Highlights
-
•
Individuals with CFRD have unique diabetes physiology and comorbidities.
-
•
Perceptions of diabetes technology use in the CF community are positive.
-
•
Limited evidence supports the clinical effectiveness of these technologies in CFRD.
-
•
Automated insulin delivery improves A1c and decreases patient burden in T1D.
-
•
Proof of benefits to device use in CFRD may improve insurance coverage and increase sustained use.
Keywords: Cystic fibrosis-related diabetes, Continuous glucose monitor, Insulin pump, Automated insulin delivery, Patient reported outcomes
Abstract
There have been tremendous advances in diabetes technology in the last decade. Continuous glucose monitors (CGM), insulin pumps, and automated insulin delivery (AID) systems aim to improve glycemic control while simultaneously decreasing the burden of diabetes management. Although diabetes technologies have been shown to decrease both hypoglycemia and hyperglycemia and to improve health-related quality of life in individuals with type 1 diabetes, the impact of these devices in individuals with cystic fibrosis-related diabetes (CFRD) is less clear. There are unique aspects of CFRD, including the different underlying pathophysiology and unique lived health care experience and comorbidities, that likely affect the use, efficacy, and uptake of diabetes technology in this population. Small studies suggest that CGM is accurate and may be helpful in guiding insulin therapy for individuals with CFRD. Insulin pump use has been linked to improvements in lean body mass and hemoglobin A1c among adults with CFRD. A recent pilot study highlighted the promise of AID systems in this population. This article provides an overview of practical aspects of diabetes technology use and device limitations that clinicians must be aware of in caring for individuals with CF and CFRD. Cost and limited insurance coverage remain significant barriers to wider implementation of diabetes technology use among patients with CFRD. Future studies exploring strategies to improve patient and CF provider education about these devices and studies showing the effectiveness of these technologies on health and patient-reported outcomes may lead to improved insurance coverage and increased rates of uptake and sustained use of these technologies in the CFRD community.
Introduction:
Cystic fibrosis (CF) is the most common life-threatening autosomal recessive disease with an estimated worldwide prevalence of approximately 1 in every 2500 live births [1]. Cystic fibrosis-related diabetes (CFRD) is the most common non-pulmonary manifestation of CF, affecting up to 30% of adolescents and 50% of adults living with CF [2]. All individuals with CF have varying degrees of abnormal glucose tolerance characterized by impairments in first phase insulin secretion [3], [4] and progressive islet cell damage and loss of insulin secretion over time [3], [5]. In addition to insulin insufficiency, CFRD is also associated with insulin resistance related to chronic inflammation, cyclic infections, glucocorticoid therapy, and an association with genetic predictors for type 2 diabetes (T2D) [6], [7], [8]. Awareness of the characteristic features of diabetes pathophysiology in CFRD and of the unique lived healthcare experiences of individuals with CF is crucial for optimizing management.
Individuals with CFRD should be seen quarterly by a specialized multidisciplinary team with expertise in diabetes and CF to support self-management practices and education [9]. CFRD management focuses on frequent blood glucose monitoring, which is recommended at least three times daily for those receiving treatment, and insulin which is the only treatment currently approved for CFRD [9], [10], [11]. Fasting glucose values between 80 and 130 mg/dL, 2-hour post-prandial values of < 180 mg/dL, and a hemoglobin A1c (A1c) target of ≤ 7% are recommended to optimize nutritional and pulmonary outcomes while also decreasing mortality [10]. Insulin requirements among individuals with CFRD may vary greatly; whereas some individuals may only require insulin during CF exacerbations others may need daily meal-time injections and/or long-acting basal insulin therapy [2].
Over the past decade we have witnessed tremendous advances in diabetes technologies, including continuous glucose monitors (CGM), insulin pumps, and automated insulin delivery (AID) systems. Among individuals with type 1 diabetes (T1D) and T2D, the use of these technologies has been associated with improvements in glycemic control, lower risk of long-term microvascular complications, and improved psychosocial well-being and treatment satisfaction [12], [13], [14], [15], [16], [17], [18], [19]. While the use of these technologies has expanded rapidly among individuals with T1D and T2D, there are limited studies exploring the use and impact of these technologies among individuals with CF and CFRD. This article aims to review existing evidence about the use of CGM, insulin pumps, and AID for CFRD management and to provide guidance about practical aspects of their use in this unique patient population.
CGM
Components of CGM
CGMs are minimally invasive devices that use a subcutaneous sensor to measure interstitial glucose concentrations every 5–15 min [20]. CGMs consist of three parts- a subcutaneous sensor that detects changes in interstitial glucose, a transmitter which relays the signal from the sensor to a receiver, and a receiver or smart device that displays the glucose value to the user. In addition to providing users with the current sensor glucose value, CGM also provides the user with trend arrows which convey the rate of sensor glucose rise or fall thereby allowing for the prediction of impending hypo- and hyperglycemia. Most CGM systems are worn on the skin for 7–14 days (Freestyle Libre, Medtronic Guardian, Dexcom), however one CGM (Eversense) uses a 90-day sensor implanted in the upper arm. Table 1 provides a summary of the unique features of each CGM system. While many CGM users elect to use this technology on a continual basis, CGM can also be worn for brief periods to support intensive data gathering or to provide additional glycemic insights during specific times (e.g., illness or steroid use).
Table 1.
Comparison of features of the currently available CGM systems.
| Dexcom G6 | FreeStyle Libre 2 | Eversense | Medtronic Guardian 3 | |
|---|---|---|---|---|
| Non-adjunctive Dosing | Approved | Approved | Approved | Not approved |
| Calibration | Not Required, Can perform | Not required, Cannot Perform | Every 12 hours | Every 12 hours |
| Sensor Warm-Up | 2 hours | 1 hour | ∼ 26 hours | Up to 2 hours |
| Sensor Wear Time | 10 days | 14 days (sensor/transmitter in one device) | 90 days | 7 days |
| Transmitter Wear Time | 90 days | 1 year | 1 year | |
| MARD | 9.0% a | 9.2% b | 8.5% c | 8.7%/ 9.1% d |
| Customizable Alarms for Hypo- / Hyperglycemia | Yes | Yes | Yes | Yes |
| Data Display | Dexcom Receiver or Smart Devices | FreeStyle Libre or Smart Devices | Dexcom Receiver or Smart Devices | 770G or Smart Device (Guardian Connect or MiniMed Mobile) |
| Follow App | Dexcom Share | LibreLinkUp | Eversense NOW | Carelink Connect |
| Interfering Substances- False Highs | Acetaminophen (>1 gram every 6 hrs) Hydroxyurea | Vitamin C | Mannitol (IV, local irrigation, peritoneal dialysis) | Acetaminophen (any dose) |
| Interfering Substances- False Lows | — | Aspirin | Tetracycline | — |
| AID Integration | Tandem Control IQ | No | No | Medtronic 770G |
*MARD, Mean Absolute Relative Difference; AID, Automated insulin delivery.
aShah VN, et al. Diabetes Technol Ther. 2018;20(6):428–433.
bFreeStyle Libre 2 User Manual.
cChristiansen MP, et al. Diabetes Technol Ther. 2019;21(5):231–237.
dChristiansen MP, et al. Diabetes Technol Ther. 2017;19(8):446–456.
Many CGM systems are factory calibrated, such that users are not required to calibrate the device with fingerstick blood glucose values, and most have non-adjunctive indications allowing users to make all diabetes treatment decisions based on CGM values alone. Whereas some CGM systems send data to a receiver in real-time (Dexcom, Medtronic), other CGMs referred to as intermittently scanned or flash CGM systems require the user to scan the transmitter with the receiver in order to see the glucose values (Freestyle).
Evidence for CGM use in CFRD
Evidence for CGM use in CFRD is limited; however, small studies have shown strong correlations between CGM and blood glucose values during oral glucose tolerance testing (r = 0.74–0.9) [21]. Although not currently used to diagnose CFRD, CGM-detected dysglycemia among individuals with CF and normal glucose tolerance correlates with early abnormalities in insulin secretion, declines in pulmonary function [22], [23], and weight loss [24]. In a prospective study among individuals with CF but without previously diagnosed CFRD, insulin therapy was initiated in 37 individuals in whom CGM glucose values were above 140 mg/dL for at least 4.5% of the time over at least 3 days of wear [25]. Compared to 22 individuals without CFRD who did not meet CGM criteria for insulin initiation, the insulin-treated group had greater improvements in weight gain and the forced expiratory volume in 1 s at 3 months that were not sustained at 12 months. At present, there are no studies investigating the impact of CGM on glycemic control, pulmonary function, weight, or quality of life in adults with CFRD already on insulin therapy.
CGM glycemic targets
CGM data from all systems are reported in a standardized format referred to the ambulatory glucose profile (AGP). The AGP captures the mean sensor glucose, glucose variability (standard deviation and coefficient of variation), percent CGM wear time, and the percentage of time in range (TIR, 70–180 mg/dL), above range (TAR, > 180 mg/dl), and below range (TBR, < 70 mg/dL). Studies have shown that 70% TIR (16.8 h per day) is correlated with an A1c of 7% and International Consensus Guidelines therefore recommend a target of 70% TIR, < 5% TBR (1.2 h per day), and < 30% TAR (7.2 h) for most individuals with T1D and T2D [26], [27]. Given the association between TIR and A1c and the recommend A1c goal of ≤ 7% for CFRD, the authors of this review recommend the use of similar CGM TIR, TBR, and TAR goals for CFRD. CGM systems also report the glucose management indicator (GMI), which provides an estimate of the A1c when ≥ 10 days of glucose data are available [28], [29]. In T1D and T2D a GMI based on 10–14 days of have been shown to provide a reliable estimate of 2–3 months of CGM data [29], though this has not been validated in CFRD to date. CGM can also support patients in attaining established glycemic targets based on fingerstick glucose monitoring, including fasting glucose values between 80 and 130 mg/dL and 2-hour post-prandial values < 180 mg/dL [9].
The implications of CGM lag time
Unlike fingerstick blood glucose, CGM measures interstitial glucose values. Glucose flows down its concentration gradient between the vascular space and the interstitial fluid. This creates a delay in CGM readings relative to blood glucose values referred to as lag time. When glucose concentrations are not changing rapidly, there is minimal difference between CGM and fingerstick blood glucose levels. However, when blood glucose levels are rising rapidly, the CGM transiently reads falsely low. Failure to recognize the delay in rise in sensor glucose values after treatment of hypoglycemia can lead to over-treatment and rebound hyperglycemia. Conversely, when blood glucose levels are falling rapidly, the CGM transiently reads falsely high. The lag time between sensor and blood glucose levels varies depending on many individual factors, including activity levels and the timing of last oral intake [30]. Studies using intravenous radiolabeled glucose isotopes suggest a lag time of 6–10 min [30]; real-world CGM data, however, has shown lag times of 5–40 min [31], [32], [33].
In addition to providing the user with a glucose value, CGM systems also display trend arrows that indicate the rate of change of sensor glucose. The ability to use CGM trend arrows to predict short term changes in glycemia has led to the development of several guidelines using trend arrows to adjust doses of rapid acting insulin. Because the trend arrows for each CGM system convey different rates of glycemic change (e.g., while a double up arrow conveys a rise of > 3 mg/dL/minute for Dexcom systems, the Freestyle Libre system does not have a double up arrow), guidelines for trend arrow-based insulin dose adjustments are specific to each system. Whereas older guidelines suggested adding or subtracting a specific number of units of rapid acting insulin to the calculated dose according to the user’s correction factor and current sensor glucose [34], [35], [36], newer simplified strategies have suggested adding or subtracting 30, 60, or 90 mg/dL to the current Dexcom sensor glucose value according to the trend arrow and predicted sensor glucose in 30 min [37]. For example, if the Dexcom sensor glucose is 200 mg/dL with a double up arrow (changing at a rate of > 3 mg/dL/minute), the sensor glucose is predicted to be ≥ 90 mg/dL higher in 30 min and so the user should use a glucose of 290 mg/dL when calculating the rapid acting insulin dose. These trend-arrow based adjustments to insulin doses can be applied when ≥ 3 h have passed since the last dose of rapid acting insulin and carbohydrate containing food intake. Table 2 displays rates of glycemic change conveyed by CGM trend arrows and proposed insulin dose adjustments based on 30-minute sensor glucose predictions for CGM systems with non-adjunctive dosing indications.
Table 2.
CGM trend arrows and rates of glycemic change by system along with proposed insulin dose adjustments for systems with non-adjunctive dosing indications when ≥ 3 h have elapsed since the last carbohydrate intake and/or dose of rapid acting insulin.
 |
*Medtronic Guardian does not have a non-adjunctive dosing indication; BG, blood glucose
CGM alarms
CGMs allow patients to utilize vibratory and/or audible alarms to facilitate the detection and treatment of actual and impending hypo- and hyperglycemia. Different thresholds for hypo- and hyperglycemia can be set by users at different times of day, and these alerts repeat at user-specified time intervals if the hypo- or hyperglycemia persists. Users can choose whether to use alerts, with the exception of urgent low alerts (<55 mg/dL) that cannot be silenced on some systems. While predictive alerts have been shown to prevent glycemic excursions among individuals with T1D and T2D [38], the decision to use alerts and the thresholds for both hypo- and hyperglycemia should be carefully considered as alarm fatigue is a common reasons for CGM discontinuation [39]. There are no formal guidelines for suggested thresholds for hypoglycemia and hyperglycemia alerts; some have suggested that CGM alarms not be used during the first several weeks of device use and others have suggested an initial hypoglycemia threshold of 70 mg/dL and hyperglycemia threshold of 250 mg/dL [40]. In patients with CFRD, the authors of this review suggest careful consideration of the patient’s A1c, hypoglycemia awareness, and lifestyle factors impacting tolerance of frequent alarms in the decision-making process.
CGM data sharing
CGM systems allow the user to upload data to a system-specific secure server either through the cloud (for smart device receivers) or by uploading receiver data to a computer. The user can personally review these data and can also share the data with their CFRD care team to facilitate data visualization and medical decision making. Many CGM systems allow users with smartphones to share their CGM data with friends and family members in real-time. While there is some evidence that data sharing may lead to modest improvements in glycemia and health-related quality of life [41], [42], qualitative data suggest that clear boundaries must be set with the follower to avoid feelings of being monitored and judged which have been linked to CGM discontinuation [43], [44], [45].
CGM accuracy and interfering substances
The Mean Absolute Relative Difference (MARD), which reflects the average of the absolute differences between reference blood glucose measurements and CGM glucose values across a wide range of glucose concentrations, is the most commonly accepted assessment of CGM accuracy. MARD values for CGM systems currently in use are all < 10% [46], [47] and provide accuracy that is comparable to or even better than home glucometers [48]. However, patients and providers must be aware of factors impacting CGM accuracy. Compression artifact or compression hypoglycemia occurs when direct external pressure on a CGM sensor leads to decrease perfusion and falsely low sensor glucose values [49], [50], [51]. Removing the external pressure will quickly normalize the sensor glucose values. Users should be mindful of where the sensor is worn so as to avoid false alerts for hypoglycemia, particularly when sleeping.
Medications may interact with CGM sensors leading to spurious hypo- and hyperglycemia (Table 1). Although hypercarbia has not been shown to impact CGM sensor accuracy [52], [53], the effects of hypoxia have not well studied. CGM has been used to titrate insulin delivery in critically ill hospitalized patients [54] and the ability to use CGM as a part of routine hospital care during the COVID-19 pandemic has also provided new insights [55], [56]. Among critically patients with COVID-19 who required insulin therapy the MARD increased by 1.2% for the Dexcom system as compared to 4.1% for the Medtronic Guardian [56], suggesting that hypoxia does not have a significant impact on CGM accuracy.
CGM and diagnostic imaging
Individuals with CF often require diagnostic imaging but all CGM systems warn against exposure to magnetic resonance imaging (MRI), x-ray and computed tomography (CT) scan, and diathermy (high frequency electromagnetic currents that are often used for physical therapy and surgical cautery). This recommendation is particularly problematic for users as CGM sensors are covered in limited supply by insurance companies and cannot be reinserted if removed. In one study, Dexcom G6 CGM sensors and transmitters were exposed to high energy x-rays (80 Gray), comparable to those used in radiation oncology or MRI. All sensors/ transmitters were able to successfully connect with the receiver after radiation or MRI exposure, and there was no significant change in CGM accuracy after radiation or MRI exposure. MRI exposure did not significantly increase the temperature of the sensor/ transmitter or generate sufficient force to dislodge the device, suggesting that sensors may not require removal for MRI or radiation.
Smart pens and pen caps
While insulin pump therapy (see Section 4) can provide dosing flexibility and support for calculating insulin doses, Bluetooth linked smart pen (InPen) and pen cap (Bigfoot Unity) technologies sync to the user’s smart phone and offer CGM/ glucometer integration and support for calculating insulin doses without a continually attached device [57]. Smart pen systems allow the user to enter personalized carbohydrate ratios and correction factors, track active insulin on board, and set reminders for insulin doses. These systems generate reports that combine glycemic data and insulin doses. Sensors in the pen can also determine when the insulin has been exposed to excessive heat or has been in use for too long, alerting the user of potential compromised potency of the insulin.
Insulin pumps
Insulin pumps provide a continuous subcutaneous insulin infusion that more closely mimic physiologic insulin secretion than injection-based therapy. Pumps infuse only rapid acting insulin, replacing basal insulin with basal rates that provide continuous background insulin delivery. Basal rates, carbohydrate ratios, correction factors, and target blood sugars can all be customized by time of day. These programmed settings allow the pump to calculate insulin doses as precise as a tenth of a unit.
There are two general classes of insulin pumps- tubed systems and patch or tubeless pumps (Fig. 1). Tubed pumps consist of a pump which includes an interactive display screen used to enter data and a cartridge that holds insulin, tubing that connects the pump to the user, and a subcutaneous cannula that infuses insulin. Infusion sites are worn for up to 3 days at a time before being changed by the user. The tubing gives the user flexibility in where they were the pump on their body (e.g., clipping to a belt or undergarment). By contrast, tubeless pumps remove the hassle of tubing but have a larger on-body presence. The tubeless pumps combine the insulin cartridge and infusion site into a single device that is attached to the body for up to 3 days at a time. Insulin delivery with tubeless pumps is operated by a separate controller.
Fig. 1.
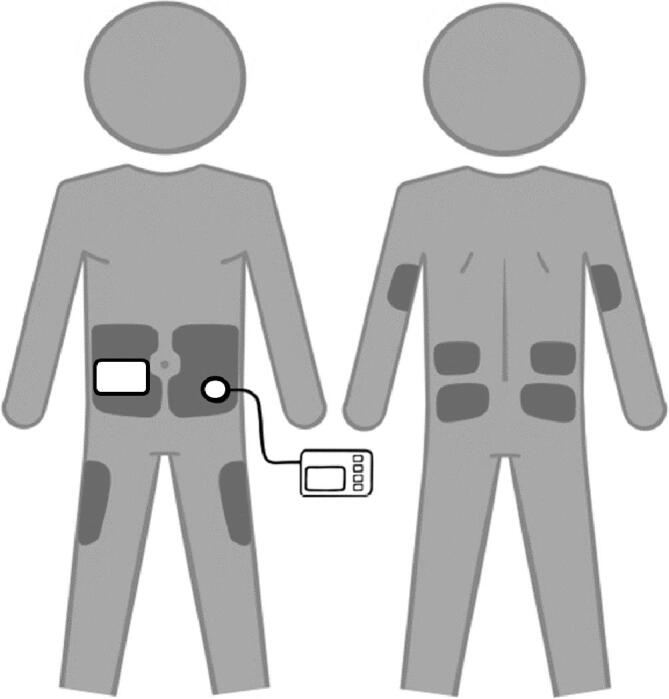
Tubed versus tubeless (or patch) insulin pumps. Shaded areas depict where the pump sites can be worn.
Evidence for insulin pump use in CFRD
While there is an abundance of evidence supporting the clinical and psychosocial benefits of insulin pump use in T1D [12], [17], evidence in CFRD is limited. In a 2009 study, Hardin and colleagues reported on 9 adults with CFRD treated with insulin pump therapy for 6 months [58]. Lean body mass increased by an average of 2.4 kg and overall weight increased by 3.6 kg. With insulin pump therapy, users were able to attain fasting and 2-hour post-prandial glycemic targets, and there was a trend noted in A1c improvement from 8.2 ± 1.9% to 7.1 ± 1.5% (p = 0.05).
Rationale for insulin pump use in CFRD
To optimize lung function and survival, individuals with CF are recommended to target a BMI ≥ 50th percentile for children or a BMI ≥ 22 kg/m2 for men and ≥ 23 kg/m2 for women [59]. The guidelines recommend 110–200% of the energy intake recommended for healthy individuals of similar age, sex, and size, particularly those not being treated with highly effective CFTR modulator therapy. Efforts to optimize caloric intake have historically relied upon high glycemic index foods, carbohydrate-containing foods that are more rapidly absorbed thereby causing more significant postprandial glycemic excursions [60].
>85% of individuals with CF require pancreatic enzyme replacement therapy to treat pancreatic insufficiency [2], [61]. Even with pancreatic enzyme replacement therapy, fat digestion often remains abnormal leading to more rapid gastric emptying and more significant post-prandial hyperglycemia [62]. Further complicating CFRD management, gastroparesis has been estimated to occur in approximately one third of those with CF [63]. As compared to multiple daily injection therapy, insulin pump therapy allows for greater insulin dosing flexibility and customization. Meal time insulin boluses can be delivered over extended periods of time by using features known as combination or extended boluses, wherein a portion of the insulin is delivered immediately, and a portion is delivered over an extended period of time. These combination boluses have been shown to improve glycemic control in T1D but have not specifically been studied in CFRD [64]. In patients eating frequent meals, pump therapy also allows for easier, more convenient insulin delivery as compared to the need for multiple injections.
In addition to variations in intestinal transit, CF is also associated with varying degrees of insulin resistance related to chronic inflammation, cyclic infections, and glucocorticoid therapy [8]. Temporary basal rates allow insulin pump users to increase or decrease basal insulin delivery by a percentage of the baseline doses for a specified time interval. Unlike adjustments to injected basal insulin doses, which typically have effects for 24 hours, temporary basal rates can be used over shorter periods of time. The ability to customize basal rates by time of day also allows for lower overnight basal insulin rates, which are commonly required given the physiology of CFRD. The use of these advanced insulin pump features may allow patients with CFRD to better customize insulin delivery in the setting of unpredictable gastric emptying, post-prandial excursions, and varying degrees of insulin resistance. Extended/ combination boluses and/ or higher temporary basal rates may be particularly useful in patients with CFRD who require tube feeds.
Sensor augmented pumps and automated insulin delivery
Sensor augmented pumps (SAP) display CGM data to facilitate dosing calculations, but do not automatically adjust insulin delivery. By contrast, low glucose suspend and predictive low glucose suspend (PLGS) systems can decrease or stop insulin delivery. Whereas PLGS systems attempt to prevent hypoglycemia by automatically suspending insulin delivery when the glucose is predicted to drop below 70 mg/dL, low glycose suspend systems can only suspend insulin delivery after hypoglycemia occurs. There have not been any interventional trials exploring the benefit of low glucose suspend or PLGS systems in CFRD. A case report exploring sensor augmented pump therapy showed improvements in glycemic control while simultaneously decreasing hypoglycemia and improving patient-reported quality of life [65].
Automated insulin delivery systems (AID), also known as hybrid closed loop (HCL) systems, use CGM glucose values to automatically increase or decrease insulin delivery to reduce glycemic excursions. AID systems increase basal insulin delivery or deliver automated correction boluses to treat or prevent hyperglycemia and can also attenuate or stop basal insulin delivery to prevent or treat hypoglycemia. Features of the currently FDA-approved AID systems (Tandem Control IQ and Medtronic 770G) are summarized in Table 3. Each AID system allows users to adjust different pump settings and alters insulin delivery using a different algorithm which considers different parameters of either user-entered (e.g., correction factor) or system-calculated values (e.g., average total daily insulin dose). AID algorithms have been shown to improve glycemic control, decrease hypoglycemia and burden of care, and improve quality of life and sleep in people with T1D [66], [67], [68], [69]. Although these AID systems have been designed for and shown to be effective in T1D, they are yet to be studied in CFRD. In a pilot study investigating a closed-loop AID device in three patients with CFRD, Sherwood and colleagues showed non-significant improvements in mean sensor glucose along with patient reported improvements in treatment satisfaction and decreased treatment burden [70]. Further studies with this device in children and adults with CFRD are currently underway.
Table 3.
Features used to calculate insulin delivery and features that the user can and cannot adjust in the currently available automated insulin delivery (AID) systems. Adapted from Messer, at al [73].
| Tandem Control IQ | Medtronic 770G | |
|---|---|---|
| CALCULATE- How does the system calculate insulin delivery? | ||
| Basal Automation | User programmed basal rates are automatically increased or decreased | System calculated basal rates based on total daily insulin dose from past 2-6 days |
| Bolus Automation | If glucose predicted to be > 180 mg/dL, 60% of the calculated dose is delivered as an hourly automated bolus | No |
| Target Glucose | 112.5-160 mg/dL | 120 mg/dL |
| ADJUST- What parameters can the user adjust? | ||
| Basal Rate | Yes | No |
| Carb Ratios | Yes | Yes |
| Correction Factor | Yes | No |
| Target Glucose | No, fixed at 110 mg/dL | No, Fixed at 120 mg/dL |
| Active Insulin Time | No, fixed at 5 hours | Yes, from 2 to 8 hours |
| Edit Recommended Bolus Doses | Yes | No |
| Combination Boluses | Yes, up to 2 hours | No |
| Unique Features | Exercise Mode- target BG 140-160 mg/dL Sleep Mode- target BG 112.5-120 mg/dL, no automated boluses |
Temp Target- Changes target glucose to 150 mg/dL |
| REVERT- When does the system stop automated insulin delivery? | ||
| When does the system revert to manual mode? | Loss of CGM data > 20 minutes | Max insulin delivery > 4hrs Minimum insulin delivery > 2.5 hours Sensor glucose > 250 mg/dL for 3hrs Sensor glucose > 300 mg/dL for 1hr |
| EDUCATE- What are the key educational points for this system? | ||
| Unique considerations specific to the system | Use Exercise mode for activity (target 140-160 mg/dL) Set sleep schedule for each night (target 112.5-120 mg/dL, no auto-corrections) |
Use temp target for activity (150 mg/dL) |
| SENSOR/ SHARE- What are unique characteristics of the CGM used in the system? | ||
| Calibration needed | No | Yes, at least every 12 hours |
| Sensor wear time | 10 days | 7 days |
| Data sharing with followers | Yes- Dexcom G6 follow app (CGM data only) |
Yes- Carelink Connect app (CGM and pump data) |
By definition, individuals with T1D have complete or near complete insulin deficiency. However, the spectrum of insulin deficiency varies widely among individuals with CFRD. Because all AID systems in clinical use have been developed specifically for T1D, it is important to recognize the differences in the pathophysiology between T1D and CFRD when considering AID algorithm function. If a person neglects to bolus for a meal or fails to administer insulin in a timely manner before eating, the CGM sensor glucose value will increase, and the AID algorithm will increase basal insulin delivery and/or deliver an automated correction bolus. This is less problematic for individuals with T1D given the complete insulin deficiency, however in individuals with CFRD the body’s own endogenous insulin secretion paired with increased AID insulin delivery may lead to reactive post-prandial hypoglycemia [71]. Reactive hypoglycemia is commonly observed among individuals with CFRD and is thought to result from delayed first phase insulin secretion and late compensatory second phase insulin secretion [72]. Fig. 2 provides an example of post-prandial hypoglycemia related to a delayed meal bolus in a person with CFRD, illustrating the importance of timely carbohydrate boluses. Similarly, due to residual endogenous insulin secretion among individuals with CFRD, the authors will often start with a less aggressive correction factor upon AID initiation when using AID systems that deliver automated correction boluses. Lower basal rates in the overnight hours may also be required for CFRD patients with significant endogenous insulin secretion.
Fig. 2.
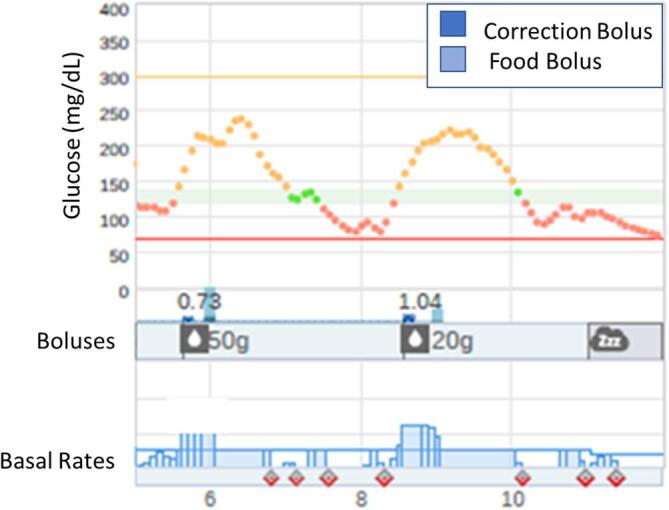
CGM tracing from an automated insulin delivery system in a patient with CFRD which captures post-prandial hypoglycemia resulting from late meal boluses. The CGM tracing captures a rise in sensor glucose beginning before the mealtime bolus for 50 g of carbohydrate at 6 pm and 20 g at 8 pm (light blue bars) were administered. This results in rapid rise in glucose to a peak of nearly 250 mg/dL (orange tracing on top), triggering an auto-bolus (dark blue) and an increase in basal insulin delivery (blue bars on the bottom), leading to reactive hypoglycemia (red tracing around 8 pm and 10 pm). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
With ongoing innovations and developments in AID, clinicians must understand which pump settings to adjust when glycemia is not optimal. Whereas the Tandem Control IQ AID system requires user programmed basal rates and correction factors, the Medtronic 770G system, when used in auto mode, determines basal rates and correction factors. Adjusting basal rates in the Medtronic 770G system will not have any impact on insulin delivery when in auto mode whereas adjusting basal rates on the Control IQ system will alter insulin delivery. With many systems already on the market and more in development, use of the CARES paradigm may help clinicians to optimally utilize each AID system (Table 3). The CARES paradigm emphasizes how each system Calculates insulin delivery, which parameters the user can Adjust, when users should Revert to open loop or manual function, critical Educational points unique to the system, and unique aspects of the CGM Sensor and data Sharing [73].
To date, systems in commercial use employ insulin only therapy, however dual hormone AID systems are being explored. Systems in development have incorporated glucagon, which raises glucose values by stimulating glycogenolysis and gluconeogenesis, and pramlintide, which slows gastric emptying allowing for better control of post-prandial hyperglycemia [74]. The use of ultra-rapid acting insulins, which add excipients to rapid acting insulins to further accelerate absorption and decrease time to onset of action, may further enhance the utility of single and dual hormone AID systems [75], particularly in CFRD given the risk for reactive hypoglycemia after post-prandial hyperglycemia.
Patient Perceptions of diabetes technology use
In a 2015 study using the German/ Austrian national diabetes patient registry, only 4.1% of individuals with CFRD reported using insulin pump therapy, and 30% discontinued use [76]. In a more recent 2021 survey of patients with CFRD in the United States, 75% of youth and adults with CFRD reported CGM use while 29% reported insulin pump use [77]. Respondents perceived significant benefits to CGM use, but greater burdens to insulin pump use. Device discontinuation rates were high; 19% of CGM users and 28% of insulin pump discontinued device use, most commonly due to concerns about embarrassment related to wearing the device, cost, increased worry about glycemia, and pain related to device use.
In an era of rapidly advancing CGM and AID technologies that hold the potential to improve glycemic control and decrease the burden of CFRD, strategies to promote device uptake and sustained use are needed. Given the perceived burdens and costs related to device use, additional studies showing clinical and psychosocial benefits to diabetes technology use may improve health insurance coverage and help to mitigate the out-of-pocket costs. Expanded CFRD-specific patient education to avoid excessive worry about glycemia and pain related to device use, which is uncommon when used properly, may also promote device use.
Conclusion
Although there is significant evidence to support the beneficial effect of diabetes technology among individuals with T1D and T2D, there are limited data exploring the impact of device use on glycemic control and health-related quality of life among individuals with CFRD who have unique diabetes physiology and lived healthcare experiences. Understanding the perceived benefits and burdens of device use and developing effective strategies to address these concerns with careful consideration of the different aspects of CFRD are needed to better support the uptake and sustained use of CGM, insulin pumps, and AID in this patient population. Future studies are needed not only to expand our knowledge of best practices in the treatment of CFRD but also to provide evidence supporting the clinical benefit of these technologies, which could be used to support improved health insurance coverage and access to these technologies for the CFRD community.
Funding Sources
This work was supported by the Cystic Fibrosis Foundation: EnVision-II CF: Emerging Leaders in CF Endocrinology [grant numbers MARKS19GEO, WILLIA19GE0, SHERWO19G0, MORAN19GE3].
CRediT authorship contribution statement
Brynn E. Marks: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. Kristen M. Williams: Conceptualization, Writing – review & editing. Jordan S. Sherwood: Conceptualization, Writing – review & editing. Melissa S. Putman: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
BEM has received investigator initiated research funding from Dexcom, Inc and Tandem Diabetes Care. MSP has received research funding from a Vertex Investigator Initiated Studies Grant. BEM, KW, JSS, and MSP are supported by the Cystic Fibrosis Foundation: EnVision-II CF: Emerging Leaders in CF Endocrinology.
Acknowledgments
Acknowledgements:
The authors would like to thank the Cystic Fibrosis Foundation for their support of the EnVision-II CF: Emerging Leaders in CF Endocrinology program, along with Kat and Mike Porco of Attain Health for the opportunity to present this information at the Cystic Fibrosis Endocrinology Summit held in Whitefish, Montana in July 2021.
Submission Declaration and Verification:
This work has not been published previously, is not under consideration for publication elsewhere, and the publication is approved by all authors. If accepted for publication, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
Contributor Information
Brynn E. Marks, Email: bmarks@childrensnational.org.
Kristen M. Williams, Email: kmw2160@cumc.columbia.edu.
Jordan S. Sherwood, Email: jssherwood@partners.org.
Melissa S. Putman, Email: msputman@partners.org.
References:
- 1.Ratjen F., Döring G. Cystic fibrosis. The Lancet. 2003;361(9358):681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 2.Foundation CF. Cystic Fibrosis Foundation Patient Registry 2019 Annual Data Report. Bethesda, Maryland; 2020.
- 3.Iannucci A., Mukai K., Johnson D., Burke B. Endocrine pancreas in cystic fibrosis: an immunohistochemical study. Hum Pathol. 1984;15(3):278–284. doi: 10.1016/s0046-8177(84)80191-4. [DOI] [PubMed] [Google Scholar]
- 4.Holl R.W., Wolf A., Thon A., Bernhard M., Buck C., Missel M., et al. Insulin Resistance with Altered Secretory Kinetics and Reduced Proinsulin in Cystic Fibrosis Patients. J Pediatr Gastroenterol Nutr. 1997;25(2):188–193. doi: 10.1097/00005176-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Moran A., Diem P., Klein D.J., Levitt M.D., Robertson R.P. Pancreatic endocrine function in cystic fibrosis. J Pediatr. 1991;118(5):715–723. doi: 10.1016/s0022-3476(05)80032-0. [DOI] [PubMed] [Google Scholar]
- 6.Blackman SM, Commander CW, Watson C, Arcara KM, Strug LJ, Stonebraker JR, et al. Genetic modifiers of cystic fibrosis-related diabetes. Diabetes. 2013;62(10):3627-35. [DOI] [PMC free article] [PubMed]
- 7.Aksit MA, Pace RG, Vecchio-Pagan B, Ling H, Rommens JM, Boelle PY, et al. Genetic Modifiers of Cystic Fibrosis-Related Diabetes Have Extensive Overlap With Type 2 Diabetes and Related Traits. J Clin Endocrinol Metab. 2020;105(5). [DOI] [PMC free article] [PubMed]
- 8.Sc N.N.M., Shoseyov D., Kerem E., Zangen D.H. Patients with cystic fibrosis and normoglycemia exhibit diabetic glucose tolerance during pulmonary exacerbation. J Cyst Fibros. 2010;9(3):199–204. doi: 10.1016/j.jcf.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Moran A., Pillay K., Becker D., Granados A., Hameed S., Acerini C.L. ISPAD Clinical Practice Consensus Guidelines 2018: Management of cystic fibrosis-related diabetes in children and adolescents. Pediatric Diabetes. 2018;19:64–74. doi: 10.1111/pedi.12732. [DOI] [PubMed] [Google Scholar]
- 10.Moran A., Pekow P., Grover P., Zorn M., Slovis B., Pilewski J., et al. Insulin therapy to improve BMI in cystic fibrosis-related diabetes without fasting hyperglycemia: Results of the cystic fibrosis related diabetes therapy trial. Diabetes Care. 2009;32(10):1783–1788. doi: 10.2337/dc09-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran A., Brunzell C., Cohen R.C., Katz M., Marshall B.C., Onady G., et al. Clinical care guidelines for cystic fibrosis-related diabetes. Diabetes Care. 2010;33(12):2697–2708. doi: 10.2337/dc10-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster N.C., Beck R.W., Miller K.M., Clements M.A., Rickels M.R., DiMeglio L.A., et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66–72. doi: 10.1089/dia.2018.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prahalad P., Tanenbaum M., Hood K., Maahs D.M. Diabetes technology: Improving care, improving patient-reported outcomes and preventing complications in young people with type 1 diabetes. Diabet Med. 2018;35(4):419–429. doi: 10.1111/dme.13588. [DOI] [PubMed] [Google Scholar]
- 14.Laffel L.M., Kanapka L.G., Beck R.W., Bergamo K., Clements M.A., Criego A., et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adolescents and Young Adults with Type 1 Diabetes: A Randomized Clinical Trial. JAMA - Journal of the American Medical Association. 2020;323(23):2388. doi: 10.1001/jama.2020.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zabeen B., Craig M.E., Virk S.A., Pryke A., Chan A.K.F., Cho Y.H., et al. Insulin pump therapy is associated with lower rates of retinopathy and peripheral nerve abnormality. PLoS ONE. 2016;11(4):e0153033. doi: 10.1371/journal.pone.0153033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller‐Godeffroy E., Vonthein R., Ludwig‐Seibold C., Heidtmann B., Boettcher C., Kramer M., et al. Psychosocial benefits of insulin pump therapy in children with diabetes type 1 and their families: The pumpkin multicenter randomized controlled trial. Pediatric Diabetes. 2018;19(8):1471–1480. doi: 10.1111/pedi.12777. [DOI] [PubMed] [Google Scholar]
- 17.Karges B., Schwandt A., Heidtmann B., Kordonouri O., Binder E., Schierloh U., et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318(14):1358. doi: 10.1001/jama.2017.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burckhardt M.-A., Abraham M.B., Mountain J., Coenen D., Paniora J., Clapin H., et al. Improvement in psychosocial outcomes in children with type 1 diabetes and their parents following subsidy for continuous glucose monitoring. Diabetes Technol Ther. 2019;21(10):575–580. doi: 10.1089/dia.2019.0149. [DOI] [PubMed] [Google Scholar]
- 19.Polonsky W.H., Hessler D., Ruedy K.J., Beck R.W. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: Further findings from the DIAMOND randomized clinical trial. Diabetes Care. 2017;40(6):736–741. doi: 10.2337/dc17-0133. [DOI] [PubMed] [Google Scholar]
- 20.Marks B.E., Wolfsdorf J.I. Monitoring of Pediatric Type 1 Diabetes. Front Endocrinol. 2020;11(March):1–16. doi: 10.3389/fendo.2020.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Riordan S.M.P., Hindmarsh P., Hill N.R., Matthews D.R., George S., Greally P., et al. Validation of continuous glucose monitoring in children and adolescents with cystic fibrosis: a prospective cohort study. Diabetes Care. 2009;32(6):1020–1022. doi: 10.2337/dc08-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan C.L., Vigers T., Pyle L., Zeitler P.S., Sagel S.D., Nadeau K.J. Continuous glucose monitoring abnormalities in cystic fibrosis youth correlate with pulmonary function decline. J Cyst Fibros. 2018;17(6):783–790. doi: 10.1016/j.jcf.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leclercq A., Gauthier B., Rosner V., Weiss L., Moreau F., Constantinescu A.A., et al. Early assessment of glucose abnormalities during continuous glucose monitoring associated with lung function impairment in cystic fibrosis patients. J Cyst Fibros. 2014;13(4):478–484. doi: 10.1016/j.jcf.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Hameed S., Morton J.R., Jaffe A., Field P.I., Belessis Y., Yoong T., et al. Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care. 2010;33(2):221–226. doi: 10.2337/dc09-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frost F., Dyce P., Nazareth D., Malone V., Walshaw M.J. Continuous glucose monitoring guided insulin therapy is associated with improved clinical outcomes in cystic fibrosis-related diabetes. J Cyst Fibros. 2018;17(6):798–803. doi: 10.1016/j.jcf.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Beck R.W., Bergenstal R.M., Cheng P., Kollman C., Carlson A.L., Johnson M.L., et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614–626. doi: 10.1177/1932296818822496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Battelino T., Danne T., Bergenstal R.M., Amiel S.A., Beck R., Biester T., et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergenstal R.M., Beck R.W., Close K.L., Grunberger G., Sacks D.B., Kowalski A., et al. Glucose management indicator (GMI): A new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275–2280. doi: 10.2337/dc18-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riddlesworth T.D., Beck R.W., Gal R.L., Connor C.G., Bergenstal R.M., Lee S., et al. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther. 2018;20(4):314–316. doi: 10.1089/dia.2017.0455. [DOI] [PubMed] [Google Scholar]
- 30.Basu A., Dube S., Veettil S., Slama M., Kudva Y.C., Peyser T., et al. Time lag of glucose from intravascular to interstitial compartment in type 1 Diabetes. J Diabetes Sci Technol. 2015;9(1):63–68. doi: 10.1177/1932296814554797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaharieva D.P., Turksoy K., McGaugh S.M., Pooni R., Vienneau T., Ly T., et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21(6):313–321. doi: 10.1089/dia.2018.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinha M., McKeon K.M., Parker S., Goergen L.G., Zheng H., El-Khatib F.H., et al. A comparison of time delay in three continuous glucose monitors for adolescents and adults. J Diabetes Sci Technol. 2017;11(6):1132–1137. doi: 10.1177/1932296817704443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keenan D.B., Mastrototaro J.J., Voskanyan G., Steil G.M. Mastrototaro Ph.D Jj, Voskanyan Ph.D G, Steil Ph.D GM. Delays in minimally invasive continuous glucose monitoring devices : A review of current technology. J Diabetes Sci Technol. 2009;3(5):1207–1214. doi: 10.1177/193229680900300528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laffel LM, Aleppo G, Buckingham BA, Forlenza GP, Rasbach LE, Tsalikian E, et al. A practical approach to using trend arrows on the dexcom G5 CGM system to manage children and adolescents with diabetes. Journal of the Endocrine Society. 2017;1(12):1461-76. [DOI] [PMC free article] [PubMed]
- 35.Aleppo G, Laffel LM, Ahmann AJ, Hirsch IB, Kruger DF, Peters A, et al. A practical approach to using trend arrows on the dexcom G5 CGM system for the management of adults with diabetes. Journal of the Endocrine Society. 2017;1(12):1445-60. [DOI] [PMC free article] [PubMed]
- 36.Kudva YC, Ahmann AJ, Bergenstal RM, Gavin JR, Kruger DF, Midyett LK, et al. Approach to using trend arrows in the FreeStyle Libre flash glucose monitoring systems in adults. Journal of the Endocrine Society. 2018;2(12):1320-37. [DOI] [PMC free article] [PubMed]
- 37.Corathers S.D., DeSalvo D.J. Therapeutic Inertia in Pediatric Diabetes: Challenges to and Strategies for Overcoming Acceptance of the Status Quo. Diabetes Spectr. 2020;33(1):22–30. doi: 10.2337/ds19-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abraham S.B., Arunachalam S., Zhong A., Agrawal P., Cohen O., McMahon C.M. Improved Real-World Glycemic Control With Continuous Glucose Monitoring System Predictive Alerts. J Diabetes Sci Technol. 2021;15(1):91–97. doi: 10.1177/1932296819859334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastrototaro J., Welsh J.B., Lee S. Practical considerations in the use of real-time continuous glucose monitoring alerts. J Diabetes Sci Technol. 2010;4(3):733–739. doi: 10.1177/193229681000400329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirsch I.B. Clinical review: Realistic expectations and practical use of continuous glucose monitoring for the endocrinologist. J Clin Endocrinol Metab. 2009;94(7):2232–2238. doi: 10.1210/jc.2008-2625. [DOI] [PubMed] [Google Scholar]
- 41.Welsh J.B., Derdzinski M., Parker A.S., Puhr S., Jimenez A., Walker T. Real-time sharing and following of continuous glucose monitoring data in youth. Diabetes Therapy. 2019;10(2):751–755. doi: 10.1007/s13300-019-0571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polonsky W.H., Fortmann A.L. Impact of Real-Time Continuous Glucose Monitoring Data Sharing on Quality of Life and Health Outcomes in Adults with Type 1 Diabetes. Diabetes Technol Ther. 2021;23(3):195–202. doi: 10.1089/dia.2020.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Litchman M.L., Allen N.A., Colicchio V.D., Wawrzynski S.E., Sparling K.M., Hendricks K.L., et al. A Qualitative Analysis of Real-Time Continuous Glucose Monitoring Data Sharing with Care Partners: To Share or Not to Share? Diabetes Technol Ther. 2018;20(1):25–31. doi: 10.1089/dia.2017.0285. [DOI] [PubMed] [Google Scholar]
- 44.Messer L.H., Johnson R., Driscoll K.A., Jones J. Best friend or spy: a qualitative meta-synthesis on the impact of continuous glucose monitoring on life with Type 1 diabetes. Diabet Med. 2018;35(4):409–418. doi: 10.1111/dme.13568. [DOI] [PubMed] [Google Scholar]
- 45.Tanenbaum M.L., Hanes S.J., Miller K.M., Naranjo D., Bensen R., Hood K.K. Diabetes device use in adults with type 1 diabetes: Barriers to uptake and potential intervention targets. Diabetes Care. 2017;40(2):181–187. doi: 10.2337/dc16-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah V.N., Laffel L.M., Wadwa R.P., Garg S.K. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6):428–433. doi: 10.1089/dia.2018.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christiansen M.P., Garg S.K., Brazg R., Bode B.W., Bailey T.S., Slover R.H., et al. Accuracy of a fourth-generation subcutaneous continuous glucose sensor. Diabetes Technol Ther. 2017;19(8):446–456. doi: 10.1089/dia.2017.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ekhlaspour L., Mondesir D., Lautsch N., Balliro C., Hillard M., Magyar K., et al. Comparative accuracy of 17 point-of-care glucose meters. J Diabetes Sci Technol. 2017;11(3):558–566. doi: 10.1177/1932296816672237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forlenza G.P., Argento N.B., Laffel L.M. Practical considerations on the use of continuous glucose monitoring in pediatrics and older adults and nonadjunctive use. Diabetes Technol Ther. 2017;19(S3):S-13–S-20. doi: 10.1089/dia.2017.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mensh B.D., Wisniewski N.A., Neil B.M., Burnett D.R. Susceptibility of interstitial continuous glucose monitor performance. J Diabetes Sci Technol. 2013;7(4):863–870. doi: 10.1177/193229681300700408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helton K.L., Ratner B.D., Wisniewski N.A. Biomechanics of the sensor-tissue interface - Effects of motion, pressure, and design on sensor performance and foreign body response - Part II: Examples and application. J Diabetes Sci Technol. 2011;5(3):647–656. doi: 10.1177/193229681100500318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adolfsson P., Örnhagen H., Jendle J. Accuracy and reliability of continuous glucose monitoring in individuals with type 1 diabetes during recreational diving. Diabetes Technol Ther. 2009;11(8):493–497. doi: 10.1089/dia.2009.0017. [DOI] [PubMed] [Google Scholar]
- 53.Adolfsson P., Örnhagen H., Eriksson B.M., Gautham R., Jendle J. In-vitro performance of the enlite sensor in various glucose concentrations during hypobaric and hyperbaric conditions. J Diabetes Sci Technol. 2012;6(6):1375–1382. doi: 10.1177/193229681200600617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agus M.S.D., Wypij D., Hirshberg E.L., Srinivasan V., Faustino E.V., Luckett P.M., et al. Tight glycemic control in critically ill children. N Engl J Med. 2017;376(8):729–741. doi: 10.1056/NEJMoa1612348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nair B.G., Dellinger E.P., Flum D.R., Rooke G.A., Hirsch I.B. A Pilot Study of the Feasibility and Accuracy of Inpatient Continuous Glucose Monitoring. Diabetes Care. 2020;43(11):e168–e169. doi: 10.2337/dc20-0670. [DOI] [PubMed] [Google Scholar]
- 56.Sadhu A.R., Serrano I.A., Xu J., Nisar T., Lucier J., Pandya A.R., et al. Continuous Glucose Monitoring in Critically Ill Patients With COVID-19: Results of an Emergent Pilot Study. J Diabetes Sci Technol. 2020;14(6):1065–1073. doi: 10.1177/1932296820964264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heinemann L, Schnell O, Gehr B, Schloot NC, Gorgens SW, Gorgen C. Digital Diabetes Management: A Literature Review of Smart Insulin Pens. J Diabetes Sci Technol. 2021:1932296820983863. [DOI] [PMC free article] [PubMed]
- 58.Hardin D.S., Rice J., Rice M., Rosenblatt R. Use of the insulin pump in treat cystic fibrosis related diabetes. J Cyst Fibros. 2009;8(3):174–178. doi: 10.1016/j.jcf.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Stallings V.A., Stark L.J., Robinson K.A., Feranchak A.P., Quinton H., Clinical Practice Guidelines on G., et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–839. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 60.Jenkins D, Wolever T, Taylor R, Barker H, Fielden H, Baldwin J, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. American Journal of Clinical Nutrition. 1981;Mar;34(3):362-6. [DOI] [PubMed]
- 61.Haupt M.E., Kwasny M.J., Schechter M.S., McColley S.A. Pancreatic enzyme replacement therapy dosing and nutritional outcomes in children with cystic fibrosis. J Pediatr. 2014;164(5):1110–1115.e1. doi: 10.1016/j.jpeds.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 62.Perano SJ, Couper JJ, Horowitz M, Martin AJ, Kritas S, Sullivan T, et al. Pancreatic enzyme supplementation improves the incretin hormone response and attenuates postprandial glycemia in adolescents with cystic fibrosis: a randomized crossover trial. J Clin Endocrinol Metab. 2014;99(7):2486-93. [DOI] [PubMed]
- 63.Corral J.E., Dye C.W., Mascarenhas M.R., Barkin J.S., Salathe M., Moshiree B. Is Gastroparesis Found More Frequently in Patients with Cystic Fibrosis? A Systematic Review. Scientifica (Cairo). 2016;2016:1–11. doi: 10.1155/2016/2918139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopez P.E., Smart C.E., McElduff P., Foskett D.C., Price D.A., Paterson M.A., et al. Optimizing the combination insulin bolus split for a high-fat, high-protein meal in children and adolescents using insulin pump therapy. Diabet Med. 2017;34(10):1380–1384. doi: 10.1111/dme.13392. [DOI] [PubMed] [Google Scholar]
- 65.Klupa T., Małecki M., Katra B., Cyganek K., Skupień J., Kostyk E., et al. Use of sensor-augmented insulin pump in patient with diabetes and cystic fibrosis: Evidence for improvement in metabolic control. Diabetes Technol Ther. 2008;10(1):46–49. doi: 10.1089/dia.2007.0238. [DOI] [PubMed] [Google Scholar]
- 66.Brown S.A., Kovatchev B.P., Raghinaru D., Lum J.W., Buckingham B.A., Kudva Y.C., et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707–1717. doi: 10.1056/NEJMoa1907863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown S.A., Forlenza G.P., Bode B.W., Pinsker J.E., Levy C.J., Criego A.B., et al. Multicenter Trial of a Tubeless, On-Body Automated Insulin Delivery System With Customizable Glycemic Targets in Pediatric and Adult Participants With Type 1 Diabetes. Diabetes Care. 2021 doi: 10.2337/dc21-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pinsker J.E., Müller L., Constantin A., Leas S., Manning M., McElwee Malloy M., et al. Real-World Patient-Reported Outcomes and Glycemic Results with Initiation of Control-IQ Technology. Diabetes Technol Ther. 2021;23(2):120–127. doi: 10.1089/dia.2020.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farrington C. Psychosocial impacts of hybrid closed-loop systems in the management of diabetes: a review. Diabet Med. 2018;35(4):436–449. doi: 10.1111/dme.13567. [DOI] [PubMed] [Google Scholar]
- 70.Sherwood J.S., Jafri R.Z., Balliro C.A., Zheng H., El-Khatib F.H., Damiano E.R., et al. Automated glycemic control with the bionic pancreas in cystic fibrosis-related diabetes: A pilot study. J Cyst Fibros. 2020;19(1):159–161. doi: 10.1016/j.jcf.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kilberg MJ, Harris C, Sheikh S, Stefanovski D, Cuchel M, Kubrak C, et al. Hypoglycemia and Islet Dysfunction Following Oral Glucose Tolerance Testing in Pancreatic-Insufficient Cystic Fibrosis. J Clin Endocrinol Metab. 2020;105(10). [DOI] [PMC free article] [PubMed]
- 72.Kilberg M.J., Sheikh S., Stefanovski D., Kubrak C., De Leon D.D., Hadjiliadis D., et al. Dysregulated insulin in pancreatic insufficient cystic fibrosis with post-prandial hypoglycemia. J Cyst Fibros. 2020;19(2):310–315. doi: 10.1016/j.jcf.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Messer L.H., Berget C., Forlenza G.P. A clinical guide to advanced diabetes devices and closed-loop systems using the CARES paradigm. Diabetes Technol Ther. 2019;21(8):462–469. doi: 10.1089/dia.2019.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boughton C.K., Hovorka R. New closed-loop insulin systems. Diabetologia. 2021;64(5):1007–1015. doi: 10.1007/s00125-021-05391-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Evans M., Ceriello A., Danne T., De Block C., DeVries J.H., Lind M., et al. Use of fast-acting insulin aspart in insulin pump therapy in clinical practice. Diabetes Obes Metab. 2019;21(9):2039–2047. doi: 10.1111/dom.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scheuing N., Badenhoop K., Borkenstein M., Konrad K., Lilienthal E., Laubner K., et al. Why is insulin pump treatment rarely used in adolescents and young adults with cystic fibrosis-related diabetes? Pediatric Diabetes. 2015;16(1):10–15. doi: 10.1111/pedi.12158. [DOI] [PubMed] [Google Scholar]
- 77.Marks BE, Kilberg M, Aliaj E, Fredkin K, Hudson J, Riva D, et al. Perceptions of Diabetes Technology Use in Cystic Fibrosis Related Diabetes Management. Diabetes Technol Ther. 2021;Online ahead of print. [DOI] [PubMed]


