Abstract
Purpose: Oncofertility care at cancer diagnosis remains underimplemented across oncology and fertility care settings, with limited tools to scale up effective implementation strategies. Using implementation science theory, we systematically assessed factors that influence oncofertility care implementation and mapped scalable strategies, particularly electronic health record (EHR)-enabled ones, that fit adult and pediatric oncology care contexts.
Methods: Using purposeful sampling, we recruited health care providers and female, reproductive-aged survivors of adolescent and young adult (AYA) cancers (AYA survivors) from a comprehensive cancer center and a freestanding children's hospital to semistructured interviews and focus groups. Using thematic analysis combining inductive codes with deductive codes using the Consolidated Framework for Implementation Research (CFIR), we characterized barriers and facilitators to care and designed responsive strategies. Two coders independently coded each transcript.
Results: We recruited 19 oncology and fertility providers and 9 cancer survivors. We identified barriers and facilitators to oncofertility care in the CFIR domains of individual, inner setting, outer setting, and process, allowing us to conceptualize oncofertility care to encompass three core components (screening, referral, and fertility preservation counseling) and map five strategies to these components that fit an adult and a children's context and bridge oncology and fertility practices. The strategies were screening using a best practice advisory, referral order, telehealth fertility counseling, provider audit and feedback, and provider education. All but provider education were EHR tools with embedded efficiencies.
Conclusion: An implementation science approach systematically assessed oncofertility care and mapped strategies to provide a theory-based approach and scalable EHR tools to support wider dissemination.
Keywords: oncofertility, implementation science, CFIR, fertility preservation
Background
Unmet reproductive health care needs are highly prevalent among the nearly 400,000 reproductive-aged, female survivors of adolescent and young adult (AYA) cancers (AYA survivors) in the United States.1 They undergo treatments such as radiation, chemotherapy, surgery, and/or endocrine therapy, which may adversely impact future fertility.2,3 AYA survivors often want to have their own families; infertility from cancer treatment significantly impairs quality of life.4,5 Because oncofertility care—fertility counseling and preservation procedures—at cancer diagnosis and post-treatment can decrease the risk of infertility,6,7 clinical guidelines from oncology and fertility societies recommend that oncologists discuss infertility risk with patients and offer fertility preservation options or referrals to reproductive specialists throughout the cancer continuum.6,8–10
Despite clinical guidelines, uptake of fertility counseling at cancer diagnosis remains highly variable. In the adult setting, 2015–2019 Quality of Oncology Practice Initiative data showed that 44% of patients younger than age 50 were counseled on reproductive risks across 400 practices.11 Continually moderate implementation of fertility counseling is attributed to heterogeneous barriers and facilitators.12 Examples include inadequate recognition of reproductive health needs by patients and providers,13,14 unclear role expectations of oncology versus fertility providers,15–18 lack of clear referral pathways,19–21 disparities in counseling between males and females,22 and lack of access to fertility programs, particularly in pediatric oncology settings.23 These research and quality improvement efforts have been limited by scope and methodology, motivating a systematic approach to assess health system barriers, map scalable strategies to address them, and describe the design process for future dissemination and adaptation.
Electronic health records (EHRs) may facilitate strategies enabling fertility counseling. EHR systems can set rule-based reminders to staff and/or providers, automate referral pathways, generate reports of fertility referral and counseling, collect patient-reported information through a patient portal, and support telehealth. For widely used EHRs, functionalities are shareable, and mobile apps can upgrade universally. Moreover, connectivity through smartphones is over 80% for AYAs, regardless of socioeconomic status, facilitating reach through such EHR apps.24
To date, an implementation science approach—the study of methods to promote integration of evidence-based practices into routine health care—has not been undertaken to address the know-do gap (i.e., gap between what we know and what we do) in fertility counseling.25 Thus, guided by the Consolidated Framework for Implementation Research (CFIR),26 we systematically assessed barriers and facilitators to fertility counseling at cancer diagnosis and use of EHR tools as strategies to integrate fertility counseling into two oncology programs, one adult and one pediatric, and to bridge oncology and fertility programs. We focused on the female AYA survivor population because of an observed gap in care in our context, compared with male survivors. We compared adult versus pediatric and inpatient versus outpatient settings. We then designed a multicomponent implementation strategy to fit these clinical contexts.
Methods
The study was approved by IRBs at the University of California San Diego (UCSD) and Rady Children's Hospital. Researchers were female oncologists, reproductive endocrinologists, implementation scientists, and medical students. Between October 2018 and May 2019, we enrolled reproductive-aged female survivors of AYA cancers and health care providers of this population to participate in semistructured interviews or focus groups. Study settings were Moores Cancer Center, an adult comprehensive cancer center, and Rady Children's Hospital, a freestanding children's hospital affiliated with UCSD. At Moores, disease teams included hematologic malignancies, gastrointestinal, neuro-oncology, breast oncology, and radiation oncology. At Rady Children's, disease teams included liquid tumor, solid tumor, bone marrow transplant, and the survivorship clinic. There is an institutional fertility program at Moores, but not at Rady Children's.
We developed CFIR-based guides for provider interviews and AYA survivor focus groups to assess multilevel ecologic factors impacting fertility counseling and explore use of EHR-based implementation strategies. The guides encompassed questions on CFIR domains (intervention, individual, inner setting, outer setting, process) and relevant domain-specific constructs.23
We conducted 19 health care provider semistructured interviews, 8 at the adult program and 11 at the pediatric program. Oncologists, advanced practice providers, nurses, and pharmacists from each disease team were approached for participation, because clinic processes varied by disease team. Program clinical and quality leaders were also recruited. After obtaining informed consent, we conducted 1-hour-long interviews in person or through video calls of each participant with two to three investigators.
We conducted four focus groups with two to three reproductive-aged AYA survivor participants each. They were recruited from the investigators' prior research studies on reproductive health in AYA survivors.27,28 Among participants who agreed to be contacted for future studies, we restricted to individuals treated at either of the two oncology programs and younger than 45. We purposefully sampled survivors at higher and lower risks of infertility (e.g., sarcoma survivors vs. thyroid cancer survivors) and at variable times since treatment to gain perspectives that may be impacted by these factors. Participants received recruitment emails, the study team answered questions, and consents were signed and returned before video focus groups. Focus groups were 1-hour long through video calls with two to three investigators.
Audio recording and note taking occurred during interviews and focus groups. Participants were compensated with e-gift cards. Recruitment was stopped when data saturation was achieved.
Data analysis
We conducted thematic analysis facilitated by MaxQDA software.29 In addition to deductive themes (e.g., CFIR constructs26,30), we identified inductive themes, those arising from the data, using the following steps: (1) two independent coders (A.D., J.G.) read the transcripts, becoming familiar with the text and developing initial codes by consensus, (2) they coded three transcripts iteratively and refined the codebook, (3) the final codebook was determined by consensus (A.D., J.G., H.I.S.), (4) all data were coded, and (5) data were summarized by theme, with systematic comparison of pediatric versus adult settings and inpatient versus outpatient settings.31
Results
We contacted 19 providers and 72 AYA survivors for participation. Ten physicians (eight medical oncologists, one surgical oncologist, and one radiation oncologist), two advanced practice providers, one pharmacist, and six nurses participated. Median (range) of years in practice was 23 (29) years. Our sample had 8 adult providers and 11 pediatric providers, with 16 women and 3 men. Among the nine AYA survivors, mean age was 33.1 (standard deviation 6.8) years, and their cancer diagnoses included thyroid, cervical, and bone cancers; leukemias; and lymphomas. Table 1 summarizes barriers and facilitators to fertility counseling by CFIR domains (i.e., individual characteristics, inner context, outer context, process) and related constructs.
Table 1.
Barriers and Facilitators of Oncofertility Care Implementation
| Construct | |
|---|---|
| Individual | |
| Knowledge and beliefs | Facilitators: |
| Variation in which oncology visit and which provider should address fertility | |
| Patient-driven requests for fertility care would prompt provider actions | |
| Barriers: | |
| Provider content knowledge gap: Treatment-related fertility risks, fertility preservation procedures, fertility care clinical guidelines | |
| Provider operational knowledge gap on how to refer to fertility care | |
| Provider belief that fertility discussions are not appropriate when patients are overwhelmed, cancer workup/treatments more pressing, cancer treatment plans unknown or prognosis is poor | |
| Self-efficacy | Facilitators: |
| Resources to support knowledge gaps on treatment-specific fertility risks: pharmacists, fertility specialists, or reliable tool | |
| Resources to support nurse-led education of survivors | |
| Inner setting | |
| Implementation climate | Facilitators: |
| Feedback: Implementation metrics of each clinical team preferred over individual provider | |
| Feedback: Peer pressure through public audit/feedback | |
| Compatibility: EHR-enabled, automated screening tools, referral pathways, collection quality metrics | |
| Barriers: | |
| Compatibility: Heterogeneous EHR templates and systems | |
| Compatibility: Automated screening protocols lack flexibility (e.g., patient overwhelmed, treatment plan unknown, too close to treatment to allow for fertility preservation procedures) | |
| Readiness for implementation | Facilitators: |
| Access to knowledge and information: Expertise on fertility risks and procedures | |
| Access: Devices and internet connectivity for in-clinic telehealth access to off-site specialists | |
| Available resources: EHR system to support screening, referrals, quality metrics, patient portal | |
| Available resources: Existing patient screening tools, templated notes or pathways, quality improvement processes that can be adapted for fertility care | |
| Barriers: | |
| Available resources: Heterogeneity in personnel resources to support screening (navigator, social work), risk counseling (pharmacist), in person translation | |
| Available resources: Lack of educational materials with depth for patients | |
| Outer setting | |
| External Policy and incentives | Facilitators: |
| Fertility care as a quality metric from accreditation organizations | |
| Clinical guidelines from oncology societies recommend fertility care | |
| Systemized delivery of fertility care as a requirement of AYA foundation funding | |
| Insurance coverage for fertility preservation services | |
| Barrier: | |
| Insurance approval of fertility preservation is inconsistent, time consuming and complex | |
| Peer pressure | Facilitator: |
| High-quality, systemized fertility care can set an oncology program apart from others | |
| Cosmopolitanism (networks) | Barrier: |
| Interorganizational networks (between oncology and fertility clinics or fertility care funding organizations and clinics) are lacking | |
| Implementation process | |
| Engaging | Facilitators: |
| Oncology team: Identify a nursing champion to support team-specific implementation procedures | |
| Organization: Engage leadership, for example, cancer cabinet or quality committee, to select fertility care as an institutional goal | |
| Patients educated about fertility care can prompt their oncology providers | |
| Planning | Facilitators: |
| Plan for providing implementation metrics/feedback to providers | |
| Adapt processes to fit each clinic, for example, determine when/which appointments appropriate for fertility screening of a newly diagnosed cancer patient, order of face-to-face consultations with adolescents and parents (together, tandem) | |
| Plan for resources needed, for example, internet access, language translators, provider educational session | |
| Stage implementation scale up: First pilot in a limited setting, for example, a few oncology teams | |
AYA, adolescent and young adult; EHR, electronic health record.
Individual characteristics
Providers had variable content knowledge about the existence of oncofertility care guidelines, fertility risks of cancer treatments, and fertility preservation procedures. Even for oncology providers who addressed fertility, depth of content knowledge was sometimes perceived to be lacking: “[I] feel as though as I am inadequately trained in this arena. Having said all of that, I do feel like we are addressing the issue and at least putting it on the table, but I don't think we're doing enough” (Advanced practice provider). This content knowledge gap limited self-efficacy; access to knowledge on fertility risks, which may be derived from a number of resources, was felt to be key to improving self-efficacy. Beyond content knowledge, oncology providers lacked knowledge on how fertility counseling and referral are operationalized in their clinic.
From the perspective of AYA survivors, fertility counseling would inform their decisions about timing of cancer treatment, and lack of knowledge about the option of fertility preservation procedures prevented AYA survivors from undertaking them: “I think before I would have liked to know that. In my opinion, I think doctors being doctors push treatment right away. And if I had known, like … if I would've been able to preserve or do something for fertility, I think I would've chosen to wait on treatment and done that” (AYA survivor).
While most providers expressed that oncofertility care is relevant to AYA survivors, a common theme was the belief that the timing of counseling at cancer diagnosis can be complicated by overwhelmed patients, competing oncology workup and/or treatment initiation needs, unclear cancer treatment plans that preclude informing patients about their reproductive risks, and poor prognosis: “I don't know if they're going to need chemo because for various reasons, and they're going to surgery first, so I don't bring it up at all. And for the patients that I'm sending for neoadjuvant, I just assume the oncologist is going to do that unless they ask me specifically. If the patient asks me about it then I'll put the referral in” (Surgical oncologist). As suggested by this oncologist, regardless of if/when a provider brings up fertility, patient-driven requests are a useful cue to action.
Preferences varied on which provider, that is, physician (oncology or fertility), advanced practice providers, or nursing, conducts the primary fertility counseling. Oncology physicians reporting self-efficacy prefer to undertake primary counseling themselves, while other providers opt for automated referrals for all survivors to fertility specialists. There was consensus that nursing has the potential for fertility education, but would require significant additional oncofertility education.
Inner context
Discussions of clinic characteristics important to delivering counseling focused on the implementation climate and readiness. EHR tools were suggested and/or endorsed by providers as compatible with automating screening for fertility needs in clinic or before visits through patient portal, referral pathways between oncology and fertility, and accrual of fertility care metrics. For providers, advantages were alleviating personnel workload, systematically selecting the targeted population (e.g., through setting age parameters and type of encounter for automated fertility needs screens) and generating shareable tools among organizations using the same EHR platform. EHR documentation, particularly with discrete fields, allows efficient collection of quality metrics for feedback and accreditation: “It's also important to have the resource of being able to pull metrics because otherwise you have this warm fuzzy feeling in your heart that we're doing super well, but then the data shows that that was an erroneous warm fuzzy feeling” (Quality leader).
Another provider theme centered on adapting available resources, including paper or EHR screening tools, EHR note templates, quality metrics reporting, and quality improvement processes for fertility counseling implementation. In addition, all levels of providers noted a lack of fertility educational materials that were sufficiently in-depth and with patient-friendly content: “Recently, for example, I had a patient … we didn't have the resources here. We had to try and pull resources and then go and discuss with the patients. I don't think as pediatric oncologists we have been trained to do that.”
Outer context
External factors influence clinic and individual provider delivery of oncofertility care. An oncology physician noted, “[At] national meetings [oncofertility] is always a big topic, something that's on our radar a lot.” Additionally, advocacy and funding organizations use oncofertility care as a quality metric and a requirement for receiving funding:
One of [a pediatric oncology non-profit's] big requirements is to have this fertility preservation talk at the very beginning, before starting chemotherapy … They require that to get their sponsorship, we have to have formal protocol in place. (Pediatric oncologist)
Insurance coverage for fertility preservation services was repeatedly discussed as a barrier by both providers and patients. Even if insurance coverage for fertility counseling is available to patients, procedures may not be covered, and authorization processes are burdensome. For example, after a fertility preservation referral order has been placed in one oncology program, insurance authorization is required and obtained by a central or provider team-specific authorization unit. The authorization is a physical letter, and to bypass the delay, the provider team always made additional calls to confirm referrals were being authorized.
Lastly, delivery of oncofertility care is complex due to involvement of different organizations, that is, oncology clinics, fertility clinics, nonprofit organizations, and insurance companies. Oncology programs do not always have a fertility program within the institution. Oncology and fertility clinics also need to join a network for fertility preservation financial support from nonprofit organizations such as LIVESTRONG. Both provider and AYA survivor participants noted a strong need to bridge these different organizations systematically and seamlessly. As an example, there was great reported burden on and lack of a streamlined, replicable process to enable oncology and fertility financial authorization staff and patients to interact with insurers to determine coverage.
Process
Many providers reflected on the types of engagement needed for effective implementation. Leadership engagement at three distinct levels were proposed: organizational level that sets oncofertility care as an institutional goal (e.g., selection of the Quality Oncology Practice Initiative quality metric32), cancer team leaders that endorse care goals for the team, and a fertility champion (often cited as nurses) within each team. Providers viewed engagement of multiple champions as a facilitator because “people feel resistance when it's like this is one person's project” (Oncologist). Moreover, engaging AYA survivors as their own advocates would effectively cue their providers.
Coplanning implementation strategies with stakeholders was endorsed for fit and buy-in. Adaptation of processes will be needed to fit clinic context, for example, which appointments are appropriate for introducing fertility needs screens. Several providers suggested piloting implementation strategies with engaged teams before full-scale implementation.
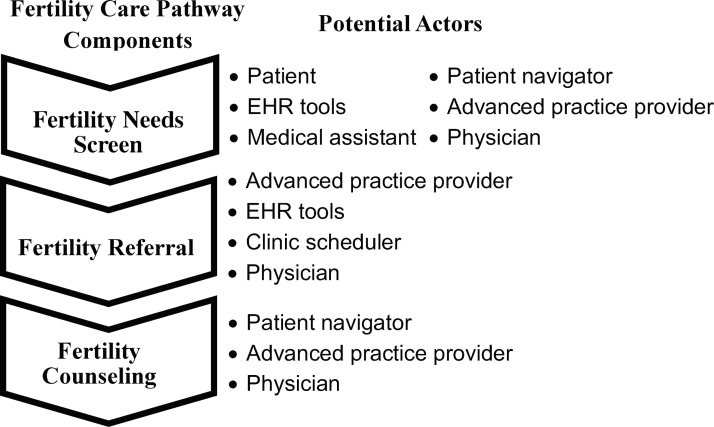
Three core components emerged as central to planning fertility counseling implementation (Fig. 1). First, patients need to be screened for fertility needs. Second, a fertility care referral needs to be placed, if appropriate. Third, patients need access to a fertility specialist for additional fertility counseling and appropriate fertility preservation strategies. Different types of individuals can participate in each stage.
FIG. 1.
Fertility care pathway involves a fertility care needs screen, a fertility referral, and fertility counseling with multiple potential actors in each step.
Differences between pediatrics and adult settings
Several themes unique to pediatrics emerged. Adolescent providers and AYA survivors similarly discussed that the timing of fertility counseling, before, concomitant, or after consultation with their parents/guardians, should be guided by the family. Some providers questioned whether adolescents would be interested in talking about future fertility, believing it was best to talk to the parents only. Pediatric providers also discussed at what age it is appropriate to talk about fertility care: “Like would the kid want to know about having children at 15 or would you talk just to the parents?” (Advanced practice provider). These concerns were echoed in the focus groups, as several patients, when asked if they initiated oncofertility conversations with their providers, shared that fertility was not on their mind when diagnosed with cancer in adolescence. Additionally, complexity of privacy laws with regard to whether adolescents and/or parents/guardians have access to their patient portal (through which telehealth and screening questionnaires may be delivered) emerged as a barrier to EHR patient portal strategies.
Investigators' mapping of strategies to fertility needs screening, referral, and specialist access
Based on barriers and facilitators reported by participants, the investigators then designed multiple strategies for the three core components, selected strategies, and identified implementors. Table 2 specifies the five selected strategies for the two oncology programs: (1) automatic fertility needs screen using a best practice advisory, (2) an opt-out fertility referral pathway through the EHR system EPIC, (3) adding an option to conduct fertility counseling through telehealth, (4) audit and feedback to providers, and (5) conducting educational meetings. Table 3 specifies other strategies that were considered but not selected. Importantly, strategies apply to both female and male patients.
Table 2.
Specification of Oncofertility Care Implementation Strategies
| Strategy | Actor | Action | Action target(s) | Temporality | Dose | Implementation and services outcomes | Justification |
|---|---|---|---|---|---|---|---|
| Mandate change | Cancer center leadership | Select fertility counseling as cancer center quality goal | Oncology teams | Once | Once | Uptake of fertility counseling by AYA survivors at diagnosis and in survivorship | (1) Prioritizes implementation; (2) access to quality improvement team and resources |
| Automate fertility needs screen: remind clinicians | EHR system EPIC | BPA pops up to (1) remind clinicians about fertility counseling, (2) shortcut to referral order | Physicians, APPs | Trigger criteria: new oncology visit, age (<42 females, <50 males), cancer diagnosis in EHR | Each provider will see BPA maximum of one time; after referral, no other providers will see BPA for 2 years | Screen all newly diagnosed and 2-year post-treatment AYA survivors for fertility needs | (1) Compatible with EHR; (2) addresses oncology provider content and operational knowledge gap |
| Automate fertility referral between clinics | EHR system EPIC | Fertility specialist referral order with cancer treatment plan automatically placed in a STAT fertility scheduler queue for insurance authorization and contacting patient within 72 hours | Oncology physicians and APPs; fertility clinic schedulers ownership of insurance authorization and patient contact; fertility specialists know proposed cancer treatments |
Each referral order | Once per referral | Fewer patients lost to care between two clinics; insurance authorization and scheduling efficiency; more precise fertility risk counseling by fertility specialists | (1) Compatible with EHR; (2) addresses oncology providers operational knowledge gap |
| Fertility counseling: add service sites | Fertility specialist, EHR system and patient portal | Televideo fertility counseling using EHR provider tool and EHR patient portal | AYA survivors at diagnosis and post-treatment | Offered to patient by fertility scheduler after fertility referral order placed | An initial 30- to 60-minute fertility counseling visit on treatment-related reproductive risks and fertility preservation options | Uptake of fertility counseling by AYA survivors; patient-centered, timely visits | (1) Compatible with EHR; (2) addresses geographic and time (multiple visits to multiple providers) barriers |
| Audit and feedback | EHR system, quality team | Metrics on screening, referral, counseling | Oncology teams; fertility teams | After initiation of screening and automated referral pathways | Monthly reports by individual provider and by clinic team | Fidelity of BPA screening and referral pathway | (1) Compatible with EHR-based screening and referral; (2) peer pressure |
| Conduct educational meetings | Implementation team, fertility specialist | 20-Minute educational session on fertility content and operationalizing implementation strategies | Cancer center cabinet and quality committee; oncology physicians and APPs at oncology team meeting; fertility clinic schedulers and administrators | After preparation of strategies | Once per group | Increase acceptability of implementation strategies | (1) Addresses oncology provider content and operational knowledge gap |
BPA, best practice advisory.
Table 3.
Characteristics of Non-Selected Implementation Strategies
| Fertility care pathway order | Strategy | Facilitators and barriers | Justification |
|---|---|---|---|
| Fertility needs screen | Involve patients: AYA survivors screened through questionnaire sent through EHR patient portal MyChart | Facilitators: | Available resource; compatibility |
| Patient time: not limited by in-person visit time | |||
| EHR patient portal uptake high in adults, low in pediatrics | |||
| Barriers: | Compatibility and available resources; compatibility; external policy and incentives; adaptability; complexity | ||
| Heterogeneity of questionnaires used by providers | |||
| Cannot be used in inpatient setting | |||
| Privacy laws do not allow parents access to patient portal in children ≥ age 12 | |||
| No Spanish version of patient portal | |||
| Wording appropriate to children and adolescents | |||
| Involve patients: Paper questionnaire in waiting room | Facilitators: | Available resource; adaptability | |
| Does not require patient to enroll in patient portal | |||
| Could be given in multiple languages | |||
| Barriers: | Complexity; available Resource; compatibility | ||
| Wording appropriate to children and adolescents | |||
| Available patient time to consider screening questions limited | |||
| Provider may miss the paper questionnaire | |||
| Revise professional roles: Dedicated fertility navigator | Facilitator: | Complexity | |
| One provider that does all the steps: screening and referral | |||
| Barriers: | Available resources; compatibility | ||
| Cost | |||
| Depending on volume, difficult to capture all new diagnoses | |||
| Technical assistance—EHR templates to document screens | Barrier: | Complexity, compatibility | |
| Heterogeneity of note templates limits automation and uniform uptake | |||
| Fertility referral | Remind clinicians: EHR inbox messages on new patients | Barrier: | Compatibility |
| Providers overwhelmed by inbox messages | |||
| Automated referral for all new patients | Facilitator: | Complexity | |
| Has one less step required of oncology providers | |||
| Barrier: | Relative advantage | ||
| Leads to unnecessary consults | |||
| Remind clinicians: added referral order to admission order set | Barrier: | Complexity | |
| Heterogeneity of order sets used by different disease teams | |||
| Would not work in outpatient setting | Compatibility |
Key differences between the inpatient and outpatient environment were identified in mapping strategies. EHR tool specifications and pathways require different programming logic between the inpatient and outpatient setting. Inpatient insurance authorizations are not required for consultations, so providers hypothesized that this would improve access to risk counseling by fertility specialists. However, female fertility preservation procedures of oocyte or embryo banking generally occur in outpatient settings, which would limit the access of hospitalized patients.
Discussion
Guided by an implementation science framework, we conducted a qualitative study with oncology providers and AYA survivors from one adult and one pediatric oncology setting to systematically identify barriers and facilitators to fertility counseling at cancer diagnosis and codesign strategies with stakeholders. We report this systemized approach to inform teams seeking to develop or adapt their implementation of oncofertility care as part of routine oncology care.
Using CFIR domains and constructs allowed systematic assessment of the facilitators and barriers that influence fertility counseling implementation and change of processes. CFIR offered a pragmatic structure for our multilevel problem and a large number of domains and constructs that we could query.26 While our qualitative guides encompassed questions based on a larger set of constructs, ultimately, the number of key relevant constructs (Table 1) was smaller and can guide the environmental scan of other clinical settings. Compatibility, feedback, available resources and planning constructs were particularly important in designing specific strategies for the two oncology programs.
Findings led to conceptualizing three core components of oncofertility care. Then, an array of implementation strategies targeting these components were evaluated, considering our findings, implementation strategies from the Expert Recommendations for Implementing Change project,33 and a priori research on components of fertility care models.12 The goals were to select strategies that fit our context and may be applied across adult and pediatric settings; we aimed to minimize the number of strategies to limit complexity and system burden. The process of describing the strategies (naming, defining, operationalizing the actor, action, action targets, temporality, dose, implementation outcomes addressed, and theoretical justification) clarified which ones were not feasible (e.g., high-quality patient educational materials on fertility risk) or did not fit (e.g., patient portal for screening).34 The findings aim to enable planning of fertility counseling implementation efforts.
Across domains, we found variability in beliefs about whose role it was to provide fertility counseling, concordant with prior reports.14 For some providers, this concern was due to lack of self-efficacy about addressing fertility, an individual characteristic, whereas others attributed to inadequate time or incompatibility with a clinical visit, an inner setting characteristic. We also found that many types of providers could perform fertility needs screening, whereas only physicians and advanced practice providers could place referrals. Hence, specifying the actors of an implementation strategy was necessary.
We sought stakeholder input on EHR tools as implementation strategies. In designing and building EHR tools, we could modify existing EHR tools such as the reporting tool for audit and feedback and the patient-friendly portal that enables secure video visits. Because of the limited number of EHR software systems, these tools could be widely disseminated and scalable. We also found that EHR tools may not be acceptable due to provider fatigue, feasible due to privacy laws, or appropriate due to low uptake of patient portal apps. Building these tools can be time and labor intensive, but a successful build in one clinical program may be disseminated to others.
This work advances prior research on oncofertility care delivery. Multiple reports highlight gaps in and interventions on provider knowledge and confidence, but show that while interventions increase providers' knowledge of fertility preservation, they largely do not increase care delivery.35–37 This is because barriers are multidimensional, for example, need for interorganizational networks and engaging oncology team on implementation procedures, as we have shown in the current study. A recent scoping review identified nine core domains for oncofertility care.38 We found that individual studies in this review contribute to one to two domains, for example, communication with patients and training providers, but lacked a systemized interrogation of a clinical system and development of a coordinated, system-based intervention that we propose in our work. Detailing our approach to evaluating two clinical systems and stakeholders and developing a coordinated intervention will enable other programs to adapt this more specific approach to their context.
Limitations include that we were limited to two oncology programs. While they were selected to reflect pediatric and adult oncology, their academic and existing single fertility clinic referral site limit generalizability. Additionally, our sampling strategy for health care providers and AYA survivors, like other qualitative work, was purposeful and not random, risking selection bias as the health care providers and AYA survivors who took part in our study may have an interest in fertility care. While the developed strategies target both sexes, female AYA survivors were specifically recruited because of the gap in care in our settings. It is possible that we did not capture additional barriers for male and childhood cancer survivors and their parents/guardians. As we developed strategies, we were limited by available resources. There is a strong need for the creation of educational resources to support fertility risk discussion, where current tools lack specificity and/or are too time intensive. Financial costs are a significant barrier that cannot be overcome with clinic-based implementation strategies alone.
In summary, we describe a systematic approach to design implementation strategies for fertility counseling at an adult and children's oncology program. We contribute data on the most salient CFIR constructs to assess and specifications on a set of potential strategies that may be adapted and deployed to improve the fertility care of adolescents and young adults with cancer.
Acknowledgments
The authors wish to thank Kris Brodsho for guidance on EHR capabilities and Nicole Stadnick, PhD, for implementation science expertise.
Authors' Contributions
Design (E.R.R., P.A., G.A.A., M.B.T., T.H., and H-C.I.S.), data collection (A.D., E.H.Y., J.G., E.R.R., P.A., M.B.T., and H.I.S.), data analysis (A.D., E.H.Y., J.G., S.A.D.R., B.N.K., and H.I.S.), article preparation (A.D., E.H.Y., and H.I.S.), article review (J.G., E.R.R., P.A., G.A.A., M.B.T., T.H., S.A.D.R., and B.N.K.).
Author Disclosure Statement
Dr. Takemoto works for ServiceNow, Inc., which did not sponsor, support, or have oversight of this research. All other authors have no competing financial interests.
Funding Information
Funding received from NIH UL1TR001442 and NIH TL1TR001443.
References
- 1. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85. [DOI] [PubMed] [Google Scholar]
- 2. van Dorp W, Haupt R, Anderson RA, et al. Reproductive function and outcomes in female survivors of childhood, adolescent, and young adult cancer: a review. J Clin Oncol. 2018;36(21):2169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barton SE, Najita JS, Ginsburg ES, et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2013;14(9):873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorman JR, Su HI, Roberts SC, et al. Experiencing reproductive concerns as a female cancer survivor is associated with depression. Cancer. 2015;121(6):935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gorman JR, Usita PM, Madlensky L, Pierce JP. Young breast cancer survivors: their perspectives on treatment decisions and fertility concerns. Cancer Nurs. 2011;34(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994–2001. [DOI] [PubMed] [Google Scholar]
- 7. Fallat ME, Hutter J; American Academy of Pediatrics Committee on Bioethics, et al. Preservation of fertility in pediatric and adolescent patients with cancer. Pediatrics. 2008;121(5):e1461–9. [DOI] [PubMed] [Google Scholar]
- 8. American Society for Reproductive Medicine; American College of Obstetricians and Gynecologists' Committee on Gynecologic Practice. Prepregnancy counseling: committee Opinion No. 762. Fertil Steril. 2019;111(1):32–42. [DOI] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: adolescent and young adult (AYA) oncology, version 1.2021-September 10, 2020. 2020. Accessed November 1, 2020 from: https://www.nccn.org/professionals/physician_gls/default.aspx#age
- 10. Patel A, Schwarz EB; Society of Family Planning. Cancer and contraception. Release date May 2012. SFP Guideline #20121. Contraception. 2012;86(3):191–8. [DOI] [PubMed] [Google Scholar]
- 11. Patel P, Kohn TP, Cohen J, et al. Evaluation of reported fertility preservation counseling before chemotherapy using the quality oncology practice initiative survey. JAMA Netw Open. 2020;3(7):e2010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anazodo A, Laws P, Logan S, et al. How can we improve oncofertility care for patients? A systematic scoping review of current international practice and models of care. Hum Reprod Update. 2019;25(2):159–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gorman JR, Bailey S, Pierce JP, Su HI. How do you feel about fertility and parenthood? The voices of young female cancer survivors. J Cancer Surviv. 2012;6(2):200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quinn GP, Vadaparampil ST, Lee JH, et al. Physician referral for fertility preservation in oncology patients: a national study of practice behaviors. J Clin Oncol. 2009;27(35):5952–7. [DOI] [PubMed] [Google Scholar]
- 15. Johnson RH, Kroon L. Optimizing fertility preservation practices for adolescent and young adult cancer patients. J Natl Compr Canc Netw. 2013;11(1):71–7. [DOI] [PubMed] [Google Scholar]
- 16. Duffy C, Allen SM, Dube C, Dickersin K. Oncologists' confidence in knowledge of fertility issues for young women with cancer. J Cancer Educ. 2012;27(2):369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Overbeek A, Van den Berg M, Louwé L, et al. Practice, attitude and knowledge of Dutch paediatric oncologists regarding female fertility. Neth J Med. 2014;72(5):264–70. [PubMed] [Google Scholar]
- 18. Ghorbani B, Madahi P, Shirazi E, et al. Iranian oncologists' attitude towards fertility preservation in a sample group. J Reprod Infertil. 2011;12(1):33. [PMC free article] [PubMed] [Google Scholar]
- 19. Loren AW, Brazauskas R, Chow EJ, et al. Physician perceptions and practice patterns regarding fertility preservation in hematopoietic cell transplant recipients. Bone Marrow Transplant. 2013;48(8):1091–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clayman ML, Harper MM, Quinn GP, et al. Oncofertility resources at NCI-designated comprehensive cancer centers. J Natl Compr Canc Netw. 2013;11(12):1504–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Warner E, Yee S, Kennedy E, et al. Oncofertility knowledge, attitudes, and practices of Canadian breast surgeons. Ann Surg Oncol. 2016;23(12):3850–9. [DOI] [PubMed] [Google Scholar]
- 22. Köhler TS, Kondapalli LA, Shah A, et al. Results from the survey for preservation of adolescent reproduction (SPARE) study: gender disparity in delivery of fertility preservation message to adolescents with cancer. J Assist Reprod Genet. 2011;28(3):269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rabah DM, Wahdan IH, Merdawy A, et al. Oncologists' knowledge and practice towards sperm cryopreservation in Arabic communities. J Cancer Surviv. 2010;4(3):279–83. [DOI] [PubMed] [Google Scholar]
- 24. Pew Research Center. Mobile fact sheet. 2018. Accessed October 10, 2018 from: www.pewinternet.org/fact-sheet/mobile
- 25. National Cancer Institute. Implementation science. Updated February 5, 2020. Accessed May 17, 2020 from: https://cancercontrol.cancer.gov/IS
- 26. Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Su HI, Stark S, Kwan B, et al. Efficacy of a web-based women's health survivorship care plan for young breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2019;176(3):579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Su HI, Kwan B, Whitcomb BW, et al. Modeling variation in the reproductive lifespan of female adolescent and young adult cancer survivors using AMH. J Clin Endocrinol Metab. 2020;105(8):2740–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patton MQ. Qualitative evaluation and research methods, 2nd ed. Thousand Oaks, CA: Sage Publications; 1990.
- 30. Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the Consolidated Framework for Implementation Research (CFIR). Implement Sci. 2013;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ulin PR RE, Tolley EE. Qualitative methods in public health: a field guide for applied research, 1st ed. San Francisco, CA: Jossey-Bass; 2005.
- 32. McNiff K. The quality oncology practice initiative: assessing and improving care within the medical oncology practice. J Oncol Pract. 2006;2(1):26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Powell BJ, Waltz TJ, Chinman MJ, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang HF, Jiang QH, Huang GY, et al. The educational program for healthcare providers regarding fertility preservation for cancer patients: a systematic review. J Cancer Educ. 2020. [DOI] [PubMed]
- 36. Quinn GP, Vadaparampil ST. Fertility preservation and adolescent/young adult cancer patients: physician communication challenges. J Adolesc Health. 2009;44(4):394–400. [DOI] [PubMed] [Google Scholar]
- 37. Forman EJ, Anders CK, Behera MA. A nationwide survey of oncologists regarding treatment-related infertility and fertility preservation in female cancer patients. Fertil Steril. 2010;94(5):1652–6. [DOI] [PubMed] [Google Scholar]
- 38. Anazodo A, Laws P, Logan S, et al. The development of an international oncofertility competency framework: a model to increase oncofertility implementation. Oncologist. 2019;24(12):e1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]