Abstract
Purpose: Reasons for the relatively low rates of adolescent and young adults (AYA) enrollment in cancer clinical trials in the United States require further empirical examination. In addition to structural factors such as lack of access and insurance barriers, attitudes toward clinical trials may be important to consider. This study aimed to evaluate and validate the Pediatric Research Participation Questionnaire (PRPQ)—a measure of attitudes to clinical trials adapted for AYA (15–29) with cancer and their caregivers.
Methods: One hundred twenty-four AYA and 94 caregivers completed the PRPQ-AYA and measures of clinical trial knowledge and developmental/emotional maturity. Factor analysis evaluated the PRPQ-AYA structure, interitem reliability was computed, and Pearson correlations examined associations of validation measures with factor scores and computed scores reflecting perceived barriers, perceived benefits, and decision balance.
Results: Confirmatory factor analysis did not confirm the prior PRPQ factor structure. Exploratory factor analysis suggested a new four-factor structure for: AYA (1) trust/mistrust, (2) barriers/costs, (3) support for participation, and (4) incentives; and caregivers (1) trust/access, (2) mistrust/costs, (3) support for participation, and (4) risks to AYA. Factor scores and barriers, benefits, and decision balance scores demonstrated acceptable interitem reliability and were significantly correlated with clinical trial knowledge and emotional maturity in the expected direction.
Conclusion: PRPQ-AYA factor structure for AYA and caregivers varied and should be interpreted cautiously due to limited power. Simple solutions of perceived benefits, perceived barriers, and decision balance were reliable and valid and provide important information to address and engage AYA through the clinical trial informed consent process.
Keywords: attitudes toward cancer clinical trials, cancer clinical trials enrollment, adolescent and young adults decision-making
Introduction
Survival of adolescent and young adults (AYA) with a history of cancer is low relative to older and younger populations1,2 due, in part, to reduced participation in therapeutic clinical trials.3–6 The AYA Committee of Children's Oncology Group has designated lack of clinical trial enrollment as a priority area for research initiatives.2 Few empirical studies have addressed this issue,7 but the limited literature and expert opinion outline factors that may influence enrollment, including limited access to trials for AYA, limited acceptability of available trials, and lack of fit between open trials and the needs of AYA.7,8 Effective communication about cancer clinical trials among AYA, primary caregivers, and health care providers may increase AYA understanding of their illness and implications of treatment choices for health-related quality of life and their involvement in treatment.9,10 Furthermore, decision-making abilities of AYA and adults may be similar when AYA are well-informed and treatment options are framed from an appropriate cognitive/developmental perspective.11–14 In this study, we evaluate a measure for assessing AYA and caregiver attitudes to cancer clinical trials for utilization by health care providers to inform efforts to enhance AYA engagement in the clinical trial decision-making process.15
The medical community encourages, and AYA and their primary caregivers value, inclusion of pediatric patients in treatment decision-making.16,17 However, cancer and its treatment significantly affect and often delay achievement of development milestones,18 resulting in increased or renewed reliance of AYA on family for support and reduced well-being.14,19,20 The process of AYA taking primary responsibility for treatment decision-making is gradual, and parents frequently remain involved through their child's young adulthood.17–19 Complicating efforts to engage AYA in clinical trial decision-making, physicians generally perceive younger AYA as not capable of meaningful participation in cancer clinical trial discussions,14,21 and recently diagnosed AYA often endorse the belief that decisions are best left to their medical team.22 Yet, parent–child collaboration in clinical trial decision-making is consistent with positive family relationships and AYA emotional maturity, and AYA who are involved in decisions may be more engaged throughout treatment.23
A limited literature connects attitudes toward clinical trials to decision-making processes. Caregivers are not satisfied with the cancer clinical trial consent process, finding the decision difficult to make and they have insufficient time to ask questions or consider options.24,25 Deficiencies in understanding are prevalent among parents who agree to enroll their child on a cancer clinical trial,24,26 and adolescent decision-makers need help distinguishing cancer clinical trials from standard treatment.27–29 AYA with cancer note additional barriers to enrollment (e.g., lack of family support, fear of toxicity, lack of concordance with their values, and a preference for standard therapy).7,30,31 However, AYA also endorse benefits of clinical trial participation such as helping themselves and others.32,33
Our own qualitative research evaluating Phase III clinical trial decision-making experiences confirms these findings.15 AYA perceived that they had no role or a minor role in clinical trial enrollment decisions. They conveyed a sense of resignation for relying on their caregivers for decision-making. In contrast, caregivers viewed decision-making as, at least in part, the responsibility of AYA while acknowledging caregivers' primary role in the final decision. Health care providers felt challenged to maintain the attention of AYA, provide balanced information and minimize coercion, and respond to preexisting developmental issues and family structure during the informed consent meeting.
This study evaluates the validity of the Pediatric Research Participation Questionnaire (PRPQ)-AYA, a modification of the PRPQ34 with a reduced item pool specific to attitudes to cancer clinical trials, to identify areas for improved decision-making processes for AYA with cancer and their caregivers.15 Using factor analyses,35 we anticipated that the factor structure of the PRPQ-AYA14 would be similar to, and confirm, the factor structure for the initial PRPQ developed for youth with health disparity conditions (sickle cell disease and asthma).34 Furthermore, related to construct validity, we expected that cancer clinical trial knowledge, age/developmental status, and emotional maturity would be associated with PRPQ-AYA subscales based on the factor structure and summary scores reflecting perceived benefits, perceived barriers, and decision balance, which were also expected to demonstrate interitem reliability. Together with decision support interventions,36 if validated, the PRPQ-AYA can serve as an important tool to systematically assess attitudes to engage AYA in cancer clinical trial decision-making.
Methods
Participants and procedures
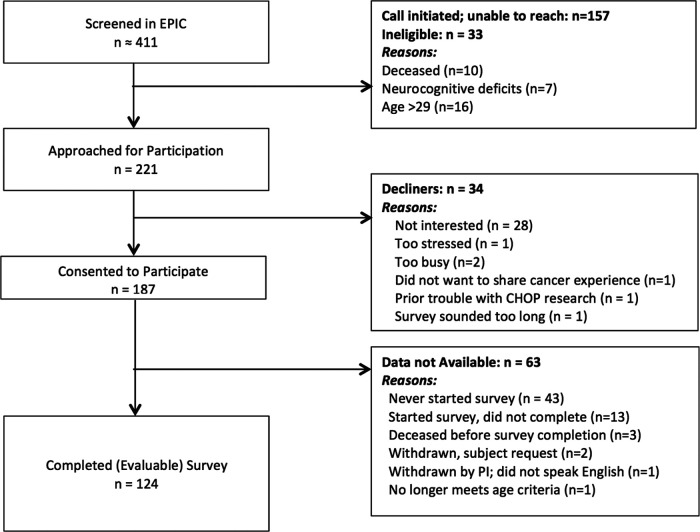
Participants were AYA with a history of cancer and their caregivers identified through cancer registries and recruited from the Children's Hospital of Philadelphia and the Hospital of the University of Pennsylvania (see Fig. 1 for CONSORT diagram for AYA). AYA were 15–29 years old, diagnosed with cancer at age 15 or older, and not in palliative care. AYA could be on active treatment or have completed treatment. Caregivers were the parents/legal guardians of consented AYA. Caregivers and AYA were excluded if they were not literate in English. Of AYA, 221 were approached for participation, 187 (85%) consented, and 124 (66%) completed the study. The most cited reason for declining to participate was “not interested” (82%), and reasons for not completing the questionnaire includes “lost to follow-up/unable to recontact” (89%). For caregivers, 136 were approached for participation, 127 (93%) consented, and 94 (74%) completed the study. Of the 94 caregivers, 67 had participating AYA. The most cited reasons for declining was too busy (44%) and not interested (33%). Subsequent to IRB approval, potential participants were approached by telephone or during outpatient oncology appointments. Following consent/assent procedures, AYA and caregivers completed separate questionnaires using a secure, web-based survey system (REDCap) and received a $25 gift card (AYA) or a $10 gift card (caregivers).
FIG. 1.
CONSORT diagram: Adolescent and young adults.
Measures
PRPQ-AYA,15,34 a modification of the PRPQ, assesses perceived barriers and benefits of cancer clinical trial participation at the patient, family, health care system, community, and societal levels based on social ecology. The original PRPQ was developed for youth with health disparity conditions (sickle cell disease and asthma) and their caregivers with input from local sickle cell and public health community agencies and medical experts. This measure was composed of 38 questions focused on medical and psychosocial research using True/False response options. It was field-tested before validation. Exploratory factor analysis of the identified four-factor structures for caregivers (N = 224) (direct benefit; mistrust of research/researchers; trust in safety of research; opportunity/cost) and for AYA (N = 76) (mistrust/no perceived benefit; safety; direct benefits/practical considerations; social support for research participation); factor analysis of the child version was not evaluable.34
To modify the PRPQ34 for AYA with cancer and their caregivers, using qualitative methods, we assessed AYA, caregiver, and provider interpretation of PRPQ questions, perceived relevance of questions to their cancer clinical trial decision-making experience, and suggestions for modified wording.15 Semistructured interviews were audiotaped and detailed notes recorded. Using discussion and consensus, the team reviewed audiotapes, notes, and summaries for each question and the overall measure to inform systematic modifications to the PRPQ. Findings suggested that the PRPQ is relevant for evaluating attitudes to cancer clinical trials, but indicated the need for revised items and response set. For the PRPQ-AYA, 20 questions were removed (e.g., “This study could provide information about my child's health I would rather not know about”), modified (e.g., “The government sometimes exposes research participants to medicine and procedures known to be harmful to one's health” changed to “Clinical trials sometimes expose research participants to medicine and procedures known to be harmful to one's health”), or added (e.g., “The following people would support my decision to participate in research: outside physician/pediatrician”), resulting in a 34-item scale for AYA and for their caregivers using a 5-point Likert response format (Strongly Disagree–Strongly Agree). AYA and caregiver forms vary only in terms of language for caregiver (e.g., my child) or AYA (e.g., my).
Knowledge about Clinical Trials37 is a 25-item measure answered true/false assessing participant understanding of clinical trials developed for adult oncology patients in a study evaluating use of National Cancer Institute (NCI)'s educational materials. For this sample, split-half reliability for % correct was moderate for AYA (r = 0.75) and Caregivers (r = 0.68).
AYA completed three measures of developmental and emotional maturity. (1) Developmental Tasks Questionnaire-YA version (8 items)38 evaluates the extent of achieving and degree of importance of developmentally appropriate tasks (e.g., independence from parents and establishing own identity). Total score for developmental tasks achieved, answered as “Not Yet,” “Working on it,” and “Already achieved,” was used to reflect developmental maturity in the analyses. (2) Emotion Regulation Questionnaire (ERQ)39 is a 10-item self-report measure assessing cognitive reappraisal and expressive suppression used to modify emotional expression. Items were rated on a 7-point Likert scale (Strongly Disagree–Strongly Agree) with higher summary scores indicating greater cognitive reappraisal or expressive suppression. (3) Consideration of Future Consequences Scale40 is a 12-item self-report measure used to evaluate time perspective/future orientation by assessing the extent to which AYA consider the potential distant outcomes of their current behaviors and the extent to which they are influenced by these potential outcomes using a 5-point Likert scale. A higher total score indicated greater time perspective/future orientation. In this sample, reliability was adequate for three developmental/emotional maturity subscales (α = 0.67–0.82), and higher scores indicated higher developmental and emotional maturity for each of the three measures.
Statistical analyses
To validate the factor structure identified in the previous PRPQ study34 (a four-factor structure of Risks/Mistrust, Benefits, Support, and Opportunity/Cost), confirmatory factor analysis (CFA) was conducted on caregiver and AYA versions of the PRPQ. Goodness of fit of the hypothesized model was assessed by various fit indices, adjusted goodness of fit index (AGFI), comparative fit index (CFI), and root mean square error of approximation (RMSEA).41 Indications of good model fit are AGFI ≥0.9, CFI ≥0.9, and RMSEA <0.06.41
Because a significant misfit was discovered, exploratory factor analyses (EFA) were conducted to identify alternative factor structure for AYA and their caregivers.35 The Scree plot was used to determine the number of factors extracted, and the oblique promax rotation was used to allow the factors to correlate with one another. We sequentially removed items with low factor loadings and repeated the analyses until all the items had a loading of ≥0.35 on at least one factor. We also sequentially removed cross-loaded items (i.e., loaded ≥0.35 on two factors and the difference between two loadings is <0.2). Following finalization of the factor analyses, reliabilities of each factor were confirmed using Cronbach's alpha, using a range of 0.70–0.95 as acceptable.42 Summated subscales, used in subsequent correlation analyses, were generated based on the identified factor structure by summing the Likert responses for each item loading on each factor with reverse coding for items with a negative loading. In addition, continuous scores were calculated from PRPQ items reflecting perceived benefits with a higher score suggesting positive attitudes and from PRPQ items reflecting perceived barriers with a higher score suggesting negative attitudes. A Decision Balance score was computed by converting the benefits and barriers scores to z scores and then subtracting the barriers z score from the benefits z score.43 Positive valence reflects more positive attitudes to clinical trials, and negative valence reflects more negative attitudes. Interitem reliability was also computed for these scales.
Pearson correlation was used to evaluate the association of subscales based on factor analysis, perceived benefits, perceived barriers and decision balance with cancer clinical trial knowledge, AYA age, developmental status, and emotional maturity. There were minimal missing data—7 AYA and 10 caregivers were excluded from the factor analyses. The sample sizes were based on the conservative estimate required for CFA.44 All analyses were performed in SAS 9.3, and a two-sided p-value <0.05 was considered statistically significant.
Results
Participants
AYA (N = 124) had a mean age of 22 years and self-identified as Non-Hispanic/Latino (94%) and White (78%) (Table 1). AYA gender and education level were evenly distributed. Mean age at diagnosis was 19.34 years, and most AYA had a leukemia or lymphoma (51%) and were off cancer treatment (73%). Caregivers (N = 94) were mostly female (84%) with a mean age of 51.19 years and self-identified as Non-Hispanic/Latino (96%) and White (85%).
Table 1.
Participant Demographics
| AYA, N = 124 | Caregivers, N = 94 | |
|---|---|---|
| Age in years | ||
| Mean (SD) (range) | 22.15 (4.22) (15–29) | 51.19 (6.51) (37–71) |
| Sex at birth | ||
| Male | 68 (55%) | 15 (16%) |
| Female | 56 (45%) | 79 (84%) |
| Ethnicity | ||
| Non-Hispanic/Latino | 117 (94%) | 90 (96%) |
| Hispanic/Latino | 7 (6%) | 3 (3%) |
| Unknown | 0 | 1 (1%) |
| Race | ||
| White | 97 (78%) | 80 (85%) |
| Black | 12 (10%) | 5 (5%) |
| Asian | 4 (3%) | 4 (4%) |
| Other/two or more | 10 (8%) | 4 (4%) |
| Did not answer | 1 (1%) | 1 (1%) |
| Education level | ||
| High school graduate or less | 45 (36%) | 34 (36%) |
| Some college | 39 (31%) | 42 (45%) |
| Bachelor's degree or more | 40 (32%) | 18 (19%) |
| Age at diagnosis in years | ||
| Mean (SD) (range) | 19.34 (4.09) (15–28) | — |
| Cancer type | ||
| Leukemia/lymphoma | 63 (51%) | — |
| Solid tumor | 48 (39%) | — |
| Brain tumor | 13 (11%) | — |
| Treatment status | ||
| On treatment | 34 (28%) | — |
AYA, adolescent and young adults.
Factor analyses
In CFA, data for this study had poor fit to the previous PRPQ models.34 The CFA model for caregivers had AGFI of 0.55, CFI of 0.61, and RMSEA of 0.13, and the CFA model for AYA had AGFI of 0.58, CFI of 0.51, and RMSEA of 0.13. EFA for AYA (n = 87) identified a four-factor structure (Table 2): (1) trust/mistrust, (2) barriers/costs, (3) support for participation, and (4) incentives. Kaiser-Meyer-Olkin Measure (KMO) of Sampling Adequacy for the final model is 0.78, suggesting that 78% of variance in the 29 items were explained by the 4 factors. EFA for caregiver (n = 117) suggested a four-factor structure (Table 3): (1) trust/access, (2) mistrust/costs, (3) support for participation, and (4) risks to AYA. KMO for this model is 0.74.
Table 2.
Adolescents and Young Adults with Cancer Exploratory Factor Analysis (N = 117)
| Factor loadings | ||||
|---|---|---|---|---|
| Item description | F1 | F2 | F3 | |
| Trust/mistrust | Barriers/costs | Support for participation | F4 Incentives | |
| I would be more likely to participate in a clinical trial if researchers learn more about my cancer and how to treat it.a | 0.79 | −0.00 | 0.12 | 0.13 |
| I would be more likely to participate in a clinical trial because I trust the medical team.b | 0.71 | 0.23 | −0.09 | 0.43 |
| I would be more likely to participate in a clinical trial if I know exactly what I will be asked to do.b | 0.71 | 0.29 | −0.01 | 0.38 |
| I would be more likely to participate in a clinical trial because I trust the medical institution.b | 0.68 | 0.24 | 0.02 | 0.37 |
| I would be more likely to participate in a clinical trial if it gives me more contact with the health care team.b | 0.61 | 0.05 | 0.20 | 0.08 |
| I would be more likely to participate in a clinical trial if it gives me access to tests/medicine and equipment that my insurance won't cover.b | 0.58 | 0.16 | 0.11 | 0.15 |
| I would be more likely to participate in a clinical trial if I get access to tests/medicine and equipment not available to the public.b | 0.58 | −0.00 | 0.10 | 0.02 |
| Researchers sometimes intentionally hide information from participants before their participation in a clinical trial.a | −0.35 | 0.15 | −0.06 | 0.14 |
| Personal and financial information given during a clinical trial does not stay private and might hurt me or people I care about.b | −0.48 | 0.13 | −0.19 | 0.04 |
| Researchers are motivated by their own career goals and not the welfare of the people who participate in their clinical trials.b | −0.47 | 0.15 | −0.03 | 0.26 |
| Clinical trials purposefully harm racial minority groups.a | −0.64 | 0.01 | −0.06 | 0.08 |
| I would be less likely to participate in a clinical trial if the trial involves randomization.b | 0.14 | 0.73 | −0.03 | 0.10 |
| I would be less likely to participate in a clinical trial if the trial requires a longer time on treatment/more cycles of chemotherapy.c | 0.07 | 0.71 | −0.04 | 0.18 |
| I would be less likely to participate in a clinical trial if the trial might cause me physical harm (i.e., more side effects).a | 0.24 | 0.70 | −0.14 | 0.25 |
| I would be less likely to participate in a clinical trial if the trial requires increased procedures.c | −0.03 | 0.67 | −0.04 | 0.19 |
| I think it's difficult to find time to participate in a clinical trial because I can't take more time off work/school.b | 0.04 | 0.67 | −0.06 | 0.06 |
| It would make me uncomfortable if my health care team wanted me to enroll in a clinical trial because the health care team will view me only as a “guinea pig” if we enroll.a | −0.10 | 0.64 | −0.04 | 0.02 |
| I think it's difficult to find time to participate in a clinical trial because it requires more time at the hospital/clinic and I spend too much time there already.c | −0.04 | 0.61 | −0.10 | 0.04 |
| I think it's difficult to find time to participate in a clinical trial because the trial site is too far away.c | 0.08 | 0.59 | −0.01 | 0.05 |
| It would make me uncomfortable if my health care team wanted me to enroll in a clinical trial because the health care team will treat me differently if we do not enroll.b | −0.16 | 0.54 | 0.00 | −0.10 |
| If I enroll in a clinical trial my outside physician/pediatrician would support our decision to participate.c | 0.07 | 0.04 | 0.85 | −0.09 |
| If I enroll in a clinical trial my community agency (e.g., pediatric cancer foundation) or others who have experience with clinical trials (e.g., support group) would support my decision to participate.a | 0.11 | −0.09 | 0.76 | −0.05 |
| If I enroll in a clinical trial my friends would support our decision to participate.b | 0.16 | −0.19 | 0.67 | 0.13 |
| If I enroll in a clinical trial my religious leader would support our decision to participate.a | 0.01 | −0.11 | 0.66 | −0.03 |
| If I enroll in a clinical trial my health care team would support our decision to participate.b | 0.21 | −0.03 | 0.63 | 0.17 |
| If I enroll in a clinical trial my family would support our decision to participate.b | 0.11 | 0.00 | 0.61 | 0.16 |
| I think more families would participate in clinical trials if the researcher provided transportation and/or housing costs.a | 0.15 | 0.11 | 0.18 | 0.86 |
| I think more families would participate in clinical trials if the researcher provided child care.b | 0.06 | 0.15 | 0.11 | 0.75 |
| I think more families would participate in clinical trials if the researcher provided money or gifts.b | 0.01 | 0.13 | 0.02 | 0.79 |
Final Patient 4-factor model is shown below with 29 items retained (originally 34 items). Seventy-eight percent of covariance explained by 4 factors. Bold values represent factor loadings >0.35.
Modified PRPQ items for cancer.
Original PRPQ items.
New items.
Items removed due to small factor loadings or cross loadings:
I would be more likely to participate in a clinical trial if the researcher from the clinical trial is from the same racial group as me.b
Clinical trials will eventually lead to a cure or improved treatments, both for trial participants and for future patients with cancer.c
Participating in a clinical trial gives us a chance to give back.b
Families don't get enough time to make an informed decision about clinical trial enrollment.c
The hospital has researchers and other members of the community review clinical trials to make sure they are safe.b
PRPQ, Pediatric Research Participation Questionnaire.
Table 3.
Caregiver Exploratory Factor Analysis (N = 87)
| Factor loadings | ||||
|---|---|---|---|---|
| Item description | F1 | F2 | F3 | F4 |
| Access/trust | Mistrust/costs | Support for participation | Risks for AYA | |
| We would be more likely to participate in a clinical trial if it gives us access to tests/medicine and equipment that my insurance won't cover.a | 0.83 | −0.00 | 0.02 | −0.23 |
| We would be more likely to participate in a clinical trial if we get access to tests/medicine and equipment not available to the public.a | 0.82 | −0.03 | 0.17 | −0.19 |
| We would be more likely to participate in a clinical trial if researchers learn more about my child's cancer and how to treat it.b | 0.76 | −0.24 | 0.10 | 0.03 |
| We would be more likely to participate in a clinical trial because we trust the medical team.a | 0.69 | −0.12 | 0.05 | 0.29 |
| We would be more likely to participate in a clinical trial if it gives us more contact with the health care team.a | 0.68 | 0.09 | 0.24 | −0.11 |
| We would be more likely to participate in a clinical trial because we trust the medical institution.a | 0.64 | −0.17 | 0.07 | 0.42 |
| I think it's difficult to find time to participate in a clinical trial because I can't take more time off work/school.a | −0.06 | 0.63 | −0.09 | 0.11 |
| I think it's difficult to find time to participate in a clinical trial because the trial site is too far away.b | 0.03 | 0.61 | −0.22 | 0.05 |
| Researchers are motivated by their own career goals and not the welfare of the people who participate in their clinical trials.a | 0.11 | 0.61 | −0.24 | −0.27 |
| I think it's difficult to find time to participate in a clinical trial because it requires more time at the hospital/clinic and I spend too much time there already.c | −0.13 | 0.56 | −0.08 | 0.18 |
| Personal and financial information given during a clinical trial does not stay private and might hurt me or people I care about.a | 0.05 | 0.54 | −0.10 | 0.12 |
| Researchers sometimes intentionally hide information from participants before their participation in a clinical trial.a | −0.07 | 0.49 | −0.14 | 0.019 |
| I think more families would participate in clinical trials if the researcher provided money or gifts.a | −0.08 | 0.48 | −0.02 | 0.09 |
| Clinical trials purposefully harm racial minority groups.a | −0.13 | 0.47 | −0.02 | 0.07 |
| We would be more likely to participate in a clinical trial if the researcher from the clinical trial is from the same racial group as me.a | −0.08 | 0.42 | 0.08 | 0.13 |
| I think more families would participate in clinical trials if the researcher provided transportation and/or housing costs.b | −0.02 | 0.37 | 0.02 | −0.11 |
| The hospital has researchers and other members of the community review clinical trials to make sure they are safe.a | −0.02 | −0.42 | 0.10 | 0.05 |
| If we enroll in a clinical trial our outside physician/pediatrician would support our decision to participate.c | 0.02 | −0.14 | 0.83 | −0.09 |
| If we enroll in a clinical trial our community agency (e.g., pediatric cancer foundation) or others who have experience with clinical trials (e.g., support group) would support our decision to participate.b | 0.08 | −0.04 | 0.77 | −0.10 |
| If we enroll in a clinical trial our religious leader would support our decision to participate.a | 0.06 | 0.07 | 0.74 | 0.01 |
| If we enroll in a clinical trial our family would support our decision to participate.a | 0.24 | −0.23 | 0.69 | −0.02 |
| If we enroll in a clinical trial our friends would support our decision to participate.a | 0.20 | −0.15 | 0.62 | 0.00 |
| If we enroll in a clinical trial our health care team would support our decision to participate.a | 0.04 | −0.35 | 0.60 | −0.08 |
| We would be less likely to participate in a clinical trial if the trial requires increased procedures.c | −0.10 | 0.29 | −0.03 | 0.73 |
| We would be less likely to participate in a clinical trial if the trial might cause my child physical harm (i.e., more side effects).a | 0.03 | −0.01 | −0.08 | 0.65 |
| We would be less likely to participate in a clinical trial if the trial requires a longer time on treatment/more cycles of chemotherapy.c | −0.19 | 0.31 | −0.10 | 0.67 |
| We would be less likely to participate in a clinical trial if the trial involves randomization.a | 0.07 | −0.02 | −0.02 | 0.50 |
Final Caregiver 4-factor model is shown below with 27 items retained (originally 34 items). Seventy-four percent of covariance explained by 4 factors. Bold represents factor loadings >0.35.
Original PRPQ items.
Modified PRPQ items for cancer.
New items.
Items removed due to small factor loadings or cross loadings:
It would make me uncomfortable if my child's health care team wanted us to enroll in a clinical trial because the health care team will treat my child differently if we do not enroll.a
It would make me uncomfortable if my child's health care team wanted us to enroll in a clinical trial because the health care team will view my child only as a “guinea pig” if we enroll.a
I think more families would participate in clinical trials if the researcher provided child care.a
Clinical trials will eventually lead to a cure or improved treatments, both for trial participants and for future patients with cancer.c
We would be more likely to participate in a clinical trial if we know exactly what we will be asked to do.a
Participating in a clinical trial gives us a chance to give back.a
Families don't get enough time to make an informed decision about clinical trial enrollment.c
Validation analyses
Interitem reliability
Acceptable reliability42 was identified for the PRPQ factors for AYA (α = 0.86 for Factor 1, α = 0.86 for Factor 2, α = 0.88 for Factor 3, and α = 0.88 for Factor 4) and caregivers (α = 0.88 for Factor 1, α = 0.80 for Factor 2, α = 0.86 for Factor 3, and α = 0.76 for Factor 4). Acceptable reliability was also confirmed for PRPQ scale scores for AYA-perceived benefits (α = 0.86) and barriers (α = 0.83) and caregivers-perceived benefits (α = 0.79) and barriers (α = 0.79).
Pearson correlations
PRPQ-AYA factor scores and computed scales were correlated with each other (Supplementary Tables S1 and S2). For AYA, trust/mistrust (Factor 1) was significantly positively correlated with cancer clinical trial knowledge and emotional maturity (reappraisal and consideration of future consequences), and support for participation (Factor 3) was significantly negatively correlated with emotion suppression (Table 4). AYA perceived benefits were significantly correlated with knowledge and emotional maturity (reappraisal, suppression, and consideration of future consequences) in the expected direction of greater knowledge and more maturity linked to higher perceived benefits. AYA perceived benefits were not significantly associated with age or developmental maturity. AYA perceived barriers were not significantly associated with any of the variables of interest. AYA decision balance was significantly associated with knowledge and emotional maturity (reappraisal) in the expected direction of more positive attitudes linked to greater knowledge and more maturity; decision balance was not significantly associated with age, developmental maturity, or emotional maturity (consideration of future consequences and suppression).
Table 4.
Pearson Correlations of PRPQ-AYA Factor Scores and Scale Scores with Validation Measures for AYA
| Validation measure | Mean (SD) | F1 | F2 | F3 | Perceived benefits | Perceived barriers | Decision balance | |
|---|---|---|---|---|---|---|---|---|
| Trust/mistrust | Barriers/costs | Support for participation | F4 Incentives | |||||
| Knowledge of clinical trials | 80.19 (14.08) | 0.45** | 0.10 | 0.13 | 0.14 | 0.35** | −0.07 | 0.27** |
| Consideration of future consequences | 3.63 (0.50) | 0.28** | 0.09 | 0.07 | 0.06 | 0.19* | −0.01 | 0.15 |
| Emotion regulation—reappraisal | 4.26 (0.98) | 0.28** | −0.08 | 0.08 | −0.02 | 0.21* | −0.16 | 0.25** |
| Emotion regulation—suppression | 2.16 (0.70) | −0.11 | 0.04 | −0.18* | −0.10 | −0.18* | 0.06 | −0.17 |
There were no significant correlations with age of AYA or developmental milestones achieved.
p < 0.05; **p < 0.01.
For caregivers, access/trust (Factor 1) was significantly positively correlated with AYA age, and mistrust/costs (Factor 2) were significantly negatively correlated with knowledge (Table 5). None of the other caregiver factor scores or caregiver perceived benefits was associated with knowledge. However, greater knowledge correlated with fewer perceived barriers and more positive attitudes toward clinical trials, and older AYA age correlated with more perceived benefits.
Table 5.
Pearson Correlations of PRPQ-AYA Factor Scores and Scale Scores with Validation Measures for Caregivers
| Validation measure | Mean (SD) | F1 | F2 | F3 | F4 | Perceived benefits | Perceived barriers | Decision balance |
|---|---|---|---|---|---|---|---|---|
| Support for participation | Risks for AYA | |||||||
| Access/trust | Mistrust/costs | |||||||
| Age of AYA | 20.62 (4.30) | 0.35** | 0.10 | 0.10 | 0.11 | 0.28** | 0.16 | 0.08 |
| Knowledge of clinical trials | 83.78 (10.44) | 0.04 | −0.29** | 0.11 | −0.08 | 0.08 | −0.23* | 0.21* |
p < 0.05; **p < 0.01.
Discussion
The literature on the challenges of limited clinical trial enrollment for AYA has focused on structural and societal barriers to participation in clinical trials and its impact on development of effective cancer therapies.1–3,8 However, AYA and their families' decision-making around cancer clinical trials, engagement of AYA, and the role of attitudes to enrollment have only recently been recognized as important components of efforts to increase AYA participation in cancer clinical trials.45,46 This study of AYA drawn from pediatric and adult cancer centers contributes to this emerging literature and suggests next steps in this important area of study. Separate four-factor structures of the PRPQ-AYA for AYA and their caregivers were identified although these scales must be interpreted with caution, as power was limited for the planned factor analyses. In contrast, findings from this study support scoring the PRPQ-AYA by summarizing perceived benefits, perceived barriers, and decision balance; these scores correlated with knowledge of clinical trials and emotional maturity for AYA and their caregivers. More research on the PRPQ-AYA is required to evaluate its utility in improving clinical trial informed consent communication and fostering positive decision-making processes for AYA.
We acknowledge that power was limited, but the factor analyses provide interesting hypotheses for future study. CFA did not support the same factor structure for AYA with cancer and their caregivers as for youth with health disparity conditions and their caregivers. This is not surprising given that important demographic (race/ethnicity, SES) and disease-related (diagnosed at birth and prior exposure to medical research) differences may shape perceptions of clinical trial research over time.47,48 It should be noted, however, that similar themes were reflected, including mistrust, social support for participation in trials, and risks (caregivers) and incentives (AYA). Furthermore, although the factors for AYA and their caregivers had some parallels (support for participation in research), caregiver responses converged around assessment of risks and evaluation of the extent to which they had trust in the provider and the institution offering the trial while AYA prioritized costs and incentives. These differences are consistent with our prior qualitative study15 and may reflect the ways in which AYA and caregiver values differentially influence clinical trial enrollment decision processes.49 Patterns of decision-making vary—from collaborative to the caregiver or AYA being the primary decision-maker—highlighting the importance of incorporating differing valuations of clinical trials around issues of benefit, risk, and incentives into the informed consent discussion process.15
Summing perceived benefits and perceived barriers on the PRPQ-AYA, as well as computing decision balance, provided psychometrically stronger and relevant information. These variables were associated with cancer clinical trial knowledge and with AYA emotional maturity, suggesting that increased knowledge can improve understanding of benefits of clinical trial enrollment. Findings from this study are consistent with but extend to those of two recent studies of AYA decision-making around cancer clinical trial enrollment in important ways.45,46 In prior studies, young adult survivors reported perceived benefits and barriers related to safety of trials.46 Furthermore, barriers to enrollment include support from the family or peer group, assessment of impact of trial enrollment on goals, and health status.45 In contrast, our results highlight the importance of accounting for trust/mistrust in research and researchers, incentives and practical considerations for participation, and community support for cancer research. PRPQ. AYA responses also underscore the need to account for attitudes of others involved in decision-making, including caregivers.
An important finding from this study on AYA clinical trial decision-making is that emotional maturity, not age or developmental milestones achieved, plays a role in understanding perceived barriers and benefits of cancer clinical trial enrollment. This is consistent with an extensive body of research in developmental science, with emerging data from neuroscience, demonstrating the role of cognitive and emotional development in understanding AYA readiness for decision-making.50 Children's Oncology Group and American Academy of Pediatrics provide children to be involved in medical decision-making to the extent they are capable,16,27 but research has primarily focused on limits related to age or cognitive developmental level.9,22 Although assessment of emotional maturity is more involved than use of age, it is a critical factor in readiness to participate in decisions about treatment in meaningful ways. Feasibility studies integrating this factor in planning for clinical trial communication can be undertaken.
AYA research is complicated by the challenges of obtaining sufficiently large diverse samples to address the study aims. There was sufficient power for correlation analyses, but sample size was more limited for CFA and dyadic analysis (AYA-caregiver pairs). The sample is representative of AYA in our cancer centers, but studies from multiple sites that oversample ethnic minority AYA and survey AYA closer in time to the clinical trial decision are necessary. We intended to assess differences by prior enrollment on a clinical trial. However, self-reported rates of participation were inconsistent and lower than the documented percentage of AYA exposed to cancer clinical trials in our centers, highlighting potential lack of understanding of clinical trials versus standard treatment.
Newly diagnosed AYA and their caregivers are given extensive complicated information on diagnosis, treatment, and clinical trials in the intense emotional context of diagnosis with short time allotted for decision-making,27 often resulting in miscomprehension.26,27 Factors contributing to AYA clinical trial enrollment are multiple and nuanced,51 and increased understanding of knowledge and attitudes to cancer and cancer clinical trials for AYA and their caregivers and their contribution to decision-making is a critical component to address this issue. Based on results of this study, use of PRPQ-AYA computed summary scales to reflect perceived barriers and benefits to clinical trial participation may serve to identify relevant attitudes that can be addressed. Additional research to further evaluate validity and reliability of the PRPQ-AYA, specifically the factor structure, closer to the informed consent process is needed.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Children's Hospital of Philadelphia Center for Childhood Cancer Research/Alex's Lemonade Stand Foundation to L.P.B.
Disclaimer
Portions of this article were presented in a poster discussion session at the 2017 World Congress of the International Society of Pediatric Oncology, Washington, DC.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Bleyer A, Viny A, Barr R. Cancer in 15- to 29-year-olds by primary site. Oncologist 2006;11:590–601. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute, LIVESTRONG Young Adult Alliance. Closing the gap: research and care imperative for adolescents and young adults with cancer, in NIH (ed). Betheseda, MD: NIH; 2006. [Google Scholar]
- 3. Burke ME, Albritton K, Marina N. Challenges in the recruitment of adolescents and young adults to cancer clinical trials. Cancer 2007;110:2385–93. [DOI] [PubMed] [Google Scholar]
- 4. Bleyer WA. Cancer in older adolescents and young adults: epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Med Pediatr Oncol 2002;38:1–10. [DOI] [PubMed] [Google Scholar]
- 5. Ferrari A, Bleyer A. Participation of adolescents with cancer in clinical trials. Cancer Treat Rev 2007;33:603–8. [DOI] [PubMed] [Google Scholar]
- 6. Stock W, La M, Sanford B, et al. . What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood 2008;112:1646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fern LA, Taylor RM. Enhancing accrual to clinical trials of adolescents and young adults with cancer. Pediatr Blood Cancer 2018;65:e27233. [DOI] [PubMed] [Google Scholar]
- 8. Fern LA, Lewandowski JA, Coxon KM, et al. . Available, accessible, aware, appropriate, and acceptable: a strategy to improve participation of teenagers and young adults in cancer trials. Lancet Oncol 2014;15:e341–50. [DOI] [PubMed] [Google Scholar]
- 9. McCabe MA. Involving children and adolescents in medical decision making: developmental and clinical considerations. J Pediatr Psychol 1996;21:505–16. [DOI] [PubMed] [Google Scholar]
- 10. Martin LR, DiMatteo MR, Lepper HS. Facilitation of patient involvement in care: development and validation of a scale. Behav Med 2001;27:111–20. [DOI] [PubMed] [Google Scholar]
- 11. Broome ME, Richards DJ, Hall J. Children in research: the experience of ill children and adolescents. J Fam Nurs 2001;7:32–49. [Google Scholar]
- 12. Kunin H. Ethical issues in pediatric life-threatening illness: dilemmas of consent, assent, and communication. Ethics Behav 1997;7:43–57. [DOI] [PubMed] [Google Scholar]
- 13. Brody JL, Annett RD, Scherer DG, et al. . Enrolling adolescents in asthma research: adolescent, parent, and physician influence in the decision-making process. J Asthma 2009;46:492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leikin S. The role of adolescents in decisions concerning their cancer therapy. Cancer 1993;71:3342–6. [DOI] [PubMed] [Google Scholar]
- 15. Barakat LP, Schwartz LA, Reilly A, et al. . Perceived barriers and benefits of Phase III clinical trials participation for adolescents and young adults with cancer (AYA): A qualitative study of AYA decision making experiences. J Adolesc Young Adult Oncol 2014;3:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Informed consent, parental permission, and assent in pediatric practice. Committee on Bioethics, American Academy of Pediatrics. Pediatrics 1995;95:314–7. [PubMed] [Google Scholar]
- 17. Unguru Y, Sill AM, Kamani N. The experiences of children enrolled in pediatric oncology research: implications for assent. Pediatrics 2010;125:e876–83. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz LA, Drotar D. Health-related hindrance of personal goal pursuit and well-being of young adults with cystic fibrosis, pediatric cancer survivors, and peers without a history of chronic illness. J Pediatr Psychol 2009;34:954–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hedstrom M, Ljungman G, von Essen L. Perceptions of distress among adolescents recently diagnosed with cancer. J Pediatr Hematol Oncol 2005;27:15–22. [DOI] [PubMed] [Google Scholar]
- 20. Richmond TS, Ulrich C. Ethical issues of recruitment and enrollment of critically ill and injured patients for research. AACN Adv Crit Care 2007;18:352–5. [DOI] [PubMed] [Google Scholar]
- 21. de Vries MC, Wit JM, Engberts DP, et al. . Pediatric oncologists' attitudes towards involving adolescents in decision-making concerning research participation. Pediatr Blood Cancer 2010;55:123–8. [DOI] [PubMed] [Google Scholar]
- 22. Stegenga K, Ward-Smith P. The adolescent perspective on participation in treatment decision making: a pilot study. J Pediatr Oncol Nurs 2008;25:112–7. [DOI] [PubMed] [Google Scholar]
- 23. Miller VA, Reynolds WW, Nelson RM. Parent-child roles in decision making about medical research. Ethics Behav 2008;18:161–81. [Google Scholar]
- 24. Truong TH, Weeks JC, Cook EF, et al. . Outcomes of informed consent among parents of children in cancer clinical trials. Pediatr Blood Cancer 2011;57:998–1004. [DOI] [PubMed] [Google Scholar]
- 25. Forcina V, Vakeesan B, Paulo C, et al. . Perceptions and attitudes toward clinical trials in adolescent and young adults with cancer: a systematic review. Adolesc Health Med Ther 2018;9:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simon CM, Siminoff LA, Kodish ED, et al. . Comparison of the informed consent process for randomized clinical trials in pediatric and adult oncology. J Clin Oncol 2004;22:2708–17. [DOI] [PubMed] [Google Scholar]
- 27. Joffe S, Fernandez CV, Pentz RD, et al. . Involving children with cancer in decision-making about research participation. J Pediatr 2006;149:862–8. [DOI] [PubMed] [Google Scholar]
- 28. Miller FG, Rosenstein DL. The therapeutic orientation to clinical trials. N Engl J Med 2003;348:1383–6. [DOI] [PubMed] [Google Scholar]
- 29. Schaeffer MH, Krantz DS, Wichman A, et al. . The impact of disease severity on the informed consent process in clinical research. Am J Med 1996;100:261–8. [DOI] [PubMed] [Google Scholar]
- 30. Mills EJ, Seely D, Rachlis B, et al. . Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol 2006;7:141–8. [DOI] [PubMed] [Google Scholar]
- 31. Ulrich CM, James JL, Walker EM, et al. . RTOG physician and research associate attitudes, beliefs and practices regarding clinical trials: implications for improving patient recruitment. Contemp Clin Trials 2010;31:221–8. [DOI] [PubMed] [Google Scholar]
- 32. Read K, Fernandez CV, Gao J, et al. . Decision-making by adolescents and parents of children with cancer regarding health research participation. Pediatrics 2009;124:959–65. [DOI] [PubMed] [Google Scholar]
- 33. Varma S, Jenkins T, Wendler D. How do children and parents make decisions about pediatric clinical research? J Pediatr Hematol Oncol 2008;30:823–8. [DOI] [PubMed] [Google Scholar]
- 34. Barakat LP, Patterson CA, Mondestin V, et al. . Initial development of a questionnaire evaluating perceived benefits and barriers to pediatric clinical trials participation. Contemp Clin Trials 2013;34:218–26. [DOI] [PubMed] [Google Scholar]
- 35. Schmitt TA. Current methodological considerations in exploratory and confirmatory factor analysis. J Psychoeduc Assess 2011;29:304–21. [Google Scholar]
- 36. Ottawa Hospital Research Institute: patient decision aids. 2015. Accessed April 7, 2019 from: https://decisionaid.ohri.ca
- 37. Campbell HM, Raisch DW, Sather MR, et al. . Impact of a clinical trials information handbook on patient knowledge, perceptions, and likelihood of participation. IRB 2008;30:6–14. [PubMed] [Google Scholar]
- 38. Seiffge-Krenke I. Chronic disease and perceived developmental progression in adolescence. Dev Psychol 1998;34:1073–84. [DOI] [PubMed] [Google Scholar]
- 39. Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol 2003;85:348–62. [DOI] [PubMed] [Google Scholar]
- 40. Strathman A, Gleicher F, Boninger DS, et al. . The consideration of future consequences: weighing immediate and distand outcomes of behavior. J Pers Soc Psychol 1994;66:742–52. [Google Scholar]
- 41. Hu L, Bentler PM. Cutoff criteria for fit inexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model 1999;6:1–55. [Google Scholar]
- 42. Tavakol M, Dennick R. Making sense of Cronbach's alpha. Int J Med Educ 2011;2:53–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rakowski W, Clark MA, Pearlman DN, et al. . Integrating pros and cons for mammography and Pap testing: extending the construct of decisional balance to two behaviors. Prev Med 1997;26:664–73. [DOI] [PubMed] [Google Scholar]
- 44. MacCallum RC, Widaman KF, Zhange S, et al. . Sample size in factor analysis. Psychol Methods 1999;4:84–9. [Google Scholar]
- 45. Gupta AA, Bell JAH, Wang K, et al. . Evaluation of adolescents and young adults (AYA) attitudes towards participation in cancer clinical trials. J Clin Oncol 2017;35:10047. [DOI] [PubMed] [Google Scholar]
- 46. Grigsby TJ, Kent EE, Montoya MJ, et al. . Attitudes toward cancer clinical trial participation in young adults with a history of cancer and a healthy college student sample: A preliminary investigation. J Adolesc Young Adult Oncol 2014;3:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patterson CA, Chavez V, Mondestin V, et al. . Clinical trial decision making in pediatric sickle cell disease: A qualitative study of perceived benefits and barriers to participation. J Pediatr Hematol Oncol 2015;37:415–22. [DOI] [PubMed] [Google Scholar]
- 48. Stevens EM, Patterson CA, Li YB, et al. . Mistrust of pediatric sickle cell disease clinical trials research. Am J Prev Med 2016;51:S78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ladd RE, Forman EN. Adolescent decision-making: giving weight to age-specific values. Theor Med 1995;16:333–45. [DOI] [PubMed] [Google Scholar]
- 50. Steinberg L, Cauffman E, Woolard J, et al. . Are adolescents less mature than adults?: minors' access to abortion, the juvenile death penalty, and the alleged APA “flip-flop”. Am Psychol 2009;64:583–94. [DOI] [PubMed] [Google Scholar]
- 51. Smith MA, Joffe S. Will My Child Do Better if She Enrolls in a Clinical Trial? Cancer 2018;124:3965–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.