Abstract
In many urban areas, elevated soil lead (Pb) concentrations are indicators of community-level Pb exposure. Here, we examine the spatial distribution and speciation of legacy soil Pb contamination in East Chicago, Ind., an industrial center with a wide range of Pb sources including a former lead smelter. In situ X-ray fluorescence spectroscopy (n=358) revealed widespread soil Pb contamination above the Environmental Protection Agency regulatory limit for soils. This soil contamination was heterogenous across all neighborhoods, and mostly uncorrelated with distance from the former smelting site. Soil Pb levels increased with decreasing median household income in East Chicago’s nine neighborhoods (r=−0.73, p=0.03). Extended X-ray absorption fine structure spectroscopy (n=44) indicated that the soil Pb was primarily adsorbed to iron and manganese oxides or humic acids, and as Pb hydroxycarbonate regardless of contamination levels. Crystalline insoluble forms of Pb, like pyromorphite, were not detected in significant concentrations. Thus, the unique chemical forms of potential Pb sources to soil, such as paint, ore and slag are not persistent and instead are extensively repartitioned into acid-soluble forms of Pb with greater bioavailability. These findings have implications for remediation efforts and human health as blood Pb levels in this community are significantly elevated.
Keywords: Heavy metals, Soil contamination, Metal Speciation, EXAFS, Environmental Health
Graphical Abstract
1. Introduction
Lead (Pb) is a ubiquitous environmental toxicant. Exposure to Pb is associated with a range of adverse neurological, renal, and developmental effects with children at elevated risk[1–5]. It is estimated that 815 million children worldwide have high blood lead levels (BLL) greater than 5 μg/dL, the current Center for Disease Control and Prevention (CDC) reference value[6]. A revision to 3.5 μg/dL is currently under review[7]. Furthermore, the current Environmental Protection Agency (EPA) soil screening guideline level of 400 mg/kg Pb which was based on a 10 μg/dL reference BLL, will likely be revised to 150 mg/kg Pb to better correspond with levels linked to exceedances of the current 5 μg/dL reference BLL[8, 9]. Today, much of modern Pb exposure is thought to result from exposure to soil[10]. Soil is a critical source of Pb exposure through both ingestion (hand-to-mouth contact) and by inhalation of resuspended fine soil particles[11]. Atmospheric soil Pb has been well documented to show seasonal fluctuations that match seasonal variation in BLL[12–15]. These data suggest that environmental exposures to soil Pb remain a critical public health challenge.
Lead contamination of soil can result from numerous anthropogenic sources. These include Pb-based paint[16], gasoline with tetraethyl Pb[17], Pb arsenate pesticides[18], firing ranges[19], coal burning[20], waste incineration[15], mining[21], and smelting[22]. The chemical speciation, or the valence, structure and reactivity of Pb within these sources is widely variable and exhibits substantial differences in stability, solubility and toxicity. For example, Pb-based paint is comprised of different, relatively soluble mineral forms of Pb depending on the pigment. “White Lead”, the most widely used paint pigment, was composed of Pb(II) carbonate (PbCO3), while yellow paint was comprised of Pb(II) chromate (PbCrO4). Organic Pb (tetraethyl Pb) was used as an additive in gasoline to reduce engine knocking. Despite being banned for use in automobiles in 1996, tetraethyl Pb is still added to non-commercial aviation gasoline[23]. Pb ore produces highly insoluble minerals such as galena (PbS) and pyromorphite (Pb5(PO4)3(Cl, F, OH). Many of these sources may not be stable once they reach soils, and may convert to other forms[21].
Urban areas can contain all of these sources, and often may have several Pb sources within a given location. Indeed, many studies have measured extensive Pb contamination in cities[24], and linked soil contamination to Pb exposure in those cities[25, 26]. Those studies have revealed highly variable levels of contamination, even at the same lot[13, 27]. Lead concentration alone is not sufficient to understand the risks posed by soil Pb because the bioaccessibility and bioavailability of different forms of Pb present in soil can vary widely[28]. The quantitative determination of the concentrations of different forms of lead species (Pb speciation) is thus central to risk assessment. Despite its significance, far fewer studies have characterized Pb speciation in residential soils [29, 30]. Some studies have probed this question but are limited to small sample sizes[31, 32], or focused on mine tailings[33] or other environments that do not fully capture the complexity of many urban environments that contain multiple and/or poorly characterized sources.
East Chicago, Indiana, is an urban community impacted by multiple legacy Pb sources including the former U.S. Smelting and Lead Refinery, Inc. (USS Lead). In 2016, it was revealed that a public housing complex and elementary school were built on the former USS Lead site without removal of highly contaminated soil[34]. This led to significantly-elevated BLL among the residents, the majority of whom are Black and Hispanic[35]. While the residents have been displaced to other neighborhoods in East Chicago, the distribution of Pb in soil in these nearby neighborhoods also remains uninvestigated.
Although East Chicago is clearly impacted by Pb contamination, significant questions remain about how extensive the Pb contamination is, the sources of Pb contamination that are most widely distributed, and to examine the relationship between potential sources and the speciation of Pb in soils. We are guided by two contrasting hypotheses that are unexplored in the literature and which have significant public health ramifications. (1) Spatial heterogeneity in the distribution of persistent sources results in highly variable Pb concentration and speciation between sites. In this case, specific sources like the smelter site would have distinct Pb concentrations (high) and speciation (for example, rich in insoluble PbS) to nearby soil, while soils affected by Pb paint or atmospheric deposition would have different Pb concentrations and speciation, and thus represent different risks. (2) Alternatively, the concentrations of Pb will be determined by the sources, but the Pb speciation will change in response to variable soil conditions. Because soils locally are reasonably homogeneous, but sources are heterogeneously distributed, this option would lead to widely variable Pb concentrations, but similar speciation. As such, the risks posed would depend on the mineral forms of Pb commonly found in soils, rather than reflection of their sources.
To test these hypotheses, we determined the bulk concentration of Pb in East Chicago residential soils using X-ray fluorescence spectroscopy (XRF) to assess the spatial structure and distribution of Pb across all neighborhoods. We also conducted molecular scale investigations using extended X-ray absorption fine structure (EXAFS) spectroscopy to determine the current chemical speciation of Pb in soils. EXAFS spectroscopy provides an element-specific probe of the local coordination environment of Pb in complex media like soils, and the data can be used to provide a non-destructive means of quantifying Pb phases, including crystalline, amorphous and poorly crystalline phases, and adsorbed or dissolved forms provided there are reference spectra available [21, 36]. EXAFS-based speciation is also less susceptible to experimental artifacts resulting from abundant mineral transformations that occur during sequential extractions, another potential method of analyzing mineral speciation [37]. Unsupervised (standardless) analysis of EXAFS spectra is also possible based on spectral similarities between samples [38, 39]. These methods of analysis become much more powerful when performed on large spectral datasets that capture a wide range of environmental variance. To our knowledge, this is the largest sample size investigation of Pb speciation in residential soils, something critical to capture the widely variable Pb concentrations in urban soils and potential variation in soils and contamination sources. Results from these measurements along with information on property history were analyzed using unsupervised hierarchical clustering analyses to reveal patterns of chemical forms of Pb: mineral vs. adsorbed, and extensive repartitioning into acid-soluble and potentially bioavailable forms of Pb. We also relate Pb contaminant levels and speciation data to community race, educational attainment, and income to highlight issues of exposome disparity and environmental justice in this largely disenfranchised community.
2. Materials and methods
2.1. Study site and sample collection
The city of East Chicago is located 30 km east of Chicago across the Illinois-Indiana border. It is a heavily industrialized area with numerous legacy and active facilities. These include the former USS Lead (an EPA Superfund Remediation Site), Anaconda Lead Products, DuPont lead-arsenate pesticide facility, and ArcelorMittal, one of the largest active steel production facilities in North America. East Chicago contains a number of neighborhoods segmented by current and historic industrial sites (Fig. 1). The study site extended 4.5 km in the E–W direction and 5.8 km in the N–S direction. Randomized soil sampling locations were pre-identified using a random points generator (www.geomidpoint.com). However, since a significant portion of East Chicago is occupied by industrial areas, not all predetermined locations could be accessed or contained soil, and those points were replaced with random locations in proximity to predetermined locations. Soils were generally classified as sandy loams or loamy sands, though most soils are formally classified as urban rather than formally assigned soil series designations[40]. The top 3 cm of soils were collected, homogenized, air-dried and stored in new plastic bags for speciation analyses (n=44). Soil cores (30 cm, 5 cm sections) were collected at selected sites using a stainless-steel soil sampling probe (n=7). Sampling campaigns were carried out in August of 2017 and 2018.
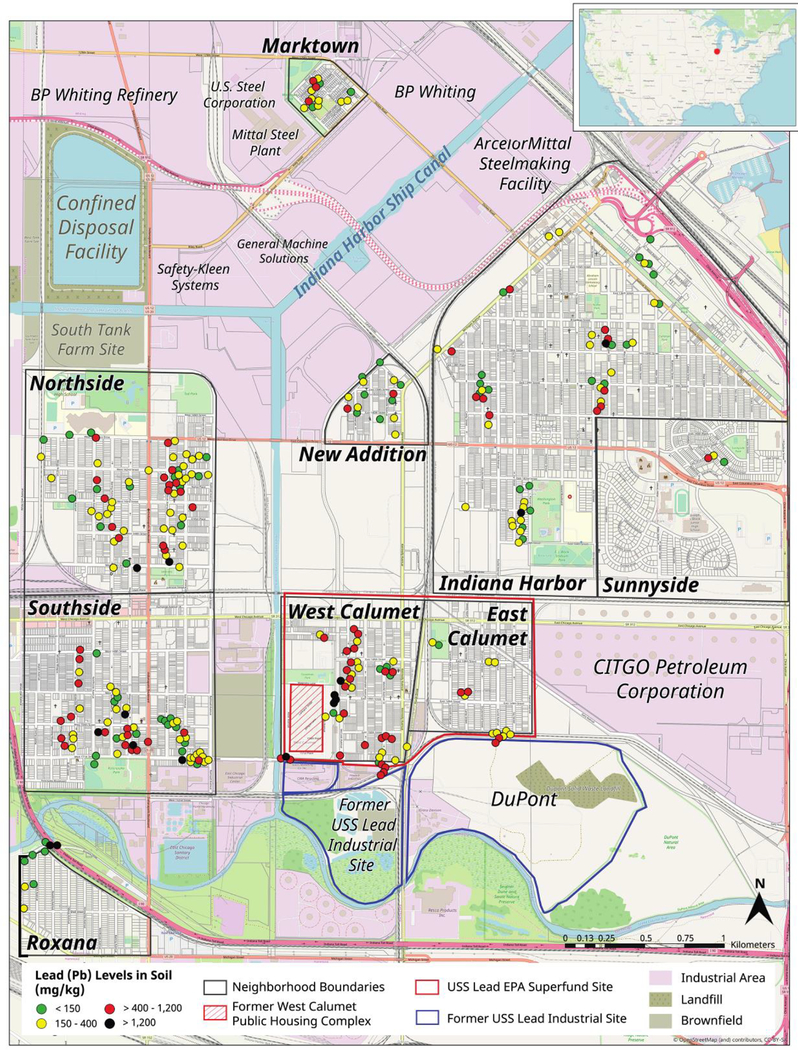
Fig. 1.
Spatial distribution of Pb concentrations in soils across East Chicago. Most neighborhoods are separated by rail, canal, major roads, and/or a range of industrial sites.
2.2. In situ soil XRF measurements
XRF is a commonly used, rapid, nondestructive technique for determining soil composition. Surficial soil samples were analyzed in situ by handheld XRF using an Olympus DELTA analyzer (Model- Innov-X DP-4000, Tokyo, Japan) and a Thermo Fisher Niton analyzer (Model- Niton XL3t Ultra, Tewskbury MA, USA) for the 2017 and 2018 sampling campaigns, respectively. XRF analysis provided solid phase concentrations of K, Ca, Ti, Cr, Mn, Fe, Cu, Zn, As, Rb, Sr, Zr, and Pb. Quality assurance and quality control (QA/QC) for XRF analysis was carried out using standard reference materials (SRM) purchased from the National Institute of Standards and Technology (NIST), EPA, and the Canadian Certified Reference Materials Project (CCRMP) yielding recoveries ranging from 72 to 117%. Detailed description of QA/QC results are presented in Table S1.
Statistical analysis of XRF concentrations were determined using R (R Project for Statistical Computing). Correlations between log10 transformed elemental concentrations were assessed using Pearson correlation coefficients. A log10 transformation produced approximately normal distributions of metal concentrations (Kolmogorov-Smirnov normality test, p ≥ 0.01).
2.3. EXAFS
Soil samples and individual Pb mineral reference standards were analyzed using extended X-ray absorption fine structure (EXAFS) spectroscopy at Stanford Synchrotron Radiation Lightsource (SSRL) on beamline 4–1. Each spectrum was collected using a Si (220) monochromator, a phi-angle of 90° and a detuned beam, with the sample chamber equipped with Soller slits and a Ge filter. Prior to measurement, a thin film of each soil sample was sealed in 25 μm thick Kapton tape. Pb L3-edge EXAFS was measured with a 100-element Ge detector as previously reported[31, 41, 42]. The spectrum of each sample was collected in fluorescence mode with a Pb calibration foil present in series to permit sample calibration on each scan. Pb L3-edge EXAFS spectra were calibrated by setting the maximum of the first derivative of a Pb foil to an energy of 13,035 eV.
Spectral averaging, background subtraction, normalization and analysis was done in SIXpack[43] (SAMware); typical chi files were saved with k-range from 1 to 8. Linear combination fitting (LCF) was performed using k3-weighted chi functions for the 44 samples. For internal consistency, all final fits used 6 reference compounds, and were restricted to positive values. SIXpack estimated errors using sensitivity analysis based on spectral noise, fit quality, and reference similarity. Normalized spectra of each soil sample, and the reference materials are included in the SI.
2.4. Statistical Analysis
Principal components analysis (PCA) was performed on the 44 EXAFS samples and six Pb standards (Fe oxide bound Pb, Mn oxide bound Pb, humic acid bound Pb, Pb hydroxycarbonate, galena, and pyromorphite) on the chi files (k3-weighted spectra, k range of 1–8 to reduce noise) with a total of 4 components accounting for 90% of the variance. The principal component loadings were exported from SIXpack for hierarchical cluster analysis performed in R to identify clustering based on the PCA loadings. Hierarchical clustering analysis was performed on principal component 1 to 4 (PC1 to PC4) based on Euclidean distance using Ward’s minimum variance method from which a dendrogram shows the splitting of different clusters. Performing hierarchical cluster analysis on the principal components of each soil sample is computationally advantageous over performing hierarchical cluster analysis on the complete spectra themselves as most of the data (~90%) can be captured in a very low number of variables and the result does not change with additional principal components after PC4. In addition, this approach was applied to the in situ XRF log10 transformed dataset. The principal component loadings from the first five components that accounted for ~90% of the variance was utilized for hierarchical clustering analysis (HCA).
Variograms were used as a geostatistical tool for assessing spatial correlations of Pb and other elements in soils via the “gstat” package in R. Variogram analysis using the robust location estimator by Cressie et al. was utilized in order to characterize the spatial correlation of Pb and several other elements measured in soil [44], [45, 46].
2.5. Demographic and property history data
Data on household income, educational attainment, and race were obtained from the U.S. Census Bureau 2018 American Community Survey (ACS) 1-Year Estimates. Household income data were obtained from Table B19001: Household Income in the Past 12 Months (in 2018 Inflation-Adjusted Dollars). Educational attainment data were obtained from Table B15003: Educational Attainment for the Population 25 Years and Over. Data regarding race were obtained from Table B02001: Race. Parcel data used for age of housing were obtained from the Real Property Assessment Data (DLGF: 20191204). USS Lead boundaries were obtained from the EPA ‘Zone 1 Amended Remedy Proposed Plan’ presentation from November 29, 2018 and February 13, 2019 (https://semspub.epa.gov/work/05/946286.pdf). Neighborhood boundaries were obtained from Northwest Indiana Regional Planning Commission[47]. The base map utilizes OpenStreetMap (https://www.openstreetmap.org). Further details are provided in the SI.
3. Results and discussion
3.1. Soil Pb concentrations
In situ XRF measurements of 358 randomly sampled soils revealed a highly heterogenous distribution of Pb concentrations across East Chicago ranging from 24 to 14,428 mg/kg (Fig. 1). The arithmetic and geometric mean Pb concentrations were 685 and 285 mg/kg, respectively. Descriptive statistics for soil Pb and other element concentrations are presented in supplementary materials (Table S2). Pb concentrations were significantly correlated with concentrations of Zn, Cu, Fe, Mn, and Cr (p<0.05, Fig. S1). Although Pb appeared to be correlated with As, this is likely spurious due to interferences from the Lα emission for Pb and the Kα emission for As[48]. Field portable XRF instruments are able to correct for this at lower Pb to As ratios, however at higher ratios, like those in this study, the deconvolution procedure is insufficient and thus leads to an overestimation of As concentrations[49]. Thus, results from our Pb and As correlation analysis are excluded.
Nearly all soils had Pb concentrations above 50 mg/kg, typical of natural background in East Chicago[50]. Concentrations of Pb were found to be above the proposed intended EPA guidance level of 150 mg/kg for 77% of all soils tested. In addition, 37% of all soils tested had Pb concentrations above the current EPA guidance level for residential soils of 400 mg/kg, and 7% tested above the 1200 mg/kg limit for industrial sites (Fig. S2). Based on these data, East Chicago appears to contain more extensive soil Pb contamination than other well-characterized urban areas of the United States. For instance, only 8% of soil samples in Los Angeles[51] (LA, n=550) and 22% of garden soils in New York City[52] (NYC, n=564) exceeded the current EPA residential soil standard. Similar to our findings, these studies in LA and NYC also found a strong correlation between Pb and Zn[52]. Investigations of soil Pb levels in other areas of Indiana revealed lower levels compared to East Chicago with an average soil Pb of 259 mg/kg (n=266) in Indianapolis[27]. Average soil Pb levels of 777 mg/kg in Philadelphia were found to be similar to those of our study although maximum values were lower than East Chicago at around 2800 mg/kg[53]. An extensive systematic review and meta-analysis by Frank et al. of Pb concentrations in soil in the US from 1996 and 2016 revealed that the mean soil Pb levels in residential yards (n=6055) near Superfund sites was 358 mg/kg[54].
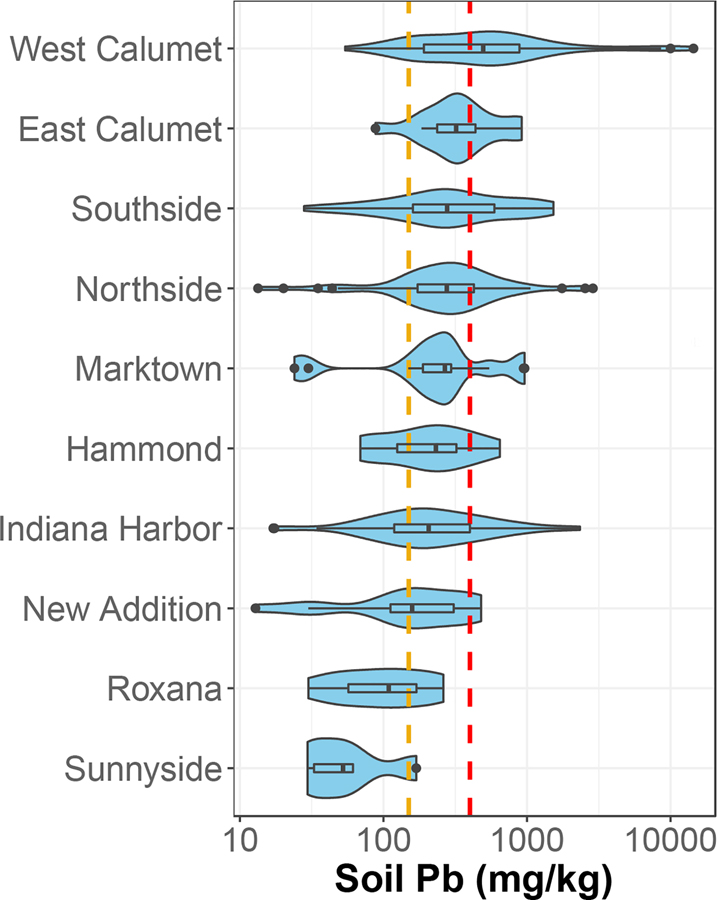
Although soil contamination was widespread, soil Pb concentrations varied considerably across neighborhoods (Fig. 2). For instance, the West Calumet neighborhood, which comprises the site of the former USS Lead smelter, had the widest range in concentrations, with 90% of samples containing soil Pb concentrations classified as elevated as assessed using the intended guidance level of 150 mg/kg. East Calumet, directly adjacent to West Calumet and also part of the Superfund remediation site, had a similar level of elevated soil Pb at 93%. The Southside and Northside neighborhoods, separated from the Calumet neighborhoods by the Grand Calumet River, had elevated soil Pb percentages of 77% and 88%, respectively despite not being a part of the Superfund site and with no recognized legacy Pb sources. The neighborhoods of Indiana Harbor, New Addition, and Marktown, which are in close proximity to active industrial sites, had elevated soil Pb levels ranging from 50% to 80%, while lowest elevated levels were observed for Roxana and Sunnyside at 30% and 20%, respectively.
Fig. 2.
Soil Pb concentrations across neighborhoods in East Chicago. Yellow line marks the 150 mg/kg intended EPA guidance level. The red line represents the 400 mg/kg current EPA guidance level for Pb in residential soils. Neighborhoods are ranked by median Pb concentration. Hammond is an adjoining city south and west of East Chicago and is included here for comparison.
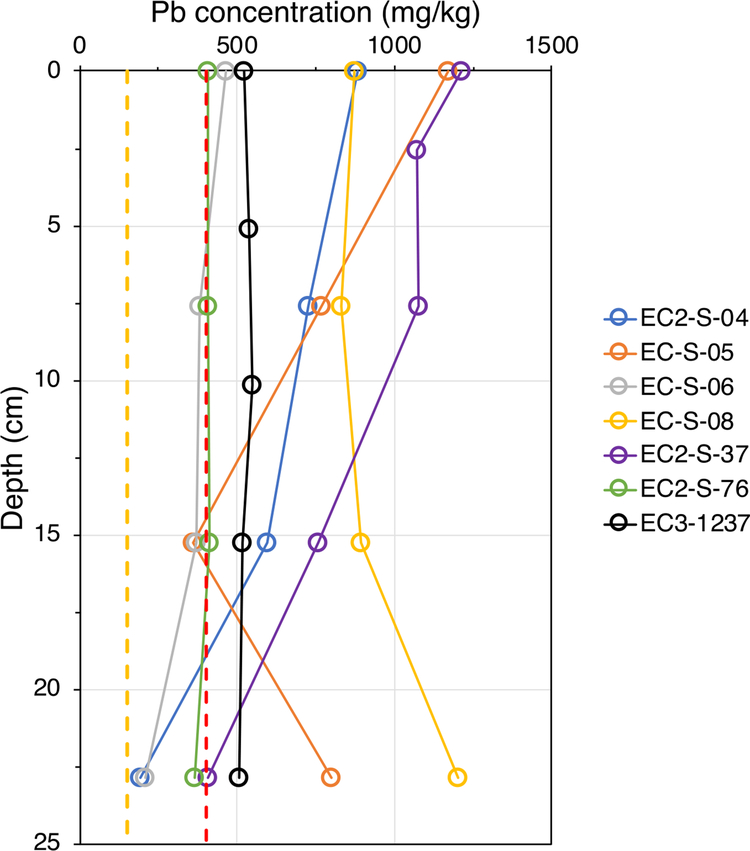
Soil cores are useful to understand if and how soil Pb has been redistributed through the soil profile by mixing, transport, or remediation. These randomly sampled cores exhibited widely different depth profiles (Fig. 3). Surface level concentrations of Pb in these core samples ranged from 400 to 1300 mg/kg. Cores EC2-S-04, and EC2-S-37 were observed to have Pb concentrations drop precipitously, typical of undisturbed, stable surficial soils that accumulate Pb contamination, with little mixing or physical transport (downward, or erosional from the top). On the other hand, cores EC-S-08, and EC-S-05 exhibited Pb concentrations that decreased from the surface to 15 cm but then increased again at depth. This may be due to mixing of contaminated soils closer to the surface from introduction of exogenous soils for gardening or yard renovation work. Cores EC-S-06, EC2-S-76, and EC3-S-1237 had relatively low surficial Pb concentrations with very little change in concentration with depth. Based on our collection of parcel data of housing it was observed that cores with higher surface Pb levels (>600 mg/kg) were from areas built between 1907 and 1926 while the lower Pb level cores were from areas built after the ban on Pb paint (1978) but both before and after the ban on leaded gasoline (1996), with the exception of core EC3–1237 built in 1927. However, the steady levels of Pb for this core with respect to depth suggests possible recent soil replacement or mixing.
Fig. 3.
Depth profile of Pb concentrations in residential soils. A wide range of Pb concentrations as well as concentration trends were observed. In cores with high surficial Pb concentrations, Pb levels decreased rapidly, while in others they remained the same, and actually increased at the deepest level. Cores with relatively low surficial Pb were observed to be constant with depth. These lower Pb cores were typically from newer properties.
3.2. Spatial distribution of Pb
The spatial distribution of soil Pb in East Chicago was highly heterogenous. Soil Pb concentrations were variable between and within neighborhoods based on diversity of the sources of Pb contamination (Fig. 1). For instance, we hypothesized West Calumet and East Calumet neighborhoods that encircle the USS Lead site would have higher levels of contamination, while soil Pb contamination within the other neighborhoods would primarily be affected by legacy sources such as Pb paint, and atmospheric deposition of leaded gasoline and smelter emissions. This hypothesis was tested by evaluating the spatial organization of Pb contamination levels using variograms of XRF-measured soil concentrations[44].
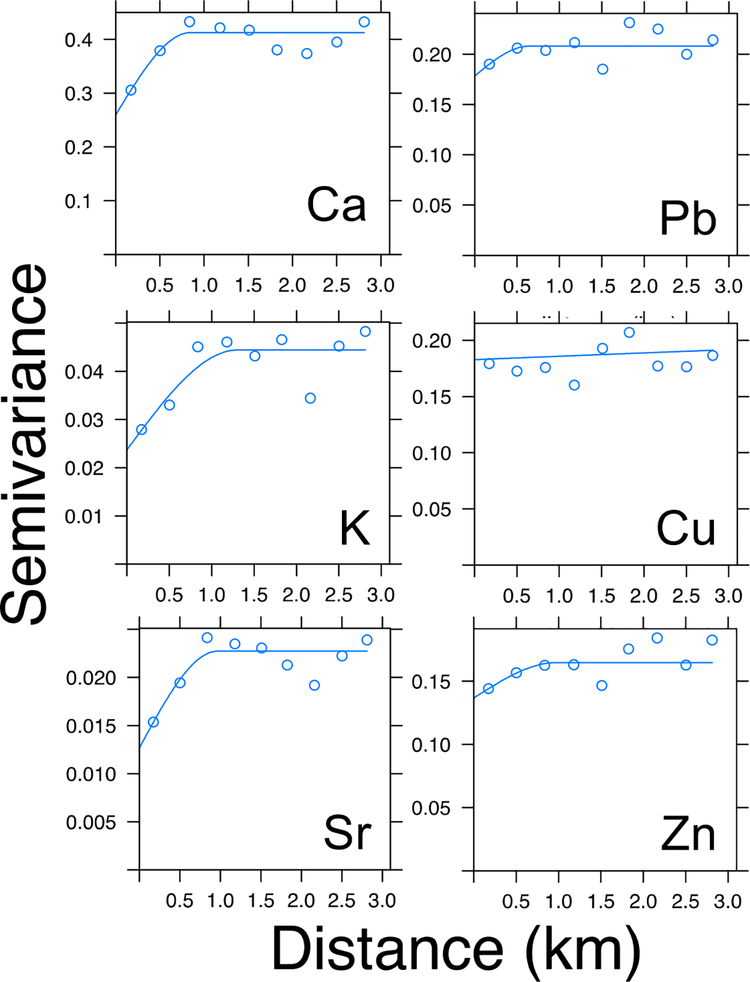
The variograms of Pb and other elements revealed distinct spatial structures for contaminants and natural constituents (Fig. 4). The variograms of Ca, K, and Sr were effectively described with spherical models, which assume that there is a length scale below which samples are close enough to have correlated concentrations, and that variance increases with radial distance. This model is indicative of the element being geogenic[55]. These elements are abundant in soil and have little or no history of industrial use in East Chicago. For elements likely to be derived from contamination, Pb, Cu and Zn for example, variance is independent of distance between samples. Thus, a pair of samples from the same yard can be just as different as a pair of samples from different neighborhoods. The lack of coherent, spatial structure is indicative of multiple, highly heterogenous anthropogenic sources, rather than a single source. This finding suggests that although remediation efforts may target a specific area, there may be other areas with similar levels of contamination in more distant neighborhoods that are not remediated. It also is indicative of the incomplete remediation of soils within the targeted area, which then contains a patchwork of remediated and unremediated soils in close proximity.
Fig. 4.
Variograms showing the variations in geostatistical correlations of metals in residential soils as a function of distance from each other. The data falling within a spherical model, as with Ca, K and Sr, are indicative of the elements being geogenic with the theoretical assumption that samples closer to each other are less dissimilar to samples further apart. Whereas a lack of fit, as observed for Cu, Zn, and Pb, is indicative of the elements being present due to anthropogenic sources and activities.
3.3. Pb speciation
There are varying sources of Pb species contributing to soil contamination in East Chicago. These include leaded paint, atmospheric deposition of tetraethyl Pb from gasoline, ore minerals for smelting, and slag. These sources of Pb have unique mineralogical signatures. Mineral forms such as galena, anglesite, pyromorphite, cerussite and hydrocerussite are common in ores, mine tailings[21, 28, 56], or in paints and other Pb products[31, 57, 58]. These mineral forms contrast with Pb2+ adsorbed to Fe(III) oxides[59, 60], Mn oxides[61], or soil organic matter (SOM) such as humic substances[32] that are often abundant in soils. Each of these have distinct Pb-O (or Pb-S) first-shell interatomic distances and second shells. Thus, Pb EXAFS is very effective and sensitive for differentiating between mineral and various adsorbed phases[33]. EXAFS also is able to account for variation in Pb hydrolysis and polymerization that can affect adsorption complex formation[62].
Although potential sources could have widely variable and easily identifiable EXAFS spectra, the spectra were remarkably similar for all samples, even for samples from widely different sources and different levels of contamination (Fig. S5). For example, a high-Pb soil near the smelter in Zone 1 vs uncontaminated soil elsewhere. Linear combination fitting of EXAFS confirmed the similarity in spectra, with fits only requiring a small collection of stable and metastable Pb species commonly found in uncontaminated soils. On average after all linear combination fitting results were normalized to a component sum of 1, 70%, of soil Pb was found in adsorbed forms, while mineral forms of Pb accounted for the remaining 30% (Table 1, Table S3 and Fig. S3). Very little primary crystalline Pb (for example, as PbS) was found in any soils, even those containing very high levels of Pb and adjacent to the USS Lead site, which processed PbS-rich ore. Fe oxide-bound Pb was the dominant form of adsorbed Pb, accounting for nearly 50% of total Pb in most samples. Humate-bound and Mn oxide-bound Pb were more variable and averaged 10% each. Although Pb adsorption to Mn oxides is energetically more stable[63], we attributed the lesser quantities of Pb adsorbed to Mn oxides to low Mn concentration in these soils, and the susceptibility of Mn oxides to reduction under anaerobic conditions[64]. Pb hydroxycarbonate was the only crystalline Pb phase found in most soils and represented about 27% of total Pb. We attribute its widespread occurrence, and that of adsorbed Pb, to the slow, partial dissolution of contaminant forms of Pb, followed by the adsorption or precipitation of dissolved Pb to common soil minerals, or circumneutral precipitation with carbonate in soil pore water[59, 65, 66]. In principle, other mineral forms such as pyromorphite also could form through similar processes, however they were usually detected at low levels (<2%). The limited formation of these insoluble forms is desirable for remediation, but is often hampered by a combination of low Pb and PO43− concentrations at neutral pH in soil solutions[67]. As a result, most of the soil Pb is bound in more bioavailable forms including adsorbed phases and hydrocerussite.
Table 1.
Descriptive statistics of linear combination fits of all 44 samples investigated using EXAFS. Results are expressed as fractional composition normalized to a component sum of 1.
| Phase | Pb species | Mean | ± 1 SD | Min | Max |
|---|---|---|---|---|---|
| Adsorbed | Fe oxide bound Pb | 0.45 | 0.14 | 0.15 | 0.83 |
| Mn oxide bound Pb | 0.10 | 0.08 | 0.00 | 0.32 | |
| Humate bound Pb | 0.11 | 0.07 | 0.00 | 0.31 | |
| Mineral | Pb hydroxycarbonate | 0.27 | 0.15 | 0.00 | 0.58 |
| Pyromorphite | 0.04 | 0.07 | 0.00 | 0.33 | |
| PbS | 0.04 | 0.05 | 0.00 | 0.20 |
Our speciation data allows us to test our proposed hypotheses. Overall, the consistency between speciation of soils containing widely different levels of Pb contamination suggest that source mineralogy is not preserved in soils, even in very contaminated sites, and that secondary mineral forms of Pb predominate. The alternative, that the speciation is similar because all of the samples are from a similar source, is highly unlikely even though there is a well-defined source (USS Lead Smelter) at the site, in part because ore and wastes from smelting would have very different mineralogies than we observed[21]. In the following sections, we examine the relationships between soil composition and speciation, both to better understand which sources of Pb are important, based on which other elements are present, and to better understand how soil conditions and composition can affect weathering and thus Pb speciation.
3.4. Integrating composition and Pb mineralogy using unsupervised hierarchical clustering analysis
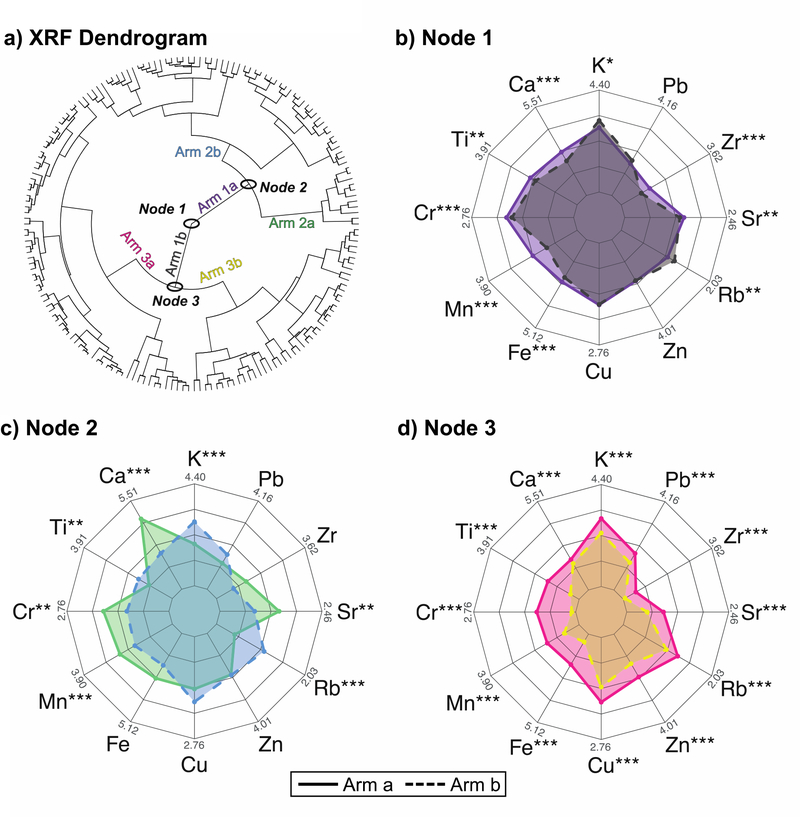
Most Pb sources contain more than Pb, and thus affect soil concentrations of several elements. XRF data measures many elements, and thus can provide a useful means of identifying signatures of specific sources. Unsupervised hierarchical clustering analysis (HCA) of principal component loadings of all XRF sample measurements was used to classify soil samples into groups based on their similar composition, and, to some extent, permit source attribution. HCA is a robust technique that determines the similarity between samples based on overall composition and without assumptions about spatial or other relationships between samples. The dendrogram produced by HCA contains three major nodes, each of which grouped samples into clusters, referred to as arms (Fig. 5a and Fig. S4). The elemental concentrations within the clusters determined by HCA were then investigated using pairwise t-tests in order to assess differences in composition between arms of the unsupervised clusters, and how they may reflect sources. The first node (Node 1) divided the soils into two arms that exhibited statistically significant differences in concentrations for K, Ca, Ti, Cr, Mn, Fe, Rb, Sr, and Zr. None of these elements are contaminants; rather they are natural and reflect differences in soil type. Although Fe, Cr, Mn and Ti could be associated with emissions from steel mills, the levels observed were not as high as would be expected[68]. Under Node 2, the arms showed significant differences in concentrations of K, Ca, Ti, Cr, Mn, Rb, and Sr. These changes are also associated with natural soil composition, and for Ca and Sr, large differences indicate differences in carbonate content of the soil[69]. Interestingly, Node 3 divided soils into arms with significant and consistent differences in concentrations for all elements as shown in Fig. 5d. In Node 3, one arm contained more of most minor and trace elements, while the other arm had much lower levels of those constituents. This indicates that Node 3 differentiates soil largely based on the dilution of most metals, likely by quartz sand given the sandy nature of the soils. This is expected given that remediation often replaces yard soil with sands from nearby, uncontaminated sandy soils.
Fig. 5.
Spider charts highlighting differences in elemental concentrations measured using XRF for the three major nodes determined using unsupervised hierarchical clustering analysis (a). Despite wide ranges of concentrations, statistically significantly different clusters were not observed for Cu, Zn, and Pb for Nodes 1 (b) and 2 (c). Significant changes were observed for all elements in Node 3 (d).
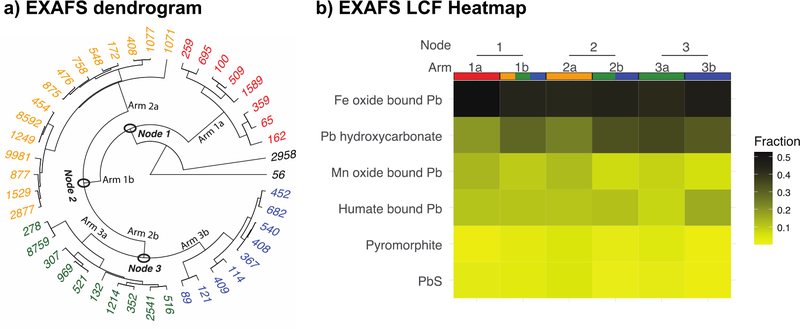
The clusters defined based on XRF data are in most cases dominated by natural soil variability rather than contaminant variation. The Pb speciation also appears to be controlled by the redistribution of Pb into secondary phases. To link soil Pb speciation to underlying soil compositional differences, we compared speciation data using pairwise t-tests between arms at each node identified using HCA of the EXAFS data (Fig. 6a and Fig. S5). Statistically significant differences were observed between arms in relative pyromorphite concentrations at Node 1 (p<0.001), galena concentrations for Node 2 (p<0.01) and Node 3 (p<0.05), and humate-bound Pb in Node 3 (p<0.001). These differences complement the findings from the XRF HCA suggesting that factors such as soil type (which affects organic matter content for example) play a significant role in controlling Pb speciation.
Fig. 6.
a) Dendrogram based on hierarchical clustering of EXAFS measurements of soils. The nodes represent the bifurcations from larger groups (Node 1) to smaller groups (Node 2 & 3). Labels represent Pb concentrations in mg/kg. Samples highlighted in black with Pb concentrations of 2958 and 56 mg/kg were not included in the nodes clustering analysis as they were significantly different from the rest of the samples. These two samples were from underneath a highway with peeling Pb paint and from a yard of a recently built property. 6b) Heatmap showing mean values of Pb species determined using linear combination fitting for each arm within the 3 nodes. The majority of the Pb (~50%) is bound to Fe oxide minerals followed by Pb hydroxycarbonate, Mn oxide bound Pb, and humate bound Pb. Trace amounts of pyromorphite and PbS were also detected. Statistically significant differences were observed within Node 1 for pyromorphite (p<0.001), within Node 2 for PbS (p<0.01), and Pb hydroxycarbonate (p<0.05), and within Node 3 for PbS (p<0.05), and humate bound Pb (p<0.001).
Our results indicate that forms of Pb in the initial sources, such as leaded gasoline, paint, ore materials are essentially absent and have been replaced by more stable, secondary forms in soil. The differences in mineralogy that are identified in HCA of XRF (Fig. 5) and EXAFS (Fig. 6) data reflect differences in secondary mineralization processes. For example, cerussite, PbCO3 from paint would dissolve slowly when added to soil, and then repartition its Pb2+ which can then be readsorbed on Fe oxides and other phases. This would decrease the equilibrium Pb2+ concentration by more than 10-fold; from 0.6 μM at pH 7 and typical soil pCO2, to ~0.06 μM depending on soil composition [70].
Pyromorphite is a highly stable, insoluble form of Pb, that, if formed, would further decrease aqueous Pb2+ concentrations. However, it is ineffectively produced in systems where Pb2+ and PO43− concentrations are low or limited by adsorption to Fe or other phases[63]. A common soil remediation technique involves the addition of phosphoric acid in order to assist in the formation of pyromorphite, by both providing a source of PO43− and also decreasing Pb2+ and PO43− adsorption[71]. The differences in pyromorphite between arms at Node 1 suggest that specific soil properties (for example, lower soil pH) may produce very limited pyromorphite formation under some conditions. Unfortunately, we lack sufficient soil characterization data to evaluate what soil properties are different between nodes. Detection of trace amounts of pyromorphite in Node 1 were found in older properties, as determined from parcel data on housing, suggesting that many of the forms detected in this study may over time be partially converted to pyromorphite.
3.5. Environmental justice
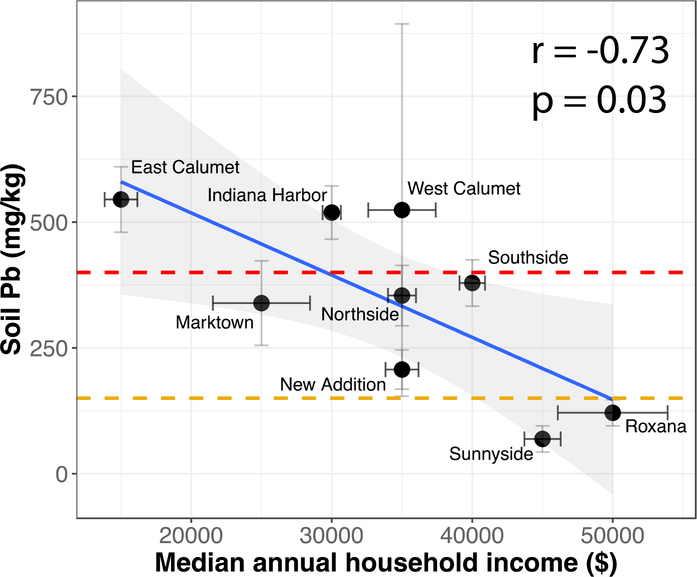
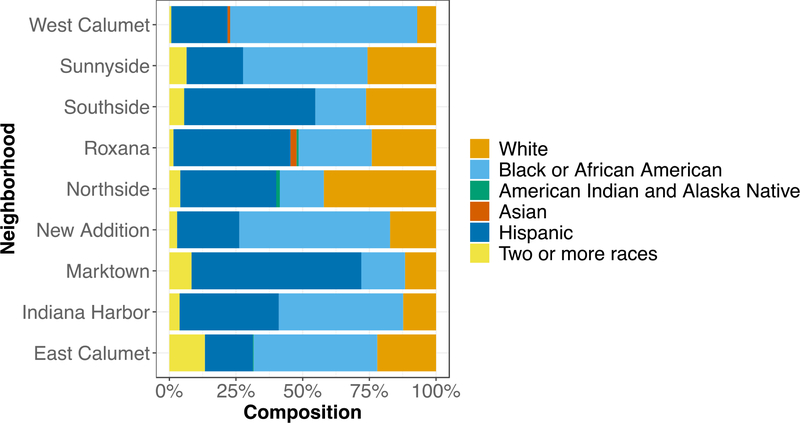
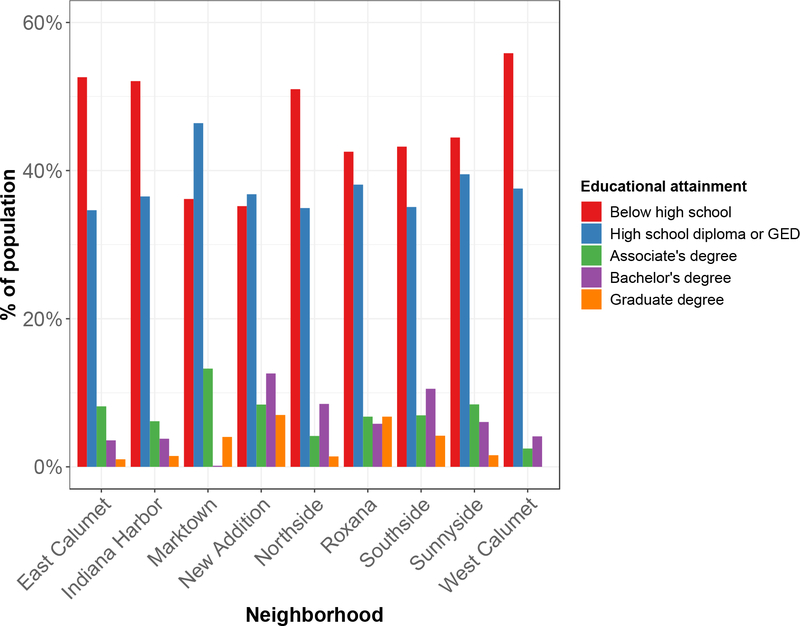
Communities of color are disproportionately affected by hazardous environmental exposures which bring to light underlying challenges with structural and institutionalized racism[72, 73]. These inequalities can then lead to multigenerational disparities in municipal zoning decisions (redlining), land use, and access to essential public health services[74]. In order to assess racial disparities in East Chicago, census tract data reflecting race, educational attainment, and annual household income stratified by neighborhood were compiled from the 2018 American Community Survey (ACS) of the U.S. Census Bureau[75]. Economic disparity was associated with Pb levels. Increasing median annual household income was significantly associated with decreasing soil Pb concentrations (Pearson’s r = −0.73, p=0.03), Fig. 7 and Fig. 8. Since race and educational attainment were more uniformly distributed across the entire city of East Chicago, neighborhood-scale correlations with soil Pb were not observed. Across all neighborhoods, Blacks and Hispanics make up a majority of the population (Fig. 9). Educational attainment was found to be low in East Chicago with 35 to 56% of the population’s educational attainment falling below high school level and only 0 to 13% holding a bachelor’s degree across all neighborhoods (Fig. 10). These findings are important to consider as studies have shown the combined effects of lead exposure and family income on childhood brain development outcomes at exposure levels similar to those observed in our study[76].
Fig. 7.
Association between median annual household income and average soil Pb concentrations of all nine neighborhoods. Error bars represent ± 1 standard error. The yellow dashes line marks the intended EPA Pb guidance value of 150 mg/kg and the current guidance value of 400 mg/kg is displayed in red. The Pearson correlation coefficient and associated p value are shown.
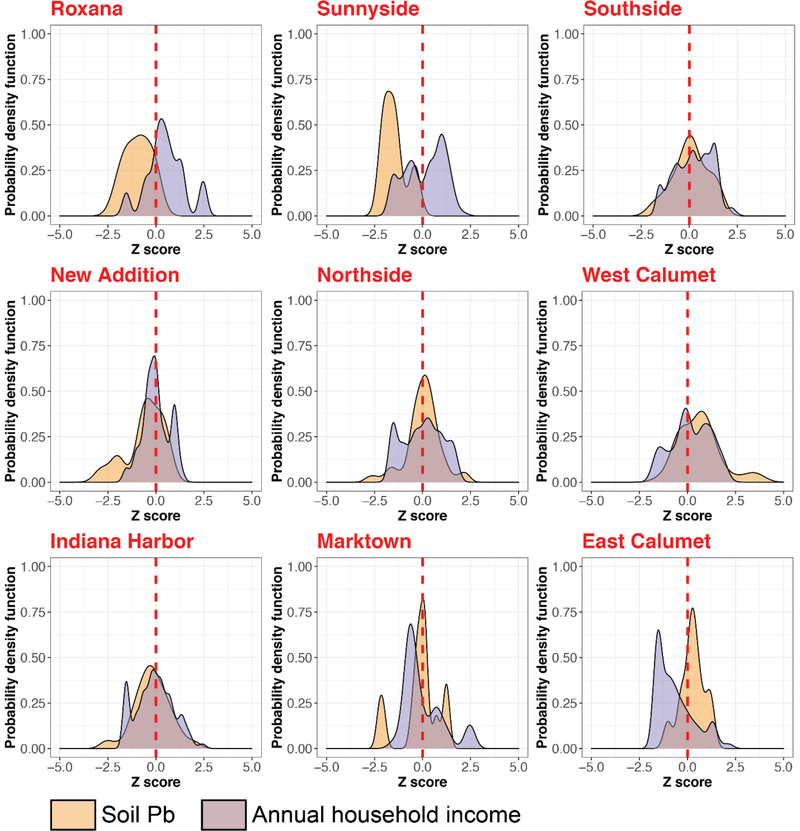
Fig. 8.
Distribution of annual household income and soil Pb for all neighborhoods in East Chicago. In order to plot both soil Pb and annual income data on a single axis, a z transformation was carried out essentially making the data unitless by allowing them to be described by their mean and standard deviation. A log10 transformation was carried out for the soil Pb data prior to z transformation to produce normality. Z score of 0 which represents the mean value is highlighted in red for all neighborhoods. The neighborhoods are ranked from left to right and top to bottom based on decreasing median annual household income. A shift is observed between the distributions of soil Pb and annual household income. This analysis also provides a more detailed visualization of the trends in income and soil Pb levels in East Chicago.
Fig. 9.
Summary of demographic data from ACS Census Bureau 2018 broken down by neighborhoods in East Chicago. Blacks and Hispanics make up a majority of the population.
Fig. 10.
Summary of educational attainment data from ACS Census Bureau 2018 broken down by neighborhoods in East Chicago. Educational attainment was found to be low in East Chicago with 35 to 56% of the population’s educational attainment falling below high school level and only 0 to 13% holding a bachelor’s degree across all neighborhoods
3.6. Health Implications
The toxicity of Pb in soil depends on whether it is ingested or inhaled[77]. Soils are generally comprised of a wide range of particles sizes that can affect their transport and inhalability. In East Chicago, the natural soil type is sandy loam which consists of sand and a mixture of silt and clay. This mixture contributes to fine particles, mostly PM2.5. We simulated disturbances in soils similar to children playing in dirt using an acoustical dry aerosol generator/elutriator system (ADAGE) to simulate particle size distributions of dusts generated from these soils as described in the SI[78]. This analysis showed that a significant fraction of soil mass (~0.5% by mass sieved <32μm) was mobilized, and that a majority, 95%, of the suspended particles were PM2.5 (Fig. S6). This might be surprising given the sandy soil texture (prevalent grain sizes of 50 μm to 2 mm), but it indicates that a significant exposure can result from dusts generated from these soils. We observed that Pb partitioned most strongly into the fine particle size fractions that are mobilized and these dusts have higher levels of Pb contamination than the bulk soil determined by a trial using a sample from Zone 1 which showed that a bulk sample with concentration of 16,970 mg/kg Pb had a dust concentration of 32,300 mg/kg Pb.
Given high soil Pb concentrations, the prevalence of more bioavailable forms of Pb[79], and the efficient generation of fine particulate matter from these soils, it is not surprising that previous work by our group has shown elevated BLL in this community[35]. Indeed, this study was undertaken to assess and respond to high documented Pb exposures in East Chicago[34]. This predominantly minority community has been grappling with environmental inequity related to elevated Pb exposures. In 2016, 1100 residents were displaced from West Calumet Housing Complex which was built atop the former USS Lead site without any soil clean-up[80]. However, these residents were displaced to other areas of East Chicago for which there was no data on soil Pb exposure. The extensive Pb contamination that is documented in our work, suggests that this strategy for Pb exposure reduction may have been ineffective.
The presence of Pb in fine particulate matter suggests that in addition to ingestion, inhalation may be an important source of Pb. Although Pb bioavailability in the stomach has been heavily studied[81, 82] there is a knowledge gap in the bioavailability of Pb in the lung, which is further complicated by the presence of diverse biochemical environments within the airway surface fluid, pulmonary epithelial cells, and alveolar macrophages. We have inhalation toxicology studies underway of East Chicago soil that seek to determine the bioavailability of various Pb species from this inhaled PM.
Our EXAFS investigation revealed that most of the diverse forms of soil Pb contamination which would be present in East Chicago has converted to metastable forms in soil, including Fe oxide-bound Pb, Mn oxide-bound Pb, organic complexes of Pb and some Pb-hydroxycarbonate, but very little primary galena and insoluble pyromorphite. The relative fraction of each species has no correlation to total Pb concentrations, meaning yards with alarmingly high Pb concentrations have a similar composition to yards with lower levels of Pb, and have bioavailabilities that are likely to be more similar as well. These findings have significant environmental and toxicological implications. Speciation provides insights into optimizing efficiency of remediation techniques while also pointing out that further work is needed to better understand the toxicity of the fine suspended soil particles in order to improve the health of the residents of East Chicago and other areas similarly impacted by Pb contamination.
Supplementary Material
Highlights.
To date, this is the largest investigation of Pb speciation in residential soils
Bulk soil Pb concentrations were spatially heterogeneous
Pb was predominantly adsorbed to Fe oxides, Mn oxides, and as Pb hydroxycarbonate
Pb speciation was not correlated to bulk concentration or location
Soil Pb was inversely correlated to household income in East Chicago neighborhoods
Acknowledgements
This work was supported by the University of Iowa’s Environmental Health Sciences Research Center, grant NIH P30ES005605 and Columbia University’s Superfund Research Program grant NIH P42ES010349. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. Dr. Thorne acknowledges support for the AESOP Study from NIH P42ES013661 for related work on polychlorinated biphenyl exposures in East Chicago. The authors thank the members of the East Chicago Community Advisory Group (CAG), Debbie Chizewer from Northwestern University’s Environmental Advocacy Clinic, Denise Abdul-Rahman from NAACP, and Sarah Rolfes from US EPA Region 5 for their collaborative assistance in working within the East Chicago community. The authors also thank Dr. Andrea Adamcakova-Dodd for laboratory assistance.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Goyer RA, Results of lead research: prenatal exposure and neurological consequences, Environmental Health Perspectives, 104 (1996) 1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Loghman-Adham M, Renal effects of environmental and occupational lead exposure, Environmental health perspectives, 105 (1997) 928–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dietrich KN, Berger OG, Succop PA, Hammond PB, Bornschein RL, The developmental consequences of low to moderate prenatal and postnatal lead exposure: intellectual attainment in the Cincinnati Lead Study Cohort following school entry, Neurotoxicology and teratology, 15 (1993) 37–44. [DOI] [PubMed] [Google Scholar]
- [4].Canfield RL, Henderson CR Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP, Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter, New England journal of medicine, 348 (2003) 1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Egan KB, Cornwell CR, Courtney JG, Ettinger AS, Blood Lead Levels in US Children Ages 1–11 Years, 1976–2016, Environmental health perspectives, 129 (2021) 037003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Burki T, Report says 815 million children have high blood lead levels, The Lancet, 396 (2020) 370. [DOI] [PubMed] [Google Scholar]
- [7].Paulson JA, Brown MJ, The CDC blood lead reference value for children: Time for a change, Environmental Health, 18 (2019) 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Widener A, EPA lowers lead dust exposure levels, Chemical & Engineering News, 96 (2018) 15–15. [Google Scholar]
- [9].Tsuji JS, Serl KM, Current uses of the EPA lead model to assess health risk and action levels for soil, Environmental Geochemistry and Health, 18 (1996) 25–33. [DOI] [PubMed] [Google Scholar]
- [10].Mielke HW, Reagan PL, Soil is an important pathway of human lead exposure, Environmental Health Perspectives, 106 (1998) 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Laidlaw MA, Filippelli GM, Resuspension of urban soils as a persistent source of lead poisoning in children: a review and new directions, Applied Geochemistry, 23 (2008) 2021–2039. [Google Scholar]
- [12].Zahran S, Laidlaw MA, McElmurry SP, Filippelli GM, Taylor M, Linking source and effect: Resuspended soil lead, air lead, and children’s blood lead levels in Detroit, Michigan, Environmental science & technology, 47 (2013) 2839–2845. [DOI] [PubMed] [Google Scholar]
- [13].Laidlaw MA, Mielke HW, Filippelli GM, Johnson DL, Gonzales CR, Seasonality and children’s blood lead levels: developing a predictive model using climatic variables and blood lead data from Indianapolis, Indiana, Syracuse, New York, and New Orleans, Louisiana (USA), Environmental Health Perspectives, 113 (2005) 793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Laidlaw MA, Zahran S, Mielke HW, Taylor MP, Filippelli GM, Re-suspension of lead contaminated urban soil as a dominant source of atmospheric lead in Birmingham, Chicago, Detroit and Pittsburgh, USA, Atmospheric Environment, 49 (2012) 302–310. [Google Scholar]
- [15].Chillrud SN, Bopp RF, Simpson HJ, Ross JM, Shuster EL, Chaky DA, Walsh DC, Choy CC, Tolley L-R, Yarme A, Twentieth century atmospheric metal fluxes into central park lake, New York City, Environmental science & technology, 33 (1999) 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jacobs DE, Clickner RP, Zhou JY, Viet SM, Marker DA, Rogers JW, Zeldin DC, Broene P, Friedman W, The prevalence of lead-based paint hazards in US housing, Environmental health perspectives, 110 (2002) A599–A606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nriagu JO, The rise and fall of leaded gasoline, Science of the total environment, 92 (1990) 13–28. [Google Scholar]
- [18].Peryea F, Creger T, Vertical distribution of lead and arsenic in soils contaminated with lead arsenate pesticide residues, Water, Air, and Soil Pollution, 78 (1994) 297–306. [Google Scholar]
- [19].Fischbein A, Rice C, Sarkozi L, Kon SH, Petrocci M, Selikoff IJ, Exposure to lead in firing ranges, JAMA, 241 (1979) 1141–1144. [PubMed] [Google Scholar]
- [20].Chow TJ, Earl JL, Lead isotopes in North American coals, Science, 176 (1972) 510–511. [DOI] [PubMed] [Google Scholar]
- [21].Ostergren JD, Brown GE, Parks GA, Tingle TN, Quantitative speciation of lead in selected mine tailings from Leadville, CO, Environmental science & technology, 33 (1999) 1627–1636. [Google Scholar]
- [22].Spear TM, Svee W, Vincent JH, Stanisich N, Chemical speciation of lead dust associated with primary lead smelting, Environmental Health Perspectives, 106 (1998) 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Miranda ML, Anthopolos R, Hastings D, A geospatial analysis of the effects of aviation gasoline on childhood blood lead levels, Environmental Health Perspectives, 119 (2011) 1513–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mielke HW, Lead in the inner cities: Policies to reduce children’s exposure to lead may be overlooking a major source of lead in the environment, American scientist, 87 (1999) 62–73. [Google Scholar]
- [25].Mielke HW, Gonzales CR, Powell E, Jartun M, Mielke PW Jr, Nonlinear association between soil lead and blood lead of children in metropolitan New Orleans, Louisiana: 2000–2005, Science of the Total Environment, 388 (2007) 43–53. [DOI] [PubMed] [Google Scholar]
- [26].Mielke HW, Gonzales CR, Powell ET, Laidlaw MA, Berry KJ, Mielke PW, Egendorf SP, The concurrent decline of soil lead and children’s blood lead in New Orleans, Proceedings of the National Academy of Sciences, 116 (2019) 22058–22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Morrison D, Lin Q, Wiehe S, Liu G, Rosenman M, Fuller T, Wang J, Filippelli G, Spatial relationships between lead sources and children’s blood lead levels in the urban center of Indianapolis (USA), Environmental geochemistry and health, 35 (2013) 171–183. [DOI] [PubMed] [Google Scholar]
- [28].Manceau A, Boisset M-C, Sarret G, Hazemann J-L, Mench M, Cambier P, Prost R, Direct determination of lead speciation in contaminated soils by EXAFS spectroscopy, Environmental science & technology, 30 (1996) 1540–1552. [Google Scholar]
- [29].McBride MB, Richards BK, Steenhuis T, Russo JJ, Sauvé S, Mobility and solubility of toxic metals and nutrients in soil fifteen years after sludge application, Soil Science, 162 (1997) 487–500. [Google Scholar]
- [30].Cho W-S, Duffin R, Thielbeer F, Bradley M, Megson IL, MacNee W, Poland CA, Tran CL, Donaldson K, Zeta potential and solubility to toxic ions as mechanisms of lung inflammation caused by metal/metal oxide nanoparticles, Toxicological Sciences, 126 (2012) 469–477. [DOI] [PubMed] [Google Scholar]
- [31].MacLean LC, Beauchemin S, Rasmussen PE, Lead speciation in house dust from Canadian urban homes using EXAFS, micro-XRF, and micro-XRD, Environmental science & technology, 45 (2011) 5491–5497. [DOI] [PubMed] [Google Scholar]
- [32].Landrot G, Khaokaew S, Lead speciation and association with organic matter in various particle-size fractions of contaminated soils, Environmental science & technology, 52 (2018) 6780–6788. [DOI] [PubMed] [Google Scholar]
- [33].Morin G, Ostergren JD, Juillot F, Ildefonse P, Calas G, Brown GE, XAFS determination of the chemical form of lead in smelter-contaminated soils and mine tailings: Importance of adsorption processes, American Mineralogist, 84 (1999) 420–434. [Google Scholar]
- [34].Goodnough A, Their Soil Toxic, 1,100 Indiana Residents Scramble to Find New Homes, New York Times, (2016). [Google Scholar]
- [35].Haque E, Moran ME, Thorne PS, Retrospective blood lead assessment from archived clotted erythrocyte fraction in a cohort of lead-exposed mother-child dyads, Science of The Total Environment, (2020) 142166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Morin G, Juillot F, Ildefonse P, Calas G, Samama J-C, Chevallier P, Brown GE Jr, Mineralogy of lead in a soil developed on a Pb-mineralized sandstone (Largentiere, France), American Mineralogist, 86 (2001) 92–104. [Google Scholar]
- [37].Scheckel KG, Impellitteri CA, Ryan JA, McEvoy T, Assessment of a sequential extraction procedure for perturbed lead-contaminated samples with and without phosphorus amendments, Environmental science & technology, 37 (2003) 1892–1898. [DOI] [PubMed] [Google Scholar]
- [38].Nghiem AA, Shen Y, Stahl M, Sun J, Haque E, DeYoung B, Nguyen KN, Thi Mai T., Trang PTK, Pham HV, Aquifer-Scale Observations of Iron Redox Transformations in Arsenic-Impacted Environments to Predict Future Contamination, Environmental Science & Technology Letters, 7 (2020) 916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nicholas SL, Erickson ML, Woodruff LG, Knaeble AR, Marcus MA, Lynch JK, Toner BM, Solid-phase arsenic speciation in aquifer sediments: A micro-X-ray absorption spectroscopy approach for quantifying trace-level speciation, Geochimica et Cosmochimica Acta, 211 (2017) 228–255. [Google Scholar]
- [40].Persinger ID, Soil survey of Lake County, Indiana, US Soil Conservation Service, 1972. [Google Scholar]
- [41].Kaste JM, Bostick BC, Friedland AJ, Schroth AW, Siccama TG, Fate and speciation of gasoline-derived lead in organic horizons of the northeastern USA, Soil Science Society of America Journal, 70 (2006) 1688–1698. [Google Scholar]
- [42].Schroth AW, Bostick BC, Kaste JM, Friedland AJ, Lead sequestration and species redistribution during soil organic matter decomposition, Environmental science & technology, 42 (2008) 3627–3633. [DOI] [PubMed] [Google Scholar]
- [43].Webb S, SIXpack: a graphical user interface for XAS analysis using IFEFFIT, Physica scripta, 2005 (2005) 1011. [Google Scholar]
- [44].Cressie N, Hawkins DM, Robust estimation of the variogram: I, Journal of the International Association for Mathematical Geology, 12 (1980) 115–125. [Google Scholar]
- [45].Cressie N, Statistics for spatial data, John Wiley & Sons, 2015. [Google Scholar]
- [46].Saby N, Arrouays D, Boulonne L, Jolivet C, Pochot A, Geostatistical assessment of Pb in soil around Paris, France, Science of the total environment, 367 (2006) 212–221. [DOI] [PubMed] [Google Scholar]
- [47].Biciunas G, Geinosky S, Creating Livable Communities in Northwest Indiana, (2015). [Google Scholar]
- [48].Thompson A, Attwood D, Gullikson E, Howells M, Kim K, Kirz J, Kortright J, Lindau I, Pianetta P, Robinson A, X-Ray Data Booklet, Lawrence Berkeley National Laboratory, Berkeley, CA, 2009, (2009) 1–2. [Google Scholar]
- [49].Parsons C, Grabulosa EM, Pili E, Floor GH, Roman-Ross G, Charlet L, Quantification of trace arsenic in soils by field-portable X-ray fluorescence spectrometry: considerations for sample preparation and measurement conditions, Journal of hazardous materials, 262 (2013) 1213–1222. [DOI] [PubMed] [Google Scholar]
- [50].Smith DB, Solano F, Woodruff LG, Cannon WF, Ellefsen KJ, Geochemical and Mineralogical Maps, with Interpretation, for Soils of the Conterminous United States, in, US Geological Survey, 2019. [Google Scholar]
- [51].Wu J, Edwards R, He XE, Liu Z, Kleinman M, Spatial analysis of bioavailable soil lead concentrations in Los Angeles, California, Environmental research, 110 (2010) 309–317. [DOI] [PubMed] [Google Scholar]
- [52].Mitchell RG, Spliethoff HM, Ribaudo LN, Lopp DM, Shayler HA, Marquez-Bravo LG, Lambert VT, Ferenz GS, Russell-Anelli JM, Stone EB, Lead (Pb) and other metals in New York City community garden soils: Factors influencing contaminant distributions, Environmental Pollution, 187 (2014) 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bradham KD, Nelson CM, Kelly J, Pomales A, Scruton K, Dignam T, Misenheimer JC, Li K, Obenour DR, Thomas DJ, Relationship between total and bioaccessible lead on children’s blood lead levels in urban residential Philadelphia soils, Environmental science & technology, 51 (2017) 10005–10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Frank JJ, Poulakos AG, Tornero-Velez R, Xue J, Systematic review and meta-analyses of lead (Pb) concentrations in environmental media (soil, dust, water, food, and air) reported in the United States from 1996 to 2016, Science of the Total Environment, 694 (2019) 133489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bachmaier M, Backes M, Variogram or semivariogram? Understanding the variances in a variogram, Precision Agriculture, 9 (2008) 173–175. [Google Scholar]
- [56].Scheckel KG, Ryan JA, Spectroscopic speciation and quantification of lead in phosphate-amended soils, Journal of Environmental Quality, 33 (2004) 1288–1295. [DOI] [PubMed] [Google Scholar]
- [57].Beauchemin S, MacLean LC, Rasmussen PE, Lead speciation in indoor dust: a case study to assess old paint contribution in a Canadian urban house, Environmental geochemistry and health, 33 (2011) 343–352. [DOI] [PubMed] [Google Scholar]
- [58].Monico L, Van der Snickt G., Janssens K, De Nolf W., Miliani C, Dik J, Radepont M, Hendriks E, Geldof M, Cotte M, Degradation process of lead chromate in paintings by Vincent van Gogh studied by means of synchrotron X-ray spectromicroscopy and related methods. 2. Original paint layer samples, Analytical chemistry, 83 (2011) 1224–1231. [DOI] [PubMed] [Google Scholar]
- [59].Trivedi P, Dyer JA, Sparks DL, Lead sorption onto ferrihydrite. 1. A macroscopic and spectroscopic assessment, Environmental science & technology, 37 (2003) 908–914. [DOI] [PubMed] [Google Scholar]
- [60].Bargar J, Brown G Jr, Parks G, Surface complexation of Pb (II) at oxide-water interfaces: III. XAFS determination of Pb (II) and Pb (II)-chloro adsorption complexes on goethite and alumina, Geochimica et Cosmochimica Acta, 62 (1998) 193–207. [Google Scholar]
- [61].Villalobos M, Bargar J, Sposito G, Mechanisms of Pb (II) sorption on a biogenic manganese oxide, Environmental science & technology, 39 (2005) 569–576. [DOI] [PubMed] [Google Scholar]
- [62].Bargar J, Brown G Jr, Parks G, Surface complexation of Pb (II) at oxide-water interfaces: II. XAFS and bond-valence determination of mononuclear Pb (II) sorption products and surface functional groups on iron oxides, Geochimica et Cosmochimica Acta, 61 (1997) 2639–2652. [Google Scholar]
- [63].Hettiarachchi GM, Pierzynski GM, Ransom MD, In situ stabilization of soil lead using phosphorus and manganese oxide, Environmental science & technology, 34 (2000) 4614–4619. [Google Scholar]
- [64].Hem JD, Redox processes at surfaces of manganese oxide and their effects on aqueous metal ions, Chemical Geology, 21 (1978) 199–218. [Google Scholar]
- [65].Kushwaha A, Rani R, Patra J, Adsorption kinetics and molecular interactions of lead [Pb (II)] with natural clay and humic acid, International Journal of Environmental Science and Technology, 17 (2020) 1325–1336. [Google Scholar]
- [66].Rhee YJ, Hillier S, Gadd GM, A new lead hydroxycarbonate produced during transformation of lead metal by the soil fungus Paecilomyces javanicus, Geomicrobiology Journal, 33 (2016) 250–260. [Google Scholar]
- [67].Ma QY, Logan TJ, Traina SJ, Lead immobilization from aqueous solutions and contaminated soils using phosphate rocks, Environmental science & technology, 29 (1995) 1118–1126. [DOI] [PubMed] [Google Scholar]
- [68].Pavilonis BT, Lioy PJ, Guazzetti S, Bostick BC, Donna F, Peli M, Zimmerman NJ, Bertrand P, Lucas E, Smith DR, Manganese concentrations in soil and settled dust in an area with historic ferroalloy production, Journal of exposure science & environmental epidemiology, 25 (2015) 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Doner HE, Lynn WC, Carbonate, halide, sulfate, and sulfide minerals, Minerals in soil environments, 1 (1989) 279–330. [Google Scholar]
- [70].Karberg NJ, Pregitzer KS, King JS, Friend AL, Wood JR, Soil carbon dioxide partial pressure and dissolved inorganic carbonate chemistry under elevated carbon dioxide and ozone, Oecologia, 142 (2005) 296–306. [DOI] [PubMed] [Google Scholar]
- [71].Unuabonah E, Adebowale K, Olu-Owolabi B, Kinetic and thermodynamic studies of the adsorption of lead (II) ions onto phosphate-modified kaolinite clay, Journal of hazardous materials, 144 (2007) 386–395. [DOI] [PubMed] [Google Scholar]
- [72].Jones CP, Toward the science and practice of anti-racism: launching a National Campaign against Racism, Ethnicity & disease, 28 (2018) 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Schell CJ, Dyson K, Fuentes TL, Des Roches S, Harris NC, Miller DS, Woelfle-Erskine CA, Lambert MR, The ecological and evolutionary consequences of systemic racism in urban environments, Science, 369 (2020). [DOI] [PubMed] [Google Scholar]
- [74].Nigra AE, Environmental racism and the need for private well protections, Proceedings of the National Academy of Sciences, 117 (2020) 17476–17478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Council NR, Using the American Community Survey: benefits and challenges, National Academies Press, 2007. [Google Scholar]
- [76].Marshall AT, Betts S, Kan EC, McConnell R, Lanphear BP, Sowell ER, Association of lead-exposure risk and family income with childhood brain outcomes, Nature medicine, 26 (2020) 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Tong S, Schirnding Y.E.v., Prapamontol T, Environmental lead exposure: a public health problem of global dimensions, Bulletin of the world health organization, 78 (2000) 1068–1077. [PMC free article] [PubMed] [Google Scholar]
- [78].Thorne PS, Experimental grain dust atmospheres generated by wet and dry aerosolization techniques, American journal of industrial medicine, 25 (1994) 109–112. [DOI] [PubMed] [Google Scholar]
- [79].Casteel SW, Weis CP, Henningsen GM, Brattin WJ, Estimation of relative bioavailability of lead in soil and soil-like materials using young swine, Environmental Health Perspectives, 114 (2006) 1162–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Goodnough A, Their soil toxic, 1,100 Indiana residents scramble to find new homes, The New York Times, (2016). [Google Scholar]
- [81].Bradham KD, Nelson CM, Diamond GL, Thayer WC, Scheckel KG, Noerpel M, Herbin-Davis K, Elek B, Thomas DJ, Dietary lead and phosphate interactions affect oral bioavailability of soil lead in the mouse, Environmental science & technology, 53 (2019) 12556–12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bradham KD, Diamond GL, Nelson CM, Noerpel M, Scheckel KG, Elek B, Chaney RL, Ma Q, Thomas DJ, Long-term in situ reduction in soil lead bioavailability measured in a mouse model, Environmental science & technology, 52 (2018) 13908–13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.