Abstract
Spirometry and testing for bronchodilator response have been recommended to detect asthma, and a bronchodilator response (BDR) of ≥12% and ≥200 mL has been suggested to confirm asthma. However, the clinical value of bronchodilation tests in newly diagnosed steroid-naïve adult patients with asthma remains unknown.
We evaluated the sensitivity of BDR in forced expiratory volume in 1 s (FEV1) as a diagnostic test for asthma in a real-life cohort of participants in the Seinäjoki Adult Asthma Study. In the diagnostic phase, 369 spirometry tests with bronchodilation were performed for 219 steroid-naïve patients. The fulfilment of each test threshold was assessed. According to the algorithm of the National Institute for Health and Care Excellence, we divided the patients into obstructive (FEV1/forced vital capacity (FVC) <0.70) and non-obstructive (FEV1/FVC ≥0.70) groups.
Of the overall cohort, 35.6% fulfilled ΔFEV1 ≥12% and ≥200 mL for the initial FEV1, 18.3% fulfilled ΔFEV1 ≥15% and ≥400 mL for the initial FEV1, and 36.1% fulfilled ΔFEV1 ≥9% of predicted FEV1 at least once. One-third (31%) of these steroid-naïve patients was obstructive (pre-bronchodilator FEV1/FVC <0.7). Of the obstructive patients, 55.9%, 26.5% and 48.5%, respectively, met the same thresholds. In multivariate logistic regression analysis, different thresholds recognised different kinds of asthma patients.
In steroid-naïve adult patients, the current BDR threshold (ΔFEV1 ≥12% and ≥200 mL) has low diagnostic sensitivity (36%) for asthma. In obstructive patients, sensitivity is somewhat higher (56%) but far from optimal. If the first spirometry test with bronchodilation is not diagnostic but asthma is suspected, spirometry should be repeated, and other lung function tests should be used to confirm the diagnosis.
Short abstract
In steroid-naïve adult patients with asthma, immediate bronchodilator response ΔFEV1 ≥12% and ≥200 mL has low diagnostic sensitivity for asthma https://bit.ly/3ut5eZ1
Introduction
The diagnosis of asthma has often been based only on a history of typical variable symptoms. The use of objective lung function measurements has been recommended to increase the precision of asthma diagnosis [1–4]. Asthma guidelines and reports present several approaches to the diagnostic work-up [2, 5, 6]. Airway obstruction in spirometry with immediate bronchodilation response (BDR) has been recommended as the main diagnostic sign [7], although the sensitivity and specificity remain obscure [8, 9]. Additional tests, such as exhaled nitric oxide (FENO), peak expiratory flow (PEF) monitoring and challenge tests, have also been recommended [2, 5, 6].
Most commonly, ΔFEV1 of the initial FEV1 ≥12% and ≥200 mL has been defined as diagnostic for asthma. Some studies prefer expressing BDR as the ΔFEV1% of the predicted FEV1 to overcome the influence of age, sex, height and pre-test obstruction [10–14]. Recently, the evidence behind the recommendation of BDR level has been evaluated [15]. In population-based studies, the upper 95th percentile of the absolute ΔFEV1BDR in healthy persons was 240–320 mL, and the ΔFEV1% of the initial FEV1 was 5.9–13.3% [15]. If measured, ΔFEV1% of the predicted FEV1 varied less (8.7–11.6%). There are few previous patient studies on the clinical value of the BDR [11, 16–20]. However, interpretation of these studies is difficult, as some of the patients included had undefined obstructive airway disease with missing data on medication and duration of possible asthma. Additional data are needed to assess the sensitivity of any ΔFEV1BDR cut-off value for diagnosing adult asthma in steroid-naïve patients [15, 21].
The Seinäjoki Adult Asthma Study (SAAS) includes patients with chronic asthma from diagnosis until a 12-year follow-up visit [22, 23]. The SAAS cohort offers a unique possibility to evaluate the diagnostics of asthma in adults because asthma diagnosis was based on typical symptoms, objective lung function measurements and clinical judgement by respiratory specialists [22]. The aim of the present study was to evaluate the sensitivity of BDR as a diagnostic tool for asthma in steroid-naïve patients in the SAAS cohort.
Methods
Study population
SAAS is a prospective, single-centre 12-year follow-up study of adult-onset asthma (ClinicalTrials.gov NCT02733016). Newly diagnosed patients were consecutively recruited from the respiratory department of the Seinäjoki Central Hospital during 1999–2002. The study covered the majority (>94%) of new adult asthma cases at the study site, representing >38% of the cases in the geographical area [24, 25]. Study patients were referred to the hospital due to suspicion of asthma mainly by primary care physicians and in most cases lung function measurements were conducted before referral. The inclusion criteria were as follows: 1) new-onset asthma, 2) asthma diagnosis confirmed by objective lung function measurements, 3) symptoms typical of asthma, and 4) age ≥15 years [22] (eTable 1). Participants gave written informed consent to the study protocol approved by the Ethics Committee of Tampere University Hospital, Tampere, Finland (R12122). The SAAS cohort included 257 newly onset adult asthma patients, of whom 203 (79%) were reached 12-years later for a follow-up visit. The basic characteristics, 12-year prognosis, phenotypes, smoking characteristics and comorbidities of the SAAS cohort have been described previously [23, 25–29]. After the 12-year follow-up, almost all patients had chronic asthma (remission rate 3%); asthma was controlled in only 34% [23], and 5.9% fulfilled the European Respiratory Society/American Thoracic Society criteria of severe asthma [25].
Study spirometries and BDR thresholds
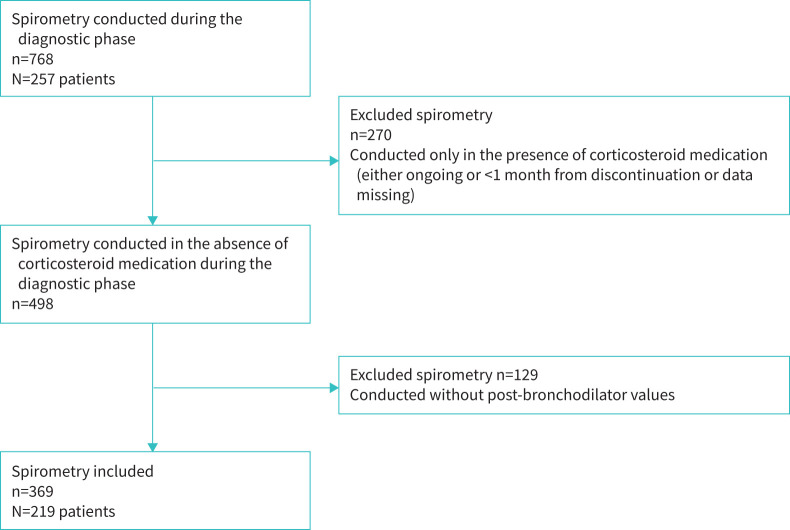
All pre-diagnostic spirometries were collected from the medical records of both primary and secondary care. A thorough chart review of the concurrent corticosteroid medication (inhaled or oral) was performed at the time of each spirometry test. Only spirometries of steroid-naïve patients were chosen, i.e. spirometries measured during corticosteroid medication or <1 month from discontinuation were excluded as well as those with insufficient medication data (n=270). Altogether, 768 spirometries were available, for an average of 2.98 per study patient. The time between spirometries of the same patient varied from days to several months. Finally, 369 spirometry tests (48%) with bronchodilation that were measured in 219 subjects without any inhaled corticosteroid/oral corticosteroid treatment during the previous 4-weeks were included, with an average of 1.68 spirometries per study patient (figure 1 and supplementary material). The three methods to calculate the BDR were absolute volume, ΔFEV1% of the initial FEV1 and ΔFEV1% of the predicted FEV1 (eTable 2). Fulfilments of the following thresholds for bronchodilator response were evaluated as follows.
FIGURE 1.
Flow chart of the study to obtain a sample of spirometry tests with bronchodilator in the Seinäjoki Adult Asthma Study study.
Absolute change:
• ≥200 mL
• ≥400 mL
ΔFEV1% of the initial FEV1 and absolute change:
• ≥12% and ≥200 mL
• ≥12% and ≥400 mL
• FEV1 ≥15% and ≥200 mL
• FEV1 ≥15% and ≥400 mL
ΔFEV1% of the predicted FEV1
• ≥8%
• ≥9%
• ≥10%
Study patients
From each patient, one spirometry (n=219) with the highest ΔFEV1% measured from the initial FEV1 was chosen. The National Institute for Health and Care Excellence (NICE) recommends pre-bronchodilator obstruction defined as FEV1/forced vital capacity (FVC) <0.7 as a starting point in the process of asthma diagnosis [6]. To test this, we divided study patients into obstructive (FEV1/FVC <0.7) or non-obstructive (FEV1/FVC ≥0.70) patients.
Statistical analysis
Continuous data are expressed as the mean (sd) or median and interquartile range. The independent-samples t-test, the Mann–Whitney U-test, and the χ2 test were used for comparisons between two groups. Multivariable binary logistic regression analysis was performed to find variables predicting the fulfilment of BDR thresholds. The correlation matrix was analysed, and the explanatory variables not strongly correlated (R<0.7) were included in the analysis. Statistical analyses were performed using IBM SPSS Statistics software, version 24 (IBM SPSS, Armonk, NY, USA). A p-value <0.05 was regarded as statistically significant. The performance of FEV1/FVC for predicting fulfilment of FEV1 reversibility threshold 12% and 200 mL was evaluated using the receiver-operator characteristic (ROC) curve.
Results
Study patients
Of the overall patient cohort, 85% (N=219) had acceptable spirometry with bronchodilation tests without corticosteroid treatment (figure 1). Their mean age was 47 years, and the majority of them were female (58%) and non-atopic (66%). One-half of patients (52%) had a history of smoking, and 21% were current smokers (table 1). Importantly, if BDR did not confirm an asthma diagnosis, PEF monitoring and additional asthma diagnostic tests were performed (eTable 3).
TABLE 1.
Characteristics of the study patients and lung function from spirometry showing the highest reversibility at the diagnostic phase in steroid-naïve patients
| Characteristics | Study patients (N=219) |
| Age, years | 47±15 |
| Age of asthma onset, years | 47±15 |
| Female | 126 (57.5%) |
| BMI, kg·m-2 | 27.1 (24.0–30.4) |
| Height, cm | 170±10 |
| Smoking history | 113 (51.6%) |
| Current smokers | 45 (20.5%) |
| Pack-years# | 15 (5–22) |
| Atopy¶ | 67 (34.3%) |
| Blood eosinophils ×109 per L | 0.25 (0.17–0.40) |
| Total IgE, kU·L−1 | 80 (34–170) |
| Pre-BD FEV1, L | 2.77±0.89 |
| Pre-BD FEV1, % predicted | 78±17 |
| Post-BD FEV1, L | 3.06±0.95 |
| Post-BD FEV1, % predicted | 86±17 |
| Pre-BD FVC, L | 3.74±1.11 |
| Pre-BD FVC, % predicted | 87±16 |
| Post-BD FVC, L | 3.95±1.12 |
| Post-BD FVC, % predicted | 92±16 |
| Pre-BD FEV1/FVC | 0.75 (0.68–0.81) |
| Post-BD FEV1/FVC | 0.79 (0.72–0.84) |
Data are presented as mean±sd, n (%) or median (interquartile range). BMI: body mass index; Ig: immunoglobulin; FEV1: forced expiratory volume in 1 s; BD: bronchodilator; FVC: forced vital capacity. #: Among those with any smoking history. ¶: At least one positive skin prick test for common allergens.
The mean and median BDRs in the study cohort are shown in table 2. As the mean (294 mL, 11.6% of the initial FEV1) and median (230 mL, 9.5% of the initial FEV1) values for the highest BDR were relatively low, the result suggests that the number of patients fulfilling, for example. ΔFEV1 ≥12% and ≥200 mL of the initial FEV1, may be low.
TABLE 2.
Bronchodilator responses in spirometry with the highest reversibility chosen from each steroid-naïve asthma patient (N=219)
| Mean±sd | Median (IQR) | Patients | |
| ΔFEV1, mL | 294±270 | 230 (130–400) | 219 |
| ΔFVC, mL | 210±354 | 130 (30–300) | 219 |
| ΔFEV1, % of the initial FEV1 | 11.6±10.7 | 9.5 (4.8–15.3) | 219 |
| ΔFVC, % of the initial FVC | 6.6±10.9 | 3.7 (0.8–8.5) | 219 |
| ΔFEV1, % of the predicted FEV1 | 8.3±7.2 | 7.0 (3.9–10.8) | 219 |
IQR: interquartile range; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity. The data are not normally distributed. The mean values are shown to make it easier to compare results with other studies.
Bronchodilator responses in all study spirometries
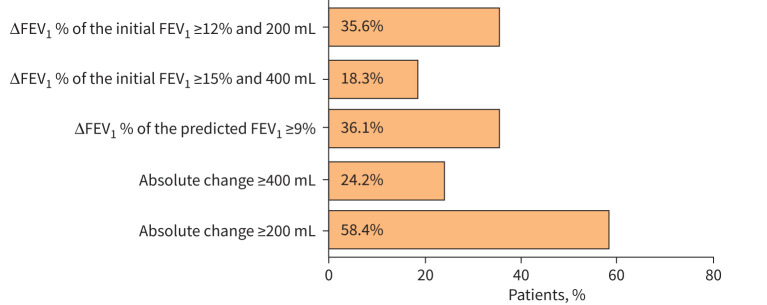
BDR in spirometries (n=369) was analysed according to the following thresholds: ≥12%, ≥15%, ≥200 mL and ≥400 mL measured from the initial FEV1 and ≥8%, ≥9% or ≥10% measured from the predicted FEV1, or their combinations. The proportion of patients fulfilling each of the most commonly used thresholds is shown in figure 2. Most of the patients fulfilled more than one criterion (44.8%), while 91 patients (41.6%) did not fulfil any of the thresholds (eTable 4).
FIGURE 2.
Percentages of asthma patients fulfilling the commonly used thresholds to define bronchodilator response. ΔFEV1: change in forced expiratory volume in 1 s; FEV1: forced expiratory volume in 1 s.
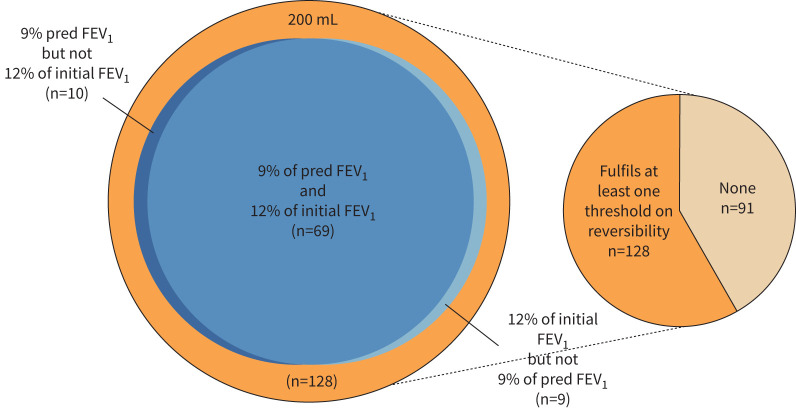
The commonly used threshold in the asthma diagnostics for BDR (ΔFEV1 ≥12% and 200 mL of the initial FEV1) was fulfilled by every third patient. Absolute BDR ≥200 mL was the most frequently fulfilled threshold (∼58%), but ≥400 mL was reached by only one-quarter of patients. Of the percentage changes, the highest proportion (>43%) of patients fulfilled the threshold of ΔFEV1% of the predicted FEV1 ≥8% (eTable 4). Nearly the same proportion fulfilled the threshold of ΔFEV1 ≥12%, and 200 mL of the initial FEV1 also fulfilled the threshold of ΔFEV1% of the predicted FEV1 ≥9% (36.1%). These two patient groups largely overlapped (figure 3). However, there was a group (n=19) of patients who fulfilled one percentage change criterion but not the other (figure 3).
FIGURE 3.
Venn diagram of the asthma patients (N=219) fulfilling the bronchodilator response thresholds of absolute volume 200 mL, change in forced expiratory volume in 1 s (ΔFEV1) ≥12% of the initial FEV1 and ΔFEV1 % of the predicted FEV1 ≥9%.
Different BDR criteria may identify different patients [8, 30]. To evaluate this, the groups fulfilling either ΔFEV1 ≥12% of the initial FEV1 and 200 mL or ΔFEV1% of the predicted FEV1 ≥9% were analysed (eTable 5). Lung function (FEV1 and FVC) was significantly better in the subgroup in which only the BDR threshold of 9% of predicted was fulfilled (n=10) compared with patients fulfilling ΔFEV1 ≥12% of the initial FEV1 and 200 mL (eTable 5). For example, the mean pre-bronchodilator FEV1 was 92±8% and 52±14%, respectively.
Predictors of the fulfilment of two thresholds
As patient-related features may be associated with diagnostic criteria, predictors of the fulfilment of the two thresholds (ΔFEV1 >9% of the predicted FEV1 and ΔFEV1 ≥12% of the initial FEV1 +200 mL) were surveyed by multivariate logistic regression analysis (table 3). An association was found between low pre-bronchodilator FEV1 (<80%) and fulfilment of both thresholds. Low total immunoglobulin E (IgE), high blood eosinophils and high FVC tended to predict the fulfilment of at least one of the thresholds (table 3).
TABLE 3.
Multivariable odds ratios for factors at the diagnostic visit associated with the fulfilment of thresholds of change in forced expiratory value in 1 s (ΔFEV1) >9% of predicted FEV1 and ΔFEV1 ≥12% and 200 mL of the initial FEV1
| ΔFEV1 ≥9% of predicted FEV1 | p-value | ΔFEV1 ≥12% of the initial FEV1+200 mL | p-value | |
| Age ≥45 years | 1.54 (0.73–3.22) | 0.258 | 1.72 (0.77–3.85) | 0.190 |
| Male | 0.71 (0.33–1.50) | 0.365 | 0.43 (0.19–1.00) | 0.050 |
| Symptoms, AQ20 | 1.10 (0.97–1.14) | 0.228 | 1.02 (0.94–1.11) | 0.630 |
| Total IgE <100 kU·L−1 | 2.06 (0.97–4.37) | 0.060 | 2.84 (1.24–6.51) | 0.014 |
| Blood eosinophils >0.25×109 per L | 1.90 (0.89–4.10) | 0.097 | 2.55 (1.10–5.88) | 0.029 |
| Post-bronchodilator FEV1/FVC<0.7 and ≥10 pack-years | 0.26 (0.60–1.11) | 0.690 | 0.39 (0.11–1.43) | 0.155 |
| Pre-bronchodilator FEV1 <80% predicted# | 6.03 (2.11–17.21) | <0.001 | 15.93 (5.00–50.80) | <0.001 |
| Pre-bronchodilator FVC >90% predicted# | 4.71 (1.68–13.18) | 0.003 | 2.90 (0.99–8.53) | 0.053 |
Ig: immunoglobulin; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; AQ20: Airways Questionnaire 20. #: Measured from the spirometry with highest reversibility. Data are presented as ORs (95% CIs). BMI and smoking were not significantly associated with the thresholds and were excluded from the model. Statistically significant associations are presented in bold.
Patients with pre-bronchodilator FEV1/FVC <0.7 versus FEV1/FVC ≥0.7
31% (n=68) of the study patients had pre-bronchodilator FEV1/FVC <0.7. They were older, more often males, and more often had a smoking history (eTable 6). However, there were no differences in blood eosinophils, IgE, symptoms, current smoking or pack-years between the groups. More patients reached the suggested criteria for ACO (asthma–COPD overlap; ≥10-pack-years and post-bronchodilator FEV1/FVC <0.7) if pre-BD FEV1/FVC was <0.7 than if pre-bronchodilator FEV1/FVC was ≥0.7, 32.3% and 2%, respectively (eTable 6). Reversibility was significantly higher in patients with pre-bronchodilator FEV1/FVC <0.7 than in those with pre-BD FEV1/FVC ≥0.7 (table 4). Diagnostic criteria in these groups also differed (eTable 7).
TABLE 4.
Bronchodilator responses in steroid-naïve asthma patients with pre-bronchodilator (pre-BD) forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) <0.7 versus FEV1/FVC ≥0.7 (N=219)
| pre-BD FEV1/FVC ≥0.7 (n=151) | pre-BD FEV1/FVC <0.7 (n=68) | p-value | |
| ΔFEV1, mL | 210 (110–370) | 285 (180–478) | 0.002 |
| ΔFVC, mL | 110 (20–240) | 200 (90–320) | 0.012 |
| ΔFEV1, % of the initial FEV1 | 7.3 (3.8–12.5) | 13.5 (9.3–19.4) | <0.001 |
| ΔFVC % of the initial FVC | 3.0 (0.5–6.6) | 6.4 (2.2–8.5) | 0.008 |
| ΔFEV1, % of the predicted FEV1 | 6.0 (3.2–9.8) | 8.9 (5.8–13.2) | 0.001 |
Data are presented as the median (interquartile range). Spirometry showing the highest reversibility chosen from each patient.
Seven of the nine BDR thresholds were fulfilled more often in patients with pre-bronchodilator FEV1/FVC <0.7 (table 5). The sensitivity of the BDR measurement (ΔFEV1 ≥12% and 200 mL of the initial FEV1 fulfilled by 55.9% of the patients) was better in obstructive patients than in the whole group (35.6%). Nevertheless, almost half of patients did not fulfil this criterion. However, even in the group of asthma patients with pre-bronchodilator FEV1/FVC <0.7, 27.9% of patients met none of the criteria (table 5).
TABLE 5.
Different thresholds of bronchodilator response in steroid-naïve asthma patients with pre-bronchodilator forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) <0.7 versus FEV1/FVC ≥0.7 measured from spirometry with the highest reversibility chosen from each patient (N=219)
| pre-BD FEV1/FVC ≥0.7 (n=151) | pre-BD FEV1/FVC <0.7 (n=68) | p-value | |
| Absolute change ≥200 mL | 80 (53.0%) | 48 (70.6%) | 0.018 |
| Absolute change ≥400 mL | 32 (21.2%) | 20 (29.4%) | 0.229 |
| ΔFEV1 % of the initial FEV1 ≥12% and 200 mL | 40 (26.5%) | 38 (55.9%) | <0.001 |
| ΔFEV1 % of the initial FEV1 ≥15% and 400 mL | 21 (13.9%) | 18 (26.5%) | 0.035 |
| ΔFEV1 % of the initial FEV1 ≥12% and 400 mL | 26 (17.2%) | 19 (27.9%) | 0.074 |
| ΔFEV1 % of the initial FEV1 ≥15% and 200 mL | 27 (17.9%) | 30 (44.1%) | <0.001 |
| ΔFEV1 % of the predicted FEV1 ≥8% | 58 (38.4%) | 38 (55.9%) | 0.019 |
| ΔFEV1 % of the predicted FEV1 ≥9% | 46 (30.5%) | 33 (48.5%) | 0.015 |
| ΔFEV1 % of the predicted FEV1 ≥10% | 37 (24.5%) | 29 (42.6%) | 0.010 |
| None of the thresholds was fulfilled | 70 (46.4%) | 19 (27.9%) | 0.012 |
Data are presented as n (%).
We performed ROC analysis to find the optimum FEV1/FVC cut-off predicting patient fulfilling criteria of ΔFEV1 ≥12% and 200 mL of the initial FEV1. The area under the curve of the model is 0.71 (p<0.001), indicating that FEV1/FVC fairly predicts this reversibility threshold. The optimum cut-off value for FEV1/FVC was 0.72, yielding sensitivity of 67.2% and specificity 74.7% (eTable 8 and eFigure 1).
Discussion
The role of bronchodilation tests to confirm the reversibility of airway obstruction in asthma diagnostics is central even though the clinical value has remained unclear. In this study, we tested different thresholds of BDR in steroid-naïve patients with asthma during the diagnostic phase. The most commonly used threshold of diagnostic BDR for asthma ΔFEV1 ≥12% and 200 mL of the initial FEV1 was fulfilled in 35.6% of the study patients. ΔFEV1 ≥9% of the predicted FEV1 was fulfilled in 36.1% of the patients, and the groups were mainly the same. Only one-third (31%) of the newly diagnosed asthma patients were obstructive, as defined by pre-bronchodilator FEV1/FVC <0.7. Among the obstructive patients, a higher proportion (55.9%) fulfilled the BDR criterion ΔFEV1 ≥12% and 200 mL of the initial FEV1. To the best of our knowledge, this is the first study to evaluate the sensitivity of the bronchodilation test and its different thresholds during the diagnostic phase in adult patients with clinically confirmed chronic asthma.
Recently, we evaluated the evidence behind the quantifiable improvement in FEV1 after short-acting bronchodilator administration as a significant change or as a diagnostic method in adult asthma [15]. Most of the previous studies included COPD patients, or the diagnosis was unclear. Most studies did not report data on steroid treatment, duration of asthma before the bronchodilator test or use of other diagnostic tests [15]. Even a short period of inhaled or oral steroid treatment can reduce BDR in spirometry [31]. In our real-life SAAS cohort including steroid-naïve patients from different phenotypes and all age groups ≥15 years, sensitivity to reach the threshold of immediate ΔFEV1BDR ≥12% and 200 mL of the initial FEV1 was 35.6%. The sensitivity of the same threshold was 13% in a Danish study involving mainly atopic young adults with minor smoking history [9] and 9% in a subgroup of asthma patients [21] both with ongoing steroid treatment. These results are in line with ours; the role of spirometry in asthma diagnostics is not nearly exclusive, especially if only the threshold of ΔFEV1BDR ≥12% and ≥200 mL of the initial FEV1 is used.
In four population-based studies of non-smoking healthy subjects, the upper 95th percentile of the ΔFEV1% of the initial FEV1 varied between 9.0–13.3%, and the ΔFEV1% of the predicted FEV1 varied less, 8.7–11.6%. [10, 12–14]. Expressing BDR as the ΔFEV1% of the predicted FEV1 [10–13] and/or as a change in the z-score [14] has been preferred to overcome the influence of age, sex, height and obstruction. For the same reason, the requirement of a fixed minimum change of >200 mL in FEV1 has been considered unrealistic [14]. It has also been suggested that ΔFEV1% of the predicted FEV1 between 9.0–10.0% may allow better discrimination between patients with asthma and COPD [11, 20, 32]. In subjects with ΔFEV1% >8% of the predicted FEV1 (diagnosis unclear, 43% on inhaled corticosteroids) has been reported to have a survival advantage because of the clinically important reversibility [33]. In our cohort, the sensitivity of the threshold of predicted FEV1 ≥9% for asthma (36.1%) was the same as for the threshold of initial FEV1 ≥12% and 200 mL (35.8%). ΔFEV1% of the predicted FEV1 ≥8% detected more subjects with asthma (43.6%). Previously, 17.9% of patients with current self-reported asthma (diagnostic method and therapy not stated) fulfilled BDR ≥9.0% of the predicted [8]. The four reversibility thresholds (ΔFEV1 ≥400 mL, ΔFEV1% of the initial FEV1 ≥12% or ≥15%, ΔFEV1% of the predicted FEV1 ≥9%) identified different kinds of patients [8]. In another study, 22% of untreated patients with mild asthma had reversibility of ≥12% and ≥200 mL, while adopting a threshold of 9% of predicted FEV1, the proportion increased to 32% [34]. In our study, the subgroup of patients with ΔFEV1BDR ≥12% and 200 mL of the initial FEV1 was almost the same as those with BDR ≥9.0% of the predicted. Patients fulfilling only the threshold of ≥9.0% of the predicted FEV1 were younger and had significantly better lung function than those showing ΔFEV1BDR ≥12% and 200 mL of the initial FEV1 but not ≥9.0% of the predicted FEV1. In a population-based study, thresholds of ΔFEV1BDR ≥12% and 200 mL were found in 17.3% of patients with self-reported asthma (therapy not stated and not withdrawn), and were associated with wheeze and atopy, total IgE and FENO [30]. Associations of the clinical features and the fulfilment of the different thresholds in our cohort were weaker. In contrast, the ΔFEV1 ≥12%+200 mL threshold in our patient population was associated with low IgE but high blood eosinophils. Adult-onset asthma is less often associated with allergy than childhood-onset asthma, but high eosinophils occur in many asthma patients at all ages. We consider that the most important clinical implication of this is that also non-atopic patients who have asthma onset later in life and present with eosinophilia may be a subgroup that can be recognised with the bronchodilator threshold of ΔFEV112%+200 mL. Our cohort included only steroid-naïve patients with newly diagnosed chronic adult-onset asthma of all severity grades, which might explain the differences against previous studies.
Recent NICE guidelines recommend objective lung function tests to diagnose adult asthma [6]. The first step in the NICE algorithm is to divide patients based on obstruction (pre-bronchodilator FEV1/FVC <0.7 or FEV1/FVC ≥0.7). According to NICE, bronchodilator tests should be performed only in obstructive (pre-bronchodilator FEV1/FVC <0.7) patients; otherwise, measurements such as FENO and PEF monitoring are recommended. One-third (31%) of the patients in our cohort had pre-bronchodilator FEV1/FVC <0.7. In this subgroup, ΔFEV1BDR≥12% and 200 mL was fulfilled in 55.9% of the patients, and other thresholds (except absolute change ≥400 mL) of BDR were more commonly fulfilled than in the subgroup of patients with pre-bronchodilator FEV1/FVC ≥0.7. However, in this latter group, reversibility was still found (ΔFEV1BDR ≥12% and 200 mL in 26.5%) and even more often if the threshold of ΔFEV1% of the predicted FEV1 ≥8% was used (38%). Our real-life cohort of steroid-naïve patients with asthma partly supports the NICE algorithm, as BDR thresholds are fulfilled more often if pre-bronchodilator FEV1/FVC is <0.7. Conversely, in the subgroup of patients with pre-bronchodilator FEV1/FVC ≥0.7, significant reversibility was found in every fourth patient, supporting the use of the bronchodilator test regardless of the pre-bronchodilator FEV1/FVC value. We also performed ROC analysis and found that FEV1/FVC only fairly predicts the fulfilment of ΔFEV1 ≥12% and 200 mL of the initial FEV1, further supporting that the recommendation to measure reversibility only in patients with FEV1/FVC <0.70 [6] is not optimal.
The main strengths of our study are asthma diagnosis based on evaluation by respiratory specialists in conjunction with symptoms, objective lung function measurements, and follow-up for 12 years with a low remission rate (3%) [23]. Thus, our results represent the clinical value of immediate BDR as a diagnostic test in steroid-naïve adult patients with chronic asthma. The availability [35] and quality [36] of the spirometry measurement were good during the collection of the study cohort. The small size of our cohort could be considered a limitation, but due to active use of lung function tests, 768 spirometry measurements were found, averaging 2.98 per study patient. The aim of our study was to evaluate BDR in steroid-naïve patients, which still provided an average of 1.7 spirometries per patient. The diagnostic threshold of BDR in our study cohort was FEV1 ≥15% and 200 mL, which might have influenced patient selection and decreased the sensitivity of the BDR test. On the other hand, subjects were included as asthmatic if they fulfilled other lung function criteria, such as excess variability or reversibility of PEF monitoring or positive challenge test. Low remission rate (3%) after follow-up for 12-years [23] ensures that patients in the SAAS cohort represent patients with chronic asthma starting at adult age. We acknowledge that the results may not be generalizable to a patient group showing temporary asthma symptoms or mild seasonal asthma that is asymptomatic most of the year.
If the diagnostic value of a test is intended to be assessed, the test should be evaluated in the diagnostic phase of the disease. While underdiagnosis and overdiagnosis are common in patients with asthma-like symptoms [3], we need retrospective studies from the diagnostic phase of patients known to have chronic asthma. Spirometry with bronchodilation tests has been the starting point if adult asthma is suspected. If the test is not diagnostic, other lung function tests, including PEF monitoring, provocation tests, and empiric steroid treatment tests, should be considered [37]. We analysed the spirometry with the highest BDR from each patient, but pre-bronchodilator FEV1/FVC <0.7 was still found in only one-third of measurements, and the sensitivity of the ΔFEV1 ≥12% and 200 mL in our adult-onset asthma patients was only 36%. Adult-onset asthma is a heterogeneous disease with several phenotypes [38, 39]. The role of diagnostic tests may vary between phenotypes due to different pathogeneses and other factors. Is it possible to enhance the sensitivity of the bronchodilation test in younger patients with milder disease, for example, by using additional thresholds of ΔFEV1% measured from the predicted FEV1 (8%–10%)? In the SAAS cohort, the fulfilment of the diagnostic threshold of immediate BDR (FEV1 ≥15% and ≥200 mL from the initial FEV1) varied between the clusters: early-onset, atopic asthma (43.6%), smokers’ asthma (42.1%), obese asthma (28%), female asthma (20%) and non-rhinitic asthma (18%) [28]. Larger studies of the clinical value of the different thresholds of immediate BDR among steroid-naïve adult asthma patients representing different phenotypes are needed.
Overall, in the SAAS cohort, the diagnostic sensitivity of the BDR test was low (35.6%) if the threshold of ΔFEV1BDR ≥12% and ≥200 mL measured from the initial FEV1 was used. Of the obstructive (pre-bronchodilator FEV1/FVC <0.7) patients, 55.9% reached the same threshold. Among non-obstructive patients, one-fourth reached significant BDR, which should be taken into account in clinical practice. The BDR test must be carried out at least once for every patient with prolonged respiratory symptoms, even though other tests are often needed before clinical conclusions.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00293-2021.SUPPLEMENT (850.6KB, pdf)
Acknowledgements
Aino Sepponen (Dept of Respiratory Medicine, Seinäjoki Central Hospital, Seinäjoki, Finland) is gratefully acknowledged for her help through all the stages of this work.
Provenance: Submitted article, peer reviewed.
This study is registered at www.ClinicalTrials.gov with identifier number NCT02733016.
This article has supplementary material available from openres.ersjournals.com
Author contributions: L.E. Tuomisto, P. Ilmarinen, M. Tommola, L. Lehtimäki, O. Niemelä and H. Kankaanranta designed the study and wrote the report. P. Ilmarinen performed the statistical analyses. All authors contributed to interpretation of the data. All authors made critical revisions of the manuscript and approved the final version of the manuscript.
Conflict of interest: L.E. Tuomisto reports personal fees and nonfinancial support from Boehringer Ingelheim, and personal fees from AstraZeneca, outside the submitted work.
Conflict of interest: P. Ilmarinen is an employee of GlaxoSmithKline, and reports personal fees from Mundipharma, AstraZeneca and Novartis, outside the submitted work.
Conflict of interest: L. Lehtimäki reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, GSK, Novartis, Mundipharma, Orion Pharma, Sanofi and Teva outside the submitted work.
Conflict of interest: O. Niemelä has nothing to disclose.
Conflict of interest: M. Tommola reports personal fees from AstraZeneca, personal fees and nonfinancial support from Boehringer Ingelheim, personal fees from Pfizer, grants from the Orion Research Foundation, and personal fees from Chiesi and GSK, outside the submitted work.
Conflict of interest: H. Kankaanranta reports grants, personal fees and nonfinancial support from AstraZeneca; personal fees from Chiesi Pharma AB; personal fees and nonfinancial support from Boehringer Ingelheim; personal fees from Novartis and Mundipharma; personal fees and nonfinancial support from Orion Pharma; personal fees from SanofiGenzyme and GlaxoSmithKline, outside the submitted work.
Support statement: This study is supported by Tampere Tuberculosis Foundation (Tampere, Finland), the Finnish Anti-Tuberculosis Association Foundation (Helsinki, Finland), the Väinö and Laina Kivi Foundation (Helsinki, Finland), the Allergy Research Foundation (Helsinki, Finland), the Research Foundation of the Pulmonary Diseases (Helsinki, Finland), the Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital (Tampere, Finland), and the Medical Research Fund of Seinäjoki Central Hospital (Seinäjoki, Finland). None of the sponsors had any involvement in the planning, execution, drafting or write-up of this study.
References
- 1.Reddel HK. Treating according to asthma control: does it work in real life? Clin Chest Med 2012; 33: 505–517. doi: 10.1016/j.ccm.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. Available from: www.ginasthma.org/. Date last accessed: February 15th 2020.
- 3.Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA 2017; 317: 269–279. doi: 10.1001/jama.2016.19627 [DOI] [PubMed] [Google Scholar]
- 4.Aaron SD, Boulet LP, Reddel HK, et al. Underdiagnosis and overdiagnosis of Asthma. Am J Respir Crit Care Med 2018; 198: 1012–1020. doi: 10.1164/rccm.201804-0682CI [DOI] [PubMed] [Google Scholar]
- 5.British Thoracic Society, Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma 2019. https://www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/. Date last accessed March, 2020.
- 6.National Institute for Health and Care Excellence (NICE) . Asthma: Diagnosis, Monitoring and Chronic Asthma Management. London, NICE, 2017. [PubMed] [Google Scholar]
- 7.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 8.Appleton SL, Adams RJ, Wilson DH, et al. Spirometric criteria for asthma: adding further evidence to the debate. J Allergy Clin Immunol 2005; 116: 976–982. doi: 10.1016/j.jaci.2005.08.034 [DOI] [PubMed] [Google Scholar]
- 9.Backer V, Sverrild A, Ulrik CS, et al. Diagnostic work-up in patients with possible asthma referred to a university hospital. Eur Clin Respir J 2015; 2: 27768. doi: 10.3402/ecrj.v2.27768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dales RE, Spitzer WO, Tousignant P, et al. Clinical interpretation of airway response to a bronchodilator. Epidemiologic considerations. Am Rev Respir Dis 1988; 138: 317–320. doi: 10.1164/ajrccm/138.2.317 [DOI] [PubMed] [Google Scholar]
- 11.Brand PL, Quanjer P, Postma DS, et al. Interpretation of bronchodilator response in patients with obstructive airways disease. The Dutch Chronic NonSpecific Lung Disease (CNSLD) Study Group. Thorax 1992; 47: 429–436. doi: 10.1136/thx.47.6.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan WC, Vollmer WM, Lamprecht B, et al. Worldwide patterns of bronchodilator responsiveness: results from the Burden of Obstructive Lung Disease study. Thorax 2012; 67: 718–726. doi: 10.1136/thoraxjnl-2011-201445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torén K, Bake B, Olin AC, et al. Measures of bronchodilator response of FEV1, FVC and SVC in a Swedish general population sample aged 50–64 years, the SCAPIS Pilot Study. Int J Chron Obstruct Pulmon Dis 2017; 12: 973–980. doi: 10.2147/COPD.S127336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quanjer PH, Ruppel GL, Langhammer A, et al. Bronchodilator response in FVC is larger and more relevant than in FEV1 in severe airflow obstruction. Chest 2017; 151: 1088–1098. doi: 10.1016/j.chest.2016.12.017 [DOI] [PubMed] [Google Scholar]
- 15.Tuomisto LE, Ilmarinen P, Lehtimäki L, et al. Immediate bronchodilator response in FEV1 as a diagnostic criterion for adult asthma. Eur Respir J 2019; 53: 1800904. doi: 10.1183/13993003.00904-2018 [DOI] [PubMed] [Google Scholar]
- 16.Nicklaus TM, Burgin WW, Jr, Taylor JR. Spirometric tests to diagnose suspected asthma. Am Rev Respir Dis 1969; 100: 153.e9. [DOI] [PubMed] [Google Scholar]
- 17.Eliasson O, Degraff AC, Jr. The use of criteria for reversibility and obstruction to define patient groups for bronchodilator trials. Influence of clinical diagnosis, spirometric, and anthropometric variables. Am Rev Respir Dis 1985; 132: 858–864. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrino R, Rodarte JR, Brusasco V. Assessing the reversibility of airway obstruction. Chest 1998; 114: 1607–1612. doi: 10.1378/chest.114.6.1607 [DOI] [PubMed] [Google Scholar]
- 19.Ouksel H, Meslier N, Badatcheff-Coat A, et al. Influence of predicted FEV1 on bronchodilator response in asthmatic patients. Respiration 2003; 70: 54–59. doi: 10.1159/000068419 [DOI] [PubMed] [Google Scholar]
- 20.Silvestri IC, Pereira CA, Rodrigues SC. Comparison of spirometric changes in the response to bronchodilators of patients with asthma or chronic obstructive pulmonary disease. J Bras Pneumol 2008; 34: 675–682. doi: 10.1590/S1806-37132008000900007 [DOI] [PubMed] [Google Scholar]
- 21.Tan DJ, Lodge CJ, Lowe AJ, et al. Bronchodilator reversibility as a diagnostic test for adult asthma: findings from the population-based Tasmanian Longitudinal Health Study. ERJ Open Res 2021; 7: 00042-2020. doi: 10.1183/23120541.00042-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kankaanranta H, Ilmarinen P, Kankaanranta T, et al. Seinajoki Adult Asthma Study (SAAS): A protocol for a 12-year real-life follow-up study of new-onset asthma diagnosed at adult age and treated in primary and specialised care. NPJ Prim Care Respir Med 2015; 25: 15042. doi: 10.1038/npjpcrm.2015.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuomisto LE, Ilmarinen P, Niemela O, et al. A 12-year prognosis of adult-onset asthma: Seinäjoki Adult Asthma Study. Respir Med 2016; 117: 223–229. doi: 10.1016/j.rmed.2016.06.017 [DOI] [PubMed] [Google Scholar]
- 24.Tuomisto LE, Erhola M, Luukkaala T, et al. Asthma programme in Finland: did the use of secondary care resources become more rational? Respir Med 2010; 104: 957–965. doi: 10.1016/j.rmed.2010.01.018 [DOI] [PubMed] [Google Scholar]
- 25.Ilmarinen P, Tuomisto LE, Niemelä O, et al. Prevalence of patients eligible for anti-IL-5 treatment in a cohort of adult-onset asthma. J Allergy Clin Immunol Pract 2019; 7: 165–174.e4doi: 10.1016/j.jaip.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Ilmarinen P, Tuomisto LE, Niemelä O, et al. Comorbidities and elevated IL-6 associate with negative outcome in adult-onset asthma. Eur Respir J 2016; 48: 10521062. doi: 10.1183/13993003.02198-2015 [DOI] [PubMed] [Google Scholar]
- 27.Tommola M, Ilmarinen P, Tuomisto LE, et al. The effect of smoking on lung function: a clinical study of adult-onset asthma. Eur Respir J 2016; 48: 1298–1306. doi: 10.1183/13993003.00850-2016 [DOI] [PubMed] [Google Scholar]
- 28.Ilmarinen P, Tuomisto LE, Niemelä O, et al. Cluster analysis on longitudinal data of patients with adult-onset asthma. J Allergy Clin Immunol Pract 2017; 5: 967–978.e3. doi: 10.1016/j.jaip.2017.01.027 [DOI] [PubMed] [Google Scholar]
- 29.Tommola M, Ilmarinen P, Tuomisto LE, et al. Differences between asthma–COPD overlap syndrome and adult-onset asthma. Eur Respir J 2017; 49: 1602383. doi: 10.1183/13993003.02383-2016 [DOI] [PubMed] [Google Scholar]
- 30.Janson C, Malinovschi A, Amaral AFS, et al. Bronchodilator reversibility in asthma and COPD: findings from three large population studies. Eur Respir J 2019; 54: 1900561. doi: 10.1183/13993003.00561-2019 [DOI] [PubMed] [Google Scholar]
- 31.Kerstjens HA, Brand PL, Quanjer PH, et al. Variability of bronchodilator response and effects of inhaled corticosteroid treatment in obstructive airways disease. Dutch CNSLD Study Group. Thorax 1993; 48: 722–729. doi: 10.1136/thx.48.7.722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meslier N, Racineux JL, Six P, et al. Diagnostic value of reversibility of chronic airway obstruction to separate asthma from chronic bronchitis: a statistical approach. Eur Respir J 1989; 2: 497–505. [PubMed] [Google Scholar]
- 33.Ward H, Cooper BC, Miller MR. Improved criterion for assessing lung function reversibility. Chest 2015; 148: 877–886. doi: 10.1378/chest.14-2413 [DOI] [PubMed] [Google Scholar]
- 34.Louis R, Bougard N, Guissard F, et al. Bronchodilation test with inhaled salbutamol versus bronchial methacholine challenge to make an asthma diagnosis: do they provide the same information? J Allergy Clin Immunol Pract 2020; 8: 618–625.e8. doi: 10.1016/j.jaip.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 35.Erhola M, Mäkinen R, Koskela K, et al. The Asthma Programme of Finland: an evaluation survey in primary health care. Int J Tuberc Lung Dis 2003; 7: 592–598. [PubMed] [Google Scholar]
- 36.Tuomisto LE, Järvinen V, Laitinen J, et al. Asthma Programme in Finland: the quality of primary care spirometry is good. Prim Care Respir J 2008; 17: 226–231. doi: 10.3132/pcrj.2008.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drake S, Wang R, Healy L, et al. Diagnosing asthma with and without aerosol-generating procedures. J Allergy Clin Immunol Pract 2021: in press [ 10.1016/j.jaip.2021.07.006]. [DOI] [PubMed] [Google Scholar]
- 38.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012; 18: 716–725. doi: 10.1038/nm.2678 [DOI] [PubMed] [Google Scholar]
- 39.Ilmarinen P, Tuomisto LE, Kankaanranta H. Phenotypes, Risk factors, and mechanisms of adult-onset asthma. Mediators Inflamm 2015; 2015: 514868. doi: 10.1155/2015/514868 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00293-2021.SUPPLEMENT (850.6KB, pdf)