Abstract
Extensive clinical data from liver-mediated gene therapy trials have shown that dose-dependent immune responses against the vector capsid may impair or even preclude transgene expression if not managed successfully with prompt immune suppression. The goal of this preclinical study was to generate an adeno-associated viral (AAV) vector capable of expressing therapeutic levels of B-domain deleted factor VIII (FVIII) at the lowest possible vector dose to minimize the potential Risk of a capsid-mediated immune response in the clinical setting. Here, we describe the studies that identified the investigational agent SPK-8011, currently being evaluated in a phase 1/2 study (NCT03003533) in individuals with hemophilia A. In particular, the potency of our second-generation expression cassettes was evaluated in mice and in non-human primates using two different bioengineered capsids (AAV-Spark100 and AAV-Spark200). At 2 weeks after gene transfer, primates transduced with 2 × 1012 vg/kg AAV-Spark100-FVIII or AAV-Spark200-FVIII expressed FVIII antigen levels of 13% ± 2% and 22% ± 6% of normal, respectively. Collectively, these preclinical results validate the feasibility of lowering the AAV capsid dose for a gene-based therapeutic approach for hemophilia A to a dose level orders of magnitude lower than the first-generation vectors in the clinic.
Keywords: hemophilia A, AAV, optimized vectors, codon optimization, low AAV dose, preclinical development
Graphical Abstract

The studies presented here represent the proof-of-concept work that generated the investigational agent SPK-8011, currently being evaluated in a phase 1/2 study (NCT03003533) for treatment of hemophilia A. Through a multi-pronged optimization approach, an adeno-associated viral vector capable of expressing therapeutic levels of hFVIII at the lowest possible vector dose was generated.
Introduction
Hemophilia is an X-linked bleeding disorder caused by a deficiency in coagulation factor VIII (FVIII; hemophilia A) or factor IX (FIX; hemophilia B). Current therapy for hemophilia is protein replacement using recombinant or plasma-derived clotting factors. The relatively short half-life of the proteins and the need for an intravenous route of delivery contribute to the burden of treatment. Gene-based therapy has the potential to markedly improve disease phenotype and to substantially lessen the burden of treatment. The current clinical trials for adeno-associated viral (AAV) vector delivery of factor IX for hemophilia B have demonstrated promising results, with average levels of factor IX activity between 5% and 33%, depending on the study.1, 2, 3
AAV delivery of FVIII for hemophilia A encounters several additional challenges compared with factor IX delivery. The major challenge is that, in contrast to factor IX and other transgene products of similar size, such as factor V, the FVIII protein is poorly expressed both in vitro and in vivo. Early studies observed low FVIII expression that was due to inefficient expression of mRNA4,5 and/or inefficient transport of protein from the endoplasmic reticulum (ER) to the Golgi.6 Transcriptional silencers and inhibitory motifs that may contribute to low FVIII expression were identified within the FVIII coding sequence.7 To overcome this challenge, several different approaches have been used. One approach is to increase FVIII transcription and translation using codon optimization.8 Another approach is to use gain-of-function variants of FVIII that have improved activity, stability, and/or secretion.9, 10, 11 Of note, the use of a hyperactive FIX variant, FIX-Padua, containing a single amino acid substitution that results in higher specific activity, has proved advantageous in the clinical gene therapy setting.2 In addition, the development of minimal regulatory promoter elements, required because the FVIII cDNA is close to the packaging limit for AAV, and the use of novel AAV capsids that improve transduction provide additional opportunities to increase FVIII expression and lower the vector dose.
Clinical experience with intravenously administered AAV vectors has revealed that the AAV vector dose may be limiting. An AAV capsid-directed CD8+ T cell response can eliminate transduced cells, leading to a self-limited transaminase rise and a rapid decline in clotting factor levels.12 Although our understanding of AAV immunogenicity is incomplete, as evidenced by differential immune responses observed among clinical trials,13 there is consensus that the immune response to the AAV capsid is less likely to be observed at low vector doses. Although investigations are still ongoing, systemic delivery of high AAV vector doses may be associated with toxicities in some patients in a clinical study of X-linked myotubular myopathy.14,15 These results have implications for other genetic diseases that are candidates for AAV gene therapy and suggest that using the lowest possible vector dose necessary to achieve therapeutic expression of the transgene product may decrease the risk for toxicity and eliciting a capsid-mediated immune response.
In order to achieve therapeutic levels of FVIII while reducing the AAV vector dose, we carried out a multi-pronged development plan to increase FVIII expression from our AAV vectors. Specifically, codon-optimized (CO) sequences were introduced into an expression cassette that uses a modified promoter element and delivered using novel AAV capsids into hemophilia A mice and non-human primates (NHPs). Variants of FVIII that have increased activity and improved secretion were also explored for further enhancement of FVIII expression after AAV delivery. We also compared the effectiveness of two novel bioengineered capsids. The studies presented here identified investigational SPK-8011, an AAV vector that contains the first bioengineered capsid to be evaluated clinically in a phase 1/2 study for the treatment of hemophilia A (NCT03003533).16
Results
Optimization of the hepatocyte-specific transthyretin promoter increases FVIII expression
The size of the B-domain deleted FVIII coding sequence is 4.37 kb, and the inverted terminal repeats (ITRs) combined account for approximately 300 bp. This imposes a stringent limitation on the size of the regulatory elements, as packaging efficiency declines precipitously above approximately 5 kb.17,18 In order to increase FVIII expression, we placed a codon-optimized, B-domain deleted FVIII sequence under the control of a hepatocyte-specific transthyretin promoter (TTRm) containing a modification of a hepatocyte nuclear factor transcription factor binding site that increases the binding affinity of the transcription factor leading to increased expression (Figure 1A).19 Delivery of AAV8 vectors using the parental TTR (TTR-hFVIII-CO) or the modified (TTRm-hFVIII-CO) promoter into immune-deficient hemophilia A (F8−/−/CD4−/−) mice resulted in levels of human FVIII (hVIII) expression that were 91 ± 24 and 435 ± 96 ng/mL, respectively (Figure S1A). Given the approximately 4-fold increased potency of the modified TTR promoter, we used it in all the subsequent studies described below.
Figure 1.
Introduction of furin variants into a first-generation codon-optimized sequence improves hFVIII expression
(A) Schematic of the B-domain deleted hFVIII expression cassettes. (B) AAV vectors were delivered at a dose of 4 × 1012 vg/kg into hemophilia A/CD4 KO mice (n = 4/group). The vectors with and without codon-optimized sequences are labeled as hFVIII-CO and hFVIII, respectively. The Δ3 and Δ4 furin variants were introduced into codon-optimized constructs, generating hFVIII-CO-Δ3 and hFVIII-CO-Δ4, respectively. Human FVIII plasma levels were assayed using ELISA. ∗p < 0.05, Kruskal-Wallis one-way ANOVA vs hFVIII. (C) Hemostatic challenge of AAV-treated animals. The tail clip assay was performed at 6 weeks after vector administration. ∗p < 0.05, Kruskal-Wallis one-way ANOVA; ns, non-significant versus wild-type (WT) PBS. (D) AAV vectors carrying either hFVIII-CO or hFVIII-CO-Δ3 cassettes were administered to hemophilia A/CD4 KO mice at three different doses (2 × 1011, 8 × 1011, and 4 × 1012 vg/kg). Shown are hFVIII levels measured using ELISA after vector administration. ∗p < 0.05, Kruskal-Wallis one-way ANOVA versus hFVIII-CO Low. Results in panels B-D are represented as mean ± standard error of the mean.
Introduction of furin variants into codon-optimized FVIII further increases FVIII expression
We have previously demonstrated that complete or partial deletion of the furin recognition site at residues 1645–1648 in the wild-type (i.e., non-codon-optimized) FVIII-BDD sequence results in increased secretion following hepatic gene transfer of AAV8 vectors.9 To determine if the increases in hFVIII expression obtained through codon optimization and the introduction of the furin variants were additive, we introduced the deletion of residues 1645–1647 (Δ3) or 1645–1648 (Δ4) into the CO cassette (Figure 1A). These constructs were packaged into the AAV-Spark100 capsid, the same capsid used in a phase 1/2 study for hemophilia B.2 The levels of FVIII expression after delivery of 4 × 1012 vg/kg (1 × 1011 vg/mouse) AAV-Spark100 were 254 ± 67 and 156 ± 18 ng/mL for CO-Δ3 and CO-Δ4, respectively, compared with 97 ± 28 ng/mL for the CO sequence alone at 8 weeks after vector administration (Figure 1B). At 24 weeks after vector administration, the CO-Δ3 and CO-Δ4 variants had levels of FVIII expression that were similar to each other and not statistically different (Figure S1B). Consistent with our previous studies with the furin variants in the context of hFVIII-BDD,9 the increased procoagulant activity of the Δ3 and Δ4 variants was due to 2- to 3-fold higher expression compared with CO and not to increased specific activity (data not shown). The hemostatic function of all the variants was assessed using a tail clip assay following the administration of 4 × 1012 vg/kg AAV-Spark100. At this vector dose, the AAV-hFVIII-BDD-treated hemophilia A mice had blood loss that was similar to that of the untreated hemophilia A mice (Figure 1C). As expected on the basis of the high levels of circulating hFVIII, the CO-, CO-Δ3-, and CO-Δ4-treated mice had significantly reduced blood loss that was similar to that of wild-type mice.
To assess whether the increased potency of the furin variants was consistent across different vector doses, the CO and CO-Δ3 cassettes were administered to immune-deficient hemophilia A mice using three different AAV-Spark100 doses: 2 × 1011, 8 × 1011, and 4 × 1012 vg/kg (Figure 1D). At 12 weeks after vector administration, the levels of hFVIII expression after AAV-CO delivery were 12 ± 2, 21 ± 3, and 104 ± 26 ng/mL for the low-, mid-, and high-dose cohorts, respectively. For the AAV-CO-Δ3 group, the levels of expression were 36 ± 12 ng/mL for the low-dose, 99 ± 26 ng/mL for the mid-dose, and 187 ± 59 ng/mL for the high-dose group. Comparison of the 4 × 1012 vg/kg dose of AAV-CO and the 8 × 1011 vg/kg dose of AAV-CO-Δ3 demonstrated that the CO-Δ3 construct achieved the same levels of expression at a 5-fold lower vector dose. Thus, the introduction of the Δ3 furin variant into the CO sequence provides a strategy for lowering the vector dose.
Second-generation codon-optimized sequences further increase FVIII expression
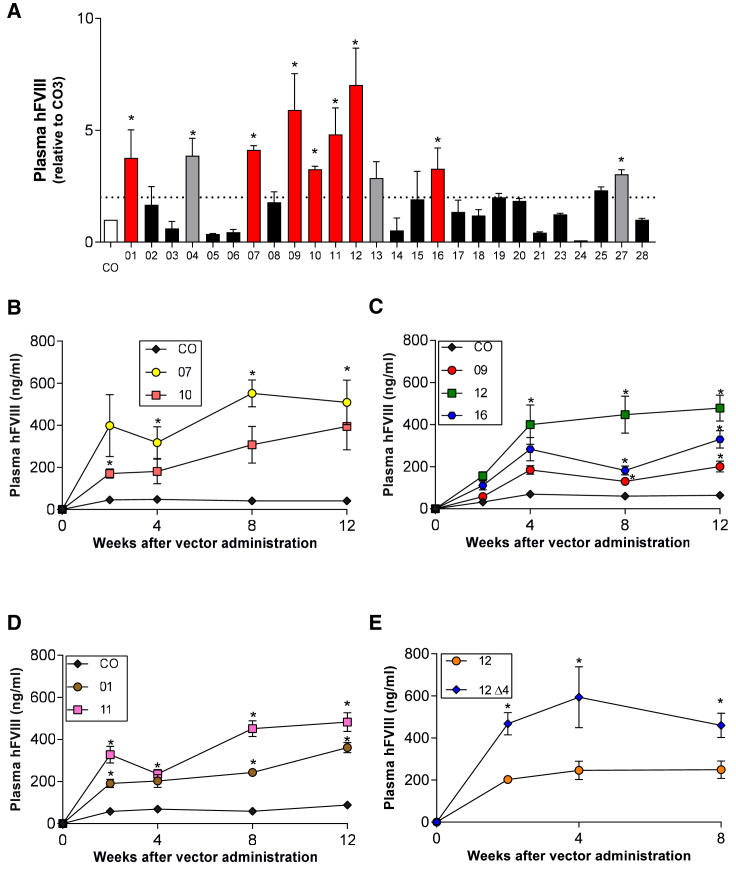
To further identify expression cassettes that may improve FVIII expression compared with CO, we screened 26 different codon-optimized sequences. Each codon-optimized sequence was introduced into the TTRm-FVIII expression cassette shown in Figure 1A. For the initial screen, the pAAV plasmids were delivered by hydrodynamic tail vein injection into wild-type mice. Ten of the 26 codon-optimized plasmids had 2- to 7-fold higher FVIII expression compared with CO (Figure 2A) and were selected for AAV production using the AAV-Spark100 capsid. Of those, 3 were discarded because of suboptimal vector yields during manufacturing. AAV vectors carrying these 7 codon-optimized sequences were delivered to immune-deficient hemophilia A mice at a dose of 4 × 1012 vg/kg. At 8 weeks after vector administration, the highest levels of hFVIII expression were observed for codon-optimized cassettes 07, 11, and 12. Specifically, circulating hFVIII levels were 550, 425, and 425 ng/mL, respectively, compared with CO (25–40 ng/mL) (Figures 2B–2D). These three constructs were also in the top half of the best performing constructs in the hydrodynamic study. Absolute quantification of human FVIII mRNA levels in the liver at the time of sacrifice showed that cassettes 07 and 12 were substantially better expressers than both the non-codon-optimized sequence and the first-generation CO sequence (Figure S2). Cassettes 01, 09, 10, and 16 expressed in the range of 175–250 ng/mL. To better understand if hydrodynamic tail vein injection may generally predict performance in the setting of AAV delivery, we also tested vector potency of two constructs that were not high expressers after plasmid injection. Perhaps not surprisingly, these two vectors had similar expression levels compared with CO (data not shown).
Figure 2.
Generation of second-generation codon-optimized FVIII cassettes
(A) Wild-type mice (n = 5/group) were hydrodynamically delivered 50 μg of plasmids carrying 1 of the 26 novel codon-optimized sequences, shown as 1–26, and benchmarked against the first-generation codon-optimized cassette (CO). Plasma was collected 24 h after hydrodynamic delivery for FVIII analysis. Average hFVIII levels in the CO group were given a relative value of 1, and all the other groups were normalized to this reference. Red bars indicate the constructs that were selected for further study as AAV vectors. Gray bars indicate the three constructs that were discarded because of low yields. The dotted line indicates 2-fold over CO. ∗p < 0.05 versus CO in groups with FVIII >2-fold difference, one-way ANOVA. (B–E) AAV delivery of second-generation codon-optimized sequences. AAV vectors using the Spark100 capsid and containing the TTRm-hFVIII-CO sequences were administered to hemophilia A/CD4 KO mice at 4 × 1012 vg/kg. Human FVIII levels were measured by ELISA and followed for 8 or 12 weeks. (B) TTRm-hFVIII-CO compared with codon-optimized sequences 07 and 10. ∗p < 0.05 versus CO, Kruskal-Wallis one-way ANOVA. (C) TTRm-hFVIII-CO compared with codon-optimized sequences 09, 12, and 16. ∗p < 0.05 versus CO, Kruskal-Wallis one-way ANOVA. (D) TTRm-hFVIII-CO compared with codon-optimized sequences 01 and 11. ∗p < 0.05 versus CO, Kruskal-Wallis one-way ANOVA. (E) TTRm-hFVIII-12 compared with a vector containing the TTRm-hFVIII-12 Δ4 furin variant. ∗p < 0.05 versus 12, unpaired t test. Results are represented as mean ± standard error of the mean.
To determine if the furin variant would result in increased expression in the context of these second-generation codon-optimized sequences that express higher levels of hFVIII, we introduced the full deletion of the furin site from 1645 to 1648 into the 12-FVIII sequence. Consistent with previous results shown above (Figure 1B), the furin variant 12-Δ4 yielded expression levels approximately 2- to 3-fold higher than the 12-FVIII sequence alone, further confirming that introduction of the furin variant into the codon-optimized sequence results in an additive effect on FVIII expression after AAV delivery even when FVIII expression is supra-physiological because of codon optimization (Figure 2E).
Efficacy of novel AAV-hFVIII vectors in non-human primates
The lead codon-optimized hFVIII transgene candidate, construct 07, was delivered to cynomolgus macaques in two different AAV capsids, AAV-Spark100 and AAV-Spark200 (originally described as AAV-LK0320), in two separate dose escalation studies. AAV-Spark100-TTRm-hFVIII-07, abbreviated SPK-8005, was administered at three different doses, 2 × 1012, 5 × 1012, and 1 × 1013 vg/kg (Figure 3). Macaques were prescreened for neutralizing antibodies against the AAV-Spark100 capsid. All treated animals were initially determined to have a <1:1 titer before vector administration. This was done to ensure successful hepatic transduction, as even low anti-AAV antibody titers inhibit vector uptake by liver cells after systemic delivery.21 All animals were also negative for the presence of neutralizing antibodies against FVIII before gene transfer. Human FVIII antigen levels were followed in the macaques for 8 weeks after AAV delivery using a transgene product-specific ELISA that does not cross-react with endogenous macaque FVIII. Antigen levels peaked at about 1 to 2 weeks following vector administration. At 1 week after gene transfer, NHPs transduced with 2 × 1012 vg/kg of SPK-8005 expressed hFVIII antigen levels of 13% ± 3% (Figure 3A). At 1 week after gene transfer, average hFVIII levels in two of the three animals in the next treatment cohort (5 × 1012 vg/kg) were 27% ± 0.2% (Figure 3B). Human FVIII could not be detected in the third macaque in that cohort at any time point. Upon re-testing of baseline plasma samples, it was determined that this animal was in fact positive for the presence of anti-AAV antibodies. Finally, at the highest tested dose of 1 × 1013 vg/kg, hFVIII antigen levels of 47% ± 23% were observed 1 week after AAV infusion (Figure 3C). As anticipated on the basis of our previous studies in NHPs expressing human FIX, hFVIII expression declined in six of the eight animals, concomitant with the appearance of anti-hFVIII antibodies in these macaques (labeled with an ε sign in Figure 3). Development of species-specific antibodies to hFVIII has been previously documented in non-human primates and is likely due to differences in several amino acid residues between the human transgene product and the endogenous cynomolgus FVIII.11
Figure 3.
Levels of hFVIII in plasma of cynomolgus macaques after SPK-8005 administration
(A–C) Animals received intravenous administration of either 2 × 1012 (A), 5 × 1012 (B), or 1 × 1013 vg/kg (C) SPK-8005, also known as Spark100-TTRm-hFVIII-07. Lines represent individual animals. Human FVIII plasma levels were assayed using ELISA and represent repeated measurements, obtained by serial bleeding, on the same group of animals during the course of the study (n = 2 or 3 animals per cohort). Human FVIII levels measured in vehicle-treated animals are shown in white squares in all three graphs. ε denotes detection of anti-FVIII antibodies.
Another cohort of cynomolgus macaques (n = 3/dose cohort) was administered AAV-Spark200-TTRm-FVIII-07, also known as SPK-8011 (Figure 4). Human FVIII antigen levels peaked at about weeks 2–3, with 22.3% ± 6% of normal hFVIII antigen levels seen in animals administered 2 × 1012 vg/kg of SPK-8011. The animals administered 6 × 1012 and 2 × 1013 vg/kg of SPK-8011 expressed hFVIII antigen levels of 62% ± 16% and 153% ± 58%, respectively. As observed in the study with SPK-8005, eight of the nine animals developed anti-hFVIII antibodies that precluded long-term efficacy follow-up. These preliminary results suggest that the AAV-Spark200 capsid more effectively transduces non-human primate hepatocytes, leading to levels of hFVIII expression that are 1.5- to 2-fold higher than AAV-Spark100.
Figure 4.
Levels of hFVIII in plasma of cynomolgus macaques after SPK-8011 administration
(A–C) Animals received intravenous administration of either 2 × 1012 (A), 6 × 1012 (B), or 2 × 1013 vg/kg (C) SPK-8011, also known as Spark200-TTRm-hFVIII-07. Lines represent individual animals. Human FVIII plasma levels were assayed using ELISA and represent repeated measurements, obtained by serial bleeding, on the same group of animals during the course of the study (n = 3 animals per cohort). ε denotes detection of anti-FVIII antibodies.
The biodistribution profile of both AAV capsids was analyzed upon sacrifice of the animals 30 days after vector administration. A total of 21 tissue samples were collected to determine the presence of vector genomes by qPCR. Three doses of AAV-Spark200 (3 × 1012, 6 × 1012, and 2 × 1013 vg/kg) and a single dose of 5 × 1012 vg/kg of AAV-Spark100 were analyzed. No signal was detected in any organs from animals infused with vehicle (data not shown). For both the AAV-Spark200 and AAV-Spark100 capsids, the highest levels of vector DNA were detected in the liver (Figure 5). For both capsids, the next most abundant vector sequences were seen in the spleen, followed by hepatic lymph nodes in the case of AAV-Spark200 and inguinal lymph nodes in the case of AAV-Spark100. Importantly, very little vector was observed in the testes and brains of the animals infused with either vector, with close to four logs lower vector copies compared with liver (Figure 5). Overall, these data are consistent with previously published data for other AAV serotypes under investigation in other liver-targeted gene therapy studies.
Figure 5.
Biodistribution of vector genomes in tissues of cynomolgus macaques after SPK-8005 and SPK-8011 administration
One microgram of genomic DNA was isolated from the indicated organs 30 days after administration of the vectors and vector genome presence was analyzed by qPCR. (A) Tissue biodistribution following administration of 5 × 1012 vg/kg of AAV-Spark100 (n = 4 macaques). (B) Tissue biodistribution following administration of 3 × 1012, 6 × 1012, or 1.2 X 1013 vg/kg AAV-Spark200 (n = 3 macaques per dose). Results are represented as mean vector copy number per haploid genome ± standard error of the mean.
In order to assess potential thrombogenesis due to expression of hFVIII in hemostatically normal cynomolgus monkeys, D-dimer antigen levels were measured in the SPK-8005 and SPK-8011 studies. In humans, the normal range for D-dimer is less than 500 ng/mL. However, reports on the clinical relevance or even the normal values of D-dimer antigen levels in cynomolgus macaques are scarce. On the basis of the levels measured before vector administration, the mean D-dimer antigen levels in the macaques at baseline were 540 and 582 ng/mL in the SPK-8005 and SPK-8011 studies, respectively. These baseline values were considered the upper limit of normal (ULN) for these two cohorts of animals. Of note, the NHPs express endogenous cynomolgus FVIII, and thus production of hFVIII as a result of hepatic gene transfer by definition results in supra-physiological levels of FVIII activity. Two criteria were used to consider a sample positive: D-dimer antigen levels above the ULN and 20% above an animal’s baseline level. Sporadic and transient elevations in D-dimer levels were observed, but overall D-dimers were higher in many NHPs at baseline than at time points post-AAV administration (Figures S3 and S4). In the cases in which elevations in D-dimer above baseline levels were observed, there was no correlation between the D-dimer levels and coagulation factor levels (data not shown). Importantly, no vector-related changes in clinical observations, body weight, or qualitatively assessed food consumption occurred, and no elevations in aspartate aminotransferase levels were observed (Figures S5 and S6). No test vector-related abnormalities were noted during physical examinations. At the terminal sacrifice, full necropsies on all animals were conducted. There were no microscopic findings that were considered direct effects of SPK-8011 and no vector-related macroscopic observations or changes in organ weight parameters. Taking all these results into account, the no adverse effect level (NOAEL) in this 30 day study was considered to be 2 × 1013 vg/kg.
Discussion
Early in the development of gene therapy approaches for hemophilia A, it became apparent that FVIII RNA and circulating protein levels were low compared with similarly sized proteins. These low FVIII levels could be explained, at least in part, by inefficient mRNA expression and presence of several transcriptional silencers and inhibitory motifs spread throughout the FVIII coding sequence.5 An additional limitation was posed by the size of the early constructs, which was significantly above the ideal packaging limit of AAV vectors. Consequently, the AAV vector dose to achieve therapeutic levels of FVIII in hemophilia A dogs was significantly higher than the AAV vector dose to achieve therapeutic levels of factor IX in hemophilia B dogs.22, 23, 24 In addition, these studies in the hemophilia A dog model used a species-specific transgene (canine FVIII [cFVIII]) to avoid an immune response to FVIII. However, we have previously shown that canine FVIII has higher specific activity and is secreted at higher levels than hFVIII.25 Therefore, it was hypothesized that the use of a human FVIII transgene might require a higher vector dose to achieve therapeutic levels of expression. Indeed, using the same expression cassette, vector doses of AAV-hFVIII that are 20-fold higher than AAV-cFVIII are required to achieve similar levels of FVIII expression with these vectors in hemophilia A mouse models.9 Despite considerable improvements in developing more potent FVIII cassettes,11 the challenge of clinical translation of AAV-FVIII delivery is illustrated by two recent clinical studies, one for the treatment of hemophilia B2 and the other for the treatment of hemophilia A,26 in which the difference in vector dose was 120-fold. Thus, developing strategies to increase hFVIII expression in the setting of gene transfer is critical to the translation of more efficient AAV-hFVIII delivery approaches. In this study, we have pursued a multi-step approach to improve expression from FVIII vectors. At the transcriptional and translational levels, we took advantage of a more potent promoter and also optimized expression through two rounds of codon optimization. We also explored the possibility of modifying the FVIII protein, using furin variants that have improved secretion. Finally, we used recently described, highly hepatotropic AAV capsids to increase vector potency. Importantly, these studies demonstrate that therapeutic levels of FVIII expression can be achieved at AAV vector doses that are comparable with the AAV vector doses used in the hemophilia B clinical studies.
Strategies to overcome the bottlenecks in FVIII expression are not new and began with the use of the B-domain deleted FVIII variant, the first modified FVIII that resulted in an increase in FVIII mRNA. Although the FVIII-BDD form had a 17-fold increase in mRNA levels compared with full-length FVIII, there was only a 30% increase in the secreted protein.6 Given the size limitation of AAV vectors, the use of an FVIII-BDD form is mandatory for all AAV-based gene therapies. Codon optimization may increase expression by improving translation but also in part by elimination of transcriptional silencers and inhibitory sequences. The first generation of codon-optimized FVIII-BDD cassettes that we developed resulted in significant increases in FVIII expression compared with the wild-type FVIII-BDD (Figure 1B). Next, we introduced further modifications at the DNA level to not only increase expression of our first-generation cassettes but reduce TLR9 signaling after gene transfer via reducing the amount of CpG dinucleotides from the coding sequence and thus minimize potential innate immune responses against the vector.27 Administration of the different codon-optimized constructs into a hemophilic mouse model demonstrated a range of hFVIII expression levels with up to a 25-fold difference between the highest and the lowest expressing cassettes.
In addition, we explored the use of variants of FVIII that have improved secretion to further overcome the barriers to FVIII expression.9,10 In our previous studies with the furin variants in the context of the wild-type hFVIII protein, the FVIII was secreted at 2- to 3-fold higher levels than the wild-type hFVIII-BDD alone.9 As codon optimization and the furin variants use two independent mechanisms to increase FVIII levels in the circulation, we combined these approaches to determine if they could be exploited together to improve FVIII expression. The introduction of the furin variants into the codon-optimized sequences also increased the expression several-fold over the codon-optimized sequence alone (Figures 1B and 1D). Not surprisingly given the high circulating levels of FVIII compared with hFVIII-BDD, gene transfer of the new variants resulted in correction of the bleeding phenotype in hemophilia A mice (Figure 1C). This demonstrates that introduction of the furin variant into the codon-optimized sequence results in an additive effect on FVIII expression after AAV delivery even when FVIII expression is supra-physiological because of codon optimization.
Despite the increased expression levels generated by the furin variants, and in order to build upon several years of clinical safety data, we made the decision to proceed with the development of a vector encoding a B-domain deleted form of FVIII (i.e., SQ) that is identical to recombinant FVIII SQ (ReFacto/Xyntha), used for hemophilia A treatment worldwide and with hemostatic activity similar to that of full-length FVIII concentrates.28 When packaged into the AAV-Spark100 capsid, the cassette with the highest potency, TTRm-hFVIII-07, known as SPK-8005, resulted in the highest expression levels in hemophilia A mice (Figure 2). On the basis of these results, we performed two studies in non-human primates using state-of-the-art hepatotropic AAV capsids (i.e., AAV-Spark100 [Figure 3] and AAV-Spark200 [Figure 4]). The AAV-Spark200 capsid, also known as AAV-LK03, was identified using a humanized mouse liver model to screen for novel AAV capsids that efficiently transduce human hepatocytes.20 Using AAV-Spark200-FVIII, we observed circulating levels of hFVIII that were 1.5- to 2-fold higher than with the AAV-Spark100 capsid. The AAV-Spark200 capsid more effectively transduces non-human primate hepatocytes and supports the approach of using a humanized mouse model for AAV library screening. As expected, vector genomes in the liver increased with increasing vector doses (Figure 5). Of note, the safety and efficacy results generated with this novel AAV vector, SPK-8011, constitute the proof of concept to support our ongoing phase 1/2 clinical study (NCT03003533) that represents the first use of a bioengineered AAV capsid in a clinical study for the treatment of hemophilia A.16
As there is a strong correlation between the AAV vector dose and the immune response to the AAV capsid that has been observed in humans, the use of the lowest possible vector dose may minimize the risk for this immune response that can lead to a reduction of the transgene expression due to CD8+ T cell elimination of transduced cells.29,30 Although the transient use of glucocorticoids has been able to attenuate this immune response in most patients, the lower AAV dose may minimize the potential immune response, and thus the goal remains, for this as for other pharmacologic agents, to achieve therapeutic levels at the lowest effective vector dose. Importantly, preliminary results from our phase 1/2 dose escalation trial appear consistent with the notion that clinically relevant levels of FVIII are achieved at doses ranging from 5 × 1011 to 2 × 1012 vg/kg, with no evidence of a cellular immune response to transduced hepatocytes.16 The ability to achieve therapeutic levels of FVIII expression at AAV vector doses that are comparable with those used in the context of AAV trials for the treatment of hemophilia B represents a significant departure from the previously held notion that AAV gene therapy for hemophilia A would require significantly higher doses. As a point of comparison, results from two gene therapy studies in severe hemophilia A subjects showed that the threshold level above which clinically relevant expression level is detected is at least 10-fold higher than for other investigational AAV vectors.26,31 The FVIII expression data in macaques appear largely consistent with preliminary clinical results using SPK-8011 and suggest that macaques are a good predictor of vector efficacy in humans for liver-targeted indications.32 Of note, the highest circulating levels of hFVIII in the primates that received 6 × 1012 vg/kg of SPK-8011 were about 100% of normal. In the clinic, targeting expression levels below 150% of normal would appear prudent, as levels of FVIII above 150% have been associated with thrombosis33,34 in normal individuals.
In summary, the preclinical results presented here with this novel AAV vector, SPK-8011, constitute the proof of concept to support our ongoing phase 1/2 clinical study for the treatment of hemophilia A (NCT03003533). Through the use of second-generation codon-optimized FVIII sequences and a bioengineered AAV capsid with specific tropism for human hepatocytes, we were able to achieve clinically relevant levels of FVIII expression in non-human primates at AAV vector doses that are comparable with those used in the context of AAV trials for the treatment of hemophilia B. Although these results are encouraging, more clinical data are required to better understand the safety and efficacy implications of using lower AAV vector doses.
Materials and methods
Factor VIII transgene constructs
A non-codon-optimized 4,374 bp nucleotide sequence encoding the B-domain deleted hFVIII-SQ variant described by Lind et al.35 was used as the primary sequence to generate three first-generation codon-optimized sequences (CO1, CO2, and CO3) using a commercially available algorithm. The second-generation codon-optimized F8 sequences were designed to reduce the amount of CpG dinucleotides from the open reading frame. The sequence of second-generation CO-07 is shown in Figure S7. Each sequence was synthesized and cloned into a pAAV plasmid containing AAV2 ITRs. Either a minimal 222 bp liver-specific transthyretin (TTR) promoter or a modified version (TTRm)19 were used along with a 108 bp synthetic intron to drive transgene expression. The TTRm promoter has a modification of a hepatocyte nuclear factor (HNF3) binding site that converts it from a weak affinity site to a site with strong affinity.19 The constructs contain a 46 bp synthetic poly(A) sequence. The furin variants were generated in the codon-optimized sequence by deletion of residues 1645–1647 (Δ3) or residues 1645–1648 (Δ4), as previously described.9
Animal models
Male hemophilia A exon 16 knockout (KO) mice36 that are also CD4 deficient (F8−/−/CD4−/−, C57BL/6) were used for AAV studies. Wild-type C57BL/6 mice were used for the hydrodynamic delivery studies. All procedures were approved by the Institutional Animal Care and Use Committee at The Children’s Hospital of Philadelphia. The studies with cynomolgus macaques were performed at Covance Laboratories. The macaques, obtained from Covance Research Products, were 2–3 years of age and weighed 3–5 kg.
Hydrodynamic delivery
Plasmid constructs (pAAV-hFVIII) were delivered into 8- to 10-week-old C57BL/6 mice (Jackson Laboratories) by hydrodynamic infusion of 50 μg of plasmid DNA in a volume of saline that was equivalent to 10% of the mouse body weight via the tail vein within five seconds.37 Three to five mice were injected per construct. Peripheral blood was collected in 3.8% sodium citrate via tail transection 24 h after hydrodynamic delivery.
Tail clip assay
The mouse tail was pre-warmed in 37°C saline, the tail was transected at a 3 mm diameter, and blood was collected for 10 min in warm saline. Total blood loss (microliters) was quantitated by lysing the red cell pellet, measuring the absorbance at 575 nm, and converting the hemoglobin content using standard curves established with known amounts of mouse whole blood.38
AAV production and delivery
Recombinant AAV vectors were produced by triple transfection of HEK293 cells, purified from clarified cell lysates by cation-exchange chromatography and by cesium chloride gradient centrifugation as previously described.39, 40, 41 Vectors were analyzed using silver staining, and titers were obtained by Taqman PCR (Applied Biosystems, Foster City, CA). AAV vectors were generated at the Research Vector Core at The Children’s Hospital of Philadelphia. The AAV-Spark100 and AAV-Spark200 (also known as AAV-LK03) capsids have been previously described.2,20 AAV vectors were administered to 8- to 12-week-old male hemophilia A/CD4 KO mice (n = 5 mice/vector) in a volume of 200 μL of phosphate-buffered saline/0.001% Pluronic via tail vein injection. Plasma samples were collected in 3.8% sodium citrate by tail sectioning. The cynomolgus macaques were administered AAV as an intravenous infusion via the saphenous vein using a calibrated infusion pump over approximately 30 min.
FVIII antigen and activity assays
hFVIII antigen and activity levels in the mice were determined as previously described.10 The levels of hFVIII transgene product in cynomolgus macaque plasma were quantified using ELISA. Plates were coated with capture antibody GMA-8024, which recognizes human FVIII and does not cross-react with endogenous cynomolgus macaque FVIII (Green Mountain Antibodies; diluted to 2 μg/mL). Plates were washed and blocked (6% BSA, 0.2% Tween 20 in PBS). Pooled cynomolgus plasma was spiked with a known concentration of recombinant B-domain deleted hFVIII (Xyntha Solofuse) for a standard curve. A biotinylated anti-hFVIII antibody (GMA-8023, Green Mountain Antibodies; diluted to 1 μg/mL) was added to the plates to bind to the captured hFVIII protein, and a peroxidase-conjugated streptavidin secondary antibody was used for detection (Thermo Fisher Scientific; 1:5,000 dilution). The peroxidase activity was revealed with 3,3′,5,5′-tetramethylbenzidine substrate (TMB). The reaction was stopped with TMB Stop Solution and then the optical density (OD) was read at 450 nm.
D-dimer assays
D-dimer levels were measured using the ASSERACHROM D-DI kit from Diagnostica Stago. Plasma samples are added to plastic microplate wells pre-coated with mouse monoclonal anti-human D-dimer antibody and incubated for 1 h at room temperature. After 5× washes, rabbit anti-human fragment D antibodies coupled with peroxidase were added and incubated for 1 h at room temperature. After addition of the TMB substrate, the reaction was stopped with a strong acid, and the intensity of the color was directly proportional to the concentration of D-dimer initially present in the plasma sample.
Digital droplet PCR
Digital droplet PCR (ddPCR) was performed on the QX100 (Bio-Rad) according to the manufacturer’s recommendations for probe-based assays. Reverse transcription was performed on RNA using the High Capacity Reverse Transcription Kit (Thermo Fisher Scientific) in 20 μL total volume. cDNA was diluted to 5 ng/μL of starting material with nuclease-free water, and 10 μL was used for ddPCR assays, each sample was run in triplicate. All ddPCR assays were multiplexed and performed in 20 μL reactions. All ddPCR assays were synthesized by IDT. FVIII assays were synthesized with a FAM reporter dye (primer/probe sequences shown in Figure S2). A HEX-containing reference assay (GusB: Mm.PT.39a.22214848) was included in each well for normalization.
Statistics
All data are shown as mean ± SEM except as otherwise indicated. Figures 3 and 4 show individual animal values. The statistical tests performed and a summary of the statistical data and analysis for Figures 1 and 2 are shown in Figure S8. Data analyses were performed using GraphPad Prism 9.
Acknowledgments
This work was supported by funding from the Delaware Valley Chapter of the National Hemophilia Foundation (to D.E.S.), NIH grant RO1 HL126850 (to D.E.S.) and Spark Therapeutics. We would like to thank Carolyn M. Yrigollen for her support with the ddPCR assays.
Author contributions
Conceptualization, D.E.S., X.M.A., L.E., and K.A.H.; Methodology, L.E., D.E.S., and X.M.A.; Investigation, L.E., S.M.A., R.T., M.D., R.J.D., G.N.N., M.W., S.K., J.S., J.F., M.C., Y.W., and C.W.; Supervision, D.E.S. and X.M.A.; Writing – Original Draft, D.E.S. and X.M.A.; Writing – Review & Editing, D.E.S., X.M.A., L.E., and K.A.H.; Funding Acquisition, K.A.H. and D.E.S.
Declaration of interests
S.M.A., R.T., M.D., M.W., S.K., J.S., J.F., M.C., Y.W., C.W., L.E., K.A.H., and X.M.A. are current or former employees of Spark Therapeutics. K.A.H., X.M.A., L.E., and D.E.S. are inventors on issued and pending patents related to AAV viral vectors for which they have received royalty payments.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2021.11.005.
Contributor Information
Denise E. Sabatino, Email: dsabatin@pennmedicine.upenn.edu.
Xavier M. Anguela, Email: xavier.anguela@vhir.org.
Supplemental information
References
- 1.Nathwani A.C., Reiss U.M., Tuddenham E.G., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D., et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J., et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miesbach W., Meijer K., Coppens M., Kampmann P., Klamroth R., Schutgens R., Tangelder M., Castaman G., Schwable J., Bonig H., et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2018;131:1022–1031. doi: 10.1182/blood-2017-09-804419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoeben R.C., Fallaux F.J., Cramer S.J., van den Wollenberg D.J., van Ormondt H., Briet E., van der Eb A.J. Expression of the blood-clotting factor-VIII cDNA is repressed by a transcriptional silencer located in its coding region. Blood. 1995;85:2447–2454. [PubMed] [Google Scholar]
- 5.Lynch C.M., Israel D.I., Kaufman R.J., Miller A.D. Sequences in the coding region of clotting factor VIII act as dominant inhibitors of RNA accumulation and protein production. Hum. Gene Ther. 1993;4:259–272. doi: 10.1089/hum.1993.4.3-259. [DOI] [PubMed] [Google Scholar]
- 6.Pittman D.D., Marquette K.A., Kaufman R.J. Role of the B domain for factor VIII and factor V expression and function. Blood. 1994;84:4214–4225. [PubMed] [Google Scholar]
- 7.Koeberl D.D., Halbert C.L., Krumm A., Miller A.D. Sequences within the coding regions of clotting factor VIII and CFTR block transcriptional elongation. Hum. Gene Ther. 1995;6:469–479. doi: 10.1089/hum.1995.6.4-469. [DOI] [PubMed] [Google Scholar]
- 8.Ward N.J., Buckley S.M., Waddington S.N., Vandendriessche T., Chuah M.K., Nathwani A.C., McIntosh J., Tuddenham E.G., Kinnon C., Thrasher A.J., et al. Codon optimization of human factor VIII cDNAs leads to high-level expression. Blood. 2011;117:798–807. doi: 10.1182/blood-2010-05-282707. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen G.N., George L.A., Siner J.I., Davidson R.J., Zander C.B., Zheng X.L., Arruda V.R., Camire R.M., Sabatino D.E. Novel factor VIII variants with a modified furin cleavage site improve the efficacy of gene therapy for hemophilia A. J. Thromb. Haemost. 2017;15:110–121. doi: 10.1111/jth.13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siner J.I., Iacobelli N.P., Sabatino D.E., Ivanciu L., Zhou S., Poncz M., Camire R.M., Arruda V.R. Minimal modification in the factor VIII B-domain sequence ameliorates the murine hemophilia A phenotype. Blood. 2013;121:4396–4403. doi: 10.1182/blood-2012-10-464164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntosh J., Lenting P.J., Rosales C., Lee D., Rabbanian S., Raj D., Patel N., Tuddenham E.G., Christophe O.D., McVey J.H., et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121:3335–3344. doi: 10.1182/blood-2012-10-462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mingozzi F., High K.A. Overcoming the host immune response to adeno-associated virus gene delivery vectors: the race between clearance, tolerance, neutralization, and escape. Annu. Rev. Virol. 2017;4:511–534. doi: 10.1146/annurev-virology-101416-041936. [DOI] [PubMed] [Google Scholar]
- 13.Herzog R.W. Complexity of immune responses to AAV transgene products – example of factor IX. Cell Immunol. 2017 doi: 10.1016/j.cellimm.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shieh P.B., Bonnemann C.G., Muller-Felber W., Blaschek A., Dowling J.J., Kuntz N.L., Seferian A.M. Re: "moving forward after two deaths in a gene therapy trial of myotubular myopathy" by wilson and flotte. Hum. Gene Ther. 2020;31:787. doi: 10.1089/hum.2020.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philippidis A. After third death, audentes' AT132 remains on clinical hold. Hum. Gene Ther. 2020;31:908–910. doi: 10.1089/hum.2020.29133.bfs. [DOI] [PubMed] [Google Scholar]
- 16.George Lindsey A., Monahan Paul E., Eyster M. Elaine, Sullivan Spencer K., Ragni Margaret V., Croteau Stacy E., et al. Multiyear Factor VIII Expression after AAV Gene Transfer for Hemophilia A. The New England Journal of Medicine. 2021;385(21):1961–1973. doi: 10.1056/NEJMoa2104205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong J.-Y., Fan P.-D., Frizzell R.A. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum. Gene Ther. 1996;7:2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 18.Grieger J.C., Samulski R.J. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J. Virol. 2005;79:9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa R.H., Grayson D.R. Site-directed mutagenesis of hepatocyte nuclear factor (HNF) binding sites in the mouse transthyretin (TTR) promoter reveal synergistic interactions with its enhancer region. Nucleic Acids Res. 1991;19:4139–4145. doi: 10.1093/nar/19.15.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lisowski L., Dane A.P., Chu K., Zhang Y., Cunningham S.C., Wilson E.M., Nygaard S., Grompe M., Alexander I.E., Kay M.A. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature. 2014;506:382–386. doi: 10.1038/nature12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang H., Couto L.B., Patarroyo-White S., Liu T., Nagy D., Vargas J.A., Zhou S., Scallan C.D., Sommer J., Vijay S., et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabatino D.E., Lange A.M., Altynova E.S., Sarkar R., Zhou S., Merricks E.P., Franck H.G., Nichols T.C., Arruda V.R., Kazazian H.H., Jr. Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors. Mol. Ther. 2011;19:442–449. doi: 10.1038/mt.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H., Lillicrap D., Patarroyo-White S., Liu T., Qian X., Scallan C.D., Powell S., Keller T., McMurray M., Labelle A., et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen G.N., Everett J.K., Kafle S., Roche A.M., Raymond H.E., Leiby J., Wood C., Assenmacher C.A., Merricks E.P., Long C.T., et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol. 2020 doi: 10.1038/s41587-020-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabatino D.E., Freguia C.F., Toso R., Santos A., Merricks E.P., Kazazian H.H., Jr., Nichols T.C., Camire R.M., Arruda V.R. Recombinant canine B-domain-deleted FVIII exhibits high specific activity and is safe in the canine hemophilia A model. Blood. 2009;114:4562–4565. doi: 10.1182/blood-2009-05-220327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., et al. AAV5–Factor VIII gene transfer in severe hemophilia A. New Engl. J. Med. 2017 doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 27.Zhu J., Huang X., Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Invest. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lusher J.M., Lee C.A., Kessler C.M., Bedrosian C.L., ReFacto Phase 3 Study G. The safety and efficacy of B-domain deleted recombinant factor VIII concentrate in patients with severe haemophilia A. Haemophilia. 2003;9:38–49. doi: 10.1046/j.1365-2516.2003.00708.x. [DOI] [PubMed] [Google Scholar]
- 29.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Rasko J., Ozelo M.C., Hoots K., Blatt P., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 30.Monahan P., Walsh C., Powell J., Konkle B., Josephson N., Escobar M., McPhee S., Litchev B., Cecerle M., Ewenstein B. Update on a phase 1/2 open-label trial of BAX335, an adeno-associated virus 8 (AAV8) vector-based gene therapy program for hemophilia B. J. Thromb. Haemost. 2015;13:87. [Google Scholar]
- 31.Leavitt A.D., Konkle B.A., Stine K., Visweshwar N., Harrington T.J., Giermasz A., Arkin S., Fang A., Plonski F., Smith L., et al. Updated follow-up of the alta study, a phase 1/2 study of giroctocogene fitelparvovec (SB-525) gene therapy in adults with severe hemophilia a. Blood. 2020;136:12. doi: 10.1182/blood-2020-137648. [DOI] [Google Scholar]
- 32.George L. International Society on Thrombosis and Haemostasis (ISTH) Congress; 2021. Phase I/II Trial of SPK-8011: Stable and Durable FVIII Expression after AAV Gene Transfer for Hemophilia A. [Google Scholar]
- 33.Koster T., Blann A.D., Briet E., Vandenbroucke J.P., Rosendaal F.R. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345:152–155. doi: 10.1016/s0140-6736(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 34.Kyrle P.A., Minar E., Hirschl M., Bialonczyk C., Stain M., Schneider B., Weltermann A., Speiser W., Lechner K., Eichinger S. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N. Engl. J. Med. 2000;343:457–462. doi: 10.1056/NEJM200008173430702. [DOI] [PubMed] [Google Scholar]
- 35.Lind P., Larsson K., Spira J., Sydow-Backman M., Almstedt A., Gray E., Sandberg H. Novel forms of B-domain-deleted recombinant factor VIII molecules. Construction and biochemical characterization. Eur. J. Biochem. 1995;232:19–27. doi: 10.1111/j.1432-1033.1995.tb20776.x. [DOI] [PubMed] [Google Scholar]
- 36.Bi L., Lawler A.M., Antonarakis S.E., High K.A., Gearhart J.D., Kazazian H.H., Jr. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat. Genet. 1995;10:119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 37.Liu F., Song Y., Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 38.Ivanciu L., Toso R., Margaritis P., Pavani G., Kim H., Schlachterman A., Liu J.H., Clerin V., Pittman D.D., Rose-Miranda R., et al. A zymogen-like factor Xa variant corrects the coagulation defect in hemophilia. Nat. Biotechnol. 2011;29:1028–1033. doi: 10.1038/nbt.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kay M.A., Manno C.S., Ragni M.V., Larson P.J., Couto L.B., McClelland A., Glader B., Chew A.J., Tai S.J., Herzog R.W., et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 40.Wright J.F., Le T., Prado J., Bahr-Davidson J., Smith P.H., Zhen Z., Sommer J.M., Pierce G.F., Qu G. Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation. Mol. Ther. 2005;12:171–178. doi: 10.1016/j.ymthe.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Sommer J.Ü.M., Smith P.H., Parthasarathy S., Isaacs J., Vijay S., Kieran J., Powell S.K., McClelland A., Wright J.F. Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol. Ther. 2003;7:122–128. doi: 10.1016/s1525-0016(02)00019-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.