Abstract
High-flow nasal cannula (HFNC) oxygen therapy has recently shown clinical benefits in hypoxaemic acute respiratory failure (ARF) patients, while the value of noninvasive ventilation (NIV) remains debated. The primary end-point was to compare alveolar recruitment using global end-expiratory electrical lung impedance (EELI) between HFNC and NIV. Secondary end-points compared regional EELI, lung volumes (global and regional tidal volume variation (VT)), respiratory parameters, haemodynamic tolerance, dyspnoea and patient comfort between HFNC and NIV, relative to face mask (FM).
A prospective randomised crossover physiological study was conducted in patients with hypoxaemic ARF due to pneumonia. They received alternately HFNC, NIV and FM.
16 patients were included. Global EELI was 4083 with NIV and 2921 with HFNC (p=0.4). Compared to FM, NIV and HFNC significantly increased global EELI by 1810.5 (95% CI 857–2646) and 826 (95% CI 399.5–2361), respectively. Global and regional VT increased significantly with NIV compared to HFNC or FM, but not between HFNC and FM. NIV yielded a significantly higher pulse oxygen saturation/inspired oxygen fraction ratio compared to HFNC (p=0.03). No significant difference was observed between HFNC, NIV and FM for dyspnoea. Patient comfort score with FM was not significantly different than with HFNC (p=0.1), but was lower with NIV (p=0.001).
This study suggests a potential benefit of HFNC and NIV on alveolar recruitment in patients with hypoxaemic ARF. In contrast with HFNC, NIV increased lung volumes, which may contribute to overdistension and its potentially deleterious effect in these patients.
Short abstract
This study found a potential benefit of HFNC and NIV on alveolar recruitment in patients with hypoxaemic ARF, but NIV also increases lung volumes, which may give rise to overdistension, reinforcing the concept of patient self-inflicted lung injury https://bit.ly/3iRcZDJ
Introduction
During severe hypoxaemic acute respiratory failure (ARF), invasive or noninvasive respiratory support allows optimised oxygenation by higher inspiratory oxygen fraction (FiO2) and alveolar recruitment with positive end-expiratory pressure (PEEP).
Although noninvasive ventilation (NIV) is widely used in intensive care units (ICUs), it remains controversial in hypoxaemic ARF and was not recommended in the most recent clinical practice guidelines [1]. Indeed, it has been suggested that NIV could be potentially deleterious in hypoxaemic ARF, even leading to poor outcomes compared to other oxygenation techniques, especially when a high tidal volume (VT) (>9 mL per kg of predicted body weight) is applied [2–4].
High-flow nasal cannula (HFNC) oxygen therapy has been developed more recently in adult ICU patients to overcome pitfalls with conventional oxygen (O2) therapy [4, 5] and, consequently, to optimise oxygenation in severe hypoxaemic ARF [4–6]. HFNC has been shown to provide a moderate PEEP effect (2–5 cmH2O), depending on the level of gas flow delivered, on whether the mouth opens or not, and on the patient's size and sex [7–11]. Nevertheless, the PEEP effect does not guarantee significant distal alveolar recruitment. Furthermore, evaluation of alveolar recruitment remains difficult at the patient's bedside outside invasive mechanical ventilation conditions.
Electrical impedance tomography (EIT), a noninvasive device, allows both dynamic visualisation of regional distribution of pulmonary ventilation during each ventilatory cycle (tidal volume variation: VT) and measurement of end-expiratory electrical impedance (EELI), which reflects expiratory lung volume and, thus, indirectly, alveolar end-expiratory recruitment [12]. EELI and VT can be measured globally or regionally from pre-defined lung quadrants. Therefore, EIT appears as an interesting technique for noninvasive alveolar recruitment assessment (EELI) that is both feasible and relevant in patients receiving invasive or noninvasive oxygenation support [13–18].
To our knowledge, no previous study has compared the alveolar recruitment effect by EIT between HFNC and NIV in patients with hypoxaemic ARF.
The aim of our study was to compare the level of alveolar recruitment between HFNC and NIV in patients with hypoxaemic ARF. Secondary objectives were to evaluate the observed difference in regional distribution of VT, respiratory rate and oxygenation, haemodynamic tolerance, dyspnoea and comfort between HFNC and NIV, relative to a face mask (FM).
Material and methods
We performed a single-centre prospective crossover physiological study in our medical ICU between February 2016 and February 2018. It was approved by the local ethics committee (CPP-SC 001/2015) and all patients received a written information letter and gave oral consent.
Study population
Eligible patients were those referred for de novo hypoxaemic ARF due to community-acquired pneumonia confirmed by chest radiography [19], responsible for hypoxaemia (arterial oxygen tension (PaO2) <60 mmHg in ambient air), without hypercapnia (arterial carbon dioxide tension <45 mmHg), requiring >6 L·min−1 of O2 on admission with high-concentration FM for a pulse oxygen saturation (SpO2) >94% and requiring HFNC and NIV alternately, based on the ICU attending physician's judgement and current literature [1, 2]. Exclusion criteria are detailed in the supplementary material.
Experimental protocol
All patients were assessed at bedside in a semi-recumbent position (45°) and alternately received standard O2, HFNC and NIV according to the study protocol (supplementary figure S1). The first patient received NIV in period 1 followed by HFNC in period 2. The following patient received HFNC and NIV in the reverse order of the previous patient, and so on. In fact, a randomisation by alternate plan was determined by the sequence order applied to the first patient. To minimise the residual effect from period 1 (“carryover effect”), patients received FM between the two periods.
Oxygenation was delivered during ≥15 min and all measurements recorded after a breathing stabilisation period of 5 min were analysed.
Standard O2 was delivered through a FM at a maximum flow rate of 15 L·min−1 for a SpO2 >94% (before HFNC and NIV treatment periods).
HFNC oxygen therapy (OptiFlow; Fisher & Paykel Healthcare, Auckland, New Zealand) was delivered at a constant flow rate of 50 L·min−1. The sizes of HFNC cannulae were chosen to maximise congruence with patients’ nostrils. Patients were asked to keep their mouth closed during HFNC periods for maximum PEEP effect. NIV was delivered with an ICU ventilator in pressure support mode. The NIV interface was a commercially available naso-buccal mask (Ultra Mirage NV; ResMed, Martinsried, Germany) individually fitted in order to reduce air leaks. The pressure support level was adjusted individually to achieve an expired tidal volume between 6–9 mL per kg of ideal body weight and an external PEEP of 5 cmH2O was applied. FiO2 was adjusted for a SpO2 >94% with both techniques.
For ethical reasons we did not perform arterial blood gas in each oxygenation condition and chose the SpO2/FiO2 ratio to compare the quality of oxygenation between devices, as reported previously [20, 21]. For FM, FiO2 was estimated using the following standardised formula: FiO2 = 0.21 + oxygen flow rate × 0.03 [20].
EIT measurements (global and regional EELI and VT values) were performed with the Pulmovista device (Dräger, Lübeck, Germany). Measurements, as well as other data collected, are detailed in the supplementary material.
Statistical analysis
The sample size was based on previous similar physiological studies in this field [17, 18, 22, 23]. Patient characteristics were described using median, first and third quartiles (Q1–Q3) for quantitative variables and absolute numbers with their percentagefor categorical variables. The median difference between HFNC and NIV, and its 95% confidence interval, were estimated usingthe Wilcoxon signed rank test or the sign test [24].
In absence of evidence for an interaction and a residual effect (supplementary tables S1 and S2), secondary aims were to compare the results observed with HFNC and NIV, relative to those observed with FM. These comparisons were carried out as described earlier for HFNC and NIV.
A p-value <0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
Study population
16 consecutive patients were included (seven women and nine men); eight received NIV first and eight HFNC first. Patient characteristics and their main outcome data are reported in table 1. Median (interquartile range (IQR)) FiO2 was 60% (48–70%) with a constant flow rate of 50 L·min−1 with HFNC. Median (IQR) values for FiO2 and pressure support level were 55% (48–70%) and 8 (8–9) cmH2O, respectively, with NIV, with a PEEP level set at 5 cmH2O for all patients.
TABLE 1.
Patient characteristics and outcome data
| Patients | Age (years) | Sex | BMI (kg·m−2) | SAPSII | SOFA | Localisation of lung consolidation (ROI1, 2, 3 or 4) | Delay between admission and inclusion (days) | Intubation | ICU length of stay (days) | Death |
| 1 | 63 | M | 25 | 27 | 4 | 3 | 0 | N | 6 | N |
| 2 | 22 | F | 28 | 25 | 3 | 4 | 3 | N | 5 | N |
| 3 | 38 | M | 22 | 22 | 4 | 2 | 4 | N | 6 | N |
| 4 | 19 | M | 17 | 15 | 2 | 1 | 6 | N | 10 | N |
| 5 | 55 | M | 33 | 31 | 3 | 1 | 3 | N | 2 | N |
| 6 | 53 | M | 40 | 56 | 9 | 3 | 15 | N | 17 | N |
| 7 | 71 | M | 26 | 27 | 1 | 3 | 2 | N | 3 | N |
| 8 | 86 | M | 25 | 47 | 4 | 1 | 2 | N | 7 | N |
| 9 | 46 | F | 36 | 16 | 3 | 3 | 6 | N | 5 | N |
| 10 | 42 | M | 21 | 32 | 1 | 4 | 2 | N | 6 | N |
| 11 | 27 | M | 23 | 12 | 6 | 1 | 2 | N | 2 | N |
| 12 | 54 | F | 21 | 21 | 5 | 3 | 2 | Y | 17 | Y |
| 13 | 47 | F | 21 | 28 | 2 | 4 | 3 | N | 4 | N |
| 14 | 69 | F | 22 | 48 | 9 | 3 | 9 | Y | 23 | N |
| 15 | 56 | F | 26 | 33 | 2 | 3 | 3 | N | 6 | N |
| 16 | 33 | F | 46 | 20 | 2 | 4 | 4 | N | 5 | N |
| Total or median (IQR) | 50 (37–58) | 7 F 9 M |
25 (22–29) | 27 (21–32) | 3 (2–4) | 4 ROI1 1 ROI2 7 ROI3 4 ROI4 |
3 (2–5) | 2 Y 14 N |
6 (5–8) | 1 Y 15 N |
BMI: body mass index; SAPSII: Simplified Acute Physiology Score II; SOFA: Sepsis-related Organ Failure Assessment; ROI: region of interest; ICU: intensive care unit; IQR: interquartile range; M: male; F: female; Y: yes; N: no.
Comparison between HFNC and NIV
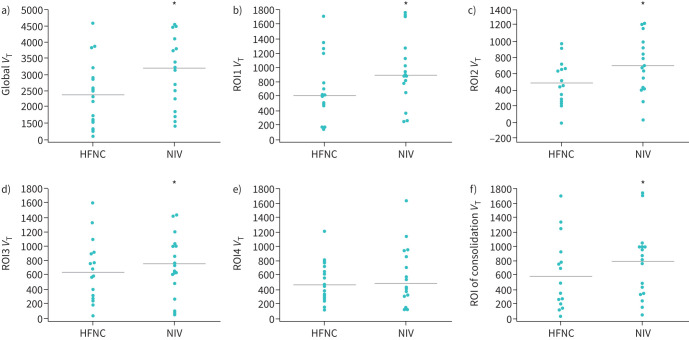
No significant difference was found for global EELI between NIV (median 4083, IQR 2928–5134) and HFNC (2921, 1706–4850), as the 95% confidence interval for the difference ranged from −1649.5 to +824.0 (p=0.4). Regional analysis of EELI (supplementary figure S1) found no significant difference between HFNC and NIV (table 2), except for region of interest (ROI)1 (95% CI −570.5– +110.0; p=0.01). Regarding VT, global VT was significantly higher with NIV (3161, 1884–3805) than with HFNC (2323, 1497–2891; p=0.001). Similarly, regional VT was found higher with NIV than with HFNC in ROI1, ROI2, ROI3 and the consolidation area, but there was no difference in ROI4 VT between NIV (444, 318–861) and HFNC (ROI4 VT NIV versus HFNC 444 (318–861) versus 450 (286–664); p=0.06) (table 2 and figure 1). The SpO2/FiO2 ratio and SpO2 were significantly higher with NIV than with HFNC (167, 143–200 versus 163, 140–200 (p=0.001) and 100, 98–100 versus 97, 96–100 (p=0.010), respectively). No significant difference was observed between HFNC and NIV for other physiological parameters (table 2).
TABLE 2.
Comparison between physiological effects of noninvasive ventilation (NIV) and high-flow nasal cannula (HFNC)
| NIV | HFNC | HFNC–NIV | ||
| Median (IQR) | Median (IQR) | Median (95% CI) | p-value # | |
| VT global (units) | 3161 (1884–3805) | 2323 (1497–2891) | −678.0 (−947.5– −322.0) | 0.001 |
| VT ROI1 (units) | 887 (657–1033) | 590 (464–774) | −204.5 (−279.5– −122.0) | 0.0007 |
| VT ROI2 (units) | 686 (413–925) | 445 (262–656) | −214.0 (−309.0– −130.0) | 0.0003 |
| VT ROI3 (units) | 743 (498–1008) | 589 (271–909) | −118.5 (−221.5–0.0) | 0.04 |
| VT ROI4 (units) | 444 (318–861) | 450 (286–664) | −93.5 (−200.0–7.5) | 0.06 |
| VT consolidation (units) | 778 (338–1002) | 489 (198–783) | −133.0 (−215.0– −53.5) | 0.004 |
| EELI global (units) | 4083 (2928–5134) | 2921 (1706–4850) | −570.5 (−1649.5–824.0) | 0.4 |
| EELI ROI1 (units) | 842 (646–1144) | 562 (215–1000) | −329.0 (−570.5– −110.0) | 0.01 |
| EELI ROI2 (units) | 960 (469–1406) | 408 (355–1152) | −174.0 (−563.0–79.5) | 0.1 |
| EELI ROI3 (units) | 767 (336–1124) | 618 (370–1251) | −101.0 (−487.0–476.0) | 0.5 |
| EELI ROI4 (units) | 846 (488–971) | 447 (373–738) | −196.0 (−491.5–733.0) | 0.4 |
| EELI consolidation (units) | 899 (767–1144) | 486 (381–946) | −322.5 (−588.5–178.5) | 0.1 |
| Respiratory rate (breaths·min −1 ) | 24 (22–27) | 23 (21–26) | −2 (−4–4) | 0.6 |
| SpO2/FiO2 ratio | 167 (143–200) | 163 (140–200) | −4.5 (−15.5– −2.0) | 0.001 |
| SpO2 (%) | 100 (98–100) | 97 (96–100) | −2 (−3–0) | 0.010 |
| Heart rate (beats·min −1 ) | 84 (68–98) | 90 (78–104) | 1 (−2–5) | 0.8 |
| SBP (mmHg) | 119 (108–131) | 125 (113–137) | 3 (−1–11) | 0.2 |
| MAP (mmHg) | 80 (76–89) | 85 (77–94) | 2 (−3–5) | 0.6 |
| Dyspnoea score (0–10) | 5 (0–5) | 5 (2–5) | 0 (−1–1) | 0.7 |
| Patient comfort score (0–10) | 4 (2–5) | 5 (4–7) | 0 (−1–4) | 0.7 |
Bold type represents statistical significance. IQR: interquartile range; VT: tidal volume variation; ROI: region of interest; EELI: end-expiratory lung impedance; SpO2: pulse oxygen saturation; FiO2: inspiratory oxygen fraction; SBP: systolic blood pressure; MAP: mean arterial pressure. #: Wilcoxon signed rank test.
FIGURE 1.
Comparative effects between high-flow nasal cannula (HFNC) and noninvasive ventilation (NIV) on global and regional tidal volume (VT) variation in all patients (n=16). Analysis of VT variation between HFNC and NIV in a) global lung, b) region of interest (ROI)1, c) ROI2, d) ROI3, e) ROI4, f) consolidation area. Data points represent VT values for individual patients with HFNC and NIV, and the horizontal line represents the median value. *: p<0.05 by Wilcoxon's test for dependent variable.
Comparison between HFNC and FM
Global EELI increased significantly with HFNC compared to FM (1444 (992–3468) versus 2921 (1706–4850); p<0.0001). Regional EELI was higher with HFNC in ROI3, ROI4 and the consolidation area, but did not differ significantly in ROI1 and ROI2 (table 3). We did not find any significant difference for global or regional VT between HFNC and FM. Mean arterial pressure increased significantly between HFNC and FM. No significant difference was observed for respiratory rate, heart rate, systolic blood pressure, SpO2/FiO2 ratio, SpO2, dyspnoea score and patient comfort (table 3).
TABLE 3.
Comparison between physiological effects of face mask (FM) and high-flow nasal cannula (HFNC)
| FM | HFNC | HFNC–FM | ||
| Median (IQR) | Median (IQR) | Median (95% CI) | p-value # | |
| VT global (units) | 2240 (1421–2752) | 2323 (1497–2891) | −3.0 (−138.5–153.5) | 0.9 |
| VT ROI1 (units) | 618 (440–692) | 590 (464–774) | −30.0 (−96.0–35.0) | 0.3 |
| VT ROI2 (units) | 408 (295–703) | 445 (262–656) | −18.5 (−63.5–37.0) | 0.4 |
| VT ROI3 (units) | 597 (287–816) | 589 (271–909) | 14.5 (−27.5–113.0) | 0.5 |
| VT ROI4 (units) | 290 (200–708) | 450 (286–664) | 15.0 (−21.0–77.0) | 0.4 |
| VT consolidation (units) | 290 (233–760) | 489 (198–783) | 2.5 (−57.0–40.5) | 0.9 |
| EELI global (units) | 1444 (992–3468) | 2921 (1706–4850) | 826.0 (399.5–2361.0) | <0.0001 |
| EELI ROI1 (units) | 278 (91–634) | 562 (215–1000) | 161.5 (−19.5–322.5) | 0.05 |
| EELI ROI2 (units) | 325 (181–531) | 408 (355–1152) | 187.5 (−31.5–464.0) | 0.1 |
| EELI ROI3 (units) | 378 (125–446) | 618 (370–1251) | 220.0 (57.0–719.0) | 0.01 |
| EELI ROI4 (units) | 309 (130–499) | 447 (373–738) | 169.5 (31.0–1104.0) | 0.01 |
| EELI consolidation (units) | 283 (125–477) | 486 (381–946) | 138.0 (24.5–613.0) | 0.01 |
| Respiratory rate (breaths·min −1 ) | 25 (23–28) | 23 (21–26) | −3 (−6–1) | 0.2 |
| SpO2/FiO2 ratio | 152 (147–152) | 163 (140–200) | 16.0 (−6.0–48.0) | 0.1 |
| SpO2 (%) | 100 (97–100) | 97 (96–100) | 0 (−2–1) | 0.5 |
| Heart rate (beats·min −1 ) | 82 (72–102) | 90 (78–104) | 1 (−2–4) | 0.4 |
| SBP (mmHg) | 118 (107–133) | 125 (113–137) | 6 (0–9) | 0.1 |
| MAP (mmHg) | 81 (74–92) | 85 (77–94) | 2 (1–7) | 0.01 |
| Dyspnoea score (0–10) | 0 (0–5) | 5 (2–5) | 0 (0–3) | 0.3 |
| Patient comfort score (0–10) | 8 (4–9) | 5 (4–7) | −2 (−4–0) | 0.1 |
Bold type reprensents statistical significance. IQR: interquartile range; VT: tidal volume variation; ROI: region of interest; EELI: end-expiratory lung impedance; SpO2: pulse oxygen saturation; FiO2: inspiratory oxygen fraction; SBP: systolic blood pressure; MAP: mean arterial pressure. #: Wilcoxon signed rank test.
Comparison between NIV and FM
Global EELI increased significantly with NIV compared to FM (1999 (764–2779) versus 4083 (2928–5134); p=0.001). Regional EELI was higher with NIV than with FM in all ROIs (table 4). Variations in global and regional EELI between NIV and FM are shown in table 4. A significantly higher global and regional VT was observed with NIV than with FM. The SpO2/FiO2 ratio was higher with NIV than with FM without any difference in SpO2. Patient comfort score was significantly lower with NIV than with FM (table 4).
TABLE 4.
Comparison between physiological effects of face mask (FM) and noninvasive ventilation (NIV)
| FM | NIV | NIV–FM | ||
| Median (IQR) | Median (IQR) | Median (95% CI) | p-value# | |
| VT global (units) | 2402 (1641–3050) | 3161 (1884–3805) | 606.0 (441.5–792.0) | <0.0001 |
| VT ROI1 (units) | 593 (566–737) | 887 (657–1033) | 182.5 (97.0–269.0) | 0.001 |
| VT ROI2 (units) | 479 (321–683) | 686 (413–925) | 182.5 (122.5–251.0) | <0.0001 |
| VT ROI3 (units) | 649 (427–836) | 743 (498–1008) | 132.5 (73.5–194.5) | 0.0002 |
| VT ROI4 (units) | 383 (259–772) | 444 (318–861) | 98.5 (17.0–178.0) | 0.02 |
| VT consolidation (units) | 593 (160–819) | 778 (338–1002) | 151.0 (58.0–215.5) | 0.009 |
| EELI global (units) | 1999 (764–2779) | 4083 (2928–5134) | 1810.5 (857.0–2646.0) | 0.001 |
| EELI ROI1 (units) | 327 (115–618) | 842 (646–1144) | 518.0 (315.5–779.0) | <0.0001 |
| EELI ROI2 (units) | 361 (135–880) | 960 (469–1406) | 457.5 (130.0–818.0) | 0.009 |
| EELI ROI3 (units) | 317 (144–567) | 767 (336–1124) | 414.0 (76.0–678.5) | 0.02 |
| EELI ROI4 (units) | 382 (115–572) | 846 (488–971) | 374.0 (108.0–670.5) | 0.01 |
| EELI consolidation (units) | 562 (160–776) | 899 (767–1144) | 404.5 (59.0–718.0) | 0.02 |
| Respiratory rate (breaths·min −1 ) | 26 (25–30) | 24 (22–27) | −2 (−6–2) | 0.6 |
| SpO2/FiO2 ratio | 152 (145–152) | 167 (143–200) | 21.0 (2.5–50.0) | 0.03 |
| SpO2 (%) | 100 (96–100) | 100 (98–100) | 0 (0–2) | 0.3 |
| Heart rate (beats·min −1 ) | 85 (79–103) | 84 (68–98) | −1 (−3–5) | 0.8 |
| SBP (mmHg) | 122 (109–129) | 119 (108–131) | −1 (−7–11) | 1.0 |
| MAP (mmHg) | 84 (77–91) | 80 ( 76–89) | −1 (−2–4) | 1.0 |
| Dyspnoea score (0–10) | 2 (0–5) | 5 (0–5) | 0 (−1–3) | 0.5 |
| Patient comfort score (0–10) | 8 (5–10) | 4 (2–5) | −3 (−5− −1) | 0.001 |
Bold type reprensents statistical significance. IQR: interquartile range; VT: tidal volume variation; ROI: region of interest; EELI: end-expiratory lung impedance; SpO2: pulse oxygen saturation; FiO2: inspiratory oxygen fraction; SBP: systolic blood pressure; MAP: mean arterial pressure. #: Wilcoxon signed rank test.
Discussion
In this study, we compared the physiological effects of different oxygenation techniques used in the noninvasive management of hypoxaemic ARF secondary to pulmonary infection. We found that both HFNC and NIV increased EELI relative to FM, but, unexpectedly, that there was no significant difference in EELI, i.e. alveolar recruitment between HFNC and NIV. Interestingly, we found that NIV increased VT relative to HFNC and FM, whereas HFNC did not increase VT relative to FM. In addition, better oxygenation (SpO2/FiO2 ratio) was observed with NIV despite similar FiO2 levels between HFNC and NIV. Patient comfort was found to be better with FM, similar to HFNC, but worse with NIV relative to FM.
One previous study showed a close relationship between EELI measured by EIT and end-expiratory lung volume (EELV) measured by an open-circuit, washout manoeuvre during a PEEP titration manoeuvre from 0 to 15 mBar in 10 patients mechanically ventilated in volume-controlled mode [14]. These results suggest that EELI measured with EIT is strongly correlated with EELV and therefore with pulmonary alveolar recruitment or overdistension. Consequently, changes in EELI results observed between the different oxygenation techniques in the present study can be interpreted as changes in EELV, i.e. alveolar recruitment rather than hyperinflation [14, 25].
We found that NIV increased EELI, i.e. alveolar recruitment, both in global lung and all dependent and nondependent lung areas (ROIs), including the consolidation area, relative to FM. To our knowledge, this is the first comparative analysis of alveolar recruitment between FM and NIV. In obese patients, three pre-oxygenation techniques were compared before intubation and a significant increase in EELI after intubation was found among those who received pre-oxygenation with NIV or NIV plus recruitment manoeuvres, relative to those receiving pre-oxygenation with a simple high-concentration FM [26]. However, this study did not provide any comparative EELI results between NIV and FM in any patient before intubation.
In parallel with increased EELI, we observed that NIV also increased VT globally throughout the lung and regionally in all dependent or nondependent ROIs. It has been demonstrated that tidal volume, measured by pneumotachograph, could be increased by nearly 300 mL with NIV in COPD patients with acute exacerbation, relative to standard O2 [27]. However, in contrast with our study, the regional distribution of this volume was not evaluated. The increase of VT in dependent (ROI3+ROI4) and nondependent regions (ROI1+ROI2) observed in our study could further explain the potentially deleterious effect of NIV in hypoxaemic ARF. Indeed, it has been suggested that NIV might increase intubation and mortality rates in more severe hypoxaemic ARF (PaO2/FiO2 ≤200 mmHg) relative to standard O2 or HFNC alone [2]. This poor NIV outcome has been linked to frequent excessive expiratory tidal volumes in hypoxaemic ARF, >9.5 mL per kg of predicted body weight [3]. In fact, as described with invasive mechanical ventilation, NIV could induce lung injury in de novo ARF related to increased expiratory tidal volume due to excessive respiratory drive. Finally, these features have led some experts to define the recent concept of patient self-inflicted lung injury (P-SILI) [28]. Therefore, our finding could add further knowledge to this new physiopathological concept, suggesting a potential excess and heterogeneity in lung volume distribution with NIV responsible for overdistension in healthy areas in hypoxaemic ARF.
Demonstrating a significant increase in EELI with HFNC relative to FM, our study also confirms a potential PEEP effect and alveolar recruitment with HFNC. Such an effect was previously demonstrated in comparison with FM, not only in healthy volunteers [28], but also in post-operative patients undergoing cardiac surgery [17], and more recently in hypoxaemic ARF patients [22]. In fact, the increase in EELI observed with HFNC appears top be proportional to the increase in the HFNC flow rate used in these studies [17, 23, 28]. By testing increasing flow rates with HFNC and measuring EELI, VT, inspiratory effort, compliance and oxygenation in 17 hypoxaemic ARF patients, Mauri et al. [22] found not only a linear increase in EELI, but also no significant change in VT with HFNC relative to FM. As in our study, EELI significantly increased in global lung and dependent ROIs, but not in nondependent ROIs. In contrast, we also specifically evaluated EELI changes in the consolidation area. Finally, our results are consistent with those of Mauri et al. [22], demonstrating that, relative to FM, HFNC can significantly increase EELI in global lung as well as dependent ROIs, but without any deleterious effect on global or regional VT. Moreover, the absence of increase in VT with HFNC suggests that alveolar recruitment can be achieved without risk of overdistension in healthy as well as pathological lung areas.
Pérez-Terán et al. [29] compared HFNC and NIV effect on EELI in healthy subjects. EELI significantly increased with HFNC and NIV, but NIV subjects showed a significant increase in nondependent regions, while the increase was more homogeneous with HFNC. To our knowledge, our study is the first to compare EELI and VT between HFNC and NIV, relative to FM in patients with hypoxaemic ARF. Only one recent study has physiologically compared HFNC with helmet-NIV [30]. These results suggested that helmet-NIV could be more effective than HFNC for moderate-to-severe hypoxaemic ARF. Nevertheless, VT measurements were not provided to exclude potential regional overdistension during helmet-NIV.
We found no evidence for differences in EELI between techniques, either in the global lung or in each ROI including the consolidation area, except in ROI1, a nondependent lung region. In fact, although we have found a potential similar PEEP effect and alveolar recruitment reflected by EELI with HFNC and NIV, these results should take into account the different settings applied in our study with these two techniques. Moreover, although NIV did not improve alveolar recruitment relative to HFNC, we found that NIV significantly increased VT by 26% in global lung, by 36% in nondependent lung regions (ROI1+2) and by 17% in dependent lung regions (ROI3+4), suggesting a higher risk of overdistension with NIV relative to HFNC. In fact, this increase in VT was observed in our study despite the use of nonaggressive NIV settings, i.e. a pressure support level for an expiratory tidal volume of 6–9 mL·kg−1, as suggested previously [3]. As mentioned earlier, this unexpected increase in lung volume associated with an excess in respiratory drive could be responsible for P-SILI [28] and, consequently, for failure and poor outcome with NIV in hypoxaemic ARF [2, 3]. In our opinion, such a risk with NIV should favour the use of HFNC given the absence of additional alveolar recruitment with NIV. Regarding oxygenation, NIV significantly increased the SpO2/FiO2 ratio when compared to HFNC, but no difference was observed between NIV and FM, or between HFNC and FM. Thesrefore, these results could appear somewhat discordant with those of previous studies, as Parke et al. [10] found better oxygenation with HFNC compared to FM. In another study comparing NIV, HFNC and mask with equal FiO2, NIV allowed the best oxygenation performance relative to HFNC and FM, and HFNC was found to be more efficient than FM [31]. The population size of these studies [10, 31] was similar to ours, but one explanation for the discrepancy in oxygenation performance could be the fact that we did not perform arterial blood gases, but only used the SpO2/FiO2 ratio at the end of each experimental period rather than the PaO2/FiO2 ratio. However, the SpO2/FiO2 ratio can exhibit some limitations, mainly if SpO2 is >95% [32].
Additionally, we evaluated patient dyspnoea and respiratory comfort. We did not find any difference in dyspnoea Borg scale between HFNC, NIV and FM, in contrast with one study reporting an improvement in dyspnoea with HFNC compared to NIV or Venturi mask [31]. Respiratory comfort was found to be similar between HFNC and NIV or HFNC and FM, but less so with NIV relative to FM. However, respiratory comfort is highly subjective and other authors reported NIV to be the least comfortable among the three techniques [7, 31].
Our study has several limitations. First, similarly to previous studies [16, 17], we did not measure lung volumes directly to objectively assess alveolar recruitment and PEEP effect or eliminate pulmonary overdistension. Indeed, EIT being an indirect and incomplete evaluation of pulmonary volumes and recruitment, a concomitant assessment of lung volumes with a pneumotachograph could be useful and relevant to optimise the dynamic evaluation of the ventilatory mechanics [23]. Second, it was a physiological study conducted in a short-time span. However, based on previous studies [17, 22], a 15-min period could be considered as sufficient to obtain a stable effect on lung volumes and gas exchanges. Third, we applied oxygenation and ventilation parameters similar to those used in the FLORALI trial [2] to perform EELI and VT measurements. However, we are aware that other settings (pressure support and PEEP level with NIV, HFNC flow rate) might give rise to other results. Fourth, comparison of a “ventilatory” support effect between HFNC and NIV would have been of additional interest. Indeed, although HFNC is primarily considered as an oxygenation technique, it was recently demonstrated that HFNC could also reduce the work of breathing in hypoxaemic ARF patients [22]. Fifth, our relative small sample size, although in line with previous physiological studies [17, 21, 22], could have underpowered the study to detect a potential difference between NIV and HFNC on EELI. Sixth, we exclusively included hypoxaemic or de novo ARF patients with pneumonia for pulmonary homogeneity considerations. Most patients had predominant unilateral pulmonary condensation on chest radiography, but some bilateral infiltrates may have introduced some heterogeneity. Last, EIT imaging displays only one horizontal part of the lungs with regard to the electrode belt, and therefore cannot measure global lung volume changes along the vertical axis. However, previous studies demonstrated good agreement between EIT measurements of lung volume changes and those obtained by other validated methods (spirometry and plethysmography) [33, 34]. Futhermore, Hinz et al. [14] have demonstrated that the cross-sectional lung electrical impedance variation was correlated with EELV during PEEP trial in mechanically ventilated adults. Moreover, the crossover design of our study might have made the comparison of EIT measures more accurate.
Conclusion
Our physiological study, comparing for the first time HFNC and NIV in hypoxaemic ARF, demonstrates an increase in EELV with both techniques, suggesting a similar potential alveolar recruitment relative to FM. In contrast with HFNC, and although NIV improves oxygenation, NIV could also increase lung volumes which may contribute to overdistension and explain its potentially deleterious effect in these hypoxaemic ARF patients.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00373-2021.SUPPLEMENT (273.4KB, pdf)
FIGURE S1 Study protocol, electrode position of the electrical impedance tomography (EIT) belt and lung volume modelization. A. Progress of the experiment: after study inclusion, the first patient included received NIV in period 1 followed by HFNC in period 2. Then, the following patient received HFNC and NIV in the reverse order of the previous patient. To minimize the residual effect from period 1, patients received FM between the 2 periods. Oxygenation was delivered during at least 15 minutes. During this time, EIT measures were recorded. Electrical impedance tomography lay out: B. 16 electrodes united within the same belt were placed on the thorax of the patient facing the alveolar zone of condensation. The reference electrode R was placed on the abdomen. C. Functional EIT images in the acquisition zone defined by the belt. D. Subdivision of the acquisition area into 4 standardized quadrants or regions of interest (ROI) numbered from 1 to 4. 00373-2021.figureS1 (353.1KB, tif)
FIGURE S2 Electrical impedance tomography recordings. EIT: electrical impedance tomography, A: respiratory rate, global and regional tidal variation (TV) in the four regions of interest (ROI) chosen. End-expiratory lung impedance (EELI) measured by EIT in global lung and in each ROI showing the evolution of EELI between B: face mask (green arrows) and NIV (pink arrows) periods; C: HFNC (red arrows) and face mask (green arrows) periods. 00373-2021.figureS2 (353.2KB, tif)
TABLE S1 Comparison of patients' characteristics at inclusion by treatment sequence 00373-2021.tableS1 (122.3KB, pdf)
TABLE S2 Effect of first treatment on patients' characteristics just before second treatment 00373-2021.tableS2 (139.7KB, pdf)
Acknowledgements
The authors are grateful to Nikki Sabourin-Gibbs (Rouen University Hospital, Rouen, France), for her help in editing the manuscript. This work was presented at the Société de Réanimation de Langue Française meeting in Paris, France, on 23–25 January 2019 and accepted at the European Respiratory Society International Congress in Madrid, Spain, on 28 September–2 October 2019.
Footnotes
This article has supplementary material available from openres.erjournals.com
Provenance: Submitted article, peer reviewed.
This manuscript has supplementary material available from openres.ersjournals.com
This study was retrospectively registered at www.clinicaltrials.gov with identifier number NCT04664322.
Data availability: All the individual participant data collected during the trial and after deidentification, including dictionaries, study protocols, the statistical analysis plan, informed consent forms and the clinical study report, will be shared immediately after publication for 3 years, with researchers who provide a methodologically sound proposal to achieve aims in the approved proposal. Proposals should be directly addressed to Christophe Girault (christophe.girault@chu-rouen.fr).
Support statement: The authors thank the Charles Nicolle Foundation for its innovative equipment funding concerning the acquisition of the Pulmovista device (Dräger, Lübeck, Germany).
Author contributions: E. Artaud-Macari, M. Bubenheim and C. Girault made substantial contributions to the conception or design of the work; E. Artaud-Macari, D. Boyer, G. Le Bouar, M. Bubenheim and C. Girault acquired, analysed or interpreted data for the work; E. Artaud-Macari, N. Bubenheim and C. Girault drafted the work or revised it critically for important intellectual content; all authors gave final approval of the version submitted for publication; and E. Artaud-Macari and C. Girault are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest: E. Artaud-Macari has nothing to disclose.
Conflict of interest: M. Bubenheim has nothing to disclose.
Conflict of interest: G. Le Bouar has nothing to disclose.
Conflict of interest: D. Carpentier has nothing to disclose.
Conflict of interest: S. Grangé has nothing to disclose.
Conflict of interest: D. Boyer has nothing to disclose.
Conflict of interest: G. Béduneau has nothing to disclose.
Conflict of interest: B. Misset has nothing to disclose.
Conflict of interest: A. Cuvelier has nothing to disclose.
Conflict of interest: F. Tamion has nothing to disclose.
Conflict of interest: C. Girault reports grants and nonfinancial support from Fischer & Paykel Healthcare and Resmed Ltd during the conduct of the study.
References
- 1.Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017; 50: 1602426. doi: 10.1183/13993003.02426-2016 [DOI] [PubMed] [Google Scholar]
- 2.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015; 372: 2185–2196. doi: 10.1056/NEJMoa1503326 [DOI] [PubMed] [Google Scholar]
- 3.Carteaux G, Millán-Guilarte T, De Prost N, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med 2016; 44: 282–290. doi: 10.1097/CCM.0000000000001379 [DOI] [PubMed] [Google Scholar]
- 4.Frat JP, Ragot S, Coudroy R, et al. Predictors of intubation in patients with acute hypoxemic respiratory failure treated with a noninvasive oxygenation strategy. Crit Care Med 2018; 46: 208–215. doi: 10.1097/CCM.0000000000002818 [DOI] [PubMed] [Google Scholar]
- 5.Spoletini G, Alotaibi M, Blasi F, et al. Heated humidified high-flow nasal oxygen in adults: mechanisms of action and clinical implications. Chest 2015; 148: 253–261. doi: 10.1378/chest.14-2871 [DOI] [PubMed] [Google Scholar]
- 6.Papazian L, Calfee CS, Chiumello D, et al. Diagnostic workup for ARDS patients. Intensive Care Med 2016; 42: 674–685. doi: 10.1007/s00134-016-4324-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frat JP, Brugiere B, Ragot S, et al. Sequential application of oxygen therapy via high-flow nasal cannula and noninvasive ventilation in acute respiratory failure: an observational pilot study. Respir Care 2015; 60: 170–178. doi: 10.4187/respcare.03075 [DOI] [PubMed] [Google Scholar]
- 8.Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care 2007; 20: 126–131. doi: 10.1016/j.aucc.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 9.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth 2009; 103: 886–890. doi: 10.1093/bja/aep280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care 2011; 56: 1151–1155. doi: 10.4187/respcare.01106 [DOI] [PubMed] [Google Scholar]
- 11.Chanques G, Riboulet F, Molinari N, et al. Comparison of three high flow oxygen therapy delivery devices: a clinical physiological cross-over study. Minerva Anestesiol 2013; 79: 1344–1355. [PubMed] [Google Scholar]
- 12.Kunst PW, Vazquez de Anda G, Böhm SH, et al. Monitoring of recruitment and derecruitment by electrical impedance tomography in a model of acute lung injury. Crit Care Med 2000; 28: 3891–3895. doi: 10.1097/00003246-200012000-00025 [DOI] [PubMed] [Google Scholar]
- 13.Hinz J, Moerer O, Neumann P, et al. Effect of positive end-expiratory-pressure on regional ventilation in patients with acute lung injury evaluated by electrical impedance tomography. Eur J Anaesthesiol 2005; 22: 817–825. doi: 10.1017/S0265021505001377 [DOI] [PubMed] [Google Scholar]
- 14.Hinz J, Hahn G, Neumann P, et al. End-expiratory lung impedance change enables bedside monitoring of end-expiratory lung volume change. Intensive Care Med 2003; 29: 37–43. doi: 10.1007/s00134-002-1555-4 [DOI] [PubMed] [Google Scholar]
- 15.Lindgren S, Odenstedt H, Olegård C, et al. Regional lung derecruitment after endotracheal suction during volume- or pressure-controlled ventilation: a study using electric impedance tomography. Intensive Care Med 2007; 33: 172–180. doi: 10.1007/s00134-006-0425-x [DOI] [PubMed] [Google Scholar]
- 16.Riera J, Pérez P, Cortés J, et al. Effect of high-flow nasal cannula and body position on end-expiratory lung volume: a cohort study using electrical impedance tomography. Respir Care 2013; 58: 589–596. doi: 10.4187/respcare.02086 [DOI] [PubMed] [Google Scholar]
- 17.Corley A, Caruana LR, Barnett AG, et al. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth 2011; 107: 998–1004. doi: 10.1093/bja/aer265 [DOI] [PubMed] [Google Scholar]
- 18.Corley A, Sharpe N, Caruana LR, et al. Lung volume changes during cleaning of closed endotracheal suction catheters: a randomized crossover study using electrical impedance tomography. Respir Care 2014; 59: 497–503. doi: 10.4187/respcare.02601 [DOI] [PubMed] [Google Scholar]
- 19.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200: e45–e67. doi: 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frat JP, Ricard JD, Coudroy R, et al. Preoxygenation with non-invasive ventilation versus high-flow nasal cannula oxygen therapy for intubation of patients with acute hypoxaemic respiratory failure in ICU: the prospective randomised controlled FLORALI-2 study protocol. BMJ Open 2017; 7: e018611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilan N, Dastranji A, Ghalehgolab Behbahani A. Comparison of the SpO2/ FiO2 ratio and the PaO2/ FiO2 ratio in patients with acute lung injury or acute respiratory distress syndrome. J Cardiovasc Thorac Res 2015; 7: 28–31. doi: 10.15171/jcvtr.2014.06 [DOI] [Google Scholar]
- 22.Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2017; 195: 1207–1215. doi: 10.1164/rccm.201605-0916OC [DOI] [PubMed] [Google Scholar]
- 23.Mauri T, Alban L, Turrini C, et al. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intensive Care Med 2017; 43: 1453–1463. doi: 10.1007/s00134-017-4890-1 [DOI] [PubMed] [Google Scholar]
- 24.Lehmann EL. Nonparametrics. Upper Saddle River, Prentice-Hall, 2018. [Google Scholar]
- 25.Eronia N, Mauri T, Maffezzini E, et al. Bedside selection of positive end-expiratory pressure by electrical impedance tomography in hypoxemic patients: a feasibility study. Ann Intensive Care 2017; 7: 76. doi: 10.1186/s13613-017-0299-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Futier E, Constantin JM, Pelosi P, et al. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: a randomized controlled study. Anesthesiology 2011; 114: 1354–1363. doi: 10.1097/ALN.0b013e31821811ba [DOI] [PubMed] [Google Scholar]
- 27.Brochard L, Isabey D, Piquet J, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med 1990; 323: 1523–1530. doi: 10.1056/NEJM199011293232204 [DOI] [PubMed] [Google Scholar]
- 28.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med 2017; 195: 438–442. doi: 10.1164/rccm.201605-1081CP [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Terán P, Marin-Corral J, Dot I, et al. Aeration changes induced by high flow nasal cannula are more homogeneous than those generated by non-invasive ventilation in healthy subjects. J Crit Care 2019; 53: 186–192. doi: 10.1016/j.jcrc.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 30.Grieco DL, Menga LS, Raggi V, et al. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2020; 201: 303–312. doi: 10.1164/rccm.201904-0841OC [DOI] [PubMed] [Google Scholar]
- 31.Schwabbauer N, Berg B, Blumenstock G, et al. Nasal high-flow oxygen therapy in patients with hypoxic respiratory failure: effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (NIV). BMC Anesthesiol 2014; 14: 66. doi: 10.1186/1471-2253-14-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/ FiO2 ratio and the PaO2/ FiO2 ratio in patients with acute lung injury or ARDS. Chest 2007; 132: 410–417. doi: 10.1378/chest.07-0617 [DOI] [Google Scholar]
- 33.Grivans C, Lundin S, Stenqvist O, et al. Positive end-expiratory pressure-induced changes in end-expiratory lung volume measured by spirometry and electric impedance tomography. Acta Anaesthesiol Scand 2011; 55: 1068–1077. doi: 10.1111/j.1399-6576.2011.02511.x [DOI] [PubMed] [Google Scholar]
- 34.van der Burg PS, Miedema M, de Jongh FH, et al. Cross-sectional changes in lung volume measured by electrical impedance tomography are representative for the whole lung in ventilated preterm infants. Crit Care Med 2014; 42: 1524–1530. doi: 10.1097/CCM.0000000000000230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00373-2021.SUPPLEMENT (273.4KB, pdf)
FIGURE S1 Study protocol, electrode position of the electrical impedance tomography (EIT) belt and lung volume modelization. A. Progress of the experiment: after study inclusion, the first patient included received NIV in period 1 followed by HFNC in period 2. Then, the following patient received HFNC and NIV in the reverse order of the previous patient. To minimize the residual effect from period 1, patients received FM between the 2 periods. Oxygenation was delivered during at least 15 minutes. During this time, EIT measures were recorded. Electrical impedance tomography lay out: B. 16 electrodes united within the same belt were placed on the thorax of the patient facing the alveolar zone of condensation. The reference electrode R was placed on the abdomen. C. Functional EIT images in the acquisition zone defined by the belt. D. Subdivision of the acquisition area into 4 standardized quadrants or regions of interest (ROI) numbered from 1 to 4. 00373-2021.figureS1 (353.1KB, tif)
FIGURE S2 Electrical impedance tomography recordings. EIT: electrical impedance tomography, A: respiratory rate, global and regional tidal variation (TV) in the four regions of interest (ROI) chosen. End-expiratory lung impedance (EELI) measured by EIT in global lung and in each ROI showing the evolution of EELI between B: face mask (green arrows) and NIV (pink arrows) periods; C: HFNC (red arrows) and face mask (green arrows) periods. 00373-2021.figureS2 (353.2KB, tif)
TABLE S1 Comparison of patients' characteristics at inclusion by treatment sequence 00373-2021.tableS1 (122.3KB, pdf)
TABLE S2 Effect of first treatment on patients' characteristics just before second treatment 00373-2021.tableS2 (139.7KB, pdf)