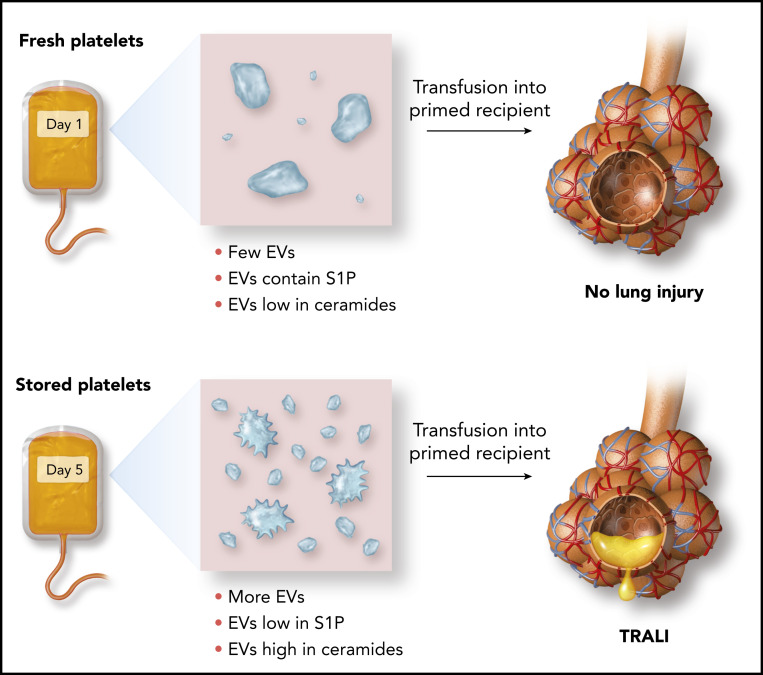
In this issue of Blood, McVey et al 1 identify storage-induced disturbances, specifically increased frequency of platelet extracellular vesicles (EVs), and sphingolipid imbalances in EVs, which together predispose to transfusion-related acute lung injury (TRALI).
Effect of storage on the release of platelet EVs and their ceramide and S1P content and susceptibility to TRALI.
Blood transfusions are life-saving interventions, but they occasionally harm recipients by causing a variety of transfusion reactions. In TRALI, the transfused blood product triggers an inflammatory response in the pulmonary vasculature of the recipient. The potentially fatal consequence is acute respiratory failure due to fluid gushing out of the bloodstream and into the lungs through leaks in the endothelial wall. 2
Recipient-reactive alloantibodies in blood products are a major cause of TRALI, and reducing the quantity of these pathogenic antibodies in plasma-rich blood products has been a successful strategy for reducing TRALI incidence. 3 However, transfusions continue to cause TRALI, sometimes in the absence of detectable alloantibodies in the blood product. Indeed, in a prospective study on the incidence of TRALI and its risk factors, the presence of alloantibodies explained only about one-half of the identified TRALI cases. 4
The suspected causative agents of “non-antibody-mediated” TRALI cases are bioactive lipids, which may be suspended in the plasma or storage medium of cellular blood products in the form of EVs. 5 During storage in blood banks, these fat-laden EVs are released from both red blood cells and platelets. Recent studies have identified that EVs arising from “storage lesions” likely act as shuttles, which traffic bioactive lipids to the pulmonary endothelium to cause TRALI. However, compared with antibody-mediated TRALI, the mechanistic basis of lung injury provoked by EVs and bioactive lipids has been more elusive.
Here, McVey and colleagues identify an EV lipid imbalance pathway through which transfusions of platelets approaching their “use by date” can lead to pulmonary endothelial injury. During storage, the quantity of EVs released from mouse or human platelets increases. Over a period of several days, the balance of sphingolipids contained in these platelet EVs shifts, with an increase in long-chain ceramides and a decrease in sphingosine-1-phosphate (S1P) (see figure). Because ceramides can cause pulmonary endothelial injury and S1P has endothelial barrier protective properties, this switch in the EV “sphingolipid rheostat” is a plausible mechanism for TRALI that results from the transfusion of aged platelets.
Using a 2-hit mouse model of stored platelet-mediated TRALI in combination with studies of human platelets and endothelial cells, McVey et al also demonstrate approaches to prevent this potentially harmful outcome of platelet transfusions. Endothelial injury caused by stored platelets could be reduced by: (a) washing platelets to remove EVs, (b) preventing ceramide-dependent EV production by blocking its production from sphingomyelin by acid sphingomyelinase, and (c) adding exogenous S1P to counteract loss of S1P from aged platelet EVs. Of these approaches, washing of stored platelets is potentially attractive, as washing is occasionally done in clinical practice to mitigate certain severe, nonhemolytic transfusion reactions, and clinical trials are already underway to test whether washing red blood cells can reduce incidence of transfusion reactions. 6 However, platelets are highly sensitive to manipulation, so the difficulty of safely exchanging platelet buffer solutions may make implementation of this strategy challenging.
As demonstrated in this article, platelet-derived signaling moieties can cause lung injury, but platelets can also play important roles in supporting the integrity of the pulmonary endothelial barrier. 7 Further research into the generation of platelet EVs and the regulation of their lipid composition may help explain this apparent paradox in platelet function. Conceptual advances are needed to answer the question of exactly how ceramides lead to endothelial barrier disruption in vivo, as unlike S1P, ceramides do not appear to signal through classical receptor-ligand interactions and instead relay information through modulating the assembly of lipid rafts. 8
Recent reports have demonstrated that platelet EVs infiltrate the lymphatic system and bone marrow during inflammation. 9,10 Given the effects identified by McVey et al in this study of EVs from stored platelets, it seems likely that the production of platelet EVs and the balance of their sphingolipid cargo may also have other important roles to play in settings beyond transfusion biology.
Supplementary Material
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1. McVey MJ, Weidenfeld S, Maishan M, et al. Platelet extracellular vesicles mediate transfusion-related acute lung injury by imbalancing the sphingolipid rheostat. Blood. 2021;137(5):690-701. [DOI] [PubMed] [Google Scholar]
- 2. Vlaar APJ, Toy P, Fung M, et al. A consensus redefinition of transfusion-related acute lung injury. Transfusion. 2019;59(7):2465-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters AL, Van Stein D, Vlaar AP. Antibody-mediated transfusion-related acute lung injury; from discovery to prevention. Br J Haematol. 2015;170(5):597-614. [DOI] [PubMed] [Google Scholar]
- 4. Toy P, Gajic O, Bacchetti P, et al; TRALI Study Group . Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119(7):1757-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silliman CC, Paterson AJ, Dickey WO, et al. The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion. 1997;37(7):719-726. [DOI] [PubMed] [Google Scholar]
- 6. Warner MA, Welsby IJ, Norris PJ, et al. Point-of-care washing of allogeneic red blood cells for the prevention of transfusion-related respiratory complications (WAR-PRC): a protocol for a multicenter randomised clinical trial in patients undergoing cardiac surgery. BMJ Open. 2017;7(8):e016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Middleton EA, Weyrich AS, Zimmerman GA. Platelets in pulmonary immune responses and inflammatory lung diseases. Physiol Rev. 2016;96(4):1211-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simmons S, Erfinanda L, Bartz C, Kuebler WM. Novel mechanisms regulating endothelial barrier function in the pulmonary microcirculation. J Physiol. 2019;597(4):997-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tessandier N, Melki I, Cloutier N, et al. Platelets disseminate extracellular vesicles in lymph in rheumatoid arthritis. Arterioscler Thromb Vasc Biol. 2020;40(4):929-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. French SL, Butov KR, Allaeys I, et al. Platelet-derived extracellular vesicles infiltrate and modify the bone marrow during inflammation. Blood Adv. 2020;4(13):3011-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.