Abstract
Purpose
To assess the effect of high ovarian response on oocyte quality and ovarian stimulation cycle outcomes.
Methods
A retrospective cohort study conducted at three IVF units. The high ovarian response (HOR) and polycystic ovary syndrome (PCOS) with HOR (PCOS HOR) groups included 151 and 13 women who underwent controlled ovarian stimulation (COS) resulting in more than 15 retrieved oocytes, for a total of 1863 and 116 cultured embryos, respectively. The normal ovarian response (NOR) group comprised 741 women with 6–15 retrieved oocytes, resulting in 4907 cultured embryos. Data collected included fresh cycle data and pregnancy rates, in addition to annotation of morphokinetic events from time of pronuclei fading to time of initiation of blastocyst formation of embryos cultured in a time lapse incubator, including occurrence of direct unequal cleavage at first cleavage (DUC-1) (less than 5 h from two to three blastomeres). Comparison was made between morphokinetic parameters between the 3 groups. Cycle outcomes were compared in the high vs. normal ovarian response groups.
Results
Oocyte maturation rate was significantly lower in the HOR vs. NOR groups (56.5% vs. 90.0%, p < 0.001), while the fertilization rates were similar (60.2% vs. 58.1%, p = 0.397). The prevalence of DUC-1 embryos was higher in the PCOS HOR and the HOR groups as compared to the NOR group (22.7% vs. 16.2% and 12.0%, respectively, p < 0.001). After exclusion of DUC-1 embryos, remaining embryos from the NOR and HOR groups reached the morphokinetic milestones at similar rates, with comparable implantation and clinical pregnancy rates, while the PCOS HOR showed shorter time to 5 blastomeres compared to the NOR and HOR groups.
Conclusions
High ovarian response might be associated with decreased oocyte quality, manifested as a higher proportion of immature oocytes and higher rate of direct uneven cleavage embryos, while embryos exhibiting normal first cleavage have similar temporal milestones and implantation potential.
Keywords: High ovarian response, Normal ovarian response, Time lapse monitoring system, Oocyte quality, Direct uneven cleavage
Introduction
The association between the number of oocytes retrieved and clinical pregnancy rate is the foundation of controlled ovarian hyperstimulation (COS). However, with the development of multiple follicles, a supraphysiological endocrine milieu is created, exhibited by elevated estradiol (E2) and progesterone levels [1, 2]. Although there is firm evidence ascribing diminished uterine receptivity [3] to this disturbed hormonal environment and other molecular processes, a controversy surrounds whether ovarian hyperstimulation also has a detrimental effect on oocyte and embryo quality, directly by yielding low-quality oocytes, or through an adverse effect attributed to the hormonal milieu.
Valbuena et al. [4] demonstrated a direct toxic effect of high E2 levels on cleavage stage embryos, consequently causing a reduced implantation rate. However, others failed to demonstrate a negative effect of ovarian hyperstimulation on embryo quality [5, 6]. By allowing continuous and detailed monitoring of embryo development, time lapse monitoring (TLM) incubators offer an important adjunct tool by which oocyte quality, represented by embryo dynamics, can be evaluated. The significance of this evaluation has been previously assessed by others, who demonstrated an association between temporal developmental milestones and blastocyst formation rate [7, 8] and implantation rate [9]. One of the phenomena assessed by TLM is the development of direct unequal cleavage at first cleavage (DUC-1) embryos, defined as the abrupt cleavage of one blastomere into three daughter blastomeres or an interval of cell cycles less than 5 h. This observation of an extremely short cell cycles might be associated with unequal distribution of DNA to blastomeres [10], consequently affecting embryo quality manifested by increased aneuploidy rate [11] and impaired implantation and clinical pregnancy rates [10, 11].
To the best of our knowledge, no data have been published describing morphokinetic evaluation of embryos obtained from women exhibiting high ovarian response (HOR). Therefore, we aimed to retrospectively compare oocyte and embryo quality, including morphokinetic events, between patients with high vs. normal ovarian response to ovarian stimulation.
Material and methods
Study population
Our study included data analysis of videos obtained from embryos cultured in time lapse incubators (EmbryoScope, Vitrolife) at three IVF units in Israel (the two campuses of the Hadassah-Hebrew University Medical Center – Ein Kerem and Mount Scopus, and Soroka University Medical Center) between January 2014 and December 2019.
The study population included 6886 embryos derived from 905 controlled ovarian stimulation (COS) cycles of women younger than 38 years—high ovarian response patients, normal ovarian response patients, and patients with polycystic ovary syndrome (PCOS) and HOR. Patients’ electronic records were examined and data regarding age, medical history, in vitro fertilization (IVF) indication, oocyte retrieval, and embryo transfer data were collected. The annotation of morphokinetic events was assessed and documented, based on the embryonic developmental milestones shown on the video files from the time lapse incubators. This analysis was performed by trained embryologists, according to accepted guidelines [12].
The study cohort included patients 20–38 years of age who underwent COS resulting in at least 6 aspirated oocytes, with complete records of treatment outcomes and embryo morphokinetics. The cohort was divided into two groups according to the number of oocytes retrieved following a COS cycle: the “high ovarian response” (HOR) group, with more than 15 oocytes aspirated [13], and the comparison “normal ovarian response” group, with retrieval of 6–15 oocytes. In a separate analysis, we included patients diagnosed with polycystic ovary syndrome (according to practiced criteria [14]) who demonstrated high ovarian response (PCOS HOR group) to stimulation, in order to evaluate the morphokinetic developmental process in this unique subgroup. Cases involving infertility factors that might affect embryo quality, including patients diagnosed with endometriosis, polycystic ovary syndrome with normal ovarian response, women undergoing preimplantation genetic testing, and male factor infertility cases, were excluded.
Intracytoplasmic sperm injection (ICSI) was used in most of the non-male factor cases in the IVF units included in the study. In order to accurately evaluate morphokinetic time events, we excluded IVF or combined IVF and ICSI cases, to avoid heterogeneity and different insemination times.
Basic clinical characteristics, IVF cycle data including estradiol levels (pg/mL) measured at ovulation trigger or 1–3 days prior to trigger oocyte maturation, and fertilization rates (normal fertilization 2 pro-nuclei (2PN) embryos) were compared between the normal and high response groups. Embryos manifesting DUC-1, defined as ≤ 5 h from the development of two blastomeres to three blastomeres, were discarded without further morphokinetic evaluation in the TLM and were not transferred, as they have been reported to demonstrate extremely low implantation rates [11]. Therefore, the subsequent morphokinetic events as well as implantation and clinical pregnancy rates did not include DUC-1 embryos. In order to assess whether a different cutoff in the definition of high ovarian response would manifest in different morphokinetic characteristics, we divided the HOR group into two subgroups and compared morphokinetic outcomes. The moderate HOR (mHOR) group was defined as 16–25 aspirated oocytes and the excessive HOR (eHOR) group was defined by more than 25 aspirated oocytes. This study was approved by the Institutional Review Board of Hadassah Hebrew University Medical Center (IRB number HMO-006–20) and the Institutional Review Board of Soroka University Medical Center (IRB number SOR -0328–17).
Oocyte aspiration and embryo culture and transfer
Controlled ovarian hyperstimulation included GnRH antagonist administration in the vast majority of cases. Following ICSI, embryos were placed in culture slides (EmbryoSlide, Unisense FertiliTech, Aarhus, Denmark) containing 12 micro-wells with culture medium, covered with mineral oil to prevent evaporation. Slides were placed in the time lapse incubator, an EmbryoScope™ system, at 37 °C, 5% O2, and 5.7% CO2 (Unisense FertiliTech, Aarhus, Denmark). According to patient and physician consultation, embryo transfer was performed at day 3–6 using either the Edwards-Wallace catheter (Classic Embryo Replacement Catheter, Smiths Medical, Hythe, Kent, U.K.) or the SIVF catheter (K-Jets-7019-SIVF; Cook IVF, Eight Miles Plains, Queensland, Australia).
Annotations of embryonic morphokinetic events
Seven-frame z-stacks, 15 μm apart, recorded at 18–20-min intervals for up to 6 days of incubation, were recorded for evaluation of oocyte fertilization and embryonic developmental events. We used the time series of these discrete events to ascertain the morphokinetic profile for each embryo, based on previously established definitions (Ciray et al., 2014). Morphokinetic events were calculated using the time of pronuclei fading (tPNf) as t0 for each morphokinetic parameter. The recorded events included cleavage of N discrete cells (tN; N = 2 to 9), morula (tM) and start of blastocyst formation (tSB).
Embryo transfer outcomes
Implantation of transferred embryos is presented as known implantation data (KID) and defined as one of three: (1) positive (KIDp)—when the number of gestational sacs is equal to the number of transferred embryos; (2) negative (KIDn)—none of the transferred embryos implanted; (3) KID unknown (KIDu)—cases in which implantation outcome was unknown, as the number of the gestational sacs was lower than the number of transferred embryos.
Implantation rate was calculated for the entire group. The numerator was the number of embryonal sacs demonstrated at 6 weeks of gestation by ultrasound divided by the overall number of embryos transferred for the entire group. Clinical pregnancy rate was calculated for the entire group. The numerator was the number of patients having at least one embryonal sac with the presence of a fetus with a heartbeat demonstrated at 6 weeks of gestation by ultrasound divided by the overall number of patients that underwent embryo transfer.
Statistical analysis
Quantitative variables (presented as mean ± standard deviation (SD)), were compared using a two-tailed Student’s t-test for two independent groups and for comparisons between 3 independent groups—the one-way analysis of variance (ANOVA) was performed. Statistical significance of between-groups comparisons of categorical variables was tested using the chi-square test. Post hoc test was additionally performed to identify between-groups statistical differences with correction to multiple comparisons. Variables that were associated with the independent variable (high vs. normal responders) were included in the multivariate logistic regression model, which was used to simultaneously assess the effect of high vs. normal ovarian response on implantation, while adjusting for morphokinetic variables and other basic and COS cycle related parameters. Each independent variable was tested for significance and the adjusted odds ratio (95% confidence interval) was calculated for the entire population. A two-sided p value < 0.05 was considered statistically significant for all analyses.
Results
Study population and COS cycle outcomes
The study population included 6886 embryos derived from 905 COS cycles of women younger than 38 years (mean age of 32.9 ± 5.3 years).
The high response group included 151 women (31.5 ± 5.4 years) with 1863 embryos and the normal response group included 741 women (33.4 ± 5.3 years) with 4907 embryos. The PCOS HOR group included 116 embryos derived from 13 women (31.2 ± 5.5 years). The treatment cycles included in this study were conducted between January 2014 and December 2019 in the three participating IVF units. Table 1 presents the comparison between the HOR and NOR groups. The HOR group was younger than the NOR group (mean of 1.9 years) and had higher peak estradiol levels. The average number of retrieved oocytes in the HOR group was more than double that of the NOR group, as expected (20.1 ± 4.2 vs. 9.6 ± 2.8; p < 0.001), and the mean number of matured oocytes also differed, though to a lesser extent (11.0 ± 2.8 vs. 8.5 ± 2.5; p < 0.001). This discrepancy was reflected in the differing maturation rates noted in the groups: 56.5 ± 16.6% in the HOR group vs. 90.0 ± 16.7% in the NOR group (p < 0.001). The mean number of fertilized oocytes in each group revealed a smaller, though significant, difference of 1.7 oocytes between the groups (6.9 ± 3.0 fertilized oocytes in the HOR vs. 5.2 ± 2.4 fertilized oocytes in the NOR group, p < 0.001), with a similar fertilization rate (60.2% vs. 58.1%, respectively).
Table 1.
Basic characteristics and ovarian stimulation cycle data of all women with high and normal ovarian response
| Parameter | High response | Normal response | p value |
|---|---|---|---|
| No. of patients | 151 | 741 | |
| Age at aspiration (years) | 31.5 ± 5.4 | 33.4 ± 5.3 | < 0.001 |
| Peak estradiol level (pg/mL) | 2173.7 ± 928.6 | 1739.8 ± 747.2 | < 0.001 |
| Mean no. of oocytes retrieved | 20.1 ± 4.2 | 9.6 ± 2.8 | < 0.001 |
| Mean no. of mature oocytes | 11.0 ± 2.8 | 8.5 ± 2.5 | < 0.001 |
| Maturation rate (%) | 56.5 ± 16.6 | 90.0 ± 16.7 | < 0.001 |
| Mean no. of fertilized oocytes | 6.9 ± 3.0 | 5.2 ± 2.4 | < 0.001 |
| Fertilization rate (%) | 60.2 ± 26.1 | 58.1 ± 27.3 | 0.397 |
| No. of embryos | 1863 | 4907 |
Data presented as Mean ± SD or n/N (%)
Morphokinetic events
The prevalence of DUC-1, an indicator for abnormal embryonic development, was evaluated and compared between the groups and was found to be higher in the HOR group compared to the NOR group (184/1134 (16.2%) embryos in the high response group vs. 347/2903 (12.0%) embryos in the normal response group respectively; p < 0.001, Fig. 1).
Fig. 1.
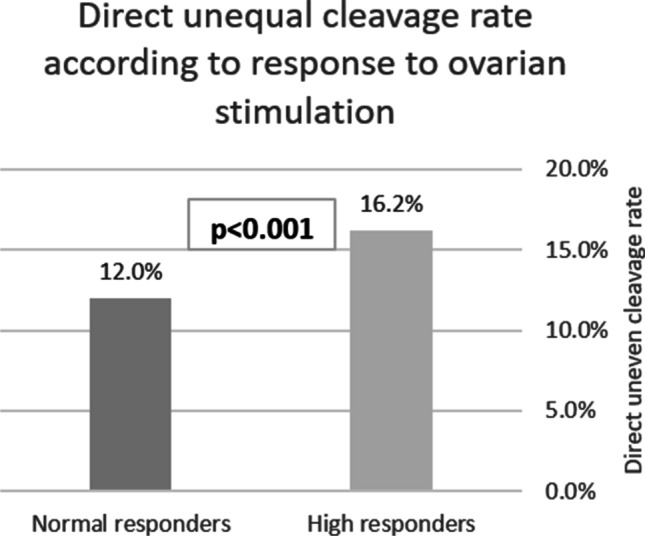
Direct unequal cleavage (less than 5 h between two and three blastomeres) at first cleavage (DUC-1) rate according to response to ovarian stimulation: normal (6–15 oocytes) and high (16 oocytes or more) response. *DUC-1 occurrence rate was 347/2903 embryos in the normal response group and 184/1134 embryos in the high response group
As noted, the morphokinetic parameters were calculated after the exclusion of DUC-1 embryos and demonstrated a similar pace of progression and acquisition of embryonic developmental milestones (t2, t3, t4, t5, t8, tM, and tSB) in the two groups (Table 2).
Table 2.
Reproductive outcomes of women with high and normal ovarian response that underwent fresh embryo transfer
| Parameter | High response | Normal response | p value |
|---|---|---|---|
| No. of patients | 121 | 658 | |
| No. of transferred embryos | 145 | 911 | |
| Mean no. of fresh embryos transferred | 1.2 ± 0.5 | 1.4 ± 0.6 | < 0.001 |
| Stage at transfer | < 0.001 | ||
| Cleavage | 88/145 (60.7%) | 774/911 (85.0%) | |
| Blastocyst | 57/145 (39.3%) | 137/911 (15.0%) | |
| Implantation rate according to stagea | |||
| Cleavage stage | 33/88 (37.5%) | 237/774 (30.6%) | 0.189 |
| Blastocyst | 29/57 (50.9%) | 71/137 (51.8%) | 0.904 |
| Clinical pregnancy ratesb | |||
| Cleavage stage | 28/66 (42.4%) | 190/531 (35.8%) | 0.292 |
| Blastocyst | 25/53 (47.2%) | 61/123 (49.6%) | 0.768 |
Data presented as Mean ± SD or n/N (%)
aImplantation rate was calculated for the entire group. The overall number of embryonal sacs demonstrated at 6 weeks of gestation by ultrasound divided by the overall number of embryos transferred
bData were available for 654/658 patients in the normal response group and for 119/121 patients in the high response group
Fresh embryo transfer outcomes
Evaluation of patients that underwent embryo transfer showed a slightly higher mean number of transferred embryos was noted in the NOR compared to the HOR group (1.4 ± 0.6 vs. 1.2 ± 0.5, respectively; p < 0.001) along with higher rate of cleavage stage of embryos transferred (85.0% vs. 60.7%, respectively; p < 0.001) compared to blastocysts transferred. Comparisons between the HOR and NOR groups according to embryo stage at transfer showed that both cleavage stage and blastocyst stage transfers had similar implantation rates (37.5% vs. 30.6% and 50.9% vs. 51.8%, p = 0.189 and 0.904, respectively) and clinical pregnancy rates (42.4% vs. 35.8% and 47.2% vs. 49.6%, p = 0.292 and 0.768, respectively) (Table 3).
Table 3.
Morphokinetic events (in hours) of all embryos obtained from women with high (15 or more retrieved oocytes) and normal (6–14 retrieved oocytes) ovarian response following the exclusion of direct uneven cleavage embryos
| Parametera | High response | Normal response | p value |
|---|---|---|---|
| No. of embryos | 950 | 2556 | |
| t2 | 2.5 ± 0.8 | 2.5 ± 0.9 | 0.064 |
| t3 | 14.6 ± 2.6 | 14.8 ± 2.8 | 0.059 |
| t4 | 15.7 ± 3.4 | 15.8 ± 3.8 | 0.222 |
| t5 | 26.6 ± 6.3 | 27.1 ± 6.5 | 0.056 |
| t8 | 34.9 ± 8.2 | 34.9 ± 8.5 | 0.952 |
| tM | 62.4 ± 9.8 | 63.3 ± 10.0 | 0.095 |
| tSB | 76.2 ± 7.9 | 76.2 ± 8.4 | 0.970 |
Data presented as Mean ± SD
aTime of pronuclei fading was used as the 0 time
t(N), cleavage of N discrete cells; tM, time to morula; tSB, time to start of blastocyst formation
A multivariable logistic regression model was generated, with implantation as the dependent variable (Table 4), in the entire study population (NOR and HOR patients). This analysis included the following parameters: patient age; peak estradiol levels; normal vs. high response to COS; maturation rate; and morphokinetic parameters. The single parameter that remained a significant independent predictor for implantation was patient age (aOR = 0.95, CI 95% 0.93–0.97, p < 0.001). A subgroup analysis was preformed between the NOR patients, the HOR group, and a third group comprised of patients diagnosed with PCOS that exhibited HOR (Table 5). The PCOS HOR group manifested the highest DUC-1 rate compared with the HOR without PCOS and NOR patients (22.7% vs. 16.2% and 12.0%, respectively, p < 0.001). A significant difference in DUC-1 rates was noted in a post-Hoc test analysis between all 3 groups (p < 0.05). Further comparison of the morphokinetic parameters after exclusion of DUC-1 embryos demonstrated that both HOR patients and PCOS patients with HOR shared similar embryonal developmental rate, except for the t5 parameter, in which the PCOS HOR embryos were faster than the HOR and NOR embryos (24.6 ± 6.4 vs 26.6 ± 6.3 and 27.1 ± 6.5 h, respectively; p = 0.002).
Table 4.
Multivariable logistic regression for known implantation data (KID) positive embryos
| aOR | Lower 95% CI | Upper 95% CI | p value | |
|---|---|---|---|---|
| Patient age | 0.95 | 0.93 | 0.97 | < 0.001 |
| Peak estradiol measured (pg/mL) | 1.00 | 1.00 | 1.00 | 0.349 |
| High ovarian response | 1.16 | 0.75 | 1.80 | 0.507 |
| Maturation rate | 0.90 | 0.41 | 1.98 | 0.797 |
Table 5.
Morphokinetic events (in hours) of all embryos obtained from women normal ovarian response (6–14 retrieved oocytes) and patients with high (15 or more retrieved oocytes) ovarian response—with and without polycystic ovary syndrome following the exclusion of direct uneven cleavage embryos
| Parametera | Normal response | High response without PCOS | PCOS with high response | p value |
|---|---|---|---|---|
| No. of embryos | 2556 | 950 | 116 | |
| t2 | 2.5 ± 0.9 | 2.5 ± 0.8 | 2.5 ± 1.1 | 0.183 |
| t3 | 14.8 ± 2.8 | 14.6 ± 2.6 | 14.5 ± 2.8 | 0.126 |
| t4 | 15.8 ± 3.8 | 15.7 ± 3.4 | 15.4 ± 3.3 | 0.250 |
| t5 | 27.1 ± 6.5* | 26.6 ± 6.3* | 24.7 ± 6.4 | < 0.001 |
| t8 | 34.9 ± 8.5 | 34.9 ± 8.2 | 34.3 ± 7.5 | 0.809 |
| tM | 63.3 ± 10.0* | 62.4 ± 9.8 | 60.6 ± 11.7 | 0.040 |
| tSB | 76.2 ± 8.4 | 76.2 ± 7.9 | 74.5 ± 6.6 | 0.412 |
Data presented as Mean ± SD
aTime of pronuclei fading was used as the 0 time
t(N), cleavage of N discrete cells; tM, time to morula; tSB, time to start of blastocyst formation; PCOS, polycystic ovary syndrome
*Significant difference (p < 0.05) shown in a post hoc test when compared to the PCOS with high response group
A comparison between the moderate HOR (mHOR) group (defined by 16–25 aspirated oocytes) and the excessive HOR (eHOR) group (more than 26 aspirated oocytes) revealed no difference in the DUC-1 rate (16.3% Vs 15.6% in the mHOR and eHOR, respectively; p = 0.851). The temporal developmental milestones were similar between the groups, except for the t8 parameter in which the eHOR embryos were faster than embryos from mHOR patients (33.2 ± 6.4 vs 35.1 ± 8.3 h, respectively; p = 0.024) and tM, in which the mHOR embryos were faster than the eHOR (62.0 ± 9.6 vs 68.2 ± 4 h, respectively; p < 0.001) (Table 6).
Table 6.
Morphokinetic events (in hours) of all embryos obtained from women with moderately high (15–25 retrieved oocytes) and excessively high (26 or more retrieved oocytes) ovarian response following the exclusion of direct uneven cleavage embryos
| Parametera | Moderate high response | Excessive high response | p value |
|---|---|---|---|
| No. of embryos | 858 | 92 | |
| t2 | 2.5 ± 0.8 | 2.3 ± 0.4 | 0.062 |
| t3 | 14.6 ± 2.6 | 14.5 ± 2.3 | 0.633 |
| t4 | 15.7 ± 3.3 | 15.5 ± 3.6 | 0.620 |
| t5 | 26.7 ± 6.3 | 26.0 ± 5.6 | 0.360 |
| t8 | 35.1 ± 8.3 | 33.2 ± 6.4 | 0.024 |
| tM | 62.0 ± 9.6 | 68.2 ± 10.4 | < 0.001 |
| tSB | 76.0 ± 7.9 | 78.3 ± 8.3 | 0.127 |
Data presented as Mean ± SD
aTime of pronuclei fading was used as the 0 time
t(N), cleavage of N discrete cells; tM, time to morula; tSB, time to start of blastocyst formation
Discussion
This study aimed to compare fresh IVF cycle outcomes, including morphokinetic parameters, between patients with high ovarian response (with and without PCOS) and normal ovarian response to ovarian stimulation. Our results showed that high ovarian response is associated with the retrieval of more incompetent oocytes, exhibited by a higher proportion of immature oocytes.
However, the matured oocytes possessed similar fertilization potential. In addition, though the proportion of DUC-1 embryos was higher in the HOR group, if these are excluded, then the remaining embryos from the HOR group reached the morphokinetic milestones at a similar rate as the NOR embryos and displayed similar implantation and pregnancy rates.
As the oocyte is considered a key factor in embryo developmental competence, we sought to assess the effect of high response to ovarian stimulation on oocyte quality.
Since we addressed different aspects of oocyte quality, both in vitro and clinical parameters were chosen as representative, including maturation and fertilization rates, morphokinetic parameters, and implantation and clinical pregnancy rates.
Time lapse analysis was used as part of our assessments, as it provides detailed and dynamic information regarding embryonal temporal milestones from very early developmental stages. This tool adds information on embryo competence. One of the phenomena exhibited by this technique is the occurrence of DUC embryos. DUC embryos show abnormal mitosis, resulting in an extremely short cell cycle, defined as an interval of 5 or fewer hours between mother and daughter cell division, or the direct cleavage of a blastomere into three or more daughter cells [11]. The clinical outcomes of DUC occurrence are well described and associated with impaired embryonal development, higher aneuploidy rate, and lower implantation rate [10, 11, 15]. We chose to assign only embryos manifesting direct uneven cleavage in the first cell cycle (DUC-1) since they were most prominently associated with these impaired outcomes. Although fertilization rates did not differ between the groups, the DUC-1 rate was significantly higher in the high response group, reinforcing our observation of possible impaired oocyte quality.
It has been previously demonstrated in a murine model that ovarian hyperstimulation has adverse effects on oocyte quality, manifested as spindle malformation and a higher rate of DNA strand breaks [16]. These observations might suggest that extrinsic factors, such as the hormonal milieu, influence meiotic maturation.
The literature regarding the effect of ovarian hyperstimulation on human oocyte quality is limited and controversial. Valbuena et al. [4] demonstrated that supraphysiologic levels of estradiol not only affect the endometrium but also have a direct effect on the embryo, impairing its developmental competence. Arce et al. displayed a positive correlation between gonadotropin (GT) doses and oocyte retrieval [6], however, with relatively few good-quality oocytes that fertilized or developed into blastocysts. They suggested that there may be a threshold level of GT above which there is a limited effect on the number of competent oocytes. Their results were in line with Koks’ work [5], which demonstrated an increase in the fraction of immature oocytes as the ovarian response increased, with a negative effect of the ovarian response on fertilization rate per oocyte obtained, but not per injected oocyte. They concluded that although there are more immature oocytes retrieved from high responders, the oocytes that do fertilize have equal quality as oocytes from normal responders. This concurs with our observation that once first mitosis was correct and even, embryos from each group displayed similar morphokinetic developmental rate and similar implantation and clinical pregnancy rates.
Since implantation rate might also be influenced by hormonal impact on the endometrium, it might not accurately reflect only embryo quality. We assessed estradiol level in a multivariate regression analysis and demonstrated that it was not predictive of implantation. This is in contrast to other reports [17] and does not exclude the influence of other hormones on endometrial receptivity and implantation rate [2]. Unfortunately, as we did not have data regarding the progesterone levels, we could not appraise its impact on our results and focused on estradiol only. Furthermore, our assessment of a possible interaction between estradiol and IVF outcomes in this population is limited since our estradiol levels were not all taken at trigger day. Moreover, the relatively low estradiol in the HOR group might be attributed to the fact that we only evaluated patients who eventually continued to embryo transfer during the fresh cycles and not treated by “freeze-all” strategy (population with higher estradiol level). Consequently, although estradiol levels were significantly higher in the high response group, they were not as high as expected and therefore the generalizability of our conclusions is limited regarding HOR patients with very high estradiol levels, including egg donors.
Due to its known and robust effect of maternal age on IVF outcomes, we included only patients younger than 38. We are aware that our results are limited by the heterogeneity between the groups regarding age and other basic characteristics, however performing subdivision to age classes would have resulted in very small numbers for analysis.
As a result of the statistically significant difference in mean patient age between the groups, age was included in the multivariate regression analysis, which showed that it was predictive for implantation. Interestingly, despite the older age and the correlation mentioned above, it seems that oocyte quality was better in the normal response group (lower DUC-1 prevalence).
Since several studies have suggested that the altered PCOS hormonal milieu might impact oocyte developmental competence by itself [18], an additional sub-analysis was preformed which included 116 embryos driven from PCOS HOR patients who demonstrated high ovarian response. PCOS HOR patients demonstrated higher DUC-1 rate compared to patients with HOR without PCOS and patients with normal ovarian response. However, once DUC-1 embryos were discarded, similar embryonal developmental rate was exhibited in the vast majority of morphokinetic parameters between the HOR and PCOS groups, reinforcing our observation that HOR mainly affects the initial stages of embryonic developments.
Limited by our practice of discarding DUC-1 embryos, no information regarding subsequent morphokinetic evaluation and implantation and clinical pregnancy rate was available. Additionally, due to its retrospective design, information regarding number of frozen and thawed embryos for each patient and the cumulative pregnancy rates were not available. Parameters that might affect results such as patients’ BMI, maximal dose of gonadotropin obtained, number of small and large follicles, and endometrial thickness at trigger were also lacking. Morphologic assessment was not collected for this cohort and blastocyst conversion rate was not available for frozen-thawed embryos and therefore not included in the analysis.
The absence of information regarding ovarian stimulation protocol might also hamper the interpretation of the results due to its possible effect on oocyte quality.
Nevertheless, our study is the first to assess oocyte quality using morphokinetic parameters in high responders and sheds more light on this unique population.
In conclusion, our results suggest that higher oocyte quantity might affect oocyte quality, manifested as higher fraction of incompetent oocytes and higher rate of embryos with direct uneven cleavage.
Whether this impairment is the result of hormonal milieu or other factors remains unanswered. Further clinical studies are needed to shed more light on this subject.
Author contribution
Natali Schachter-Safrai and Gilad Karavani have contributed substantially to the conception and design of the study, analysis and interpretation of data, and drafting the article. Efrat Esh-Broder and Eliahu Levitas have contributed substantially to the acquisition, analysis and interpretation of data, and revision of the article. Tamar Wainstock has contributed substantially to the conception and design of the study, analysis and interpretation of data, and revision of the article. Iris Har-Vardi and Assaf Ben-Meir have contributed substantially to the conception and design of the study, acquisition, analysis and interpretation of data, and drafting and revision of the article. All authors have approved the final version of the study.
Funding
Israel Innovation Authority – Kamin grant 55326.
Data availability
Available upon request.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Natali Schachter-Safrai and Gilad Karavani should be considered as equal contribution first authors.
Iris Har-Vardi and Assaf Ben-Meir should be considered as equal contribution last authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kosmas IP, Kolibianakis EM, Devroey P. Association of estradiol levels on the day of hCG administration and pregnancy achievement in IVF: a systematic review. Hum Reprod. 2004;19:2446–2453. doi: 10.1093/humrep/deh473. [DOI] [PubMed] [Google Scholar]
- 2.Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update. 2013;19:433–457. doi: 10.1093/humupd/dmt014. [DOI] [PubMed] [Google Scholar]
- 3.Valbuena D, Jasper M, Remohí J, Pellicer A, Simón C. Ovarian stimulation and endometrial receptivity. Hum Reprod. 1999;14(Suppl 2):107–111. doi: 10.1093/humrep/14.suppl_2.107. [DOI] [PubMed] [Google Scholar]
- 4.Valbuena D, Martin J, de Pablo JL, Remohí J, Pellicer A, Simón C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76:962–968. doi: 10.1016/S0015-0282(01)02018-0. [DOI] [PubMed] [Google Scholar]
- 5.Kok JD, Looman CW, Weima SM, te Velde ER. A high number of oocytes obtained after ovarian hyperstimulation for in vitro fertilization or intracytoplasmic sperm injection is not associated with decreased pregnancy outcome. Fertil Steril. 2006;85:918–924. doi: 10.1016/j.fertnstert.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 6.Arce JC, Andersen AN, Fernández-Sánchez M, Visnova H, Bosch E, García-Velasco JA, Barri P, de Sutter P, Klein BM, Fauser BC. Ovarian response to recombinant human follicle-stimulating hormone: a randomized, antimullerian hormone-stratified, dose-response trial in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil Steril. 2014;102:1633–40.e5. doi: 10.1016/j.fertnstert.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Motato Y, de los Santos MJ, Escriba MJ, Ruiz BA, Remohí J, Meseguer M. Morphokinetic analysis and embryonic prediction for blastocyst formation through an integrated time-lapse system. Fertil Steril. 2016;105:376–84.e9. doi: 10.1016/j.fertnstert.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Kirkegaard K, Kesmodel US, Hindkjær JJ, Ingerslev HJ. Time-lapse parameters as predictors of blastocyst development and pregnancy outcome in embryos from good prognosis patients: a prospective cohort study. Hum Reprod. 2013;28:2643–2651. doi: 10.1093/humrep/det300. [DOI] [PubMed] [Google Scholar]
- 9.Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohí J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26:2658–2671. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 10.Rubio I, Kuhlmann R, Agerholm I, Kirk J, Herrero J, Escriba MJ, et al. Limited implantation success of direct-cleaved human zygotes: a time-lapse study. Fertil Steril. 2012;98:1458–1463. doi: 10.1016/j.fertnstert.2012.07.1135. [DOI] [PubMed] [Google Scholar]
- 11.Zhan Q, Ye Z, Clarke R, Rosenwaks Z, Zaninovic N. Direct Unequal cleavages: embryo developmental competence, genetic constitution and clinical outcome. PLoS One. 2016;11:e0166398. doi: 10.1371/journal.pone.0166398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciray HN, Campbell A, Agerholm IE, Aguilar J, Chamayou S, Esbert M, et al. Time-Lapse User Group. Proposed guidelines on the nomenclature and annotation of dynamic human embryo monitoring by a time-lapse user group. Hum Reprod. 2014;29:2650–60. doi: 10.1093/humrep/deu278. [DOI] [PubMed] [Google Scholar]
- 13.Simón C, Cano F, Valbuena D, Remohí J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum oestradiol concentrations in high and normal responder patients. Hum Reprod. 1995;10:2432–2437. doi: 10.1093/oxfordjournals.humrep.a136313. [DOI] [PubMed] [Google Scholar]
- 14.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 15.Athayde Wirka K, Chen AA, Conaghan J, Ivani K, Gvakharia M, Behr B, et al. Atypical embryo phenotypes identified by time-lapse microscopy: high prevalence and association with embryo development. Fertil Steril. 2014;101:1637–1648. doi: 10.1016/j.fertnstert.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 16.Van Blerkom J, Davis P. Differential effects of repeated ovarian stimulation on cytoplasmic and spindle organization in metaphase II mouse oocytes matured in vivo and in vitro. Hum Reprod. 2001;16:757–764. doi: 10.1093/humrep/16.4.757. [DOI] [PubMed] [Google Scholar]
- 17.Fauser BC, Devroey P. Reproductive biology and IVF: ovarian stimulation and luteal phase consequences. Trends Endocrinol Metab. 2003;14:236–242. doi: 10.1016/S1043-2760(03)00075-4. [DOI] [PubMed] [Google Scholar]
- 18.Wood JR, Dumesic DA, Abbott DH, Strauss JF. Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon request.


