Abstract
The stability of cyclooxygenase 2 (Cox-2) mRNA is regulated positively by proinflammatory stimuli acting through mitogen-activated protein kinase (MAPK) p38 and negatively by anti-inflammatory glucocorticoids such as dexamethasone. A tetracycline-regulated reporter system was used to investigate mechanisms of regulation of Cox-2 mRNA stability. Dexamethasone was found to destabilize β-globin–Cox-2 reporter mRNAs by inhibiting p38. This inhibition occurred at the level of p38 itself: stabilization of reporter mRNA by a kinase upstream of p38 was blocked by dexamethasone, while stabilization by a kinase downstream of p38 was insensitive to dexamethasone. Inhibition of p38 activity by dexamethasone was observed in a variety of cell types treated with different activating stimuli. Furthermore, inhibition of p38 was antagonized by the anti-glucocorticoid RU486 and was delayed and actinomycin D sensitive, suggesting that ongoing glucocorticoid receptor-dependent transcription is required.
In spite of their deleterious effects on extracellular matrix, immune function, and carbohydrate metabolism, glucocorticoids (GCs) are still widely used in the treatment of inflammatory diseases such as rheumatoid arthritis and asthma (3), largely because they down-regulate “proinflammatory” genes, such as those encoding tumor necrosis factor alpha (56), interleukin -1α (IL-1α) and IL-1β (1, 32), IL-6 and IL-8, granulocyte-macrophage colony-stimulating factor (58), beta interferon (19, 40, 41), monocyte chemottractant protein 1 (42), inducible nitric oxide synthase (29, 59), and cyclooxygenase-2 (Cox-2) (4, 11, 37, 38, 49). Both positive and negative regulation of gene expression are mediated by the GC receptor (GR), which is retained in the cytoplasm in the absence of its ligand and transported to the nucleus following ligand activation (5). Several mechanisms have been suggested for the negative regulation of gene expression by GCs (36). For example, GR is proposed to interact with and inhibit transactivation by the transcription factors NF-κB and AP-1 (26, 28, 34, 45, 53, 63), which are positive regulators of many of the genes in question. Both of these transcription factors may also be subject to less direct negative regulatory mechanisms. GCs are reported to induce the expression of IκB, a cytoplasmic inhibitor of NF-κB (2, 52), and to inhibit cJun N-terminal kinases (JNKs), which are required for the expression and function of AP-1 components (7, 20, 56). In several cases, GCs inhibit gene expression at the level of mRNA turnover (1, 32, 41, 42). For example, inhibition of Cox-2 gene expression was partly mediated by destabilization of Cox-2 mRNA. This effect required ongoing transcription and translation, suggesting an involvement of dexamethasone-induced gene expression (38, 49).
In response to various proinflammatory stimuli, mitogen-activated protein kinase (MAPK) p38 is activated by MAPK kinase 6 (MKK6) (12, 23, 44). In turn, p38 phosphorylates and activates a number of substrates, including MAPK-activated protein kinase 2 (MAPKAPK-2) (16, 50). This signal transduction cascade is required for the expression of several proinflammatory genes and exerts its effects at least partly at the level of mRNA stability (60, 61). An involvement in the control of Cox-2 mRNA stability was demonstrated by means of actinomycin D chase experiments (14, 47) and confirmed using a tetracycline-regulated mRNA stability assay (31). In this case, the stability of a chimeric β-globin–Cox-2 reporter mRNA was altered by coexpression of constitutively active or dominant negative components of the p38 pathway (31).
The control of mRNA stability is frequently mediated by the 3′ untranslated region (UTR) of the transcript in question. In particular, the 3′ UTRs of unstable cytokine, growth factor, and proto-oncogene mRNAs often contain AU-rich elements, with repeats of the so-called destabilizing motif AUUUA (9, 10, 55). The most abundant Cox-2 transcript contains an exceptionally long 3′ UTR of 2.5 kb, which contains 22 copies of the AUUUA motif (24, 39). Using the tetracycline-regulated reporter assay, a p38-responsive element was mapped to a short, evolutionarily conserved AU-rich region which lies immediately 3′ to the translation termination codon of Cox-2 mRNA and contains six AUUUA repeats (31). Although some 3′ UTRs have been implicated as targets of dexamethasone (17, 25, 41), reporter mRNAs containing the Cox-2 3′ UTR were not responsive to GCs in previous studies (15, 21, 38, 49). The mechanism of regulation of Cox-2 mRNA stability by GCs is therefore unclear.
The tetracycline-regulated reporter system was used to investigate the mechanism of regulation of Cox-2 mRNA stability by the synthetic GC dexamethasone in HeLa Tet-off (HeLa-TO) cells. Dexamethasone destabilized Cox-2 mRNA by inhibiting MAPK p38. The site of action of dexamethasone was downstream of MKK6, and upstream of MAPKAPK-2. The MAPK p38 pathway controls the expression of several genes at the level of mRNA stability (31, 35, 60, 61). The findings described here suggest that GCs may negatively regulate gene expression by means of p38 inhibition and consequent mRNA destabilization. This represents an important novel point of convergence of two signal transduction pathways, which profoundly influence proinflammatory gene expression.
MATERIALS AND METHODS.
Materials.
All the DNA constructs used in this work were previously described (31). SB203580 was from Calbiochem-Novabiochem. Salmonella enterica serovar Typhimurium lipopolysaccharide, dexamethasone, RU486, and all-trans-retinoic acid were from Sigma-Aldrich Co. The rabbit antiserum to the C-terminal peptide of p38α MAPK used for both immunoprecipitation and Western blotting has been described previously (51). The rabbit anti-human antibodies against the phosphorylated forms of p38 MAPK and MKK6 were from New England Biolabs (no. 9211 and 9231, respectively). The rabbit antiserum to JNK was described previously (13). Rabbit anti-human Cox-2 antibody was from Oxford Biomedical Research. A rabbit polyclonal antiserum to the N terminus of MKK6 was raised against the synthetic peptide MELGRGAYGVVEKMR.
Cell culture and transfection.
HeLa-TO cells (Clontech) were maintained in Dulbecco's modified Eagle's medium–10% fetal calf serum supplemented with G418 (100 ng/ml; Life Technologies). Cells were seeded in six-well plates at a density of 1.5 × 105cells/well. The following day, the cells were transfected using Superfect (Qiagen). The amount of total transfected DNA was kept constant within all experiments by addition of appropriate empty expression vectors and/or Bluescript plasmid (Stratagene). After 24 h, tetracycline (Sigma) was added at a final concentration of 100 ng/ml, and the cells were harvested in guanidine thiocyanate lysis buffer (Ambion) at different intervals, as indicated in each figure. Lysates were passed through Shredder columns (Qiagen) and stored frozen at −20°C. In some experiments, dexamethasone, RU486, or SB203580 were added to cells 1 or 2 h prior to the addition of tetracycline. HeLa (American Type Culture Collection), human skin fibroblast, and RAW264.7 cells were maintained in Dulbecco's modified Eagle's medium–10% fetal calf serum. Human peripheral blood T cells were prepared from the buffy coat fraction of a unit of blood from a single donor. Mononuclear cells were prepared by Ficoll-Hypaque centrifugation on a Lymphoprep gradient, and T cells were isolated by centrifugal elutriation on a Beckman JE6 elutriator, using RPMI 1640 medium containing 1% fetal calf serum. T-cell purity was assessed by forward- and side-scatter flow cytometry in a Becton Dickinson FACScan, and cells of >90% purity were collected. Cells were rested in RPMI 1640–1% fetal calf serum for 2 h before treatments.
RNase protection assay.
Riboprobes were prepared as previously described (31), and RNase protection assays were carried out using the Direct Protect kit (Ambion) as described previously (31). Each experiment was performed at least twice.
Immunoprecipitation and assay of p38 MAPK and JNK.
HeLa-TO cells were incubated in the absence or presence of dexamethasone, the culture medium was removed, and the culture dishes were washed in phosphate-buffered saline. The cells were irradiated in a Stratalinker (40 J/m2; UV-C) (Stratagene), and the original culture medium was replaced. After the period indicated in each figure, the cells were lysed for 10 min on ice in lysis buffer (20 mM HEPES [pH 7.4], 50 mM sodium β-glycerophosphate, 2 mM EGTA, 1% Triton X-100, 10% glycerol, 150 mM NaCl, 10 mM NaF, 1 mM sodium orthovanadate, 2 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride, 3 μg of aprotinin per ml, 10 μM E64, 2 μg of pepstatin per ml). Samples were clarified by centrifugation at 4°C for 10 min at 16,000 × g. p38 MAPK was immunoprecipitated by the addition of 5 μl of rabbit antiserum to the C terminus of p38 MAPK. Samples were incubated at 4°C for 1 h with mixing before the addition of 30 μl of a 50% slurry of protein A-coated beads in lysis buffer and incubation for an additional 2 h. The beads were washed four times in 1 ml of wash buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 25 mM sodium β-glycerophosphate, 10 mM sodium tetrapyrophosphate, 1 mM sodium orthovanadate, 2 mM DTT, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) and twice in 1 ml of kinase assay buffer (20 mM HEPES [pH 7.5], 20 mM sodium β-glycerophosphate, 200 mM NaCl, 2 mM DTT, 10 mM MgCl2, 10 mM NaF, 0.1 mM sodium orthovanadate, 0.5 mM EDTA, 0.5 mM EGTA, 0.05% Brij 35). p38 MAPK was assayed using 1 μg of recombinant MAPKAPK-2 as the substrate in kinase assay buffer with 20 μM ATP and 4 μCi of [γ-32P]ATP in an assay volume of 30 μl. Samples were incubated at room temperature for 20 min with agitation, and the reaction was stopped by the addition of 4× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer. The samples were separated by SDS-PAGE (10% polyacrylamide). Gels were stained with Coomassie brilliant blue R-250, and 32P incorporation into MAPKAPK-2 was quantified on a phosphorimager (Fuji FLA-2000).
JNK immunoprecipitation was performed in an identical fashion but with 5 μl of rabbit antiserum to JNK and protein A-coated beads. Samples were assayed for kinase activity as described above using 1 μg of glutathione S-transferase–ATF-2(18–96) as substrate.
Western blotting.
HeLa-TO cells in p100 plates were transfected with or without constitutively active MKK6 expression vector and left for 24 h as described above. Some of the cells were UV irradiated as described above and left for 30 min after the stimulus. Then the cells were harvested in radioimmunoprecipitation assay buffer, separated by SDS-PAGE, and electrophoretically transferred to nitrocellulose (Sartorius). The membranes were probed with rabbit polyclonal antibodies to Cox-2, phospho-p38 MAPK, or p38α MAPK and then with a peroxidase-coupled second antibody (Dako). Proteins were detected using the enhanced chemiluminescence system (Amersham).
RESULTS
Dexamethasone inhibits the expression of Cox-2 protein and steady-state mRNA in HeLa-TO cells.
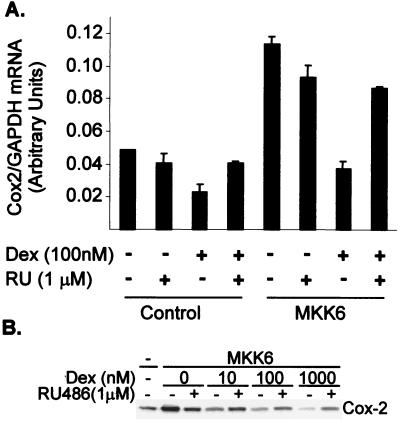
In pulmonary epithelial cells, induction of the Cox-2 gene by IL-1 was blocked by dexamethasone at the transcriptional and posttranscriptional levels (38). The HeLa-TO cells used throughout this study responded poorly to IL-1; however, endogenous Cox-2 mRNA was induced by expression of a constitutively active form of MKK6, an activator of p38 (31) (Fig. 1A). The induction was blocked by dexamethasone, and this effect was antagonized by the anti-steroid RU486, indicating that inhibitory effects of dexamethasone are mediated by the glucocorticoid receptor. A slight inhibition of basal Cox-2 expression by dexamethasone was observed.
FIG. 1.
Dexamethasone inhibits the expression of Cox-2 protein and steady-state mRNA. (A) HeLa-TO cells were transfected with 100 ng of MKK6 expression vector or empty vector control (pcDNA3). After 22 h, dexamethasone (100 nM Dex), RU486 (1 μM RU) or both were added. After a further 2 h, cells were harvested and RNase protection assays were performed to quantify Cox-2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Mean Cox-2/GAPDH mRNA ratios from a representative experiment are shown (with standard deviations; n = 4). (B) Hela-TO cells were transfected as above and incubated for 24 h in the absence or presence of the indicated concentrations of Dex alone or with 1 μM RU486. After 24 h, cells were harvested and Western blotting was performed using an antibody against Cox-2.
Cox-2 protein expression was also induced by the expression of MKK6, and the induction was blocked by dexamethasone in a dose-dependent manner, with a 50% inhibitory concentration (IC50) close to 10 nM (Fig. 1B). The effects of dexamethasone were again antagonized by RU486. In these experiments, RU486 alone partly blocked the induction of Cox-2 mRNA and protein, probably due to the weak agonistic properties of this reagent.
Dexamethasone reverses the stabilization of β-globin–Cox-2 mRNA by MKK6.
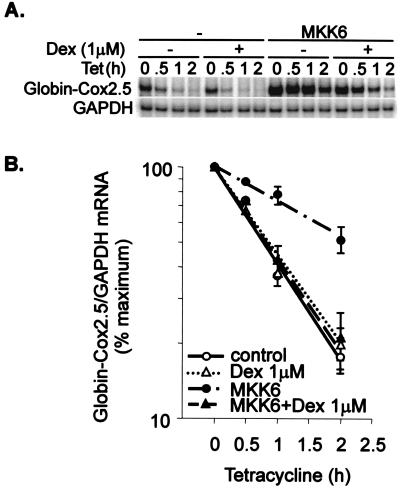
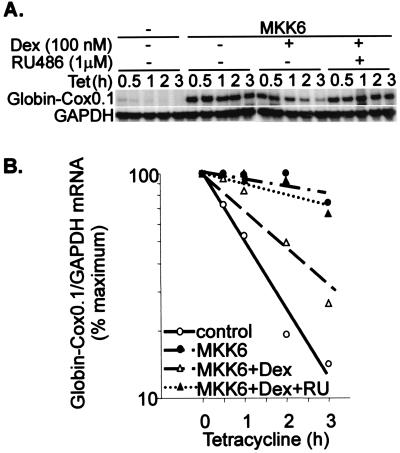
The effects of dexamethasone on Cox-2 steady-state mRNA levels in the absence or presence of MKK6 (Fig. 1) may reflect transcriptional or posttranscriptional regulation. The Cox-2 promoter contains binding sites for both Ap1 and NF-κB, which are known to be negatively regulated by the GR. To investigate the regulation of Cox-2 mRNA stability in isolation from transcriptional effects, a tetracycline-responsive globin reporter system was used, as described previously (31, 61, 62). The β-globin reporter transcript was expressed under the control of a tetracycline-regulated promoter, whose transcription can be rapidly switched off by the addition of 100 ng of tetracycline per ml to the tissue culture medium. Insertion of the 2.5-kb Cox-2 3′UTR destabilized the globin transcript, and the chimeric transcript was stabilized by coexpression of a constitutively active MKK6 mutant, as previously reported (31) (Fig. 2). Dexamethasone did not influence basal β-globin–Cox2.5 mRNA stability but reversed the MKK6-dependent stabilization of this reporter mRNA. A β-globin reporter mRNA containing part of the IL-8 3′ UTR is stabilized by activation of the p38 pathway (61). The stabilization of the β-globin–IL-8 transcript by MKK6 was reversed by dexamethasone (data not shown). The stability of the control β-globin transcript, lacking any inserted 3′ UTR fragment, was not influenced by dexamethasone (data not shown). The destabilizing effects of dexamethasone were less clear when it was added simultaneously with tetracycline (data not shown), and the steroid was added to cells 2 h prior to tetracycline in the experiments illustrated.
FIG. 2.
Dexamethasone reverses the stabilization of β-globin–Cox-2 mRNA by MKK6. HeLa-TO cells were transfected with 50 ng of pGL3c, 20 ng of pTetBBB-Cox2.5, and 100 ng of MKK6 expression vector or empty vector control (pcDNA3). After 22 h, vehicle control or dexamethasone (1 μM Dex) was added. After a further 2 h, tetracycline (Tet) was added to a final concentration of 100 ng/ml. Cells were harvested at the time intervals shown, and RNase protection assays were performed to quantify luciferase, Cox-2, β-globin–Cox2.5, and GAPDH mRNAs. (A) A representative experiment. Only the β-globin reporter and GAPDH loading control are shown. (B) Graphical representation of means and standard deviations of 11 independent experiments. β-globin/GAPDH ratios were plotted as percentages of the maximum value at the time of tetracycline addition.
Dexamethasone sensitivity is conferred by a short, AU-rich region of the Cox-2 3′ UTR.
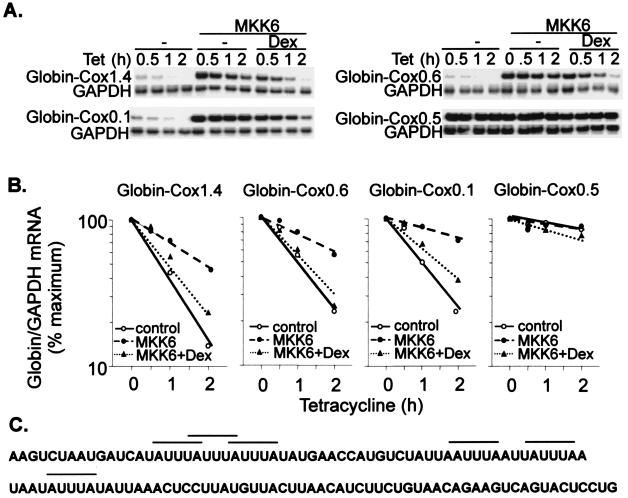
The 3′ UTR of the most abundant Cox-2 transcript is exceptionally long (2.5 kb). Cryptic polyadenylation sites give rise to minor transcripts having 3′ UTRs of 1.4 and 0.6 kb. Using a series of β-globin–Cox-2 reporter constructs, it was demonstrated that p38-dependent regulation of mRNA stability is mediated by a relatively short (123 nucleotides) and evolutionarily conserved AU-rich region lying immediately 3′ to the translation termination signal (31). The same deletion series was used to map dexamethasone-sensitive sites within the Cox-2 3′ UTR (Fig. 3). Transcripts containing 3′ UTR nucleotides 1 to 1440 (Cox1.4), 1 to 603 (Cox0.6) or 1 to 123 (Cox0.1) were similarly destabilized by dexamethasone in the presence of MKK6. In contrast, a transcript containing nucleotides 124 to 603 (Cox0.5) was unresponsive to MKK6 and dexamethasone. Under these conditions, dexamethasone and SB203580 exerted very similar effects on the stability of β-globin–Cox reporter mRNAs (Table 1). Therefore the conserved AU-rich element (Fig. 3C) appears to mediate the regulation of mRNA stability not only by the p38 pathway but also by dexamethasone.
FIG. 3.
Dexamethasone sensitivity is conferred by a short, AU-rich region of the Cox-2 3′ UTR. Transfections and RNase protection assays were performed as described in the legend to Fig 2, using the reporter constructs pTetBBB-Cox1.4, pTetBBB-Cox0.6, pTetBBB-Cox0.5, and pTetBBB-Cox0.1. Dexamethasone (Dex) (1 μM) was added 22 h after the transfection, and tetracycline (Tet) was added after a further 2 h. (A) Representative experiments. Only the β-globin reporter and GAPDH loading control are shown. (B) Graphical representation of the experiments in panel A. Each transfection was performed at least twice, with qualitatively identical results. (C) Sequence of the 123-nucleotide p38-responsive region of the Cox-2 3′ UTR. AUUUA repeats are indicated by horizontal bars.
TABLE 1.
Half-lives of β-globin Cox-2 reporter mRNAs in the presence of MKK6, dexamethasone, or SB203580a
| Transcript | Half-lifeb of transcript (h)
|
|||
|---|---|---|---|---|
| Control | MKK6 | MKK6+Dex | MKK6+SB | |
| β-globin–Cox2.5 | 1.16 | 2.00 | 1.08 | 1.32 |
| β-globin–Cox1.4 | 1.24 | 2.06 | 1.29 | 1.07 |
| β-globin–Cox0.6 | 1.02 | 2.24 | 1.31 | 1.15 |
| β-globin–Cox0.1 | 1.26 | 4.16 | 1.65 | 1.64 |
| β-globin–Cox0.5 | NMc | NM | NM | NM |
Transfections, treatments, and RNase protection assays were essentially as described in the legend to Fig. 2. SB203580 (1 μM) was added 30 min prior to the addition of tetracycline, and dexamethasone (1 μM) was added 2 h prior to the addition of tetracycline.
Half-lives were calculated from plots of β-globin/GAPDH ratio against time. The half-lives shown are from two representative experiments.
NM, not measurable.
Regulation of mRNA stability by dexamethasone is dose dependent and GR mediated.
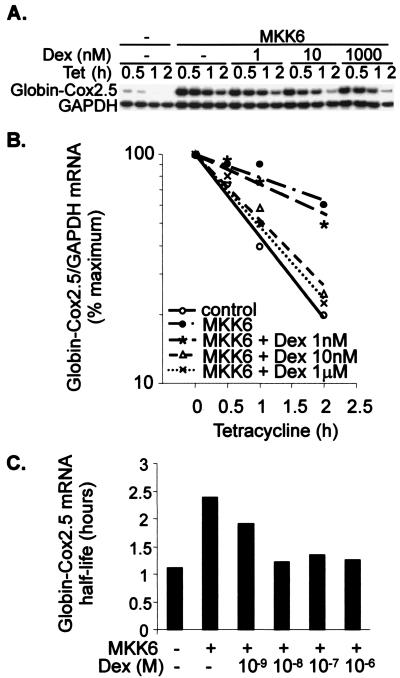
The dose dependence of mRNA stability regulation was tested using a range of dexamethasone concentrations between 1 nM and 1 μM. In the presence of MKK6, 1 nM dexamethasone weakly destabilized β-globin–Cox2.5, whereas a 10 nM dose was as effective as the maximum 1 μM dose (Fig. 4). The IC50 for the regulation of mRNA stability by dexamethasone was estimated to lie between 1 and 10 nM (Fig. 4C). Destabilization of a reporter mRNA by 100 nM dexamethasone was completely reversed by 1 μM RU486 (Fig. 5); therefore, dexamethasone exerts its effects through the GR. All-trans-retinoic acid, a ligand for another member of the nuclear hormone receptor superfamily, did not influence reporter mRNA stability (data not shown). The regulation of reporter mRNA stability mirrors the regulation of endogenous gene expression, suggesting that this is a significant mechanism for the control of Cox-2 gene expression.
FIG. 4.
The regulation of mRNA stability by dexamethasone is dose dependent. Transfections and RNase protection assays were performed as described in the legend to Fig 2, using the reporter construct pTetBBB-Cox2.5. Dexamethasone (Dex) was added 22 h after the transfection, and tetracycline (Tet) was added after a further 2 h. (A) Representative experiment. Only the β-globin reporter and GAPDH loading control are shown. (B) Graphical representation of the experiment in panel A. Each transfection was performed at least twice, with qualitatively identical results. (C) Approximate half-lives of β-globin–Cox2.5 reporter mRNA in the presence of different concentrations of dexamethasone.
FIG. 5.
The regulation of mRNA stability by dexamethasone is GR mediated. Transfections and RNase protection assays were performed as described in the legend to Fig 2, using the reporter construct pTetBBB-Cox0.1. Dexamethasone (Dex) (100 nM), alone or with RU486 (1 μM), was added 22 h after the transfection, and tetracycline (Tet) was added after a further 2 h. (A) Representative experiment. Only the β-globin reporter and GAPDH loading control are shown. (B) Graphical representation of the experiment in panel A. Each transfection was performed at least twice, with qualitatively identical results.
Dexamethasone exerts its effects at the level of MAPK p38.
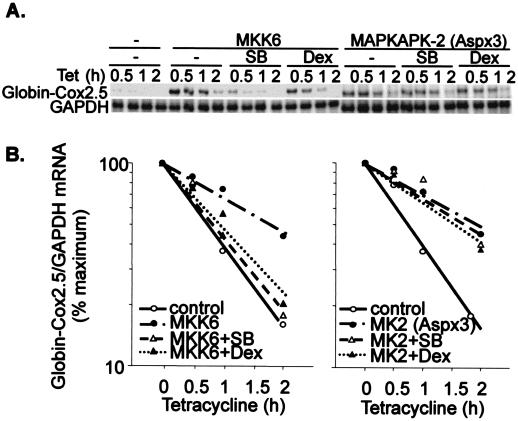
The kinase MAPKAPK-2 is phosphorylated and activated by p38. We previously showed that a constitutively active mutant of MAPKAPK-2 stabilized a β-globin–Cox2.5 reporter mRNA while two dominant negative mutants of MAPKAPK-2 interfered with the stabilization of the transcript by MKK6 (31). Regulation of mRNA stability by the p38 pathway is thus mediated by the p38 substrate MAPKAPK-2. To further investigate the mode of action of dexamethasone, β-globin–Cox2.5 was stabilized by coexpression of a constitutively active mutant of MAPKAPK-2. As reported, MKK6-dependent mRNA stabilization was sensitive to both SB203580 and dexamethasone; however, MAPKAPK2-dependent stabilization was sensitive to neither drug (Fig. 6). Therefore the effects of dexamethasone are proximal to p38 itself. The steroid must either reverse the activation of p38 or block this activation, even when the stimulus is provided by a constitutively active upstream kinase.
FIG. 6.
Dexamethasone exerts its effects at the level of MAPK p38. HeLa-TO cells were transfected with 50 ng of pGL3c, 20 ng of pTetBBB-Cox2.5, and 100 ng of MKK6 expression vector (left panel) or constitutively active MAPKAPK-2 (MK2 Aspx3) expression vector (right panel). Dexamethasone (Dex) (1 μM) or SB203580 (SB) (1 μM) was added 22 h after the transfection, and tetracycline (Tet) was added after a further 2 h. RNase protection assays were performed as described in the legend to Fig. 2. (A) Representative experiment. Only the β-globin reporter and GAPDH loading control are shown. (B) Graphical representation of the experiment in panel A. Each transfection was performed four times, with qualitatively identical results.
Dexamethasone decreases both the phosphorylation and activity of MAPK p38.
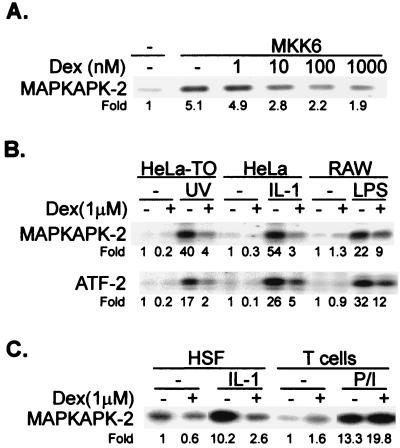
To test the effects of dexamethasone on the p38 pathway, HeLa-TO cells were transfected with a vector expressing constitutively active MKK6 and then treated with dexamethasone, and endogenous p38 enzyme activity was measured in immune complex kinase assays 2 h later. Kinase activity was stimulated fivefold by MKK6 and inhibited in a dose-dependent manner, with a maximum inhibition of approximately 60% in the presence of 1 μM dexamethasone (Fig. 7A). Half-maximal inhibition of p38 occurred at a dexamethasone concentration of approximately 10 nM. Similar results were obtained following cotransfection of MKK6 with an epitope-tagged p38 expression vector and immunoprecipitation of the exogenous p38 (data not shown).
FIG. 7.
Dexamethasone decreases the activity of MAPK p38 and JNK. (A) HeLa-TO cells in 10-cm dishes were mock transfected or transfected with 0.5 μg of MKK6 expression vector and were treated with dexamethasone (Dex) as indicated 22 h later. After a further 2 h, the cells were harvested and endogenous p38 activity was measured in immune complex kinase assays. (B) HeLa-TO, HeLa, or RAW 264.7 cells were incubated for 2 h in the presence or absence of 1 μM dexamethasone and then stimulated with UV radiation (40 J/m2), IL-1 (20 ng/ml), or lipopolysaccharide (LPS) (10 ng/ml), respectively. After 15 min (HeLa cells) or 30 min (HeLa-TO and RAW 264.7 cells), p38 MAPK activity (upper panel) or JNK activity (lower panel) was measured in immune complex assays. Fold activation compared to unstimulated cells is shown under each panel. (C) Primary human skin fibroblasts (HSF) or elutriated human peripheral blood T cells were incubated for 2 h in the presence or absence of 1 μM dexamethasone. The fibroblasts were then stimulated for 15 min with IL-1 (20 ng/ml), and the T cells were stimulated for 10 min with 50 ng of PMA per ml and 5 μg of ionomycin per ml (P/I). Assays of p38 activity were as described above.
The effects of dexamethasone in a variety of cell lines or primary cells stimulated with different p38-activating stimuli were investigated. In HeLa-TO cells treated with UV light, in another HeLa cell line stimulated with IL-1, or in RAW264.7 macrophages treated with bacterial lipopolysaccharide, the activation of p38 was strong and was potently inhibited by 1 μM dexamethasone. In these cells the activation of JNK was similarly affected by dexamethasone (Fig. 7B).
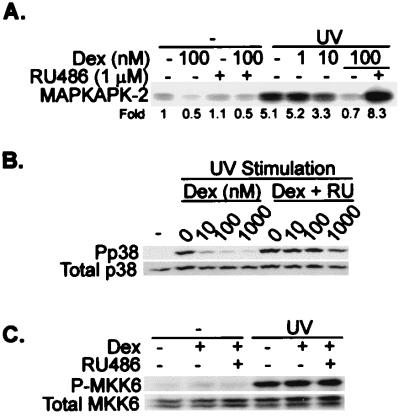
In human skin fibroblasts stimulated with IL-1, the activation of p38 was blocked by dexamethasone, while in human peripheral blood lymphocytes stimulated with phorbol myristate acetate (PMA) and ionomycin, the activation of p38 was slightly augmented by dexamethasone (Fig. 7C). Inhibition of p38 activity in UV-stimulated HeLa-TO cells was dose dependent, with an IC50 close to 10 nM, and was antagonized by RU486 (Fig. 8A). Under the same conditions, phosphorylated p38 could be detected using an antibody against the doubly phosphorylated TGY activation motif. As measured by this method, dexamethasone inhibited the phosphorylation of p38 but had no effect on p38 expression (Fig. 8B). The inhibition of p38 phosphorylation was dose dependent and reversible by RU486. In UV-stimulated HeLa-TO cells, phosphorylated MKK6 could also be detected using a phospho-specific antibody. This phosphorylation was unaffected by dexamethasone (Fig. 8C), consistent with the hypothesis that the site of action of dexamethasone is downstream of MKK6.
FIG. 8.
Dexamethasone decreases both the phosphorylation and activity of MAPK p38 in a dose-dependent manner. (A) HeLa-TO cells were incubated in the absence or presence of the indicated concentrations of dexamethasone (Dex), alone or with 1 μM RU486. After 2 h, the cells were UV stimulated and p38 MAPK activity was measured in immune complex assays. Fold activation compared to unstimulated cells is shown under the panel. The experiment was performed twice, with qualitatively identical results. (B) Treatments and stimulation of the cells were performed as described above. At 30 min after the UV stimulation, the cells were harvested and Western blotting was performed using antibodies against phospho-p38 MAPK (Pp38) or p38α MAPK (total p38). (C) HeLa-TO cells were pretreated for 2 h with 100 nM dexamethasone and/or 1 μM RU486 as indicated and then stimulated with UV. After 30 min, the cells were harvested and Western blotting was performed using antibodies against phospho-MKK6 or total MKK6.
The down-regulation of p38 activity by dexamethasone is delayed and requires ongoing transcription.
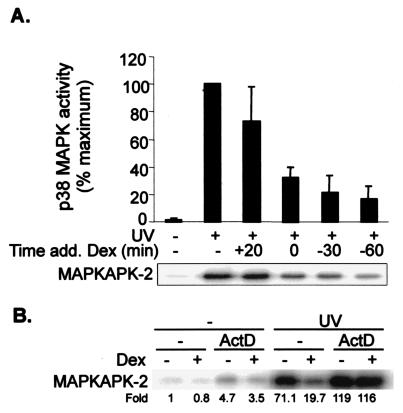
To determine the time dependence of the inhibitory activity of dexamethasone, cells were stimulated with UV and harvested 30 min later for assay of p38 activity (Fig. 9A). Dexamethasone was added to these cells 30 min or 1 h prior to the stimulus, at the same time as the stimulus, or 20 min after it (that is, 10 min before harvesting). When present for 10 min prior to harvesting, dexamethasone weakly and rather inconsistently inhibited UV-stimulated p38 activity. Longer incubation with dexamethasone resulted in gradually increasing inhibition. In comparison, the inhibition of p38 activity by 1 μM SB203580 was more or less complete and instantaneous, as expected for a low-molecular-weight direct inhibitor (data not shown).
FIG. 9.
The down-regulation of p38 activity by dexamethasone is delayed and requires ongoing transcription. (A) HeLa-TO cells were UV stimulated, and p38 activity was measured after 30 min, with or without dexamethasone (Dex) treatment as shown. The times of addition of dexamethasone are indicated with respect to the UV stimulus at t = 0. (B) HeLa-TO cells were incubated in the absence or presence of actinomycin D (ActD; 5 μg/ml) 15 min before the addition of 1 μM dexamethasone. After 2 h, cells were UV stimulated, and p38 MAPK activity was measured in immune complex assays 30 min later. Fold activation compared to unstimulated cells is shown under the panel. Each experiment was performed twice, with qualitatively identical results.
The somewhat gradual inhibition of p38 activity is consistent with a requirement for dexamethasone-dependent gene expression. To determine whether de novo gene expression was required for the action of dexamethasone, cells were stimulated with UV in the absence or presence of the transcriptional inhibitor actinomycin D and in the absence or presence of dexamethasone (Fig. 9B). Actinomycin D weakly activated p38 on its own and slightly synergized with UV. The inhibition of UV-stimulated p38 activity by dexamethasone was completely blocked by actinomycin D, confirming that de novo gene expression is required for the action of dexamethasone.
The p38 pathway plays a dominant role in the regulation of Cox-2 gene expression.
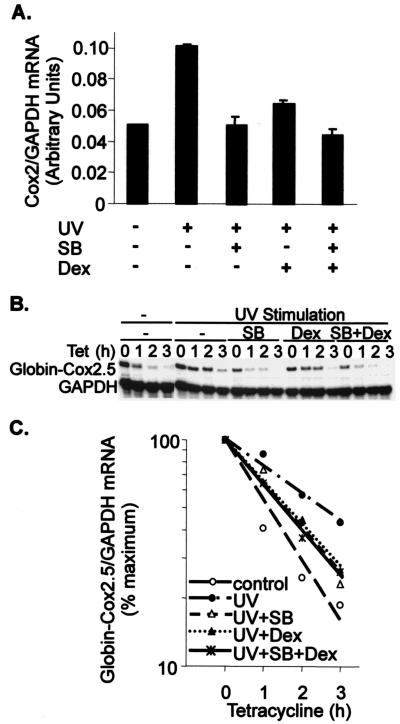
In all of the kinase experiments described above, JNK and p38 behaved almost identically (Fig. 7B and data not shown). To this point, studies of mRNA stability regulation were performed with MKK6, a specific stimulus of the p38 pathway. To investigate the role of JNKs in the control of Cox-2 mRNA turnover, endogenous Cox-2 gene expression was induced by UV stimulation, which activated both p38 and JNK pathways (Fig. 7B). Cells were pretreated with 1 μM dexamethasone (which inhibits both p38 and JNK [Fig. 7B]), with 1 μM SB203580 (which specifically inhibits p38) (47), or with both drugs. Each drug inhibited Cox-2 mRNA induction, and no synergistic inhibition was observed (Fig. 10A).
FIG. 10.
The p38 pathway plays a dominant role in the regulation of Cox-2 gene expression. (A) HeLa-TO cells were incubated in the absence or presence of 1 μM SB203580 (SB), 1 μM dexamethasone (Dex), or both together for 1 h prior to UV stimulation. After a further 1 h, cells were harvested and RNase protection assays were performed to quantify Cox-2 and GAPDH mRNAs. Mean Cox-2/GAPDH mRNA ratios from a representative experiment are shown (with standard deviations, n = 4). (B) HeLa-TO cells were transfected as described in the legend to Fig. 2, using the reporter construct pTetBBB-Cox2.5. After 24 h, the cells were incubated with 1 μM dexamethasone, 1 μM SB203580, or both for 1 h and then stimulated with UV (40 J/m2). After a further 1 h, tetracycline (Tet) was added, the cells were harvested at the time intervals shown, and RNase protection assays were performed as described in the legend to Fig. 2. Only the β-globin reporter and GAPDH loading control are shown. (C) Graphical representation of the experiment in panel A. Each transfection was performed three times, with qualitatively identical results.
The β-globin–Cox2.5 reporter mRNA was stabilized by UV stimulation. Pretreatment of cells with 1 μM dexamethasone or 1 μM SB203580 for 1 h prior to the tetracycline chase antagonized this stabilization very similarly (Fig. 10B and C). The rates of decay were almost identical in the presence of either drug, although the levels of β-globin–Cox2.5 mRNA at the zero time points differed, reflecting the slightly delayed action of dexamethasone. No additive effects on reporter mRNA stability were observed in the presence of both drugs. Therefore, although dexamethasone is an inhibitor of both p38 and JNK signal transduction pathways, the p38 pathway plays a dominant role in the regulation of Cox-2 gene expression.
DISCUSSION
In common with other proinflammatory genes such as those encoding tumor necrosis factor alpha IL-1, IL-6, IL-8 and granulocyte-macrophage colony-stimulating factor, Cox-2 expression is regulated by both GCs (4, 11, 37, 38, 49) and the MAPK p38 signal transduction cascade (14, 22, 30, 31, 33, 43, 46–48). Having used a tetracycline-regulated assay system to characterize the regulation of Cox-2 mRNA stability by p38 (31), we used the same system to study the effects of a synthetic GC, dexamethasone. Several lines of evidence suggested that dexamethasone regulates Cox-2 gene expression by means of inhibition of MAPK p38. First, the stability of reporter mRNAs was sensitive to dexamethasone only in the presence of factors or treatments which stimulated p38 activity. Second, a β-globin–IL-8 reporter mRNA, which was previously shown to be sensitive to p38 (61), was also found to be sensitive to dexamethasone. Third, dexamethasone sensitivity mapped to precisely the same small region of the Cox-2 3′UTR shown to mediate the regulation of stability by p38. Fourth, dexamethasone and SB203580 did not display additive or synergistic effects on endogenous Cox-2 gene expression or reporter mRNA stability. Fifth, the stabilization of β-globin–Cox2.5 mRNA by constitutively active MAPKAPK-2 was not sensitive to dexamethasone, and so the GC cannot exert its effects at a downstream level of the stabilization process, for example at the level of RNA-protein interactions. If this were the case, MKK6-dependent and MAPKAPK-2-dependent stabilization would be expected to be equally dexamethasone sensitive.
It was subsequently demonstrated that dexamethasone down-regulated the activity but not the expression of p38 in HeLa-TO cells. The activity of JNK was similarly down-regulated by dexamethasone, as others have reported (7, 20, 56). However the lack of additive effects of dexamethasone and SB203580 on the expression of the endogenous Cox-2 gene or the stability of β-globin–Cox-2 reporter mRNAs suggests that the p38 pathway plays a dominant role in the regulation of Cox-2 mRNA stability. Other investigators have concluded that dexamethasone has no effect on p38 activity (20, 56), while one recent publication described a modest inhibition of p38 activity (54) and another described the activation of p38 (65) by dexamethasone. In primary human lymphocytes, dexamethasone slightly augmented the activation of p38 by PMA and ionomycin (Fig. 7). These contrasting observations presumably reflect cell-specific differences in the cross talk between the GC and p38 signaling pathways.
Dexamethasone effectively destabilized β-globin–Cox-2 reporter transcripts at 1 to 10 nM concentrations, whereas the IC50 for the inhibition of p38 activity appeared slightly higher (approximately 10 nM). However, the dependence of reporter mRNA stability on p38 activity is unknown and may be quite complex. For example, the slight discrepancy of IC50s could reflect stabilization of mRNA above a critical threshold of p38 activity.
Most importantly, these results establish that dexamethasone can block signaling events downstream of MKK6, an activator of p38 (12, 23, 44), and upstream of MAPKAPK-2, a substrate and effector of p38 (16, 50). While the phosphorylation state of MKK6 was not sensitive to dexamethasone, the inhibition of p38 activity was accompanied by a decrease in its phosphorylation, as judged by Western blotting with phosphospecific antibodies. JNK activity was similarly affected by dexamethasone. One hypothetical mechanism of action of dexamethasone is the disruption of interactions between MAPKs and their upstream MKKs. JNK signaling cascades are thought to be organized by scaffold proteins such as JNK interacting protein (6, 18, 64). While little is known about the organization of the p38 signaling cascade, there is no evidence that the same scaffold proteins are involved. It is thus unclear whether this hypothesis can account for the parallel inhibition of p38 and JNK by dexamethasone. A second hypothesis is that the activation of p38 and JNK is reversed by the action of phosphatase(s). The activity of MAPK signaling pathways is regulated by a large family of dual-specificity MAPK phosphatases (MKPs), some of which display substrate specificity for members of the p38 and JNK families in vitro (8, 27). Several of these MKPs are regulated at the level of gene expression or activation. Since inhibition of p38 and JNK activity is delayed and appears to depend on de novo gene expression, it is possible that the effects of dexamethasone are mediated by transcriptional upregulation of an MKP or another p38/JNK-inactivating phosphatase (57).
Multiple mechanisms are apparently employed by GCs to negatively regulate the expression of proinflammatory genes (36). Many of the same genes are also controlled by the p38 signal transduction cascade, which is activated by several proinflammatory stimuli (14, 31, 35, 47, 48, 60, 61). Although some phosphatases inactivate p38 in vitro, little is known about off-mechanisms of the p38 cascade in vivo. It is possible that the targeting of such off-mechanisms is an important means by which GCs block or dampen proinflammatory responses. In future work, we aim to establish whether p38 dephosphorylation is involved in the destabilization of Cox-2 mRNA by dexamethasone and, if so, to identify the phosphatase(s) responsible.
ACKNOWLEDGMENTS
We are grateful to C. J. Marshall, A.-B. Shyu, J. Han, and M. Kracht for provision of reagents.
M. Lasa was supported for part of this work by a grant from the Nuffield Foundation Oliver Bird Fund. We are also grateful to the Medical Research Council and Arthritis Research Campaign for support.
REFERENCES
- 1.Amano Y, Lee S W, Allison A C. Inhibition by glucocorticoids of the formation of interleukin-1 alpha, interleukin-1 beta, and interleukin-6: mediation by decreased mRNA stability. Mol Pharmacol. 1993;43:176–182. [PubMed] [Google Scholar]
- 2.Auphan N, DiDonato J A, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 3.Barnes P J. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- 4.Barrios-Rodiles M, Tiraloche G, Chadee K. Lipopolysaccharide modulates cyclooxygenase-2 transcriptionally and posttranscriptionally in human macrophages independently from endogenous IL-1 beta and TNF-alpha. J Immunol. 1999;163:963–969. [PubMed] [Google Scholar]
- 5.Beato M, Truss M, Chavez S. Control of transcription by steroid hormones. Ann N Y Acad Sci. 1996;784:93–123. doi: 10.1111/j.1749-6632.1996.tb16231.x. [DOI] [PubMed] [Google Scholar]
- 6.Burack W R, Shaw A S. Signal transduction: hanging on a scaffold. Curr Opin Cell Biol. 2000;12:211–216. doi: 10.1016/s0955-0674(99)00078-2. [DOI] [PubMed] [Google Scholar]
- 7.Caelles C, Gonzalez-Sancho J M, Munoz A. Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev. 1997;11:3351–3364. doi: 10.1101/gad.11.24.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- 9.Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Identification of a common nucleotide sequence in the 3′-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci USA. 1986;83:1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C Y, Shyu A B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- 11.Crofford L J, Wilder R L, Ristimaki A P, Sano H, Remmers E F, Epps H R, Hla T. Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester, and corticosteroids. J Clin Investig. 1994;93:1095–1101. doi: 10.1172/JCI117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuenda A, Alonso G, Morrice N, Jones M, Meier R, Cohen P, Nebreda A R. Purification and cDNA cloning of SAPKK3, the major activator of RK/p38 in stress- and cytokine-stimulated monocytes and epithelial cells. EMBO J. 1996;15:4156–4164. [PMC free article] [PubMed] [Google Scholar]
- 13.Davis W, Stephens L R, Hawkins P T, Saklatvala J. Synergistic activation of JNK/SAPK by interleukin-1 and platelet-derived growth factor is independent of Rac and Cdc42. Biochem J. 1999;338:387–392. [PMC free article] [PubMed] [Google Scholar]
- 14.Dean J L, Brook M, Clark A R, Saklatvala J. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J Biol Chem. 1999;274:264–269. doi: 10.1074/jbc.274.1.264. [DOI] [PubMed] [Google Scholar]
- 15.Dixon D A, Kaplan C D, McIntyre T M, Zimmerman G A, Prescott S M. Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3′-untranslated region. J Biol Chem. 2000;275:11750–11757. doi: 10.1074/jbc.275.16.11750. [DOI] [PubMed] [Google Scholar]
- 16.Freshney N W, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Gras E A, Chi P, Thompson E A. Glucocorticoid-mediated destabilization of cyclin D3 mRNA involves RNA-protein interactions in the 3′-untranslated region of the mRNA. J Biol Chem. 2000;275:22001–22008. doi: 10.1074/jbc.m001048200. [DOI] [PubMed] [Google Scholar]
- 18.Garrington T P, Johnson G L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 19.Gessani S, McCandless S, Baglioni C. The glucocorticoid dexamethasone inhibits synthesis of interferon by decreasing the level of its mRNA. J Biol Chem. 1988;263:7454–7457. [PubMed] [Google Scholar]
- 20.Gonzalez M V, Gonzalez-Sancho J M, Caelles C, Munoz A, Jimenez B. Hormone-activated nuclear receptors inhibit the stimulation of the JNK and ERK signalling pathways in endothelial cells. FEBS Lett. 1999;459:272–276. doi: 10.1016/s0014-5793(99)01257-0. [DOI] [PubMed] [Google Scholar]
- 21.Gou Q, Liu C H, Ben-Av P, Hla T. Dissociation of basal turnover and cytokine-induced transcript stabilization of the human cyclooxygenase-2 mRNA by mutagenesis of the 3′-untranslated region. Biochem Biophys Res Commun. 1998;242:508–512. doi: 10.1006/bbrc.1997.7994. [DOI] [PubMed] [Google Scholar]
- 22.Guan Z, Buckman S Y, Miller B W, Springer L D, Morrison A R. Interleukin-1beta-induced cyclooxygenase-2 expression requires activation of both c-Jun NH2-terminal kinase and p38 MAPK signal pathways in rat renal mesangial cells. J Biol Chem. 1998;273:28670–28676. doi: 10.1074/jbc.273.44.28670. [DOI] [PubMed] [Google Scholar]
- 23.Han J, Lee J D, Jiang Y, Li Z, Feng L, Ulevitch R J. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 24.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoover R R, Floros J. SP-A 3′-UTR is involved in the glucocorticoid inhibition of human SP-A gene expression. Am J Physiol. 1999;276:L917–L924. doi: 10.1152/ajplung.1999.276.6.L917. [DOI] [PubMed] [Google Scholar]
- 26.Jonat C, Rahmsdorf H J, Park K K, Cato A C, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 27.Keyse S M. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 28.Konig H, Ponta H, Rahmsdorf H J, Herrlich P. Interference between pathway-specific transcription factors: glucocorticoids antagonize phorbol ester-induced AP-1 activity without altering AP-1 site occupation in vivo. EMBO J. 1992;11:2241–2246. doi: 10.1002/j.1460-2075.1992.tb05283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunz D, Walker G, Eberhardt W, Pfeilschifter J. Molecular mechanisms of dexamethasone inhibition of nitric oxide synthase expression in interleukin 1 beta-stimulated mesangial cells: evidence for the involvement of transcriptional and posttranscriptional regulation. Proc Natl Acad Sci USA. 1996;93:255–259. doi: 10.1073/pnas.93.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaPointe M C, Isenovic E. Interleukin-1beta regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p42/44 and p38 MAPK signaling pathways in cardiac myocytes. Hypertension. 1999;33:276–282. doi: 10.1161/01.hyp.33.1.276. [DOI] [PubMed] [Google Scholar]
- 31.Lasa M, Mahtani K R, Finch A, Brewer G, Saklatvala J, Clark A R. Regulation of cyclooxygenase 2 mRNA stability by the mitogen-activated protein kinase p38 signaling cascade. Mol Cell Biol. 2000;20:4265–4274. doi: 10.1128/mcb.20.12.4265-4274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S W, Tsou A P, Chan H, Thomas J, Petrie K, Eugui E M, Allison A C. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci USA. 1988;85:1204–1208. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuura H, Sakaue M, Subbaramaiah K, Kamitani H, Eling T E, Dannenberg A J, Tanabe T, Inoue H, Arata J, Jetten A M. Regulation of cyclooxygenase-2 by interferon gamma and transforming growth factor alpha in normal human epidermal keratinocytes and squamous carcinoma cells. Role of mitogen-activated protein kinases. J Biol Chem. 1999;274:29138–29148. doi: 10.1074/jbc.274.41.29138. [DOI] [PubMed] [Google Scholar]
- 34.McKay L I, Cidlowski J A. Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: mechanisms of mutual antagonism. Mol Endocrinol. 1998;12:45–56. doi: 10.1210/mend.12.1.0044. [DOI] [PubMed] [Google Scholar]
- 35.Miyazawa K, Mori A, Miyata H, Akahane M, Ajisawa Y, Okudaira H. Regulation of interleukin-1beta-induced interleukin-6 gene expression in human fibroblast-like synoviocytes by p38 mitogen-activated protein kinase. J Biol Chem. 1998;273:24832–24838. doi: 10.1074/jbc.273.38.24832. [DOI] [PubMed] [Google Scholar]
- 36.Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000;55:603–613. doi: 10.1136/thorax.55.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton R, Kuitert L M, Slater D M, Adcock I M, Barnes P J. Cytokine induction of cytosolic phospholipase A2 and cyclooxygenase-2 mRNA is suppressed by glucocorticoids in human epithelial cells. Life Sci. 1997;60:67–78. doi: 10.1016/s0024-3205(96)00590-5. [DOI] [PubMed] [Google Scholar]
- 38.Newton R, Seybold J, Kuitert L M, Bergmann M, Barnes P J. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post-transcriptional mechanisms involving loss of polyadenylated mRNA. J Biol Chem. 1998;273:32312–32321. doi: 10.1074/jbc.273.48.32312. [DOI] [PubMed] [Google Scholar]
- 39.Newton R, Seybold J, Liu S F, Barnes P J. Alternate COX-2 transcripts are differentially regulated: implications for post-transcriptional control. Biochem Biophys Res Commun. 1997;234:85–89. doi: 10.1006/bbrc.1997.6586. [DOI] [PubMed] [Google Scholar]
- 40.Peppel K, Baglioni C. Deadenylation and turnover of interferon-beta mRNA. J Biol Chem. 1991;266:6663–6666. [PubMed] [Google Scholar]
- 41.Peppel K, Vinci J M, Baglioni C. The AU-rich sequences in the 3′ untranslated region mediate the increased turnover of interferon mRNA induced by glucocorticoids. J Exp Med. 1991;173:349–355. doi: 10.1084/jem.173.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poon M, Liu B, Taubman M B. Identification of a novel dexamethasone-sensitive RNA-destabilizing region on rat monocyte chemoattractant protein 1 mRNA. Mol Cell Biol. 1999;19:6471–6478. doi: 10.1128/mcb.19.10.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.43. Pouliot M, Baillargeon J, Lee J C, Cleland L G, James M J. Inhibition of prostaglandin endoperoxide synthase-2 expression in stimulated human monocytes by inhibitors of p38 mitogen-activated protein kinase. J Immunol. 1997;158:4930–4937. [PubMed] [Google Scholar]
- 44.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ray A, Prefontaine K E. Physical association and functional antagonism between the p65 subunit of transcription factor NF-κ B and the glucocorticoid receptor. Proc Natl Acad Sci USA. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiser C O, Lanz T, Hofmann F, Hofer G, Rupprecht H D, Goppelt-Struebe M. Lysophosphatidic acid-mediated signal-transduction pathways involved in the induction of the early-response genes prostaglandin G/H synthase-2 and Egr-1: a critical role for the mitogen-activated protein kinase p38 and for Rho proteins. Biochem J. 1998;330:1107–1114. doi: 10.1042/bj3301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridley S H, Dean J L, Sarsfield S J, Brook M, Clark A R, Saklatvala J. A p38 MAP kinase inhibitor regulates stability of interleukin-1-induced cyclooxygenase-2 mRNA. FEBS Lett. 1998;439:75–80. doi: 10.1016/s0014-5793(98)01342-8. [DOI] [PubMed] [Google Scholar]
- 48.Ridley S H, Sarsfield S J, Lee J C, Bigg H F, Cawston T E, Taylor D J, DeWitt D L, Saklatvala J. Actions of IL-1 are selectively controlled by p38 mitogen-activated protein kinase: regulation of prostaglandin H synthase-2, metalloproteinases, and IL-6 at different levels. J Immunol. 1997;158:3165–3173. [PubMed] [Google Scholar]
- 49.Ristimaki A, Narko K, Hla T. Down-regulation of cytokine-induced cyclo-oxygenase-2 transcript isoforms by dexamethasone: evidence for post-transcriptional regulation. Biochem J. 1996;318:325–331. doi: 10.1042/bj3180325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 51.Saklatvala J, Rawlinson L, Waller R J, Sarsfield S, Lee J C, Morton L F, Barnes M J, Farndale R W. Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue. J Biol Chem. 1996;271:6586–6589. doi: 10.1074/jbc.271.12.6586. [DOI] [PubMed] [Google Scholar]
- 52.Scheinman R I, Cogswell P C, Lofquist A K, Baldwin A S., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 53.Scheinman R I, Gualberto A, Jewell C M, Cidlowski J A, Baldwin A S., Jr Characterization of mechanisms involved in transrepression of NF-κB by activated glucocorticoid receptors. Mol Cell Biol. 1995;15:943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shalom-Barak T, Quach J, Lotz M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem. 1998;273:27467–27473. doi: 10.1074/jbc.273.42.27467. [DOI] [PubMed] [Google Scholar]
- 55.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 56.Swantek J L, Cobb M H, Geppert T D. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–6282. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takekawa M, Maeda T, Saito H. Protein phosphatase 2Calpha inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J. 1998;17:4744–4752. doi: 10.1093/emboj/17.16.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tobler A, Meier R, Seitz M, Dewald B, Baggiolini M, Fey M F. Glucocorticoids downregulate gene expression of GM-CSF, NAP-1/IL-8, and IL-6, but not of M-CSF in human fibroblasts. Blood. 1992;79:45–51. [PubMed] [Google Scholar]
- 59.Walker G, Pfeilschifter J, Kunz D. Mechanisms of suppression of inducible nitric-oxide synthase (iNOS) expression in interferon (IFN)-gamma-stimulated RAW 264.7 cells by dexamethasone. Evidence for glucocorticoid-induced degradation of iNOS protein by calpain as a key step in post-transcriptional regulation. J Biol Chem. 1996;271:16679–16687. doi: 10.1074/jbc.272.26.16679. [DOI] [PubMed] [Google Scholar]
- 60.Wang S W, Pawlowski J, Wathen S T, Kinney S D, Lichenstein H S, Manthey C L. Cytokine mRNA decay is accelerated by an inhibitor of p38-mitogen-activated protein kinase. Inflamm Res. 1999;48:533–538. doi: 10.1007/s000110050499. [DOI] [PubMed] [Google Scholar]
- 61.Winzen R, Kracht M, Ritter B, Wilhelm A, Chen C Y, Shyu A B, Muller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu N, Loflin P, Chen C Y, Shyu A B. A broader role for AU-rich element-mediated mRNA turnover revealed by a new transcriptional pulse strategy. Nucleic Acids Res. 1998;26:558–565. doi: 10.1093/nar/26.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang-Yen H F, Chambard J C, Sun Y L, Smeal T, Schmidt T J, Drouin J, Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990;62:1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- 64.Yasuda J, Whitmarsh A J, Cavanagh J, Sharma M, Davis R J. The JIP group of mitogen-activated protein kinase scaffold proteins. Mol Cell Biol. 1999;19:7245–7254. doi: 10.1128/mcb.19.10.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang J P, Wong C K, Lam C W. Role of caspases in dexamethasone-induced apoptosis and activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase in human eosinophils. Clin Exp Immunol. 2000;122:20–27. doi: 10.1046/j.1365-2249.2000.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]