Abstract
Purpose
Does semi-automated vitrification have lower inter-operator variability and better clinical outcomes than manual vitrification?
Methods
Retrospective analyses of 282 patients whose embryos had been cryopreserved, manually with Irvine®-CBS® (MV) or semi-automatically vitrified with the GAVI® method (AV) (from November 2017 to September 2020). Both techniques were performed during the same period by 5 operators. Inter-operator variability was statistically analyzed between operators who performed the vitrification and those who performed the warming process to compare the intact survival rate (% embryos with 100% intact blastomeres) and the positive survival rate (at least 50% intact blastomeres). Additionally, the complete vitrification time was assessed for the 2 techniques according to the number of vitrified embryos.
Results
Manual vitrification involved warming 338 embryos in 266 cycles for 181 couples compared to 212 embryos in 162 AV cycles for 101 patients. The positive survival rate was higher (p < 0.05) after MV (96%; 323/338) than after AV (90%; 191/212). The intact survival rate (86 vs 84%) and the clinical pregnancy rate (27 vs 22%) were not significantly different between MV and AV. Regarding the inter-operator variability, no significant difference in positive and intact survival rate was evident between the 5 technicians, neither by vitrification nor by warming steps with MV and AV. Concerning time-saving, the MV technique proved to be quicker than AV (minus 11 ± 9 min).
Conclusions
Manual vitrification exhibited favorable total survival rates and was more time efficient, while both MV and AV cooling and warming treatments showed little operator variability.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02346-3.
Keywords: Vitrification, Embryo, Automation, Variability, Quality management, Survival rate
Introduction
Vitrification is the most efficient and commonly used technique for oocyte and embryo cryopreservation in assisted reproductive technologies (ARTs). As a rapid freezing procedure, vitrification prevents the formation of ice crystals which may induce cellular damage [1]. In contrast to conventional slow freezing, a metastable glass phase devoid of detectable ice is achieved, in part, by the use of higher concentrations of cryoprotective agents and higher warming rates than cooling rates. Overall, improved clinical outcomes and other organizational advantages are achieved with vitrification [2]. Without question, the efficacy of embryo vitrification has single-handedly contributed to improving the cumulative pregnancy rates per oocyte retrieval. Meanwhile, the reliability as a freeze-all strategy has mitigated concerns of ovarian hyperstimulation syndrome [3]. The efficacy of vitrification has also facilitated the widespread application of preimplantation genetic testing and fertility preservation in patients who receive gonadotoxic therapies for cancer and other diseases [4].
As a manual and time-sensitive method, vitrification requires precision, coordination, and speed to achieve consistently high embryo survival rates. As with other laboratory procedures, vitrification requires operator training to be performed successfully and it is generally accepted that vitrification has a learning curve [5]. Depending on the device and solution used, steps that might vary include contact time of the embryo with the final vitrification solution, the volume of vitrification solution used, and how it is loaded onto or into the device [2]. Conversely, the warming process is also operator-dependent. The rate of warming should be as rapid as possible and the embryo or oocyte(s) should be successfully recovered [6, 7]. The inter-relationship between rapid cooling and faster warming is subject to user variation which can impact survivability [8].
Oddly enough, embryo vitrification is very often qualified as an operator-dependent procedure in the literature, yet to our knowledge there is no study on the inter-operator variability neither with manual nor with automated techniques. One study [9] mentions a lack of reproducibility in survival outcomes for oocyte vitrification/warming from three different cycles of the same patient. However, it is difficult to assess whether the variability was due to different technicians or to a batch cycle effect.
Automation of the vitrification process is expected to standardize different steps, such as the contact time of cryoprotectants, the volume of solution (i.e., cooling rate), and to decrease inter-operator variability. Recently and for the first time, a semi-automated system for vitrification was developed: Gavi® (Genea, Sydney, Australia). The Gavi is a closed-type vitrification system that controls stepwise exposure to vitrification solutions, timing, temperature, and the duration of exposure to the vitrification medium. Automated embryo vitrification has been programmed to decrease inter-operator variability and reduce working time.
The novelty of the current study is the comparison of the inter-operator variability of manual MV and semi-automated AV vitrification by analyzing embryo survival rates independently, according to the technician performing the vitrification or the warming events. In addition, we evaluated the working time according to the number of embryos vitrified with both techniques, and strived to compare clinical outcomes between MV and AV for cleavage stage embryos.
Materials and methods
Study design
This retrospective study included 282 patients from Toulouse University Hospital who had their embryos cryopreserved by MV or AV between November 2017 and September 2020, then warmed from March 2018 to December 2020. Patients’ demographic data are presented in Table 1.
Table 1.
Patient characteristics and results of vitrified/warmed embryo cycles with both techniques. The transfer score includes age, attempt rank, ovarian response to stimulation and the number and morphology of embryos, and is highly correlated with the implantation rate in fresh cycles [10]
| Manual vitrification | GAVI vitrification | Statistical comparisons | |
|---|---|---|---|
| Number of couples | 181 | 101 | |
| Number of warming cycles | 266 | 162 | |
| Women’s age at vitrification | 33.1 ± 4.6 | 33.2 ± 4.1 | NS |
| Women’s age at warming | 33.6 ± 4.6 | 33.7 ± 4.1 | NS |
| Transfer score | 92 ± 46 | 94 ± 37 | NS |
| Type of cycle (%) | |||
|
Stimulated Hormonal replacement |
126 (47) 140 (53) |
91 (56) 71 (44) |
NS |
| Number of warmed embryos | 338 | 212 | |
| Number of embryos lost during warming (%) | 2 (0.6) | 3 (1.4) | |
| Number of embryos with at least 50% of intact blastomeres (= positive survival rate) (%) | 323/338 (96) | 191/212 (90) | p < 0.05 |
| Number of embryos with 100% intact blastomeres (= intact survival rate) (%) | 292/338 (86) | 178/212 (84) | NS |
| Number of embryo transfers (% cycles) | 266/266 (100) | 162/162 (100) | NS |
| Number of transferred embryos, mean ± SD | 1.2 ± 0.4 | 1.2 ± 0.4 | NS |
| Number of implanted embryos/Number of warmed embryos (implantation rate %) | 80/338 (24) | 36/212 (17) | NS |
| Clinical pregnancies (% cycles) | 73/266 (27) | 36/162 (22) | NS |
Embryos
Day 2 or day 3 embryos with less than 20% fragments and with at least 3 cells or 6 cells respectively were cryopreserved after conventional IVF or ICSI. Based on the availability of the AV apparatus, all embryos from a couple were frozen using the same technique (manual or semi-automated vitrification). To evaluate the vitrification-warming process using both techniques, we assessed the Key Performance Indicators (KPI) in accordance with the Vienna consensus [11] and the Alpha consensus on cryopreservation [12]: positive survival rate (embryos with ≥ 50% morphologically intact blastomeres) and intact survival rate (embryos with 100% morphologically intact blastomeres). Survival rates were analyzed in relation to cell stage before cryopreservation and immediately after thawing/warming.
Manual vitrification-warming procedure
Vitrification was performed using closed CryoBioSystem vitrification (CBS-VIT) High Security (HS) straws (Cryobiosystem, Saint-Ouen-sur-Iton, France) and the Vitrification Freeze Kit with dimethylsulfoxide (DMSO)-ethylene glycol (EG)-sucrose as cryoprotectants (FUJIFILM Irvine Scientific, Santa Ana, CA) according to the manufacturer’s guidelines. The vitrification procedure was carried out at room temperature. Embryos were vitrified one by one. They were transferred to a 50 µL droplet of equilibration solution for 10 min, then into a 50 µL droplet of vitrification solution containing 15% (v/v) dimethyl sulfoxide and 15% (v/v) ethylene glycol. Embryos were then immediately loaded onto the CBS HS straw device in the smallest possible volume of vitrification solution (less than 1 µL). The straw was heat-sealed before being plunged directly into liquid nitrogen. The total time needed to vitrify embryos starting from the vitrification solution droplet to loading of the straw and plunging into liquid nitrogen did not exceed 110 s.
Warming was performed the day of embryo transfer (2 to 3 h prior to transfer) using the Vitrification Thaw Kit (FUJIFILM Irvine Scientific, Santa Ana, CA). A Nunc 4-well dish (Nunc A/S, Roskilde, Denmark) with one well of 500 µL thawing solution (1.0 M sucrose in HEPES buffered human tubal fluid [HTF] medium) was maintained at 37 °C. After cutting the straw, the dish containing the thawing solution at 37 °C was removed at the last moment from the bench-top incubator, and the capillary was removed from the straw; the gutter-end was then immediately placed in the droplet of warm solution, allowing the embryos to be released and eluted for 1 min. The embryos were then incubated for 4 min at room temperature in 50 µL of dilution solution (0.5 M sucrose in HEPES buffered HTF medium). Finally, the embryos were sequentially washed in two 50 µL-droplets of washing solution (HEPES buffered HTF medium) for 4 min each, before being transferred to a culture dish with 20% protein supplemented culture medium Global Total® (Life Global, Trumbull, USA) (HSA Irvine Scientific, Santa Ana, CA) for 2 h. Embryo morphology was assessed according to the Istanbul Consensus [13].
Semi-automated vitrification and warming with the GAVI® system (Fig. 1)
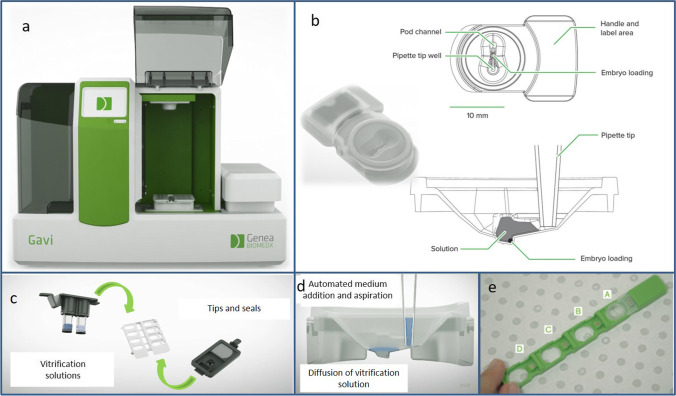
Fig. 1.
GAVI® Apparatus. General view (a), loading pod (b), medium and consumables (c), automated medium addition and aspiration (d), storage cassette can hold up to 4 pods (e).
Source: Genea Biomedx
The instrument uses a closed device called a “pod” which is inserted into a “cassette” that can hold up to four pods (Fig. 1). The embryo is held in a cavity at the bottom of the pod for the duration of the vitrification process and storage. As stated by Roy et al. [14], the vitrification and warming solutions included the following: Vitrification Solution 1 containing 8% v/v ethylene glycol and 8% v/v DMSO; Vitrification Solution 2 containing 16% v/v ethylene glycol, 16% v/v DMSO and 0.68 M trehalose; Warming Solution 1 containing 1 M trehalose; and Warming Solution 2 containing 0.5 M trehalose.
The vitrification steps using GAVI® were followed according to the protocol specified by the manufacturer (Genea, Sydney, Australia) [14]. In brief, after a 5-min incubation step at 37 °C in HEPES buffer medium (Vitbase®, Genea), embryos were deposited at the bottom of the pod with a small amount of Vitbase® and the pod was then attached to the cassette. The embryologist then pressed the button to start the cleavage stage vitrification mode. During the process, the GAVI® system utilized automated pipetting, and single-use pipette tips to automatically and sequentially replace the equilibration and dehydration media. At the end of the process, after discarding the dehydration medium, each GAVI pod was heat-sealed with a lid. The cassette containing the pod(s) was then dipped in liquid nitrogen by the embryologist. The duration of the GAVI® system program is 15 min for cleavage stage embryos (for 1 to 4 embryos).
The GAVI® cryopreserved embryos was manually warmed using Gems Warming solutions® (Genea, Sydney, Australia) according to the manufacture’s guidelines [14]. Gems Warming solutions 1 to 3 (Genea, Sydney, Australia) were equilibrated to room temperature in a four-well dish (solution 3 was set in two wells). A GAVI pod was retrieved from the cassette in liquid nitrogen and immersed into a 37 °C deionized water bath for 3 s. Under a microscope, the lid seal was removed and 10 µL of warming solution 1 was carefully added to the pod at room temperature. At this point, the embryo must be located in the pod, which is somewhat delicate because the embryo moves in the pod after warming solution 1 is added. After 1 min, the embryos were transferred from the Gavi Pod to warming solution 1 in a four-well dish (well 1) and incubate for another 1 min. The embryos were then moved to warming solution 2 (well 2) and incubated for 3 min, then moved to warming solution 3 (well 3) and incubated for 5 min. Finally, the embryos were washed and incubated for 1 min in warming solution 3 (well 4). The embryos were then transferred to a culture dish and assessed as previously described for MV embryos [13].
Demographic characteristics of the operators
The operators included in this study were ART laboratory technicians who had an average of 6-year experience working in an ART laboratory (4, 4, 12, 8, and 2 years for technicians A, B, C, D, and E, respectively). The average age of the technicians was 41.4 years (26–49 years).
Vitrification-warming qualification of the operators
Since there is no internationally accepted practical training to acquire cryopreservation skills [15], we established our own training and qualification program. In our center, technicians are allowed to perform unsupervised vitrification/warming procedures after having been trained and having performed at least 30 embryo vitrifications using non-transferred embryos (tripronucleate zygotes 3PN, reformed embryos) which yielded a survival rate of at least 80%. This learning process is consistent with that described by Dessole et al. [5]. The duration of training and qualification was 2.8 ± 1.1 months for the GAVI system and 3.8 ± 2.4 months for vitrification-warming (NS).
Endometrial preparation
When patients had normal ovulatory cycles, they had mild ovarian stimulation with an rFSH and GnRH antagonist (stimulated cycle) followed by hCG to trigger ovulation and intra-vaginal progesterone during the luteal phase. In patients with amenorrhea or oligomenorrhea, hormonal replacement with percutaneous estradiol and intra-vaginal progesterone was used.
Measured outcomes
Comparison of outcomes for the two techniques
The outcome parameters were: intact survival rate (percentage of embryos with 100% intact blastomeres), positive survival rate (percentage of embryos with at least 50% intact blastomeres), implantation rate, and clinical pregnancy rate. The implantation rate was measured by the ratio of the number of intrauterine gestational sacs observed on the transvaginal ultrasound scan at least 5 weeks after embryo transfer to the number of thawed/warmed embryos. Considering that implantation rates depend not only on the freeze/thawing technique but also on the parameters of the IVF attempt, we compared the transfer score of the IVF attempt during which the embryos were frozen for both techniques (Table 1). This score includes female age, attempt rank, ovarian response to stimulation, as well as the number and morphology of embryos, and is highly correlated to the implantation rate in fresh cycles [10].
Assessment of inter-operator variability
To compare the inter-operator variability, we compared the survival rates (positive survival rate and intact survival rate) by independently considering the technician who performed vitrification and who performed warming for both techniques. Since survival rates could depend on both the vitrification-warming technique as well as the parameters of the IVF attempt, they were adjusted (through logistic regression) for the transfer score of the IVF attempt during which the embryos were frozen [10].
Assessment of completion time
For both techniques, we loaded only one embryo per straw (MV) or per pod (AV). We precisely measured the times for both techniques according to the number of embryos to be vitrified (from 1 to 4 embryos). Beyond 4 embryos and up to 10 embryos, the technical times were extrapolated. The threshold of 4 was chosen because up to 4 embryos can be individually vitrified during the same cycle with AV. For MV, when there were several embryos to be vitrified, they were initiated every 1 min, and by a maximum of 3 at a time, i.e., we waited until the 3rd embryo was vitrified before beginning the vitrification protocol for the 4th embryo. These times were measured for the vitrification of 1 to 4 embryos for the same patient and do not take into account the assessment of embryo morphology before vitrification, the preparation times of the administrative file, the preparation of small equipment and consumables, and the storage of the embryos in tanks, because these factors are independent of both techniques and are specific to the organization of each laboratory.
Statistical analysis
Statistical analyses were performed using the StatView software (Abacus Concepts Inc., Berkeley, CA). Data are means ± SD. Percentages were compared by the χ2 test. Means were compared using the Student’s t-test.
Ethics approval
Data were retrospectively extracted from the Medifirst clinical database used in our department. This database is approved for clinical research by the French National Commission for Information Technology and Civil Liberties (CNIL). According to French law (2012–300), patients are aware that their data can be used for anonymous clinical studies unless they specifically state otherwise. This information is detailed in posters in the rooms in the center and patients can inform the center in writing if they do not wish to participate in clinical studies.
Results
Over a period of 34 months, 338 embryos were warmed after MV in 266 cycles for 181 couples, while 212 embryos were warmed after AV in 162 cycles for 101 couples.
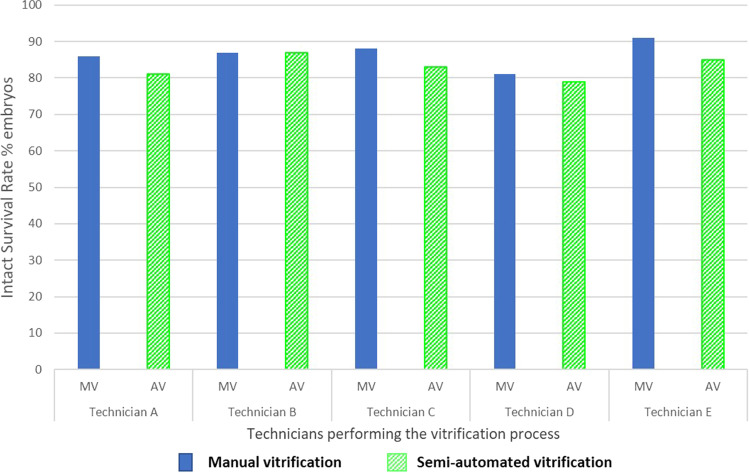
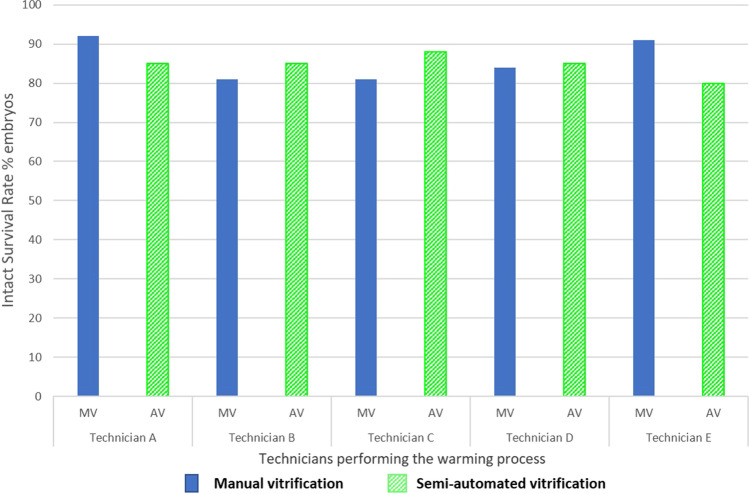
There appeared to be no inter-operator variation in the performance of vitrification or warming using MV or the AV system. There was no significant difference in positive survival rate and intact survival rate neither for technicians who performed vitrification nor those who performed warming (Tables 2 and 3, Figs. 2 and 3). After logistic regression and adjustment for transfer score, intact survival rates by technicians who performed vitrification or warming with MV and AV were not statistically different (p > 0.05), when each technician was compared to the technician with the best result (Supplementary Table 1).
Table 2.
Results of vitrified/warmed embryo cycles considering the operator who performed the vitrification or warming, using the semi-automated GAVI® device. Data with the same superscript are significantly different
| Vitrification operator | A | B | C | D | E | _ | _ | _ | _ | _ |
| Warming operator | _ | _ | _ | _ | _ | A | B | C | D | E |
| Mean female age at vitrification | 33.7 ± 3.9a,b | 32.6 ± 3.7c | 33.8 ± 4.0d,e | 36.4 ± 3.6a,c,d,f | 31.2 + 4.2b,e,f | 33.6 ± 3.5 | 33.9 ± 3.5 g | 33.1 ± 5.2 | 32.0 + 4.1 g | 35.9 ± 4.3 |
| Number of warming cycles | 18 | 56 | 31 | 23 | 34 | 21 | 50 | 17 | 26 | 48 |
| Number of transfers | 18 | 56 | 31 | 23 | 34 | 21 | 50 | 17 | 26 | 48 |
| Number of warmed embryos | 21 | 71 | 42 | 39 | 39 | 26 | 65 | 24 | 33 | 64 |
| Number of lost embryos during warming | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 0 | 1 |
| Positive survival rate (%) (% embryos with at least 50% of intact blastomeres) | 18/21 (86) | 66/71 (93) | 38/42 (90) | 34/39 (87) | 35/39 (90) | 23/26 (88) | 59/65 (91) | 23/24 (96) | 29/33 (88) | 57/64 (89) |
| Intact survival rate (% embryos with 100% of intact blastomeres) | 17/21 (81) | 62/71 (87) | 35/42 (83) | 31/39 (79) | 33/39 (85) | 22/26 (85) | 55/65 (85) | 21/24 (88) | 28/33 (85) | 51/64 (80) |
a,b,d,e,gp < 0.05; c,fp < 0.0001
Table 3.
Results of vitrified/warmed embryo cycles considering the operator who performed the manual vitrification or warming with Irvine®-CBS®
| Vitrification operator | A | B | C | D | E | _ | _ | _ | _ | _ |
| Warming operator | _ | _ | _ | _ | _ | A | B | C | D | E |
| Mean female age at vitrification | 33.4 ± 4.9 | 33.5 ± 4.2 | 32.9 ± 5.2 | 32.6 ± 4.5 | 32.5 ± 3.3 | 33.3 ± 4.8 | 33.7 ± 4.4 | 33.2 ± 4.0 | 32.7 ± 4.6 | 32.6 ± 4.8 |
| Number of thawing cycles | 65 | 71 | 55 | 45 | 30 | 57 | 67 | 23 | 54 | 65 |
| Number of transfers | 65 | 71 | 55 | 45 | 30 | 57 | 67 | 23 | 54 | 65 |
| Number of thawed embryos | 81 | 91 | 72 | 59 | 35 | 73 | 85 | 36 | 63 | 81 |
| Number of lost embryos during thawing | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Positive survival rate (%) (% embryos with at least 50% of intact blastomeres) | 79/81 (97) | 87/91 (96) | 68/72 (94) | 54/59 (92) | 35/35 (100) | 73/73 (100) | 81/85 (95) | 32/36 (89) | 59/63 (94) | 78/81 (96) |
| Intact survival rate (% embryos with 100% of intact blastomeres) | 70/81 (86) | 79/91 (87) | 63/72 (88) | 48/59 (81) | 32/35 (91) | 67/73 (92) | 69/85 (81) | 29/36 (81) | 53/63 (84) | 74/81 (91) |
Fig. 2.
Intact survival rate with the 2 techniques (MV: manual vitrification with Irvine-CBS and AV: semi-automated with the GAVI system) after vitrification/warming according to the technician who performed the vitrification process. There was no significant difference in intact survival rates between technicians
Fig. 3.
Intact survival rate with the 2 techniques (MV: manual vitrification with Irvine-CBS and AV: semi-automated with the GAVI system) after vitrification/warming according to the technician who performed the warming process. There was no significant difference in intact survival rates between technicians
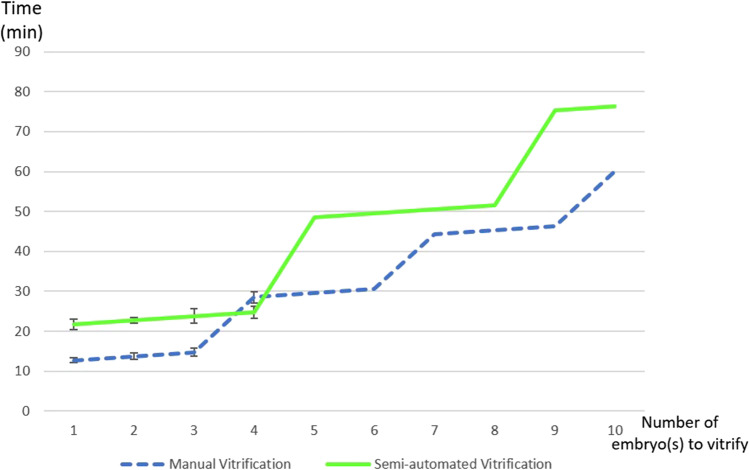
The duration of the vitrification processes are indicated in Fig. 4. For example, to vitrify 1 embryo, the time was 12 min 45 s with MV and 21 min 45 s with AV. For 4 embryos from the same couple, the time was 28 min 50 s with MV and 24 min 45 s with AV. For 10 embryos from the same couple, the time was 60 min with MV and 76 min 15 s with AV.
Fig. 4.
Times for the vitrification process with both techniques according to the number of embryo(s) to be vitrified for the same patient. We precisely measured the times with both techniques according to the number of embryos to be vitrified (from 1 to 4 embryos). Beyond 4 embryos and up to 10 embryos, the technical times were extrapolated. The threshold of 4 was chosen because up to 4 embryos can be individually vitrified during the same cycle with AV. For manual vitrification, when there were several embryos to be vitrified, they were initiated every 1 min and by a maximum of 3 at a time
For all the technicians combined, Table 1 shows that the positive survival rate was higher after MV than after AV (respectively 96% (323/338) and 90% (191/212); p < 0.05). The percentages of fully intact embryos and clinical pregnancies were not significantly different for MV and AV; and the implantation rates showed a non-significant trend in favor of MV (p = 0.06).
Discussion
Vitrification is a highly technical ART procedure. In this study, we wanted to evaluate the theoretical advantages of automating vitrification by measuring inter-operator reproducibility and technique time compared to manual vitrification. This study novelly compared the inter-technician variability of both techniques by independently considering vitrification and warming. Survival rates and intact survival rates are reliable criteria to define a successful thawing procedure performed by individual operators in the lab [12], but it cannot alleviate errors in embryo recovery. In terms of cleavage stage embryo survival rates, both semi-automated and MV showed nonstatistically significant variability according to the technician who performed vitrification or who performed warming. In light of these results, the manual feature of the technique should not be presented as a weakness. The GAVI system allows control of vitrification variables such as temperature, the exact duration of incubation, the diffusion gradients of solutions at the embryo level and the cooling rate [14]. Our biological and clinical results show that these critical steps can be sufficiently standardized with low inter-operator variability using MV.
These reassuring data reveal that, in a center with qualified technicians or embryologists, the success rate is not dependent on the operator who vitrifies or warms. Low variability can be easily achieved and should be monitored and maintained by each laboratory. In light of our results, we are convinced that the main technical challenge of the manual vitrification/warming process is not the difficulty of accomplishing manual steps but rather a good and detailed definition and description of all the steps in the process: conditions of use and details on media aliquots, the time and temperature to equilibrate solutions, how to transfer the embryo from one media drop to another with as little fluid as possible, the size of the drop containing the embryo and how to load the embryo in the straw. The warming process is also very important [16]: the volume of the warming solution and minimizing the time of exposure to the warming solution outside the incubator are very important (affects the warming rate). Once all these steps are clearly specified in the vitrification/warming procedure and are familiar to all technicians, they present no particular difficulty for an experienced embryologist. In AV, the results could be modified by some steps that remain manual during the semi-automated process: filling the pod with medium, depositing embryos in the pod before placement in the machine and all the manual warming steps. The necessity to consider several quality controls factors to integrate a novel vitrification program in an IVF lab has been shown [8]. Many technical factors related to the homogeneity of the amount of vitrification solution surrounding the embryo in the straw, labelling, cryogenic storage safety, sealing and visualization of the embryo upon warming can ultimately influence the final outcome [8]. Besides the need for technical accuracy, precision, and well-defined operating modes as explained above, there are several alternative protocols (including different solutions, devices, volumes, temperatures and time of equilibration) which, by themselves, could influence results regardless of the technician’s skill.
In this study, we did not attempt to evaluate the learning time required to achieve a good level of experience in vitrification, but rather the inter-operator reliability once the operator was qualified. We showed that when carried out by trained embryologists, MV and AV show low inter-operator variability. In relation to other ART techniques, ICSI for example, the published data shows that after the operator has become competent, performance indicators (fertilization rates for ICSI) were comparable between operators [17]. We showed that this also holds true for manual vitrification-warming.
It should be noted that there was non-recovery of 3 out of 212 (1.4%) embryos using AV and 2 out of 338 (0.6%) using MV (NS). Although this difference is nonstatistically significant, it raises an important question since such events must absolutely be avoided. We do not know the cause of these events: increased loss with automation or difficulty to visualize the embryo in the device upon warming?
Vitrification is a time-consuming task. One of the questions asked regarding the potential benefits of using AV is the time saved compared to the manual technique. As shown in Fig. 4, apart from vitrifying 4 embryos, for which the GAVI technique is shorter by 3 min 45 s, the MV technique is quicker in all other cases (minus 11 ± 9 min) with a difference of up to 29 min if 9 embryos are vitrified. The GAVI system was capable of vitrifying up to 4 embryos from the same couple simultaneously (with 1 embryo per pod in order to perform a single-embryo-transfer). Therefore, the “stepped” form of Fig. 4 is linked to the fact that it is possible to load up to 4 embryos from the same patient at the same time during one vitrification cycle with GAVI, and for MV we initiated vitrification every minute (maximum of 3 embryos at one time). Therefore, we waited for the end of the vitrification of the 3rd embryo before initiating the 4th. It is important to consider the fact that we measured these times in cases where all the embryos belonged to the same couple. When this is not the case, only one patient should be initiated per automated cycle, unlike with MV for which we can initiate vitrification for several patients’ embryos with intervals of 1 min on different workstations. As such, when vitrifying 3 embryos from 3 different couples, the GAVI technique requires 69 min 15 s (3 automated cycles with 1 embryo per cycle and 2 min between 2 cycles when there is only one GAVI apparatus) instead of 14 min 45 s with MV. Besides the longer total technique time with the GAVI technique, we can nevertheless highlight the peace of mind it provides, with the possibility to attend to other tasks during the 15-min automated cycle (for cleavage stage embryos). We found that technicians had some difficulty completing other tasks in less than 15 min (during the automated cycle).
One limitation of our study is that it was not carried out on all embryo stages. Further studies are required to assess inter-operator variability at the blastocyst stage. However, there is no reason to believe that differences in method efficiency would be observed as blastocyst survival is equally high (> 95%). The advantage of evaluating inter-operator variability for early-stage embryos is the ability to objectively and easily evaluate the endpoint criteria, i.e., blastomere survival. Survival rate does not allow us to assess the impact of technique on the actual ability of the embryo to develop. Therefore, the implantation rate is very useful to assess real embryo quality but it could be influenced by many other clinical and biological factors.
The strength of our study is that both techniques were performed during the same time period and by the same operators. All the lab technicians were trained and qualified to perform both techniques. Furthermore, our design allowed us to distinguish the impact of the vitrification process from that of warming. We think that both processes involve critical steps that can influence performance and outcomes.
Our study is the first to report on clinical outcomes after vitrification-warming of early human embryos using a semi-automated system. Despite positive survival rates favoring MV, the semi-automated system provided results similar to those of MV which confirms the feasibility of GAVI reaching quality benchmarks established by others [12]: positive survival rate and intact survival rate were 96% and 86%, respectively, after manual vitrification-warming (benchmark values 95% and 85%) and 90% and 84% after semi-automated vitrification-warming (Table 1). Moreover, our positive and fully intact survival rates of cleavage stage embryos using MV were quite higher than those already published with the same vitrification medium and device [18]. We found no significant difference in intact survival rate between the two techniques. It has been shown that full intact embryos have better developmental potential than non-intact embryos [19]. The cooling and warming rates in the GAVI pod estimated at 14,100 °C/min and 11,000 °C/min, respectively [14] which are significantly higher than those reported for closed vitrification systems with high security CBS straws (1000 to 2000 °C/min) [20]. Those cooling rates were also validated in another closed CBS straw system [21]. Therefore, the potential benefit of the higher cooling rate with closed system GAVI pods compared to conventional closed-type systems such as HSV CBS on embryo viability is considered small, and as a result may not have affected clinical outcomes in this study. A high cooling rate could be a benefit for oocyte vitrification as stated by others [6] but we did not evaluate AV on this point. Moreover, the importance of warming rates to prevent recrystallization events that could adversely affect cellular survival of vitrified embryos has been highlighted by some authors [16, 22, 23]. To avoid an excess of ice crystals, it must exceed the cooling rate to ensure a high survival rate [8] (not reached with GAVI). The GAVI showed comparable clinical outcomes (implantation and pregnancy rate) to MV for human cleavage stage embryo. Additionally, in terms of efficiency, we must consider the cost of vitrification (including the medium and devices) which is 3 to 4 times more expensive with AV than with MV (excluding the purchasing cost of the GAVI apparatus).
In conclusion, while there are many questions concerning the need to standardize and automate the technique due to several critical steps involved in the manual technique, our study is the first to show that both semi-automated vitrification (AV) and manual vitrification (MV) have low inter-operator variability. Contrary to what was assumed by other authors [24, 25], we showed, with measured data, that the GAVI method does not reduce inter-operator variability and does not reduce the time required for multiple embryo vitrification compared to manual vitrification.
We provided the first clinical results on semi-automated vitrification of cleavage stage embryos. This study shows that the automated GAVI system can successfully vitrify human cleavage stage embryos with high survival rates (> 90%) as with the manual technique. The GAVI showed comparable clinical outcomes (implantation and pregnancy rate) to MV for human cleavage stage embryo. Further randomized trials are required to evaluate pregnancy and childbirth.
Given the major importance of oocyte and embryo vitrification in ART laboratories, automation should be developed. However, this study confirms the need for caution before using new automated techniques which should be evaluated in terms of time saved, cost and clinical efficiency in comparison to the benchmark technique.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
No specific funding was sought for the study. The University Hospital supported the authors throughout the study period and manuscript preparation.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liebermann J, Dietl J, Vanderzwalmen P, Tucker MJ. Recent developments in human oocyte, embryo and blastocyst vitrification: where are we now? Reprod Biomed Online. 2003;7(6):623–633. doi: 10.1016/s1472-6483(10)62084-6. [DOI] [PubMed] [Google Scholar]
- 2.Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23(2):139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mourad S, Brown J, Farquhar C. Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;1:CD012103. doi: 10.1002/14651858.CD012103.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet. 2014;384(9950):1302–1310. doi: 10.1016/S0140-6736(14)60834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dessolle L, Biau DJ, de Larouziere V, Ravel C, Antoine JM, Darai E, et al. Learning curve of vitrification assessed by cumulative summation test for learning curve (LC-CUSUM) Fertil Steril. 2009;92(3):943–945. doi: 10.1016/j.fertnstert.2009.01.133. [DOI] [PubMed] [Google Scholar]
- 6.Vajta G, Rienzi L, Ubaldi FM. Open versus closed systems for vitrification of human oocytes and embryos. Reprod Biomed Online. 2015;30(4):325–333. doi: 10.1016/j.rbmo.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Jin B, Mazur P. High survival of mouse oocytes/embryos after vitrification without permeating cryoprotectants followed by ultra-rapid warming with an IR laser pulse. Sci Rep. 2015;5:9271. doi: 10.1038/srep09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiewe M. Quality control factors influencing the successful and reliable implementation of oocyte and embryo vitrification. Cryopreservation in Eukaryotes. 2016;181–98. 10.5772/65332.
- 9.Tannus S, Dahan MH, Tan J, Tan SL. Issues related to human oocyte vitrification: a consideration of the facts. J Assist Reprod Genet. 2018;35(7):1157–1158. doi: 10.1007/s10815-018-1184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gatimel N, Ladj M, Teston C, Lesourd F, Fajau C, Cohade C, et al. How many embryos should be transferred? A validated score to predict ongoing implantation rate. Eur J Obstet Gynecol Reprod Biol. 2017;212:30–36. doi: 10.1016/j.ejogrb.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Eshre Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod Biomed Online. 2017;35(5):494–510. doi: 10.1016/j.rbmo.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Alpha Scientists In Reproductive Medicine The Alpha consensus meeting on cryopreservation key performance indicators and benchmarks: proceedings of an expert meeting. Reprod Biomed Online. 2012;25(2):146–167. doi: 10.1016/j.rbmo.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Alpha Scientists in Reproductive Medicine and Eshre Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83. 10.1093/humrep/der037. [DOI] [PubMed]
- 14.Roy TK, Brandi S, Tappe NM, Bradley CK, Vom E, Henderson C, et al. Embryo vitrification using a novel semi-automated closed system yields in vitro outcomes equivalent to the manual Cryotop method. Hum Reprod. 2014;29(11):2431–2438. doi: 10.1093/humrep/deu214. [DOI] [PubMed] [Google Scholar]
- 15.Kovacic B, Plas C, Woodward BJ, Verheyen G, Prados FJ, Hreinsson J, et al. The educational and professional status of clinical embryology and clinical embryologists in Europe. Hum Reprod. 2015;30(8):1755–1762. doi: 10.1093/humrep/dev118. [DOI] [PubMed] [Google Scholar]
- 16.Seki S, Mazur P. The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure. Cryobiology. 2009;59(1):75–82. doi: 10.1016/j.cryobiol.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durban M, Garcia D, Obradors A, Vernaeve V, Vassena R. Are we ready to inject? Individualized LC-CUSUM training in ICSI. J Assist Reprod Genet. 2016;33(8):1009–1015. doi: 10.1007/s10815-016-0686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debrock S, Peeraer K, Fernandez Gallardo E, De Neubourg D, Spiessens C, D’Hooghe TM. Vitrification of cleavage stage day 3 embryos results in higher live birth rates than conventional slow freezing: a RCT. Hum Reprod. 2015;30(8):1820–30. 10.1093/humrep/dev134. [DOI] [PubMed]
- 19.Yu L, Jia C, Lan Y, Song R, Zhou L, Li Y, et al. Analysis of embryo intactness and developmental potential following slow freezing and vitrification. Syst Biol Reprod Med. 2017;63(5):285–293. doi: 10.1080/19396368.2017.1362060. [DOI] [PubMed] [Google Scholar]
- 20.AbdelHafez F, Xu J, Goldberg J, Desai N. Vitrification in open and closed carriers at different cell stages: assessment of embryo survival, development, DNA integrity and stability during vapor phase storage for transport. BMC Biotechnol. 2011;11:29. doi: 10.1186/1472-6750-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiewe MC, Zozula S, Anderson RE, Fahy GM. Validation of microSecure vitrification (muS-VTF) for the effective cryopreservation of human embryos and oocytes. Cryobiology. 2015;71(2):264–272. doi: 10.1016/j.cryobiol.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Rall WF. Factors affecting the survival of mouse embryos cryopreserved by vitrification. Cryobiology. 1987;24(5):387–402. doi: 10.1016/0011-2240(87)90042-3. [DOI] [PubMed] [Google Scholar]
- 23.Mazur P, Seki S. Survival of mouse oocytes after being cooled in a vitrification solution to -196 degrees C at 95 degrees to 70,000 degrees C/min and warmed at 610 degrees to 118,000 degrees C/min: A new paradigm for cryopreservation by vitrification. Cryobiology. 2011;62(1):1–7. doi: 10.1016/j.cryobiol.2010.10.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miwa A, Noguchi Y, Hosoya K, Mori Y, Sato T, Kasahara Y, et al. Equivalent clinical outcome after vitrified-thawed blastocyst transfer using semi-automated embryo vitrification system compared with manual vitrification method. Reprod Med Biol. 2020;19(2):164–170. doi: 10.1002/rmb2.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dal Canto M, Moutier C, Brambillasca F, Guglielmo MC, Bartolacci A, Fadini R, et al. The first report of pregnancies following blastocyst automated vitrification in Europe. J Gynecol Obstet Hum Reprod. 2019;48(7):537–540. doi: 10.1016/j.jogoh.2019.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.