Highlights
-
•
Long-term Candida infection contributes to oral cancer development.
-
•
Candida species contribute to tumor induction along the gastrointestinal tract through production of carcinogenic nitrosamines.
-
•
Voriconazole is involved in the development of squamous cell carcinoma in lung transplant patients.
Keywords: Cancer, Fungal dysbiosis, Fungal pathogen, Metastasis, Human mycobiota
Abstract
The role and impact of commensal and pathogenic fungi in different parts of the human body are being increasingly appreciated, unveiling the importance of such microorganisms in human health. A key function is the involvement of the mycobiota in cross-kingdom interactions within the microbiome. Any disturbance in the functionality of the microbiota could alter metabolic reactions, have a negative impact on homeostasis or induce diseases. The association of fungi with cancer development is the focus of this review. Several studies have reported direct or indirect involvement of fungal pathogens and mycobiome dysbiosis in induction of carcinogenesis. Most studies focused on cancers of the gastrointestinal tract. However, researchers are now investigating other organs, such as the skin, where the significant results obtained confirm the involvement of fungal pathogens and administration of antifungal drugs in development of cancer. This review gives an overview of the different organs affected and describes the mechanisms used by these eukaryotes or antifungals to induce oncogenesis.
Graphical abstract
1. Introduction
The human microbiota is made up of a complex community of different microbes such as bacteria, fungi, protozoa, viruses and archaeal species (Raimondi et al., 2019; Richard and Sokol 2019). With recent advances in molecular technologies and culture-independent methods, researchers have been able to study and appreciate the role of microbial communities in human health (Konturek et al., 2015). The microbiome is involved in the immune response, homeostasis, metabolism and development of various diseases and inflammatory disorders (Hall and Noverr 2017; Hoggard et al., 2018). Although the focus has been on the bacterial part of the microbiome, there have been an increasing number of studies on the other components of the microbiome in the last ten years (Richard and Sokol 2019).
Fungi are non-phototrophic eukaryotes with a unique cell wall composed mainly of chitin. These organisms can be subdivided based on their fruiting bodies and their life cycle and can be classified as yeasts, which are single-celled microorganisms that reproduce via mating or budding, mushrooms and moulds. Mushrooms have an easily visible multi-cellular fruiting body above the ground whereas moulds have long multi-cellular filamentous structures known as hyphae growing by apical extension (McGinnis and Tyring 1996). Candida, Malassezia and Saccharomyces yeasts are commonly found throughout the human body which also harbor a variety of moulds belonging to the following genera: Aspergillus, Alternaria, Cladosporium and Epicoccum (Underhill and Iliev 2014). The mycobiota is present in almost every part or surface of the human body, from the skin to internal organs such as the gastrointestinal tract and vagina (Ghannoum et al., 2010; Drell et al., 2013; Findley et al., 2013; Hallen-Adams and Suhr 2017).
Even though fungi are less abundant compared to their bacterial counterpart, these eukaryotic organisms still play a key role in human health as the mycobiome can contain both pathogens and beneficial fungi (Huffnagle and Noverr 2013). Although anyone is vulnerable to fungal diseases, immunocompromised people are particularly prone to invasive infections (Suhr 2015; Chin et al., 2016; Hallen-Adams and Suhr 2017; Ksiezopolska and Gabaldón 2018). Recent studies have found increasing evidence that presence of fungal pathogens, invasive infections or fungal dysbiosis within organs could lead to life-threatening diseases and disorders within the human body (Rizzetto et al., 2014; Underhill and Iliev 2014; Zhang et al., 2020).
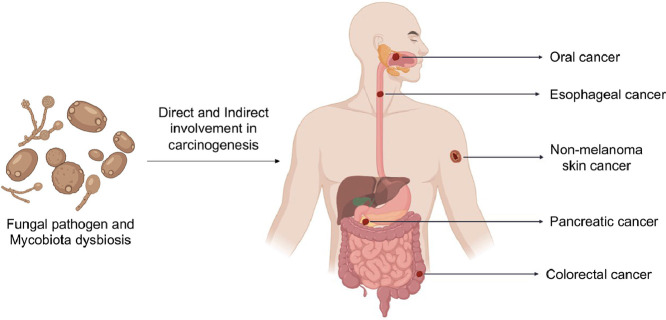
Cancer is one of the prime causes of death worldwide according to the World Health Organization (WHO), with 10 million cases of death recorded in 2020 (World Health Organization 2021). More and more studies are revealing the association and possible involvement of specific fungal species, such as the commensal pathobionts Candida species, in the induction of carcinogenesis and metastasis in different organs of the human body (Chung et al., 2017; Kaźmierczak-Siedlecka et al., 2020b; Zhang et al., 2020). In this review, we will focus on the evidence for perturbations of the mycobiota and their association with human health, particularly in relation to the development of cancers of the gastrointestinal tract and the integumentary system, considered to be among the common and deadliest cancers occurring worldwide.
2. Cancers of the digestive system
2.1. Oral cancer
Oral cancer can develop in different parts of a human mouth, including the tongue, cheeks, lips, gums, or palate. The salivary glands, tonsils and pharynx (oropharynx) are also prone to cancer but such cases occur less frequently (Ellington et al., 2020). In 2020, there were 377,713 cases of lip and oral cancer worldwide, with highest frequency obtained from Papua New Guinea (incidence rate: >34.1 per 100,000 individuals) (Sung et al., 2021). High numbers of cases were also observed in South Central Asian countries (incidence rate of 17.9 per 100,000 individuals) (Ferlay et al., 2020), Australia and New Zealand (incidence rate of 12.1 per 100,000 individuals), and Eastern Europe (11.1 per 100,000 individuals) (Sung et al., 2021). The major risk factors, enhancing development of oral tumourigenesis, were found to be betel nut (Areca nut) chewing practices, heavy alcohol intake, human papillomavirus (HPV) infection, cigarette smoking and overexposure to ultraviolet (UV) light for lip cancer (Rigel 2008; Bagnardi et al., 2015; Han et al., 2016; Gupta et al., 2018) as well as poor oral hygiene and diet (Arzmi et al., 2019).
Fungi such as Candida species are usually present in the mouth and are harmless under normal conditions. However, specific host factors could trigger these fungal species to switch to a pathogenic form (Underhill and Iliev 2014; Forbes et al., 2016; Liang and Bennett 2020; Zhai et al., 2020), thus causing infections. Oral candidiasis is the main fungal infection that occurs in the human oral cavity and is usually caused by Candida albicans or other Candida species (Chung et al., 2017; Sharma 2019). These can range from mild to severe invasive infections depending on host immunity (Chin et al., 2016; Ksiezopolska and Gabaldón 2018; Sharma 2019).
Candidiasis has long been associated with cancer treatment or therapy complications (Chung et al., 2017). It was later discovered that this disease might as well be involved in the development and progression of mouth cancer. A study in Taiwan revealed that individuals suffering from a Candida infection were significantly more at risk of developing subsequent oral cancer compared to their control cohort (Chung et al., 2017). Carcinogenic viruses and bacteria, such as HPV, Helicobacter pylori, and other known microbial carcinogens (Karin et al., 2006; Snow and Laudadio 2010; Momin and Richardson 2012; Mesri et al., 2014; Kumar et al., 2020), were the focal points of research for the past decade. Now, the focus is being drawn on identifying any potential relationship between fungal pathogens and oral cancer, especially in immunocompromised host (Bakri et al., 2010; Salazar et al., 2012; Sankari et al., 2015; Chung et al., 2017).
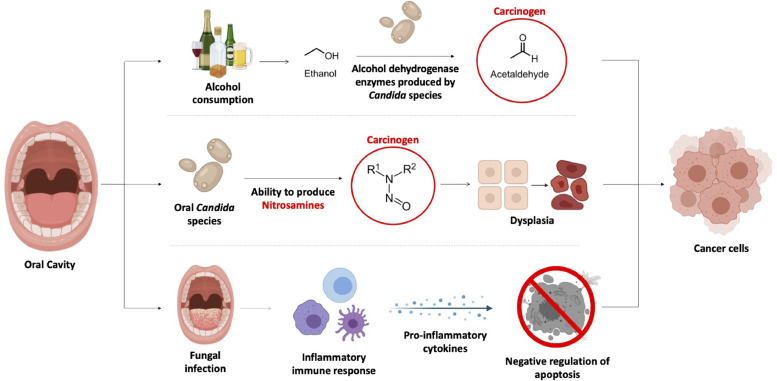
C. albicans have been associated with cancer development through the production of specific hydrolytic enzymes metabolizing alcohol to acetaldehyde (ACH). ACH is known as a group 1 human carcinogen when associated with heavy alcohol intake (Alnuaimi et al., 2016; Nieminen and Salaspuro 2018). Candida species possess alcohol dehydrogenase (ADH) enzymes, necessary for the conversion of ethanol to ACH. ADH is a cell wall protein essential for fungal growth and metabolism (Liu et al., 2019). Furthermore, ADH may also interact with host cell proteins and trigger an immune response (Reid and Fewson 1994; Klotz et al., 2001; Chaffin 2008; Liu et al., 2019). The impact of ACH on cells mainly results in oxidative stress and cell damage (Clavijo-Cornejo et al., 2014; Simoni-Nieves et al., 2018), induction of pro-inflammatory cytokines and mediators leading to inflammation (Hernández et al., 2008; Reyes-Gordillo et al., 2014; Simoni-Nieves et al., 2018), and formation of covalent adducts in protein or DNA residues (Setshedi et al., 2010), DNA cross-linking or chromosomal aberrations (Homann et al., 1997; Poschl and Seitz 2004). These events may trigger mutations within-host DNA, and impairment of protein function or degradation of protein molecules essential for normal host function and cell division (Setshedi et al., 2010). This, in turn, could lead to tumor development and progression (Fig. 1) (Alnuaimi et al., 2016; Kaźmierczak-Siedlecka et al., 2020b).
Fig. 1.
A summary of the different means by which fungal species are involved in development of oral cancer.
Another mechanism by which Candida species help in tumor induction comes from their ability to invade host tissue and produce nitrosamines such as N-nitrosobenzylmethylamine (NBMA) which have been found to cause cancer in animal models (Bakri 2011; Shukla et al., 2019). Nitrosamines compounds were reported to potentially activate proto-oncogenes (Scully 2011) and cause dysplasia (Fig. 1), which is the abnormal enlargement, development and differentiation of cells (Daftary et al., 1972; Hornstein et al., 1979; Krogh et al., 1987; Sanjaya et al., 2011; Shukla et al., 2019). Hojatti et al. (1987) demonstrated that nitrosamine compounds, that have been activated, can mutate a proto-oncogene in vitro. They made use of pEC plasmids containing c-Ha-ras-I proto-oncogene, involved in regulating cell division in the human body, and NIH 3T3 cells as in vitro host. The authors reported that esterase enzymes, also commonly found in the human body, converted N-nitrosomethyl(acetoxymethyl)amine into a methylating agent known as α-hydroxynitrosodimethylamine which in turn reacted with c-Ha-ras-I gene via methylation. The methylated DNA within the gene lead to the conversion of the normal gene to the oncogene form. Nitrosamines, produced by Candida species within the mouth, may easily interact with premalignant lesions such as oral epithelial dysplasia and leukoplakias, worsening the risk of developing oral cancer (Saigal et al., 2011; Hettmann et al., 2015). Krogh et al. (1987) isolated various strains of Candida species from leukoplakia and erytholeukoplakia, both precancerous oral lesions, and assessed the catalytic ability of these yeasts to produced NBMA via High-performance liquid chromatography (HPLC) and gas chromatography–mass spectrometry (GC–MS). The authors demonstrated that the isolates were producing NBMA from its precursors N-benzylmethylamine and nitrite at rates ranging from 0 to 1.2 microgram per 106 cells. It was also observed that C. albicans with high capacity to produce nitrosamines were mainly obtained from lesions showing more precancerous features (Krogh et al., 1987). Moreover, C. albicans isolated from precancerous lesions had higher ability to produce nitrosamine compounds compared to isolates obtained from lesions and normal mucosa (Korgh et al. 1987), further supporting the role of nitrosamines in induction of cancerous cells.
Moreover, members of the Candida genus have the ability to produce biofilm, easily adhere to cell surfaces and invade epithelial cells within the oral cavity. These features are considered as virulent factors that might play a key role in development of oncogenesis, mainly by-way-of infections and inflammation (Alnuaimi et al., 2016; Kaźmierczak-Siedlecka et al., 2020b). During the course of an inflammation, cytokines such as IL-6 and IL-17A are produced by T-helper cells 17 (Th17) which in turn activates the Wnt signaling pathway and nuclear factor kappa B (NF-κB) signaling pathway. Overexpression of pro-inflammatory cytokines, such as IL-6, is a pivotal point in tumourigenesis via negative regulation of apoptosis which highly affects cell cycles (Fig. 1) (Dongari-Bagtzoglou and Fidel 2005; Chen et al., 2015; Dai et al., 2019; Kaźmierczak-Siedlecka et al., 2020b).
2.2. Esophageal cancer
Esophageal cancer (EC) occurs in the esophagus, which is the fibromuscular tube transporting food (bolus) from the pharynx to the stomach (Peters et al., 2019). EC has the seventh-highest incidence rate of cases worldwide, with 604,000 new cases in 2020 with 70% of the cases occurring in men (Arnold et al., 2020; Sung et al., 2021). This type of cancer is prevalent in Eastern Asia, especially China. Moreover, it was reported that EC is the prime cause of cancer death within the Bangladeshi population irrespective of sexes and in Malawian men (Sung et al., 2021).
EC has two main histologic subtypes with distinct etiologies, namely esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) (Conteduca et al., 2012; Peters et al., 2019; Sung et al., 2021). People living in Asia, especially in China, have a greater risk of suffering from ESCC (Blot and Tarone 2017). The major risk factors involved in the initiation and spread of ESCC are heavy alcohol consumption, tobacco smoking, poor diet with nutrient deficiencies, heavy consumption of pickled vegetables and hot beverages, betel quid chewing practices and ingestion of nitrosamines (Blot and Tarone 2017). EAC is most prevalent in countries with high income and its induction is attributed to obesity, gastroesophageal reflux disease (GERD) and Barrett's esophagus which is a complication of GERD (Blot and Tarone 2017), resulting in the transformation of normal esophageal epithelium cells to intestinal-like epithelium tissue (Conteduca et al., 2012; Peters et al., 2019).
The involvement of fungi in EC have not been extensively studied, however, some findings showed that specific fungal species might have a key role in the development of ESCC in patients suffering from autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) (Zhu et al., 2017). APECED is an autoimmune disease that is caused by mutations in the autoimmune regulator (AIRE) gene which induces defective T-cell central tolerance, hampering the elimination of T lymphocytes reactive to host cells (Xing and Hogquist 2012). A weakened or defective central tolerance makes APECED patients more prone to chronic fungal infections (Rautemaa et al., 2007; Mathis and Benoist 2009; Manley et al., 2011) as innate immunity and T-helper cells are crucial in host defense against fungal infection, invasion and expansion in the human body (Stoner and Gupta 2001; Zhu et al., 2017). Among 92 patients diagnosed with APECED, six individuals were identified with oral carcinoma as well and five out of six patients with oral carcinoma reported long-term oral candidiasis (Rautemaa et al., 2007).
It was reported that ESCC patients frequently suffer from fungal lesions, ulcerations and chronic inflammation of the esophagus, suggesting a potential link between environmental fungi ingestion from the mouth and development of ESCC (Stoner and Gupta 2001). The mechanisms by which they could do so are still understudied. However, a study using kinase-dead IkB kinase alpha (Ikka) knock-in (IkkaKA/KA) mice developing and mimicking similar impaired central tolerance and phenotypes as APECED patients, demonstrated that autoreactive CD4 T-cells promoted chronic fungal infection, inflammation, and epithelium tissue damage. These alterations, together with elevated epidermal growth factor receptor (EGFR) activity have key roles in the carcinogenesis of ESCC in mice (Zhu et al., 2012, 2017). The authors also found similarities between the hallmarks of ESCC from both non-APECED patients and IkkaKA/KA mice (Zhu et al., 2017). Moreover, IkkaKA/KA mice orally infected with Cladosporium cladosporioides once every fortnight, for four infections in total, demonstrated 60% incidence for esophageal or oral tumor development (Zhu et al., 2017). These findings confirm the importance of both the host genetics and fungal pathogens in cancer development.
Previously published case-study reports demonstrated a potential involvement of fungi, especially Candida species, with development of esophageal cancer. Patients with chronic Candida infections, known as chronic mucocutaneous candidiasis (CMC) have increased risk of developing esophageal cancer. A case series study found that two out of six family members with CMC developed and died from ESCC (Koo et al., 2017). Domingues-Ferreira et al. (2009) suggested that individuals suffering from CMC have deficient immune systems which consequently lead to persistent invasive Candida infections in different parts of the human body such as skin, mucous membranes and appendages. The authors hypothesized that NBMA, the carcinogen produced by fungal cells, mentioned earlier, might play a major role in cancer development (Domingues-Ferreira et al., 2009). This hypothesis is consistent with the demonstration by Hsia et al. (1981) that C. albicans have the ability to produce the carcinogenic NBMA in the esophagus. Another case study of two patients, pointed out the impact of a defective immunity and fungal infection on esophageal oncogenesis (Delsing et al., 2012). The authors implied that long-term Candida infection increases risk of ESCC based upon their inspections on both patients, suggesting that mutations within the signal transducer and activator of transcription 1 (STAT1) gene and impaired function of T-lymphocytes were reported to promote the induction and spread of cancer in the esophagus, especially in CMC patients. They also attributed promotion of carcinogenesis to the production of carcinogenic nitrosamine compounds by fungal species in the presence of high alcohol level, as described in previous section (Delsing et al., 2012).
Nonetheless, the mechanism of action and involvement of pathogenic fungi has not been well documented. There are gaps to be filled in our knowledge of the eukaryotic biota in the human body. More emphasis should be placed on coming up with new technologies, techniques and approaches to uncover all the hidden aspects of the human gut mycobiome and its involvement with esophageal cancer in non-autoimmune individuals.
2.3. Pancreatic cancer
Pancreatic cancer is one of the most lethal types of cancers around the globe (Simoes et al., 2017; Kaźmierczak-Siedlecka et al., 2020b). Sung et al. (2021) reported that 466,000 individuals of a total of 496,000 diagnosed pancreatic cancer patients worldwide (94%) died in 2020. This burden is mainly attributed to the fact that the likelihood of recovery is relatively low, and that the majority of patients are diagnosed at stages III or IV of cancer (Eissa et al., 2019). Europe, Australia, New Zealand and North America recorded the highest rates of pancreatic cancer cases in 2020 (Arnold et al., 2020; Sung et al., 2021). A study performed in 28 countries in Europe, predicted that by 2025, there will be a 25% increase in number of pancreatic cancer deaths and thus, will overtake breast cancer death rate (Ferlay et al., 2016). Obesity, poor diet, diabetes, chronic pancreatitis and heavy alcohol consumption were found to be associated with pancreatic oncogenesis (Meng et al., 2018; Arnold et al., 2020; Sung et al., 2021). Till now, treatment for pancreatic cancer mainly involves radical dissection followed by chemotherapy (Orth et al., 2019). Early diagnosis is quite rare and no well-established and standard early screening procedures or biomarkers that are readily accessible worldwide (Meng et al., 2018).
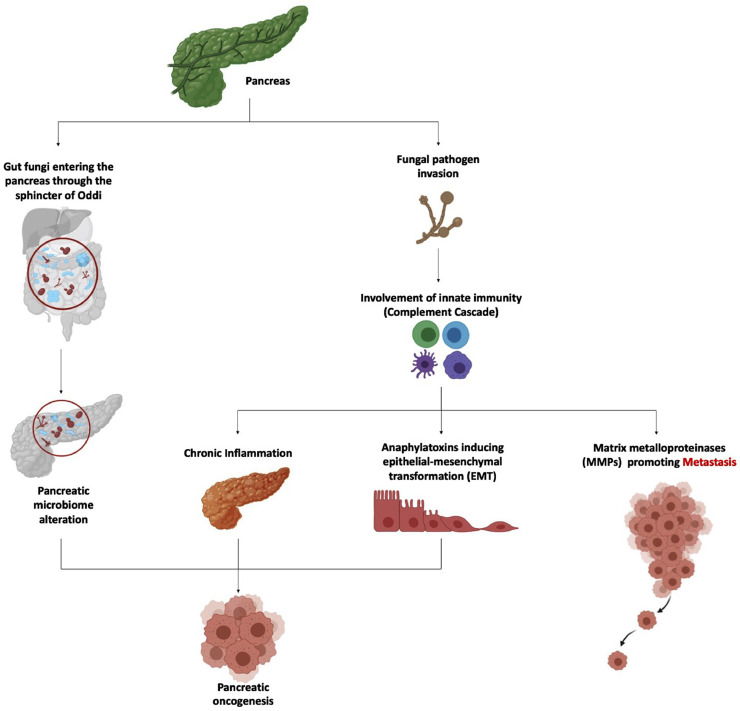
Pancreatic ductal adenocarcinoma (PDAC) is the prevalent type of cancer occurring in this organ (Simoes et al., 2017). Different bacteria have been associated with the development of PDAC such as Helicobacter pylori, Porphyromonas gingivalis, Neisseira elongata, Streptococcus mitis and various viruses affecting the human liver (Wei et al., 2019). Dysbiosis within the human bacteriome have been associated with occurrence and spread of PDAC. One study by Aykut et al. (2019) focused on the possible link between the gut mycobiome and pancreatic cancer (Fig. 2). It was speculated that gut fungi can enter the pancreas through the sphincter of Oddi, thereby altering the pancreatic microbiome. This change in microflora is implicated in development of PDAC. Pancreatic tumor samples from both mice and humans were analysed and compared to healthy pancreatic tissues. It was found that there was a 3000-fold increase in fungal density in tumor tissues. Malassezia globosa, Saccharomyces cerevisiae and Aspergillus species were enriched in these cancer tissue samples. Increase in fungal load and increase in abundance of specific fungal species originating from the gut could accelerate pancreatic tumor growth (Aykut et al., 2019; Richard and Sokol 2019).
Fig. 2.
Overview of the implications of gut mycobiome dysbiosis and fungal pathogen in the induction and spread of pancreatic cancer.
Mannose-binding lectin (MBL) activation also contributes to induction and spread of pancreatic cancer (Luan et al., 2015; Arzmi et al., 2019) as discussed below. MBL is a recognition molecule that has a key role in innate immunity and host defense against pathogenic fungi (Van Asbeck et al. 2008). This pattern recognition receptor will bind to glycans found on fungal cell wall and initiate the complement cascade that will eventually lead to pancreatic oncogenesis (Luan et al., 2015; Arzmi et al., 2019; Aykut et al., 2019). The complement cascade can prevent tumor by eliminating carcinogenic pathogens and is involved in apoptosis and necrosis of cancer cells (Pio et al., 2014; Martin and Blom 2016; Kochanek et al., 2018). On the other hand, when chronic inflammation is involved, the complement cascade supports cell proliferation and activation of proto-oncogenes (Fig. 2) (Bamberg et al., 2010; Rutkowski et al., 2010; Mamidi et al., 2017; Kochanek et al., 2018; Cedzynski and Swierzko 2020).
Epithelial-mesenchymal transformation (EMT) may also be instigated by anaphylatoxins C3a and C5a, major components of the complement system, during the course of inflammation caused by pathogens (Nitta et al., 2013; Liu et al., 2017; Kochanek et al., 2018; Cedzynski and Swierzko 2020). EMT is a process by which the normal epithelial cells switch to mesenchymal form by losing their intrinsic asymmetry and cell to cell adhesion and by undergoing metabolic changes (Puthiyaveetil et al., 2016). In addition, transition from tumor cells to metastasis may be promoted by the action of matrix metalloproteinases (MMPs), known as enzymes that degrade the extracellular matrix (ECM). C3a and C5a have been reported to activate such enzymes during inflammatory response to fungal pathogens (Fig. 2) (Löffek et al., 2011; Nitta et al., 2013; Sayegh et al., 2014; Jabłońska-Trypuć et al., 2016; Cedzynski and Swierzko 2020).
2.4. Colorectal cancer
Colorectal cancer (CRC), including bowel, colon or rectal cancer, is the third most common cancer diagnosed worldwide (Brennan and Garrett 2016) with over 1.9 million cases and an incidence rate of 935,000 deaths in 2020 (Sung et al., 2021). The majority of cases were estimated to originate from Europe (mainly Norway and Hungary), Australia, New Zealand and Northern American countries (Sung et al., 2021). Researchers determined that heavy alcohol intake, tobacco smoking, sedentary lifestyle, heavy animal-source food consumption (especially red or processed meat) and obesity are the leading risk factors involved in the induction and spread of CRC (Siegel et al., 2020; Sung et al., 2021).
Diet highly influences the gut microbiome which in turn can have a key role in the development and spread of CRC (Gao et al., 2017). The involvement of bacteria such as Escherichia coli, Helicobacter hepaticus, Helicobacter pylori, Enterococcus faecalis, Fusobacterium nucleatum, Bacteroides fragilis and Streptococcus bovis, have been studied for the past decade and associated with CRC (Gagnière et al., 2016; Dai et al., 2019; Kaźmierczak-Siedlecka et al., 2020a). With the use of metagenomics and bioinformatics tools, researchers were able to analyze the gut mycobiota composition of patients suffering from CRC, colon polyps or adenoma tissues, and control subjects to determine whether the gut mycobiota could potentially impact on the formation and expansion of colon cancer (Luan et al., 2015; Gao et al., 2017; Coker et al., 2019). A study on three large cohorts, with a total of 274 CRC patients, 239 adenoma patients, and 270 healthy controls reported evidence that fungal dysbiosis within the human gut may lead to CRC (Coker et al., 2019). The authors observed an increased Basidiomycota/Ascomycota ratio and higher abundance of Malasseziomycete fungi in participants suffering from CRC compared to the control group. Basidiomycota/Ascomycota ratio has previously been reported as an index for fungal dysbiosis in ecosystems (Kuramae et al., 2013), and Malassezia-synthesised aryl hydrocarbon receptor ligands have also been suggested to cause basal cell carcinoma through UV radiation–induced carcinogenesis (Gaitanis et al., 2012). On the other hand, this study reported a decrease in richness of Saccharomyces cerevisiae, Lipomyces starkeyi and Pneumocystidomycetes fungal species. This depletion in S. cerevisiae richness may potentially advantage the spread of tumor within the human gut (Coker et al., 2019). This yeast species is considered as a probiotic which provides lots of benefits to host health (Fakruddin et al., 2017). A study demonstrated that S. cerevisiae stimulates the humoral and cell-mediated immune response by initiating the production of anti-inflammatory IL-10 cytokines (Jawhara et al., 2012; Fakruddin et al., 2017) and by lowering of tumor necrosis factor- alpha (TNFα) inflammatory cytokine (Jawhara et al., 2012), essential for suppression of cancer through necrosis and apoptosis (Idriss and Naismith 2000; Fakruddin et al., 2017).
The mycobiota of adenomas and adjacent tissues from CRC patients were sampled and analysed by Luan et al. (2015) using high-throughput sequencing technologies. The outcome of the analysis revealed that a decrease in fungal diversity was observed in the adenoma tissue samples (Luan et al., 2015). Gao et al. (2017) investigated the diversity and density of gut fungi among patients suffering from CRC and patients with colon polyps compared to control subjects. The authors reported an association between fungal dysbiosis, through loss of beneficial yeasts and increase in fungal pathogens, within the human gut and colon polyps and CRC. An increased Basidiomycota/Ascomycota ratio and higher level of opportunistic fungal species from the Trichosporon and Malassezia genera were observed in patients suffering from CRC. This study also demonstrated that fungal diversity was much lower in cancer patients compared to the healthy control, specially at an early stage of oncogenesis. These mycobiota alterations were proposed to play a major role in CRC (Gao et al., 2017). For now, broad conclusions cannot be drawn due to limited studies and low sample size. However, the findings stated above strongly suggest that CRC is associated with fungal dysbiosis in the human gut mycobiome.
Mutations within the human genes involved in immune response have been associated with alteration of the human mycobiome. Overgrowth of Candida species is prevalent in individuals with mutations within genes coding for proteins involved in helper T cells (Th17 and Th1) immune response (Patel and Kuchroo 2015; Lai et al., 2018). Recurrent vulvovaginal candidiasis (RVVC) and onychomycosis are highly prevalent in individuals having specific mutations reducing the function of C-Type lectin domain containing 7A (CLEC7A), involved in innate immunity, or caspase recruitment domain family member 9 (CARD9) proteins, regulating inflammation and programmed cell death. These mutations and impaired functions consequently lead to increase abundance of Candida species in the patients’ body (Glocker et al., 2009; Carvalho et al., 2012; Drewniak et al., 2013; Wang et al., 2016). Malik et al. (2018) reported that a non-defective SKY-CARD9 signaling pathway provide protection against colitis disorder and colon cancer by promoting production of inflammasomes and IL-18 cytokines. This pathway induces T cell anti-tumor responses and helps to maintain a balanced and healthy mycobiota within the human GI tract (Malik et al., 2018). Another study performed using a murine model, reported a higher abundance of mycobiota with increase in Candida tropicalis in CARD9-deficient mice with impaired immunity against fungi (Wang et al., 2018). It was found that this fungal dysbiosis induced the build-up and accumulation of myeloid-derived suppressor cells (MDSCs) which are immature myeloid cells that amplify the risk of carcinogenesis by promoting immune suppression (Gabrilovich and Nagaraj 2009; Wang et al., 2018). Fungal dysbiosis have shown to play a crucial role in human health in terms of control of inflammation disorders and cancer (Conche and Greten 2018; Malik et al., 2018; Wang et al., 2018; Zhang et al., 2020).
3. Indirect involvement of fungi in cancers of the integumentary system
Skin cancers commonly occur in individuals around the world and can be categorised into melanoma skin cancer (MSC) or non-melanoma skin cancer (NMSC). In the United States of America alone, it is estimated that melanoma will account for approximately 150,000 cases and will have an expected death rate of 11,500 individuals in 2021 (American Cancer Society 2021). MSC develops as melanocytes, cells that produce pigments, mutate, and divide uncontrollably. Hence, melanoma can take place at any region of the skin (Riker et al., 2010). The major risk factor of melanoma is exposure to sunlight for prolonged periods, as confirmed by a study estimating that 86% of melanoma are caused by ultraviolet radiation from the sun (Parkin et al., 2011). Non-melanoma skin cancers are more common and accounted for more than one million new patients in 2020. It was reported that men were two times more prone to this type of cancer and that highest frequency of NMSC was observed in Australia and New Zealand (Sung et al., 2021). Prolonged exposure to sunlight contribute to development of NMSC and the use of sunscreens can reduce up to 40% risk of developing skin cancer (Green et al., 1999).
Other than exposure to sunlight and UV radiations, recent studies have found that skin cancers could be subsequent consequences of solid organ transplants. Solid organ transplant patients have a 65-fold higher risk of developing a non-melanoma skin cancer known as squamous cell carcinoma (SCC) which tends to develop rapidly leading to further surgeries and increased risk of death (Lindelöf et al., 2006). Lung transplant patients are especially at higher risk for the development of SCC due to high dose of immunosuppression required for the transplant procedure (Lindelöf et al., 2006). Fungal infections are also prominent among lung transplant patients due to their immunocompromised status. These individuals can easily get infected by various fungal pathogens such as Aspergillus, Candida and Cryptococcus species which could lead to invasive and chronic inflammatory infections (Shoham and Marr 2012). Hence, most lung transplant procedures will be accompanied by antifungal medication such as voriconazole, which is a triazole drug, to treat and prevent occurrence or spread of fungal infections (Herbrecht et al., 2002). Recent studies have found that voriconazole significantly increases the risk of developing SCC by approximately 2.6 fold depending on dose (Singer et al., 2012). The dose of antifungal drug will depend on the severity and drug resistance of the causative biological agent. Despite the effectiveness of voriconazole on fungal infections (Steinbach and Dvorak 2012), another study confirmed that exposure to voriconazole post-surgery could increase the risk of SCC by 73% when administering at standard dose. Additionally, the risk increases by 3% for every 30-day exposure to voriconazole (Mansh et al., 2016). Voriconazole is associated with cutaneous toxicities such as photosensitivity (Haylett et al., 2013) and metabolites voriconazole N-oxide (VNO), which leads to keratinocytes sensitivity to ultraviolet A light which may induce DNA damage and inhibit repair mechanisms (Ona and Oh 2015) thus leading to oncogenesis.
4. Concluding remarks
Researchers have found significant evidence of associations between specific fungi with the development of the human GI tract cancers, as well as indirect involvement of fungi via the action of an antifungal agent, in the development of skin cancer in lung transplant patients. As stated in this review, fungal pathogens may induce inflammatory responses, which greatly contributes to tumourigenesis. Could these immune responses to fungi put other organs at risk of cancer? More focus should be placed on full characterization of the human mycobiota worldwide, to effectively investigate commensal fungi, cross-kingdom interactions within organs and to get better insights into the implications of the association of fungal infections or fungal dysbiosis with cancers across the human body systems.
Availability of data and material
Not applicable.
Funding
School of Science, Monash University Malaysia.
Code availability
Not applicable
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
All authors approved the final version to be published.
CRediT authorship contribution statement
Marie Andrea Laetitia Huët: Visualization, Conceptualization. Chuen Zhang Lee: Visualization, Conceptualization. Sadequr Rahman: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors wish to thank Monash University Malaysia (Subang Jaya, Malaysia) for their support.
References
- Alnuaimi A.D., Ramdzan A.N., Wiesenfeld D., O'Brien-Simpson N.M., Kolev S.D., Reynolds E.C., McCullough M.J. Candida virulence and ethanol-derived acetaldehyde production in oral cancer and non-cancer subjects. Oral Dis. 2016;22:805–814. doi: 10.1111/odi.12565. [DOI] [PubMed] [Google Scholar]
- American Cancer Society (2021) Cancer facts & Figures 2021. Atlanta.
- Arnold M., Abnet C.C., Neale R.E., Vignat J., Giovannucci E.L., McGlynn K.A., Bray F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159:335–349. doi: 10.1053/j.gastro.2020.02.068. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzmi M.H., Dashper S., McCullough M. Polymicrobial interactions of Candida albicans and its role in oral carcinogenesis. J. Oral Pathol. Med. 2019;48:546–551. doi: 10.1111/jop.12905. [DOI] [PubMed] [Google Scholar]
- Aykut B., Pushalkar S., Chen R., Li Q., Abengozar R., Kim J.I., Shadaloey S.A., Wu D., Preiss P., Verma N., Guo Y., Saxena A., Vardhan M., Diskin B., Wang W., Leinwand J., Kurz E., Kochen Rossi J.A., Hundeyin M., Zambrinis C., Li X., Saxena D., Miller G. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019;574:264–267. doi: 10.1038/s41586-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnardi V., Rota M., Botteri E., Tramacere I., Islami F., Fedirko V., Scotti L., Jenab M., Turati F., Pasquali E., Pelucchi C., Galeone C., Bellocco R., Negri E., Corrao G., Boffetta P., La Vecchia C. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br. J. Cancer. 2015;112:580–593. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakri M. University of Otago; New Zealand: 2011. The Expression of Candida albicans Acetaldehyde Producing Enzymes in C. Albicans Infected Mucosal lesions: a Potential Role in Some Oral Cancers. [Google Scholar]
- Bakri M.M., Hussaini H.M., Holmes A., Cannon R.D., Rich A.M. Revisiting the association between candidal infection and carcinoma, particularly oral squamous cell carcinoma. J. Oral Microbiol. 2010;2:1–6. doi: 10.3402/jom.v2i0.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberg C.E., Mackay C.R., Lee H., Zahra D., Jackson J., Lim Y.S., Whitfeld P.L., Craig S., Corsini E., Lu B., Gerard C., Gerard N.P. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J. Biol. Chem. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot W.J., Tarone R.E. Oxford University Press; 2017. Esophageal Cancer. In: Cancer Epidemiology and Prevention. [Google Scholar]
- Brennan C.A., Garrett W.S. Gut microbiota, inflammation, and colorectal cancer. Annu. Rev. Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A., Giovannini G., De Luca A., D'Angelo C., Casagrande A., Iannitti R.G., Ricci G., Cunha C., Romani L. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell Mol. Immunol. 2012;9:276–286. doi: 10.1038/cmi.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedzynski M., Swierzko A. Components of the lectin pathway of complement in haematologic malignancies. Cancers (Basel) 2020;12 doi: 10.3390/cancers12071792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin W.L. Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 2008;72:495–544. doi: 10.1128/mmbr.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhang F., Tsai Y., Yang X., Yang L., Duan S., Wang X., Keng P., Lee S.O. IL-6 signaling promotes DNA repair and prevents apoptosis in CD133+ stem-like cells of lung cancer after radiation. Radiat. Oncol. 2015;10:227. doi: 10.1186/s13014-015-0534-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chin V.K., Lee T.Y., Rusliza B., Chong P.P. Dissecting candida albicans infection from the perspective of c. Albicans virulence and omics approaches on host–pathogen interaction: a review. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung L.M., Liang J.A., Lin C.L., Sun L.M., Kao C.H. Cancer risk in patients with candidiasis: a nationwide population-based cohort study. Oncotarget. 2017;8:63562–63573. doi: 10.18632/oncotarget.18855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo-Cornejo D., Gutiérrez-Carrera M., Palestino-Domínguez M., Dominguez-Perez M., Nuño N., Souza V., Miranda R.U., Kershenobich D., Gutiérrez-Ruiz M.C., Bucio L., Gómez-Quiroz L.E. Acetaldehyde targets superoxide dismutase 2 in liver cancer cells inducing transient enzyme impairment and a rapid transcriptional recovery. Food Chem. Toxicol. 2014;69:102–108. doi: 10.1016/j.fct.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Coker O.O., Nakatsu G., Dai R.Z., Wu W.K.K., Wong S.H., Ng S.C., Chan F.K.L., Sung J.J.Y., Yu J. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut. 2019;68:654–662. doi: 10.1136/gutjnl-2018-317178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conche C., Greten F.R. Fungi enter the stage of colon carcinogenesis. Immunity. 2018;49:384–386. doi: 10.1016/j.immuni.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Conteduca V., Sansonno D., Ingravallo G., Marangi S., Russi S., Lauletta G., Dammacco F. Barrett’s esophagus and esophageal cancer: an overview. Int. J. Oncol. 2012;41:414–424. doi: 10.3892/ijo.2012.1481. [DOI] [PubMed] [Google Scholar]
- Daftary D.K., Mehta F.S., Gupta P.C., Pindborg J.J. The presence of Candida in 723 oral leukoplakias among Indian villagers. Scand. J. Dent. Res. 1972;80:75–79. doi: 10.1111/j.1600-0722.1972.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Dai Z., Zhang J., Wu Q., Chen J., Liu J., Wang L., Chen C., Xu J., Zhang H., Shi C., Li Z., Fang H., Lin C., Tang D., Wang D. The role of microbiota in the development of colorectal cancer. Int. J. Cancer. 2019;145:2032–2041. doi: 10.1002/ijc.32017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsing C.E., Bleeker-Rovers C.P., van de Veerdonk F.L., Tol J., van der Meer J.W.M., Kullberg B.J., Netea M.G. Association of esophageal candidiasis and squamous cell carcinoma. Med. Mycol. Case Rep. 2012;1:5–8. doi: 10.1016/j.mmcr.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues-Ferreira M., Grumach A.S., Duarte A.J.D.S., De Moraes-Vasconcelos D. Esophageal cancer associated with chronic mucocutaneous candidiasis. Could chronic candidiasis lead to esophageal cancer? Med. Mycol. 2009;47:201–205. doi: 10.1080/13693780802342545. [DOI] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A., Fidel P.L. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J. Dent. Res. 2005;84:966–977. doi: 10.1177/154405910508401101. [DOI] [PubMed] [Google Scholar]
- Drell T., Lillsaar T., Tummeleht L., Simm J., Aaspõllu A., Väin E., Saarma I., Salumets A., Donders G.G.G., Metsis M. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age estonian women. PLoS ONE. 2013;8:e54379. doi: 10.1371/journal.pone.0054379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewniak A., Gazendam R.P., Tool A.T.J., Van Houdt M., Jansen M.H., Van Hamme J.L., Van Leeuwen E.M.M., Roos D., Scalais E., De Beaufort C., Janssen H., Van Den Berg T.K., Kuijpers T.W. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013;121:2385–2392. doi: 10.1182/blood-2012-08-450551. [DOI] [PubMed] [Google Scholar]
- Eissa M.A.L., Lerner L., Abdelfatah E., Shankar N., Canner J.K., Hasan N.M., Yaghoobi V., Huang B., Kerner Z., Takaesu F., Wolfgang C., Kwak R., Ruiz M., Tam M., Pisanic T.R., Iacobuzio-Donahue C.A., Hruban R.H., He J., Wang T.H., Wood L.D., Sharma A., Ahuja N. Promoter methylation of ADAMTS1 and BNC1 as potential biomarkers for early detection of pancreatic cancer in blood. Clin. Epigenetics. 2019;11:1–10. doi: 10.1186/s13148-019-0650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington T.D., Henley S.J., Senkomago V., O'neil M.E., Wilson R.J., Singh S., Thomas C.C., Wu M., Richardson L.C. Morbidity and mortality weekly report trends in incidence of cancers of the oral cavity and Pharynx-United States 2007-2016. Cent. Dis. Control Prev. 2020;69 doi: 10.15585/mmwr.mm6915a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakruddin M., Hossain M.N., Ahmed M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Complement Altern. Med. 2017;17:1–11. doi: 10.1186/s12906-017-1591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. (2020) Cancer Today (powered by GLOBOCAN 2018).
- Ferlay J., Partensky C., Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. (Madr) 2016;55:1158–1160. doi: 10.1080/0284186X.2016.1197419. [DOI] [PubMed] [Google Scholar]
- Findley K., Oh J., Yang J., Conlan S., Deming C., Meyer J.A., Schoenfeld D., Nomicos E., Park M., Kong H.H., Segre J.A. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013;498:367–370. doi: 10.1038/nature12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes J.D., Van Domselaar G., Bernstein C.N. The gut microbiota in immune-mediated inflammatory diseases. Front. Microbiol. 2016;7:1–18. doi: 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnière J., Raisch J., Veziant J., Barnich N., Bonnet R., Buc E., Bringer M.A., Pezet D., Bonnet M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016;22:501–518. doi: 10.3748/wjg.v22.i2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitanis G., Magiatis P., Hantschke M., Bassukas I.D., Velegraki A. The Malassezia genus in skin and systemic diseases. Clin. Microbiol. Rev. 2012;25:106–141. doi: 10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Kong C., Li H., Huang L., Qu X., Qin N., Qin H. Dysbiosis signature of mycobiota in colon polyp and colorectal cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2017;36:2457–2468. doi: 10.1007/s10096-017-3085-6. [DOI] [PubMed] [Google Scholar]
- Ghannoum M.A., Jurevic R.J., Mukherjee P.K., Cui F., Sikaroodi M., Naqvi A., Gillevet P.M. Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker E.-.O., Hennigs A., Nabavi M.N., Schäffer A.A., Al E. A Homozygous CARD9 Mutation in a Family with Susceptibility to Fungal Infections. N. Engl. J. Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A., Williams G., Nèale R., Hart V., Leslie D., Parsons P., Marks G.C., Gaffney P., Battistutta D., Frost C., Lang C., Russell A. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354:723–729. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- Gupta S., Gupta R., Sinha D.N., Mehrotra R. Relationship between type of smokeless tobacco & risk of cancer: a systematic review. Indian J. Med. Res. 2018:56–76. doi: 10.4103/ijmr.IJMR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R.A., Noverr M.C. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr. Opin. Microbiol. 2017;40:58–64. doi: 10.1016/j.mib.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallen-Adams H.E., Suhr M.J. Fungi in the healthy human gastrointestinal tract. Virulence. 2017;8:352–358. doi: 10.1080/21505594.2016.1247140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han A.Y., Kuan E.C., Clair J.M.S., Alonso J.E., Arshi A., St John M.A. Epidemiology of squamous cell carcinoma of the lip in the United States a population-based cohort analysis. JAMA Otolaryngol. Head Neck Surg. 2016;142:1216–1223. doi: 10.1001/jamaoto.2016.3455. [DOI] [PubMed] [Google Scholar]
- Haylett A.K., Felton S., Denning D.W., Rhodes L.E. Voriconazole-induced photosensitivity: photobiological assessment of a case series of 12 patients. Br. J. Dermatol. 2013;168:179–185. doi: 10.1111/j.1365-2133.2012.11196.x. [DOI] [PubMed] [Google Scholar]
- Herbrecht R., Denning D.W., Patterson T.F., Bennett J.E., Greene R.E., Oestmann J.-.W., Kern W.V., Marr K.A., Ribaud P., Lortholary O., Sylvester R., Rubin R.H., Wingard J.R., Stark P., Durand C., Caillot D., Thiel E., Chandrasekar P.H., Hodges M.R., Schlamm H.T., Troke P.F., de Pauw B. Voriconazole versus Amphotericin B for Primary Therapy of Invasive Aspergillosis. N. Engl. J. Med. 2002;347:408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- Hernández E., Bucio L., Souza V., Escobar M.C., Gómez-Quiroz L.E., Farfán B., Kershenobich D., Gutiérrez-Ruiz M.C. Pentoxifylline downregulates α (I) collagen expression by the inhibition of Iκbα degradation in liver stellate cells. Cell Biol. Toxicol. 2008;24:303–314. doi: 10.1007/s10565-007-9039-5. [DOI] [PubMed] [Google Scholar]
- Hettmann A., Demcsák A., Decsi G., Bach Á., Pálinkó D., Rovó L., Nagy K., Takács M., Minarovits J. Infectious agents associated with head and neck carcinomas. Adv. Microbiol. Infect. Dis. Public Health. 2015:63–80. doi: 10.1007/5584_2015_5005. [DOI] [PubMed] [Google Scholar]
- Hoggard M., Vesty A., Wong G., Montgomery J.M., Fourie C., Douglas R.G., Biswas K., Taylor M.W. Characterizing the human mycobiota: a comparison of small subunit rRNA, ITS1, ITS2, and large subunit rRNA genomic targets. Front. Microbiol. 2018;9:1–14. doi: 10.3389/fmicb.2018.02208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojatti H., Milligan J.R., Archer M.C., RS-H . In: Relevance Of N-Nitroso Compounds To Human Cancer: Exposures And Mechanisms. Bartsch TKO H., editor. World Health Organization; Lyon, France: 1987. Activation Of the human C-Ha-Ras-1 proto-oncogene by in-vitro reaction with N-Nitrosomethyl(Acetoxymethyl)Amine. [Google Scholar]
- Homann N., Jousimies-Somer H., Jokelainen K., Heine R., Salaspuro M. High acetaldehyde levels in saliva after ethanol consumption: methodological aspects and pathogenetic implications. Carcinogenesis. 1997;18:1739–1743. doi: 10.1093/carcin/18.9.1739. [DOI] [PubMed] [Google Scholar]
- Hornstein O.P., Grässel R., Schirner E. Prevalence rates of candidosis in leukoplakias and carcinomas of the oral cavity. Arch. Dermatol. Res. 1979;266:99–102. doi: 10.1007/BF00412869. [DOI] [PubMed] [Google Scholar]
- Hsia C.C., Sun T.T., Wang Y.Y., Anderson L.M., Armstrong D., Good R.A. Enhancement of formation of the esophageal carcinogen benzylmethylnitrosamine from its precursors by Candida albicans. Proc. Natl. Acad. Sci. 1981;78:1878–1881. doi: 10.1073/pnas.78.3.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle G.B., Noverr M.C. The emerging world of the fungal microbiome. Trends Microbiol. 2013;21:334–341. doi: 10.1016/j.tim.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriss H.T., Naismith J.H. TNF-alpha and the TNF Receptor Superfamily: structure-Function Relationship(s) Microsc. Res. Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Jabłońska-Trypuć A., Matejczyk M., Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016;31:177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- Jawhara S., Habib K., Maggiotto F., Pignede G., Vandekerckove P., Maes E., Dubuquoy L., Fontaine T., Guerardel Y., Poulain D. Modulation of intestinal inflammation by yeasts and cell wall extracts: strain dependence and unexpected anti-inflammatory role of glucan fractions. PLoS ONE. 2012;7:1–15. doi: 10.1371/journal.pone.0040648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Lawrence T., Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kaźmierczak-Siedlecka K., Daca A., Fic M., van de Wetering T., Folwarski M., Makarewicz W. Therapeutic methods of gut microbiota modification in colorectal cancer management–fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes. 2020;11:1518–1530. doi: 10.1080/19490976.2020.1764309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaźmierczak-Siedlecka K., Dvořák A., Folwarski M., Daca A., Przewłócka K., Makarewicz W. Fungal gut microbiota dysbiosis and its role in colorectal, oral, and pancreatic carcinogenesis. Cancers (Basel) 2020;12:1–13. doi: 10.3390/cancers12051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S.A., Pendrak M.L., Hein R.C. Antibodies to α5β1 and αvβ3 integrins react with Candida albicans alcohol dehydrogenase. Microbiology. 2001;147:3159–3164. doi: 10.1099/00221287-147-11-3159. [DOI] [PubMed] [Google Scholar]
- Kochanek D.M., Ghouse S.M., Karbowniczek M.M., Markiewski M.M. Complementing cancer metastasis. Front. Immunol. 2018;9:1–11. doi: 10.3389/fimmu.2018.01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek P.C., Haziri D., Brzozowski T., Hess T., Heyman S., Kwiecien S., Konturek S.J., Koziel J. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J. Physiol. Pharmacol. 2015;66:483–491. [PubMed] [Google Scholar]
- Koo S., Kejariwal D., Al-Shehri T., Dhar A., Lilic D. Oesophageal candidiasis and squamous cell cancer in patients with gain-of-function STAT1 gene mutation. United Eur. Gastroenterol. J. 2017;5:625–631. doi: 10.1177/2050640616684404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh P., Hald B., Holmstrup P. Possible mycological etiology of oral mucosal cancer: catalytic potential of infecting Candida aibicans and other yeasts in production of N -nitrosobenzylmethylamine. Carcinogenesis. 1987;8:1543–1548. doi: 10.1093/carcin/8.10.1543. [DOI] [PubMed] [Google Scholar]
- Ksiezopolska E., Gabaldón T. Evolutionary emergence of drug resistance in candida opportunistic pathogens. Genes (Basel) 2018;9 doi: 10.3390/genes9090461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Metz D.C., Ellenberg S., Kaplan D.E., Goldberg D.S. Risk factors and incidence of gastric cancer after detection of helicobacter pylori infection: a large cohort study. Gastroenterology. 2020;158:527–536. doi: 10.1053/j.gastro.2019.10.019. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramae E.E., Hillekens R.H.E., de Hollander M., van der Heijden M.G.A., van den Berg M., van Straalen N.M., Kowalchuk G.A. Structural and functional variation in soil fungal communities associated with litter bags containing maize leaf. FEMS Microbiol. Ecol. 2013;84:519–531. doi: 10.1111/1574-6941.12080. [DOI] [PubMed] [Google Scholar]
- Lai G.C., Tan T.G., Pavelka N. The mammalian mycobiome: a complex system in a dynamic relationship with the host. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018:1–22. doi: 10.1002/wsbm.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S.H., Bennett R.J. The impact of gene dosage and heterozygosity on the diploid pathobiont candida albicans. J. Fungi. 2020;6 doi: 10.3390/jof6010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindelöf B., Jarnvik J., Ternesten-Bratel A., Granath F., Hedblad M. Mortality and clinicopathological features of cutaneous squamous cell carcinoma in organ transplant recipients: a study of the Swedish cohort. Acta Derm. Venereol. 2006;86:219–222. doi: 10.2340/00015555-0069. [DOI] [PubMed] [Google Scholar]
- Liu X.Y., Wang X.Y., Li R.Y., Jia S.C., Sun P., Zhao M., Fang C. Recent progress in the understanding of complement activation and its role in tumor growth and anti-tumor therapy. Biomed. Pharmacother. 2017;91:446–456. doi: 10.1016/j.biopha.2017.04.101. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ou Y., Sun L., Li W., Yang J., Zhang X., Hu Y. Alcohol dehydrogenase of Candida albicans triggers differentiation of THP-1 cells into macrophages. J. Adv. Res. 2019;18:137–145. doi: 10.1016/j.jare.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffek S., Schilling O., Franzke C.W. Series “matrix metalloproteinases in lung health and disease” edited by J. Müller-Quernheim and O. Eickelberg number 1 in this series: biological role of matrix metalloproteinases: a critical balance. Eur. Respir. J. 2011;38:191–208. doi: 10.1183/09031936.00146510. [DOI] [PubMed] [Google Scholar]
- Luan C., Xie L., Yang X., Miao H., Lv N., Zhang R., Xiao X., Hu Y., Liu Y., Wu N., Zhu Y., Zhu B. Dysbiosis of fungal microbiota in the intestinal mucosa of patients with colorectal adenomas. Sci. Rep. 2015;7980:1–9. doi: 10.13040/IJPSR.0975-8232.9(10).4201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A., Sharma D., Malireddi R.K.S., Guy C.S., Chang T.C., Olsen S.R., Neale G., Vogel P., Kanneganti T.D. SYK-CARD9 signaling axis promotes gut fungi-mediated inflammasome activation to restrict colitis and colon cancer. Immunity. 2018;49:515–530. doi: 10.1016/j.immuni.2018.08.024. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi S., Höne S., Kirschfink M. The complement system in cancer: ambivalence between tumour destruction and promotion. Immunobiology. 2017;222:45–54. doi: 10.1016/j.imbio.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Manley N.R., Richie E.R., Blackburn C.C., Condie B.G., Sage J. Structure and function of the thymic microenvironment. Front. Biosci. 2011;16:2461. doi: 10.2741/3866. [DOI] [PubMed] [Google Scholar]
- Mansh M., Binstock M., Williams K., Hafeez F., Kim J., Glidden D., Boettger R., Hays S., Kukreja J., Golden J., Asgari M.M., Chin-Hong P., Singer J.P., ST A.r.r.o.n. Voriconazole exposure and risk of cutaneous squamous cell carcinoma, aspergillus colonization, invasive aspergillosis and death in lung transplant recipients. Am. J. Transpl. 2016;16:262–270. doi: 10.1111/ajt.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M., Blom A.M. Complement in removal of the dead-balancing inflammation. Immunol. Rev. 2016;274:218–232. doi: 10.1111/imr.12462. [DOI] [PubMed] [Google Scholar]
- Mathis D., Benoist C. Aire. Annu. Rev. Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- McGinnis M.R., Tyring S.K. (1996) Introduction to Mycology. In: Baron S (ed) Medical Microbiology. 4th Ed. The University of Texas Medical Branch at Galveston, Texas, USA. [PubMed]
- Meng C., Bai C., Brown T.D., Hood L.E., Tian Q. Human gut microbiota and gastrointestinal cancer. Genom. Proteom. Bioinform. 2018;16:33–49. doi: 10.1016/j.gpb.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesri E.A., Feitelson M., Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe. 2014;15:266–282. doi: 10.1016/j.chom.2014.02.011.HUMAN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momin B., Richardson L. An analysis of content in comprehensive cancer control (CCC) plans that address chronic Hepatitis B and C Virus infections as major risk factors for liver cancer. J. Commun. Health. 2012;37:912–916. doi: 10.1007/s10900-011-9507-y.An. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen M.T., Salaspuro M. Local acetaldehyde-an essential role in alcohol-related upper gastrointestinal tract carcinogenesis. Cancers (Basel) 2018;10:1–23. doi: 10.3390/cancers10010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta H., Wada Y., Kawano Y., Murakami Y., Irie A., Taniguchi K., Kikuchi K., Yamada G., Suzuki K., Honda J., Wilson-Morifuji M., Araki N., Eto M., Baba H., Imamura T. Enhancement of human cancer cell motility and invasiveness by anaphylatoxin C5a via aberrantly expressed C5a receptor (CD88) Clin. Cancer Res. 2013;19:2004–2013. doi: 10.1158/1078-0432.CCR-12-1204. [DOI] [PubMed] [Google Scholar]
- Ona K., Oh D.H. Voriconazole N-oxide and its ultraviolet B photoproduct sensitize keratinocytes to ultraviolet A. Br. J. Dermatol. 2015;173:751–759. doi: 10.1111/bjd.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth M., Metzger P., Gerum S., Mayerle J., Schneider G., Belka C., Schnurr M., Lauber K. Pancreatic ductal adenocarcinoma: biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019;14:1–20. doi: 10.1186/s13014-019-1345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin D.M., Mesher D., Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br. J. Cancer. 2011;105:S66–S69. doi: 10.1038/bjc.2011.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D.D., Kuchroo V.K. Th17 Cell Pathway in Human Immunity: lessons from Genetics and Therapeutic Interventions. Immunity. 2015;43:1040–1051. doi: 10.1016/j.immuni.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Peters Y., Al-Kaabi A., Shaheen N.J., Chak A., Blum A., Souza R.F., Di Pietro M., Iyer P.G., Pech O., Fitzgerald R.C., Siersema P.D. Barrett oesophagus. Nat. Rev. Dis. Prim. 2019;5 doi: 10.1038/s41572-019-0086-z. [DOI] [PubMed] [Google Scholar]
- Pio R., Corrales L., Lambris J.D. The role of complement in tumor growth. Adv. Exp. Med. Biol. 2014;772:229–262. doi: 10.1007/978-1-4614-5915-6_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschl G., Seitz H.K. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- Puthiyaveetil J.S.V., Kota K., Chakkarayan R., Chakkarayan J., Thodiyil A.K.P. Epithelial-Mesenchymal interactions in tooth development and the significant role of growth factors and genes with emphasis on mesenchyme-a review. J. Clin. Diagn. Res. 2016;10:ZE05–ZE09. doi: 10.7860/JCDR/2016/21719.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S., Amaretti A., Gozzoli C., Simone M., Righini L., Candeliere F., Brun P., Ardizzoni A., Colombari B., Paulone S., Castagliuolo I., Cavalieri D., Blasi E., Rossi M., Peppoloni S. Longitudinal survey of fungi in the human gut: ITS profiling, phenotyping, and colonization. Front. Microbiol. 2019;10:1–12. doi: 10.3389/fmicb.2019.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautemaa R., Hietanen J., Niissalo S., Pirinen S., Perheentupa J. Oral and oesophageal squamous cell carcinoma-a complication or component of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED, APS-I) Oral Oncol. 2007;43:607–613. doi: 10.1016/j.oraloncology.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Reid M.F., Fewson C.A. (1994) Molecular characterization of microbial alcohol dehydrogenases. [DOI] [PubMed]
- Reyes-Gordillo K., Shah R., Arellanes-Robledo J., Hernández-Nazara Z., Rincón-Sánchez A.R., Inagaki Y., Rojkind M., Lakshman M.R. Mechanisms of action of acetaldehyde in the up-regulation of the human α2(I) collagen gene in hepatic stellate cells: key roles of Ski, SMAD3, SMAD4, and SMAD7. Am. J. Pathol. 2014;184:1458–1467. doi: 10.1016/j.ajpath.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M.L., Sokol H. The gut mycobiota: insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2019;16:331–345. doi: 10.1038/s41575-019-0121-2. [DOI] [PubMed] [Google Scholar]
- Rigel D.S. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J. Am. Acad. Dermatol. 2008;58 doi: 10.1016/j.jaad.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Riker A.I., Zea N., Trinh T. The epidemiology, prevention, and detection of melanoma. Ochsner J. 2010;10:56–65. [PMC free article] [PubMed] [Google Scholar]
- Rizzetto L., De Filippo C., Cavalieri D. Richness and diversity of mammalian fungal communities shape innate and adaptive immunity in health and disease. Eur. J. Immunol. 2014;44:3166–3181. doi: 10.1002/eji.201344403. [DOI] [PubMed] [Google Scholar]
- Rutkowski M.J., Sughrue M.E., Kane A.J., Ahn B.J., Fang S., Parsa A.T. The complement cascade as a mediator of tissue growth and regeneration. Inflamm. Res. 2010;59:897–905. doi: 10.1007/s00011-010-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S., Bhargava A., Mehra S., Dakwala F. Identification of Candida albicans by using different culture medias and its association in potentially malignant and malignant lesions. Contemp. Clin. Dent. 2011;2:188. doi: 10.4103/0976-237x.86454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar C.R., Francois F., Li Y., Corby P., Hays R., Leung C., Bedi S., Segers S., Queiroz E., Sun J., Wang B., Ho H., Craig R., Cruz G.D., Blaser M.J., Perez-perez G., Hayes R.B., Dasanayake A., Pei Z., Chen Y. Association between oral health and gastric precancerous lesions. Carcinogenesis. 2012;33:399–403. doi: 10.1093/carcin/bgr284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjaya P.R., Gokul S., Gururaj Patil B., Raju R. Candida in oral pre-cancer and oral cancer. Med. Hypotheses. 2011;77:1125–1128. doi: 10.1016/j.mehy.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Sankari S.L., Gayathri K., Balachander N., Malathi L. Candida in potentially malignant oral disorders. J. Pharm. Bioallied Sci. 2015;7:S162–S164. doi: 10.4103/0975-7406.155886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayegh E.T., Bloch O., Parsa A.T. Complement anaphylatoxins as immune regulators in cancer. Cancer Med. 2014;3:747–758. doi: 10.1002/cam4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully C. Oral cancer aetiopathogenesis; past, present and future aspects. Med. Oral Patol. Oral Cir. Bucal. 2011;16:306–311. doi: 10.4317/medoral.16.e306. [DOI] [PubMed] [Google Scholar]
- Setshedi M., Wands J.R., De La Monte S.M. Acetaldehyde adducts in alcoholic liver disease. Oxid. Med. Cell Longev. 2010;3:178–185. doi: 10.4161/oxim.3.3.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A. Oral Candidiasis: an Opportunistic Infection of AIDS. Int. J. Appl. Dent. Sci. 2019;5:23–27. doi: 10.4172/2155-6113.1000i101. [DOI] [Google Scholar]
- Shoham S., Marr K.A. Invasive fungal infections in solid organ transplant recipients. Future Microbiol. 2012;7:639–655. doi: 10.2217/fmb.12.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla K., Vun I., Lov I., Laparidis G., McCamley C., Ariyawardana A. Role of Candida infection in the malignant transformation of oral leukoplakia: a systematic review of observational studies. Transl. Res. Oral Oncol. 2019;4 doi: 10.1177/2057178x19828229. 2057178x1982822. [DOI] [Google Scholar]
- Siegel R.L., Miller K.D., Goding Sauer A., Fedewa S.A., Butterly L.F., Anderson J.C., Cercek A., Smith R.A., Jemal A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- Simoes P.K., Olson S.H., Saldia A., Kurtz R.C. Epidemiology of pancreatic adenocarcinoma. Chin. Clin. Oncol. 2017;6:1–9. doi: 10.21037/cco.2017.06.32. [DOI] [PubMed] [Google Scholar]
- Simoni-Nieves A., Clavijo-Cornejo D., Gutiérrez-Ruiz M., Gomez-Quiroz L. (2018) Acetaldehyde effects on cellular redox state. In: Liver Oxid. Stress Dietary Antioxid.. pp 63–70.
- Singer J.P., Boker A., Metchnikoff C., Binstock M., Boettger R., Golden J.A., Glidden D.V., Arron S.T. High cumulative dose exposure to voriconazole is associated with cutaneous squamous cell carcinoma in lung transplant recipients. J. Hear Lung. Transpl. 2012;31:694–699. doi: 10.1016/j.healun.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow A.N., Laudadio J. Human papillomavirus detection in head and neck squamous cell carcinoma. Adv. Anat. Pathol. 2010;17:394–403. doi: 10.3332/ecancer.2014.475. [DOI] [PubMed] [Google Scholar]
- Steinbach W.J., Dvorak C.C. 4th Ed. Elsevier; 2012. Antifungal Agents. In: Principles and Practice of Pediatric Infectious Diseases; pp. 1484–1492. e5. [Google Scholar]
- Stoner G.D., Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737–1746. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- Suhr M.J. University of Nebraska - Lincoln; 2015. Characterization and Investigation of Fungi Inhabiting the Gastrointestinal Tract of Healthy and Diseased Humans. [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;0:1–41. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Underhill D.M., Iliev I.D. The mycobiota: interactions between commensal fungi and the host immune system. Nat. Rev. Immunol. 2014;14:405–416. doi: 10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Asbeck E.C., Hoepelman A.I.M., Scharringa J., Herpers B.L., Verhoef J. Mannose binding lectin plays a crucial role in innate immunity against yeast by enhanced complement activation and enhanced uptake of polymorphonuclear cells. BMC Microbiol. 2008;8:1–10. doi: 10.1186/1471-2180-8-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Fan C., Yao A., Xu X., Zheng G., You Y., Jiang C., Zhao X., Hou Y., Hung M.C., Lin X. The adaptor protein CARD9 protects against colon cancer by restricting mycobiota-mediated expansion of myeloid-derived suppressor cells. Immunity. 2018;49:504–514. doi: 10.1016/j.immuni.2018.08.018. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Pan D., Zhou Z., You Y., Jiang C., Zhao X., Lin X. Dectin-3 deficiency promotes colitis development due to impaired antifungal innate immune responses in the gut. PLoS Pathog. 2016;12:1–22. doi: 10.1371/journal.ppat.1005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M.Y., Shi S., Liang C., Meng Q.C., Hua J., Zhang Y.Y., Liu J., Zhang B., Xu J., Yu X.J. The microbiota and microbiome in pancreatic cancer: more influential than expected. Mol. Cancer. 2019;18:1–15. doi: 10.1186/s12943-019-1008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2021) Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed 24 Apr 2021.
- Xing Y., Hogquist K.A. T-Cell tolerance: central and peripheral. Cold Spring Harb. Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a006957. a006957–a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai B., Ola M., Rolling T., Tosini N.L., Joshowitz S., Littmann E.R., Amoretti L.A., Fontana E., Wright R.J., Miranda E., Veelken C.A., Morjaria S.M., Peled J.U., van den Brink M.R.M., Babady N.E., Butler G., Taur Y., Hohl T.M. High-resolution mycobiota analysis reveals dynamic intestinal translocation preceding invasive candidiasis. Nat. Med. 2020;26:59–64. doi: 10.1038/s41591-019-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Wang Y., Shen S., Hou Y., Chen Y., Wang T. The mycobiota of the human body: a spark can start a prairie fire. Gut Microbes. 2020;11:655–679. doi: 10.1080/19490976.2020.1731287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Willette-Brown J., Song N.Y., Lomada D., Song Y., Xue L., Gray Z., Zhao Z., Davis S.R., Sun Z., Zhang P., Wu X., Zhan Q., Richie E.R., Hu Y. Autoreactive T Cells and chronic fungal infection drive esophageal carcinogenesis. Cell Host Microbe. 2017;21:478–493. doi: 10.1016/j.chom.2017.03.006. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Phan Q.T., Boontheung P., Solis N.V., Loo J.A., Filler S.G. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc. Natl. Acad. Sci. 2012;109:14194–14199. doi: 10.1073/pnas.1117676109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.