Abstract
Background:
In middle-aged adults with depression, cerebral vasodilatory reactivity is blunted; however, this has not been examined in treatment-naïve adults with major depressive disorder (MDD). We tested the hypothesis that cerebrovascular reactivity would be blunted in young adults (18–30 yrs) with MDD compared to healthy non-depressed adults (HA) and would be attenuated to a greater extent in adults with symptomatic MDD (sMDD) compared to adults with MDD in remission (euthymic MDD; eMDD).
Methods:
Sixteen adults with MDD [21±3yrs; n = 8 sMDD (6 women); n = 8 eMDD (5 women)] and 14 HA (22±3yrs; 9 women) participated. End-tidal carbon dioxide concentration (PETCO2; capnograph), beat-to-beat mean arterial pressure (MAP; finger photoplethysmography), middle cerebral artery blood velocity (MCAv; transcranial Doppler ultrasound), and internal carotid artery (ICA) diameter and blood velocity (Doppler ultrasound) were continuously measured during baseline and rebreathing-induced hypercapnia. Cerebrovascular reactivity was calculated as the relative increase in vascular conductance during hypercapnia.
Results:
In adults with MDD, cerebrovascular reactivity in the MCA (Δ39±9 HA vs. Δ31±13% MDD, p = 0.04), but not the ICA (Δ36±24 HA vs. Δ34±18% MDD, p = 0.84), was blunted compared to HA. In the MCA, cerebrovascular reactivity was reduced in adults with sMDD compared to adults with eMDD (Δ36±11 eMDD vs. Δ25±13% sMDD, p = 0.02).
Limitations:
The cross-sectional nature approach limits conclusions regarding the temporal nature of this link.
Conclusion:
These data indicate that MCA cerebrovascular reactivity is blunted in young adults with MDD and further modulated by current depressive symptomology, suggesting that the management of depressive symptomology may secondarily improve cerebrovascular health.
Keywords: Depression, Cerebral blood flow, Transcranial Doppler ultrasound, Middle cerebral artery velocity, vasomotor reactivity
1. Introduction
Major depressive disorder (MDD) is a mood disorder that is episodic in nature, highly recurrent, characterized by persistently depressed mood and/or anhedonia that causes clinically significant functional impairments in daily life (2013), and a leading cause of worldwide disability and overall global disease burden, surpassing both cardiovascular disease and cancer (Brody et al., 2018; Malhi and Mann, 2018). MDD manifests in ~10–15% of adults in the US (Kessler et al., 2005; Whiteford et al., 2013). Alarmingly, in young adults (18–25 yrs), the incidence rate of a major depressive episode has increased by nearly 65% in the past decade alone (Twenge et al., 2019). Compelling evidence directly links depressive disorders to an increased risk of developing cerebrovascular disease and cognitive impairments (Alexopoulos et al., 1997; Taylor et al., 2013). Although the mechanisms are undoubtedly multifactorial, peripheral and cerebral vascular dysfunction, both of which are linked to brain health and function, are likely primary contributing factors to cerebrovascular disease progression in adults with depression (Direk et al., 2012; Taylor et al., 2013).
Precise and coordinated adjustments in cerebral blood flow are necessary for cerebral metabolic homeostasis and adequate perfusion. In this way, alterations in the partial pressure of arterial carbon dioxide (PaCO2) lead to rapid and robust changes in cerebral vascular smooth muscle tone and subsequent increases or decreases in cerebral blood flow (Hoiland et al., 2019). In normal physiological conditions, hypercapnia increases and hypocapnia decreases cerebral blood flow (Ide et al., 2003). Given this tight regulation, alterations in PaCO2 and the ensuing changes in cerebral vascular resistance and blood flow (i.e., cerebrovascular reactivity) are commonly used to provide an index of cerebrovascular function (Hurr et al., 2017; Ide et al., 2003; Ringelstein et al., 1988). Importantly, blunted cerebrovascular reactivity is an independent predictor of ischemic stroke risk (Markus and Cullinane, 2001) and is evident in adults with established cerebrovascular and neurological diseases (Beishon et al., 2017; Cantin et al., 2011; Markus and Cullinane, 2001; Smolinski and Czlonkowska, 2016; Vicenzini et al., 2007). Mounting evidence demonstrates marked abnormalities in cerebral blood flow regulation in middle-aged and older adults with depressive disorders (Direk et al., 2012; Dotson et al., 2009; Greenstein et al., 2010; Lui et al., 2009; Monkul et al., 2012; Ota et al., 2014; Paranthaman et al., 2010; Taylor et al., 2013; Tiemeier et al., 2002). In middle-aged adults with MDD, cerebrovascular reactivity in response to pharmacologically induced (e.g., acetazolamide) increases in PaCO2 is blunted, with some evidence for improved responsiveness following remission of depressive symptoms (de Castro et al., 2008; Lemke et al., 2010; Neu et al., 2004). Although peripheral vascular dysfunction is evident in young, otherwise healthy, adults with MDD and is graded in relation to symptom severity (Cooper et al., 2010; Fiedorowicz et al., 2015; Greaney et al., 2020, 2019a, 2019b; Rajagopalan et al., 2001), whether active depressive symptomology is also linked to blunted cerebrovascular reactivity to physiologically-induced increases in PaCO2 in young adults with MDD has not yet been examined.
The aim of the present investigation was to examine cerebrovascular reactivity in treatment-naïve, otherwise healthy, young adults (18–30 yrs) with MDD. In an attempt to account for the heterogeneity in patterns of cerebral vasoreactivity (Coverdale et al., 2015), we simultaneously measured arterial diameter and blood velocity in the internal carotid artery (ICA) using duplex Doppler ultrasound and middle cerebral artery (MCA) blood velocity using transcranial Doppler ultrasound before and during rebreathing-induced hypercapnia. We hypothesized that cerebral vasodilatory responsiveness to hypercapnia would be blunted in both the ICA and MCA in young adults with MDD compared to healthy non-depressed young adults (HA). Using a cross-sectional approach, we further hypothesized that the magnitude of hypercapnia-induced cerebral vasodilation would be attenuated in adults with symptomatic MDD (sMDD) compared to young adults with MDD in remission (euthymic MDD; eMDD).
2. Methods
The Institutional Review Board at The University of Texas at Arlington approved the experimental procedures (2019–0256). The study was conducted in accordance with the guidelines set forth by the Declaration of Helsinki except for registration in a database. The study benefits, procedures, and risks were explained to the subjects, and verbal and written informed consent were obtained voluntarily from all participants prior to participation.
2.1. Neuropsychiatric assessment
Participants were recruited from The University of Texas at Arlington and the surrounding Dallas-Fort Worth metroplex using common means of recruitment. Participants were screened to determine study eligibility prior to enrollment. All participants underwent a structured diagnostic clinical interview, the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998), to determine presence or absence of DSM-5 major psychiatric illness, including MDD. Thirty-three otherwise healthy non-medicated adults were screened for MDD; of these, 16 patients (11 women) met the criteria for study enrollment (Table 1). Consistent with standard DSM-5 diagnostic criteria (2013), adults with sMDD (n = 8; 6 women) were defined by the presence of 5 out of 9 depressive symptoms within 2 weeks prior to interview, and adults with eMDD (n = 8; 5 women) were defined by fewer than 5 depressive symptoms for at least 2 weeks prior to interview. Adults with sMDD and eMDD were classified using the MINI diagnostic algorithm (Sheehan et al., 1998). Fourteen healthy adults (HA; 9 women) without any history or evidence of major psychiatric illness served as the control group. Participants were excluded for any of the following: co-morbid current psychiatric disorders (e.g., schizophrenia, bipolar disorder, psychosis, post-traumatic stress disorder, panic disorder), the recent or current use of psychoactive or psychopharmacological drugs, and active suicidal ideation.
Table 1.
Subject Characteristics
| Characteristic | HA | MDD | p-value |
|---|---|---|---|
| N (M/F) | 14 (5/9) | 16 (5/11) | |
| Age (yr) | 22 ± 3 | 21 ± 3 | 0.38 |
| Height (cm) | 164.1 ± 9.3 | 162.3 ± 6.2 | 0.54 |
| Mass (kg) | 66.6 ± 12.1 | 60.8 ± 10.9 | 0.18 |
| BMI (kg/m2) | 25.0 ± 2.5 | 23.4 ± 4.4 | 0.33 |
| Heart Rate (bpm) | 71 ± 8 | 74 ± 11 | 0.18 |
| Systolic BP (mmHg) | 117 ± 11 | 114 ± 13 | 0.77 |
| Diastolic BP (mmHg) | 73 ± 5 | 71 ± 7 | 0.76 |
| Habitual Physical Activity (MET-mins/wk) | 5820 ± 4317 | 4719 ± 5718 | 0.57 |
| Blood Biochemistry | |||
| HbA1c (%) | 5.2 ± 0.2 | 5.1 ± 0.3 | 0.76 |
| Total Cholesterol (mg/dl) | 160 ± 28 | 161 ± 22 | 0.77 |
| HDL (mg/dl) | 57 ± 13 | 62 ± 11 | 0.40 |
| LDL (mg/dl) | 83 ± 25 | 83 ± 20 | 0.62 |
| Triglycerides (mg/dl) | 99 ± 49 | 84 ± 34 | 0.54 |
| Depression Assessment | |||
| PROMIS (raw score) | 14 ± 5 | 25 ± 6 | <0.01 |
| PROMIS (T-score) | 51 ± 8 | 63 ± 6 | <0.01 |
| PHQ-9 (au) | 3 ± 3 | 12 ± 7 | <0.001 |
| Number of Lifetime Depressive Episodes | – | 9 (range 2–30) | |
| Emotional Assessment | |||
| Negative Affect (T-score) | 48 ± 13 | 64 ± 9 | <0.01 |
| Social Satisfaction (T-score) | 52 ± 10 | 39 ± 9 | <0.01 |
| Psychological Well-Being (T-score) | 54 ± 9 | 39 ± 1 | <0.01 |
HA, healthy non-depressed adults; MDD, Major Depressive Disorder; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PROMIS, patient-reported outcome measurement information system; PHQ-9, Patient Health Questionnaire (symptom severity: 0–4, minimal; 5–9, mild; 10–14, moderate; 15–19, moderately severe; 20–27, severe). Emotional and Assessments were derived from the NIH Toolbox (Salsman et al., 2013; Weintraub et al., 2013). Values are mean ± standard deviation.
Depressive symptom severity was evaluated by both the National Institutes of Health Patient-Reported Outcomes Measurement Information System (PROMIS; emotional distress – depression, short-form) (Schalet et al., 2016) and the Patient Health Questionnaire-9 (PHQ-9) (Spitzer et al., 1999). Both the PROMIS and PHQ-9 provide a valid and sensitive index of depressive symptomology (Choi et al., 2014; Kroenke et al., 2001; Spitzer et al., 1999). The PROMIS consists of 8 items with a 7-day time frame and a 5-point scale (1= never to 5=always); item content focuses on the emotional, cognitive, and behavioral manifestations of depression (Choi et al., 2014). Raw scores are converted to a T-score, standardized to reflect a mean score of 50 with a standard deviation of 10, and centered around the US general population. (Choi et al., 2014) Thus, a PROMIS depression T-score of 60 indicates the presence of depressive symptoms that are one standard deviation higher than the national average. The PHQ-9 is a 9-item instrument based directly on the diagnostic criteria for depressive disorders outlined in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) (2013; Kroenke et al., 2001). Participants rated the presence of depressive symptoms in the previous 2 weeks using a 4-point Likert scale (0=not at all to 3=nearly every day). Scores of 5, 10, 15, and 20 represent cut points for mild, moderate, moderately severe, and severe depression, respectively.
All participants also completed a medical health history, which included anthropometric measurements, resting blood pressure and heart rate measurements (Connex Spot Monitor; Welch Allyn, Skaneateles Falls, NY, USA), and blood chemistry and lipid profile (LabCorp; Dallas, TX). Participants completed the International Physical Activity Questionnaire to estimate habitual physical activity. All participants were familiarized with the experimental protocol at the initial screening visit.
All participants were free of cardiovascular, metabolic, or renal disease. In addition, all were recreationally active, non-obese (body mass index < 30 kg/m2), did not use tobacco products, and were not taking prescription medications, except for hormonal contraception (n = 1 HA). We did not control for menstrual cycle phase at the experimental visit, because, from an ethical perspective, every attempt was made to test MDD patients within ~1 week of qualification and enrollment to facilitate expedient follow-up with a mental healthcare provider. This approach has been employed in our previous investigations in a similar patient population (Greaney et al., 2020, 2019a, 2019b). A urine pregnancy test confirmed the absence of pregnancy at the experimental visit.
2.2. Assessment of overall emotional health and cognitive function
In addition to the surveys quantifying depressive symptom severity, participants also completed the National Institutes of Health Toolbox Cognition and Emotion Batteries (iPad platform), as previously described (Salsman et al., 2013; Weintraub et al., 2013). Briefly, the Toolbox Cognition Battery entails seven tests that assess five major cognitive subdomains: processing speed, executive function, working memory, episodic memory, and language. A fluid composite cognition score is a combination of the tests assessing the subdomains of fluid cognitive ability (processing speed, executive function, working memory, and episodic memory); a crystallized composite cognition score is a combination of the tests assessing the subdomains of crystallized cognitive ability (language). A total cognitive function composite score is calculated based upon both fluid and crystallized composites and reflects general cognitive function (Weintraub et al., 2013). Composite scores are calculated as 1) raw scores standardized to a normative mean of 100 (standard deviation of 15), 2) an age-adjusted percentile score [relative to all adults in the US within each age group (binned by year) enrolled in the Toolbox National Norming Study (Beaumont et al., 2013)], and 3) a fully corrected T-score (adjusted for age, sex, race, ethnicity, and highest level of education).
The Emotion Battery is an ~20 min self-administered assessment of emotional functioning across 17 scales (general life satisfaction, meaning and purpose, positive affect, friendship, emotional support, instrumental support, loneliness, perceived rejection, perceived stress, anger-affect, anger-hostility, sadness, fear-affect, fear-somatic arousal, perceived hostility, anger-physical aggression, and self-efficacy). Participants rated their emotional state in the previous week using a 5-point Likert scale (1=never to 5=always). Composite scores are calculated within the major subdomains central to emotional health: negative affect, psychological well-being, and social satisfaction (Salsman et al., 2013). Problematic emotions were defined as more than 1 standard deviation below the mean (T<40) for positive emotion scales and more than 1 standard deviation above the mean (T>60) for negative emotion scales (Babakhanyan et al., 2018).
2.3. Assessment of cerebrovascular function
Participants abstained from food for 3 hr and from caffeine, alcohol, and strenuous exercise for 24 hr prior to the experimental visit. Heart rate (HR) was continuously measured via a single-lead electrocardiogram (Cardio Card; Nasiff Associates, Central Square, NY). Beat-to-beat arterial blood pressure was measured using finger photoplethysmography (Finometer Pro; Finapres Medical Systems, Enschede, NL) obtained from the right hand positioned at heart level. Automated brachial artery blood pressure (Tango M2; Suntech Medical Inc., Morrisville, NC) was measured intermittently throughout the protocol and was used to verify absolute Finometer measurements. Respiratory excursions were monitored using a strain gage pneumograph placed over the abdomen (Pneumotrace II; UFI, Morro Bay, CA).
MCA mean blood velocity (MCAv) was measured by transcranial Doppler ultrasound (TCD), as previously described in detail (Claassen et al., 2007; Hurr et al., 2015a, 2015b, 2017; Patik et al., 2018). A 2-MHz TCD probe (Neurovision TOC; Multigon Industries Inc, Yonkers, NY) was placed over the left temporal window and attached via headband to prevent probe shifts during the protocol. Adjustments to the probe angle and insonation depth settings were made, as appropriate, until an optimal signal was acquired. Diameter of the right ICA was measured using duplex Doppler ultrasound (Logiq P5; GE, Milwaukee, WI) with an adjustable (10–13 MHz) linear array transducer. Optimal B-mode signals of the ICA were obtained for clear delineation between the lumen and vessel walls. Duplex mode (at a pulsed frequency of 5 MHz) was used for the simultaneous measurement of diameter and velocity. The velocity cursor was set midvessel, approximately 1.0–1.5 cm distal to the carotid sinus, with a 60° angle of insonation, and sample volume was adjusted to encompass the entire vessel lumen without extending into the surrounding tissue. All Doppler-derived measurements were performed by a single operator with extensive training and experience in acquiring high-quality images (Akins et al., 2021; Barbosa et al., 2018; Hurr et al., 2015a, 2015b, 2017; Kaur et al., 2021; Patik et al., 2018).
Participants were then fitted with a nose-clip and breathed through a mouthpiece attached to a Y-valve (V2; Hans Rudolph, Shawnee, KS). One end of the Y-valve was connected to a 5-L bag and the other was open to room air, allowing for an instantaneous switch from room air to rebreathing from the bag. The rebreathing bag was pre-filled with the participant's expired air. End-tidal CO2 tension (PETCO2), a proxy for PaCO2, was measured continuously via a sample line connecting the mouthpiece to a capnograph (Capnocheck Plus; Smiths Medical, Dublin, OH). Peripheral oxygen saturation (SpO2) was monitored throughout the protocol using a digital pulse oximeter (Capnocheck Plus).
Following instrumentation, participants rested quietly in the supine position for 20 min. Thereafter, cerebrovascular reactivity to rebreathing-induced hypercapnia was assessed (Claassen et al., 2007; Hurr et al., 2015a, 2015b, 2017; Patik et al., 2018). Baseline data were collected for 3 min, during which participants breathed room air. Following baseline, subjects then performed a deep inspiration, upon which the Y-valve was switched so that participants expired into and inspired out of the 5-L bag (i.e., rebreathing). Rebreathing was performed for 3 min or until subjects indicated they could no longer tolerate the stimulus. During rebreathing, medical grade oxygen was bled into the bag in order to maintain arterial normoxia (SpO2 = ~97%); this was calculated for each participant based on their basal metabolic rate estimated using the Harris-Benedict equation (Claassen et al., 2007; Hurr et al., 2015a, 2015b, 2017; Patik et al., 2018). Cardiovascular and cerebrovascular outcome variables were measured continuously throughout baseline and the rebreathing protocol.
2.4. Data and statistical analysis
All data were recorded at 40–1000 Hz and stored offline for analysis (Powerlab 16/35 and LabChart; ADInstruments, Colorado Springs, CO). Mean arterial pressure (MAP) was calculated as (2 • diastolic blood pressure/3)+(systolic blood pressure/3). Cerebral vascular conductance index (CVCi = MCAv • MAP−1) was calculated for the MCA. Second-by-second ICA blood velocity and diameter were analyzed using automated edge-detection software (CardioSuite; Quipu, Pisa, IT). ICA diameter (D) was assessed along a section of artery with clearly defined vessel walls while ICA blood velocity (VICA) was analyzed as the entire velocity spectrum. ICA blood flow () was subsequently calculated as [π(D/2)2] • VICA • 60. Cerebral vascular conductance in the ICA was also calculated (CVC = • MAP−1). In 4 participants (2 MDD), adequate ICA images could not be maintained for the duration of the rebreathing protocol; these individuals were excluded from the analysis of ICA-derived variables. Average values for all variables were determined during the last 60 s of baseline. During hypercapnia, all data were analyzed on a breath-by-breath basis. The absolute breath-by-breath increase in PETCO2 throughout rebreathing was determined for each individual. The data were averaged and analyzed over 3 breaths at each 3 mmHg increase in PETCO2 up to, and including, the highest common magnitude of hypercapnia achieved by all subjects (ΔPETCO2 = 9 mmHg) (Hurr et al., 2015b; Patik et al., 2018). Cerebrovascular reactivity was quantified as the percent increase in CVCi and CVC from baseline.
An a priori power analysis (α = 0.05, β = 0.80, ƒ = 0.25, 2 groups) indicated a sample size of 12 participants per group would be required to detect a meaningful physiological difference (Hurr et al., 2015b). Student's unpaired t-tests were used to compare participant characteristics. All cardiovascular and cerebrovascular outcome variables were analyzed using two-way (group x. hypercapnia) mixed-model ANOVA, with post hoc corrections (Tukey) applied for specific planned comparisons when appropriate (SAS v9.4; Cary, NC). Data are presented as mean±standard deviation, effect sizes are reported as partial eta squared (ηp2), and significance was set at α < 0.05.
3. Results
There were no group differences in anthropometric characteristics, volume of habitual physical activity, resting blood pressure, or blood biochemistry (Table 1; all p>0.05). By study design, adults with MDD reported clinically significant depressive symptoms of mild-moderate severity (Table 1; p<0.001). In addition, indices of overall emotional health and well-being were lower and negative affect was greater in adults with MDD compared to HA (Table 1; all p<0.01). Total, fluid, and crystallized cognitive function composite scores were not different between groups (Table 2; all p>0.05).
Table 2.
Cognitive Function.
| Cognition Assessment | HA | MDD | p-value |
|---|---|---|---|
| Total Composite Score | |||
| Raw (unadjusted) | 113 ± 7 | 110 ± 5 | 0.19 |
| Age-adjusted (%) | 66 ± 23 | 56 ± 19 | 0.32 |
| Fully-adjusted (T-score) | 55 ± 8 | 53 ± 5 | 0.40 |
| Fluid Composite Score | |||
| Raw (unadjusted) | 117 ± 9 | 114 ± 8 | 0.30 |
| Age-adjusted (%) | 62 ± 29 | 53 ± 31 | 0.41 |
| Fully-adjusted (T-score) | 53 ± 9 | 50 ± 9 | 0.44 |
| Crystallized Composite Score | |||
| Raw (unadjusted) | 105 ± 5 | 104 ± 3 | 0.54 |
| Age-adjusted (%) | 63 ± 23 | 56 ± 19 | 0.55 |
| Fully-adjusted (T-score) | 55 ± 8 | 54 ± 4 | 0.67 |
HA, healthy non-depressed adults; MDD, Major Depressive Disorder. Cognition Assessments were derived from the NIH Toolbox (Salsman et al., 2013; Weintraub et al., 2013). Values are mean ± standard deviation.
There were no differences in respiratory or cardiovascular parameters at baseline or during rebreathing-induced hypercapnia between groups (Table 3; all p>0.05). Respiratory rate did not change during rebreathing in either subject group (Table 3; p>0.05). Hypercapnia elicited small, but significant, increases in blood pressure and heart rate in adults with MDD (Table 3 and Fig 1A), but these responses were not different between HA and adults with MDD (p>0.05).
Table 3.
Respiratory and Cardiovascular Responses to Hypercapnia.
| Variable | baseline | PETCO2 = 3 mmHg | PETCO2 = 6 mmHg | PETCO2 = 9 mmHg | group p-value (ηp2) | hypercapnia p-value (ηp2) | interaction p-value (ηp2) |
|---|---|---|---|---|---|---|---|
| Respiratory Rate (breaths•min−1) | |||||||
| HA | 14 ± 4 | 12 ± 2 | 12 ± 3 | 12 ± 3 | 0.79(0.003) | 0.13(0.07) | 0.37(0.04) |
| MDD | 12 ± 4 | 12 ± 5 | 12 ± 4 | 12 ± 3 | |||
| Heart Rate (bpm) | |||||||
| HA | 69 ± 6 | 73 ± 7 | 72 ± 7 | 72 ± 9 | 0.67(0.007) | <0.01(0.23) | 0.25(0.05) |
| MDD | 68 ± 12 | 72 ± 13* | 69 ± 12 | 71 ± 13* | |||
| Systolic BP (mmHg) | |||||||
| HA | 124 ± 12 | 122 ± 14 | 126 ± 13 | 127 ± 13 | 0.51(0.02) | <0.01(0.19) | 0.08(0.08) |
| MDD | 120 ± 9 | 122 ± 10 | 122 ± 10 | 123 ± 10 | |||
| Diastolic BP (mmHg) | |||||||
| HA | 70 ± 6 | 68 ± 7 | 72 ± 7 | 73 ± 8* | 0.79(0.003) | <0.01(0.46) | 0.34(0.04) |
| MDD | 70 ± 4 | 70 ± 6 | 71 ± 6 | 73 ± 6* | |||
| MAP (mmHg) | |||||||
| HA | 88 ± 7 | 86 ± 9 | 90 ± 8 | 90 ± 9 | 0.85(0.001) | <0.01(0.37) | 0.17(0.06) |
| MDD | 87 ± 4 | 87 ± 6 | 88 ± 6 | 89 ± 7* | |||
HA, healthy non-depressed adults; MDD, major depressive disorder; BP, blood pressure; MAP, mean arterial pressure; PETCO2, end-tidal carbon dioxide. Values are mean ± SD; effect sizes are reported as partial eta squared (ηp2).
p<0.05 vs. baseline.
Fig. 1.
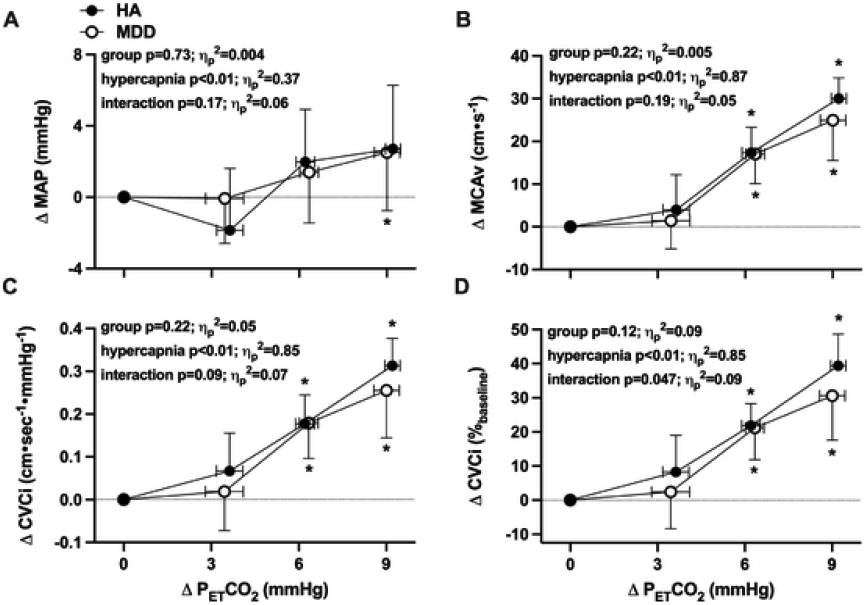
The increase in mean arterial pressure (MAP; Panel A), middle cerebral artery blood velocity (MCAv; Panel B), and absolute (Panel C) and relative (Panel D) cerebrovascular conductance index during rebreathing-induced increases in end-tidal carbon dioxide (PETCO2) in healthy non-depressed adults (HA; n = 14; 9 women) and adults with major depressive disorder (MDD; n = 16; 11 women). The absolute breath-by-breath increase in PETCO2 throughout rebreathing was determined for each individual and presented at each 3 mmHg increase in PETCO2 up to, and including, the highest common magnitude of hypercapnia achieved by all subjects (ΔPETCO2 = 9 mmHg). Outcome variables were analyzed using two-way (group x. hypercapnia) mixed-model ANOVA, with post hoc corrections (Tukey) applied for specific planned comparisons when appropriate. Data are presented as mean±standard deviation; effect sizes are reported as partial eta squared (ηp2). *p<0.05 vs. baseline.
Cerebrovascular outcome variables were not different between groups at baseline (Table 4; all p>0.05). Hypercapnia elicited progressive increases in both MCAv and CVCi (Fig 1B-D). Absolute MCAv and CVCi were not different between groups during rebreathing (Table 4; both p>0.05); however, the relative increase in CVCi during hypercapnia was blunted in adults with MDD (Fig 1D). Hypercapnia had no effect on ICA D in either group (Table 4 and Fig 2A; p>0.05). Rebreathing-induced increases in and CVC were not different between HA and adults with MDD (Table 4 and Fig 2B-D; all p>0.05).
Table 4.
Cerebrovascular Responses to Hypercapnia in the MCA and ICA.
| Variable | baseline | PETCO2 =3 mmHg | PETCO2 =6 mmHg | PETCO2 =9 mmHg | group p-value (ηp2) | hypercapnia p-value (ηp2) | interaction p-value (ηp2) |
|---|---|---|---|---|---|---|---|
| MCAv (cm•sec−1) | |||||||
| HA | 71.7 ± 16.0 | 75.7 ± 18.6 | 89.0 ± 19.5* | 101.7 ± 17.9* | 0.86(0.001) | <0.01(0.87) | 0.19(0.05) |
| MDD | 74.8 ± 15.7 | 76.2 ± 16.5 | 91.8 ± 18.9* | 99.7 ± 19.5* | |||
| CVCi (cm•sec−1•mmHg−1) | |||||||
| HA | 0.82 ± 0.2 | 0.89 ± 0.2 | 1.00 ± 0.2* | 1.14 ± 0.2* | 0.86(0.001) | <0.01(0.85) | 0.09(0.07) |
| MDD | 0.86 ± 0.2 | 0.88 ± 0.2 | 1.04 ± 0.2* | 1.12 ± 0.2* | |||
| ICA D (mm) | |||||||
| HA | 4.8 ± 0.7 | 4.8 ± 0.8 | 4.8 ± 0.8 | 4.8 ± 0.8 | 0.52(0.02) | 0.16(0.07) | 0.42(0.04) |
| MDD | 5.0 ± 0.8 | 5.0 ± 0.6 | 4.9 ± 0.7 | 5.1 ± 0.7 | |||
| (ml•min−1) | |||||||
| HA | 444 ± 173 | 414 ± 210 | 490 ± 256 | 614 ± 257* | 0.85(0.001) | <0.01(0.68) | 0.46(0.04) |
| MDD | 422 ± 116 | 411 ± 138 | 500 ± 161* | 576 ± 162* | |||
| CVC (ml•min−1•mmHg−1) | |||||||
| HA | 5.10 ± 2.0 | 4.89 ± 2.4 | 5.57 ± 3.0 | 6.94 ± 3.2* | 0.80(0.02) | <0.01(0.62) | 0.44(0.04) |
| MDD | 4.85 ± 1.3 | 4.75 ± 1.6 | 5.66 ± 1.8* | 6.41 ± 1.6* | |||
MCA, middle cerebral artery; ICA, internal carotid artery; MCAv, MCA mean velocity; CVCi, cerebrovascular conductance index; ICA D, ICA diameter; : ICA blood flow; CVC, cerebrovascular conductance; HA, healthy non-depressed adults; MDD, major depressive disorder; PETCO2, end-tidal carbon dioxide. Values are mean ± SD; effect sizes are reported as partial eta squared (ηp2).
p<0.05 vs. baseline.
Fig. 2.
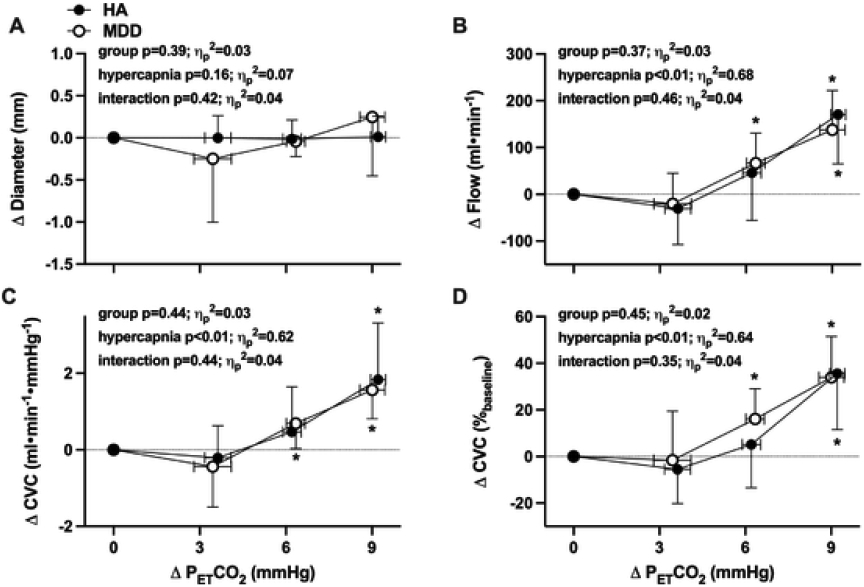
The increase in internal carotid artery diameter (Panel A), blood flow (Panel B), and absolute (Panel C) and relative (Panel D) cerebrovascular conductance during rebreathing-induced increases in end-tidal carbon dioxide (PETCO2) in healthy non-depressed adults (HA; n = 12; 8 women) and adults with major depressive disorder (MDD; n = 14; 9 women). The absolute breath-by-breath increase in PETCO2 throughout rebreathing was determined for each individual and presented at each 3 mmHg increase in PETCO2 up to, and including, the highest common magnitude of hypercapnia achieved by all subjects (ΔPETCO2 = 9 mmHg). Outcome variables were analyzed using two-way (group x. hypercapnia) mixed-model ANOVA, with post hoc corrections (Tukey) applied for specific planned comparisons when appropriate. Data are presented as mean±standard deviation; effect sizes are reported as partial eta squared (ηp2). *p<0.05 vs. baseline.
In adults with MDD, the relative increase in CVCi (at ΔPETCO2 = 9 mmHg) was negatively related to the number of lifetime depressive episodes (r = −0.62 (95% confidence interval −0.87 – −0.14), p = 0.02) but not to depressive symptom severity (r = −0.16 (95% confidence interval −0.61 – 0.37), p = 0.56). However, there was no relation between either number of depressive episodes or symptom severity and the relative increases in CVC [episodes: r = −0.35 (95% confidence interval −0.77 – 0.28), p = 0.26; severity: r = 0.27 (95% confidence interval −0.30 – 0.70), p = 0.34]. There was no relation between any indices of cognitive function and hypercapnia-induced vasodilation (data not shown).
To further assess the influence of current depressive symptomology on cerebrovascular responsiveness to hypercapnia, data were analyzed separately in adults with eMDD (n = 8; 5 women) and adults with sMDD (n = 8; 6 women), a classification determined by the outcome of the initial MINI, and compared to HA. Adults with eMDD reported depressive symptoms of mild-to-moderate severity [median PHQ-9 score 8 (interquartile range 3–15)], whereas adults with sMDD reported symptoms of moderate severity [median PHQ-9 score 16 (interquartile range 7–18]. Adults with sMDD had greater symptom severity scores on the PROMIS (raw score: 22±6 eMDD vs. 29±3 sMDD, p = 0.015; T-score: 60±6 eMDD vs. 67±3 sMDD, p = 0.016). During hypercapnia, the mean arterial pressure response was not different between the three groups (group p = 0.93, hypercapnia p<0.01, interaction p = 0.33). Hypercapnia-induced increases in CVCi were significantly blunted in adults with sMDD but not different between HA and adults with eMDD (Fig 3A-B). However, there were no differences between groups in ICA vasodilatory responsiveness (Fig 3C-D). There were no differences in absolute MCAv, ICA D, or between the three groups (data not shown).
Fig. 3.
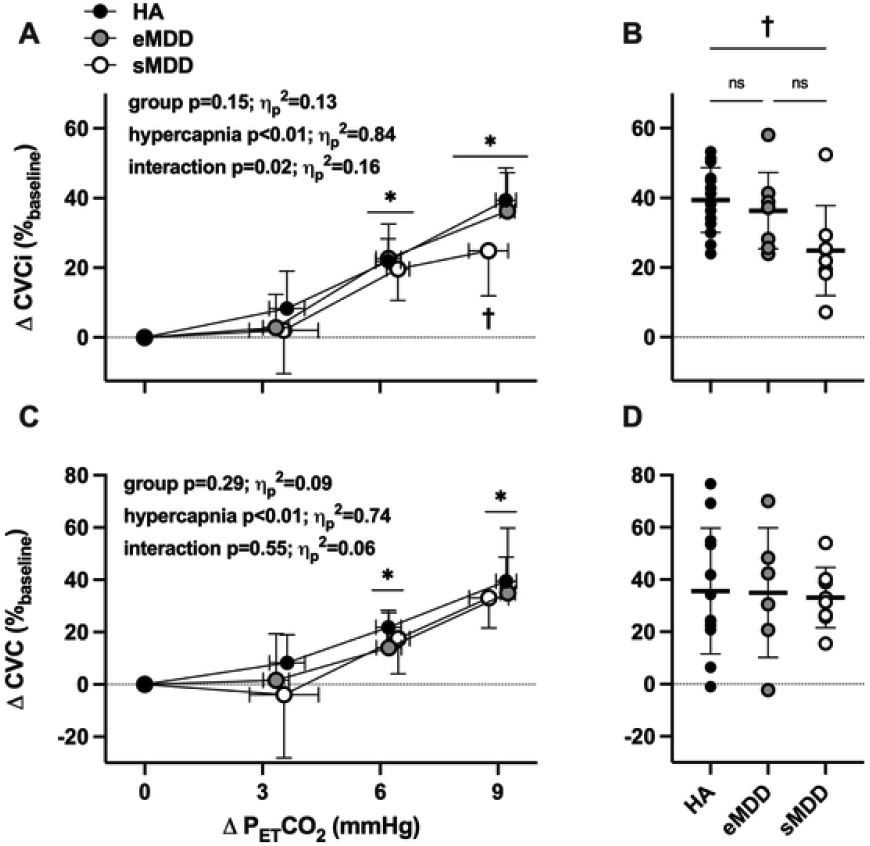
The relative increase in cerebrovascular conductance index (CVCi; Panel A) and cerebrovascular conductance (CVC; Panel C) during rebreathing-induced increases in end-tidal carbon dioxide (PETCO2) in healthy non-depressed adults (HA; n = 14; 9 women), adults with major depressive disorder in remission (euthymic; eMDD; n = 8; 5 women), and adults with symptomatic MDD (sMDD; n = 8; 6 women). The absolute breath-by-breath increase in PETCO2 throughout rebreathing was determined for each individual and presented at each 3 mmHg increase in PETCO2 up to, and including, the highest common magnitude of hypercapnia achieved by all subjects (ΔPETCO2 = 9 mmHg). Outcome variables were analyzed using two-way (group x. hypercapnia) mixed-model ANOVA, with post hoc corrections (Tukey) applied for specific planned comparisons when appropriate. Individual data at ΔPETCO2 = 9 mmHg for each are shown in Panels B and D. ns, not significant. Effect sizes are reported as partial eta squared (ηp2). *p<0.05 vs. baseline; †p<0.05 vs. HA.
4. Discussion
The primary novel finding of this study is that the relative increase in cerebral vascular conductance in the MCA, but not the ICA, during rebreathing-induced hypercapnia was blunted in otherwise healthy non-medicated young adults with MDD. Further, hypercapnia-induced increases in CVCi were attenuated in adults with sMDD, but not in adults with eMDD, compared to HA. There was no association between current depressive symptomology and ICA responsiveness. These findings add to the growing body of literature linking mood disorders to alterations in cerebrovascular function and suggest a potential mechanistic link between the neurobiology of depressive symptomology and cerebrovascular vasodilatory capacity in young adults with MDD. In addition, these data lend additional preliminary support to the concept that the management of depressive symptomology may secondarily confer vasculoprotective effects.
There is a large body of evidence for impaired cerebral blood flow regulation at rest and in response to vasodilatory stimuli in middle-aged and older adults with depressive disorders (de Castro et al., 2008; Direk et al., 2012; Dotson et al., 2009; Greenstein et al., 2010; Lemke et al., 2010; Neu et al., 2004; Ota et al., 2014; Paranthaman et al., 2010; Taylor et al., 2013; Tiemeier et al., 2002). In these studies, administration of acetazolamide, an inhibitor of carbonic anhydrase was used to increase PaCO2 secondary to the accumulation of carbonic acid, thus acting as a pharmacological stimulus for cerebrovascular vasodilation (Leaf and Goldfarb, 2007); MCAv was concurrently measured using TCD (de Castro et al., 2008; Lemke et al., 2010; Neu et al., 2004). Confirming and extending these findings, the present investigation demonstrates blunted MCA vascular responsiveness to a physiological stimulus in young adults with MDD compared to non-depressed adults. Importantly, rebreathing-induced alterations in respiratory or blood pressure responses were not different between groups. Because impairments in cerebrovascular reactivity have been linked to cognitive decline (Sabayan et al., 2012; Yang et al., 2017), we also assessed cognitive function. In the present study, blunted hypercapnia-induced increases in CVCi were noted in the absence of any detectable decline in cognitive function. Although this is perhaps not surprising given that majority of participants were enrolled in undergraduate and graduate degree programs at a university, these data have potential implications for the temporal association between alterations in cerebrovascular function and the development of cognitive impairment. Future studies more specifically investigating neurovascular coupling (e.g., the cerebrovascular response to a cognitive stimulus) in adults with MDD are warranted.
To date, no studies have examined ICA vasodilatory capacity in response to rebreathing-induced hypercapnia in adults with MDD. In the present investigation, we demonstrated a significant increase in ICA blood flow and conductance, without any changes in vessel diameter, in young adults both with and without MDD. The magnitude of this increase (~30%) was largely consistent with reported ICA responsiveness to steady-state hypercapnia (+9 mmHg PETCO2) (Coverdale et al., 2015; Hoiland et al., 2016; Willie et al., 2012). An increase in ICA blood flow without an increase in diameter is thought to reflect the delay between the onset of ICA shear stress and diameter change, which can occur upwards of 2 min after hypercapnia onset, as well as the relatively small shear rate stimulus (Carr et al., 2020; Carter et al., 2016). Interestingly, ICA vasodilation was not different between groups, even at the highest degree of hypercapnia. This was a somewhat unexpected finding, given the blunted response observed in the MCA. Although the reason(s) for these differences between vessels are not readily apparent, one potential interpretation of these data is that regional blood flow distribution within the cerebral vascular network is altered in adults with depression. Although the MCA is the largest terminal branch of the ICA, the ICA also feeds the ophthalmic artery and anterior cerebral artery (ACA) (Charlick and J, 2021). It is therefore plausible that blood flow to the ophthalmic artery and ACA is greater, with concomitant reductions in blood flow in the MCA, in adults with depression. Providing some support for this concept are studies that have used arterial spin labeling magnetic resonance imaging (MRI) to measure regional cerebral blood flow in adults with depression (Lui et al., 2009; Ota et al., 2014). These studies report hypoperfusion in the prefrontal area and anterior cingulate cortices in adults with depression, despite a preservation in global cerebral flow (Lui et al., 2009; Ota et al., 2014). Importantly, the MCA supplies both the frontal and temporal lobes. The data presented here, therefore, may be broadly consistent with, and supportive of, the literature demonstrating region-specific alterations in cerebral vascular regulation in depression.
There is some evidence in middle-aged adults with MDD that cerebrovascular reactivity is improved following remission of depressive symptoms (de Castro et al., 2008; Lemke et al., 2010; Neu et al., 2004). Using a longitudinal approach, Lemke et al. (Lemke et al., 2010) measured acetazolamide-induced increases in MCAv in middle-aged (~43 yrs) patients in an acute episode of depressive illness and again 21 months under euthymic conditions secondary to treatment with antidepressant, antipsychotic, and mood stabilizing medications; a healthy non-depressed group was investigated at comparable time reports. These authors report a reduction in cerebrovascular reactivity in acutely depressed patients and improved responsiveness following treatment and remission. Although it is challenging to isolate the effect of mood improvement from the potential effects of pharmacological treatment on cerebrovascular function in these studies (de Castro et al., 2008; Lemke et al., 2010; Neu et al., 2004), these results nevertheless provide compelling support for an important role for current depressive symptomology in driving blunted cerebrovascular reactivity.
To begin to address whether active depressive symptomology is associated with cerebrovascular dilatory responsiveness to a physiological stimulus in young non-medicated adults with MDD, we conducted ancillary analyses assessing cerebrovascular reactivity separately in adults with eMDD and adults with sMDD. Using this cross-sectional approach, we report blunted hypercapnia-induced increases in relative CVCi in adults with sMDD compared to both adults with eMDD and HA; responsiveness was not different between adults with eMDD and HA. There were no differences in ICA vasodilatory responsiveness between groups. Interestingly, cerebrovascular reactivity was not related to depressive symptom severity, suggesting that current depressive symptomology influences vascular function, regardless of the perceived severity of the current episode. These data are broadly consistent with the studies outlined above and potentially implicate non-pharmacological strategies for the management of depressive symptoms as secondarily preserving vascular health in young adults with MDD.
5. Perspectives
The present findings demonstrate blunted cerebrovascular reactivity in the MCA, but not the ICA, in young, otherwise healthy non-medicated adults with MDD. Further, attenuated cerebral vasodilatory responsiveness was evident in adults with sMDD but not in adults with eMDD. Collectively, these data suggest that cerebrovascular function is altered early in the course of depression and provide an experimental basis for conducting interventional studies targeting the management of depressive symptomology as a means to improve cerebrovascular health and reduce the risk of cerebrovascular disease and cognitive impairment in adults with clinical depression. Importantly, the association between cerebrovascular function and depressive symptoms is bidirectional. That is, there is evidence that cerebrovascular disease may predispose, precipitate, or perpetuate some depressive diseases in the elderly (Alexopoulos et al., 1997). Based on this, it is tempting to speculate that blunted cerebrovascular reactivity, as an index of cerebral vascular health, could also conceivably contribute to the pathogenesis of depression in young adults via its effects on structural and functional neurocircuitry. This intriguing possibility, which could have profound clinical implications, warrants future investigation.
Acknowledgements
We greatly appreciate the effort expended by the volunteer participants. We thank Jeremiah Campbell, MS, Zachary Martin, MS, Sherri Pham, BS, and Salwa Shoaid, BS for their assistance with data acquisition.
7. Funding
This work was supported by National Institutes of Health (NIH) awards HL133414 and MH123928 (JLG).
Footnotes
Declaration of Competing Interest
None.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 2013. 5th ed. American Psychiatric Association, Arlington, VA. [Google Scholar]
- Akins JD, Curtis BM, Patik JC, Olvera G, Nasirian A, Campbell JC, Shiva S, Brothers RM, 2021. Blunted hyperemic response to mental stress in young, non-Hispanic black men is not impacted by acute dietary nitrate supplementation. J. Appl. Physiol 130 (1985), 1510–1521. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M, 1997. Vascular depression’ hypothesis. Arch. Gen. Psychiatry 54, 915–922. [DOI] [PubMed] [Google Scholar]
- Babakhanyan I, McKenna BS, Casaletto KB, Nowinski CJ, Heaton RK, 2018. National Institutes of Health Toolbox Emotion Battery for English- and Spanish-speaking adults: normative data and factor-based summary scores. Patient Relat. Outcome Meas. 9, 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa TC, Kaur J, Stephens BY, Akins JD, Keller DM, Brothers RM, Fadel PJ, 2018. Attenuated forearm vascular conductance responses to rhythmic handgrip in young African-American compared with Caucasian-American men. Am. J. Physiol. Heart Circ. Physiol 315, H1316–H1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont JL, Havlik R, Cook KF, Hays RD, Wallner-Allen K, Korper SP, Lai JS, Nord C, Zill N, Choi S, Yost KJ, Ustsinovich V, Brouwers P, Hoffman HJ, Gershon R, 2013. Norming plans for the NIH Toolbox. NeurologyNeurology 80, S87–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beishon L, Haunton VJ, Panerai RB, Robinson TG, 2017. Cerebral hemodynamics in mild cognitive impairment: a systematic review. J. Alzheimers Dis 59, 369–385. [DOI] [PubMed] [Google Scholar]
- Brody DJ, Pratt LA, Hughes JP, 2018. Prevalence of depression among adults aged 20 and over: United States. 2013-2016. NCHS Data Brief 1–8. [PubMed] [Google Scholar]
- Cantin S, Villien M, Moreaud O, Tropres I, Keignart S, Chipon E, Le Bas JF, Warnking J, Krainik A, 2011. Impaired cerebral vasoreactivity to CO2 in Alzheimer’s disease using BOLD fMRI. Neuroimage 58, 579–587. [DOI] [PubMed] [Google Scholar]
- Carr J, Hoiland RL, Caldwell HG, Coombs GB, Howe CA, Tremblay JC, Green DJ, Ainslie PN, 2020. Internal carotid and brachial artery shear-dependent vasodilator function in young healthy humans. J. Physiol 598, 5333–5350. [DOI] [PubMed] [Google Scholar]
- Carter HH, Atkinson CL, Heinonen IH, Haynes A, Robey E, Smith KJ, Ainslie PN, Hoiland RL, Green DJ, 2016. Evidence for Shear Stress-Mediated Dilation of the Internal Carotid Artery in Humans. Hypertension 68, 1217–1224. [DOI] [PubMed] [Google Scholar]
- Charlick M, J MD, 2021. Anatomy, Head and Neck, Internal Carotid Arteries. StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Choi SW, Schalet B, Cook KF, Cella D, 2014. Establishing a common metric for depressive symptoms: linking the BDI-II, CES-D, and PHQ-9 to PROMIS depression. Psychol. Assess 26, 513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen JA, Zhang R, Fu Q, Witkowski S, Levine BD, 2007. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J. Appl. Physiol 102 (1985), 870–877. [DOI] [PubMed] [Google Scholar]
- Cooper DC, Milic MS, Tafur JR, Mills PJ, Bardwell WA, Ziegler MG, Dimsdale JE, 2010. Adverse impact of mood on flow-mediated dilation. Psychosom. Med 72, 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coverdale NS, Lalande S, Perrotta A, Shoemaker JK, 2015. Heterogeneous patterns of vasoreactivity in the middle cerebral and internal carotid arteries. Am. J. Physiol. Heart Circ. Physiol 308, H1030–H1038. [DOI] [PubMed] [Google Scholar]
- de Castro AG, Bajbouj M, Schlattmann P, Lemke H, Heuser I, Neu P, 2008. Cerebrovascular reactivity in depressed patients without vascular risk factors. J. Psychiatr. Res 42, 78–82. [DOI] [PubMed] [Google Scholar]
- Direk N, Koudstaal PJ, Hofman A, Ikram MA, Hoogendijk WJ, Tiemeier H, 2012. Cerebral hemodynamics and incident depression: the Rotterdam Study. Biol. Psychiatry 72, 318–323. [DOI] [PubMed] [Google Scholar]
- Dotson VM, Beason-Held L, Kraut MA, Resnick SM, 2009. Longitudinal study of chronic depressive symptoms and regional cerebral blood flow in older men and women. Int. J. Geriatr. Psychiatry 24, 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedorowicz JG, Ellingrod VL, Kaplan MJ, Sen S, 2015. The development of depressive symptoms during medical internship stress predicts worsening vascular function. J. Psychosom. Res 79, 243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney JL, Dillon GA, Saunders EFH, Alexander LM, 2020. Peripheral microvascular serotoninergic signaling is dysregulated in young adults with major depressive disorder. J. Appl. Physiol 128, 100–107. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney JL, Koffer RE, Saunders EFH, Almeida DM, Alexander LM, 2019a. Self-reported everyday psychosocial stressors are associated with greater impairments in endothelial function in young adults with major depressive disorder. J. Am. Heart Assoc 8, e010825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaney JL, Saunders EFH, Santhanam L, Alexander LM, 2019b. Oxidative stress contributes to microvascular endothelial dysfunction in men and women with major depressive disorder. Circ. Res 124, 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein AS, Paranthaman R, Burns A, Jackson A, Malik RA, Baldwin RC, Heagerty AM, 2010. Cerebrovascular damage in late-life depression is associated with structural and functional abnormalities of subcutaneous small arteries. Hypertension 56, 734–740. [DOI] [PubMed] [Google Scholar]
- Hoiland RL, Fisher JA, Ainslie PN, 2019. Regulation of the cerebral circulation by arterial carbon dioxide. Compr. Physiol 9, 1101–1154. [DOI] [PubMed] [Google Scholar]
- Hoiland RL, Tymko MM, Bain AR, Wildfong KW, Monteleone B, Ainslie PN, 2016. Carbon dioxide-mediated vasomotion of extra-cranial cerebral arteries in humans: a role for prostaglandins?. J. Physiol 594, 3463–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurr C, Harrison ML, Brothers RM, 2015a. Acute flavanol consumption improves the cerebral vasodilatory capacity in college-aged African Americans. Exp. Physiol 100, 1030–1038. [DOI] [PubMed] [Google Scholar]
- Hurr C, Kim K, Harrison ML, Brothers RM, 2015b. Attenuated cerebral vasodilatory capacity in response to hypercapnia in college-aged African Americans. Exp. Physiol 100, 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurr C, Patik JC, Kim K, Brothers RM, 2017. Blunted cerebral vascular responsiveness to hypercapnia in obese individuals. Exp. Physiol 102, 1300–1308. [DOI] [PubMed] [Google Scholar]
- Ide K, Eliasziw M, Poulin MJ, 2003. Relationship between middle cerebral artery blood velocity and end-tidal PCO2 in the hypocapnic-hypercapnic range in humans. J. Appl. Physiol 95 (1985), 129–137. [DOI] [PubMed] [Google Scholar]
- Kaur J, Barbosa TC, Nandadeva D, Young BE, Stephens BY, Brothers RM, Fadel PJ, 2021. Attenuated Rapid-Onset Vasodilation to Forearm Muscle Contraction in Black Men. Med. Sci. Sports Exerc 53, 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, 2001. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med 16, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaf DE, Goldfarb DS, 2007. Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J. Appl. Physiol 102 (1985), 1313–1322. [DOI] [PubMed] [Google Scholar]
- Lemke H, de Castro AG, Schlattmann P, Heuser I, Neu P, 2010. Cerebrovascular reactivity over time-course - from major depressive episode to remission. J. Psychiatr. Res 44, 132–136. [DOI] [PubMed] [Google Scholar]
- Lui S, Parkes LM, Huang X, Zou K, Chan RC, Yang H, Zou L, Li D, Tang H, Zhang T, Li X, Wei Y, Chen L, Sun X, Kemp GJ, Gong QY, 2009. Depressive disorders: focally altered cerebral perfusion measured with arterial spin-labeling MR imaging. Radiology 251, 476–484. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Mann JJ, 2018. Depression. Lancet 392, 2299–2312. [DOI] [PubMed] [Google Scholar]
- Markus H, Cullinane M, 2001. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 124, 457–467. [DOI] [PubMed] [Google Scholar]
- Monkul ES, Silva LA, Narayana S, Peluso MA, Zamarripa F, Nery FG, Najt P, Li J, Lancaster JL, Fox PT, Lafer B, Soares JC, 2012. Abnormal resting state corticolimbic blood flow in depressed unmedicated patients with major depression: a (15)O-H(2)O PET study. Hum. Brain Mapp 33, 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu P, Schlattmann P, Schilling A, Hartmann A, 2004. Cerebrovascular reactivity in major depression: a pilot study. Psychosom. Med 66, 6–8. [DOI] [PubMed] [Google Scholar]
- Ota M, Noda T, Sato N, Hattori K, Teraishi T, Hori H, Nagashima A, Shimoji K, Higuchi T, Kunugi H, 2014. Characteristic distributions of regional cerebral blood flow changes in major depressive disorder patients: a pseudo-continuous arterial spin labeling (pCASL) study. J. Affect. Disord 165, 59–63. [DOI] [PubMed] [Google Scholar]
- Paranthaman R, Greenstein AS, Burns AS, Cruickshank JK, Heagerty AM, Jackson A, Malik RA, Scott ML, Baldwin RC, 2010. Vascular function in older adults with depressive disorder. Biol. Psychiatry 68, 133–139. [DOI] [PubMed] [Google Scholar]
- Patik JC, Tucker WJ, Curtis BM, Nelson MD, Nasirian A, Park S, Brothers RM, 2018. Fast-food meal reduces peripheral artery endothelial function but not cerebral vascular hypercapnic reactivity in healthy young men. Physiol. Rep 6, e13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Brook R, Rubenfire M, Pitt E, Young E, Pitt B, 2001. Abnormal brachial artery flow-mediated vasodilation in young adults with major depression. Am. J. Cardiol 88, 196–198. A197. [DOI] [PubMed] [Google Scholar]
- Ringelstein EB, Sievers C, Ecker S, Schneider PA, Otis SM, 1988. Noninvasive assessment of CO2-induced cerebral vasomotor response in normal individuals and patients with internal carotid artery occlusions. Stroke 19, 963–969. [DOI] [PubMed] [Google Scholar]
- Sabayan B, Jansen S, Oleksik AM, van Osch MJ, van Buchem MA, van Vliet P, de Craen AJ, Westendorp RG, 2012. Cerebrovascular hemodynamics in Alzheimer’s disease and vascular dementia: a meta-analysis of transcranial Doppler studies. Ageing Res. Rev 11, 271–277. [DOI] [PubMed] [Google Scholar]
- Salsman JM, Butt Z, Pilkonis PA, Cyranowski JM, Zill N, Hendrie HC, Kupst MJ, Kelly MA, Bode RK, Choi SW, Lai JS, Griffith JW, Stoney CM, Brouwers P, Knox SS, Cella D, 2013. Emotion assessment using the NIH Toolbox. Neurology 80, S76–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalet BD, Pilkonis PA, Yu L, Dodds N, Johnston KL, Yount S, Riley W, Cella D, 2016. Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. J. Clin. Epidemiol 73, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 (Suppl 20), 22–33. quiz 34-57. [PubMed] [Google Scholar]
- Smolinski L, Czlonkowska A, 2016. Cerebral vasomotor reactivity in neurodegenerative diseases. Neurol. Neurochir. Pol 50, 455–462. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, 1999. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 282, 1737–1744. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS, 2013. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol. Psychiatry 18, 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier H, Bakker SL, Hofman A, Koudstaal PJ, Breteler MM, 2002. Cerebral haemodynamics and depression in the elderly. J. Neurol. Neurosurg. Psychiatry 73, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge JM, Cooper AB, Joiner TE, Duffy ME, Binau SG, 2019. Age, period, and cohort trends in mood disorder indicators and suicide-related outcomes in a nationally representative dataset, 2005-2017. J. Abnorm. Psychol 128, 185–199. [DOI] [PubMed] [Google Scholar]
- Vicenzini E, Ricciardi MC, Altieri M, Puccinelli F, Bonaffini N, Di Piero V, Lenzi GL, 2007. Cerebrovascular reactivity in degenerative and vascular dementia: a transcranial Doppler study. Eur. Neurol 58, 84–89. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K, Fox NA, Beaumont JL, Mungas D, Nowinski CJ, Richler J, Deocampo JA, Anderson JE, Manly JJ, Borosh B, Havlik R, Conway K, Edwards E, Freund L, King JW, Moy C, Witt E, Gershon RC, 2013. Cognition assessment using the NIH Toolbox. Neurology 80, S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T, 2013. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382, 1575–1586. [DOI] [PubMed] [Google Scholar]
- Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA, Ainslie PN, 2012. Regional brain blood flow in man during acute changes in arterial blood gases. J. Physiol 590, 3261–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Sun Y, Lu Z, Leak RK, Zhang F, 2017. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res. Rev 34, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]


