Abstract
Airway inflammation, mucus hyperproduction and epithelial remodelling are hallmarks of many chronic airway diseases, including asthma, COPD and cystic fibrosis. While several cytokines are dysregulated in these diseases, most studies focus on the response of airways to interleukin (IL)-4 and IL-13, which have been shown to induce mucus hyperproduction and shift the airway epithelium towards a hypersecretory phenotype.
We hypothesised that other cytokines might induce the expression of chloride (Cl−) channels/transporters, and regulate epithelial differentiation and mucus production. To this end, fully differentiated human airway basal cells (BCi-NS1.1) were treated with cytokines identified as dysregulated in those diseases, namely IL-8, IL-1β, IL-4, IL-17A, IL-10 and IL-22, and tumour necrosis factor-α.
Our results show that the cystic fibrosis transmembrane conductance regulator (CFTR) is the main Cl− channel modulated by inflammation, in contrast to transmembrane protein 16A (TMEM16A), whose levels only changed with IL-4. Furthermore, we identified novel roles for IL-10 and IL-22 by influencing epithelial differentiation towards ciliated cells and away from pulmonary ionocytes. In contrast, IL-1β and IL-4 reduced the number of ciliated cells while increasing club cells. Interestingly, while IL-1β, IL-4 and IL-10 upregulated CFTR expression, IL-4 was the only cytokine that increased both its function and the number of CFTR-expressing club cells, suggesting that this cell type may be the main contributor for CFTR function. Additionally, all cytokines assessed increased mucus production through a differential upregulation of MUC5AC and MUC5B transcript levels.
This study reveals a novel insight into differentiation resulting from the cross-talk of inflammatory mediators and airway epithelial cells, which is particularly relevant for chronic airway diseases.
Short abstract
Disease-specific inflammatory cytokines induce airway mucus hyperproduction and changes in chloride channels/transporters, and modulate epithelial differentiation https://bit.ly/2WaeV1z
Introduction
The human airway epithelium is a barrier that maintains fluid homeostasis, clears particles/pathogens and recruits immune cells during infection [1]. These functions are achieved by a heterogeneous population of epithelial cells, namely ciliated cells, which drive clearance through ciliary beating; goblet cells, which secrete mucus to trap pathogens; club cells, which produce protective surfactants and antiproteases; and basal cells, which differentiate into the other cell types [2].
Efficient mucociliary clearance (MCC) depends on a regular airway surface liquid that is regulated by epithelial ion channels and transporters [3]. Proper airway hydration is achieved by apical sodium (Na+) absorption and chloride (Cl−) secretion, with water flowing according to the osmotic gradient. Apical Cl− secretion is driven by the cystic fibrosis transmembrane conductance regulator (CFTR), transmembrane protein 16A (TMEM16A, also Anoctamin1/ANO1) and exchangers of the solute carrier family 26 (SCL26A9 and SLC26A4, also Pendrin), while the epithelial sodium channel (ENaC) mediates Na+ absorption [3]. Additionally, several Cl− channels/transporters are permeable to bicarbonate (HCO3−), essential for mucus release and expansion [4].
Upon infection, airway epithelial cells are exposed to inflammatory mediators that act as signals to increase mucus and fluid secretion [5]. Previous studies have established a link between inflammation and ion transport. However, the focus has been on the response to interleukins (IL)-4 and -13, which upregulate CFTR and TMEM16A while inhibiting ENaC activity [6, 7]. Regarding other inflammatory mediators, the results are contradictory. For example, IL-1β and tumour necrosis factor (TNF)-α are described as upregulating [8, 9] and downregulating [10, 11] CFTR, whereas no information is available about other Cl− channels/transporters. Furthermore, it remains unknown how certain cytokines modulate the airways, namely chemokines (e.g. IL-8), which recruit immune cells to the site of infection [12], and anti-inflammatory cytokines (e.g. IL-10) [13].
Inflammatory mediators also regulate tissue homeostasis, modulating the distribution of airway epithelial cell types. Airway remodelling is a key feature of airway diseases, including cystic fibrosis (CF), COPD and asthma. Although several cytokines are unbalanced in these conditions [14, 15], most reports focus on IL-4 and IL-13, which increase goblet cells at the expense of ciliated cells [16, 17]. Notably, there is no information about the potential effect of inflammation on pulmonary ionocytes, a rare cell type highly enriched in CFTR expression [18], although their contribution to CFTR function is controversial [19].
This study aimed to determine the effect of different inflammatory mediators on airway epithelial cells in Cl− channels/transporters, epithelial differentiation and mucus production. To this end, we assessed cytokines that are secreted upon infection (IL-8, IL-1β, TNF-α, IL-17A) [20, 21], anti-inflammatory (IL-10) [13] or tissue-protective (IL-22) [22]. IL-4 was included, as its effect on mucus production and epithelial Cl− transport is well described [7, 23].
Our data, using a human respiratory basal cell line (BCi-NS1.1) with the ability to multi-differentiate into the various airway epithelial cell types [24], demonstrate that CFTR is the main Cl− channel modulated by inflammation, with all cytokines increasing its expression, except for TNF-α. Remarkably, some cytokines also regulate the percentage of ciliated cells (IL-1β, IL-4, IL-10, IL-22), club cells (IL-1β, IL-4) and ionocytes (IL-10, IL-22). Furthermore, we observed that IL-4 upregulated CFTR activity, which may be explained by the increase in the number of CFTR-expressing club cells and/or TMEM16A, an enhancer of CFTR function [25]. All cytokines upregulated mucus production by increasing the transcript levels of MUC5AC (IL-4, IL-10, IL-22), MUC5B (IL-1β, TNF-α, IL-17A) or both (IL-8). Finally, we grouped the different cytokines according to their overall effect on the airway epithelium.
Materials and methods
Cell culture
Basal Cell Immortalised Non-Smoker 1.1 (BCi-NS1.1) cells [24] were cultured in Pneumacult-Ex medium (Stemcell Technologies, Vancouver, Canada) and differentiated under air–liquid interface (ALI) as described previously [26], using 6.5- or 12-mm diameter transwells with 0.4-μm pore polyester membrane inserts (Corning, New York, NY, USA) previously coated with human type IV collagen (Sigma-Aldrich, St. Louis, MO, USA).
Exposure to cytokines
BCi-NS1.1 cells (ALI day 27) were exposed to 10 ng·mL−1 IL-8 (R&D Systems, Minneapolis, MN, USA), IL-1β, TNF-α, IL-17A, IL-10, IL-22 (Stemcell Technologies) and IL-4 (ThermoFisher Scientific, Waltham, MA, USA) from the basolateral media for 72 h.
Quantitative reverse transcriptase PCR
mRNA expression was quantified as described previously [26], using primer pairs MUC5AC (fwd:5′-CCTGAGGGGACGGTGCTT-3′, rv:5′-ACGAGGTGCAGTTGGTGC-3′), MUC5B (fwd:5′-GTGAGGAGGACTCCTGTCAAGT-3′, rv:5′-CCTCGCAGAAGGTGATGTTG-3′), SLC26A9 (fwd:5′-GACTACATCATTCCTGACCTGC-3′, rv:5′-AGGAGTAGAGGCCATTGACTG-3′), GAPDH (fwd:5′-ATGGGGAAGGTGAAGGTCG-3′, rv:5′-GGGGTCATTGATGGCAACAATA-3′). Mean relative levels of expression were calculated for the two target genes using the ΔΔCT method, where fold change=2(–ΔΔCT).
Primary antibodies
The primary antibodies used were anti-CFTR (596,570) (CFF), anti-TMEM16A (SP31), anti-SLC26A4, anti-GAPDH, anti-βTubulinIV (Abcam, Cambridge, UK), anti-DNAI1, anti-FOXI1 (Sigma-Aldrich), anti-CC16 (BioVendor, Brno, Czech Republic) and anti-Calnexin (BD Biosciences, San Jose, CA, USA).
Western blot
Protein lysates were prepared as described previously [26] and loaded in acrylamide gels for electrophoresis. Membranes were blocked with 5% non-fat milk (NFM)-PBS with Tween-20 and probed with anti-SLC26A4, anti-DNAI1 (1:1000), anti-CC16 (1:500), anti-GAPDH and anti-calnexin (1:10 000) diluted in blocking buffer. Membranes probed for TMEM16A, CFTR and FOXI1 were blocked with 1% NFM-TBS with Tween-20 and incubated with respective antibodies (1:500) diluted in blocking buffer. Incubation with secondary antibodies was performed as described previously [26].
Immunofluorescence
Immunofluorescence was performed as described previously [26] using primary antibodies at a 1:100 dilution (anti-CFTR (570), anti-βTubulin IV, anti-CC16, anti-FOXI1). Nuclei were stained with Hoechst 33342 solution (200 ng·mL−1; Sigma-Aldrich). Transwells were imaged with a Leica TCS SP8 confocal microscope. Cell types were quantified by averaging values obtained from at least five different images in each transwell, using the Fiji software with maximum intensity projections and confirming co-staining with xz images. Nuclei were quantified in maximum X–Y image projections using the StarDist plugin in Fiji [27].
Ussing chamber
Monolayers of BCi-NS1.1 cells were analysed under open-circuit conditions in a micro-Ussing chamber as described previously [26]. 30 μM amiloride was added apically to block ENaC. CFTR activity was measured by co-applying 2 μM forskolin and 100 μM 3-isobutyl-1-methylxanthine (IBMX), followed by 30 μM CFTR-inh172 (all compounds from Sigma-Aldrich).
Data analyses
Data are presented as mean±sem. Statistical analysis was performed with unpaired t-tests (p<0.05).
Heatmap was generated in Rstudio (1.2.5042), using the heatmap.2 function (package gplots) and rescaling data between 0 and 1.
Results
Effect of inflammatory mediators on airway epithelial Cl− channels/transporters
Fully differentiated BCi-NS1.1 cells were exposed to different cytokines, and the CFTR, TMEM16A and SLC26A4 protein levels were analysed using Western blotting. SLC26A9 expression was analysed using quantitative reverse transcriptase (qRT)-PCR, as no Western blot signal was detected with two different antibodies (data not shown).
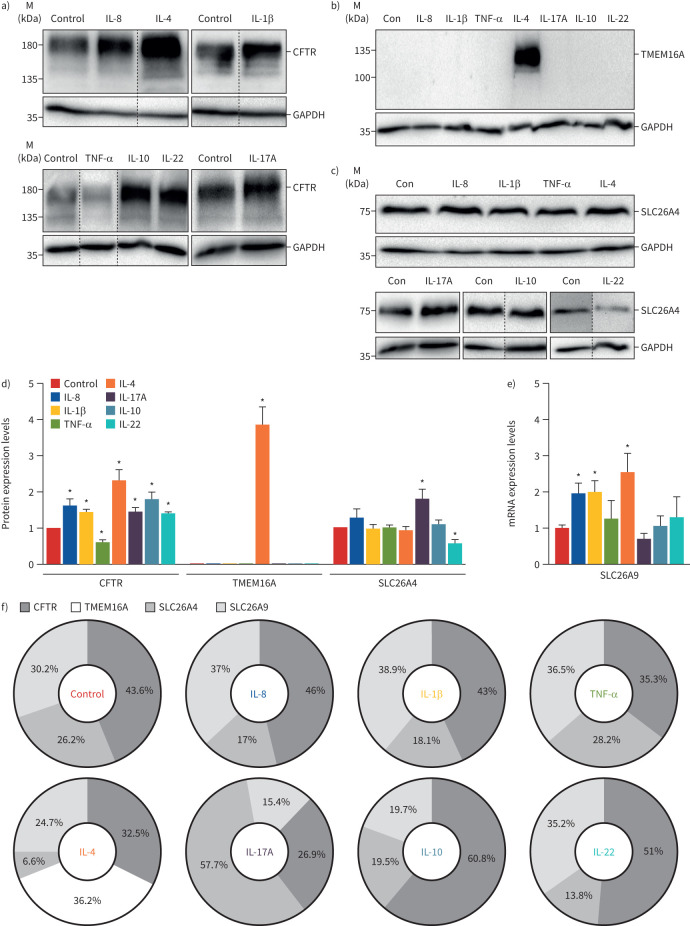
Our results show that all cytokines upregulate CFTR expression, except for TNF-α, which had the opposite effect (figure 1a,d). In contrast, IL-4 was the only cytokine increasing TMEM16A expression, which is undetectable in control BCi-NS1.1 cells (figure 1b,d), as described previously [26]. Moreover, SLC26A4 expression was significantly upregulated by IL-17A and downregulated by IL-22 (figure 1c,d). Conversely, these cytokines did not affect the SLC26A9 transcript levels, which increased with IL-8, IL-1β and IL-4 (figure 1e).
FIGURE 1.
Effect of inflammatory mediators on the expression of airway chloride channels and transporters in differentiated BCi-NS1.1 cells. Western blot of endogenous a) cystic fibrosis transmembrane conductance regulator (CFTR) (∼180 kDa), b) transmembrane protein 16A (TMEM16A) (∼120 kDa) and c) solute carrier family 26 member SLC26A4 (∼85 kDa) expression following exposure to cytokines. GAPDH (∼36 kDa) was used as a loading control. For each cytokine, representative blots are shown. Dashed lines indicate lanes run on the same gel but noncontiguous. IL: interleukin; TNF: tumour necrosis factor; Con: control. d) CFTR, TMEM16A and SLC26A4 protein expression levels were detected by Western blotting, quantified by densitometry and normalised to the loading control and control untreated cells. Data are presented as mean±sem (n=3–4). *: p<0.05 versus control, unpaired t-test. e) SLC26A9 mRNA expression levels following exposure to cytokines, quantitatively measured using quantitative reverse transcriptase PCR and normalised to the housekeeping gene GAPDH. Fold change values are mean±sem, relative to control untreated cells (n=3–4). *: p<0.05 versus control, unpaired t-test. f) Contribution of CFTR, TMEM16A, SLC26A4 and SLC26A9 for total chloride channels/transporters expression following exposure to each cytokine. Values were calculated by dividing the fold change of each channel's protein/mRNA expression levels shown in d) and e) by the sum of all.
Furthermore, the contribution of each protein for total Cl− channel/transporter expression was calculated by dividing the fold change of each channel's protein/mRNA expression levels (figure 1d,e) by the sum of all (figure 1f). IL-4 was the only cytokine inducing TMEM16A (figure 1b,d), accounting for ∼36% of total Cl− channel/transporter expression (figure 1f). CFTR contribution was highest (∼61%) in IL-10-treated cells, while SLC26A4 and SLC26A9 had a maximal expression with IL-17A and IL-1β (∼58% and 39%, respectively) (figure 1f).
Effect of inflammatory mediators on epithelial cell differentiation
Previous studies identified CFTR expression in ciliated cells, club cells and ionocytes [16, 18, 27]. Thus, we asked whether these cytokines change differentiation into these cell types, as inflammatory mediators were reported to regulate epithelial differentiation [16, 17].
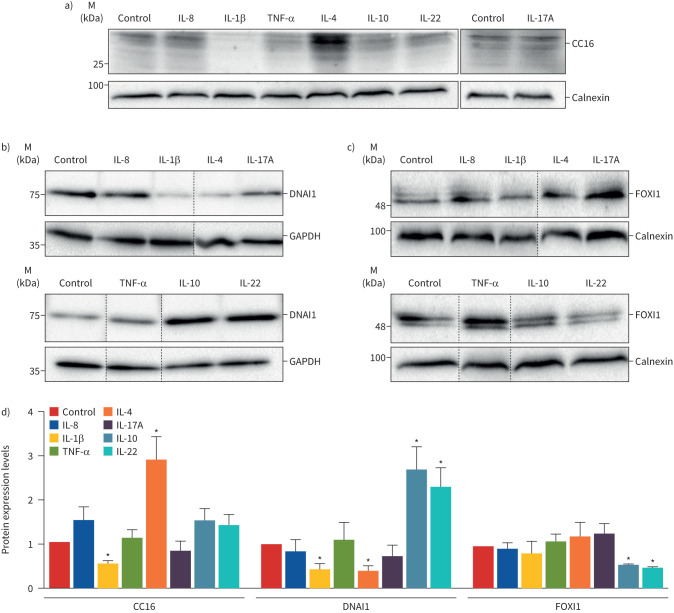
To this end, we compared protein expression of markers for club cells (CC16), ciliated (DNAI1) and ionocytes (FOXI1). Western blot results demonstrate that while IL-1β downregulated CC16 expression, IL-4 significantly increased (∼three-fold) (figure 2a,d). Moreover, IL-1β and IL-4 decreased DNAI1 levels, in contrast with IL-10 and IL-22, which increased its expression by ∼2.5-fold (figure 2b,d). Regarding pulmonary ionocytes, IL-10 and IL-22 were the only cytokines downregulating FOXI1 protein levels (figure 2c,d).
FIGURE 2.
Effect of inflammatory mediators on cell-type markers specific for ciliated cells, club cells and pulmonary ionocytes. Western blot comparing the endogenous expression of the a) club cell marker CC16 (∼25 kDa), b) ciliated cell marker DNAI1 (∼75 kDa) and c) pulmonary ionocyte marker FOXI1 (∼40 kDa) following exposure to cytokines. GAPDH (∼36 kDa) and calnexin (∼90 kDa) were used as loading controls. For each cytokine, representative blots are shown. Dashed lines indicate lanes run on the same gel but noncontiguous. IL: interleukin; TNF: tumour necrosis factor. d) DNAI1, CC16 and FOXI1 protein expression levels were detected by Western blotting, quantified by densitometry and normalised to the loading control and control untreated cells. Data are shown as mean±sem (n=3–4). *: p<0.05 versus control, unpaired t-test.
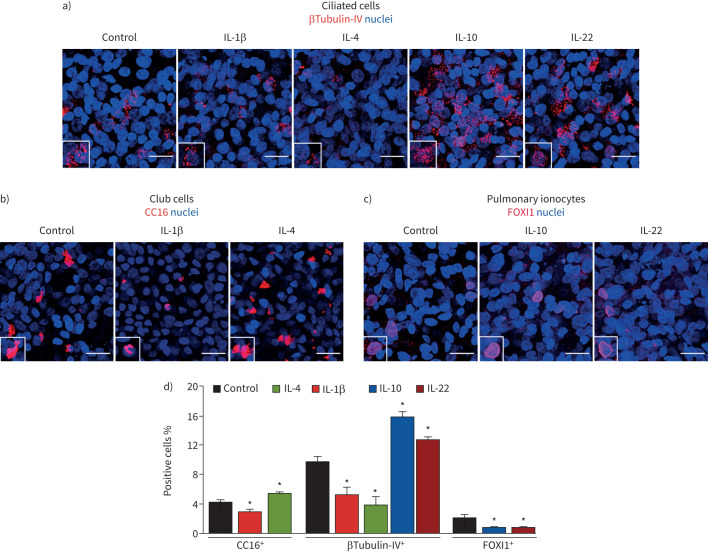
To determine whether changes in protein levels correspond to equivalent differences in the percentage of ciliated cells, club cells and ionocytes, we performed immunofluorescence with antibodies against βTubulin-IV (another ciliated-cell marker) CC16 and FOXI1, co-staining each marker with CFTR to determine its distribution under different inflammatory conditions.
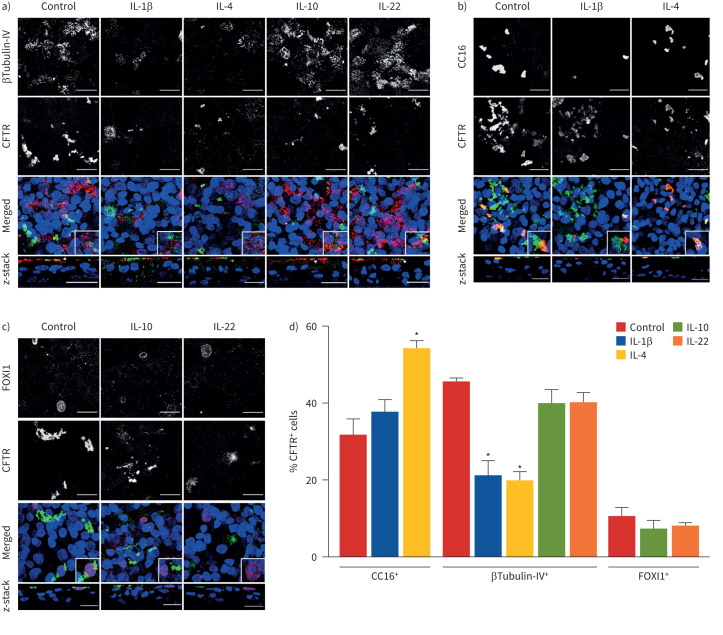
Indeed, we observed matching differences in the percentage of ciliated cells, club cells and ionocytes relative to the expression of cell-type markers (figures 2 and 3). In agreement with previous studies, we also detected CFTR expression in ciliated cells, club cells and pulmonary ionocytes resulting from differentiation of BCi-NS1.1 cells (figure 4), similar to those cells present in the native epithelium. Moreover, co-staining of βTubulin-IV and CFTR demonstrated that, although IL-10 and IL-22 increased ciliated cells (∼1.5-fold), the total number of CFTR-expressing βTubulin-IV+ cells did not change (figure 4a,d). Conversely, IL-1β and IL-4 decreased ciliated cells by half and reduced its contribution to total CFTR expression (figure 4a,d). Interestingly, co-labelling of CFTR and CC16 showed that IL-4 increased the percentage of club cells and the number of CFTR-expressing club cells (figure 4b,d), suggesting that the IL-4-induced CFTR upregulation (figure 1a,d) occurs in club cells. In contrast, IL-1β did not change the percentage of cells co-labelled with CFTR and CC16, despite the decrease in club cells (figure 4b,d). Furthermore, the IL-10- and IL-22-induced downregulation of FOXI1 protein levels (figure 2c,d) was also associated with a significant decrease in the percentage of pulmonary ionocytes, although the number of CFTR-expressing FOXI1+ cells did not change (figure 4c,d).
FIGURE 3.
Modulation of epithelial differentiation towards ciliated, club cells and pulmonary ionocytes by interleukin (IL)-1β, IL-4, IL-10 and/or IL-22 in BCi-NS1.1 cells. Confocal immunofluorescence microscopy images showing a) ciliated cells (labelled with βTubulin-IV), b) club cells (labelled with CC16) or c) pulmonary ionocytes (labelled with FOXI1) in permeabilised fully differentiated BCi-NS1.1 cells (air–liquid interface day 30) exposed to IL-1β, IL-4, IL-10 or IL-22. Nuclei were stained with Hoechst. Images are maximum projections (and insets) of an amplified region. Scale bars=20 μm. Images were acquired with a Leica TCS SP8 confocal microscope (objective 63× oil, numerical aperture 1.4). d) Quantification of the percentages of ciliated cells, club cells and pulmonary ionocytes (stained with the βTubulin-IV, CC16 and FOXI1 antibodies, respectively, shown in a–c). The number of each cell type was normalised to the total number of nuclei. *: p<0.05.
FIGURE 4.
Cystic fibrosis transmembrane conductance regulator (CFTR) distribution under different inflammatory conditions. Confocal immunofluorescence microscopy images showing CFTR (green) and a) ciliated cells (labelled with βTubulin-IV in red), b) club cells (labelled with CC16 in red) or c) pulmonary ionocytes (labelled with FOXI1 in red) in permeabilised fully differentiated BCi-NS1.1 cells (air–liquid interface day 30) exposed to interleukin (IL)-1β, IL-4, IL-10 or IL-22. Nuclei were stained with Hoechst. Images are maximum projections (and insets) or z-stacks of an amplified region. Scale bars=20 μm. Images were acquired with a Leica TCS SP8 confocal microscope (objective 63× oil, numerical aperture 1.4). White arrowheads in z-stack images (a) show cells that are co-stained with CFTR and βTubulin-IV. d) Quantification of the percentages of cells co-stained with CFTR and CC16, βTubulin-IV or FOXI1 normalised to the total number of CFTR+ cells. Quantifications were performed in 15 images randomly acquired in three independent experiments (five images per experiment). Values are presented as mean±sem (n=3). *: p<0.05 versus control, unpaired t-test.
Modulation of CFTR activity by IL-1β, IL-4 and IL-10
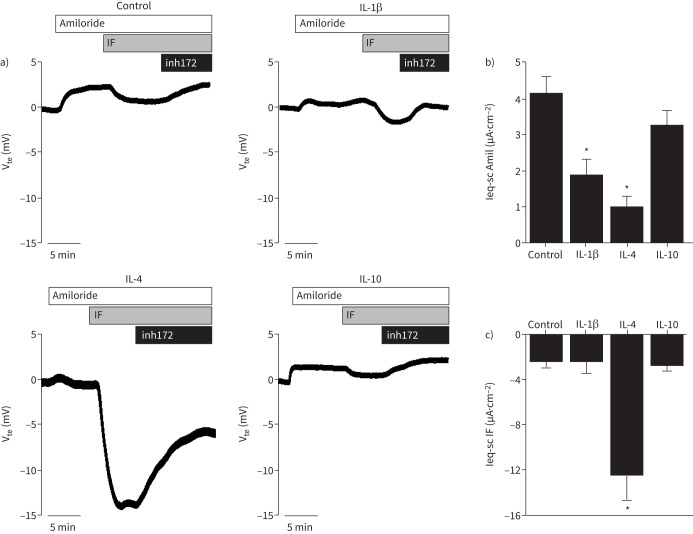
While IL-1β, IL-4 and IL-10 upregulated CFTR expression (figure 1a,d), they showed different effects in ciliated, club cells and pulmonary ionocytes (figure 2a–d). Thus, by comparing CFTR function with these cytokines, we can conclude which cell type contributes more to total CFTR function. Ussing chamber results demonstrated that IL-4 was the only cytokine that significantly increased the cAMP-activated currents (figure 5a,c), elicited by IBMX and forskolin. These data suggest an appreciable contribution of club cells to CFTR function, as this cell type was induced by IL-4 (figures 2 and 3). Additionally, we observed that IL-1β and IL-4 significantly decreased the amiloride-sensitive Na+ absorption currents (figure 5a,b), downregulating ENaC function.
FIGURE 5.
Effect of interleukin (IL)-1β, IL-4 and IL-10 on the cAMP-activated and amiloride-sensitive currents in polarised BCi-NS1.1 cells. a) Representative Ussing chamber tracings of transepithelial voltage measurements (Vte) for untreated BCi-NS1.1 cells (control) and treated with IL-1β, IL-4 and IL-10. A positive deflection is observed after stimulation with the epithelial sodium channel inhibitor amiloride (30 μM), which is followed by a negative deflection after stimulation with apical 3-isobutyl-1-methylxanthine (IBMX) (100 μM) and forskolin (2 μM) (IF). This effect was reversed upon the addition of the cystic fibrosis transmembrane conductance regulator inhibitor-172 (inh172; 30 μM). b) Summary of the equivalent short-circuit current (Ieq-sc) (μA·cm−2) calculated for the response to amiloride (Amil). c) Summary of the Ieq-sc (μA·cm−2) calculated for the response to IBMX+forskolin (IF). Values are presented as mean±sem (n=3–5). *: p<0.05 versus control, unpaired t-test.
Regulation of mucin gene expression by inflammatory mediators
Next, we assessed MUC5AC and MUC5B transcript levels, the two main mucins in human airway mucus [28]. As these mucins are essential components of the MCC machinery, it is relevant to determine the ratio between them to define how mucus properties change with inflammation.
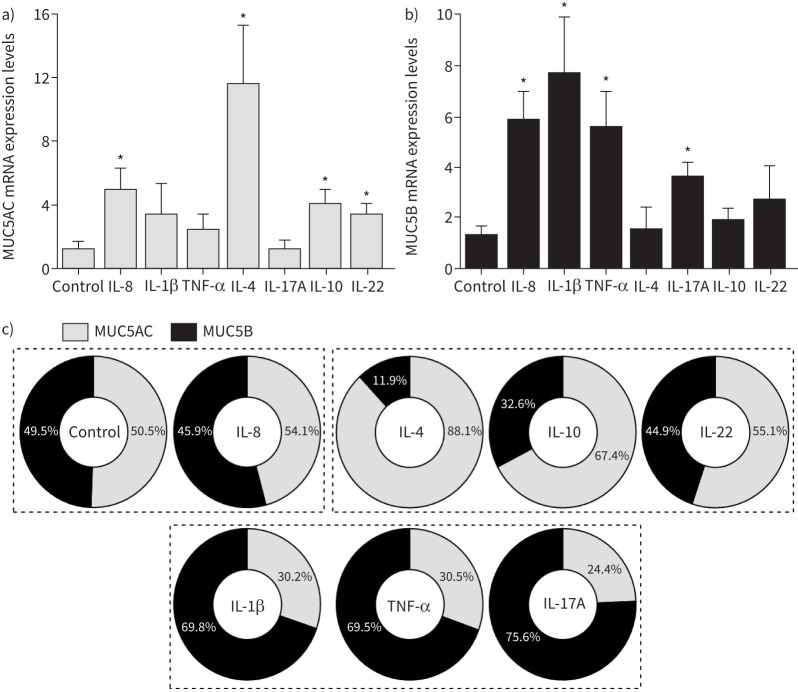
Our qRT-PCR results demonstrate that all cytokines increased MUC5AC and/or MUC5B transcripts, albeit at different levels, resulting in different ratios. IL-8, IL-4, IL-10 and IL-22 upregulated MUC5AC transcripts (figure 6a), with IL-4 inducing the greatest unbalance as it had no significant effect on MUC5B (∼12-fold in MUC5AC versus MUC5B). MUC5B levels were higher with IL-1β (∼eight-fold), followed by IL-8, TNF-α and IL-17A (figure 6b).
FIGURE 6.
MUC5AC and MUC5B mRNA expression in differentiated BCi-NS1.1 cells following exposure to cytokines. a) MUC5AC and b) MUC5B mRNA expression levels following exposure to cytokines, measured by quantitative reverse transcriptase PCR and normalised to the housekeeping gene GAPDH. Fold-change values are presented as mean±sem, relative to control untreated cells (n=3–5). *: p<0.05 versus control, unpaired t-test. c) Contribution of MUC5AC and MUC5B for mucin expression. Values were calculated by dividing each mucin's fold-change expression levels by the sum of the MUC5AC and MUC5B expression levels shown in a) and b). Dashed lines indicate groups of treatments that have matching contributions of MUC5AC or MUC5B expression. IL: interleukin; TNF: tumour necrosis factor.
Additionally, the relative expression of each mucin was calculated by dividing the MUC5AC or MUC5B fold-change expression levels by the sum of the two (figure 6c). While IL-8 significantly increased the MUC5AC and MUC5B mRNA expression levels (figure 6a,b), the relative percentage of each mucin is similar to control. In contrast, IL-4, IL-10 and IL-22 mainly upregulated MUC5AC, whereas MUC5B represents >60% of mucin mRNAs with IL-1β, TNF-α or IL-17A (figure 6c).
Summary of the effect of different cytokines on Cl− channels/transporters, epithelial differentiation and mucus production
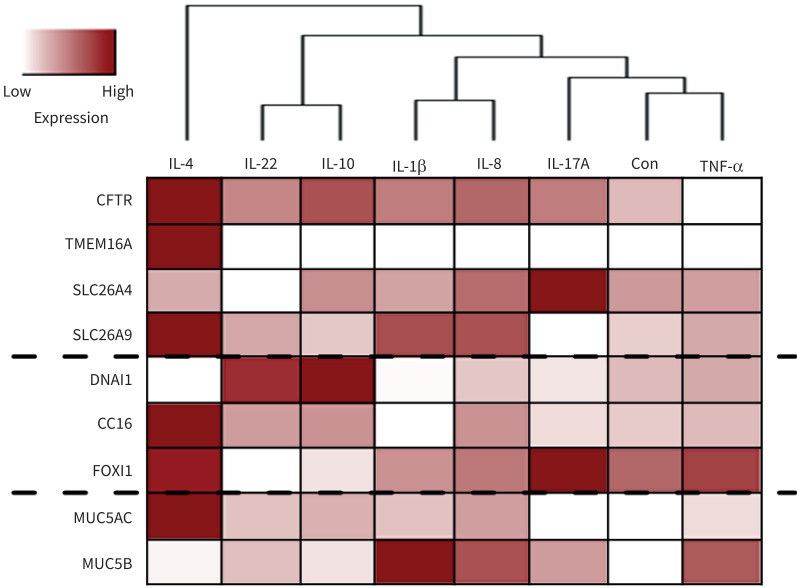
The observed effects on the expression of Cl− channels/transporters, epithelial differentiation and mucus production (figures 1, 2 and 6) are summarised in a heatmap (figure 7) where cytokines are clustered according to their effect on the airway epithelium properties. Altogether, we observed that IL-4 induced the most significant changes in all these parameters. Moreover, IL-10 and IL-22 showed similar effects by upregulating CFTR, DNAI1 and MUC5AC, while decreasing FOXI1. IL-1β and IL-8 are in the same cluster as both increased CFTR, SLC26A9 and MUC5B expression. Finally, IL-17A and TNF-α were the cytokines with fewer effects on BCi-NS1.1 cells (figure 7).
FIGURE 7.
Summary of the observed changes in different chloride channels/transporters expression and differentiation markers upon exposure to cytokines. The heatmap was generated based on the results shown in figures 1, 2 and 6. Data were rescaled between 0 and 1, with lower and higher expression levels represented in white and red, respectively. Inflammatory mediators are grouped into clusters according to the relative expression levels of all the proteins analysed. Dashed lines separate the chloride channels/transporters, differentiation markers and mucins. IL: interleukin; Con: control; TNF: tumour necrosis factor; CFTR: cystic fibrosis transmembrane conductance regulator; TMEM16A: transmembrane protein 16A; SLC26A: solute carrier family 26.
Discussion
Airway inflammation is a hallmark of many airway obstructive diseases, occurring with mucus hyperproduction and epithelial remodelling. Under these conditions, there is a complex cross-talk between airway epithelial cells and the immune system to ensure efficient MCC. When MCC fails, e.g. due to airway surface liquid dehydration, mucus plugging and airway clogging arise, followed by progressive lung failure. To fully understand the pathogenesis caused by inflammation, it is essential to characterise how airway epithelial cells respond to inflammatory stimuli. Although several cytokines are dysregulated upon disease [14, 15], most studies focus on the response of airway epithelial cells to IL-4 and IL-13 due to their involvement in asthma [17, 29]. In this study, we screened a panel of seven cytokines that are dysregulated in CF, COPD and/or asthma (IL-8, IL-1β, TNF-α, IL-4, IL-17A, IL-10, IL-22 [14, 15]) and determined their effect on airway epithelial cells, using BCi-NS1.1 cells that, like primary cells, differentiate into a mucociliated epithelium, but with an extended number of passages [24].
Increasing evidence supports the concept that inflammatory stimuli modulate transepithelial Cl− transport, which is essential to regulate the airway surface liquid, maintaining the epithelium barrier's homeostasis and host defence [5]. Therefore, we first evaluated the expression of CFTR, TMEM16A, SCL26A4 and SLC26A9. Overall, our results show that CFTR is the primary Cl− channel modulated by inflammation, with all cytokines increasing its expression, except for TNF-α (figure 1a,d). Interestingly, while these observations agree with previous studies [5], they also establish novel roles for IL-8, IL-10 and IL-22 as regulators of CFTR protein expression. Interestingly, previous studies showed that wild-type CFTR suppresses IL-8 secretion from airway epithelial cells [30]; here we observed that IL-8 also has an autocrine effect, upregulating CFTR and SLC26A9 (figure 1a,d,e).
Furthermore, although all cytokines increased mucus production (figure 6), IL-4 was the only one upregulating TMEM16A (figure 1b,d,f). This observation may shed some light on the current controversy on whether TMEM16A is required for mucus production [31]. Indeed, we have shown previously that IL-4-mediated TMEM16A upregulation does not drive mucus hyperproduction, but instead, both events result from an increase in cell proliferation [26].
Besides regulating Cl− channels/transporters, we also show that cytokines modulate epithelial differentiation. Particularly, our results demonstrate that while IL-1β and IL-4 decrease ciliated cells, they have the opposite effect on club cells (figures 2 and 3a,b,d). These effects should be considered when designing therapies to reduce mucus production in chronic airway diseases, as they might not be sufficient if the airways lack ciliated cells to achieve proper MCC. This is particularly relevant for CF, a disease characterised by mucus plugging and stasis, in which IL-1β is the main cytokine driving mucus hyperproduction [32]. In contrast, IL-10 and IL-22 biased the airway epithelium towards ciliated cells and away from ionocytes (figures 2 and 3), suggesting that these cytokines might support mucosal defence by directly regulating MUC5AC production (figure 6) and ciliated-cell differentiation (figures 2 and 3), ensuring efficient MCC. Moreover, the reduction in pulmonary ionocytes induced by IL-10 and IL-22 (figure 3c,d) may help understand this new cell-type function.
Interestingly, our results showed that although IL-1β, IL-4 and IL-10 upregulated CFTR expression (figure 1a,d), IL-4 was the only cytokine significantly increasing its function (figure 5a,c). These results may be explained by opposite changes in ciliated cells, club cells and ionocytes (figures 2 and 3). Indeed, IL-4 increased the percentage of CFTR-expressing club cells (figure 4b,d), suggesting that this cell type is the main contributor to CFTR function in IL-4-treated cells. This conclusion is supported by other studies describing the contribution of club cells to CFTR expression/function [32, 33]. Additionally, this effect may also be explained by TMEM16A upregulation, an enhancer of CFTR function [25]. Furthermore, whereas IL-1β decreased club cells, it did not affect the contribution of this cell type to CFTR expression (figure 4) or function (figure 5a,c). Our results on IL-10-treated cells also show that pulmonary ionocytes do not necessarily mediate CFTR function, as this cytokine decreased their percentage (figure 3c,d) while not affecting cAMP-activated currents (figure 5a,c).
Inflammatory cytokines are among the main factors inducing mucin hyperproduction [34]. Here, all cytokines increased mucus production by upregulating mRNA expression of either MUC5AC (IL-4, IL-10, IL-22), MUC5B (IL-1β, TNF-α, IL-17A) or both (IL-8) (figure 6). These results corroborate previous reports describing a differential induction of MUC5AC and MUC5B due to activation of different transcription factors and signalling pathways [35, 36]. These mucins play distinct roles in maintaining lung homeostasis: MUC5B is essential to regulate MCC and avoid infection, while MUC5AC is considered an “acute response mucin”, and its levels rapidly increase after an allergic inflammatory challenge [28]. Furthermore, MUC5AC is a key controller of mucus transport by anchoring MUC5B-containing strands secreted from submucosal glands [37]. Thus, the differential induction of both mucins will probably affect mucus transport. These effects are particularly relevant for CF, where MUC5B-mucus strands remain tethered to submucosal glands, leading to mucus stasis [38]. Thus, by increasing MUC5B mRNA expression, IL-8, IL-1β, TNF-α and IL-17A may worsen mucus stasis in CF. Nonetheless, the overall cytokine-mediated mucus hyperproduction likely occurs as a protective response of airway epithelial cells to ensure proper clearance by trapping the inhaled pathogens in the secreted mucus. However, when CFTR (a key channel ensuring airway surface liquid hydration) is deficient, the effect of mucus hypersecretion is noxious.
While it is widely established that pro-inflammatory mediators stimulate mucus production [34], less information is available about anti-inflammatory cytokines. In this study, MUC5AC transcript levels were also upregulated by IL-10 (figure 6), a potent anti-inflammatory cytokine [13]. Interestingly, IL-10 was identified to regulate MUC2 expression, the prominent intestinal mucin [39], through the activation of the transcription factors STAT1 and STAT3, the latter being involved in MUC5AC transcription [40]. Moreover, IL-22 induced a similar effect (figure 6), a cytokine that maintains the mucosal cell barrier by promoting tissue regeneration [22]. IL-22 belongs to the IL-10 family, and both cytokines activate similar pathways, including STAT3 [22]. Thus, further studies are required to determine how IL-10 and IL-22 regulate MUC5AC gene expression and ultimately address whether this effect is translated into an increase in mucin protein expression and percentage of secretory cells.
In summary, by assessing Cl− channels/transporters, epithelial differentiation status and mucus production, we grouped these cytokines according to their effect on the airway epithelium (figure 7). Overall, we observed that IL-4 induced the most extensive modifications, upregulating CFTR, TMEM16A and SLC26A9 (figure 1), club-cell differentiation (figures 2 and 3), MUC5AC hyperproduction (figure 6a) and decreasing the number of ciliated cells (figure 3a,d). Therefore, as IL-4 also increased CFTR function (figure 5a,c), it would be interesting to elucidate the mechanisms behind this effect, which may lead to the identification of novel strategies to increase CFTR expression and function in CF cells.
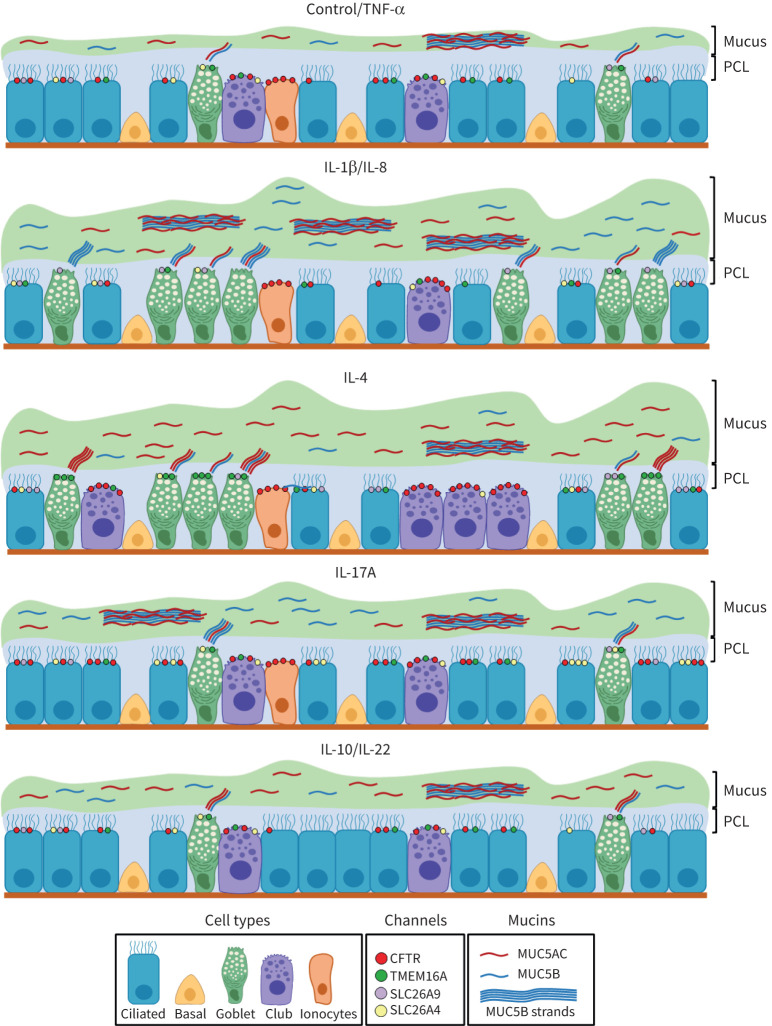
Taking together all data in this study, we propose a model for the effect of each cytokine on airway epithelial cells. For simplicity, cytokines grouped on the same cluster are represented together (figures 7 and 8). In summary, these models describe the effects of the different cytokines on the differential induction of MUC5AC and MUC5B, the percentage of different airway epithelial cell types, and expression of Cl− channels/transporters. Overall, these results will help to understand the impact of inflammatory mediators on airway epithelium properties. Further studies are required to fully characterise the signalling pathways activated by these cytokines that may improve MCC so as to identify druggable targets for the treatment of chronic pulmonary diseases while reducing the impact of inflammation. Additionally, this knowledge may also allow predicting a specific individual's pathophysiology according to the circulating levels of these cytokines.
FIGURE 8.
Models for the impact of distinct cytokines on the airway epithelium in terms of differentiation status, mucus production and expression of chloride channels/transporters. Cytokines are represented based on the heatmap clusters (figure 7): interleukin (IL)-8 and IL-22 induced similar effects as IL-1β and IL-10, respectively; tumour necrosis factor (TNF)-α is not shown, as it was grouped with control untreated cells. Mucus hyperproduction is represented by a higher thickness of the mucus gel layer (green) above the periciliary layer (PCL; blue), resulting from the upregulation of MUC5AC (red threads) or MUC5B (blue threads). MUC5B strands are also shown, usually secreted from submucosal glands and coated in the surface epithelium by MUC5AC. MUC5AC hyperproduction was prevalent in IL-4- and IL-10-treated cells, while IL-1β and IL-17A increased MUC5B. The cytokine's effects on epithelial differentiation are displayed by changes in the number of ciliated cells (higher in IL-10, lower in IL-1β and IL-4), club cells (higher in IL-4, lower in IL-1β) and pulmonary ionocytes (lower in IL-10). The number of cystic fibrosis transmembrane conductance regulator (CFTR), transmembrane protein 16A (TMEM16A) and solute carrier family 26 (SLC26A4 and SLC26A9) channels/transporters vary according to the observed differences following exposure to cytokines (figure 1) and have a cell-type distribution matching what is described for the human airway epithelium.
Acknowledgements
The authors thank Ronald G. Crystal (Dept of Genetic Medicine, Weill Cornell Medical College, New York, NY, USA) for kindly providing the BCi-NS1.1 cell line. The microscopy images presented in this study were acquired at the faculty of sciences of the University of Lisbon's microscopy facility (Lisbon, Portugal), a node of the Portuguese Platform of BioImaging (ref. PPBI-POCI-01–0145-FEDER-022122).
Provenance: Submitted article, peer reviewed.
Conflict of interest: The authors have no conflicting financial interests to disclose.
Support statement: This work was supported by UIDB/04046/2020 and UIDP/04046/2020, centre grants both from FCT/MCTES Portugal (to BioISI); and research grant SRC 013 from CF Trust UK (to M.D. Amaral). F.B. Simões is a recipient of a fellowship from the BioSys PhD programme (PD/BD/114393/2016, ref. SFRH/PD/BD/131008/2017) from FCT (Portugal). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology 2003; 8: 432–446. doi: 10.1046/j.1440-1843.2003.00493.x [DOI] [PubMed] [Google Scholar]
- 2.Crystal RG, Randell SH, Engelhardt JF, et al. . Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc 2008; 5: 772–777. doi: 10.1513/pats.200805-041HR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollenhorst MI, Richter K, Fronius M. Ion transport by pulmonary epithelia. J Biomed Biotechnol 2011; 2011: 174306. doi: 10.1155/2011/174306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen EYT, Yang N, Quinton PM, et al. . A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol 2010; 299: L542–L549. doi: 10.1152/ajplung.00180.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanke F. The contribution of the airway epithelial cell to host defense. Mediators Inflamm 2015; 2015: 463016. doi: 10.1155/2015/463016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danahay H, Atherton H, Jones G, et al. . Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2002; 282: L226–L236. doi: 10.1152/ajplung.00311.2001 [DOI] [PubMed] [Google Scholar]
- 7.Galietta LJ, Pagesy P, Folli C,et al. . IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. J Immunol 2002; 168: 839–845. doi: 10.4049/jimmunol.168.2.839 [DOI] [PubMed] [Google Scholar]
- 8.He Q, Tsang LL, Ajonuma LC, et al. . Abnormally up-regulated cystic fibrosis transmembrane conductance regulator expression and uterine fluid accumulation contribute to Chlamydia trachomatis-induced female infertility. Fertil Steril 2010; 93: 2608–2614. doi: 10.1016/j.fertnstert.2010.01.040 [DOI] [PubMed] [Google Scholar]
- 9.Brouillard F, Bouthier M, Leclerc T, et al. . NF-κB mediates up-regulation of CFTR gene expression in Calu-3 cells by interleukin-1β. J Biol Chem 2001; 276: 9486–9491. doi: 10.1074/jbc.M006636200 [DOI] [PubMed] [Google Scholar]
- 10.Baudouin-Legros M, Hinzpeter A, Jaulmes A, et al. . Cell-specific posttranscriptional regulation of CFTR gene expression via influence of MAPK cascades on 3′UTR part of transcripts. Am J Physiol Cell Physiol 2005; 289: 1240–1250. doi: 10.1152/ajpcell.00595.2004 [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran S, Karp PH, Osterhaus SR, et al. . Post-transcriptional regulation of cystic fibrosis transmembrane conductance regulator expression and function by microRNAs. Am J Respir Cell Mol Biol 2013; 49: 544–551. doi: 10.1165/rcmb.2012-0430OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jundi K, Greene CM. Transcription of interleukin-8: how altered regulation can affect cystic fibrosis lung disease. Biomolecules 2015; 5: 1386–1398. doi: 10.3390/biom5031386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest 2000; 117: 1162–1172. doi: 10.1378/chest.117.4.1162 [DOI] [PubMed] [Google Scholar]
- 14.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J 2001; 18: Suppl. 34, 50s–59s. doi: 10.1183/09031936.01.00229701 [DOI] [PubMed] [Google Scholar]
- 15.Courtney JM, Ennis M, Elborn JS. Cytokines and inflammatory mediators in cystic fibrosis. J Cyst Fibros 2004; 3: 223–231. doi: 10.1016/j.jcf.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 16.Scudieri P, Caci E, Bruno S, et al. . Association of TMEM16A chloride channel overexpression with airway goblet cell metaplasia. J Physiol 2012; 590: 6141–6155. doi: 10.1113/jphysiol.2012.240838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laoukili J, Perret E, Willems T, et al. . IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest 2001; 108: 1817–1824. doi: 10.1172/JCI200113557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montoro DT, Haber AL, Biton M, et al. . A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018; 560: 319–324. doi: 10.1038/s41586-018-0393-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scudieri P, Musante I, Venturini A, et al. . Ionocytes and CFTR chloride channel expression in normal and cystic fibrosis nasal and bronchial epithelial cells. Cells 2020; 9: 2090. doi: 10.3390/cells9092090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toews GB. Cytokines and the lung. Eur Respir J 2001; 18: Suppl. 34, 3s–17s. doi: 10.1183/09031936.01.00266001 [DOI] [PubMed] [Google Scholar]
- 21.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol 2009; 123: 986–994. doi: 10.1016/j.jaci.2009.03.033 [DOI] [PubMed] [Google Scholar]
- 22.Dudakov JA, Hanash AM, van den Brink MRM. Interleukin-22: immunobiology and pathology. Annu Rev Immunol 2015; 33: 747–785. doi: 10.1146/annurev-immunol-032414-112123.Interleukin-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabbagh K, Takeyama K, Lee HM, et al. . IL-4 induces mucin gene expression and goblet cell metaplasia in vitro and in vivo. J Immunol 1999; 162: 6233–6237. [PubMed] [Google Scholar]
- 24.Walters MS, Gomi K, Ashbridge B, et al. . Generation of a human airway epithelium derived basal cell line with multipotent differentiation capacity. Respir Res 2013; 14: 135. doi: 10.1186/1465-9921-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedetto R, Ousingsawat J, Wanitchakool P, et al. . Epithelial chloride transport by CFTR requires TMEM16A. Sci Rep 2017; 7: 12397. doi: 10.1038/s41598-017-10910-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simões FB, Quaresma MC, Clarke LA, et al. . TMEM16A chloride channel does not drive mucus production. Life Sci Alliance 2019; 2: e201900462. doi: 10.26508/LSA.201900462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt U, Weigert M, Broaddus C, et al. . Cell detection with star-convex polygons. In: Frangi A, Schnabel J, Davatzikos C, et al., eds. Medical Image Computing and Computer Assisted Intervention – MICCAI 2018. Lecture Notes in Computer Science. Vol. 11071. Cham, Springer, 2018; pp. 265–273. doi: 10.1007/978-3-030-00934-2_30 [DOI] [Google Scholar]
- 28.Ridley C, Thornton DJ. Mucins: the frontline defence of the lung. Biochem Soc Trans 2018; 46: 1099–1106. doi: 10.1042/BST20170402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okuda K, Dang H, Kobayashi Y, et al. . Secretory cells dominate airway CFTR expression and function in human airway superficial epithelia. Am J Respir Crit Care Med 2021; 203: 1275–1289. doi: 10.1164/rccm.202008-3198OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veit G, Bossard F, Goepp J, et al. . Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol Biol Cell 2012; 23: 4188–4202. doi: 10.1091/mbc.E12-06-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danahay H, Fox R, Lilley S, et al. . Potentiating TMEM16A channel function does not stimulate airway mucus secretion or bronchial and pulmonary arterial smooth muscle contraction. FASEB Bioadv 2020; 2: 464–477. doi: 10.1096/fba.2020-00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G, Sun L, Kato T, et al. . IL-1β dominates the promucin secretory cytokine profile in cystic fibrosis. J Clin Invest 2019; 129: 4433–4450. doi: 10.1172/JCI125669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rehman T, Thornell IM, Pezzulo AA, et al. . TNFα and IL-17 alkalinize airway surface liquid through CFTR and pendrin. Am J Physiol Cell Physiol 2020; 319: C331–C344. doi: 10.1152/ajpcell.00112.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thai P, Loukoianov A, Wachi S, et al. . Regulation of airway mucin gene expression. Annu Rev Physiol 2008; 70: 405–429. doi: 10.1146/annurev.physiol.70.113006.100441.Regulation [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bautista MV, Chen Y, Ivanova VS, et al. . IL-8 regulates mucin gene expression at the posttranscriptional level in lung epithelial cells. J Immunol 2009; 183: 2159–2166. doi: 10.4049/jimmunol.0803022 [DOI] [PubMed] [Google Scholar]
- 36.Enss ML, Cornberg M, Wagner S, et al. . Proinflammatory cytokines trigger MUC gene expression and mucin release in the intestinal cancer cell line LS180. Inflamm Res 2000; 49: 162–169. doi: 10.1007/s000110050576 [DOI] [PubMed] [Google Scholar]
- 37.Ermund A, Meiss LN, Rodriguez-Pineiro AM, et al. . The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin. Biochem Biophys Res Commun 2017; 492: 331–337. doi: 10.1016/j.bbrc.2017.08.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoegger MJ,Fischer AJ, McMenimen JD, et al. . Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 2014; 345: 818–822. doi: 10.1002/dev.21214.Developmental [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasnain SZ, Tauro S, Das I, et al. . IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 2013; 144: 357–368. doi: 10.1053/j.gastro.2012.10.043 [DOI] [PubMed] [Google Scholar]
- 40.Hao W, Wang J, Zhang Y, et al. . Leptin positively regulates MUC5AC production and secretion induced by interleukin-13 in human bronchial epithelial cells. Biochem Biophys Res Commun 2017; 493: 979–984. doi: 10.1016/j.bbrc.2017.09.106 [DOI] [PubMed] [Google Scholar]