Abstract
Cholestatic and non-alcoholic fatty liver disease (NAFLD) share several key pathophysiological mechanisms which can be targeted by novel therapeutic concepts that are currently developed for both areas. Nuclear receptors (NRs) are ligand-activated transcriptional regulators of key metabolic processes including hepatic lipid and glucose metabolism, energy expenditure and bile acid (BA) homoeostasis, as well as inflammation, fibrosis and cellular proliferation. Dysregulation of these processes contributes to the pathogenesis and progression of cholestatic as well as fatty liver disease, placing NRs at the forefront of novel therapeutic approaches. This includes BA and fatty acid activated NRs such as farnesoid-X receptor (FXR) and peroxisome proliferator-activated receptors, respectively, for which high affinity therapeutic ligands targeting specific or multiple isoforms have been developed. Moreover, novel liver-specific ligands for thyroid hormone receptor beta 1 complete the spectrum of currently available NR-targeted drugs. Apart from FXR ligands, BA signalling can be targeted by mimetics of FXR-activated fibroblast growth factor 19, modulation of their enterohepatic circulation through uptake inhibitors in hepatocytes and enterocytes, as well as novel BA derivatives undergoing cholehepatic shunting (instead of enterohepatic circulation). Other therapeutic approaches more directly target inflammation and/or fibrosis as critical events of disease progression. Combination strategies synergistically targeting metabolic disturbances, inflammation and fibrosis may be ultimately necessary for successful treatment of these complex and multifactorial disorders.
Keywords: inflammation, fibrosis
Key message.
Cholestatic and non-alcoholic fatty liver disease share several key pathophysiological mechanisms which can be targeted by novel therapeutic concepts that are currently developed for both areas.
Pharmacological ligands for nuclear (hormone) receptors (NRs) regulate key metabolic pathways such as bile acid (BA), lipid, glucose and energy metabolism, as well as inflammation and fibrosis placing them at the forefront of current therapeutic developments.
Therapeutic modulation of BA signalling through pharmacological ligands for the nuclear bile acid receptor (farnesoid-X receptor (FXR)), mimetics of the FXR-induced gut hormone ‘fibroblast growth factor 19’ and side-chain modified BA (nor-ursodeoxycholic acid) have shown promising clinical results.
Novel pharmacological ligands for specific isoforms of peroxisome proliferator-activated receptors and liver-specific ligands for thyroid hormone receptor beta 1 complete the spectrum of currently available NR-targeted drugs.
Combination strategies synergistically targeting metabolic disturbances, intestinal dysbiosis, inflammation and fibrosis may be necessary for successful treatment of these complex and multifactorial disorders.
Introduction
Although cholestatic and non-alcoholic fatty liver disease (NAFLD) are aetiologically different, they share several key pathophysiological mechanisms which may be amenable to novel therapeutic interventions. Notably, therapeutic concepts for both disease areas have often been promoted by eminent scientists who were active on both sides with ursodeoxycholic acid (UDCA) being a common denominator for a considerable amount of time.1 2 Much has changed in recent years due to our significant advances in understanding the molecular mechanism of bile acid (BA) and lipid metabolism, their regulation and what goes awry in disease.3–6 NAFLD has become the most common chronic liver disease globally affecting up to 30% of the adult population and cirrhosis due to non-alcoholic steatohepatitis (NASH) as potentially progressive variant has become the second leading indication of liver transplantation with numbers further rising, expected to surpass all other indications in the near future.7 Intriguingly, rare (orphan) immune-mediated liver diseases (such as primary sclerosing cholangitis (PSC) have meanwhile become the third leading indication for liver transplantation in Europe already outnumbering hepatitis C,8 reflecting the huge unmet medical need in PSC and other immune-mediated liver diseases with effective therapeutic strategies still significantly lagging behind other areas of hepatology. While numbers of patients with primary biliary cholangitis (PBC) listed for liver transplantation, have decreased by almost 50% despite opposing epidemiological trends, possibly due to the therapeutic impact of UDCA, PSC has become the leading indication for liver transplantation among patients with immune-mediated liver diseases.8 9 Thus, both cholestatic and fatty liver diseases urgently require novel and effective therapies to prevent or at least reduce the growing burden of liver transplantation and death.
Shared pathogenetic principles as targets for therapeutic interventions
NAFLD is considered the hepatic manifestation of metabolic syndrome10 where lipid overload as a result of increased fatty acid influx due to insulin resistance in the adipose tissue leads to metabolically stressed hepatocytes with activation of cell death and proinflammatory signalling pathways.6 NASH may therefore be grossly viewed as an influx problem due to increased fatty acid load with consecutive lipotoxicity,11 12 while cholestasis may be considered an efflux problem resulting in accumulation of potentially cytotoxic and proinflammatory BAs and other cholephiles.13 14 In both scenarios failed or inadequate metabolic adaptation to substrate overload results in sublethal cell stress or even cell death with release of mediators and extracellular vesicles driving inflammation and fibrosis15–17 (figure 1). Interestingly, BA toxicity as result of impaired hepatobiliary excretory function18 19 and functional (micro)cholestasis20 may also be involved in the pathogenesis of NASH.12 Given the central role of BAs in regulating hepatic lipid metabolism, dysregulation of BA homoeostasis and signalling may further contribute to abnormal lipid metabolism and lipotoxicity in NAFLD.12 Liver fibrosis is the consequence of a sustained woundhealing process caused by unresolved chronic cell injury and is characterised by excessive accumulation of extracellular matrix components produced by activated hepatic stellate cells (HSC).21 Since fibrosis represents an important prognostic turning point in the evolution of virtually any liver disease, direct antifibrotic strategies have ever since received much attention.22 The ideal drug would be expected to impact on all critical steps involved in the progression of liver diseases, ranging from the initial (metabolic) insult, over cellular stress/death, inflammation to fibrosis (figure 1). Nuclear (hormone) receptors (NRs) are ligand-activated transcription factors which control a broad spectrum of genes involved in (BA) metabolism, inflammation, cell proliferation and tissue repair including fibrosis (figures 2 and 3)23 which makes them highly attractive targets for treatment of metabolic and cholestatic disorders (see below). Direct antifibrotic therapeutic actions may become more critical when liver disease is already too advanced to allow sufficient time for primarily causal therapeutics to act, since clinical progression of advanced fibrosis may occur more rapidly than previously anticipated.24
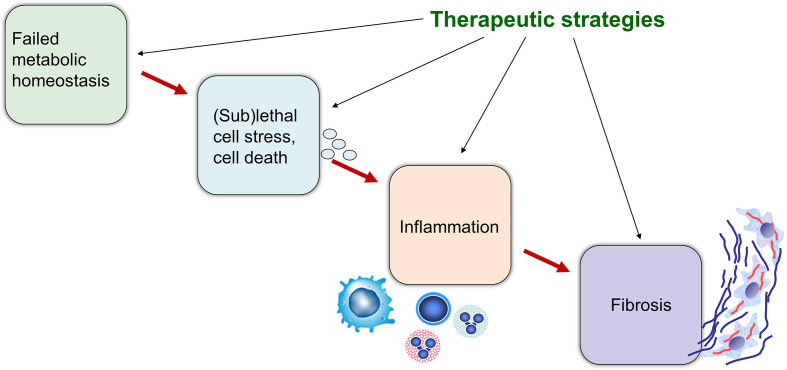
Figure 1.
Failed metabolic homoeostasis results in sublethal cell stress, inflammation and fibrosis. In both non-alcoholic fatty liver disease and cholestasis, inadequate metabolic adaptation to substrate overload results in sublethal cell stress or even cell death with release of mediators (eg, cytokines, chemokines, microRNAs), in part as cargo of extracellular vesicles, driving inflammation and fibrosis. The ideal therapeutic strategy would be expected to impact on several if not all critical steps involved in the initiation and progression of liver diseases. Combination strategies synergistically targeting metabolic disturbances, inflammation and fibrosis may be ultimately necessary for successful treatment of complex cholestatic and metabolic liver diseases.
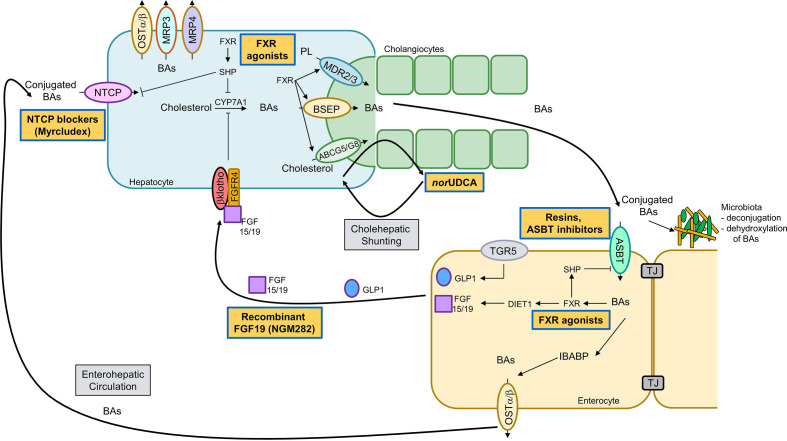
Figure 2.
Therapeutic strategies along the enterohepatic and cholehepatic bile acid (BA) circulation. After hepatic synthesis via cytochrome P450 7A1 (CYP7A1) and excretion into bile through the bile salt export pump (BSEP/ABCB11) BAs undergo an enterohepatic circulation, that is, they are reabsorbed in the ileum by apical sodium-dependent bile acid transporter (ASBT/SLC10A2) and transported back to the liver through portal blood where hepatic reuptake of conjugated BAs is mediated via sodium/taurocholate cotransporting polypeptide (NTCP/SLC10A1) and organic anion transporting polypeptides (OATPs) for unconjugated BAs (not shown). In hepatocytes, farnesoid-X receptor (FXR) induces the transcriptional repressor SHP which in turn inhibits CYP7A1 (BA synthesis) and NTCP transcription (BA uptake). FXR induces BSEP, phospholipid export pump/floppase (MDR3/ABCB4; Mdr2 in mice) and cholesterol export pump (ABCG5/8). At the basolateral membrane organic solute transporter (OSTα/OSTβ), multidrug resistance-related proteine (MRP)3 and MRP4 facilitate alternative hepatic BA pump which is also in part induced by FXR (not shown). After uptake of BAs via ASBT into enterocytes (lower panel), BA-activated FXR induces sinusoidal OSTα/OSTβ heterodimer for BA efflux into portal blood. Intestinal FXR via DIET1 induces fibroblast growth factor (FGF) 19, which circulates to the liver and binds to its receptor FGFR4, subsequently inhibiting BA synthesis. Gut microbiota deconjugate and dehydroxylate primary BAs into secondary BAs. Enterohepatic drugs acting within the gut-liver axis: (non-)steroidal FXR agonists (eg, obeticholic acid) and FGF19 mimetics. Cholehepatic drugs, such as nor-ursodeoxycholic acid (norUDCA), undergo cholehepatic shunting between hepatocytes and cholangiocytes, thereby cutting short the enterohepatic circulation. Transport blockers: ASBT inhibitors and BA sequestrants as well as NTCP inhibitors, prevent intestinal or hepatic BA reuptake. TJ, tight junction.
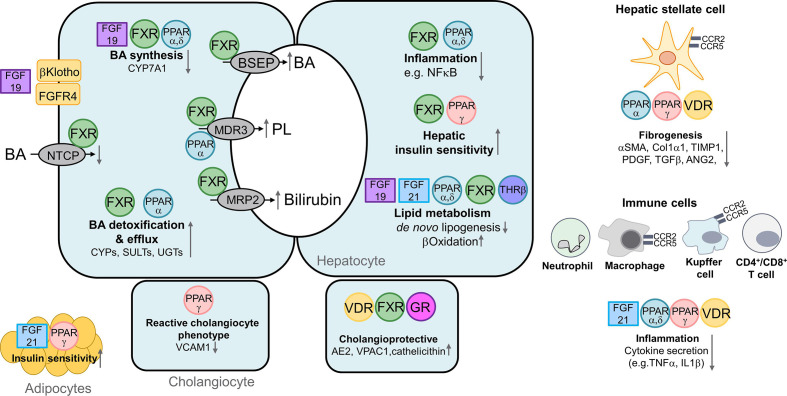
Figure 3.
Nuclear receptors as therapeutic targets regulating metabolism and inflammation. Hepatocyte, left panel: farnesoid X receptor (FXR) represses hepatic bile acid (BA) uptake sodium/taurocholate cotransporting polypeptide (NTCP) and BA synthesis cytochrome P450 7A1 (CYP7A1) via induction of the transcriptional repressor SHP (not shown). Moreover intestine‐derived fibroblast growth factor (FGF) 19 (binding to the FGFR4/βKlotho dimer) also downregulates CYP7A1 expression. Conversely, FXR promotes biliary excretion of BAs, phospholipids (PL) and bilirubin via induction of canalicular bile salt export pump (BSEP), multidrug resistant protein 3 (MDR3) and multidrug resistance-related protein 2 (MRP2), respectively (centre), and also facilitates BA elimination via alternative basolateral BA transporter such as organic solute transporter (OSTα/β) (not shown). BA detoxification by phase 1 and 2 enzymes is stimulated through FXR and peroxisome proliferator-activated receptor (PPAR)α. PPARα stimulates phospholipid secretion (via MDR3), thus counteracting intrinsic bile toxicity. Right panel: FXR as well as PPAR α and δ reduce inflammation via suppression of NFκΒ. FXR and PPARγ improve hepatic insulin sensitivity. FGF19, FGF21, FXR and thyroid hormone receptor beta (THRβ) suppress de novo lipogenesis, while PPAR α and δ stimulate β−oxidation. In cholangiocytes (lower panel), activation of FXR, vitamin D receptor (VDR) and glucocorticoid receptor (GR) exert cholangioprotective effects via upregulation of vasoactive intestinal polypeptide receptor 1 (VPAC1), anion exchanger (AE) 2 and cathelicidin. Activation of PPARγ in cholangiocytes reduces vascular cell adhesion molecule (VCAM‐1) expression, thereby counteracting reactive cholangiocyte phenotype. Anti-fibrotic effects of nuclear receptors (NRs) in hepatic stellate cells (HSCs, far right panel): PPARα and γ and VDR reduce expression of profibrogenic genes such as alpha smooth muscle actin (αSMA), Collagen 1a1 (Col1α1), TIMP1, platelet-derived growth factor (PDGF), transforming growth factor beta (TGFβ) and angiopoietin-2 (ANG2). Furthermore, NRs reduce migration, proliferation as well as trans-differentiation of HSC into myofibroblasts. Anti-inflammatory effects of NRs are related to their activation in immune cells such as macrophages and Kupffer cells (as well as adaptive immune cells, not shown). Activation of FGF21, PPARα, γ, δ and VDR reduce expression of proinflammatory cytokines such as tumour necrosis factor alpha (TNFα) and interleukin 1 beta (IL1β) (lower right panel). Cenicriviroc (CVC) an antagonist for C-C chemokine receptor type 2 and 5 (CCR2/5) on macrophages, Kupffer cells and HSCs exerts anti-inflammatory and anti-fibrotic effects, As result of FGF21 and PPARγ activation in adipocytes, insulin sensitivity is increased (lower left panel).
Both cholestatic and fatty liver diseases are characterised by complex cellular interaction of hepatocytes with cholangiocytes, HSCs and proinflammatory cells, such as monocyte-derived macrophages, resident Kupffer cells and lymphocytes. Fatty acid (FA)-induced lipotoxicity results in cellular stress of hepatocytes,11 but also cholangiocytes25 and HSCs.26 BA-induced cell stress may occur both at the level of hepatocytes (eg, via BA retention) and cholangiocytes (eg, via toxic bile composition and/or biliary stasis).5 14 Another interesting convergence point are cytoskeletal alterations with formation of Mallory-Denk bodies in response to (FA and BA-induced) cell stress observed in both NASH and cholestasis.27 In addition to direct activation of inflammatory and profibrogenic cells by toxic BAs and fatty acids,5 6 12 21 there is intensive cellular crosstalk through proinflammatory and profibrogenic mediators (partly as cargo of extracellular vesicles) released from stressed hepatocytes and cholangiocytes as part of the ‘reactive cholangiocyte phenotype’,28 further perpetuating inflammatory and profibrotic responses.17 29 Importantly, NRs are broadly expressed in all relevant liver cellular compartments, including hepatocytes, cholangiocytes, HSCs, macrophages and other immune cells, making them highly suitable therapeutic targets for both cholestatic and metabolic liver diseases.27
Together with environmental factors and lifestyle, genetic factors are key determinants for the pathogenesis and progression of liver diseases.30 Although originally discovered in the context of NAFLD/NASH, genetic variants of the patatin-like phospholipase domain-containing 3 (PNPLA3), also known as adiponutrin, play a key role in the progression of liver diseases of virtually any aetiology.31 32 Despite the clinical importance of the PNPLA3 (I148M) variant for progression to NASH, fibrosis and hepatocellular cancer (HCC) the function of PNPLA3 is still controversial.32 In addition to hepatocytes and HSCs,32 PNPLA3 is also highly expressed in cholangiocytes.33 Interestingly patients with PBC carrying the PNPLA3 I148M variant reported less pruritus,34 which could be due to the role of this enzyme in metabolism of lipid metabolites linked to the pathogenesis of cholestatic pruritus.35 In male patients with PSC with bile duct stenosis requiring intervention, the PNPLA3 I148M variant may be a risk factor for reduced survival36 while another study found no impact.37 Knockdown/inhibition of PNPLA3 variants is currently receiving considerable attention as novel treatment strategy for NASH.38 Variants of the ABCB4 gene encoding the hepatobiliary phosphatiylcholine floppase not only confer risk of bile duct injury and cholestatic liver disease by altering bile toxicity,39 but large-scale whole-genome sequencing uncovered a common ABCB4 variant as a general risk factor for elevated aminotransferases and higher impact variants as potential determinants of early-onset gallstone disease, cholestasis of pregnancy, liver cirrhosis and hepatobiliary cancer.40 As pointed out below, ABCB4 expression can be stimulated by a wide range of NR ligands and chaperones can (partly) restore impaired mutant function.39 These examples may demonstrate, how genetic variants initially considered only in a specific context may have more global impact on progression of liver diseases irrespective of their specific aetiology.
BAs have emerged as important pathogenetic factors and therapeutic targets in both cholestatic and metabolic liver diseases.4 13 41 Since BAs are potentially cytotoxic and proinflammatory at higher pathophysiological concentrations14 it is important to maintain their homoeostasis by controlling BA transport and metabolism (figure 2).13 Besides their well-established role in dietary lipid absorption, BAs have recently been recognised to act as hormone-like signalling molecules that serve as ligands for NRs such as the farnesoid X receptor (FXR/NR1H4) as main NR for BAs.42–44 In addition to FXR also other NRs such as the pregnane X receptor (PXR/NR1I2), the constitutive androstane receptor (CAR/NR1I3) and the vitamin D receptor (NR1I1) are activated via certain BAs.45 Through these NRs BA control their own transport and metabolism, lipid and glucose metabolism as well as innate/adaptive immunity.41 Additional critical NRs for the control of metabolism include peroxisome proliferator-activated receptors (PPARs) α, γ and δ as well as thyroid hormone receptor (THR) β. Another key BA receptor is the Takeda G protein-coupled receptor TGR5 (GP-BAR or M-BAR) a G-protein coupled receptor (GPCR).46 In contrast to NRs, GPCRs are localised at the cell membrane and cellular BA uptake/transport is not required for the activation of these receptors. Initial studies uncovered a key role of TGR5 in mediating immunosuppressive effects of BAs on macrophages.46 Moreover, TGR5 has an important role in regulating energy expenditure and lipid metabolism,47 again highlighting the potential role of BAs as key regulators of immunometabolism. Impaired TGR5 expression and signalling may contribute to the pathogenesis of cholangiopathies such as PBC and PSC, since TGR5 also protects cholangiocytes by stimulating bicarbonate secretion.48 49 However, persistent stimulation of TGR5 may predispose to gallstone formation through changes in gallbladder motility and promotes proliferation of cholangiocellular carcinoma (CCC) cells and polycystic cholangiocytes which overexpress TGR5, thus raising safety concerns for clinical development of TGR5 agonists.48 Therefore, depending on the clinical context and the underlying disease either activation or inhibition of TGR5 may have beneficial effects. The entero-endocrine signalling function of BAs is further expanded by stimulation of metabolically active gut hormones such as fibroblast growth factor 15 (murine analogue to fibroblast growth factor 19) (FGF15/19)50 and glucagon-like peptide1 (GLP-1)46 (figure 2). Thus, BA signalling in the liver and the gut has broad implications for both cholestatic disorders and NAFLD.12 23
BAs undergo an enterohepatic circulation (4–12 times per day) and are effectively conserved by reabsorption in the terminal ileum (figure 2); thus only 3%–5% BAs are lost via stool and need to be replaced by daily BA synthesis.13 There is an intensive bidirectional interaction between BAs and intestinal microbiota.51 On one hand, BAs control and modify the composition of gut bacteria through their detergent and signalling properties while on the other hand BAs are deconjugated and metabolised to secondary BAs by the intestinal microbiota.51
Changes in gut microbiota (dysbiosis) may play a key role in the pathogenesis of NAFLD/NASH and cholestasis52–54 and may also impact on BA composition and signalling.51 55 Conversely, BAs and FXR ligands alter intestinal microbiota which may add to their therapeutic effects in both entities.53 56 Specific gut pathobionts such as Klebsiella pneumoniae may disrupt the intestinal epithelial barrier and initiate a hepatic T helper 17 cell immune response in PSC.57 Interestingly, NAFLD pathogenesis has been linked to high-alcohol-producing K. pneumoniae.58 Treating dysbiosis with restoration of its immunological and metabolic function holds much promise in both disease areas. Various absorbable/systemic and non-absorbable antibiotics may act via modulation of gut microbiota and have shown to improve liver biochemistry in PSC, with vancomycin as one of the most promising agents.59 Probiotics have been rather disappointing in treatment of NASH60 and cholestatic disorders such as PSC,61 but faecal microbiota transplantation from lean donors improves insulin resistance in individuals with metabolic syndrome62 and has shown first promising results in PSC.63 Importantly, gut microbiota may not only serve as a trigger of liver injury but may also have protective actions. As such total elimination of intestinal microbiome in germ-free mice has been shown to aggravate liver injury in mouse models of liver fibrosis and PSC.64 65
These specific examples may emphasise the multiple, partly unexpected mechanistic similarities and shared principles between metabolic and cholestatic liver diseases, which have cross-fertilised our pathogenetic understanding for both areas with imminent implications for the development of joint therapeutic strategies.
Nuclear receptor pathways as a new therapeutic frontier
The NR superfamily is the largest group of transcriptional regulators and consists of 48 members in humans.23 Ligands include both endogenous and exogenous molecules such as hormones, fatty acids, BAs, other intermediary products of metabolism, drugs and toxins.23 27 NRs typically induce transcriptional programmes involved in metabolism or transport of the ligand, thus providing a feedback mechanism to maintain cellular homoeostasis.23 27 Many of the recently developed drugs for cholestatic and metabolic liver diseases are high affinity ligands for these NRs (in the nanomolar to low micromolar range), thus avoiding the toxicity otherwise associated with administration of their natural ligands. Thus, NRs such as FXR, PPARs and THR have become key therapeutic targets for the development of new drugs for cholestatic and fatty liver diseases (table 1, figure 3). Moreover, xenobiotic sensors such as CAR and PXR might be interesting future pharmacological targets since they also have a critical role in BA and lipid metabolism.27
Table 1.
Novel therapeutic approaches for both cholestatic disorders and NASH—key clinical trials
| Compound class | Cholestasis | NASH |
| Steroidal FXR agonist |
Obeticholic acid (OCA):
|
Obeticholic acid (OCA):
|
| Non-steroidal FXR agonists |
Cilofexor:
Tropifexor:
|
Cilofexor:
Tropifexor
MET409
|
| FGF19 mimetic | Aldafermin/NGM282: |
Aldafermin/NGM282:
|
| PPARα agonists |
Bezafibrate:
|
No larger clinical trials in this indication. |
| PPARγ agonist | No systematic clinical trials in this indication. |
Pioglitazone:
|
| PPARδ agonist |
Seladelpar:
|
Seladelpar:
|
| PPARα/δ agonist |
Elafibranor:
|
Elafibranor:
|
| PPARα/γ agonist |
Saroglitazar:
|
Saroglitazar:
|
| FGF21 mimetics | No clinical trials in this indication. |
Pegbelfermin:
Efruxifermin (EFX):
|
| THR β1 agonists | Despite preclinical effects on biliary homoeostasis not yet tested clinically. |
Resmetirom (MGL-3196)
VK2809
|
| Norucholic acid (norUDCA; nor-ursodeoxycholic acid) |
|
|
| CCR2/CCR5 antagonist | Cenicriviroc:
|
Cenicriviroc:
|
| LOXL2 inhibitor |
Simtuzumab:
|
Simtuzumab:
|
ACC, Acetyl-CoA Carboxylase; ALP, alkaline phosphatase; ALT, alkaline phosphatase; ASK, apoptosis signal-regulating kinase; AST, aspartate aminotransferase; CVC, cenicriviroc; FGF, fibroblast growth factor; FXR, farnesoid-X receptor; HDL, high density lipoprotein; LDL, low densitiy lipoprotein; LOXL2, lysyl oxidase-like 2; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; norUDCA, nor-ursodeoxycholic acid; PBC, primary biliary cholangitis; PDFF, proton density fat fraction; PPAR, peroxisome proliferator-activated receptor; PSC, primary sclerosing cholangitis; T2DM, type 2 diabetes mellitus; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
In PBC, UDCA is used as first-line treatment,2 66 67 but 30%–50% of patients respond insufficiently (based on biochemical criteria) and require a second-line therapy with FXR ligands with the approved drug obeticholic acid (OCA)68 69, off-label use of PPAR ligands such as fibrates70 or glucocorticoid receptor (GR)-ligands such as budesonide71 which is mainly used in the context overlap syndromes.66 67 72 NR targeted therapies may be viewed as immunometabolic drugs targeting both biliary homoeostasis and inflammation/immunity (figure 3) which makes them highly attractive targets for immune-mediated cholestatic disorders such as PBC and PSC where both aspects may contribute to disease pathogenesis and progression. Even budesonide may have more than anti-inflammatory and immunosuppressive effects via GR since recent data indicate that it also controls bile detoxification (via PXR) and bicarbonate secretion.73 Interestingly biologics in this presumably autoimmune disorder have been quite disappointing so far, although several appealing immunomodulatory concepts (eg, Janus Kinase (JAK)1/2 inhibitors (baricitinib), anti-interleukin-17, etrasimod (S1P receptor 1,4,5 modulator), anti-CX3CL1/anti-Fractalkine are still tested in ongoing trials.74
In PSC no established drug treatment exists so far and we face the challenges of the high malignancy risk and its association with inflammatory bowel disease.75 Intriguingly, NR could not only address hepatobiliary homoeostasis but also gut inflammation and microbiota in PSC.76 As such NR (FXR, PPAR)-targeted therapies could even represent a one stop shopping for both liver and gut.
In NASH clinical trials with OCA (targeting FXR)77 78 and pioglitazone (targeting PPARγ)79–82 have already demonstrated efficacy of NRs ligands through control of lipid metabolism, inflammation and fibrosis83 (table 1). Another promising emerging NR target is THR which now can be targeted with liver specific THR-β ligands (table 1).6
Farnesoid X receptor
As major intracellular BA receptor and key regulator of BA homoeostasis,42–44 as well as lipid and glucose metabolism,41 FXR has become a central therapeutic target for cholestatic and fatty liver diseases. Moreover, FXR modulates liver regeneration, carcinogenesis, inflammation and intestinal microbiota.12 13 41 45 While the effects on intestinal integrity and microbiota are mediated via FXR regulated antimicrobial peptides and tight junction proteins, the anti-inflammatory effects of FXR are mainly due to nuclear factor kappa B (NFκB) antagonising effects.84 Mice lacking FXR show severely disturbed BA homoeostasis85 and genetic variants of FXR in humans cause a subtype 5 of severe progressive familial intrahepatic cholestasis,86 emphasising the key role of FXR in BA homoeostasis and pathogenesis of cholestasis. Expression of FXR is also reduced by inflammation and in various cholestatic diseases such as in PBC.87 Conversely, pharmacological activation of FXR restores BA homoeostasis by stimulation of transcription of the bile salt export pump that mediates the rate-limiting step in hepatocellular BA excretion across the canalicular membrane. Together with a reduction of BA uptake systems Na +- taurocholate cotransporting polypeptide (NTCP) and synthesis (CYP7A1) as well as stimulation of alternative basolateral export systems (OSTα/ß), this reduces hepatic BA load and alleviates BA-mediated cellular stress in cholestasis,45 which makes FXR an attractive key target for treatment of cholestasis (figures 2 and 3).
In NAFLD BA-activated FXR pathways are involved in the regulation of hepatic lipogenesis and lipoprotein metabolism, gluconeogenesis, glycogen synthesis and insulin sensitivity12 27 41 88 (figure 3). In addition to the physiological hormonal function of BAs in the regulation of lipid and glucose metabolism there is also convincing evidence for disturbed BA homoeostasis in NASH. With increasing severity of NASH and fibrosis, BA levels tend to increase and the composition of BAs changes, which may partly result from insulin resistance,89 impaired hepatobiliary excretory function18–20 and altered microbial BA metabolism caused by NASH-associated dysbiosis.55 Despite increased BA levels, FXR and FGF19 signalling is impaired, resulting in increased BA synthesis90 91 and BA toxicity could contribute to the hepatocellular injury and even carcinogenesis in NASH.4 12 92 Thus, restoration of FXR signalling and BA and lipid homoeostasis may explain at least part of the therapeutic effects of FXR ligands in NAFLD/NASH. In addition, broader anti-inflammatory and antifibrotic effects as well as improved endothelial function mediated by FXR may also contribute.93
Obeticholic acid as first in class steroidal FXR agonist
OCA, a 6α-ethyl derivate of chenodeoxycholic acid, is a steroidal FXR agonist (still maintaining its BA structure) and first-in-class therapeutic FXR ligand.94 In patients with PBC, OCA as monotherapy or add-on therapy to UDCA in those with insufficient biochemical response, improved biochemical markers of cholestasis68 69 95 96 and has been conditionally approved as second-line therapy for PBC by the US Food and Drug Administration (FDA) and European Medicines Agency.66 67 In line with the beneficial effects of OCA in PBC, encouraging results were also observed in patients with PSC97 (table 1). The main side effect in PBC and PSC was dose-dependent pruritus. Although long-term efficacy of OCA on biochemical parameters and stabilisation of inflammation and fibrosis in PBC has been demonstrated as extension of the phase 3 registration trial up to 5 years,69 97 a benefit for ‘hard’ clinical endpoints still needs to be demonstrated (table 1). Real world data confirmed the efficacy and safety of OCA in PBC with lower efficacy and reduced tolerability in patients with cirrhosis.98 99 Notably, use of OCA was associated with an increase in hepatic decompensation in patients with compensated PBC cirrhosis.100 which led to an update of the PBC label with a contraindication for use in patients with decompensated cirrhosis, a prior decompensation event or with compensated cirrhosis and evidence of portal hypertension.
A phase 2a study in patients with type 2 diabetes and NAFLD showed improved insulin sensitivity as well as gamma glutamyl transferase (γGT) and alanine amino transferase (ALT) by OCA.101 A subsequent phase 2b trial in patients with NASH revealed an improvement in the NAS score as predefined primary endpoint after OCA treatment over 72 weeks. Compared with placebo no greater proportion of patients showed resolution of NASH, but more patients showed improved of fibrosis in the OCA group.77 An 18-month interim analysis of a recent phase 3 study showed significant improvement of fibrosis but not NASH in patients receiving OCA.78 The main side effects of OCA included pruritus and dyslipidaemia (table 1).
Non-steroidal FXR agonists—the next generation of FXR agonists
In addition to OCA, several non-steroidal FXR ligands with even higher affinity for FXR have been developed. Some of which have already been tested in phase 2 clinical studies for the treatment of PBC, PSC and NASH102 (table 1). In contrast to steroidal FXR agonists, non-steroidal FXR ligands no longer have a BA structure and therefore, may have different pharmacokinetic profiles, efficacy and safety profiles.13 102 Some of these agonists may operate as gut-preferential FXR ligands, with shorter hepatic and limited systemic exposure which has raised expectations to limit prototypic side effects such as dyslipidaemia and pruritus.13 102 In patients with PSC the non-steroidal FXR agonist cilofexor improved cholestatic liver enzymes and non-invasive markers of liver fibrosis without aggravation of pruritus.103 Similarly, cilofexor improved liver enzymes in patients with PBC, but pruritus was observed in this study.104 The precursor compound of cilofexor, PX-104, improved insulin sensitivity and liver enzymes in patients with non-diabetic NAFLD.105 Cilofexor reduced hepatic fat content (MR-PDFF) and serum γGT in patients with NASH, again dose-dependent pruritus was noted as a side effect106 (table 1). Interestingly, no significant changes in lipid parameters were observed with cilofexor in this study. Cilofexor has also been investigated in combination with the Acetyl-CoA carboxylase (ACC) inhibitor firsocostat107 and/or semaglutide (GLP-1RA)108 (table 1).
Tropifexor, another highly potent non-steroidal FXR agonist109 has been explored in a phase 2 clinical trial which showed improvement of liver enzymes and hepatic fat content (table 1). Serum levels of low density lipoprotein (LDL)-cholesterol were moderately increased while high density lipoprotein (HDL)-cholesterol was reduced, and pruritus was reported as side effect,110 again demonstrating that non-steroidal FXR ligands may have a side effect profile similar to steroidal FXR ligands in line with experimental data.111 Tropifexor reduced γGT (but not alkaline phosphatase (ALP)) in patients with PBC and pruritus was noted.112 The absence of ALP reduction might be explained by potent FXR-related activation of ALP transcription through the high affinity ligand tropifexor.109 Tropifexor has also been tested in combination with cenicriviroc (CCR2/5 blocker, see below) in patients with NASH and F2/F3 fibrosis (TANDEM, NCT03517540). Another study explores the combination of tropifexor and licogliflozin (a dual inhibitor of both SGLT1 and SGLT2) in NASH (ELIVATE, NCT04065841).
In a recent smaller phase 1b study the non-steroidal, fexeramine-derived FXR agonist MET409 improved fat content (MRI-proton density fat fraction (PDFF)) over 12 weeks113 and is further evaluated alone or in combination with empagliflozin (SGLT2 inhibitor) in patients with both NASH and type 2 diabetes (NCT04702490). Dose-dependent pruritus, increases in LDL cholesterol, fasting glucose and transient ALT elevations were observed with MET409, which could be partly overcome by choosing a lower dose which still effectively reduced liver fat.113
Some non-steroidal FXR agonists may act exclusively in the gut, effects which may be mediated largely by stimulation of intestinal FGF19 expression.114 As such, the intestine-restricted FXR agonist fexaramine, reduced weight gain, decreased insulin resistance and improved hepatic steatosis in mice.115 Interestingly, an opposing strategy with intestinal FXR antagonism through specific BA species (eg, taurine or glycine-β-muricholic acid) also has beneficial effects against hepatic steatosis in mice; these effects were mediated through inhibition of intestinal FXR-dependent ceramide production which contributes to obesity and hepatic steatosis.116 117 However, such beneficial effects of FXR antagonism may be restricted to the intestine, since inhibition of hepatic FXR gives rise to cholestasis, steatosis or even liver cancer as suggested from FXR knockout studies in mice.85
UDCA—FXR agonist or antagonist?
UDCA can be viewed as the first available ‘enterohepatic drug’ circulating with the endogenous BA pool enriched by UDCA.118 UDCA is used for treatment of a broad range of cholestatic disorders with proven survival benefit as first-line treatment of PBC.2 In addition to its cytoprotective properties, UDCA acts as a secretagogue in hepatocytes and cholangiocytes, stimulating the vesicular targeting of transporters to the canalicular membrane.2 UDCA is a weak FXR agonist in vitro119 which competes with stronger FXR-agonistic signalling of endogenous BAs in man thus acting as weak agonistic antagonist.120 UDCA had only limited therapeutic efficacy in NASH in larger randomised controlled studies,121–124 although improvement of hepatic insulin resistance was observed in one high-dose study.123 Short-term, high-dose UDCA (given prior to bariatric surgery to morbidly obese patients with NAFLD), showed FXR-antagonistic effects in vivo, thereby stimulating BA synthesis and inducing lipid accumulation in liver as well as visceral white adipose tissue.120 Although enhanced triglyceride (TG) storage could be viewed beneficial as reflection of lipid partitioning counteracting lipotoxicity, it may be counterintuitive to stimulate both BA and TG synthesis as two deranged key pathways in the pathogenesis of NASH. In line with these findings, UDCA is no longer recommended for treatment of NAFLD/NASH.125
Fibroblast growth factor 19
FXR stimulates expression of intestinal FGF19 in the terminal ileum, which—after reaching the liver via the portal circulation and binding to FGFR4/ßKlotho receptor complex—inhibits hepatic BA synthesis through repression of CYP7A1.50 126 FGF19 is also a key regulator of postprandial lipid and glucose metabolism, as reflected by suppression of lipogenesis and gluconeogenesis, but promotion of fatty acid oxidation and glycogen synthesis.50 126 In addition to its role in regulating BA homoeostasis and metabolic pathways (figures 2 and 3), FGF19 also stimulates cell proliferation in the liver and molecular alterations of FGF19-FGFR4 signalling which raises potential concerns for hepatic carcinogenesis, when overstimulated by FXR ligands.127 Notably, aberrant FGF19-FGFR4 signalling has been identified in HCC127 but not in CCC where other pathways/genomic alterations including FGFR2 (intrahepatic CCC)128 or TP53 and KRAS (PSC-associated extrahepatic/perihilar CCC)129 among others are involved. Importantly, non-tumorigenic variants of FGF19, such as M52/M70/NGM282 (aldafermin),130 131 have been developed which can be used therapeutically with beneficial effects on metabolism without promoting or perhaps even counteracting carcinogenesis.132 133
The FGF19 mimetic M70/NGM282 improved liver injury in the Mdr2/Abcb4–/– mouse model of sclerosing cholangitis.130 Moreover, M52, protected Mdr2/Abcb4–/– and Fxr –/– mice from spontaneous hepatic fibrosis, cellular proliferation and HCC formation.134 In patients with PBC with insufficient response to UDCA NGM282/aldafermin mildly improved cholestatic liver enzymes, but mainly gastrointestinal side effects such as diarrhoea, abdominal pain and nausea (but not pruritus) were observed131 (table 1). Interestingly, NGM282/aldafermin improved non-invasive serum markers of hepatic fibrosis without reducing cholestatic liver enzymes such as ALP in patients with PSC135 possibly reflecting direct anti-inflammatory and antifibrotic actions. Whether NGM282/aldafermin-related anti-inflammatory and antifibrotic effects may reduce the risk of malignancies in PSC remains open.
FGF19 is also an attractive therapeutic target in NASH (table 1) where NGM282/aldafermin, significantly reduced hepatic fat content, serum aminotransferase levels and non-invasive fibrosis markers while histological improvement of fibrosis or resolution of NASH did not reach statistical significance after 24 weeks of treatment.136 Interestingly, enrichment of Veillonella, a BA-sensitive bacteria whose enrichment is enabled by NGM282/aldafermin, may be a marker for therapeutic response.137 Side effects of NGM282 are mostly of gastrointestinal origin but also include increases in LDL-cholesterol,138 which can be managed by statins.139 These findings emphasise that FXR/FGF-19-mediated suppression of BA synthesis will result in increased hepatocellular cholesterol levels with subsequent downregulation of LDL-receptor and increased serum cholesterol levels.88 This is an important consideration for any FXR-pathway targeted therapy in NASH and associated cardiovascular risk.7
Peroxisome proliferator-activated receptors
PPARs are a group of NRs that fine tune lipid and glucose metabolism and regulate inflammation and fibrosis.140 141 The three isoforms, PPARα, PPARγ and PPARδ (also known as ß), are expressed in different parenchymal and non-parenchmal liver cell compartments, making them highly attractive targets for therapy of metabolic142 and cholestatic liver diseases.143 Various drugs target PPARα (fibrates), PPARγ (thiazolidinediones/glitazones), PPARδ (seladelpar) or simultaneously two PPAR isoforms (PPARα/γ—glitazars and PPARα/δ—elafibranor). Recently more broadly acting pan-PPAR ligands such as lanifibranor have been propagated. Bezafibrate is a strong and predominant PPARα ligand with activity for other isoforms and therefore is sometimes also referred to as pan PPAR ligand.140 141
PPAR alpha
Despite their key role in lipid metabolism and management of dyslipidaemia,140 PPARα ligands (fibrates) did not show convincing efficacy in NASH in smaller pilot studies.144 However, several studies have demonstrated beneficial effects of fibrates (bezafibrate and fenofibrate) in cholestasis which seem to be mediated by repression of BA synthesis, stimulation of phospholipid excretion (counteracting intrinsic BA toxicity on bile ducts) and anti-inflammatory effects (via suppression of NFκB signalling).143 Bezafibrate 400 mg/day over 24-month resulted in complete biochemical response in 31% of patients with PBC with an incomplete response to UDCA70 (table 1). In 67% of patients even complete normalisation of alkaline phosphatase was observed, which is more rarely obtained with FXR ligands (7% with OCA)96 or budesonide (35%).71 Importantly, pruritus, liver stiffness and prognostic scores of PBC improved, in line with other recent studies confirming the positive effects on pruritus145 146 and long-term outcomes from the vast Japanese experience.147 Side effects include increases in serum creatinine, myalgia and hepatotoxicity (ALT elevations). Increases in creatinine appear to be a pharmacodynamic effect without nephropathy148 and and usually do not require cessation of treatment. Bezafibrate is currently not approved for the treatment of cholestatic liver diseases and thus used off-label when prescribed to patients with PBC or PSC. Moreover, bezafibrate is not available in the USA, where fenofibrate with a narrower PPARα-spectrum could be used since it has also demonstrated beneficial effects in smaller studies.149 150 Encouraging first results with fibrates have also been reported in PSC.151
PPAR gamma
In line with their key metabolic effects on insulin sensitivity, glucose and lipid metabolism152 thiazolidinediones/glitazones (PPARγ agonists) such as pioglitazone have been shown to improve NASH and fibrosis79–82 (table 1). Although so far no licensed drug therapy for NASH exists, they can be used alone or in combination with vitamin E according to current treatment guidelines.125 153 Glitazones also have shown beneficial effects in preclinical models of cholestasis by inhibiting bile duct proliferation and fibrosis in a rat model of chronic cholestasis154 but these have clinically never been followed systematically except single case reports.155 In contrast to PPARα a direct role for PPARγ in the regulation of BA metabolism has not yet been reported. However, targeting PPARγ may of particular interest in inflammatory cholestasis because of its crucial role in attenuation of inflammation-mediated transporter and enzyme changes.156 These findings suggest unexpected anti-cholestatic actions of classic metabolic drugs, although these mechanisms may not justify repurposing of these drugs for these indications. Notably, the plant extract curcumin, the yellow pigment of the spice turmeric, reduces liver damage, cholangitis and biliary fibrosis in Mdr2/Abcb4–/– mice by blocking proinflammatory cholangiocyte activation in a PPARγ-dependent fashion.157 However, curcumin did not result in significant improvements in cholestasis or symptoms in a recent pilot study in patients with PSC.158
PPAR delta
PPAR-δ is is ubiquitously expressed and profoundly influences BA and lipid metabolism, as well as inflammation and fibrosis159 making it an attractive therapeutic target for metabolic and cholestatic liver diseases. However, PPAR-δ-triggered mechanisms could promote cancer cell survival and cancer progression, which has raised concerns for their clinical development.159 Seladelpar is currently the only PPARδ ligand clinically developed for treatment of PBC, PSC and NASH, with the most robust results obtained in PBC so far160 (table 1). Development of this drug was transiently halted due to safety signals in follow-up biopsies in the NASH programme. After an in-depth investigation and comprehensive safety evaluation, all holds on seladelpar for ongoing clinical studies in were lifted.161 However, the results in NASH were rather controversial with a disconnection of no significant fat reduction (MR-PDFF) and dose-dependent histological improvements which however did not reach statistical significance162 (table 1).
Dual PPAR agonists
In addition to agonists for specific isoforms, drugs that activate more than one PPAR have been developed. Dual PPARα/γ agonists, termed glitazars, improve insulin resistance, dyslipidaemia163 and fatty liver164 in rodents. Saroglitazar was shown to improve ALT, liver fat content, insulin resistance and atherogenic dyslipidaemia in patients with NASH165 with encouraging histological signals.166 However, it has to be kept in mind that some compounds of this class of drugs were also shown to have cardiovascular and renal side effects.167 Of note, in patients with PBC with inadequate response to UDCA 16 weeks of saroglitazar treatment improved the primary endpoint ALP.168
The dual PPARα/δ agonists elafibranor showed beneficial effects on NASH resolution in a post-hoc analysis (with modified endpoint criteria) of the phase 2b GOLDEN-505 study,169 but had no significant impact on the main endpoint of NASH resolution without an increase in fibrosis in the phase 3 RESOLVE-IT trial.170 Elafibranor has also shown first results with improvement of cholestatic liver enzymes in patients with PBC not responding to UDCA171 (table 1).
In line with preclinical data,172 the pan PPAR agonist lanifibranor showed encouraging phase 2 data with NASH resolution without worsening of fibrosis over 24 weeks in addition to improved liver enzymes and a beneficial lipid profile with increased HDL cholesterol and reduced triglycerides.173
Fibroblast growth factor 21
FGF21 is another member of the FGF19 subfamily and is physiologically induced mainly in the liver during fasting through a mechanism dependent on PPARα.174 FGF21 is a key metabolic messenger regulating glucose and lipid metabolism, insulin sensitivity, energy homoeostasis, macronutrient preference and also exerts anti-inflammatory actions via inhibition of c-Jun N-terminal kinase (JNK) and NF-κB signalling pathways.174 175 The interest in therapeutic applications for FGF21 in NASH was stimulated by its ability to correct metabolic dysfunction and decrease body weight in diabetes and obesity.174 Pegbelfermin, a pegylated FGF21 analogue with prolonged half life,176 and efruxifermin, a fusion polypeptide of FGF21 with human IgG1 Fc,177 both reduced hepatic lipid content (MRI-PDFF) in pilot phase 2a studies patients with NASH (table 1). Efruxifermin was also shown to improve markers of fibrosis in F1-F2177 and F4 patients with NASH with compensated cirrhosis178 (table 1). Other approaches include humanised bispecific or monoclonal antibodies activating the FGFR1/β-Klotho complex and have been shown to improved liver fat content and serum lipids.175
Thyroid hormone receptor
Thyroid hormones stimulate hepatic fatty acid β‐oxidation as well as cholesterol and phospholipid excretion into bile.179 180 THR include different isoforms and in liver THR-β1 isoform represents 80% of THRs, while 20% are represented by THR-α1.181 Selective modulation of THR-β1 allows targeting hepatic genes without cardiac side effects.181 182 Numerous studies have linked subclinical hypothyroidism and low thyroid function with NAFLD, NASH and fibrosis as well as cardiovascular mortality.182 183 Low-dose thyroid hormone treatment reduced hepatic lipid content in patients with diabetes with NAFLD184 Two promising thyromimetic compounds specifically targeting THR-β1 in the liver, resmetirom (MGL-3196) and VK2809 are currently studied and have shown already beneficial effects in phase 2 studies in NASH with additional beneficial impact on associated cardiometabolic risk profiles (table 1).185
PBC is frequently associated with other autoimmune diseases including Hashimoto’s thyroiditis and hypothyroidism may aggravate cholestasis in PBC186 187 since thyroid hormones and THR-β1 regulate BA homoeostasis and bile formation180 188 including stimulation of biliary phospholipid excretion via ABCB4.188 Notably, defective ABCB4 expression and function results in bile duct injury and various cholestatic syndromes39 emphasising its relevance as therapeutic target. Expression of ABCB4 is also controlled by FXR and PPARα189 190 and the effects of FXR with THR-ß1 may be synergistic,188 suggesting that these drugs could be combined in the treatment of cholestatic disorders.
Enterohepatic bile acid circulation as therapeutic target
In addition to direct FXR agonists and FGF19 mimetics, pharmacological modulation of BA transport within their enterohepatic circulation may also indirectly alter BA signalling along the FXR-FGF19 axis (figure 2). Blocking ileal BA absorption depletes the body from primary BAs and—as a consequence of the compensatory increase of BA synthesis—also cholesterol, making this an attractive therapeutic approach for both metabolic and cholestatic liver diseases.11 13 BA sequestrants/resins have originally been developed as treatment for hypercholesterolaemia when additional effects on glucose and lipid metabolism such as reductions in hemoglobin A1c (HbA1c) but increases in serum triglycerides—which now can be explained by the role of BA signalling in control of lipid and glucose homoeostasis12 41—have already been noted in earlier clinical studies.191 Similar observations have been made in patients with intestinal resections191 and the rationale for apical sodium-dependent bile acid transporter (ASBT) inhibitors is also based on the beneficial effects of surgical interruption of the enterohepatic circulation in patients with paediatric cholestasis.192 Resins bind BAs in the intestinal lumen, but resin-bound BAs are still able to signal through TGR5 which stimulates secretion of GLP1 from enteroendocrine L-cells.193 The high potential of GLP-1 receptor agonists has recently been demonstrated by phase 2 studies with liraglutide194 and semaglutide,195 effects which may be largely mediated by its effects on insulin sensitivity and body weight. Apart from its metabolic effects, GLP-1 has also cholangioprotective effects against apoptosis and attenuating the reactive cholangiocyte phenotype.196 197 The BA sequestrant colesevelam and ASBT inhibitors (lopixibat and A4250) completely reversed liver and bile duct injury in Mdr2/Abcb4–/– mice,198–200 indicating that interruption of enterohepatic circulation of BAs may have therapeutic potential for attenuating cholestatic liver injury beyond their currently explored role in treatment of pruritus.189
Inhibition of ileal BA uptake protects against hepatic steatosis and restored insulin sensitivity in high-fat diet-fed mice, effects which are mediated by a marked shift in hepatic BA composition, with a reduction in hydrophilic, FXR antagonistic species and an increase in FXR agonistic BAs.201 However, clinical studies in human NASH with resins and ASBT inhibitors have so far been disappointing.202
Inhibition of NTCP by Myrcludex B (bulevirtide), a small peptide inhibitor originally designed to prevent hepatitis B virus uptake via NTCP, may also reduce intrahepatic BA levels.203 However, small molecule inhibitors of NTCP can also prevent HBV infection without interrupting BA uptake.204 Moreover, several drugs such as rosiglitazone, zafirlukast and sulfasalazine inhibit NTCP.205 In a chemical mouse model of sclerosing cholangitis NTCP inhibition by Myrcludex B improved hepatic inflammation and fibrosis.206 However, this therapeutic approach has so far not yet been tested clinically for cholestasis. Notably, genetic absence of NTCP in mice and men results in increased serum levels of unconjugated BAs, but is well tolerated without pruritus, fat malabsorption or liver dysfunction.207 208
norUDCA AS PARADIGM CHOLEHEPATIC DRUG
Nor-ursodeoxycholic acid (norUDCA, recently assigned the new international non-proprietary name norucholic acid) is a side-chain-shortened derivate of UDCA and is resistant to side-chain conjugation with glycine and taurine.209 Consequently, norUDCA—in contrast to its parent compound UDCA—undergoes cholehepatic shunting between cholangiocytes and hepatocytes, which results in the generation of a HCO3 – rich hypercholeresis and high intrahepatic enrichment.209 210 norUDCA has shown anti-cholestatic, anti-inflammatory, immunomodulatory and anti-fibrotic actions in animal models and improves cholestatic liver and bile duct injury in the Mdr2/Abcb4 –/– mouse model of sclerosing cholangitis.33 211 Clinically, norUDCA improved biochemical markers of cholestasis in a recent phase 2 clinical trial in PSC irrespective of prior exposure and response to UDCA212 (table 1). Since norUDCA reinforces the HCO3 – umbrella2 it may also be a therapeutic approach in other cholangiopathies with defective HCO3 – secretion such as PBC.213
In addition to its beneficial effects on the biliary tree, norUDCA has also direct hepatoprotective, anti-inflammatory and antifibrotic effects in mouse models of NASH,13 213 214 making it a promising therapeutic agent in NAFLD/NASH with first encouraging phase 2a data215 (table 1). Notably, norUDCA does not act via FXR or other NRs,213 making it an attractive combination partner for drugs targeting NRs within the enterohepatic circulation (figure 2).
Targeting inflammation and fibrosis via chemokine receptors CCR2/CCR5
Macrophages have emerged as essential players in acute and chronic liver injury and in a wide range of liver diseases including NASH216 and more recently also PSC.217 Cenicriviroc (CVC) is a dual CCR2/CCR5 chemokine receptor antagonist that is able to block CCR2/5 on macrophages and HSC (figure 3) and is currently developed as anti-inflammatory/anti-fibrotic treatment in NASH and other indications.218 Despite encouraging interim data after 1 year,219 final analysis after 2 years revealed that a no significant antifibrotic effect in NASH (table 1).220 A long-term phase 3 study (AURORA, NCT03028740) was terminated early due to lack of efficacy, but CVC is still investigated in combination strategies (eg, FXR agonist tropifexor/TANDEM trial see above).
Pharmacological or genetic inhibition of peribiliary macrophage recruitment attenuated liver injury and fibrosis in mouse models of PSC.221 Interestingly, microbe-stimulated monocytes drive Th17 differentiation in vitro and induce cholangiocytes to produce chemokines mediating recruitment of Th17 cells and more monocytes into portal tracts.222 Interestingly, Th17 cells may also be involved in NASH progression223 and, secukinumab, a monoclonal antibody against IL17A, is currently investigated in patients with psoriasis and coexisting NAFLD (NCT04237116). In a rodent model combination of all-trans retinoic acid and CVC synergistically reduced liver fibrosis and bile duct injury, although a reduction in neutrophils and T cells but not macrophages was observed.224 Recently, an open label, proof of concept phase 2 study with CVC in patients with PSC (PERSEUS trial, NCT02653625) has been completed with negative results (table 1).
Simtuzumab as antifibrotic strategy
Simtuzumab is a humanised monoclonal antibody against lysyl oxidase-like 2 (LOXL2), which has raised much hope for treatment of fibrosis in both NASH and PSC, since LOXL2 contributes to fibrogenesis by cross-linkage of collagen and regulates bile duct permeability.225 226 In patients with PSC and NASH increased serum levels of LOXL2 correlate with more advanced fibrosis and severity of portal hypertension.226 227 Despite these encouraging findings, simtuzumab failed to show antifibrotic effects in NASH patients with bridging fibrosis or compensated cirrhosis227 and in patients with PSC228 (table 1). However, despite being ‘negative’ the biomaterial and data obtained during these clinical trials have provided important pathogenetic and clinical insights into these diseases.24 229 Other antifibrotic strategies have recently been reviewed elsewhere in more detail.230 231
Conclusions
A better understanding of the pathogenesis of cholestatic and metabolic liver diseases has crossfertilised drug development for both disease areas. Several novel therapeutic approaches for cholestatic and fatty liver diseases currently investigated in phase 2 and 3 clinical trials are based on shared pathogenetic and therapeutic principles (table 1). Due to the central role of NRs and BAs as integrators of metabolism and inflammation, targeting these pathways has great potential. Some of these approaches have already resulted in first encouraging results (table 1) and even conditional approvement of novel therapies, but their long-term efficacy, tolerability and safety still needs to be evaluated. Apart from therapeutic efficacy a positive impact on pruritus in cholestatic disorders (seen with fibrates) and beneficial cardiometabolic profile in NASH (seen with PPAR and THR-β1 ligands) is an important aspect for considering long-term treatment with these drugs. Based on the complex pathophysiology of cholestatic and fatty liver diseases and the multiple pathways involved in their progression, multifactorial treatments or combination therapies that engage different targets are urgently required. Several of the currently explored drugs (eg, NR ligands) simultaneously target multiple key pathogenetic processes and may show even synergistic effects when combined in the management of these disorders, for example, combining FXR and PPAR ligands in PBC232 233 and NASH.234 Interestingly, in extension of dual PPARα/γ and α/δ agonists, dual FXR/PPARδ agonists are currently developed for treatment of NASH.235 As broadly acting drugs NR (eg, FXR) ligands are also explored in combination with anti-diabetic (eg, GLP-1 RA; SGLT2 inhibitors) or anti-inflammatory drugs (eg, CVC) to achieve a higher therapeutic efficacy in NASH which is still in an unsatisfactory range for single agents.236 Apart from combining existing drugs, the development of dual GLP-1/glucagon receptor or GLP-1/GIP agonists or even triagonists237 with first promising results in NASH237–239 may show up another promising approach in drug development. Notably, these peptide hormones can be combined with NR ligands thus increasing the efficacy in target organs while at the same time restricting side effects.237 A major challenge will be to test the plethora of therapeutic options which requires innovative designs such as basket trials.240
Footnotes
Correction notice: This article has been corrected since it published Online First. The corresponding address has been amended and the heading 'norUDCA as paradigm cholehepatic drug' added.
Contributors: Concept and supervision: MT; drafting of the manuscript: MT; critical revision of the manuscript for important intellectual content: MT and CDF; obtained funding: MT; material support: CDF and MT. The authors thank Alexandra Weisgram for editorial assistance.
Funding: This work was supported by the grants F7310‐B21 and I2755-B30 from the Austrian Science Foundation (to MT).
Competing interests: MT: Consulting: Albireo, BiomX, Boehringer Ingelheim, Falk, Genfit, Intercept, Janssen, MSD, Gilead, Novartis, Shire, Phenex, Regulus. Speakers bureau: Falk Foundation, Gilad, Intercept, MSD. Grants: Albireo, Cymabay, Falk, Gilead, Intercept, MSD, Takeda, Alnylam, Ultragenyx. Travel grants: Abbvie, Falk, Gilead, Intercept. Intellectual property rights: Co Inventor or Patent on Medical Use nor UDCA. CDF received travel grants from Falk and Gilead.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid 'mechanisms of action and clinical use in hepatobiliary disorders'. J Hepatol 2001;35:134–46. 10.1016/s0168-8278(01)00092-7 [DOI] [PubMed] [Google Scholar]
- 2. Beuers U, Trauner M, Jansen P, et al. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol 2015;62:S25–37. 10.1016/j.jhep.2015.02.023 [DOI] [PubMed] [Google Scholar]
- 3. Trauner M, Meier PJ, Boyer JL. Molecular pathogenesis of cholestasis. N Engl J Med 1998;339:1217–27. 10.1056/NEJM199810223391707 [DOI] [PubMed] [Google Scholar]
- 4. Arab JP, Arrese M, Trauner M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu Rev Pathol 2018;13:321–50. 10.1146/annurev-pathol-020117-043617 [DOI] [PubMed] [Google Scholar]
- 5. Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology 2010;139:1481–96. 10.1053/j.gastro.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 6. Friedman SL, Neuschwander-Tetri BA, Rinella M, et al. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908–22. 10.1038/s41591-018-0104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2019;69:2672–82. 10.1002/hep.30251 [DOI] [PubMed] [Google Scholar]
- 8. Williams R, Alexander G, Armstrong I, et al. Disease burden and costs from excess alcohol consumption, obesity, and viral hepatitis: fourth report of the Lancet standing Commission on liver disease in the UK. Lancet 2018;391:1097–107. 10.1016/S0140-6736(17)32866-0 [DOI] [PubMed] [Google Scholar]
- 9. Webb GJ, Rana A, Hodson J, et al. Twenty-Year comparative analysis of patients with autoimmune liver diseases on transplant Waitlists. Clin Gastroenterol Hepatol 2018;16:278–87. 10.1016/j.cgh.2017.09.062 [DOI] [PubMed] [Google Scholar]
- 10. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202–9. 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 11. Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 2010;52:774–88. 10.1002/hep.23719 [DOI] [PubMed] [Google Scholar]
- 12. Arab JP, Karpen SJ, Dawson PA, et al. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology 2017;65:350–62. 10.1002/hep.28709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trauner M, Fuchs CD, Halilbasic E, et al. New therapeutic concepts in bile acid transport and signaling for management of cholestasis. Hepatology 2017;65:1393–404. 10.1002/hep.28991 [DOI] [PubMed] [Google Scholar]
- 14. Jansen PLM, Ghallab A, Vartak N, et al. The ascending pathophysiology of cholestatic liver disease. Hepatology 2017;65:722–38. 10.1002/hep.28965 [DOI] [PubMed] [Google Scholar]
- 15. Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell 2015;160:816–27. 10.1016/j.cell.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017;542:177–85. 10.1038/nature21363 [DOI] [PubMed] [Google Scholar]
- 17. Hirsova P, Ibrabim SH, Gores GJ, et al. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res 2016;57:1758–70. 10.1194/jlr.R066357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pizarro M, Balasubramaniyan N, Solís N, et al. Bile secretory function in the obese Zucker rat: evidence of cholestasis and altered canalicular transport function. Gut 2004;53:1837–43. 10.1136/gut.2003.037689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geier A, Dietrich CG, Grote T, et al. Characterization of organic anion transporter regulation, glutathione metabolism and bile formation in the obese Zucker rat. J Hepatol 2005;43:1021–30. 10.1016/j.jhep.2005.05.031 [DOI] [PubMed] [Google Scholar]
- 20. Segovia-Miranda F, Morales-Navarrete H, Kücken M, et al. Three-Dimensional spatially resolved geometrical and functional models of human liver tissue reveal new aspects of NAFLD progression. Nat Med 2019;25:1885–93. 10.1038/s41591-019-0660-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017;14:397–411. 10.1038/nrgastro.2017.38 [DOI] [PubMed] [Google Scholar]
- 22. Trautwein C, Friedman SL, Schuppan D, et al. Hepatic fibrosis: concept to treatment. J Hepatol 2015;62:S15–24. 10.1016/j.jhep.2015.02.039 [DOI] [PubMed] [Google Scholar]
- 23. Karpen SJ, Trauner M. The new therapeutic frontier--nuclear receptors and the liver. J Hepatol 2010;52:455–62. 10.1016/j.jhep.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 24. Sanyal AJ, Harrison SA, Ratziu V, et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the Simtuzumab trials. Hepatology 2019;70:1913–27. 10.1002/hep.30664 [DOI] [PubMed] [Google Scholar]
- 25. Natarajan SK, Ingham SA, Mohr AM, et al. Saturated free fatty acids induce cholangiocyte lipoapoptosis. Hepatology 2014;60:1942–56. 10.1002/hep.27175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bruschi FV, Claudel T, Tardelli M, et al. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatology 2017;65:1875–90. 10.1002/hep.29041 [DOI] [PubMed] [Google Scholar]
- 27. Rudraiah S, Zhang X, Wang L. Nuclear receptors as therapeutic targets in liver disease: are we there yet? Annu Rev Pharmacol Toxicol 2016;56:605–26. 10.1146/annurev-pharmtox-010715-103209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guicciardi ME, Trussoni CE, LaRusso NF, et al. The spectrum of reactive cholangiocytes in primary sclerosing cholangitis. Hepatology 2020;71:741–8. 10.1002/hep.31067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu R, Li X, Zhu W, et al. Cholangiocyte-Derived exosomal long noncoding RNA H19 promotes hepatic stellate cell activation and cholestatic liver fibrosis. Hepatology 2019;70:1317–35. 10.1002/hep.30662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karlsen TH, Lammert F, Thompson RJ. Genetics of liver disease: from pathophysiology to clinical practice. J Hepatol 2015;62:S6–14. 10.1016/j.jhep.2015.02.025 [DOI] [PubMed] [Google Scholar]
- 31. Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 2008;40:1461–5. 10.1038/ng.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tardelli M, Bruschi FV, Trauner M. The role of metabolic lipases in the pathogenesis and management of liver disease. Hepatology 2020;72:1117–26. 10.1002/hep.31250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moustafa T, Fickert P, Magnes C, et al. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology 2012;142:140–51. 10.1053/j.gastro.2011.09.051 [DOI] [PubMed] [Google Scholar]
- 34. Krawczyk M, Milkiewicz M, Marschall H-U, et al. Variant adiponutrin confers genetic protection against cholestatic itch. Sci Rep 2014;4:6374. 10.1038/srep06374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kremer AE, Martens JJWW, Kulik W, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology 2010;139:1008–18. 18 e1. 10.1053/j.gastro.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 36. Friedrich K, Rupp C, Hov JR, et al. A frequent PNPLA3 variant is a sex specific disease modifier in PSC patients with bile duct stenosis. PLoS One 2013;8:e58734. 10.1371/journal.pone.0058734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kruk B, Liebe R, Milkiewicz M, et al. Pnpla3 p.I148M and TM6SF2 p.E167K variants do not predispose to liver injury in cholestatic liver diseases: a prospective analysis of 178 patients with PSC. PLoS One 2018;13:e0202942. 10.1371/journal.pone.0202942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lindén D, Ahnmark A, Pingitore P, et al. Pnpla3 silencing with antisense oligonucleotides ameliorates nonalcoholic steatohepatitis and fibrosis in PNPLA3 I148M knock-in mice. Mol Metab 2019;22:49–61. 10.1016/j.molmet.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stättermayer AF, Halilbasic E, Wrba F, et al. Variants in Abcb4 (mdr3) across the spectrum of cholestatic liver diseases in adults. J Hepatol 2020;73:651–63. 10.1016/j.jhep.2020.04.036 [DOI] [PubMed] [Google Scholar]
- 40. Gudbjartsson DF, Helgason H, Gudjonsson SA, et al. Large-Scale whole-genome sequencing of the Icelandic population. Nat Genet 2015;47:435–44. 10.1038/ng.3247 [DOI] [PubMed] [Google Scholar]
- 41. Lefebvre P, Cariou B, Lien F, et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 2009;89:147–91. 10.1152/physrev.00010.2008 [DOI] [PubMed] [Google Scholar]
- 42. Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science 1999;284:1362–5. 10.1126/science.284.5418.1362 [DOI] [PubMed] [Google Scholar]
- 43. Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science 1999;284:1365–8. 10.1126/science.284.5418.1365 [DOI] [PubMed] [Google Scholar]
- 44. Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 1999;3:543–53. 10.1016/s1097-2765(00)80348-2 [DOI] [PubMed] [Google Scholar]
- 45. Zollner G, Trauner M. Nuclear receptors as therapeutic targets in cholestatic liver diseases. Br J Pharmacol 2009;156:7–27. 10.1111/j.1476-5381.2008.00030.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pols TWH, Noriega LG, Nomura M, et al. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol 2011;54:1263–72. 10.1016/j.jhep.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006;439:484–9. 10.1038/nature04330 [DOI] [PubMed] [Google Scholar]
- 48. Keitel V, Häussinger D. Role of TGR5 (GPBAR1) in liver disease. Semin Liver Dis 2018;38:333–9. 10.1055/s-0038-1669940 [DOI] [PubMed] [Google Scholar]
- 49. Reich M, Spomer L, Klindt C, et al. Downregulation of TGR5 (GPBAR1) in biliary epithelial cells contributes to the pathogenesis of sclerosing cholangitis. J Hepatol 2021;75:634–46. 10.1016/j.jhep.2021.03.029 [DOI] [PubMed] [Google Scholar]
- 50. Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2005;2:217–25. 10.1016/j.cmet.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 51. Ridlon JM, Kang D-J, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2006;47:241–59. 10.1194/jlr.R500013-JLR200 [DOI] [PubMed] [Google Scholar]
- 52. Hov JR, Karlsen TH. The microbiome in primary sclerosing cholangitis: current evidence and potential concepts. Semin Liver Dis 2017;37:314–31. 10.1055/s-0037-1608801 [DOI] [PubMed] [Google Scholar]
- 53. Tang R, Wei Y, Li Y, et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut 2018;67:534–41. 10.1136/gutjnl-2016-313332 [DOI] [PubMed] [Google Scholar]
- 54. Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014;146:1513–24. 10.1053/j.gastro.2014.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Puri P, Sanyal AJ. The intestinal microbiome in nonalcoholic fatty liver disease. Clin Liver Dis 2018;22:121–32. 10.1016/j.cld.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 56. Friedman ES, Li Y, Shen T-CD, et al. FXR-Dependent modulation of the human small intestinal microbiome by the bile acid derivative obeticholic acid. Gastroenterology 2018;155:1741–52. 10.1053/j.gastro.2018.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nakamoto N, Sasaki N, Aoki R, et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol 2019;4:492–503. 10.1038/s41564-018-0333-1 [DOI] [PubMed] [Google Scholar]
- 58. Yuan J, Chen C, Cui J, et al. Fatty liver disease caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab 2019;30:1172. 10.1016/j.cmet.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 59. Damman JL, Rodriguez EA, Ali AH, et al. Review article: the evidence that vancomycin is a therapeutic option for primary sclerosing cholangitis. Aliment Pharmacol Ther 2018;47:886–95. 10.1111/apt.14540 [DOI] [PubMed] [Google Scholar]
- 60. Meroni M, Longo M, Dongiovanni P. The role of probiotics in nonalcoholic fatty liver disease: a new insight into therapeutic strategies. Nutrients 2019;11:2642. 10.3390/nu11112642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vleggaar FP, Monkelbaan JF, van Erpecum KJ. Probiotics in primary sclerosing cholangitis: a randomized placebo-controlled crossover pilot study. Eur J Gastroenterol Hepatol 2008;20:688–92. 10.1097/MEG.0b013e3282f5197e [DOI] [PubMed] [Google Scholar]
- 62. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012;143:913–6. 10.1053/j.gastro.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 63. Allegretti JR, Kassam Z, Carrellas M, et al. Fecal microbiota transplantation in patients with primary sclerosing cholangitis: a pilot clinical trial. Am J Gastroenterol 2019;114:1071–9. 10.14309/ajg.0000000000000115 [DOI] [PubMed] [Google Scholar]
- 64. Mazagova M, Wang L, Anfora AT, et al. Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. Faseb J 2015;29:1043–55. 10.1096/fj.14-259515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tabibian JH, O'Hara SP, Trussoni CE, et al. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology 2016;63:185–96. 10.1002/hep.27927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver . EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67:145–72. 10.1016/j.jhep.2017.03.022 [DOI] [PubMed] [Google Scholar]
- 67. Lindor KD, Bowlus CL, Boyer J, et al. Primary biliary cholangitis: 2018 practice guidance from the American association for the study of liver diseases. Hepatology 2019;69:394–419. 10.1002/hep.30145 [DOI] [PubMed] [Google Scholar]
- 68. Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med 2016;375:631–43. 10.1056/NEJMoa1509840 [DOI] [PubMed] [Google Scholar]
- 69. Trauner M, Nevens F, Shiffman ML, et al. Long-Term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol Hepatol 2019;4:445–53. 10.1016/S2468-1253(19)30094-9 [DOI] [PubMed] [Google Scholar]
- 70. Corpechot C, Chazouillères O, Rousseau A, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med 2018;378:2171–81. 10.1056/NEJMoa1714519 [DOI] [PubMed] [Google Scholar]
- 71. Hirschfield GM, Beuers U, Kupcinskas L, et al. A placebo-controlled randomised trial of budesonide for pBC following an insufficient response to UDCA. J Hepatol 2021;74:321–9. 10.1016/j.jhep.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 72. Corpechot C. Primary biliary cirrhosis beyond ursodeoxycholic acid. Semin Liver Dis 2016;36:15–26. 10.1055/s-0035-1571273 [DOI] [PubMed] [Google Scholar]
- 73. Arenas F, Hervias I, Uriz M, et al. Combination of ursodeoxycholic acid and glucocorticoids upregulates the AE2 alternate promoter in human liver cells. J Clin Invest 2008;118:695–709. 10.1172/JCI33156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gao L, Wang L, Woo E, et al. Clinical management of primary biliary Cholangitis-Strategies and evolving trends. Clin Rev Allergy Immunol 2020;59:175–94. 10.1007/s12016-019-08772-7 [DOI] [PubMed] [Google Scholar]
- 75. Karlsen TH, Folseraas T, Thorburn D, et al. Primary sclerosing cholangitis - a comprehensive review. J Hepatol 2017;67:1298–323. 10.1016/j.jhep.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 76. Vesterhus M, Karlsen TH. Emerging therapies in primary sclerosing cholangitis: pathophysiological basis and clinical opportunities. J Gastroenterol 2020;55:588–614. 10.1007/s00535-020-01681-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (Flint): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956–65. 10.1016/S0140-6736(14)61933-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394:2184–96. 10.1016/S0140-6736(19)33041-7 [DOI] [PubMed] [Google Scholar]
- 79. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med Overseas Ed 2010;362:1675–85. 10.1056/NEJMoa0907929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cusi K, Orsak B, Bril F, et al. Long-Term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus. Ann Intern Med 2016;165:305–15. 10.7326/M15-1774 [DOI] [PubMed] [Google Scholar]
- 81. Musso G, Cassader M, Paschetta E, et al. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis. JAMA Intern Med 2017;177:633–40. 10.1001/jamainternmed.2016.9607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ratziu V, Caldwell S, Neuschwander-Tetri BA. Therapeutic trials in nonalcoholic steatohepatitis: insulin sensitizers and related methodological issues. Hepatology 2010;52:2206–15. 10.1002/hep.24042 [DOI] [PubMed] [Google Scholar]
- 83. Fuchs C, Traussnigg S, Trauner M. Nuclear receptor modulation for the treatment of nonalcoholic fatty liver disease. Semin Liver Dis 2016;36:069–86. 10.1055/s-0036-1571296 [DOI] [PubMed] [Google Scholar]
- 84. Wang Y-D, Chen W-D, Wang M, et al. Farnesoid X receptor antagonizes nuclear factor κB in hepatic inflammatory response. Hepatology 2008;48:1632–43. 10.1002/hep.22519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sinal CJ, Tohkin M, Miyata M, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 2000;102:731–44. 10.1016/S0092-8674(00)00062-3 [DOI] [PubMed] [Google Scholar]
- 86. Gomez-Ospina N, Potter CJ, Xiao R, et al. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat Commun 2016;7:10713. 10.1038/ncomms10713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zollner G, Fickert P, Silbert D, et al. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol 2003;38:717–27. 10.1016/S0168-8278(03)00096-5 [DOI] [PubMed] [Google Scholar]
- 88. Chávez-Talavera O, Tailleux A, Lefebvre P, et al. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 2017;152:1679–94. 10.1053/j.gastro.2017.01.055 [DOI] [PubMed] [Google Scholar]
- 89. Legry V, Francque S, Haas JT, et al. Bile acid alterations are associated with insulin resistance, but not with NASH, in obese subjects. J Clin Endocrinol Metab 2017;102:3783–94. 10.1210/jc.2017-01397 [DOI] [PubMed] [Google Scholar]
- 90. Nobili V, Alisi A, Mosca A, et al. Hepatic farnesoid X receptor protein level and circulating fibroblast growth factor 19 concentration in children with NAFLD. Liver Int 2018;38:342–9. 10.1111/liv.13531 [DOI] [PubMed] [Google Scholar]
- 91. Jiao N, Baker SS, Chapa-Rodriguez A, et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018;67:1881–91. 10.1136/gutjnl-2017-314307 [DOI] [PubMed] [Google Scholar]
- 92. Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-Induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013;499:97–101. 10.1038/nature12347 [DOI] [PubMed] [Google Scholar]
- 93. Fiorucci S, Cipriani S, Mencarelli A, et al. Farnesoid X receptor agonist for the treatment of liver and metabolic disorders: focus on 6-ethyl-CDCA. Mini Rev Med Chem 2011;11:753–62. 10.2174/138955711796355258 [DOI] [PubMed] [Google Scholar]
- 94. Pellicciari R, Fiorucci S, Camaioni E, et al. 6α-Ethyl-Chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J Med Chem 2002;45:3569–72. 10.1021/jm025529g [DOI] [PubMed] [Google Scholar]
- 95. Kowdley KV, Luketic V, Chapman R, et al. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology 2018;67:1890–902. 10.1002/hep.29569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hirschfield GM, Mason A, Luketic V, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology 2015;148:751–61. 10.1053/j.gastro.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 97. Bowlus CL, Pockros PJ, Kremer AE, et al. Long-Term obeticholic acid therapy improves histological endpoints in patients with primary biliary cholangitis. Clin Gastroenterol Hepatol 2020;18:1170–8. 10.1016/j.cgh.2019.09.050 [DOI] [PubMed] [Google Scholar]
- 98. Wilde A-CB, Lieb C, Leicht E, et al. Real-World clinical management of patients with primary biliary Cholangitis-A retrospective multicenter study from Germany. J Clin Med 2021;10:1061. 10.3390/jcm10051061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. D'Amato D, De Vincentis A, Malinverno F, et al. Real-World experience with obeticholic acid in patients with primary biliary cholangitis. JHEP Rep 2021;3:100248. 10.1016/j.jhepr.2021.100248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. John BV, Schwartz K, Levy C, et al. Impact of obeticholic acid exposure on decompensation and mortality in primary biliary cholangitis and cirrhosis. Hepatol Commun 2021;5:1426–36. 10.1002/hep4.1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013;145:574–82. 10.1053/j.gastro.2013.05.042 [DOI] [PubMed] [Google Scholar]
- 102. Gege C, Hambruch E, Hambruch N, et al. Nonsteroidal FXR ligands: current status and clinical applications. Handb Exp Pharmacol 2019;256:167–205. 10.1007/164_2019_232 [DOI] [PubMed] [Google Scholar]
- 103. Trauner M, Gulamhusein A, Hameed B, et al. The nonsteroidal farnesoid X receptor agonist Cilofexor (GS-9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology 2019;70:788–801. 10.1002/hep.30509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kowdley KV, Minuk GY, Pagadala MR. The nonsteroidal farnesoid X receptor (FXR) agonist Cilofexor improves liver biochemistry in patients with primary biliary cholangitis (pBC): a phase 2, randomized, placebo-controlled trial. Hepatology 2019;70:31a–2. [Google Scholar]
- 105. Traussnigg S, Halilbasic E, Hofer H, et al. Open-label phase II study evaluating safety and efficacy of the non-steroidal farnesoid X receptor agonist PX-104 in non-alcoholic fatty liver disease. Wien Klin Wochenschr 2021;133:441–51. 10.1007/s00508-020-01735-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Patel K, Harrison SA, Elkhashab M, et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology 2020;72:58–71. 10.1002/hep.31205 [DOI] [PubMed] [Google Scholar]
- 107. Loomba R, Noureddin M, Kowdley KV, et al. Combination therapies including Cilofexor and Firsocostat for bridging fibrosis and cirrhosis attributable to NASH. Hepatology 2021;73:625–43. 10.1002/hep.31622 [DOI] [PubMed] [Google Scholar]
- 108. Alkhouri N, Herring R, Kabler H. Safety and efficacy of combination therapies including Semaglutide, Cilofexor, and Firsocostat in patients with NASH. Hepatology 2020;72:131A. [Google Scholar]
- 109. Tully DC, Rucker PV, Chianelli D, et al. Discovery of Tropifexor (LJN452), a highly potent Non-bile acid FXR agonist for the treatment of cholestatic liver diseases and nonalcoholic steatohepatitis (NASH). J Med Chem 2017;60:9960–73. 10.1021/acs.jmedchem.7b00907 [DOI] [PubMed] [Google Scholar]
- 110. Lucas KJ, Lopez P, Lawitz EJ. Safety and efficacy of tropifexor in patients with fibrotic nonalcoholic steatohepatitis: 48‐week results from part C of the phase 2 flight‐fxr study. Hepatology 2020;72:101A–2. [Google Scholar]
- 111. Papazyan R, Liu X, Liu J, et al. Fxr activation by obeticholic acid or nonsteroidal agonists induces a human-like lipoprotein cholesterol change in mice with humanized chimeric liver. J Lipid Res 2018;59:982–93. 10.1194/jlr.M081935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Schramm C, Hirschfield G, Mason AL, et al. Early assessment of safety and efficacy of tropifexor, a potent non bile-acid FXR agonist, in patients with primary biliary cholangitis: an interim analysis of an ongoing phase 2 study. J Hepatol 2018;68:S103. 10.1016/S0168-8278(18)30426-4 [DOI] [Google Scholar]
- 113. Harrison SA, Bashir MR, Lee K-J, et al. A structurally optimized FXR agonist, MET409, reduced liver fat content over 12 weeks in patients with non-alcoholic steatohepatitis. J Hepatol 2021;75:25–33. 10.1016/j.jhep.2021.01.047 [DOI] [PubMed] [Google Scholar]
- 114. Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med 2015;3:5. 10.3978/j.issn.2305-5839.2014.12.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med 2015;21:159–65. 10.1038/nm.3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Jiang C, Xie C, Lv Y, et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun 2015;6:10166. 10.1038/ncomms10166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gonzalez FJ, Jiang C, Patterson AD. An intestinal Microbiota-Farnesoid X receptor axis modulates metabolic disease. Gastroenterology 2016;151:845–59. 10.1053/j.gastro.2016.08.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med 1999;159:2647–58. 10.1001/archinte.159.22.2647 [DOI] [PubMed] [Google Scholar]
- 119. Lew J-L, Zhao A, Yu J, et al. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J Biol Chem 2004;279:8856–61. 10.1074/jbc.M306422200 [DOI] [PubMed] [Google Scholar]
- 120. Mueller M, Thorell A, Claudel T, et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol 2015;62:1398–404. 10.1016/j.jhep.2014.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 2004;39:770–8. 10.1002/hep.20092 [DOI] [PubMed] [Google Scholar]
- 122. Leuschner UFH, Lindenthal B, Herrmann G, et al. High-Dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology 2010;52:472–9. 10.1002/hep.23727 [DOI] [PubMed] [Google Scholar]
- 123. Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J Hepatol 2011;54:1011–9. 10.1016/j.jhep.2010.08.030 [DOI] [PubMed] [Google Scholar]
- 124. Dufour J-F, Oneta CM, Gonvers J-J, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin E in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2006;4:1537–43. 10.1016/j.cgh.2006.09.025 [DOI] [PubMed] [Google Scholar]
- 125. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. 10.1016/j.jhep.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 126. Degirolamo C, Sabbà C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov 2016;15:51–69. 10.1038/nrd.2015.9 [DOI] [PubMed] [Google Scholar]
- 127. Raja A, Park I, Haq F, et al. FGF19-FGFR4 Signaling in Hepatocellular Carcinoma. Cells 2019;8:536. 10.3390/cells8060536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557–88. 10.1038/s41575-020-0310-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Goeppert B, Folseraas T, Roessler S, et al. Genomic characterization of cholangiocarcinoma in primary sclerosing cholangitis reveals therapeutic opportunities. Hepatology 2020;72:1253–66. 10.1002/hep.31110 [DOI] [PubMed] [Google Scholar]
- 130. Zhou M, Learned RM, Rossi SJ, et al. Engineered fibroblast growth factor 19 reduces liver injury and resolves sclerosing cholangitis in Mdr2-deficient mice. Hepatology 2016;63:914–29. 10.1002/hep.28257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mayo MJ, Wigg AJ, Leggett BA, et al. NGM282 for treatment of patients with primary biliary cholangitis: a multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Commun 2018;2:1037–50. 10.1002/hep4.1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Luo J, Ko B, Elliott M, et al. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med 2014;6:247ra100. 10.1126/scitranslmed.3009098 [DOI] [PubMed] [Google Scholar]
- 133. Zhou M, Wang X, Phung V, et al. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res 2014;74:3306–16. 10.1158/0008-5472.CAN-14-0208 [DOI] [PubMed] [Google Scholar]
- 134. Gadaleta RM, Scialpi N, Peres C, et al. Suppression of hepatic bile acid synthesis by a non-tumorigenic FGF19 analogue protects mice from fibrosis and hepatocarcinogenesis. Sci Rep 2018;8:17210. 10.1038/s41598-018-35496-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hirschfield GM, Chazouillères O, Drenth JP, et al. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol 2019;70:483–93. 10.1016/j.jhep.2018.10.035 [DOI] [PubMed] [Google Scholar]
- 136. Harrison SA, Neff G, Guy CD, et al. Efficacy and safety of Aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology 2021;160:219–31. 10.1053/j.gastro.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 137. Loomba R, Ling L, Dinh DM, et al. The commensal microbe Veillonella as a marker for response to an FGF19 analog in NASH. Hepatology 2021;73:126–43. 10.1002/hep.31523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Harrison SA, Rinella ME, Abdelmalek MF, et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2018;391:1174–85. 10.1016/S0140-6736(18)30474-4 [DOI] [PubMed] [Google Scholar]
- 139. Rinella ME, Trotter JF, Abdelmalek MF, et al. Rosuvastatin improves the FGF19 analogue NGM282-associated lipid changes in patients with non-alcoholic steatohepatitis. J Hepatol 2019;70:735–44. 10.1016/j.jhep.2018.11.032 [DOI] [PubMed] [Google Scholar]
- 140. Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol 2015;62:720–33. 10.1016/j.jhep.2014.10.039 [DOI] [PubMed] [Google Scholar]
- 141. Dubois V, Eeckhoute J, Lefebvre P, et al. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Invest 2017;127:1202–14. 10.1172/JCI88894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Francque S, Szabo G, Abdelmalek MF, et al. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol 2021;18:24-39. 10.1038/s41575-020-00366-5 [DOI] [PubMed] [Google Scholar]
- 143. Ghonem NS, Assis DN, Boyer JL. Fibrates and cholestasis. Hepatology 2015;62:635–43. 10.1002/hep.27744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Laurin J, Lindor KD, Crippin JS, et al. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology 1996;23:1464–7. 10.1002/hep.510230624 [DOI] [PubMed] [Google Scholar]
- 145. Reig A, Sesé P, Parés A. Effects of bezafibrate on outcome and pruritus in primary biliary cholangitis with suboptimal ursodeoxycholic acid response. Am J Gastroenterol 2018;113:49–55. 10.1038/ajg.2017.287 [DOI] [PubMed] [Google Scholar]
- 146. de Vries E, Bolier R, Goet J, et al. Fibrates for Itch (FITCH) in Fibrosing Cholangiopathies: A Double-Blind, Randomized, Placebo-Controlled Trial. Gastroenterology 2021;160:734–43. 10.1053/j.gastro.2020.10.001 [DOI] [PubMed] [Google Scholar]
- 147. Honda A, Tanaka A, Kaneko T, et al. Bezafibrate improves globe and UK-PBC scores and long-term outcomes in patients with primary biliary cholangitis. Hepatology 2019;70:2035–46. 10.1002/hep.30552 [DOI] [PubMed] [Google Scholar]
- 148. Scott R, Best J, Forder P, et al. Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study: baseline characteristics and short-term effects of fenofibrate [ISRCTN64783481]. Cardiovasc Diabetol 2005;4:13. 10.1186/1475-2840-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Cheung AC, Lapointe-Shaw L, Kowgier M, et al. Combined ursodeoxycholic acid (UDCA) and fenofibrate in primary biliary cholangitis patients with incomplete UDCA response may improve outcomes. Aliment Pharmacol Ther 2016;43:283–93. 10.1111/apt.13465 [DOI] [PubMed] [Google Scholar]
- 150. Grigorian AY, Mardini HE, Corpechot C, et al. Fenofibrate is effective adjunctive therapy in the treatment of primary biliary cirrhosis: a meta-analysis. Clin Res Hepatol Gastroenterol 2015;39:296–306. 10.1016/j.clinre.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 151. Lemoinne S, Pares A, Reig A, et al. Primary sclerosing cholangitis response to the combination of fibrates with ursodeoxycholic acid: French-Spanish experience. Clin Res Hepatol Gastroenterol 2018;42:521–8. 10.1016/j.clinre.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 152. Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004;351:1106–18. 10.1056/NEJMra041001 [DOI] [PubMed] [Google Scholar]
- 153. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology 2018;67:328–57. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 154. Marra F, DeFranco R, Robino G, et al. Thiazolidinedione treatment inhibits bile duct proliferation and fibrosis in a rat model of chronic cholestasis. World J Gastroenterol 2005;11:4931–8. 10.3748/wjg.v11.i32.4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Okai T, Mouri H, Yamaguchi Y, et al. Beneficial hepatic effect of troglitazone in a patient with antimitochondrial antibody-negative primary biliary cirrhosis. Am J Gastroenterol 2002;97:209–10. 10.1111/j.1572-0241.2002.05411.x [DOI] [PubMed] [Google Scholar]
- 156. Ghose R, Mulder J, von Furstenberg RJ, et al. Rosiglitazone attenuates suppression of RXRalpha-dependent gene expression in inflamed liver. J Hepatol 2007;46:115–23. 10.1016/j.jhep.2006.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Baghdasaryan A, Claudel T, Kosters A, et al. Curcumin improves sclerosing cholangitis in Mdr2-/- mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut 2010;59:521–30. 10.1136/gut.2009.186528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Eaton JE, Nelson KM, Gossard AA, et al. Efficacy and safety of curcumin in primary sclerosing cholangitis: an open label pilot study. Scand J Gastroenterol 2019;54:633–9. 10.1080/00365521.2019.1611917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Liu Y, Colby JK, Zuo X, et al. The role of PPAR-δ in metabolism, inflammation, and cancer: many characters of a critical transcription factor. Int J Mol Sci 2018;19. 10.3390/ijms19113339. [Epub ahead of print: 26 Oct 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Jones D, Boudes PF, Swain MG, et al. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol 2017;2:716–26. 10.1016/S2468-1253(17)30246-7 [DOI] [PubMed] [Google Scholar]
- 161. FDA lifts all clinical holds on Seladelpar. Available: https://www.globenewswire.com/news-release/2020/07/23/2066548/0/en/FDA-Lifts-All-Clinical-Holds-on-Seladelpar.html
- 162. Harrison SA, Gunn NT, Khazanchi A. A 52-week multi-center double-blind randomized phase 2 study of Seladelpar, a potent and selective peroxisome proliferator-activated receptor delta (PPAR-delta) agonist, in patients with nonalcoholic steatohepatitis (NASH). Hepatology 2020;72:1043A–4.31849085 [Google Scholar]
- 163. Harrity T, Farrelly D, Tieman A, et al. Muraglitazar, a novel dual (alpha/gamma) peroxisome proliferator-activated receptor activator, improves diabetes and other metabolic abnormalities and preserves beta-cell function in db/db mice. Diabetes 2006;55:240–8. 10.2337/diabetes.55.01.06.db05-0648 [DOI] [PubMed] [Google Scholar]
- 164. Ye J-M, Iglesias MA, Watson DG, et al. Pparalpha /gamma ragaglitazar eliminates fatty liver and enhances insulin action in fat-fed rats in the absence of hepatomegaly. Am J Physiol Endocrinol Metab 2003;284:E531–40. 10.1152/ajpendo.00299.2002 [DOI] [PubMed] [Google Scholar]
- 165. Gawrieh S, Noureddin M, Loo N, et al. Saroglitazar, a PPAR-α/γ agonist, for treatment of NAFLD: a randomized controlled double-blind phase 2 trial. Hepatology 2021. 10.1002/hep.31843. [Epub ahead of print: 02 Apr 2021]. [DOI] [PubMed] [Google Scholar]
- 166. Siddiqui MS, Idowu MO, Parmar D, et al. A phase 2 double blinded, randomized controlled trial of Saroglitazar in patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2020. 10.1016/j.cgh.2020.10.051. [Epub ahead of print: 02 Nov 2020]. [DOI] [PubMed] [Google Scholar]
- 167. Rubenstrunk A, Hanf R, Hum DW, et al. Safety issues and prospects for future generations of PPAR modulators. Biochim Biophys Acta 2007;1771:1065–81. 10.1016/j.bbalip.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 168. Vuppalanchi R, González-Huezo MS, Payan-Olivas R, et al. A multicenter, open-label, single-arm study to evaluate the efficacy and safety of Saroglitazar in patients with primary biliary cholangitis. Clin Transl Gastroenterol 2021;12:e00327. 10.14309/ctg.0000000000000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-α and -δ, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016;150:1147–59. 10.1053/j.gastro.2016.01.038 [DOI] [PubMed] [Google Scholar]
- 170. Harrison SA, Ratziu V, Bedossa P. RESOLVE-IT phase 3 of Elafibranor in NASH: final results of the week 72 interim surrogate efficacy analysis. Hepatology 2020;72:LP23. [Google Scholar]
- 171. Schattenberg JM, Pares A, Kowdley KV, et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J Hepatol 2021;74:1344–54. 10.1016/j.jhep.2021.01.013 [DOI] [PubMed] [Google Scholar]
- 172. Boyer-Diaz Z, Aristu-Zabalza P, Andrés-Rozas M, et al. Pan-Ppar agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J Hepatol 2021;74:1188–99. 10.1016/j.jhep.2020.11.045 [DOI] [PubMed] [Google Scholar]
- 173. Francque S, Bedossa P, Ratziu V. The PanPPAR agonist lanifibranor induces both resolution of NASH and regression of fibrosis after 24 weeks of treatment in non-cirrhotic NASH: results of the native phase 2B trial. Hepatology 2020;72:9A–11. [Google Scholar]
- 174. Flippo KH, Potthoff MJ. Metabolic messengers: FGF21. Nat Metab 2021;3:309–17. 10.1038/s42255-021-00354-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Tillman EJ, Rolph T. Fgf21: an emerging therapeutic target for non-alcoholic steatohepatitis and related metabolic diseases. Front Endocrinol 2020;11:601290. 10.3389/fendo.2020.601290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Sanyal A, Charles ED, Neuschwander-Tetri BA, et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2A trial. Lancet 2019;392:2705–17. 10.1016/S0140-6736(18)31785-9 [DOI] [PubMed] [Google Scholar]
- 177. Harrison SA, Ruane PJ, Freilich BL, et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2A trial. Nat Med 2021;27:1262–71. 10.1038/s41591-021-01425-3 [DOI] [PubMed] [Google Scholar]
- 178. Harrison SA, Ruane P, Freilich B. Efruxifermin (EFX) improved markers of fibrosis, liver injury andmetabolism in F4 NASH patients with compensated cirrhosis. J Hepatol 2021;75:S204. [Google Scholar]
- 179. Sinha RA, Bruinstroop E, Singh BK, et al. Nonalcoholic fatty liver disease and hypercholesterolemia: roles of thyroid hormones, metabolites, and agonists. Thyroid 2019;29:1173–91. 10.1089/thy.2018.0664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Layden TJ, Boyer JL. The effect of thyroid hormone on bile salt-independent bile flow and Na+, K+ -ATPase activity in liver plasma membranes enriched in bile canaliculi. J Clin Invest 1976;57:1009–18. 10.1172/JCI108342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Yuan C, Lin JZH, Sieglaff DH, et al. Identical gene regulation patterns of T3 and selective thyroid hormone receptor modulator gC-1. Endocrinology 2012;153:501–11. 10.1210/en.2011-1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Sinha RA, Bruinstroop E, Singh BK, et al. Thyroid hormones and thyromimetics: a new approach to nonalcoholic steatohepatitis? Hepatology 2020;72:770–1. 10.1002/hep.31204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and Low-Normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol 2018;16:123–31. 10.1016/j.cgh.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 184. Bruinstroop E, Dalan R, Cao Y, et al. Low-Dose levothyroxine reduces intrahepatic lipid content in patients with type 2 diabetes mellitus and NAFLD. J Clin Endocrinol Metab 2018;103:2698–706. 10.1210/jc.2018-00475 [DOI] [PubMed] [Google Scholar]
- 185. Harrison SA, Bashir MR, Guy CD, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019;394:2012–24. 10.1016/S0140-6736(19)32517-6 [DOI] [PubMed] [Google Scholar]
- 186. Floreani A, Mangini C, Reig A, et al. Thyroid dysfunction in primary biliary cholangitis: a comparative study at two European centers. Am J Gastroenterol 2017;112:114–9. 10.1038/ajg.2016.479 [DOI] [PubMed] [Google Scholar]
- 187. Silveira MG, Mendes FD, Diehl NN, et al. Thyroid dysfunction in primary biliary cirrhosis, primary sclerosing cholangitis and non-alcoholic fatty liver disease. Liver Int 2009;29:1094–100. 10.1111/j.1478-3231.2009.02003.x [DOI] [PubMed] [Google Scholar]
- 188. Gautherot J, Claudel T, Cuperus F, et al. Thyroid hormone receptor β1 stimulates ABCB4 to increase biliary phosphatidylcholine excretion in mice. J Lipid Res 2018;59:1610–9. 10.1194/jlr.M084145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Shoda J, Inada Y, Tsuji A, et al. Bezafibrate stimulates canalicular localization of NBD-labeled PC in HepG2 cells by PPARalpha-mediated redistribution of ABCB4. J Lipid Res 2004;45:1813–25. 10.1194/jlr.M400132-JLR200 [DOI] [PubMed] [Google Scholar]
- 190. Liu Y, Binz J, Numerick MJ, et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest 2003;112:1678–87. 10.1172/JCI18945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Claudel T, Staels B, Kuipers F. The farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol 2005;25:2020–30. 10.1161/01.ATV.0000178994.21828.a7 [DOI] [PubMed] [Google Scholar]
- 192. Karpen SJ, Kelly D, Mack C, et al. Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders. Hepatol Int 2020;14:677–89. 10.1007/s12072-020-10070-w [DOI] [PubMed] [Google Scholar]
- 193. Harach T, Pols TWH, Nomura M, et al. Tgr5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci Rep 2012;2:430. 10.1038/srep00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (lean): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679–90. 10.1016/S0140-6736(15)00803-X [DOI] [PubMed] [Google Scholar]
- 195. Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous Semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021;384:1113–24. 10.1056/NEJMoa2028395 [DOI] [PubMed] [Google Scholar]
- 196. Marzioni M, Alpini G, Saccomanno S, et al. Exendin-4, a glucagon-like peptide 1 receptor agonist, protects cholangiocytes from apoptosis. Gut 2009;58:990–7. 10.1136/gut.2008.150870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Marzioni M, Alpini G, Saccomanno S, et al. Glucagon-Like peptide-1 and its receptor agonist exendin-4 modulate cholangiocyte adaptive response to cholestasis. Gastroenterology 2007;133:244–55. 10.1053/j.gastro.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 198. Baghdasaryan A, Fuchs CD, Österreicher CH, et al. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J Hepatol 2016;64:674–81. 10.1016/j.jhep.2015.10.024 [DOI] [PubMed] [Google Scholar]
- 199. Miethke AG, Zhang W, Simmons J, et al. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology 2016;63:512–23. 10.1002/hep.27973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Fuchs CD, Paumgartner G, Mlitz V, et al. Colesevelam attenuates cholestatic liver and bile duct injury in Mdr2-/- mice by modulating composition, signalling and excretion of faecal bile acids. Gut 2018;67:1683–91. 10.1136/gutjnl-2017-314553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Rao A, Kosters A, Mells JE, et al. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet–fed mice. Sci Transl Med 2016;8:357ra122. 10.1126/scitranslmed.aaf4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Newsome PN, Palmer M, Freilich B, et al. Volixibat in adults with non-alcoholic steatohepatitis: 24-week interim analysis from a randomized, phase II study. J Hepatol 2020;73:231–40. 10.1016/j.jhep.2020.03.024 [DOI] [PubMed] [Google Scholar]
- 203. Verrier ER, Colpitts CC, Sureau C, et al. Hepatitis B virus receptors and molecular drug targets. Hepatol Int 2016;10:567–73. 10.1007/s12072-016-9718-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Kaneko M, Futamura Y, Tsukuda S, et al. Chemical array system, a platform to identify novel hepatitis B virus entry inhibitors targeting sodium taurocholate cotransporting polypeptide. Sci Rep 2018;8:2769. 10.1038/s41598-018-20987-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Donkers JM, Zehnder B, van Westen GJP, et al. Reduced hepatitis B and D viral entry using clinically applied drugs as novel inhibitors of the bile acid transporter Ntcp. Sci Rep 2017;7:15307. 10.1038/s41598-017-15338-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Slijepcevic D, Roscam Abbing RLP, Fuchs CD, et al. Na + -taurocholate cotransporting polypeptide inhibition has hepatoprotective effects in cholestasis in mice. Hepatology 2018;68:1057–69. 10.1002/hep.29888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Slijepcevic D, Kaufman C, Wichers CGK, et al. Impaired uptake of conjugated bile acids and hepatitis b virus pres1-binding in na(+) -taurocholate cotransporting polypeptide knockout mice. Hepatology 2015;62:207–19. 10.1002/hep.27694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Vaz FM, Paulusma CC, Huidekoper H, et al. Sodium taurocholate cotransporting polypeptide (SLC10A1) deficiency: conjugated hypercholanemia without a clear clinical phenotype. Hepatology 2015;61:260–7. 10.1002/hep.27240 [DOI] [PubMed] [Google Scholar]
- 209. Yoon YB, Hagey LR, Hofmann AF, et al. Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology 1986;90:837–52. 10.1016/0016-5085(86)90859-0 [DOI] [PubMed] [Google Scholar]
- 210. Halilbasic E, Fiorotto R, Fickert P, et al. Side chain structure determines unique physiologic and therapeutic properties of norursodeoxycholic acid in Mdr2-/- mice. Hepatology 2009;49:1972–81. 10.1002/hep.22891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Fickert P, Wagner M, Marschall H-U, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in mdr2 (ABCB4) knockout mice. Gastroenterology 2006;130:465–81. 10.1053/j.gastro.2005.10.018 [DOI] [PubMed] [Google Scholar]
- 212. Fickert P, Hirschfield GM, Denk G, et al. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol 2017;67:549–58. 10.1016/j.jhep.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 213. Halilbasic E, Steinacher D, Trauner M. Nor-Ursodeoxycholic acid as a novel therapeutic approach for cholestatic and metabolic liver diseases. Dig Dis 2017;35:288–92. 10.1159/000454904 [DOI] [PubMed] [Google Scholar]
- 214. Beraza N, Malato Y, Sander LE, et al. Hepatocyte-Specific NEMO deletion promotes NK/NKT cell– and TRAIL-dependent liver damage. J Exp Med 2009;206:1727–37. 10.1084/jem.20082152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Traussnigg S, Schattenberg JM, Demir M, et al. Norursodeoxycholic acid versus placebo in the treatment of non-alcoholic fatty liver disease: a double-blind, randomised, placebo-controlled, phase 2 dose-finding trial. Lancet Gastroenterol Hepatol 2019;4:781–93. 10.1016/S2468-1253(19)30184-0 [DOI] [PubMed] [Google Scholar]
- 216. Lefere S, Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: crosstalk with metabolism. JHEP Reports 2019;1:30–43. 10.1016/j.jhepr.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Cadamuro M, Girardi N, Gores GJ, et al. The emerging role of macrophages in chronic cholangiopathies featuring biliary fibrosis: an attractive therapeutic target for orphan diseases. Front Med 2020;7:115. 10.3389/fmed.2020.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Lefere S, Devisscher L, Tacke F. Targeting CCR2/5 in the treatment of nonalcoholic steatohepatitis (NASH) and fibrosis: opportunities and challenges. Expert Opin Investig Drugs 2020;29:89–92. 10.1080/13543784.2020.1718106 [DOI] [PubMed] [Google Scholar]
- 219. Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018;67:1754–67. 10.1002/hep.29477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ratziu V, Sanyal A, Harrison SA, et al. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2B CENTAUR study. Hepatology 2020;72:892–905. 10.1002/hep.31108 [DOI] [PubMed] [Google Scholar]
- 221. Guicciardi ME, Trussoni CE, Krishnan A, et al. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J Hepatol 2018;69:676–86. 10.1016/j.jhep.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Kunzmann LK, Schoknecht T, Poch T, et al. Monocytes as potential mediators of pathogen-induced T-helper 17 differentiation in patients with primary sclerosing cholangitis (PSC). Hepatology 2020;72:1310–26. 10.1002/hep.31140 [DOI] [PubMed] [Google Scholar]
- 223. Rau M, Schilling A-K, Meertens J, et al. Progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis is marked by a higher frequency of Th17 cells in the liver and an increased Th17/Resting regulatory T cell ratio in peripheral blood and in the liver. J.i. 2016;196:97–105. 10.4049/jimmunol.1501175 [DOI] [PubMed] [Google Scholar]
- 224. Yu D, Cai S-Y, Mennone A, et al. Cenicriviroc, a cytokine receptor antagonist, potentiates all-trans retinoic acid in reducing liver injury in cholestatic rodents. Liver Int 2018;38:1128–38. 10.1111/liv.13698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Chen W, Yang A, Jia J, et al. Lysyl oxidase (LOX) family members: rationale and their potential as therapeutic targets for liver fibrosis. Hepatology 2020;72:729–41. 10.1002/hep.31236 [DOI] [PubMed] [Google Scholar]
- 226. Pollheimer MJ, Racedo S, Mikels-Vigdal A, et al. Lysyl oxidase-like protein 2 (LOXL2) modulates barrier function in cholangiocytes in cholestasis. J Hepatol 2018;69:368–77. 10.1016/j.jhep.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 227. Harrison SA, Abdelmalek MF, Caldwell S, et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology 2018;155:1140–53. 10.1053/j.gastro.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 228. Muir AJ, Levy C, Janssen HLA, et al. Simtuzumab for primary sclerosing cholangitis: phase 2 study results with insights on the natural history of the disease. Hepatology 2019;69:684–98. 10.1002/hep.30237 [DOI] [PubMed] [Google Scholar]
- 229. Gindin Y, Chung C, Jiang Z, et al. A Fibrosis-Independent hepatic transcriptomic signature identifies drivers of disease progression in primary sclerosing cholangitis. Hepatology 2021;73:1105–16. 10.1002/hep.31488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med 2019;65:37–55. 10.1016/j.mam.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 231. Lemoinne S, Friedman SL. New and emerging anti-fibrotic therapeutics entering or already in clinical trials in chronic liver diseases. Curr Opin Pharmacol 2019;49:60–70. 10.1016/j.coph.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 232. Soret P-A, Lam L, Carrat F, et al. Combination of fibrates with obeticholic acid is able to normalise biochemical liver tests in patients with difficult-to-treat primary biliary cholangitis. Aliment Pharmacol Ther 2021;53:1138–46. 10.1111/apt.16336 [DOI] [PubMed] [Google Scholar]
- 233. Smets L, Verbeek J, Korf H, et al. Improved markers of cholestatic liver injury in patients with primary biliary cholangitis treated with obeticholic acid and bezafibrate. Hepatology 2021;73:2598–600. 10.1002/hep.31613 [DOI] [PubMed] [Google Scholar]
- 234. Roth JD, Veidal SS, Fensholdt LKD, et al. Combined obeticholic acid and elafibranor treatment promotes additive liver histological improvements in a diet-induced ob/ob mouse model of biopsy-confirmed NASH. Sci Rep 2019;9:9046. 10.1038/s41598-019-45178-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Schierle S, Neumann S, Heitel P, et al. Design and structural optimization of dual FXR/PPARδ activators. J Med Chem 2020;63:8369–79. 10.1021/acs.jmedchem.0c00618 [DOI] [PubMed] [Google Scholar]
- 236. Dufour J-F, Caussy C, Loomba R. Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut 2020;69:1877–84. 10.1136/gutjnl-2019-319104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Müller TD, Clemmensen C, Finan B, et al. Anti-Obesity therapy: from rainbow pills to Polyagonists. Pharmacol Rev 2018;70:712–46. 10.1124/pr.117.014803 [DOI] [PubMed] [Google Scholar]
- 238. Hartman ML, Sanyal AJ, Loomba R, et al. Effects of novel dual GIP and GLP-1 receptor agonist Tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care 2020;43:1352–5. 10.2337/dc19-1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Nahra R, Wang T, Gadde KM, et al. Effects of Cotadutide on metabolic and hepatic parameters in adults with overweight or obesity and type 2 diabetes: a 54-Week randomized phase 2B study. Diabetes Care 2021;44:1433–42. 10.2337/dc20-2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Ampuero J, Romero-Gomez M. Stratification of patients in NASH clinical trials: a pitfall for trial success. JHEP Rep 2020;2:100148. 10.1016/j.jhepr.2020.100148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Kowdley KV, Vuppalanchi R, Levy C, et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol 2020;73:94–101. 10.1016/j.jhep.2020.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Harrison SA, Wong VW-S, Okanoue T, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol 2020;73:26–39. 10.1016/j.jhep.2020.02.027 [DOI] [PubMed] [Google Scholar]
- 243. Harrison SA, Rossi SJ, Paredes AH, et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology 2020;71:1198–212. 10.1002/hep.30590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Hirschfield G, Kowdley KV, Shiffman ML. Enhance: safety and efficacy of Seladelpar in patients with primary biliary cholangitis (pBC) -A phase 3 international, randomized, placebo-controlled study. Hepatology 2020;72:LO11. [PMC free article] [PubMed] [Google Scholar]
- 245. Harrison SA, Bashir M, Moussa SE, et al. Effects of Resmetirom on noninvasive endpoints in a 36-Week phase 2 active treatment extension study in patients with NASH. Hepatol Commun 2021;5:573–88. 10.1002/hep4.1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Loomba R, Neutel J, Mohseni R, et al. LBP-20-VK2809, a novel liver-directed thyroid receptor beta agonist, significantly reduces liver fat with both low and high doses in patients with non-alcoholic fatty liver disease: a phase 2 randomized, placebo-controlled trial. J Hepatol 2019;70:e150–1. 10.1016/S0618-8278(19)30266-X [DOI] [Google Scholar]
- 247. Eksteen B, Bowlus CL, Montano-Loza AJ, et al. Efficacy and safety of Cenicriviroc in patients with primary sclerosing cholangitis: PERSEUS study. Hepatol Commun 2021;5:478–90. 10.1002/hep4.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]