were classified as LLV or MVR. In our study population, the lower limit of HBV DNA detection was 100 IU/mL due to the long study population inclusion period and the limited sensitivity of HBV DNA detection kits in southwest China at that time. Patients who experienced LLV were defined by persistent or intermittent episodes of detectable HBV DNA (<2,000 IU/mL) after 1 year of NUC treatment. Among the 203 patients with LLV, 16 never achieved a complete virological response (CVR) (<100 IU/mL), which remained between 100 and 1,999 IU/mL throughout the follow-up period (persistent LLV). The remaining 187 patients achieved CVR but had intermittent episodes of detectable HBV DNA levels in the serum (between 100 and 1,999 IU/mL, intermittent LLV). Patients who maintained MVR were defined by persistently undetectable HBV DNA (<100 IU/mL) after 1 year of NUC treatment. MVR was observed for 471 patients.
Clinical outcome
The primary outcome was the development of end-stage liver disease after 1 year of NUC treatment. The follow-up period was calculated as time elapsed between commencement of antiviral treatment and the last date of NUC therapy or the last date of follow-up (reference August 31, 2020), whichever came first. End-stage liver disease included decompensated liver cirrhosis and HCC. Liver cirrhosis was defined by the presence of atrophy or a nodular liver parenchyma pattern with or without splenomegaly from the ultrasound examination or the existence of esophageal varices or gastric varices observed during upper endoscopy.13 Compensatory cirrhosis was defined by the absence of severe complications, such as ascites, esophageal and gastric varices bleeding or hepatic encephalopathy, with mostly Child-Pugh A liver function. Decompensated cirrhosis was diagnosed as cirrhosis once severe complications such as ascites, esophageal and gastric varices hemorrhage or hepatic encephalopathy occurred, with mostly Child-Pugh B or C liver function.14 HCC diagnosis was based on histology findings in liver biopsy or imaging, including computed tomography or magnetic resonance imaging, accompanied by tumor markers.15
The secondary endpoint of this study was cirrhosis improvement after long-term antiviral treatment. Improvement of liver cirrhosis was observed when the imaging did not indicate the presence of cirrhosis and the liver stiffness measurement (LSM) was <7.4 kPa (TBIL≤ULN, ALT≤5ULN) or the LSM was <6 kPa (TBIL, ALT≤ULN) after long-term antiviral therapy.13,16
The last endpoint of this study was comparison of the long-term outcome of LLV patients who changed treatments or continued with the original treatment.
HCC risk scores and cutoff values for risk stratification
We adopted four published HCC risk scores: CU-HCC, GAG-HCC, REACH-B, and PAGE-B, which were based on patients’ clinical and laboratory parameters (Supplementary Table 1). Patients were divided into low- and high-risk HCC by cutoff values of 5, 82, 8 and 10 for CU-HCC, GAG-HCC, REACH-B and PAGE-B scores, respectively, according to previous studies.17–20
Laboratory methods
Routine liver biochemical tests and platelet counts were performed using commercially available autoanalyzers, and hepatitis serological markers were assayed using commercially available enzyme-linked immunoassays. According to the instructions of the manufacturer, the ULN for ALT was 40 U/L. HBV DNA was detected by real-time PCR assay, and the detection limit was 100 IU/mL. HBeAg was measured by electrochemiluminescence immunoassay.
LSMs were performed by experienced operators using TE (Fibroscan; Echosens, Paris, France), based on standard procedures.21 Only LSM values with 10 valid measurements, a success rate of 60%, and an interquartile range (IQR) and median of 30% were considered reliable.
Statistical analysis
The chi-square test or Fisher’s exact test was used to compare frequencies and proportions. Continuous variables were expressed as the median and interquartile range (25th to 75th percentile) and compared by the Mann-Whitney U test. Univariate logistic regression analysis was used to compare the difference in clinical data between MVR and LLV. Multivariate logistic regression analysis was performed on all the factors associated with LLV in the univariate analysis (2-sided p-value of <0.05). The cumulative rates of newly developed end-stage liver disease (decompensated cirrhosis and HCC) and reverse liver cirrhosis during NUC treatment between MVR and LLV were plotted using a Kaplan-Meier curve and compared using the log-rank test. A Cox proportional hazard model was used to analyze factors associated with newly developed end-stage liver disease and HCC, and significant factors with p of <0.1 in the univariate analysis were subjected to multivariate analysis to determine independent predictive factors.
To reduce the impact of potential confounding effects between groups, significant differences in baseline characteristics were adjusted by propensity score (PS) matching. We used nearest-neighbor matching with a caliper size of 0.02 and matched the patients in a 1:1 ratio.
Statistical tests were performed using the IBM SPSS Statistics package for Windows (Armonk, NY, USA), version 25.0. Statistical significance was defined as two-sided p-values of <0.05.
Results
Comparison of patient characteristics at baseline between LLV and MVR
The baseline characteristics of the 674 patients are shown in Table 1. All patients continued to take oral antiviral drug throughout the follow-up period. During a median follow-up period of 42 (27, 61) months, LLV was found in 203 of the 674 patients with HBV infection (30.12%). The LLV group had a higher proportion of HBeAg positivity, higher HBV DNA levels, higher albumin levels, lower ALT and AST levels and a lower proportion using first-line drugs (p<0.05).
Table 1. Characteristics of the study population at baseline.
| All (n=674) | LLV (n=203) | MVR (n=471) | p | |
|---|---|---|---|---|
| Age, years | 44 (34, 52) | 44 (35, 52) | 44 (34, 52) | 0.199 |
| Male, % | 488 (72.4%) | 153 (75.4%) | 335 (71.1%) | 0.258 |
| HBeAg positive, % | 380 (56.7%) | 136 (67.0%) | 244 (52.2%) | 0.000 |
| HBV DNA, log10 IU/L | 5.64 (3.99, 6.90) | 6.11 (4.29, 7.30) | 5.44 (3.85, 6.76) | 0.001 |
| Platelet, 109/L | 153 (108, 191) | 150 (100, 188) | 154 (112, 192) | 0.279 |
| ALT, U/L | 80 (40, 170) | 68 (38, 115) | 89 (40, 188) | 0.003 |
| AST, U/L | 55 (34, 104) | 48 (34, 74) | 59 (34, 121) | 0.002 |
| Bilirubin, mmol/L | 14.9 (11.20, 20.25) | 15.15 (11.60, 20.65) | 14.70 (11.00, 20.10) | 0.373 |
| Albumin, mg/L | 44.70 (42.60, 46.75) | 45.05 (42.98, 47.63) | 44.60 (42.30, 46.40) | 0.016 |
| Cirrhosis, % | 200 (29.7%) | 60 (29.6%) | 140 (29.7%) | 0.965 |
| Treatment-naïve, % | 545 (80.9%) | 158 (77.8%) | 387 (82.2%) | 0.190 |
| Treatment | 0.000 | |||
| First-line drugs, % | 499 (74.0%) | 118 (58.1%) | 381 (80.9%) | |
| Non-first-line drugs, % | 157 (23.3%) | 73 (36.0%) | 84 (17.8%) | |
| Non-first-line drugs combined with first-line drugs, % | 18 (2.7%) | 12 (5.9%) | 6 (1.3%) |
LLV, low-level viremia; MVR, maintained virological response; HBeAg, hepatitis B e antigen; HBV, hepatitis B virus; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Comparison of the long-term outcome between LLV patients and MVR patients
LLV patients had a higher risk of end-stage liver disease
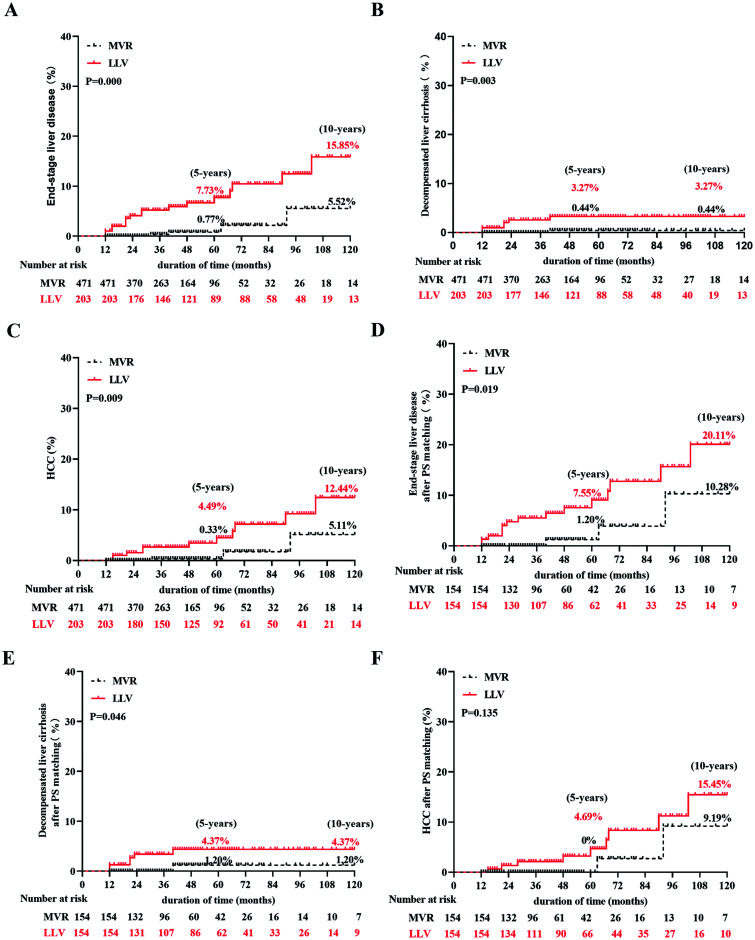
When comparing the long-term outcome during the 10-year follow-up, we found that 16 (2.37%) of the 674 patients developed decompensated cirrhosis and 14 (2.08%) developed HCC. Thirteen (81.25%) cases of decompensated cirrhosis and 11 (78.57%) cases of HCC occurred in the LLV group (2 patients with persistent LLV and 9 patients with intermittent LLV). Because of the small number of patients with intermittent LLV, we performed a follow-up analysis of patients with persistent and intermittent LLV as a whole. Cumulative incidence rates of end-stage liver disease were 0.77% and 5.52% at 5 and 10 years in patients with MVR, respectively, which were lower than those in patients with LLV (7.73% and 15.85% at 5 and 10 years, respectively; p=0.000; Fig. 1A). In addition, the cumulative incidence rates of decompensated cirrhosis and HCC were lower in patients with MVR than in patients with LLV (0.44% and 0.44% vs. 3.27% and 3.27% in decompensated cirrhosis at 5 and 10 years, 0.33% and 5.11% vs. 4.49% and 12.44% in HCC at 5 and 10 years, respectively; p=0.003, p=0.009; Fig. 1B, C). To reduce the impact of potential confounding effects between groups, we used PS matching to yield 154 matched pairs of patients from the MVR and LLV groups. Within this matched cohort, there were no significant differences between the MVR and LLV groups in any baseline characteristic (Supplementary Table 2). The long-term outcomes, including the development of end-stage liver disease and decompensated cirrhosis, were consistent with previous analyses after PS matching (p=0.019 in end-stage liver disease; p=0.046 in decompensated cirrhosis; Fig. 1D, E). No significant difference was found in HCC occurrence between the two groups after PS matching (p=0.135; Fig. 1F). We also compared the risk of LLV and end-stage liver disease between patients treated with entecavir (ETV) and patients treated with tenofovir disoproxil fumarate (TDF). Among the 497 patients who received first-line drugs, 77 patients used TDF and 420 used ETV. The proportion of LLV and the risk of developing end-stage liver disease were similar in both groups during a nearly 60-month follow-up (p>0.05; Supplementary Fig. 2).
Fig. 1. Cumulative incidence rates of long-term clinical outcome according to virological response.
(A) End-stage liver disease. (B) Decompensated cirrhosis. (C) HCC. (D) End-stage liver disease after PS matching. (E) Decompensated cirrhosis after PS matching. (F) HCC after PS matching. LLV, low-level viremia; MVR, maintained virological response; HCC, hepatocellular carcinoma; PS matching, propensity score matching.
LLV patients had a higher risk of HCC in the high-risk group of four HCC risk models
Because HCC variability occurred significantly between pre-and post-PS matching in patients with LLV and patients with MVR, to further compare the risk of HCC, we introduced four HCC risk models. We screened high-risk populations for HCC between the MVR group and LLV group based on the predefined cutoff values of four HCC risk models (CU-HCC, GAG-HCC, REACH-B and PAGE-B) and compared their risk of developing HCC. Within the CU-HCC model, the cumulative incidence rates of HCC were 0.78% and 9.31% at 5 and 10 years in patients with MVR, respectively, which were lower than those in patients with LLV (10.91% and 24.91% at 5 and 10 years, respectively; p=0.006; Fig. 2A). The cumulative incidence rates of HCC between the MVR and LLV groups in the other three models were similar to those in the previous model (p=0.008 within GAG-HCC, p=0.009 within REACH-B, p=0.008 within PAGE-B, respectively; Fig. 2B–D). Thus, we found significant differences in virological response states for the development of HCC in high-risk patients.
Fig. 2. Cumulative incidence rates of HCC in the high-risk group of four HCC risk models.
(A) HCC in the CU-HCC model. (B) HCC in the GAG-HCC model. (C) HCC in the PEACH-B model. (D) HCC in the PAGE-B model. LLV, low-level viremia; MVR, maintained virological response; HCC, hepatocellular carcinoma.
Subgroup analyses showed patients with cirrhosis had a poor long-term outcome in the LLV group
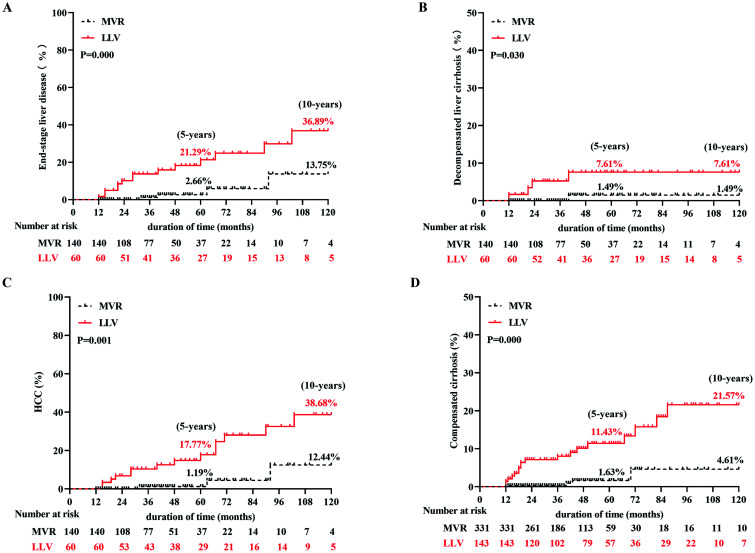
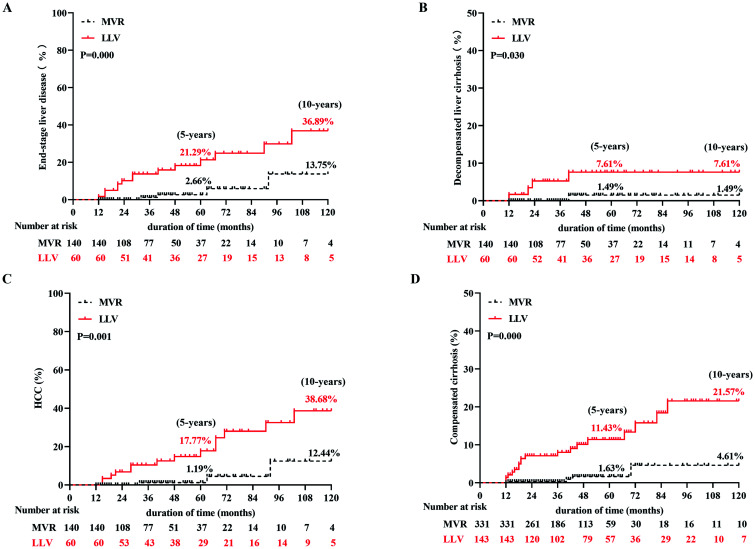
Of the 200 patients with compensatory cirrhosis, MVR was obtained in 140 (70%) patients. Among these patients, end-stage liver disease developed in 18 (9%) (5 patients with decompensated cirrhosis and 13 patients with HCC). The cumulative incidence rates of end-stage liver disease, including decompensated cirrhosis and HCC, were lower in patients with MVR than in patients with LLV (2.66% and 13.75% vs. 21.29% and 36.89% in end-stage liver disease at 5 and 10 years, 1.49% and 1.49% vs. 7.61% and 7.61% in decompensated cirrhosis at 5 and 10 years, 1.19% and 12.44% vs. 17.77% and 38.68% in HCC at 5 and 10 years, respectively; p=0.000, p=0.030, p=0.001; Fig. 3A–C). In addition, of the 474 patients with chronic hepatitis, 331 (69.83%) patients obtained MVR and 143 (30.17%) patients developed LLV. No patient in the MVR group developed end-stage liver disease, while only single (0.7%) patient in the LLV group developed HCC; however, 22 (4.64%) patients developed compensatory cirrhosis, including 4 (1.21%) in the MVR group and 18 (12.59%) in the LLV group. The cumulative incidence rates of compensated cirrhosis were lower in patients with MVR than in patients with LLV (1.63% and 4.61% vs. 11.43% and 21.57% at 5 and 10 years, respectively, p=0.000; Fig. 3D). In general, the clinical outcome was better in the MVR group.
Fig. 3. Cumulative incidence rates of long-term clinical outcome according to virological response in compensatory cirrhosis and chronic hepatitis patients.
(A) End-stage liver disease in compensatory cirrhosis patients. (B) Decompensated cirrhosis in compensatory cirrhosis patients. (C) HCC in compensatory cirrhosis patients. (D) Compensated cirrhosis in chronic hepatitis patients. LLV, low-level viremia; MVR, maintained virological response; HCC, hepatocellular carcinoma.
A higher percentage of cirrhosis patients in the MVR group reversed after long-term antiviral therapy
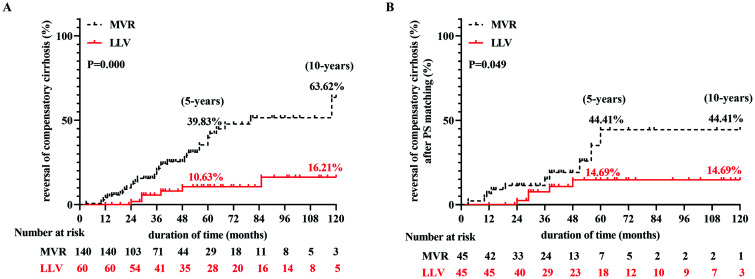
Among the 200 patients with cirrhosis, 44 (22%) showed reversal after long-term antiviral therapy. Of these, 39 (27.86%) patients were in the MVR group. A comparison of the incidence of cirrhosis reversal in the compensatory period between the two groups revealed that the incidence was 39.83% and 63.62% at 5 and 10 years, respectively, in the MVR group which was higher than the LLV group (10.63% and 16.21% at 5 and 10 years, respectively; p=0.000; Fig. 4A). The trend after PS matching was the same as the overall trend (44.41% and 44.41% vs. 14.69% and 14.69% at 5 and 10 years, respectively, p=0.014; Fig. 4B, Supplementary Tables 3, 4). Therefore, continuous MVR is helpful in reversing the disease.
Fig. 4. Cumulative incidence rates of reversed cirrhosis after long-term antiviral therapy.
(A) Reversal of compensatory cirrhosis. (B) Reversal of compensatory cirrhosis after PS matching. LLV, low-level viremia; MVR, maintained virological response; PS matching, propensity score matching.
Independent risk factors for LLV, end-stage liver disease and HCC
Treatment containing non-first-line drugs, lower ALT and higher HBV DNA levels at baseline, and HBV DNA levels at 6 months were independent risk factors for LLV
During follow-up, LLV was observed in 203 patients (30.12%). The treatment regimen, ALT and HBV DNA levels at baseline, HBeAg status and HBV DNA levels at 6 months were associated with LLV in unadjusted analysis. The incidence of LLV was higher in patients on non-first-line drugs than first-line drugs (p=0.000), in patients with an intermediate ALT level (<100 U/L) than in patients with high ALT levels (≥100 U/L, p=0.000), in patients with a high viral load (>6 log10 IU/L) than in patients with an intermediate viral load (≤6 log10 IU/L, p=0.007), in patients who were HBeAg-positive than in patients who were HBeAg-negative (p=0.000) and in patients with HBV DNA ≥3 log10 IU/L than in patients with HBV DNA <3 log10 IU/L at 6 months (p=0.000). In multivariable logistic regression models, treatment regimen, ALT, HBV DNA levels at baseline and HBV DNA levels at 6 months were the independent factors associated with LLV (p<0.05) (Table 2).
Table 2. Factors associated with LLV.
| Univariate |
Multivariable model |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Male sex | 0.805 | 0.552–1.173 | 0.259 | |||
| Age | 1.009 | 0.995–1.023 | 0.207 | |||
| Treatment-naïve | 1.312 | 0.874–1.971 | 0.190 | |||
| Treatment | ||||||
| First-line drugs | reference | reference | ||||
| Non-first-line drugs | 2.806 | 1.928–4.085 | 0.000 | 2.929 | 1.925–4.455 | 0.000 |
| Non-first-line drugs combined with first-line drugs | 6.458 | 2.372–17.580 | 0.000 | 5.846 | 2.014–16.970 | 0.001 |
| Baseline ALT<100, U/L | 1.946 | 1.366–2.773 | 0.000 | 2.544 | 1.680–3.852 | 0.000 |
| Baseline HBV DNA > 6 log10, IU/L | 1.578 | 1.133–2.198 | 0.007 | 2.200 | 1.437–3.370 | 0.000 |
| Cirrhosis | 0.992 | 0.692–1.423 | 0.965 | |||
| HBeAg-positive | 1.855 | 1.315–2.618 | 0.000 | 1.400 | 0.936–2.094 | 0.102 |
| Month 6 HBV DNA >3 log10, IU/L | 5.609 | 3.107–10.126 | 0.000 | 3.343 | 1.754–6.372 | 0.000 |
LLV, low-level viremia; OR, odds ratio; CI, confidence interval; ALT, alanine aminotransferase; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen.
LLV and cirrhosis were independent risk factors for end-stage liver disease and HCC
Independent risk factors of end-stage liver disease are helpful to reduce adverse prognosis. In this study, age, PLT count at baseline, LLV and cirrhosis were predictive factors of end-stage liver diseases in the univariate analysis. In the multivariable Cox regression analysis, all the factors that were p<0.1 in the univariate analysis were included in the multivariable analyses, including male sex, age, PLT count at baseline, LLV, and cirrhosis. LLV and cirrhosis were independent risk factors for end-stage liver disease (hazard ratio [HR]=6.280, confidence interval [CI]=2.081–18.951, p=0.001; HR=6.378, CI=1.623–25.074, p=0.008, respectively). In HCC patients, age, PLT count at baseline, LLV and cirrhosis were also predictive factors in the univariate analysis. LLV and cirrhosis were significantly associated with a higher risk of HCC. (HR=5.108, CI=1.392–18.737, p=0.014; HR=18.316, CI=2.005–167.307, p=0.010, respectively; Table 3).
Table 3. Risk of end-stage liver disease and HCC.
| End-stage liver disease |
HCC |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariable model |
Univariate |
Multivariable model |
|||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Male sex | 6.575 (0.881–49.053) | 0.066 | 5.298 (0.692–40.526) | 0.108 | 30.721 (0.152–6,191.832) | 0.206 | ||
| Age | 1.060 (1.024–1.097) | 0.001 | 1.039 (0.997–1.083) | 0.067 | 1.062 (1.018–1.109) | 0.005 | 1.033 (0.982–1.088) | 0.211 |
| Treatment-naïve | 1.094 (0.400–2.994) | 0.861 | 1.301 (0.407–4.161) | 0.658 | ||||
| Treatment | 1.026 (0.466–2.262) | 0.949 | 1.222 (0.459–3.255) | 0.688 | ||||
| Baseline ALT, U/L | 0.527 (0.191–1.453) | 0.216 | 0.996 (0.988–1.004) | 0.314 | ||||
| Baseline HBV DNA, log10IU/L | 1.139 (0.472–2.749) | 0.773 | 0.915 (0.317–2.639) | 0.869 | ||||
| PLT, 109/L | 0.982 (0.972–0.991) | 0.000 | 0.991 (0.980–1.003) | 0.146 | 0.982 (0.971–0.994) | 0.003 | 0.996 (0.982–1.010) | 0.569 |
| HBeAg-positive | 1.622 (0.682–3.854) | 0.274 | 2.119 (0.709–6.332) | 0.179 | ||||
| LLV | 6.695 (2.225–20.141) | 0.001 | 6.280 (2.081–18.951) | 0.001 | 4.847 (1.323–17.756) | 0.017 | 5.108 (1.392–18.737) | 0.014 |
| Cirrhosis | 13.271 (3.902, 45.137) | 0.000 | 6.378 (1.623–25.074) | 0.008 | 27.168 (3.546–208.166) | 0.001 | 18.316 (2.005–167.307) | 0.010 |
HCC, hepatocellular carcinoma; HR, hazard ratio; CI, confidence interval; ALT, alanine aminotransferase; HBV, hepatitis B virus; PLT, platelets; HBeAg, hepatitis B e antigen; LLV, low-level viremia.
Changing the treatment of LLV patients can enhance the CVR rate
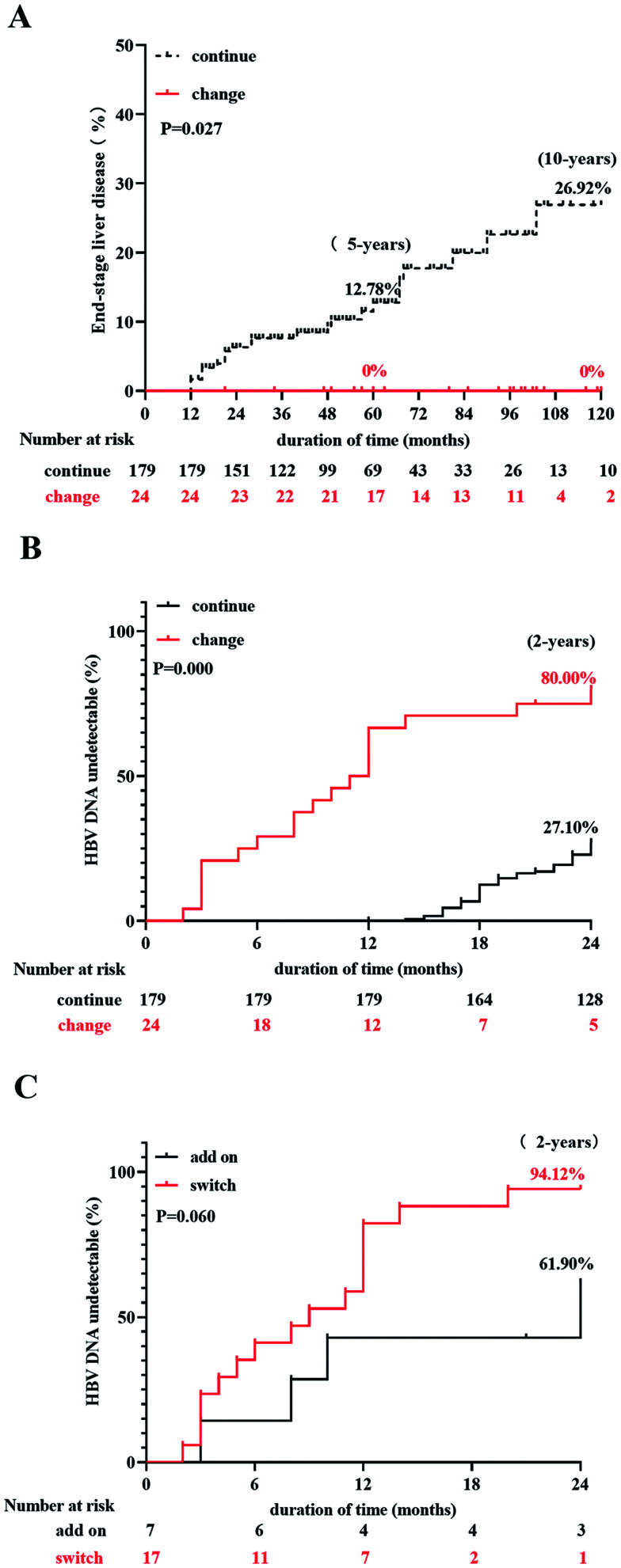
Of the 203 patients with LLV, 24 changed their treatment plans when the HBV DNA was detectable (100–1,999 IU/mL). These patients were not tested for drug resistance sites. The cumulative incidence rate of end-stage liver disease was 0% and 0% at 5 and 10 years in patients with changed treatment, respectively, which were lower than those in patients who continued the original treatment (12.78% and 26.95% at 5 and 10 years, respectively, p=0.027; Fig. 5A). The cumulative incidence rate of complete inhibition of HBV DNA was 80.00% at 24 months in patients who changed treatments, which was higher than that in patients who continued with the original treatment (27.10% at 24 months, p=0.000; Fig. 5B). Seven of 24 patients who changed the treatment took another medicine plus the original regimen. When comparing the difference in the complete inhibition rate of HBV DNA between patients who changed and added a drug, we found no statistical difference between the two groups (p=0.084; Fig. 5C). The current results were only a preliminary comparison because of possible bias due to the small number of patients that changed and added drugs to the original regimen. The change group included the switch to ETV from adefovir (ADV) in five patients and ETV+ADV to ETV+TDF in five patients. Lamivudine (LAM)+ADV combination therapy was replaced by ETV monotherapy in two patients, LAM was changed to ETV+ADV in one patient, LDT was changed to TDF in two patients, and ETV was changed to TDF in two patients. The addition group included switching ETV to ETV+ADV in four patients, ADV+ETV combined therapy instead of ADV monotherapy in one patient, switching from LDT monotherapy to LDT+ADV combined therapy in one patient and switching from LDT monotherapy to LDT+ADV and then to ETV+TDF in one patient.
Fig. 5. Comparison of treatment regimen on patient outcomes.
(A) Adjusted treatment vs. continuation of original treatment. (B) Complete virological suppression rate between original treatment continuation vs. adjusted treatment. (C) CVR between switching a drug and adding a drug. LLV, low-level viremia; MVR, maintained virological response; CVR, complete virological response.
Discussion
Through a systematic retrospective analysis of previous studies, we found that the rate of achieved hepatitis B surface antigen (commonly known as HBsAg) loss was very low regardless of whether IFNs or IFNs combined with NUC were used.22 Therefore, maintaining the virological response of HBV DNA remains our top research focus. Real-world studies have suggested that 20-40% of patients will still develop LLV, even with first-line drugs.10–12 In this study, 30.12% of patients developed LLV, and end-stage liver disease developed more frequently in patients who experienced LLV than MVR (7.73% and 15.85% vs. 0.77% and 5.52% at 5 and 10 years, respectively, p=0.000), indicating that LLV is harmful. In addition, whether differences in efficacy exist between first-line drugs remains a popular research topic. In this study, no significant difference was found in the risk of LLV and the development of end-stage liver disease between the two groups, which is consistent with some previous clinical studies and systematic retrospective analyses.23–25
Patients with cirrhosis and patients in high-risk HCC models had a significantly increased risk of developing HCC in the LLV group than in the MVR group, which is consistent with previous studies.3,7,10 Although current guidelines recommend antiviral therapy for cirrhosis patients regardless of baseline HBV DNA levels, we should focus on the virological response of this population because they are likely to get the most benefit from MVR.2,9,26 As long as LLV occurs, the long-term outcome of patients is not as good as MVR patients, regardless of whether first-line drugs or non-first-line drugs are used. Therefore, monitoring HBV DNA levels should be emphasized in clinical practice.
We also found that the cirrhosis improvement rate was lower in LLV patients during long-term treatment. During antiviral therapy, the fibrosis burden is known to be a dynamic process, and fibrosis regression has been reported following extended NUC treatment;16 however, LLV is related to persistent low-grade inflammation27,28 and may affect dynamic changes in liver fibrosis. Therefore, a high percentage of patients with liver cirrhosis had low levels of HBV DNA detected during follow-up. Maintaining a long-term virological response during NUC treatment is beneficial for reversing liver fibrosis.
We then evaluated factors associated with LLV. Patients with high levels of ALT, moderate HBV viral load, potent antiviral drugs and DNA levels dropping below 3 log10 after 6 months of treatment were more likely to obtain MVR, which is consistent with prior reports.29–32 LLV tended to occur when using non-first-line drugs or when first-line drugs were combined with non-first-line drugs. These findings confirmed the existing guideline recommendations of first-line treatment agents for CHB patients.2,9,13 Higher ALT levels at baseline may indicate higher host immune activity that assists in viral clearance.33 At the same time, patients with a high viral load were also more prone to a poor response.34 We also found that LLV was an independent risk factor for the development of end-stage liver disease and HCC by multivariable Cox regression model analysis. Another factor affecting the progression of liver disease was cirrhosis, which has been a well-known risk factor for the development of HCC. Therefore, obtaining a complete virological response may benefit more patients .
When a patient develops LLV, whether they should continue monotherapy or switch to other drugs is inconclusive. Guidelines recommend that patients with partial virological responses (also referred to as PVR) who used non-first-line medication should switch to the most effective antiviral agent that does not share cross-resistance.2,9,35,36 The American Association for the Study of Liver Diseases (i.e. AASLD) recommendation for patients with LLV suggests that patients treated with ETV or TDF monotherapy should continue monotherapy, although the quality and certainty of evidence are low.9 The European Association for the Study of the Liver (i.e. EASL) do not recommend changing the initial treatment strategy in patients with low levels (HBV DNA <69 IU/mL) and/or declining HBV DNA concentrations on potent NUC monotherapy; if HBV DNA is in a platform state (69<DNA<2,000 IU/mL), the possibility of switching to another drug or a combination of ETV+TDF/TAF should be considered.2 In our study, 203 patients in the LLV group had intermittent HBV DNA detection, so only 24 patients changed their treatment regimen. We found that patients who changed their treatment were more likely to achieve complete virological suppression than those who continued the original treatment, and their long-term clinical outcome was better. At the same time, we also found no significant difference in the CVR between switching to another drug and adding on a drug; however, due to our limited data, these conclusions may be biased and require further validation in a larger population. In a recent study in which patients with LLV were treated with ETV or NUC combination therapy, almost all patients achieved complete virological suppression when switching to TAF treatment after 48 weeks, suggesting that patients with LLV need more effective treatment.11 The guidelines and recent research have provided valuable references for the follow-up treatment of LLV patients but more clinical studies are needed.
This study has a few limitations. First, this study involved a limited number of cases. A large-sample prospective study needs to be performed for further confirmation. Second, we did not use the internationally recommended 20 IU/mL as the lower detection limit of HBV DNA due to the limitation of the sensitivity of HBV DNA testing at that time, but this did not affect the analysis or final conclusions.
In conclusion, this retrospective and real-world cohort demonstrated the importance of MVR during NUC therapy. LLV was associated with an increased risk of end-stage liver disease. In particular, cirrhosis patients who had LLV had a significantly increased risk of developing HCC, which indicates that LLV is harmful. Changing the treatment plan was an effective way to treat LLV. More prospective studies are needed to clarify the treatment plan for this patient population in the future.
Supporting information
A total of 674 adult patients with hepatitis B virus infection treated with NUCs for more than 1 year were analyzed. LLV, low-level viremia; MVR, maintained virological response; ETV, entecavir; TDF, tenofovir disoproxil fumarate; ADV, adefovir; LAM, lamivudine; LDT, telbivudine.
A. The proportion of LLV in the treatment of ETV or TDF. B. The risk of end-stage liver disease in the ETV or TDF group. LLV, low-level viremia; MVR, maintained virological response; ETV, entecavir; TDF, tenofovir disoproxil fumarate.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ADV
adefovir
- Alb
albumin
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CHB
chronic hepatitis B
- CI
confidence interval
- CVR
complete virological response
- EASL
European Association for the Study of the Liver
- ETV
entecavir
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- HBV
chronic hepatitis B virus
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- IFNs
interferons
- IQR
interquartile range
- LAM
lamivudine
- LDT
telbivudine
- LLV
low-level viremia
- LSM
liver stiffness measurement
- MVR
maintained virological response
- NUCs
nucleoside/nucleotide analogs
- OR
odds ratio
- PLT
platelet
- PS matching
propensity score matching
- PVR
partial virological responses
- TBIL
total bilirubin
- TE
transient elastography
- TDF
tenofovir disoproxil fumarate
- ULN
upper limit of normal
Data sharing statement
No additional data are available.
References
- 1.Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. Lancet. 2014;384(9959):2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 4.Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51(2):422–430. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 5.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 6.Singal AK, Salameh H, Kuo YF, Fontana RJ. Meta-analysis: the impact of oral anti-viral agents on the incidence of hepatocellular carcinoma in chronic hepatitis B. Aliment Pharmacol Ther. 2013;38(2):98–106. doi: 10.1111/apt.12344. [DOI] [PubMed] [Google Scholar]
- 7.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351(15):1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 8.Jang JW, Choi JY, Kim YS, Woo HY, Choi SK, Lee CH, et al. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology. 2015;61(6):1809–1820. doi: 10.1002/hep.27723. [DOI] [PubMed] [Google Scholar]
- 9.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Clin Liver Dis (Hoboken) 2018;12(1):33–34. doi: 10.1002/cld.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JH, Sinn DH, Kang W, Gwak GY, Paik YH, Choi MS, et al. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology. 2017;66(2):335–343. doi: 10.1002/hep.28916. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa E, Nomura H, Nakamuta M, Furusyo N, Koyanagi T, Dohmen K, et al. Tenofovir alafenamide after switching from entecavir or nucleos(t)ide combination therapy for patients with chronic hepatitis B. Liver Int. 2020;40(7):1578–1589. doi: 10.1111/liv.14482. [DOI] [PubMed] [Google Scholar]
- 12.Xing M, Sen L, Jane D, Andrew GI. Chronic hepatitis B management in clinical practice in Fuzhou Province, China: retrospective cross-sectional analysis of electronic medical record data. Abstracts. Hepatol Int. 2020;14:1–470. doi: 10.1007/s12072-020-10030-4. [DOI] [Google Scholar]
- 13.Chinese Society of Hepatology, Chinese Medical Association Chinese guidelines on the management of liver cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2019;27(11):846–865. doi: 10.3760/cma.j.issn.1007-3418.2019.11.008. Chinese. [DOI] [PubMed] [Google Scholar]
- 14.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Kanwal F, Singal AG. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology. 2019;157(1):54–64. doi: 10.1053/j.gastro.2019.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YP, Liang XE, Zhang Q, Peng J, Zhu YF, Wen WQ, et al. Larger biopsies evaluation of transient elastography for detecting advanced fibrosis in patients with compensated chronic hepatitis B. J Gastroenterol Hepatol. 2012;27(7):1219–1226. doi: 10.1111/j.1440-1746.2012.07122.x. [DOI] [PubMed] [Google Scholar]
- 17.Wong VW, Chan SL, Mo F, Chan TC, Loong HH, Wong GL, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol. 2010;28(10):1660–1665. doi: 10.1200/JCO.2009.26.2675. [DOI] [PubMed] [Google Scholar]
- 18.Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50(1):80–88. doi: 10.1016/j.jhep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12(6):568–574. doi: 10.1016/S1470-2045(11)70077-8. [DOI] [PubMed] [Google Scholar]
- 20.Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64(4):800–806. doi: 10.1016/j.jhep.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 21.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48(5):835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Qiu K, Liu B, Li SY, Li H, Chen ZW, Luo AR, et al. Systematic review with meta-analysis: combination treatment of regimens based on pegylated interferon for chronic hepatitis B focusing on hepatitis B surface antigen clearance. Aliment Pharmacol Ther. 2018;47(10):1340–1348. doi: 10.1111/apt.14629. [DOI] [PubMed] [Google Scholar]
- 23.Idilman R, Gunsar F, Koruk M, Keskin O, Meral CE, Gulsen M, et al. Long-term entecavir or tenofovir disoproxil fumarate therapy in treatment-naïve chronic hepatitis B patients in the real-world setting. J Viral Hepat. 2015;22(5):504–510. doi: 10.1111/jvh.12358. [DOI] [PubMed] [Google Scholar]
- 24.Kim SU, Seo YS, Lee HA, Kim MN, Lee YR, Lee HW, et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naïve chronic hepatitis B in South Korea. J Hepatol. 2019;71(3):456–464. doi: 10.1016/j.jhep.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Liu X, Dang Z, Yu L, Jiang Y, Wang X, et al. Nucleos(t)ide analogues for reducing hepatocellular carcinoma in chronic hepatitis B patients: a systematic review and meta-analysis. Gut Liver. 2020;14(2):232–247. doi: 10.5009/gnl18546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia JD, Hou JL, Wei L, Zhuang H. Highlights of the guidelines of prevention and treatment for chronic hepatitis B (2019 version) Zhonghua Gan Zang Bing Za Zhi. 2020;28(1):21–23. doi: 10.3760/cma.j.issn.1007-3418.2020.01.006. Chinese. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, Wu X, Zhou J, Meng T, Wang B, Chen S, et al. Persistent low level of hepatitis B virus promotes fibrosis progression during therapy. Clin Gastroenterol Hepatol. 2020;18(11):2582–2591.e6. doi: 10.1016/j.cgh.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Lok AS, Hadziyannis SJ, Weller IV, Karvountzis MG, Monjardino J, Karayiannis P, et al. Contribution of low level HBV replication to continuing inflammatory activity in patients with anti-HBe positive chronic hepatitis B virus infection. Gut. 1984;25(11):1283–1287. doi: 10.1136/gut.25.11.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu H, Geng DY, Shen F, Zhang JY, Lu B, Ma LX. Optimization of adefovir therapy in chronic hepatitis B according to baseline predictors and on-treatment HBV DNA: a 5-year prospective study. Virol J. 2011;8:444. doi: 10.1186/1743-422X-8-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuen MF, Fung J, Seto WK, Wong DK, Yuen JC, Lai CL. Combination of baseline parameters and on-treatment hepatitis B virus DNA levels to start and continue patients with lamivudine therapy. Antivir Ther. 2009;14(5):679–685. [PubMed] [Google Scholar]
- 31.Yoo EH, Cho HJ. Clinical response to long-term tenofovir monotherapy in Korean chronic hepatitis B patients. Clin Chim Acta. 2017;471:308–313. doi: 10.1016/j.cca.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Lovett GC, Nguyen T, Iser DM, Holmes JA, Chen R, Demediuk B, et al. Efficacy and safety of tenofovir in chronic hepatitis B: Australian real world experience. World J Hepatol. 2017;9(1):48–56. doi: 10.4254/wjh.v9.i1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Le AK, Chaung KT, Henry L, Hoang JK, Cheung R, et al. Fatty liver is not independently associated with the rates of complete response to oral antiviral therapy in chronic hepatitis B patients. Liver Int. 2020;40(5):1052–1061. doi: 10.1111/liv.14415. [DOI] [PubMed] [Google Scholar]
- 34.Desalegn H, Aberra H, Berhe N, Mekasha B, Stene-Johansen K, Krarup H, et al. Treatment of chronic hepatitis B in sub-Saharan Africa: 1-year results of a pilot program in Ethiopia. BMC Med. 2018;16(1):234. doi: 10.1186/s12916-018-1229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association The guidelines of prevention and treatment for chronic hepatitis B (2019 version) Zhonghua Gan Zang Bing Za Zhi. 2019;27(12):938–961. doi: 10.3760/cma.j.issn.1007-3418.2019.12.007. Chinese. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A total of 674 adult patients with hepatitis B virus infection treated with NUCs for more than 1 year were analyzed. LLV, low-level viremia; MVR, maintained virological response; ETV, entecavir; TDF, tenofovir disoproxil fumarate; ADV, adefovir; LAM, lamivudine; LDT, telbivudine.
A. The proportion of LLV in the treatment of ETV or TDF. B. The risk of end-stage liver disease in the ETV or TDF group. LLV, low-level viremia; MVR, maintained virological response; ETV, entecavir; TDF, tenofovir disoproxil fumarate.