Abstract
Background
Alectinib, a second-generation anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor (TKI), is highly effective in advanced ALK-rearranged non-small-cell lung cancer and represents a standard first-line therapy. New strategies are needed, however, to delay resistance. We conducted a phase I/II study to assess the safety and efficacy of combining alectinib with bevacizumab, a monoclonal antibody against vascular endothelial growth factor.
Patients and methods
Patients with advanced ALK-rearranged non-squamous non-small-cell lung cancer were enrolled. The phase I portion employed a dose de-escalation strategy with alectinib and bevacizumab starting at the individual standard doses. The primary objective was to determine the recommended phase II dose (RP2D). In phase II, the primary objective was to evaluate the safety of the combination at the RP2D; the secondary objective was to determine extracranial and intracranial efficacy.
Results
Eleven patients were enrolled between September 2015 and February 2020. Most patients (82%) had baseline brain metastases. Six patients (55%) were treatment-naive; five (46%) had received prior ALK TKIs (crizotinib, n = 3; ceritinib, n = 1; crizotinib then brigatinib, n = 1). No dose-limiting toxicities occurred. RP2D was determined as alectinib 600 mg orally twice daily plus bevacizumab 15 mg/kg intravenously every 3 weeks. Three patients experienced grade 3 treatment-related adverse events: pneumonitis related to alectinib, proteinuria related to bevacizumab, and hypertension related to bevacizumab. Treatment-related intracranial hemorrhage was not observed. Six (100%) of six treatment-naive patients and three (60%) of five ALK TKI-pretreated patients had objective responses; median progression-free survival was not reached (95% confidence interval, 9.0 months-not reached) and 9.5 months (95% confidence interval, 4.3 months-not reached), respectively. Intracranial responses occurred in four (100%) of four treatment-naive and three (60%) of five TKI-pretreated patients with baseline brain metastases. The study was stopped prematurely because of slow accrual.
Conclusions
Alectinib plus bevacizumab was well tolerated without unanticipated toxicities or dose-limiting toxicities.
Key words: alectinib, bevacizumab, anaplastic lymphoma kinase, non-small-cell lung cancer, targeted therapy
Highlights
-
•
Alectinib plus bevacizumab is safe in patients with advanced ALK-rearranged non-small-cell lung cancer.
-
•
Bevacizumab combined with alectinib does not increase risk of intracranial hemorrhage in patients with brain metastases.
-
•
Enrollment challenges with this trial highlight important considerations for future studies of ALK inhibitor combinations.
Introduction
Anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKIs) have significantly improved outcomes of patients with advanced ALK-rearranged non-small-cell lung cancer (NSCLC). Crizotinib, a first-generation ALK TKI, became the initial standard first-line therapy for advanced ALK-rearranged NSCLC after demonstrating superior efficacy compared with platinum-pemetrexed chemotherapy.1 Several randomized phase II trials have since demonstrated, however, that more potent and central nervous system (CNS)-active second-generation ALK TKIs (alectinib, brigatinib, and ensartinib) are superior to crizotinib in the first-line setting.2, 3, 4 Alectinib in particular has been commonly used on the basis of the phase III global ALEX trial, which demonstrated significantly prolonged progression-free survival (PFS) compared with crizotinib with a median PFS of 25.7 months versus 10.4 months (by independent review committee assessment).2 Despite this efficacy, drug resistance develops, and disease progression is inevitable in most patients.5, 6, 7
As one approach to improve upon the efficacy of second-generation ALK inhibitors, a third-generation ALK TKI, lorlatinib, was evaluated in patients with untreated advanced ALK-rearranged NSCLC in the phase III CROWN study.8 The comparator here, however, was crizotinib—rather than a second-generation ALK TKI—with markedly superior PFS in favor of lorlatinib. An alternative strategy being explored to delay resistance and disease progression is combination therapy, built upon the foundation of an ALK inhibitor. To date, various partners including anti-programmed cell death protein 1/programmed death-ligand 1 checkpoint inhibitors,9, 10, 11, 12 heat shock protein 90 inhibitors,13 and inhibitors of MET,14,15 MEK,16,17 or SHP218,19 have been combined with an ALK TKI. A combination regimen that offers additive clinical benefit in advanced ALK-rearranged NSCLC is yet to be established.
Bevacizumab is an anti-angiogenic monoclonal antibody targeting the vascular endothelial growth factor (VEGF) signaling pathway, which has shown efficacy when combined with platinum-based chemotherapy in advanced non-squamous NSCLC.20 Despite initial concerns related to the potential risk of intracranial hemorrhage limiting its use in patients with brain metastases, multiple studies have now demonstrated the safety and efficacy of bevacizumab in treating brain metastases in NSCLC when combined with either chemotherapy or targeted therapy.21, 22, 23 This is particularly relevant in ALK-rearranged NSCLC given its propensity to form CNS metastases.24,25 Furthermore, in advanced NSCLC harboring epidermal growth factor receptor (EGFR) gene mutations, a combination of erlotinib, a first-generation EGFR TKI, with bevacizumab significantly improved PFS compared with erlotinib alone.26, 27, 28 The combination of bevacizumab and erlotinib was granted approval by the European Medicines Agency for the first-line treatment of patients with advanced EGFR-mutant NSCLC. More recently, in a phase I/II study, bevacizumab combined with osimertinib, a third-generation CNS-active EGFR inhibitor, was found to be well tolerated and met the study’s prespecified endpoint for effectiveness,29 providing the rationale for an ongoing phase III trial of osimertinib with or without bevacizumab in advanced untreated EGFR-mutant NSCLC (ClinicalTrials.gov identifier NCT04181060).
Here, we carried out a phase I/II trial to evaluate the safety and efficacy of bevacizumab plus alectinib in patients with advanced ALK-rearranged NSCLC.
Materials and methods
Study design and participants
This study was an investigator-initiated, single-institution, open-label, single-arm phase I/II clinical trial. Eligible patients had locally advanced or metastatic non-squamous NSCLC with ALK rearrangements as established using ALK FISH, immunohistochemistry, or next-generation sequencing. ALK FISH was considered positive if >15% of tumor cells demonstrated abnormal signals. Patients who were treatment-naive in the advanced setting, as well as those who had disease progression on previous treatments including ALK TKI(s), were permitted to enroll; however, prior alectinib or anti-angiogenic therapy was not permitted. While patients were initially required to have at least one measurable CNS lesion per modified RECIST version 1.130 in addition to measurable extracranial disease by RECIST version 1.1, the protocol was later amended to allow enrollment with or without measurable CNS disease. Other key eligibility criteria included age 18 years or older, Eastern Cooperative Oncology Group performance status of 0-2, and adequate end-organ function. Patients who had clinically stable or asymptomatic untreated, non-hemorrhagic CNS metastases were eligible.
Key exclusion criteria included: squamous cell or mixed, predominantly squamous adenosquamous histology; history of hemoptysis; tumor infiltrating into large vessels or proximal tracheobronchial network; intracranial hemorrhage; history of or genetic predisposition to a bleeding diathesis or coagulopathy; arterial or venous thromboembolic events within 6 months of enrollment; and poorly controlled arterial hypertension (systolic >150 mm Hg and/or diastolic > 100 mm Hg). The protocol was approved by the local Institutional Review Board, and all patients provided written informed consent before screening.
Study procedures
In the phase I portion of the study, a 3 + 3 dose de-escalation design was used to determine the recommended phase II dose (RP2D) of alectinib and bevacizumab. Starting alectinib dose of 600 mg twice daily administered orally (p.o.) and bevacizumab dose of 15 mg/kg every 3 weeks administered intravenously (i.v.) were selected based on the individual RP2Ds for each medication and anticipation for minimal overlapping toxicities. If no dose-limiting toxicities (DLTs) were observed at the initial dose level, the cohort was expanded to a total of six patients to determine the final dose level established as RP2D. DLTs were defined as adverse events (AEs) occurring within the first cycle of treatment (21 days) attributed to the study drugs. The RP2D for the combination of alectinib and bevacizumab was defined as either (i) the highest dosage cohort in which less than a third of patients experienced a DLT, or (ii) alectinib at the previously defined RP2D as a single agent (600 mg twice daily) plus bevacizumab at the highest tolerated dose investigated for the indication (15 mg/kg every 21 days), whichever was the lower dose. In the phase II portion of the study, all patients received alectinib plus bevacizumab at the RP2D determined in the phase I portion.
Cycles were 21 days long. Treatment was continued until there was evidence of progressive disease, death, or unacceptable toxicity. Patients were allowed to continue study drugs beyond progression if deemed clinically beneficial at the investigator’s discretion. Intra-patient dose modification of bevacizumab was not permitted. Dose holds and reductions of alectinib were permitted in the event of protocol-specified treatment-related AEs. Patients could remain on alectinib alone despite discontinuation of bevacizumab, provided they were tolerating alectinib. Of note, this study period spanned the COVID-19 pandemic.31 During the pandemic, the protocol was amended to allow bevacizumab infusions to be held in the absence of toxicity at the investigator’s discretion, to minimize patient exposures.
Safety assessments were carried out in all patients at baseline, on day 1 and day 15 of the first cycle, and every 3 weeks thereafter. For patients who were holding or had permanently discontinued bevacizumab, safety assessments could be carried out every 6 weeks. AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Computed tomography scans of the chest and abdomen (and pelvis if clinically indicated) and brain magnetic resonance imaging (MRI) scans were obtained at baseline, every 6 weeks for the first 10 cycles of study treatment, and every 12 weeks thereafter. Patients in the phase I portion who had no evidence of intracranial metastases on brain MRI screening were not required to undergo subsequent brain MRI. Response assessment was conducted according to the RECIST version 1.1. For patients with brain metastases, intracranial response was assessed using modified RECIST version 1.1.30
Outcomes
The primary objectives of this study were to determine the RP2D of the combination of alectinib and bevacizumab (phase I) and to evaluate the safety and tolerability of alectinib and bevacizumab at the RP2D as assessed using the CTCAE version 4.0 (phase II). The secondary endpoints were safety, tolerability, and DLTs (in phase I); CNS objective response rate (ORR), CNS disease control rate (DCR; defined as the rate of complete response, partial response, and stable disease), CNS PFS, overall (intra- and extra-CNS) ORR, overall (intra- and extra-CNS) DCR, PFS, and patient-reported functioning and impact on disease/treatment-related symptoms of brain metastases and global QOL assessed using European Organisation for Research and Treatment of Cancer quality of life questionnaire (QLQ)-C30 and QLQ-BN20. PFS was measured as the time from the start of the study drug treatment until disease progression or death.
Statistical analysis
For the dose-finding, phase I portion of the study, no hypothesis was pre-established, and sample size was not predefined. For the phase II portion of the study, the combination of alectinib and bevacizumab was to be deemed unsafe if two or more patients were observed to have grade 2 or higher CNS hemorrhagic events. We initially planned to enroll 20 patients, which would guarantee that the study treatment discontinuation rate was not higher than 28% based upon the upper bound of the 95% exact binomial confidence interval (CI). Because of slow accrual, however, the decision was made to stop the study prematurely in July 2021.
Safety data are summarized for all patients who received at least one dose of study treatment. Efficacy data are reported for all patients with a baseline scan and at least one post-treatment scan, or in patients who had a best overall response of progressive disease or death before the end of cycle 1. CNS and overall PFS rates were estimated using the Kaplan-Meier method. Data were pooled for the phase I and phase II cohorts. Statistical analyses were carried out using SAS version 9.4. This study is registered with ClinicalTrials.gov (identifier NCT02521051).
Results
Patient characteristics
Between September 2015 and February 2020, a total of 11 patients with advanced ALK-rearranged NSCLC were enrolled and received at least one dose of the study drugs. One additional patient initially provided consent but withdrew thereafter with the preference of starting commercial alectinib. Baseline demographics and disease characteristics for the 11 patients who received alectinib plus bevacizumab are presented in Table 1. The data cut-off for these analyses was 31July 2021. The median age at the time of study entry was 46 years (range, 25-66 years). Most patients were never smokers (72.7%), and all had adenocarcinoma. Most patients (81.8%) had active brain metastases at baseline. Two of the nine patients with baseline brain metastases had previously received brain radiation [either whole brain radiotherapy (n = 1) completed 17.0 months before study start, or stereotactic radiosurgery (n = 1) completed 3.5 months before study start], with definite evidence of subsequent CNS disease progression before study enrollment.
Table 1.
Baseline patient characteristics
| Baseline characteristic | n = 11 |
|---|---|
| Age, years | |
| Median (range) | 46 (25-66) |
| Sex, n (%) | |
| Male | 5 (45.5) |
| Female | 6 (54.5) |
| Race, n (%) | |
| White | 8 (72.7) |
| Asian | 2 (18.2) |
| Unknown | 1 (9.1) |
| ECOG performance status, n (%) | |
| 0 | 7 (63.6) |
| 1 | 3 (27.3) |
| 2 | 1 (9.1) |
| Smoking status, n (%) | |
| Never | 8 (72.7) |
| Light (<10 pack-years) | 3 (27.3) |
| Histology, n (%) | |
| Adenocarcinoma | 11 (100) |
| Brain metastases, n (%) | |
| Presenta | 9 (81.8) |
| Absent | 2 (18.2) |
| ALK testing, n (%) | |
| IHC | 2 (18.2) |
| FISH | 9 (81.8) |
| Line of therapy | |
| Median (range) | 1 (1-3) |
| Prior ALK TKIs, n (%) | |
| Yesb | 5 (45.5) |
| No | 6 (54.5) |
ALK, anaplastic lymphoma kinase; ECOG, Eastern Cooperative Oncology Group; IHC, immunohistochemistry; TKI, tyrosine kinase inhibitor.
Two patients had received prior brain radiation (whole brain radiotherapy, one; stereotactic radiosurgery, one) with subsequent progression in the central nervous system and active brain metastases before study enrollment.
Three patients had received prior crizotinib; one had received prior ceritinib; and one had received prior crizotinib followed by brigatinib.
Six patients (54.5%) were treatment-naive. The remaining 5 patients (45.5%) had received the following prior ALK TKI(s): crizotinib only (n = 3), ceritinib only (n = 1), or crizotinib followed by brigatinib (n = 1). All patients discontinued their preceding ALK TKI because of disease progression (CNS and extra-CNS progression, n = 2; CNS only, n = 2; extra-CNS only, n = 1). The median time interval between the last ALK TKI dose and the initiation of alectinib plus bevacizumab was 8 days (range, 5-10). None of the patients had received prior chemotherapy or immunotherapy.
Safety and toxicity
All 11 patients were evaluated for toxicity. No DLTs were identified in the initial dosing cohort of six patients enrolled in the phase I portion of the study. Therefore, dosing de-escalation was not pursued, and RP2D was established as p.o. alectinib 600 mg twice a day plus i.v. bevacizumab 15 mg/kg every 21 days.
The most common treatment-related AEs occurring in more than one patient enrolled in phase I/II portions were: myalgia (n = 7; 63.6%), fatigue (n = 5, 45.5%), diarrhea (n = 5, 45.5%), hypertension (n = 4, 36.4%), proteinuria (n = 4, 36.4%), edema of the limbs (n = 4, 36.4%), increased alanine aminotransferase (n = 4, 36.4%), constipation (n = 4, 36.4%), increased creatine phosphokinase (CPK) (n = 3, 27.3%), increased aspartate aminotransferase (AST) (n = 3, 27.3%), epistaxis (n = 3, 27.3%), nausea (n = 3, 27.3%), hypophosphatemia (n = 3, 27.3%), increased bilirubin (n = 2, 18.2%), and maculopapular rash (n = 2, 18.2%) (Table 2). Three patients experienced grade 3 serious AEs that were deemed related to study treatment: pneumonitis related to alectinib (n = 1), proteinuria related to bevacizumab (n = 1), and hypertension related to bevacizumab (n = 1). No grade 4 or 5 AEs occurred. The patient who experienced grade 3 pneumonitis attributed to alectinib discontinued study treatment because of this toxicity. Seven patients (63.6%) discontinued bevacizumab early (but continued alectinib monotherapy on the study), after a median of 9 cycles of treatment (range, 4-48). A total of 4 patients (36.4%) stopped bevacizumab due to treatment-related AEs [proteinuria (n = 3), fatigue (n = 1)]. The remaining 3 patients discontinued bevacizumab following diverticular abscess not related to study treatment (n = 1), following radiation to a progressive disease site (n = 1), or per patient preference (after cycle 48 on the study without disease progression or toxicity, n = 1). All seven patients who discontinued bevacizumab early remained on the study and continued with alectinib monotherapy. Overall, treatment-naive patients (n = 6) received a median of 17.5 cycles of bevacizumab (range, 4-48), and patients with prior ALK TKI treatment (n = 5) received a median of 7 cycles of bevacizumab (range, 7-34). One patient required dose reductions of alectinib to 300 mg twice daily because of grade 2 fatigue possibly related to study treatment.
Table 2.
Treatment-related adverse events
| Treatment-related adverse events, n (%) | Any grade | Grade 3/4 |
|---|---|---|
| Myalgia | 7 (64) | 0 |
| Fatigue | 5 (46) | 0 |
| Diarrhea | 5 (46) | 0 |
| Hypertension | 4 (36) | 1 (9) |
| Proteinuria | 4 (36) | 1 (9) |
| Edema limbs | 4 (36) | 0 |
| ALT increased | 4 (36) | 0 |
| Constipation | 4 (36) | 0 |
| CPK increased | 3 (27) | 0 |
| AST increased | 3 (27) | 0 |
| Epistaxis | 3 (27) | 0 |
| Nausea | 3 (27) | 0 |
| Hypophosphatemia | 3 (27) | 0 |
| Bilirubin increased | 2 (18) | 0 |
| Rash, maculopapular | 2 (18) | 0 |
| Pneumonitis | 1 (9) | 1 (9) |
| Anemia | 1 (9) | 0 |
| Hemoptysis | 1 (9) | 0 |
| Gingival bleeding | 1 (9) | 0 |
| Hematoma | 1 (9) | 0 |
| Tongue sensitivity | 1 (9) | 0 |
| Headache | 1 (9) | 0 |
| Dysgeusia | 1 (9) | 0 |
| Bloating | 1 (9) | 0 |
| Sinus congestion | 1 (9) | 0 |
| Palpitations | 1 (9) | 0 |
| Memory impairment | 1 (9) | 0 |
| Influenza-like syndrome | 1 (9) | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine phosphokinase.
No treatment-related deaths were reported. Of note, although the majority of patients (81.8%) had untreated brain metastases at baseline, intracranial hemorrhagic events were not observed. One patient (9.1%) had grade 1 hemoptysis deemed possibly related to bevacizumab, and one patient (9.1%) had grade 1 hematoma related to bevacizumab. Serious or fatal hemorrhagic events did not occur in this study.
Efficacy
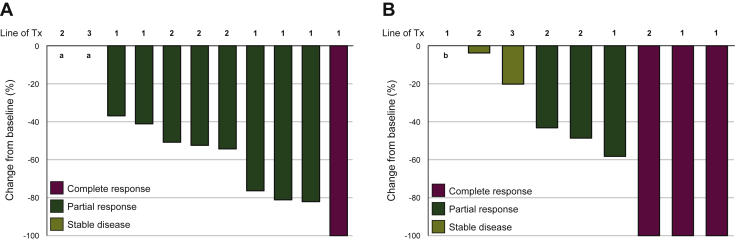
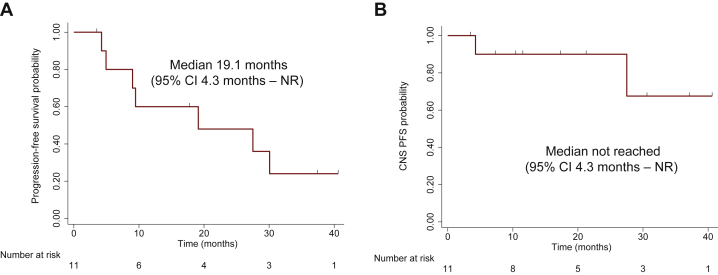
All patients were included in the efficacy analysis. Overall, nine patients (81.8%) had confirmed objective responses, of which one (9.1%) was a complete response (Figure 1A). All had disease control (DCR 100%). The median PFS of the overall population was 19.1 months (95% CI 4.3 months-not reached) after a median follow-up of 34.9 months (median follow-up for patients still alive by the end of the study: 37 months) (Figure 2A).
Figure 1.
Antitumor activity of alectinib plus bevacizumab in patients with advanced ALK-rearranged non-small-cell lung cancer.
(A) Overall antitumor activity of alectinib plus bevacizumab as shown in the waterfall plot of best percentage change from baseline in the sum of longest tumor diameters. (B) Intracranial antitumor activity of alectinib plus bevacizumab in patients with measurable and non-measurable brain metastases at baseline (n = 9).
Line of Tx, line of study treatment of advanced lung cancer.
a Patients who had stable disease with 0% tumor shrinkage as best response.
b Patients who had complete intracranial response with resolution of non-measurable baseline brain metastasis.
Figure 2.
Progression-free survival and central nervous system (CNS) progression-free survival of patients treated with alectinib plus bevacizumab.
Kaplan–Meier curves depicting (A) progression-free survival and (B) CNS progression-free survival of 11 patients enrolled in the study.
CI, confidence interval; NR, not reached; PFS, progression-free survival.
Among treatment-naive patients who received alectinib plus bevacizumab as initial treatment (‘first-line cohort,’ n = 6), the ORR and DCR were both 100% (6/6 confirmed objective responses), and the median PFS was not reached (95% CI 9.0 months-not reached) (Supplementary Figure S1A and Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100342). Among patients who had received prior ALK TKI(s) (‘ALK TKI-pretreated cohort,’ n = 5), the ORR was 60% (3/5), DCR 100% (5/5), and the median PFS 9.5 months (95% CI 4.3 months-not reached), respectively (Supplementary Figure S1A and Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100342). Of the three responders who had received prior ALK TKIs, two had experienced prior disease progression on crizotinib, whereas one patient had experienced prior CNS and extracranial disease progression on first-line ceritinib.
At data cut-off, four patients (36%) had died. The 2-year OS rate for the entire cohort was 63.6%.
CNS efficacy
Among 9 patients with measurable or non-measurable CNS lesions at baseline, the CNS ORR was 77.8% (7/9) and CNS DCR was 100% (9/9) (Figure 1B; Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100342). Of note, four patients (44.4%) had CNS complete responses. Among 8 patients with measurable CNS target lesions at baseline, the CNS ORR was 75.0% (6/8; 3 CNS complete responses) and CNS DCR was 100% (8/8). The median CNS PFS was not reached (95% CI 4.3 months-not reached) (Figure 2B).
In the first-line cohort, the CNS ORR and CNS DCR among 4 patients with measurable or non-measurable baseline brain metastases were 100% (4 CNS responses, 3 of which were CNS complete responses) (Supplementary Figure S1B and Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100342). The median CNS PFS was not reached (95% CI 27.5 months-not reached). In the ALK TKI-pretreated cohort, among 5 patients, all of whom had measurable baseline brain metastases, the CNS ORR was 60% (3/5), CNS DCR was 100% (5/5), and the median CNS PFS was not reached (95% CI 4.3 months-not reached) (Supplementary Figure S1B and Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100342). The three CNS responders in the ALK TKI-pretreated cohort included one crizotinib-pretreated patient with a CNS complete response, another crizotinib-pretreated patient with a CNS partial response, and a ceritinib-pretreated patient with prior CNS progression who then had CNS partial response to alectinib plus bevacizumab.
Discussion
To our knowledge, this is the first phase I/II trial to be published reporting on the safety and activity of adding the anti-VEGF inhibitor bevacizumab to alectinib in patients with advanced ALK-rearranged NSCLC. In this study, the combination was found to be tolerable with no new safety signals identified. Based on the safety assessment, p.o. alectinib 600 mg twice daily combined with i.v. bevacizumab 15 mg/kg every 3 weeks was determined to be the recommended phase II doses for the combination.
With five ALK inhibitors currently approved for first-line use in advanced ALK-rearranged NSCLC, anticipated toxicities influence the selection of first-line regimen. The most frequent treatment-related AEs of alectinib plus bevacizumab were highly concordant with the toxicities anticipated from each individual agent—such as myalgia, increased CPK, and edema associated with alectinib,2,32,33 or hypertension, proteinuria, and epistaxis associated with bevacizumab.34,35 The overall safety of combining bevacizumab with alectinib in this study is in line with a previously presented Japanese phase II study of alectinib plus bevacizumab in patients with ALK-rearranged NSCLC who had progressed on prior alectinib,36 and with prior trials assessing tolerability of bevacizumab when combined with chemotherapy or with other targeted therapies such as crizotinib or EGFR TKIs erlotinib or osimertinib.20,21,26,29,37,38 The rate of discontinuation of bevacizumab due to AEs is also comparable to that reported in prior studies, with proteinuria representing the most common treatment-related AE resulting in bevacizumab discontinuation which likely reflects the dose-dependent effect of bevacizumab on the development of proteinuria and the long treatment of patients on combination regimen.28,34,35,39,40 It is worth noting that despite the presence of brain metastases at baseline in the majority of patients enrolled in this study, intracranial hemorrhagic events did not occur. Whereas this finding supports the safety of using bevacizumab in patients with ALK-rearranged NSCLC who have known high incidence of brain metastases, further investigation is warranted given the small size of this study cohort.
In this study, the combination of alectinib plus bevacizumab was highly efficacious in both TKI-naive and TKI-pretreated patients (ORR 100% and 60%, respectively; and DCR 100% and 100%, respectively), as anticipated on the basis of the known efficacy of alectinib alone in these patient populations.2,33 The CNS ORR among treatment-naive patients with measurable and non-measurable CNS metastases at baseline was 100% with a CNS complete response rate of 75% (compared with 59% and 45%, respectively, with alectinib monotherapy in the global ALEX trial2), although the number of patients was very small and further investigation of CNS and extracranial efficacy is required. It is possible that anti-angiogenic therapy with bevacizumab may improve TKI delivery and efficacy in the CNS through modulation of the tumor vasculature.41 Furthermore, of note, bevacizumab has additionally demonstrated efficacy in treating brain radiation necrosis,42,43 which is not uncommon in ALK-rearranged NSCLC given the frequency of CNS metastases which may require local therapies.
Our study raises several questions regarding the future clinical development of ALK-VEGF inhibitor combinations in ALK-rearranged NSCLC. First, could the combination of anti-VEGF therapy plus an ALK TKI augment efficacy beyond that achieved with an ALK TKI alone? Further studies are needed in order to provide the rationale for pursuing randomized trials. Second, what is the optimal line of therapy (i.e. first-line versus later-line) in which VEGF inhibition may be combined with an ALK TKI? Prior studies have demonstrated at least some clinical benefit from the addition of bevacizumab to alectinib or lorlatinib after disease progression on ALK TKI monotherapy;36,44 however, whether the combination is best pursued upfront versus following disease relapse on an ALK inhibitor remains undetermined. And third, in such studies focused on assessing the efficacy of ALK-VEGF inhibitor combinations, how do we select the best endpoint (PFS versus OS)? In the context of advanced, treatment-naive EGFR-mutant NSCLC, the randomized phase III NEJ026 trial demonstrated that bevacizumab plus erlotinib significantly prolonged PFS compared with erlotinib monotherapy, but this did not translate into OS benefit.27,45 The multicenter phase III ARTEMIS-CTONG1509 study in Chinese patients with untreated EGFR-mutant NSCLC similarly showed PFS benefit from the combination of bevacizumab plus erlotinib, and while the OS data remained immature, the mature 2-year and 3-year OS rates were not significantly different between the combination arm and the erlotinib monotherapy arm.26 The potential benefit of adding bevacizumab to a more CNS-active and potent, third-generation EGFR TKI osimertinib, however, remains to be determined, and is currently being evaluated in an ongoing randomized phase III trial (ClinicalTrials.gov identifier NCT04181060). Furthermore, whether the observations made in the EGFR-mutant NSCLC patient population will be mirrored in ALK-rearranged NSCLC is unknown.
Finally, what is the optimal duration of bevacizumab when combining with an ALK inhibitor in patients with advanced ALK-rearranged lung cancer, who may continue on the same line of treatment for several years? Indeed, the median PFS with first-line treatment using second-generation ALK inhibitors including alectinib exceeds 2 years.2, 3, 4 Whereas four patients (36%) in this study discontinued bevacizumab early due to treatment-related AEs, seven patients (63.6%) overall discontinued bevacizumab early due to either treatment-related AEs, per investigator discretion, or per patient preference, after a median of 9 cycles of bevacizumab (range, 4-48). Long-term addition of an agent that must be administered i.v. and will inevitably increase toxicities is certainly a weighty consideration for patients who may otherwise be treated with an oral TKI alone, as is the added cost of such combination regimens. It will be important to consider these questions on the combination strategy of ALK TKI plus anti-VEGF therapy, in addition to other combination approaches (i.e. an ALK inhibitor plus chemotherapy), which collectively will provide the framework for informing potential avenues to augment efficacy and offer more durable disease control in patients with ALK-rearranged lung cancer.
This study had several limitations. As mentioned, the sample size was small owing to the slow accrual and premature closure of the study. Multiple factors likely contributed to the limited accrual. First, ALK-rearranged NSCLC remains a small subset of lung cancer overall. Second, although our institution is a major referral center for patients with ALK-rearranged lung cancers, the availability of multiple approved first-line ALK inhibitors meant that the majority of patients were not being referred for first-line clinical trial options. Third, this was a single-institution study, impacting access. Fourth, the trial regimen incorporated i.v. bevacizumab infusions, necessitating more frequent visits than would typically be required for patients receiving standard-of-care p.o. ALK TKIs—a difference further exacerbated when patients remain on treatment for years. Other contributors included the availability of other competing clinical trials and the COVID-19 pandemic which occurred during the study period.
Another limitation of our study was that the patient population enrolled in this study was heterogeneous and included both TKI-naive and TKI-pretreated patients, as the primary objective of the study was to assess the safety and tolerability of the combination. Although the toxicity profile was overall reassuring without significant new safety signals in this analysis, there may not have been sufficient power to capture an increase in relatively rare events, which requires further study. Additionally, the efficacy assessment requires ongoing investigation. Some patients were remaining on the combination therapy and the PFS and OS data were immature; in this setting, it is not yet feasible to determine subsequent patient outcomes after disease progression. This study was also not designed to formally evaluate mechanisms of resistance to alectinib plus bevacizumab, given the small sample size and the non-randomized nature of the study (in terms of comparing with resistance mechanisms to alectinib alone).
Conclusions
In summary, to the best of our knowledge, this study suggests that the combination of bevacizumab with a second-generation ALK inhibitor alectinib is safe and tolerable in patients with advanced ALK-rearranged NSCLC, including in patients with baseline brain metastases. Toxicities in line with those anticipated from each individual agent were observed from the combination. More broadly, this trial raises important considerations for the design of future studies of ALK inhibitor combinations, particularly in the first-line setting. Multicentered clinical trials will help enhance patient access, and modifications to eligibility criteria—such as allowing the enrollment of patients who received up to a limited duration of single-agent ALK TKI without evidence of disease progression—may further provide flexibility for referrals and enrollment. An abbreviated course of the combination partner could be considered where appropriate (e.g. with an anti-angiogenic agent or chemotherapy). Finally, incorporation of telehealth into trial design could ameliorate the burden patients incur related to travel for on-site visits, although this may be more relevant and feasible for studies without i.v. infusion requirements.
Acknowledgements
We are grateful to the patients, their families and caregivers, and all of the investigators and staff who participated in this study.
Funding
This work was supported by Genentech, United States/Roche (no grant number) who provided study drugs and funding, the Targeting a Cure for Lung Cancer Research Fund and Be A Piece of the Solution at MGH.
Disclosure
JJL has served as a compensated consultant for Genentech, C4 Therapeutics, Blueprint Medicines, Nuvalent, Bayer, Novartis, and Turning Point Therapeutics; received honorarium and travel support from Pfizer; received institutional research funds from Roche/Genentech, Hengrui Therapeutics, Turning Point Therapeutics, Neon Therapeutics, Relay Therapeutics, Bayer, Elevation Oncology, Linnaeus Therapeutics, and Novartis; received CME funding from OncLive, MedStar Health, and Northwell Health. CGA has participated in advisory boards with Pfizer, Regeneron/Sanofi-Genzyme, AstraZeneca, Takeda, Daiichi-Sankyo, Jazz, and Bristol-Myers Squibb. LVS has received consulting fees from AstraZeneca, Genentech, Pfizer, and Janssen, and has received institutional research support from Boehringer-Ingelheim, Novartis, AstraZeneca, and Genentech. IDJ has received honoraria from Foundation Medicine, American Lung Association, ASCO Post, OncLive, DAVA Oncology, Creative Education Concepts, Medscape, and Total Health Conferencing; consulting fees from Bayer, Boehringer Ingelheim, BostonGene, AstraZeneca, Pfizer, Xcovery, Catalyst, Novocure, Genentech, and Syros; research support from Array, Genentech, Novartis, Pfizer, and Guardant Health; and travel support from Array and Pfizer. ATS has served as a compensated consultant or received honoraria from Achilles, Archer, Ariad/Takeda, Bayer, Blueprint Medicines, Chugai, Daiichi-Sankyo, EMD Serono, Foundation Medicine, Guardant, Ignyta, KSQ Therapeutics, Loxo Oncology, Natera, Novartis, Pfizer, Roche-Genentech, Servier, Syros, Taiho Pharmaceutical, and TP Therapeutics; received institutional research funding from Ariad, Ignyta, Novartis, Pfizer, Roche-Genentech, and TP Therapeutics; received travel support from Genentech and Pfizer; and is currently employed by and owns stock in Novartis. JFG has served as a compensated consultant or received honoraria from Bristol-Myers Squibb, Genentech/Roche, Ariad/Takeda, Loxo/Lilly, Blueprint, Oncorus, Regeneron, Gilead, AstraZeneca, Pfizer, Incyte, Karyopharm, Novartis, Merck, Agios, Amgen, iTeos, GlydeBio, Moderna, and Array; research support from Novartis, Genentech/Roche, and Ariad/Takeda; institutional research support from Bristol-Myers Squibb, Tesaro, Moderna, Blueprint, Jounce, Array Biopharma, Merck, Adaptimmune, Novartis, and Alexo; and has an immediate family member who is an employee of Ironwood Pharmaceuticals. All remaining authors have declared no potential conflicts of interest.
Contributor Information
A.T. Shaw, Email: ashaw1@mgh.harvard.edu.
J.F. Gainor, Email: jgainor@mgh.harvard.edu.
Supplementary data
References
- 1.Solomon B.J., Mok T., Kim D.W., et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 2.Peters S., Camidge D.R., Shaw A.T., et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 3.Camidge D.R., Kim H.R., Ahn M.J., et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379(21):2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- 4.Horn L., Wang Z., Wu G., et al. Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase-positive non-small cell lung cancer: a randomized clinical trial. JAMA Oncol. 2021;7:1617–1625. doi: 10.1001/jamaoncol.2021.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gainor J.F., Dardaei L., Yoda S., et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6(10):1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin J.J., Riely G.J., Shaw A.T. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017;7(2):137–155. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoda S., Lin J.J., Lawrence M.S., et al. Sequential ALK inhibitors can select for lorlatinib-resistant compound ALK mutations in ALK-positive lung cancer. Cancer Discov. 2018;8(6):714–729. doi: 10.1158/2159-8290.CD-17-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw A.T., Bauer T.M., de Marinis F., et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 9.Felip E., de Braud F.G., Maur M., et al. Ceritinib plus nivolumab in patients with advanced ALK-rearranged non-small cell lung cancer: results of an open-label, multicenter, phase 1B study. J Thorac Oncol. 2020;15(3):392–403. doi: 10.1016/j.jtho.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Spigel D.R., Reynolds C., Waterhouse D., et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of anaplastic lymphoma kinase translocation – positive advanced non-small cell lung cancer (CheckMate 370) J Thorac Oncol. 2018;13(5):682–688. doi: 10.1016/j.jtho.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 11.Kim D.-W., Gadgeel S.M., Gettinger S.N., et al. Safety and clinical activity results from a phase Ib study of alectinib plus atezolizumab in ALK+ advanced NSCLC (aNSCLC) J Clin Oncol. 2018;36(suppl):9009. [Google Scholar]
- 12.Shaw A.T., Lee S.-H., Ramalingam S.S., et al. Avelumab (anti-PD-L1) in combination with crizotinib or lorlatinib in patients with previously treated advanced NSCLC: phase 1b results from JAVELIN Lung 101. J Clin Oncol. 2018;36(suppl):9008. doi: 10.1016/j.jtocrr.2024.100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sang J., Acquaviva J., Friedland J.C., et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov. 2013;3(4):430–443. doi: 10.1158/2159-8290.CD-12-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagogo-Jack I., Yoda S., Lennerz J.K., et al. MET alterations are a recurring and actionable resistance mechanism in ALK-positive lung cancer. Clin Cancer Res. 2020;26(11):2535–2545. doi: 10.1158/1078-0432.CCR-19-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji J., Mitra A., Camidge D.R., Riess J.W. Early alectinib resistance from MET amplification in ALK-rearranged NSCLC: response to crizotinib with re-response to alectinib and crizotinib. Clin Lung Cancer. 2021;22:e851–e855. doi: 10.1016/j.cllc.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Hrustanovic G., Olivas V., Pazarentzos E., et al. RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat Med. 2015;21(9):1038–1047. doi: 10.1038/nm.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shrestha N., Nimick M., Dass P., Rosengren R.J., Ashton J.C. Mechanisms of suppression of cell growth by dual inhibition of ALK and MEK in ALK-positive non-small cell lung cancer. Sci Rep. 2019;9(1):18842. doi: 10.1038/s41598-019-55376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dardaei L., Wang H.Q., Singh M., et al. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nat Med. 2018;24(4):512–517. doi: 10.1038/nm.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kano H., Ichihara E., Watanabe H., et al. SHP2 inhibition enhances the effects of tyrosine kinase inhibitors in preclinical models of treatment-naive ALK-, ROS1-, or EGFR-altered non-small cell lung cancer. Mol Cancer Ther. 2021;20(9):1653–1662. doi: 10.1158/1535-7163.MCT-20-0965. [DOI] [PubMed] [Google Scholar]
- 20.Sandler A., Gray R., Perry M.C., et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 21.Socinski M.A., Langer C.J., Huang J.E., et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol. 2009;27(31):5255–5261. doi: 10.1200/JCO.2009.22.0616. [DOI] [PubMed] [Google Scholar]
- 22.Besse B., Le Moulec S., Mazieres J., et al. Bevacizumab in patients with nonsquamous non-small cell lung cancer and asymptomatic, untreated brain metastases (BRAIN): a nonrandomized, phase II study. Clin Cancer Res. 2015;21(8):1896–1903. doi: 10.1158/1078-0432.CCR-14-2082. [DOI] [PubMed] [Google Scholar]
- 23.Reck M., von Pawel J., Zatloukal P., et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21(9):1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gainor J.F., Tseng D., Yoda S., et al. Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non-small-cell lung cancer. JCO Precis Oncol. 2017;2017 doi: 10.1200/PO.17.00063. PO.17.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drilon A., Lin J.J., Filleron T., et al. Frequency of brain metastases and multikinase inhibitor outcomes in patients with RET-rearranged lung cancers. J Thorac Oncol. 2018;13(10):1595–1601. doi: 10.1016/j.jtho.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Q., Xu C.R., Cheng Y., et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): a multicenter phase 3 study. Cancer Cell. 2021;39:1279–1291. doi: 10.1016/j.ccell.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Saito H., Fukuhara T., Furuya N., et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 28.Seto T., Kato T., Nishio M., et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 29.Yu H.A., Schoenfeld A.J., Makhnin A., et al. Effect of osimertinib and bevacizumab on progression-free survival for patients with metastatic EGFR-mutant lung cancers: a phase 1/2 single-group open-label trial. JAMA Oncol. 2020;6(7):1048–1054. doi: 10.1001/jamaoncol.2020.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long G.V., Trefzer U., Davies M.A., et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 31.Piper-Vallillo A.J., Mooradian M.J., Meador C.B., et al. Coronavirus disease 2019 infection in a patient population with lung cancer: incidence, presentation, and alternative diagnostic considerations. JTO Clin Res Rep. 2021;2(1):100124. doi: 10.1016/j.jtocrr.2020.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gadgeel S.M., Gandhi L., Riely G.J., et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15(10):1119–1128. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 33.Ou S.H., Ahn J.S., De Petris L., et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016;34(7):661–668. doi: 10.1200/jco.2015.63.9443. [DOI] [PubMed] [Google Scholar]
- 34.Niho S., Kunitoh H., Nokihara H., et al. Randomized phase II study of first-line carboplatin-paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer. 2012;76(3):362–367. doi: 10.1016/j.lungcan.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Reck M., von Pawel J., Zatloukal P., et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe S., Matsumoto N., Koshio J., et al. MA21.05 phase II trial of the combination of alectinib with bevacizumab in ALK-positive nonsquamous non-small cell lung cancer. J Thorac Oncol. 2019;14(10):S336. [Google Scholar]
- 37.Akamatsu H., Toi Y., Hayashi H., et al. Efficacy of osimertinib plus bevacizumab vs osimertinib in patients with EGFR T790M-mutated non-small cell lung cancer previously treated with epidermal growth factor receptor-tyrosine kinase inhibitor: West Japan Oncology Group 8715L phase 2 randomized clinical trial. JAMA Oncol. 2021;7(3):386–394. doi: 10.1001/jamaoncol.2020.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Z., Xiong Q., Cui Z., et al. Efficacy and safety of crizotinib plus bevacizumab in ALK/ROS-1/c-MET positive non-small cell lung cancer: an open-label, single-arm, prospective observational study. Am J Transl Res. 2021;13(3):1526–1534. [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu X., Wu S., Dahut W.L., Parikh C.R. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49(2):186–193. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 40.Lafayette R.A., McCall B., Li N., et al. Incidence and relevance of proteinuria in bevacizumab-treated patients: pooled analysis from randomized controlled trials. Am J Nephrol. 2014;40(1):75–83. doi: 10.1159/000365156. [DOI] [PubMed] [Google Scholar]
- 41.Jain R.K., di Tomaso E., Duda D.G., Loeffler J.S., Sorensen A.G., Batchelor T.T. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 42.Khan M., Zhao Z., Arooj S., Liao G. Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: a systematic review & meta-analysis. BMC Cancer. 2021;21(1):167. doi: 10.1186/s12885-021-07889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanigawa K., Mizuno K., Kamenohara Y., Unoki T., Misono S., Inoue H. Effect of bevacizumab on brain radiation necrosis in anaplastic lymphoma kinase-positive lung cancer. Respirol Case Rep. 2019;7(7):e00454. doi: 10.1002/rcr2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choudhury N.J., Young R.J., Sellitti M., Miller A., Drilon A. Lorlatinib and bevacizumab activity in ALK-rearranged lung cancers after lorlatinib progression. JCO Precis Oncol. 2020;4 doi: 10.1200/PO.20.00271. PO.20.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maemondo M., Fukuhara T., Saito H., et al. NEJ026: final overall survival analysis of bevacizumab plus erlotinib treatment for NSCLC patients harboring activating EGFR-mutations. J Clin Oncol. 2020;38(suppl):9506. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.